Abstract
Lassa virus (LASV), the causative agent of Lassa fever (LF), was first identified in 1969. Since then, outbreaks in the endemic countries of Nigeria, Liberia, and Sierra Leone occur on an annual basis resulting in a case-fatality rate of 15-70% in hospitalized patients. There is currently no licensed vaccine and there are limited animal models to test vaccine efficacy. An estimated 37.7 million people are at risk of contracting LASV; therefore, there is an urgent need for the development of a safe, effective vaccine against LASV infection. The LF endemic countries are also inflicted with HIV, Ebola, and malaria infections. The safety in immunocompromised populations must be considered in LASV vaccine development. The novel adenovirus vector-based platform, Ad5 (E1-,E2b-) has been used in clinical trial protocols for treatment of immunocompromised individuals, has been shown to exhibit high stability, low safety risk in humans, and induces a strong cell-mediated and pro-inflammatory immune response even in the presence of pre-existing adenovirus immunity. To this nature, our lab has developed an Ad5 (E1-,E2b-) vector-based vaccine expressing the LASV-NP or LASV-GPC. We found that guinea pigs vaccinated with two doses of Ad5 (E1-,E2b-) LASV-NP and Ad5 (E1-,E2b-) LASV-GPC were protected against lethal LASV challenge. The Ad5 (E1-,E2b-) LASV-NP and LASV-GPC vaccine represents a potential vaccine candidate against LF.
Keywords: Lassa virus, Lassa fever, vaccine, adenovirus vector
INTRODUCTION
Lassa virus (LASV), a member of the Arenavirdae family, is the causative agent of Lassa fever (LF) [1]. LASV is endemic in West Africa and the hospitalized case-fatality rate ranges from 15-70% depending on the outbreak [2-5]. After a 2 to 21day incubation period, LF begins with flu like symptoms before progressing to more severe symptoms such as facial edema, high fever, bleeding from mucosal and gastrointestinal tracts. Death is proceeded by shock and coma and typically occurs 14 days after the onset of symptoms [6,7]. The antiviral Ribavirin has been shown to be effective when administered early, but is not readily available in high incidence areas [8]. Approximately one-third of Lassa fever survivors develop sensorineural hearing loss which is often permanent [9]. An estimated 37.7 million people are at risk of contracting LASV, therefore, the development of a safe and effective vaccine is a crucial medical need [10].
Arenaviruses, including LASV, have bi-segmented negative single stranded RNA as their genomes. Each segment contains 2 open-reading frames of viral proteins in ambisense manner. Large (L)-segment codes RNA-dependent RNA polymerase (L) and Z protein, which plays a role of matrix protein in other enveloped-RNA viruses. Small (S)-segment codes nucleoprotein (NP), which is the most abundant protein in arenavirus infected cells, and glycoprotein precursor (GPC) [1]. GPC is cleaved into stable signal peptide (SSP), GP1 and GP2 [11,12]. GPC is the only membrane-anchored surface protein of arenaviruses and, therefore, the main target of neutralizing antibodies [13-15].
The immune response in severe and fatal cases of LF is considered immunosuppressive [16,17]. LASV primary targets are antigen-presenting cells (APCs) such as dendritic cells and macrophages. LASV infects APCs and fails to activate the cells resulting in a diminished T-cell response [18]. Neutralizing antibodies are not induced in fatal LF cases or survivors’ until the convalescence stage [16,17,19]. Lassa fever survivors are able to produce a strong, early T cell response [20]. LASV NP or GPC-specific T-cells have been detected in LF survivors many years after infection [21-23]. Therefore, a vaccine that elicits not only antibodies but also a robust LASV-specific T-cell response is ideal.
The non-replicative adenovirus serotype 5 vector [Ad5 (E1-, E2b-)], which has deletions in the early 1 (E1), early 2b (E2b) and early 3 (E3) gene regions, has been shown to be safe and well tolerated in human clinical trials even in the presence of pre-existing immunity [24-28]. The novel Ad5 (E1-, E2b-) vector is able to infect dendritic cells resulting in the upregulation of costimulatory molecules and the presentation of antigens to the immune response. This ultimately leads to the induction of B-cells and T-cells against the transgene expressed protein resulting in the production of antibodies and specific-T-cell responses [29,30]. Additionally, the LF endemic countries are also inflicted with human immunodeficiency virus (HIV), human papilloma virus, tuberculosis, Ebola virus and malaria infections [16,31-34]. Safety in immunocompromised populations should be considered in LASV vaccine development. The adenovirus vector-based platform, Ad5 (E1-,E2b-) has been used in immunocompromised individuals, has been shown to exhibit high stability, low safety risk in humans, and induce a strong cell-mediated and pro-inflammatory immune responses even in the presence of pre-existing adenovirus immunity [24,30,35]. Therefore, based on the need for an effective T-cell response and the safety of the Ad5 (E1-, E2b-) vector, we have utilized this novel gene delivery Ad5 vector platform to develop a vaccine against LF [16,17,20]. Single-antigen vector vaccines expressing either the LASV GPC or LASV NP were constructed. In a lethal LF guinea pig challenge model, vaccination with both the vaccines were shown to be protective.
MATERIALS AND METHODS
Cells and viruses.
Vero, Vero E6, and E.C7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and L-glutamine. LASV strain LF2384, which was isolated from a fatal LF case during a 2012 outbreak in Sierra Leone, was propagated in Vero cells and virus-containing cell culture supernatant was stored in an −80°C freezer until use [36,37]. All work with infectious LASV was performed in biosafety level 4 (BSL-4) facility in Galveston National Laboratory (GNL), The University of Texas Medical Branch (UTMB) in accordance with institutional guidelines.
Ad5 (E1-, E2b-) vector based vaccine expressing LASV-GPC or -NP
The Ad5 (E1-, E2b-) was generated as previously described [27-29,38,39]. The LASV NP or LASV GPC single vectored vaccines were constructed utilizing the GPC and NP gene sequence of LASV Josiah strain. Purified Ad5 (E1-, E2b-) LASV-GPC or -NP infectious unit (IU) was determined by Foci forming unit (FFU) assay on E.C7 cell monolayers. Briefly, 100μl of 10-fold dilution of viral stock with 10% FBS DMEM was inoculated into monolayer of E.C7 cells in 24-well plates, and incubated in a CO2 incubator for 1 hour at 37°C. After washing inoculum out, 10% FBS DMEM was added to each well. The cells were fixed with 100% methanol 40-48hpi. The goat anti-hexon-HRP conjugated antibody (1:500) (Pierce) was incubated on cells for 1 hour at 37°C then stained using the ImmPACT DAB Peroxidase Substrate Kit (Vector) according to manufactures instructions.
Western Blot
Western blot analysis was performed at previously described [40]. Briefly, E.C7 cells were transfected with the Ad5 (E1-, E2b-) GPC or NP constructs using X-tremeGene 9 (Roche). Cell lysates were prepared at 24 hours post-transfection with 2x Laemmli sample buffer (BioRad) with 5% β-Mercaptoethanol then boiled at 95°C for 5 minutes. The protein samples were electrophoresed by SDS-PAGE, and then transferred to PVDF membrane using Mini Trans-Blot Electrophoretic Transfer Cell apparatus according to manufactures instructions (Bio-Rad). The membranes were incubated with the anti-NP monoclonal antibody NA05-AG12 (1:1000) (BEI Resources) or anti-GP-2 monoclonal antibody (1:1000) (ProSci) overnight at 4°C and with appropriate secondary antibodies conjugated with HRP (1:3000) (Cell Signaling) for 1 hour at room temperature. Proteins were visualized with ECL-2 Western Blotting Detection Reagents (Thermo Scientific) according to the manufacturer’s instruction.
Animal experiments
Five- to 7-week-old female Hartley guinea pigs were purchased from Charles River. All animals were housed in ABSL2 and ABSL4 facilities in GNL, UTMB, All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at UTMB and were carried out according to the National Institutes of Health guidelines. Measuring of body temperature and weight were performed with subcutaneously implanted BMDS IPTT-300 transponders and a DAS-6007 transponder reader (Bio Medic Data Systems). Guinea pigs were intramuscularly vaccinated with mixed adenovirus vector expressing LASV GPC or NP (1.0×1010 IU each), or adenovirus vector expressing H1N1 subtype influenza A virus hemagglutinin as control in 100 μl of PBS at 56 and 40 days before challenge. Guinea pigs were intraperitoneally inoculated with 8.0×104 PFU of LASV strain LF2384 in 100 μl of PBS, and monitored daily for 21 days after inoculation. Animals were humanely euthanized once they showed neurological symptom, were not able to access their food or water, or lost more than 15% of their body weight. Blood and tissues (brain, lung, liver, spleen, and kidney) were collected for virological and pathological study.
Enzyme-linked immunosorbent assay (ELISA)
LASV GPC or NP were used for ELISA antigens. GPC or NP open reading frame of LASV Josiah strain were cloned into pCAGGS expression plasmid. The plasmids were transfected into HEK293T cells and the cells were collected at 72 hour-post transfection and washed with PBS. LASV GPC was extracted by Mem-PER Plus Membrane Protein Extraction Kit (Thermo Scientific) according to manufacturer instruction. LASV NP was extracted with cell lysis buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 0.5% Triton X-100) [41]. Empty vector transfected cells were used as negative control. ELISA plates were coated with 1:100 diluted antigens at 4°C overnight. After discarded antigens, plates were blocked with PBS containing 0.05% Tween20 and 3% skim milk for 1 hour at room temperature. After washing with PBS containing 0.05% Tween20 (PBST), plates were incubated with serial diluted sera at 4°C overnight. Plates were washed with PBST 3 times and then 1:10,000 diluted Goat Anti-Guinea pig IgG H&L (HRP) (Abcam) was added and incubated 1 hour at room temperature. After 3 times wash with PBST, the reaction was visualized by adding 3,3’,5,5’-tetramethylbenzidineLiquid Substrate, Supersensitive, for ELISA (Sigma) and stopped with 1M phosphoric acid. The optical density at 450 nm (OD450) was measured and standardized with OD450 of negative control antigens. The cut-off OD value was 0.1.
Detection of neutralizing antibody
Neutralizing antibody was detected by plaque reduction neutralizing test. LASV was diluted with 2% FBS DMEM to yield 80 PFU, and mixed with serial-diluted heat-inactivated serum. After 30 minutes incubation in 37°C, mixture was inoculated into monolayer of Vero cells in 12-well plates, and incubated in a CO2 for 30 minutes at 37°C. After washing inoculum out, MEM with 0.6% Tragacanth (Sigma) and 2% FBS was added as overlay. After incubation for 5-6 days, cells were fixed with 10% formalin and plaques were visualized by crystal violet staining. Antibody titer was presented as 50% plaque reduction titer (PRNT50).
Virus titration
Tissue samples were homogenized by a TissueLyser system (Qiagen) to yield 10% homogenate in Phosphate buffered saline (PBS). Blood was collected in EDTA tubes. Plaque assay was performed to detect virus from tissue homogenates and whole blood samples. Briefly, 100 μl of 10-fold diluted samples with 2% FBS DMEM was inoculated into monolayer of Vero E6 cells in 12-well plates, and incubated in a CO2 incubator for 30 minutes at 37°C. After washing inoculum out, MEM with 0.6% Tragacanth (Sigma) and 2% FBS was added as overlay. After incubation for 5-6 days, cells were fixed with 10% formalin and plaques were visualized by crystal violet staining.
Histology
Tissues were collected at the time of euthanasia and fixed in 10% buffered formalin for at least 21 days. The tissues were then trimmed and embedded in paraffin. Thin sections (5.0 μM) of the brain, liver, lung, spleen, and kidney were stained with hematoxylin and eosin.
Statistical analysis
Statistical analyses were performed with GraphPad Prism Software. The geometric mean of neutralizing antibody titers and statistically significant differences in the mean weight, mean temperature, ELISA data between groups of animals were determined by Student’s t-test (*: p<0.05). Log-rank (Mantel-Cox) test was used for survival curve comparison.
RESULTS
Ad5 (E1-, E2b-) vector based vaccine construction and target protein expression
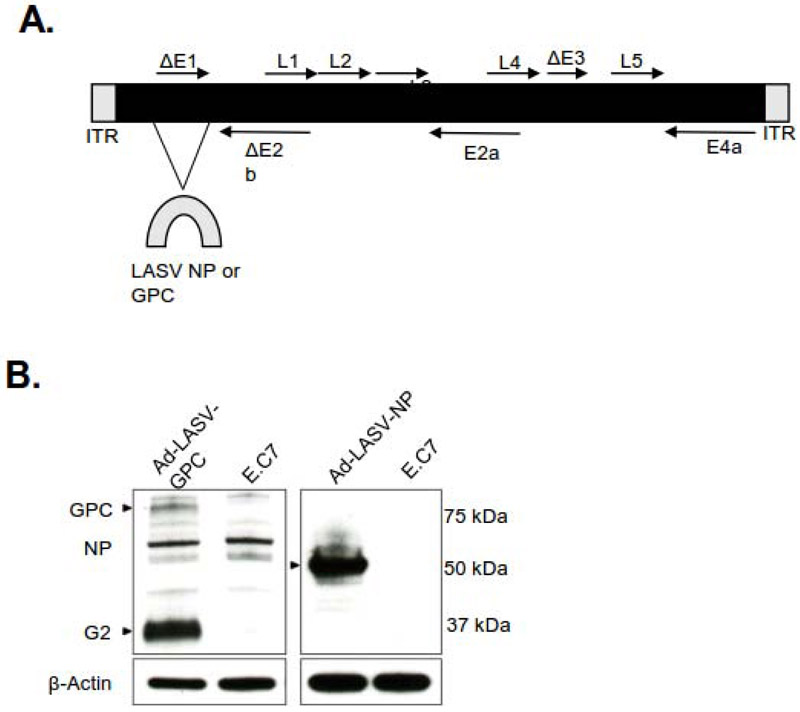
The novel Ad5 (E1-, E2b-) platform has been constructed using various transgenes and tested in clinical trials of patients having colorectal cancer, prostate cancer, HER2 positive breast cancer and has been shown to be absent of Serious Adverse Events (SAE). The Ad5 (E1-, E2b-) carrying various transgenes has been tested in animals for protection against HIV, influenza A virus infection [24,26,27,30,38] and HPV infection (unpublished). The deletion in the structural E1 gene and E2b gene render the vector non-replicative and deletions in the E3 gene allow the vector to be effective even in the presence of pre-existing Ad5 immunity [25,29]. The LASV GPC or LASV NP were inserted into the Ad5 (E1-, E2b-) vector based platform (Figure 1A). The product was then amplified in E.C7 cells, (HEK293 cells) which constitutively express the Ad polymerase and preterminal protein, before undergoing concentration and purification [27-29,38,39]. The protein expression of LASV GPC or NP in E.C7 cells transfected with Ad5 (E1-, E2b-) LASV-GPC or - NP vector were confirmed via western blot (Figure 1B).
Figure 1. Schematic of Ad5 (E1-,E2b-) expressing LASV GPC or NP.
(A) Schematic of the adenovirus genome with mutations in the E1, E3 and E2b domains. (B) Western blotting of GPC and NP.
Vaccination against LASV sing the Ad5 (E1-, E2b-) vaccines
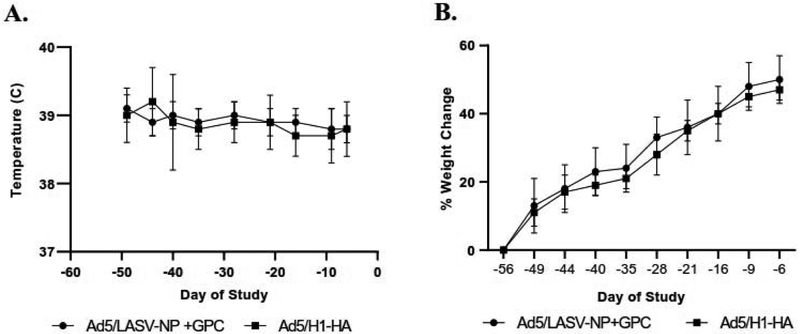
The Ad5 (E1-, E2b-) single vector vaccines expressing LASV NP or LASV GPC were tested in a lethal guinea pig challenge model to determine their efficacy and immunogenicity. According to our experimental design (Figure 2), guinea pigs were immunized twice on days -56 and day -46 with 1×1010 infectious units (IU) of both the single vectored Ad5 (E1-, E2b-) LASV-GPV and - NP vaccines (n=8). As a control, the mock vaccinated group were immunized on day -56 and day -40 with 1×1010 IU of Ad5 (E1-, E2b-)-H1-HA (n=6). None of the guinea pigs showed clinical symptoms nor abnormal change of body temperature or weight after vaccination (Figure 3A and 3B), although transient weight and temperature changes in first 24-72 hours after vaccination were not monitored because the data was not available. There are no significant differences between groups at any time points in body or temperature changes.
Figure 2. Schematic of experimental design.
Schematic of immunization, challenge, and bleeding schedule.
Figure 3. Average temperature and weight change after vaccination.
(A) Average temperature and (B) weight parameters after vaccination and prior to LASV challenge. There are no significant differences between groups at any time points in body weight or temperature changes.
Antibody response after vaccination
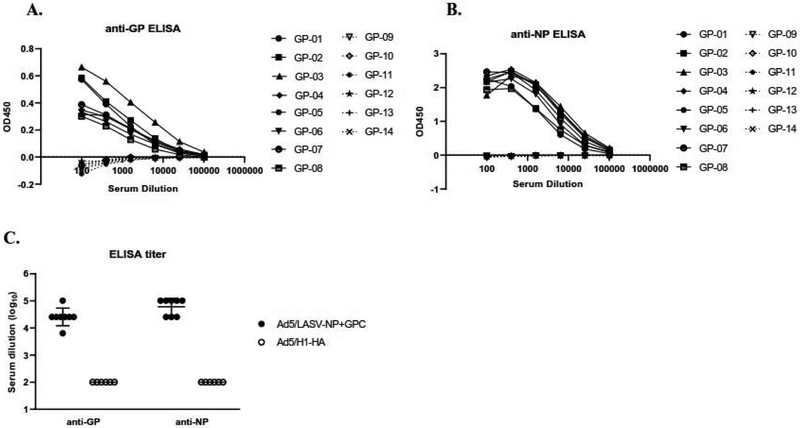
To determine the antibody level after vaccination, enzyme-linked immunosorbent assay (ELISA) against the LASV GP and the LASV NP was performed using sera collected at day -6. All guinea pigs vaccinated with Ad5 (E1-, E2b-) LASV GPC or -NC exhibited antibodies to the GP (Figure 4A) and NP (Figure 4B); whereas, none of the mock-vaccinated group expressed antibodies against LASV. ELISA titer of sera from vaccinated animals against LASV GPC and NP were 1:6400 to 1:102400 and 1:25600 to 1:102400, respectively (Figure 4C).
Figure 4. Non-neutralizing antibody response after vaccination.
The presences of (A) anti-GP, (B) anti-NP antibodies or (C) antibody titers were measured via ELISA from serum collected at Day -6. Statistical analysis between vaccinated and mock group was performed with student’s t-test (p<0.05). (A and B) Solid lines: Ad5 (E1-, E2b-) LASV-GPC and -NP. Broken lines: Ad5 (E1-, E2b-)-H1-HA. (C) Broken line indicated detection limit (1:<100).
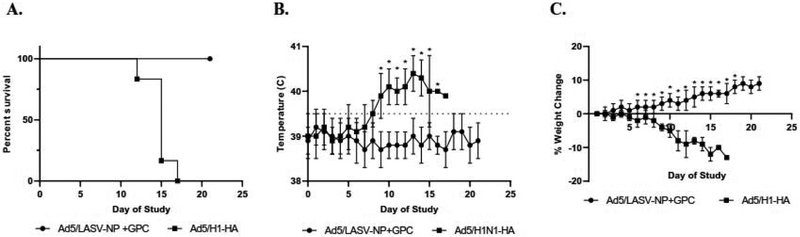
Protection effect of vaccination against LASV challenge
Vaccinated guinea pigs were inoculated with a lethal dose (over 5000 LD50) of the fatal LASV clinical isolate LF2834 [37,42]. All guinea pigs vaccinated with Ad5 (E1-, E2b-) LASV-GPV and -NP survived the challenge and did not exhibit any signs of disease (Fig. 5A). The Ad5 (E1-, E2b-) H1-HA vaccinated animals became febrile, more than 40°C, at 8 days post inoculation (d.p.i.) (Figure 5B) and began losing weight at 6 d.p.i. (Figure 5C). The guinea pigs began developing classic disease symptoms such as lethargy and loss of appetite at 11 d.p.i. Prior to death, guinea pigs developed hypothermia and hind leg paralysis. All animals succumbed to disease at 13 to 17 d.p.i. (Figure 5A). The Ad5 (E1-, E2b-) LASV-GPV and -NP vaccinated guinea pig temperature remained steady throughout the course of the study (Figure 5B) and continued to gain weight after challenge (Figure 5C).
Figure 5. Dynamics of weight and temperature change after lethal LF challenge.
(A) Survival curve of guinea pigs following two doses of Ad5 (E1-,E2b-) LASV GPC and-NP vaccination and lethal LF challenge. (B) The average temperature after challenge. The dotted line represents the limit of normal guinea pig temperature range (*: p<0.05). (C) The average weight change after challenge (*: p<0.05). Survival curve was significantly different by Log-rank (Mantel-Cox) (p<0.001).
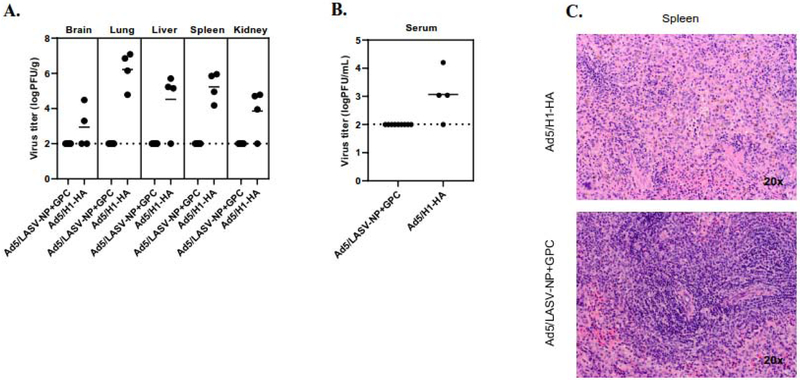
Viral load in LASV-challenged guinea pigs
To assess the dissemination of LASV to organs and viremia after challenge, brain, lung, liver, spleen, kidney, and blood samples were collected from all the Ad5 (E1-,E2b-) LASV-GPC and - NP vaccinated and 4/6 Ad5 (E1-,E2b-) H1-HA vaccinated animals at euthanasia. All guinea pigs vaccinated with Ad5 (E1-, E2b-) LASV-GPC and -NP were euthanized at the end of study (day+21) (Figure 2), and 4/6 guinea pigs vaccinated with Ad5 (E1-, E2b-) H1-HA were euthanized at day+13 and +15 after reaching the humane endpoint criteria (Figure 5A). LASV was readily detected in the spleen and the lung with the highest viral titers in the lung in all Ad5 (E1-, E2b-) H1-HA inoculated animals tested. LASV was also detected in the liver and kidney of 3/4 animals tested and the brain (Figure 6A) and blood of 2/4 guinea pigs Ad5 (E1-, E2b-) H1-HA inoculated animals tested (Figure 6B). No LASV was detectable in the brain, lung, liver, spleen, kidney or blood samples of the Ad5 (E1-, E2b-) LASV-GPC and -NP vaccinated guinea pigs (Figure 6A and 6B). Histology samples were taken from the liver, lung, kidney, brain and spleen. In the liver, lung, kidney, and brain no dramatic differences were observed between them. In the spleen, a depletion of neutrophils was observed in the red pulp of Ad5 (E1-,E2b-) H1-HA compared to Ad5 (E1-, E2b-) LASV-GPC and -NP vaccinated guinea pigs.
Figure 6. Efficacy of Ad5(E1-,E2b-) LASV-GPC and -NP vaccine.
(A) Organs or (B) blood were harvested at the time of euthanasia and titrated. Guinea pigs vaccinated with Ad5 (E1-, E2b-) LASV-GPC and -NP were euthanized at day+21. Guinea pigs vaccinated with Ad5 (E1-, E2b-) H1-HA were euthanized at day+13 or +15. (C) H&E staining of the spleen at the time of euthanasia. Representative images shown.
Neutralizing antibody against LASV in vaccinated guinea pigs
To detect neutralizing antibody, serum samples were collected at pre-vaccination (day-56), post-vaccination (day-6), and the time of euthanasia (Final). As expected, no guinea pigs in the Ad5 (E1-, E2b-) H1-HA vaccinated group exhibited neutralizing antibodies to LASV at day -6 (Table 1). In the Ad5 (E1-, E2b-) LASV-GPC and -NP vaccinated group, however, 2/8 guinea pigs had a PRNT50 at 1:10 dilution and one at 1:20 (Table 1). At the time of euthanasia, one guinea tested in the mock vaccine group had a PRNT50 of 1:10 dilutions (Table 1). All guinea pigs expressed high neutralizing antibody titers at euthanasia (1:20 to 1:160) with a geometric mean of 1:61.6 (Table 1).
Table 1.
PRNT50 titer against LASV
| Vaccine | Animal ID |
PRNT50 | ||
|---|---|---|---|---|
| day-56 | day-6 | Final | ||
| Ad5 (E1-, E2b-) LASV-GPC+NP |
GP01 | UD | UD | 1:160 |
| GP02 | UD | 1:10 | 1:80 | |
| GP03 | UD | UD | 1:160 | |
| GP04 | UD | UD | 1:80 | |
| GP05 | UD | 1:10 | 1:20 | |
| GP06 | UD | UD | 1:160 | |
| GP07 | UD | 1:10 | 1:20 | |
| GP08 | UD | UD | 1:20 | |
| Ad5 (E1-, E2b-) H1-HA |
GP09 | UD | UD | NT |
| GP10 | UD | UD | <1:10 | |
| GP11 | UD | UD | <1:10 | |
| GP12 | UD | UD | NT | |
| GP13 | UD | UD | 1:10 | |
| GP14 | UD | UD | NT | |
UD: under detection limit (1:<10)
NT: not tested
DISCUSSION
Lassa virus can cause severe hemorrhagic fever illness with a high case fatality rate [3,4]. Approximately one-third of survivors develop sensorineural hearing loss, leading to an impact to their quality of life; thus, an effective and safe vaccine is of high medical need [9]. To this end, we have developed a LASV vaccine utilizing the novel and clinically tested Ad5 (E1-, E2b-) vector. The development of Ad5 vector vaccines has been hindered due to pre-existing immunity to Ad5. To subvert this, a novel Ad5 vector platform with deletions in the E1, E2, and E3 genes has been developed and shown to induce both an antibody and cell-mediated immune response even in the presence of pre-existing immunity [25,29,30]. Vaccines have been developed using this platform against infectious diseases caused by HIV-1 and influenza A virus, and immunotherapies for antigenic cancer targets [26,38,39,43].
As expected, all guinea pigs vaccinated herein with Ad5 (E1-, E2b-) LASV-GPC and -NP successfully produced antibodies against LASV GPC and NP after 2 doses (Fig. 3). However, only 37.5% of them had neutralizing antibodies although all the vaccinated guinea pigs were protected against lethal LASV challenge. This result indicates that neutralizing antibody existence before LASV infection is not essential for protection. Recently, Abreu-Mota et al. found that non-neutralizing antibodies against LASV GPC provide protection against LASV infection [44]. It is hypothesized that antibodies inducing antibody-dependent cellular cytotoxicity may play an important role for protection. Additionally, all survivors had neutralizing titer in their sera (Table 1). It is indicated that the 2-dose vaccination schedule induces an early neutralizing antibody response, leading to the elimination of LASV before development of symptoms.
An early, strong T-cell response has been shown to contribute to LF survival [19,20,45]. Antibodies to LASV are not induced during infection, even in survivors, until late in the covalence stage [23]. Vaccines that only induce an antibody-mediated response have been reported to lack protective against LF challenge [16]. Previous research has shown that the Ad5 (E1-, E2b-) vector elicits a robust, protective cell and antibody-mediated response following immunization [29,30]. The full characterization of a T-cell mediated response induced by the Ad5 (E1-,E2b-) single vector vaccines expressing LASV NP or LASV GPC are under investigation.
There are currently no vaccines under clinical development against LF; however, several vaccine candidates have been tested preclinically. Recombinant viral vector vaccines with mainly the GP have been successful in animal testing using the vesicular stomatitis virus (VSV), yellow fever 17D, Rabies, and Vaccinia (Lister and NYBH) [44,46]. The most promising and well characterized Lassa fever vaccine candidates are ML-29 and the VSV-LASV GPC. ML-29 is a reassortment of the pathogenic LASV and non-pathogenic Arenavirus Mopeia virus (MOPV). This live attenuated vaccine candidate consists of the LASV S segment, which encodes the NP and GPC, and the MOPV L segment, encoding the Z protein and L protein. The current Ad5 platform has several advantages over the ML-29 and VSV-LASV GPC vaccine candidates [47]. Ad5 (E1-, E2b-) vector vaccines have been used safely in human clinical trials [21,27]. The vaccines are manufactured in a human E,C7 cell line at high dose levels.. Also, the Ad5 platform used here is non-replicating and can be used in immunocompromised individuals without concern of Ad5 infection [24,30]. This is important for the endemic region because there are a high number of HIV and other immunocompromised co-infections [31,32,34].
The Ad5 (E1-, E2b-) single vector vaccines expressing LASV NP or LASV GPC represent a promising vaccine candidate against Lassa fever. Further research will be needed to understand if the vaccine is fully protective with one dose, or vaccination target is only NP or GPC. More research is needed to be done to understand the cell-mediated immune response induced by the Ad5 (E1-, E2b-) single vector vaccines expressing LASV NP or LASV GPC to understand the correlates of protection. Non-the-less we believe that this LF vaccine should be tested in human clinical trials.
Acknowledgments:
We would like to thank the UTMB Pathology Core and Animal Resources Center (ARC). J.M. is supported by Japan Society for the Promotion of Science (JSPS). E.J.M is supported by the National Institutes of Health [T32 AI0075256]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Buchmeier MJ, de la Torre J-C, Peters CJ. Arenaviridae: The Viruses and Their Replication In: Knipe DM, Howley PM, editors. Fields Virology: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- [2].Shehu NY, Gomerep SS, Isa SE, Iraoyah KO, Mafuka J, Bitrus N, et al. Lassa Fever 2016 Outbreak in Plateau State, Nigeria-The Changing Epidemiology and Clinical Presentation. Front Public Heal 2018;6:232. doi: 10.3389/fpubh.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buba MI, Dalhat MM, Nguku PM, Waziri N, Mohammad JO, Bomoi IM, et al. Mortality Among Confirmed Lassa Fever Cases During the 2015–2016 Outbreak in Nigeria. Am J Public Health 2018;108:262–4. doi: 10.2105/AJPH.2017.304186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geisbert TW. Predicting outcome and improving treatment for Lassa fever. Lancet Infect Dis 2018;18:594–5. doi: 10.1016/S1473-3099(18)30116-6. [DOI] [PubMed] [Google Scholar]
- [5].Shaffer JG, Grant DS, Schieffelin JS, Boisen ML, Goba A, Hartnett JN, et al. Lassa Fever in Post-Conflict Sierra Leone. PLoS Negl Trop Dis 2014;8:e2748. doi: 10.1371/journal.pntd.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yun NE, Walker DH, Yun NE, Walker DH. Pathogenesis of Lassa Fever. Viruses 2012;4:2031–48. doi: 10.3390/v4102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ 2003;327:1271–5. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, et al. Lassa Fever. N Engl J Med 1986;314:20–6. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- [9].Mateer EJ, Huang C, Shehu NY, Paessler S. Lassa fever-induced sensorineural hearing loss: A neglected public health and social burden. PLoS Negl Trop Dis 2018;12:e0006187. doi: 10.1371/journal.pntd.0006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mylne AQN, Pigott DM, Longbottom J, Shearer F, Duda KA, Messina JP, et al. Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg 2015;109:483–92. doi: 10.1093/trstmh/trv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burri DJ, Palma JR, Kunz S, Pasquato A. Envelope Glycoprotein of Arenaviruses. Viruses 2012;4:2162–81. doi: 10.3390/v4102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burri DJ, Pasqual G, Rochat C, Seidah NG, Pasquato A, Kunz S. Molecular characterization of the processing of arenavirus envelope glycoprotein precursors by subtilisin kexin isozyme-1/site-1 protease. J Virol 2012;86:4935–46. doi: 10.1128/JVI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahmutovic S, Clark L, Levis SC, Briggiler AM, Enria DA, Harrison SC, et al. Molecular Basis for Antibody-Mediated Neutralization of New World Hemorrhagic Fever Mammarenaviruses. Cell Host Microbe 2015;18:705–13. doi: 10.1016/J.CHOM.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robinson JE, Hastie KM, Cross RW, Yenni RE, Elliott DH, Rouelle JA, et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat Commun 2016;7:11544. doi: 10.1038/ncomms11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hastie KM, Zandonatti MA, Kleinfelter LM, Heinrich ML, Rowland MM, Chandran K, et al. Structural basis for antibody-mediated neutralization of Lassa virus. Science (80- ) 2017;356:923–8. doi: 10.1126/science.aam7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol 2017;17:195–207. doi: 10.1038/nri.2016.138. [DOI] [PubMed] [Google Scholar]
- [17].Russier M, Pannetier D, Baize S. Immune responses and Lassa virus infection. Viruses 2012;4:2766–85. doi: 10.3390/v4112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot M-C, Deubel V. Lassa Virus Infection of Human Dendritic Cells and Macrophages Is Productive but Fails to Activate Cells. J Immunol 2004;172:2861–9. doi: 10.4049/JIMMUNOL.172.5.2861. [DOI] [PubMed] [Google Scholar]
- [19].Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, et al. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J Virol 2009;83:5890–903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Flatz L, Rieger T, Merkler D, Bergthaler A, Regen T, Schedensack M, et al. T Cell-Dependence of Lassa Fever Pathogenesis. PLoS Pathog 2010;6:e1000836. doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meulen J ter, Badusche M, Satoguina J, Strecker T, Lenz O, Loeliger C, et al. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology 2004;321:134–43. doi: 10.1016/J.VIROL.2003.12.013. [DOI] [PubMed] [Google Scholar]
- [22].ter Meulen J, Badusche M, Kuhnt K, Doetze A, Satoguina J, Marti T, et al. Characterization of human CD4(+) T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J Virol 2000;74:2186–92. doi: 10.1128/jvi.74.5.2186-2192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Günther S, Kühle O, Rehder D, Odaibo GN, Olaleye DO, Emmerich P, et al. Antibodies to Lassa virus Z protein and nucleoprotein co-occur in human sera from Lassa fever endemic regions. Med Microbiol Immunol 2001;189:225–9. [DOI] [PubMed] [Google Scholar]
- [24].Balint JP, Gabitzsch ES, Rice A, Latchman Y, Xu Y, Messerschmidt GL, et al. Extended evaluation of a phase 1/2 trial on dosing, safety, immunogenicity, and overall survival after immunizations with an advanced-generation Ad5 [E1-, E2b-]-CEA(6D) vaccine in late-stage colorectal cancer. Cancer Immunol Immunother 2015;64:977–87. doi: 10.1007/s00262-015-1706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, et al. Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Cancer Gene Ther 2009;16:673–82. doi: 10.1038/cgt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, Jones FR. Novel Adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine 2009;27:6394–8. doi: 10.1016/J.VACCINE.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gabitzsch ES, Xu Y, Balint JP, Balcaitis S, Sanders-Beer B, Jones FR. Induction and comparison of SIV immunity in Ad5 naive and Ad5 immune non-human primates using an Ad5 [E1-, E2b-] based vaccine. Vaccine 2011;29:8101–7. doi: 10.1016/J.VACCINE.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gabitzsch ES, Xu Y, Balint JP, Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother 2010;59:1131–5. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Gayle RB, Amalfitano A, et al. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant-based vaccine used to induce cell mediated immune responses. Immunol Lett 2009;122:44–51. doi: 10.1016/J.IMLET.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morse MA, Chaudhry A, Gabitzsch ES, Hobeika AC, Osada T, Clay TM, et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother 2013;62:1293–301. doi: 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Djomand G, Quaye S, Sullivan PS. HIV epidemic among key populations in west Africa. Curr Opin HIV AIDS 2014;9:506–13. doi: 10.1097/COH.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, Banura C, et al. The Burden of Human Papillomavirus Infections and Related Diseases in Sub-Saharan Africa. Vaccine 2013;31:F32–46. doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ajayi IO, Ughasoro MD, Ogunwale A, Odeyinka O, Babalola O, Sharafadeen S, et al. A qualitative exploration of malaria operational research situation in Nigeria. PLoS One 2017;12:e0188128. doi: 10.1371/journal.pone.0188128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gehre F, Otu J, Kendall L, Forson A, Kwara A, Kudzawu S, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa: preparing for large-scale tuberculosis research and drug resistance surveillance. BMC Med 2016;14:160. doi: 10.1186/s12916-016-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabitzsch ES, Balint-Junior JP, Xu Y, Balcaitis S, Sanders-Beer B, Karl J, et al. Control of SIV infection and subsequent induction of pandemic H1N1 immunity in rhesus macaques using an Ad5 [E1-, E2b-] vector platform. Vaccine 2012;30:7265–70. doi: 10.1016/J.VACCINE.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yun NE, Ronca S, Tamura A, Koma T, Seregin AV., Dineley KT, et al. Animal Model of Sensorineural Hearing Loss Associated with Lassa Virus Infection. J Virol 2016;90:2920–7. doi: 10.1128/JVI.02948-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maruyama J, Manning J, Mateer E, Sattler R, Bukreyeva N, Huang C, et al. Lethal infection of Lassa virus isolated from a human clinical sample in outbred guinea pigs without adaption. mSphere. 2019. In Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jones FR, Gabitzsch ES, Xu Y, Balint JP, Borisevich V, Smith J, et al. Prevention of influenza virus shedding and protection from lethal H1N1 challenge using a consensus 2009 H1N1 HA and NA adenovirus vector vaccine. Vaccine 2011;29:7020–6. doi: 10.1016/j.vaccine.2011.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gabitzsch ES, Xu Y, Balcaitis S, Balint JP, Jones FR. An Ad5[E1-, E2b-]-HER2/neu vector induces immune responses and inhibits HER2/neu expressing tumor progression in Ad5 immune mice. Cancer Gene Ther 2011;18:326–35. doi: 10.1038/cgt.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Manning JT, Seregin AV., Yun NE, Koma T, Huang C, Barral J, et al. Absence of an N-Linked Glycosylation Motif in the Glycoprotein of the Live-Attenuated Argentine Hemorrhagic Fever Vaccine, Candid #1, Results in Its Improper Processing, and Reduced Surface Expression. Front Cell Infect Microbiol 2017;7:1–12. doi: 10.3389/fcimb.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Koma T, Huang C, Aronson JF, Walker AG, Miller M, Smith JN, et al. The Ectodomain of Glycoprotein from the Candid#1 Vaccine Strain of Junin Virus Rendered Machupo Virus Partially Attenuated in Mice Lacking IFN-αβ/γ Receptor. PLoS Negl Trop Dis 2016; 10:e0004969. doi: 10.1371/journal.pntd.0004969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yun NE, Seregin A V, Walker DH, Popov VL, Walker AG, Smith JN, et al. Mice lacking functional STAT1 are highly susceptible to lethal infection with Lassa virus. J Virol 2013;87:10908–11. doi: 10.1128/JVI.01433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gabitzsch ES, Tsang KY, Palena C, David J, Fantini M, Kwilas A, et al. The generation and analyses of a novel combination of recombinant adenovirus vaccines targeting three tumor antigens as an immunotherapeutic. Oncotarget 2015;6:31344–59. doi: 10.18632/oncotarget.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Abreu-Mota T, Hagen KR, Cooper K, Jahrling PB, Tan G, Wirblich C, et al. Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever. Nat Commun 2018;9:4223. doi: 10.1038/s41467-018-06741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McElroy AK, Akondy RS, Harmon JR, Ellebedy AH, Cannon D, Klena JD, et al. A Case of Human Lassa Virus Infection With Robust Acute T-Cell Activation and Long-Term Virus-Specific T-Cell Responses. J Infect Dis 2017;215:1862–72. doi: 10.1093/infdis/jix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hallam HJ, Hallam S, Rodriguez SE, Barrett ADT, Beasley DWC, Chua A, et al. Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development. Npj Vaccines 2018;3:11. doi: 10.1038/s41541-018-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Warner BM, Safronetz D, Stein DR. Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Des Devel Ther 2018;12:2519–27. doi: 10.2147/DDDT.S147276. [DOI] [PMC free article] [PubMed] [Google Scholar]