Abstract
Changes to arm swing and gait symmetry are symptomatic of several pathological gaits associated with reduced stability. The purpose of this study was to examine the relative contributions of arm swing and gait symmetry towards gait stability. We theorized that actively increasing arm swing would increase gait stability, while asymmetric walking would decrease gait stability. Fifteen healthy, young adults (23.4 ± 2.8 yrs) walked on a split-belt treadmill under symmetric (1.2 m/s) and asymmetric walking (left/right, 5:4 speed ratio) with three different arm swings: held, normal, and active. Trunk local dynamic stability, inter-limb coordination, and spatiotemporal gait variability and symmetry were measured. Active arm swing resulted in improved local trunk stability, increased gait variability, and decreased inter-limb coordination (p < .013). The changes in local trunk stability and gait variability during active arm swing suggests that these metrics quantify fundamentally different aspects of stability and are not always comparable. Split-belt walking caused reduced local trunk stability, increased gait variability, and increased lower limb asymmetry (p < .003). However, the arm swing symmetry was unaffected by gait asymmetry, this suggests that the decreases in gait stability are linked to the increases in gait asymmetry rather than increases in arm swing asymmetry.
Introduction
Arm swing during gait has been shown to have both passive and active components [1, 2]. While the exact interplay between these components in arm swing is still unknown, the small torques calculated at the shoulder indicate that passive, pendular like oscillations are likely dominant in the formation of normal arm swing. However, muscle activity at the shoulder is persistent, even during conditions of restricted arm movements (e.g. bound arms) [2]. This activity is thought to arise from central pattern generators important in the formation of normal gait patterns. Indeed, there appears to be a neurological link between the oscillatory movements of the arms and legs [3, 4]. The purpose of this neural connection, and more broadly of the ubiquitous presence of arm swing in healthy human gait, is presently unclear. Past research has linked arm swing during gait to decreased metabolic cost [5], decreased vertical ground reaction forces [6], and increased stability [7, 8].
Stable gait can be defined as gait which maintains equilibrium despite the presence of internal and external perturbations [9]. Interestingly, Ortega et al. [5] found that an increased metabolic cost due to walking without arm swing was mitigated by applying external lateral stabilization. Therefore, it was posited that the increased metabolic rate when walking without both arm swing and external stabilization was due to an increased effort in maintaining stability [5]. Further studies into this link between arm swing and gait stability have found conflicting results. Some studies have found decreases in stability when arm swing was prevented (either by binding the arms or holding them still) as compared to normal swing by analysing step width variability [10], harmonic ratios [11], and local dynamic stability [8]. Yet, others found no effect on stability when comparing bound and normal swing using local dynamic stability [7] and harmonic ratios [11].
Decreases in arm swing amplitude have been found in older adults [12] and people with PD [13–15]. Simultaneously, while some level of gait variability is common in healthy subjects, both younger and older [16], increases in gait spatiotemporal variability—often used indicators of gait stability and measured as the standard deviation of step or stride width, length, or time—are associated with increased fall risk in older adults as well as people with Parkinson’s disease (PD) [17–20]. It has been previously suggested that deliberately emphasized arm swing could have a positive effect on trunk stability, and initial results demonstrated improved mediolateral trunk stability using harmonic ratios in older adults [11]. Other work has found increases in local dynamic stability when comparing active arm swing (e.g. increased swing amplitude) conditions to normal swing [21]. It remains unclear whether consciously increasing arm swing amplitude could have a beneficial aspect on gait stability. It is possible that increased attentional demands of active arm swing, in switching from largely passive and automatic control to conscious control, may have negative effects on interlimb coordination. Continuous relative phase (CRP) is a common method used to measure coordination among body segments [22–24] where interstride variability of CRP is an indicator of the stability of the coordination pattern [23]. To our knowledge, no previous studies have compared the effects of normal, held and active arm swing conditions on gait stability within the same population.
A concept from dynamical systems theory, local dynamic stability uses an estimate of the maximal Lyapunov exponent (MLE), a measure of chaos, to quantify stability where a smaller MLE represents an increase in stability and a larger MLE represents a decrease in stability. Dingwell et al. [25] were the first to use the MLE to quantify stability during steady-state gait and found it to be a more sensitive measure of stability than traditional gait variability measures when comparing overground walking to walking on treadmills in a population of healthy young adults [25]. Further studies have supported its use as a measure of stability; van Schooten et al. [26] demonstrated the feasibility of MLE as an indicator of gait stability by using galvanic vestibular stimulation to reduce the stability of healthy young adults [26]. Looking at the local dynamic stability of lower limb joint angles [27] and of the trunk velocity [28], researchers also reported greater trunk stability with increased walking speed. Finally, older adults show significant decreases in gait stability compared to young adults when measured with the MLE [29].
Asymmetry in gait has also been linked to decreases in gait stability. Individuals with pathological gait, such as those with PD, often demonstrate asymmetry of the lower limbs as well as changes in arm swing amplitude and symmetry when compared to age-matched controls [20]. Studies also link this asymmetric gait pattern to increased risk of falls [30]. In healthy young adults, asymmetric gait induced via split-belt treadmills is associated with decreased stability measured using margins of stability [31, 32]. However, the relationship between asymmetry in the upper and lower limbs and changes in stability is not well understood.
The purpose of this study was to further examine the role of arm swing and lower limb symmetry on gait stability in healthy young adults using common gait variability measures (mean and standard deviation of step length and width) and local dynamic stability. We hypothesized that active arm swing would lead to greater stability, that asymmetric walking would be less stable than symmetric walking, and that active arm swing would mitigate stability decreases caused by asymmetric walking.
Materials and methods
Subjects
An a priori power analysis performed using SigmaPlot 12.5 (Systat Software, San Jose, CA) indicated that 12 participants would result in adequate statistical power when set at β = 0.8 and estimated with the local dynamic stability results from Bruijn et al. (2010). To account for possible attrition, fifteen healthy, young adults (8 male, 23.4 ± 2.8 years (mean ± s.d.); 72.3 ± 13.5 kg; 170.2 ± 8.1 cm) from the Ottawa area were recruited. Subjects were excluded based on the presence of any recent (< 6 months) musculoskeletal injuries, or any chronic neurological or orthopaedic disorders that could affect gait. All participants but one (who reported as ambidextrous) self-reported as right handed. The study was carried out in compliance with the Tri-Council Policy statement; Ethical Conduct for Research Involving humans; The International Conference on Harmonization—Good Clinical practice: Consolidated Guideline; and the provisions of the Personal Health Information Protection Act 2004. The study was approved by the Ottawa Health Science Network Research Ethics Board (20170291-01H) as well as by the University of Ottawa Research Ethics Board (A06-17-03). All subjects gave written, informed consent prior to participation.
Protocol
A 57 marker set was used to capture kinematic data [33] in the Computer Assisted Rehabilitation Environment (CAREN) (CAREN-Extended, Motekforce Link, Amsterdam, NL) at the Ottawa Hospital Rehabilitation Centre. The CAREN system combines a 6 degree of freedom platform with an integrated instrumented split-belt treadmill (Bertec Corp., Columbus, OH) and a 12 camera Vicon motion capture system (Vicon 2.6, Oxford, UK). Kinematic data was captured at 100Hz, and kinetic data was collected at 1000Hz. Participants were asked to walk on the split-belt treadmill at a speed of 1.2 m/s. Each trial had a duration of 200s, and the first 25s of each trial were removed to allow the treadmill to reach the set speed and the participant to reach a steady-state. The three arm swing conditions were: held, normal, and active. For the held swing condition, participants were instructed to hold their arms at their sides in a relaxed manner, without swinging or stiffness. The instructions for the active swing condition were to swing their arms such that the arm was horizontal when each forward swing peaked. For the asymmetric walking condition, the right treadmill belt speed was set to 80% of the left side, or 0.96 m/s; a smaller treadmill asymmetry ratio than previous studies was chosen to cause gait asymmetries while only functioning as a minor continuous perturbation. The combination of all arm swing and symmetry conditions were randomized for each participant; each condition was performed once.
Analysis
All data was imported into Visual3D v6 (C-Motion, Germantown, MD) and filtered using a 4th order, zero-lag low-pass Butterworth filter with a cutoff frequency of 12Hz and 10Hz for the kinematic and kinetic data, respectively. Heel strikes were identified with a logistic classification model which used ground reaction forces from both force plates and kinematic data (feet position relative to the pelvis, feet velocity–relative to both the pelvis and the laboratory, feet acceleration, and both knee angles); all heel strikes were manually verified and corrected as necessary. Trunk linear and angular velocities, feet centre of mass positions, and left and right shoulder and hip angles in the sagittal plane were exported from Visual3D and further data analyses were done in Julia (v1.0.3) [34] using custom code. All measures were calculated using 125 strides of data.
Arm swing amplitude was calculated as the average sagittal range of motion of the shoulder angle to confirm adherence to condition instructions. CRP between contralateral arm-leg pairs was calculated as recommended by Lamb and Stockl [35] by first centering the amplitude of shoulder and hip angles, then calculating the phase angle for each signal using the signal and its Hilbert transform, shown in Eqs (1) and (2). Finally, the CRP was calculated between the two signals as shown in Eq (3) [35]. CRP calculated with non-sinusoidal signals will vary across a period, therefore it is necessary to calculate the average of the ensemble standard deviation of CRP, hereafter referred to as MSDCRP, to quantify the stability of coordination patterns as the interstride variability of CRP [22, 35].
| (1) |
| (2) |
| (3) |
Note that the extrema, min and max, in Eq (1) were the average extrema of the 125 strides analysed. In Eq (2), H(X) represents the imaginary part of the analytic signal produced by the Hilbert transform. Circular means and standard deviations were used to reduce the CRP data to MSDCRP [36].
Average step width and step width variability were calculated as the average and standard deviation of the mediolateral distance between successive heel strikes. Average step length and step length variability were calculated as the average and standard deviation of the anteroposterior distance between successive heel strikes [18]. Step length metrics were analysed separately for each foot. This was necessary to avoid confounding effects in the asymmetric gait condition, where the left and right steps will naturally have 2 distinct lengths. Left steps are defined as the left heel strike following a right leg stance; right steps are defined oppositely.
Arm swing asymmetry, and step asymmetry–both temporal and spatial–were calculated as
| (4) |
where L and R are the metrics for the left and right sides, and where a result of zero represents perfect symmetry [37]. Temporal step asymmetry was calculated using step time, and spatial step asymmetry was calculated using step length.
Local dynamic stability
Local dynamic stability, measured as the maximum finite time Lyapunov exponent (MLE), characterizes the average logarithmic divergence of two trajectories from infinitesimally close initial conditions. The MLE was calculated with Rosenstein’s method [38] using 125 strides of data interpolated to a length of 12,500 samples for an average stride length of roughly 100 points [39]. Note that 125 strides were analysed instead of the more common number of 140 strides because an unacceptable number of trials in the active swing condition did not contain an adequate number of strides. Data was reconstructed in a 12D state space of the form
| (5) |
using the linear and angular velocity of the trunk and their 25 samples delayed signals [40]. To account for the differing units, the linear and angular velocities were normalized to unit variance [41]. Only the “short term” maximum finite time Lyapunov exponent was used, which is defined as the slope of the divergence curve from 0 to 0.5 strides, inclusive [39, 40].
Statistical analysis
A two-way (Arms×Symmetry, all swing conditions vs. both symmetry conditions) repeated-measures ANOVA was performed on the following variables: left and right arm swing amplitude, arm swing asymmetry, average right and left step length and width, spatial and temporal step asymmetry, right and left step length and width variability, and MLE. One outlier was identified using boxplots in SPSS—which denote outliers as samples 3 times the interquartile range or more away from the median—and removed for left step length variability and temporal step asymmetry. A separate two-way (Arms×Symmetry, normal and active swing vs. both symmetry conditions) repeated-measures ANOVA was performed on MSDCRP for both arm-leg pairs; MSDCRP in the held condition was not analysed as there was no expectation of a stable coordination pattern between the motionless arms and leg swing. Significance level was set a priori at α = 0.05. A Bonferroni correction was used for all post-hoc tests performed. Assumption of normality was confirmed using a Kolmogorov-Smirnov test and a Greenhouse-Geisser p was reported when Mauchly’s Test of Sphericity was violated.
Results
Arm swing range of motion and coordination
Table 1 contains the results of the arm swing range of motion (RoM) and MSDCRP for both contralateral limb pairs. A significant effect of arm swing was found for the left (F(2, 28) = 387.36, p < .001, ), and right (F(2, 28) = 362.65, p < .001, ) swing amplitudes. Treadmill symmetry conditions had no effect on arm swing asymmetry (p > .05) (Tables 2 and 3).
Table 1. Summary of arm swing and contralateral limb coordination.
| Arm swing | ||||
|---|---|---|---|---|
| Symmetry | Held | Normal | Active | |
| Left arm RoM (°) | Symmetric | 7.34 ± 2.16 | 25.9 ± 10.3 | 95.8 ± 17.3 |
| Asymmetric | 7.73 ± 2.37 | 25.4 ± 7.29 | 89.5 ± 18.8 | |
| Right arm RoM (°) | Symmetric | 6.45 ± 2.05 | 25.3 ± 9.61 | 95.4 ± 16.1 |
| Asymmetric | 6.85 ± 1.75 | 27.5 ± 8.88 | 91.1 ± 16.2 | |
| MSDCRPleft shoulder, right hip (°) | Symmetric | 13.1 ± 11.4 | 30.8 ± 31.3 | |
| Asymmetric | 12.1 ± 4.93 | 29.2 ± 34.6 | ||
| MSDCRPright shoulder, left hip (°) | Symmetric | 13.7 ± 11.9 | 31.2 ± 31.8 | |
| Asymmetric | 11.4 ± 3.80 | 29.5 ± 36.7 | ||
Table 2. Summary of asymmetry measures.
| Arm swing | ||||
|---|---|---|---|---|
| Symmetry | Held | Normal | Active | |
| Arm swing asymmetry | Symmetric | 21.6 ± 18.9 | 22.1 ± 14.1 | 9.24 ± 6.96 |
| Asymmetric | 23.0 ± 12.8 | 24.6 ± 26.2 | 10.9 ± 7.91 | |
| Spatial step asymmetry | Symmetric | 2.88 ± 2.42 | 3.73 ± 2.31 | 3.75 ± 2.82 |
| Asymmetric | 16.4 ± 6.18 | 15.9 ± 5.65 | 13.9 ± 5.69 | |
| Temporal step asymmetry | Symmetric | 3.23 ± 2.19 | 3.23 ± 2.02 | 3.63 ± 2.89 |
| Asymmetric | 1.94 ± 1.24 | 1.71 ± 1.46 | 3.41 ± 2.12 | |
Table 3. Summary of main and interaction effects.
| Main Effect | |||
|---|---|---|---|
| Arm Swing | Symmetry | Arm Swing × Symmetry | |
| Arm swing asymmetry | F(2, 28) = 5.36, p = .011, | n.s. | n.s. |
| Spatial step asymmetry | n.s. | F(1, 14) = 90.37, p < .001, | F(2, 28) = 5.00, p = .014, |
| Temporal step asymmetry* | n.s. | n.s. | n.s. |
| MLE | F(2, 28) = 18.31, p < .001, | F(1, 14) = 37.55, p < .001, | n.s. |
| Step width (m) | n.s. | F(1, 14) = 27.36, p < .001, | n.s. |
| Step width SD† (m) | F(2, 28) = 17.16, p < .001, | n.s. | n.s. |
| Left step length (m) | F(2, 28) = 40.69, p < .001, | F(1, 14) = 175.38, p < .001, | n.s. |
| Left step length SD (m) | F(2, 26) = 4.95, p = .035, | F(1, 13) = 13.42, p = .003, | n.s. |
| Right step length (m) | F(2, 28) = 18.05, p < .001, | n.s. | n.s. |
| Right step length SD (m) | F(2, 28) = 7.70, p = .002, | F(1, 14) = 33.07, p < .001, | n.s. |
n.s. stands for not significant.
*Temporal step asymmetry approached significance for main effects of both arm swing (p = .064) and symmetry (p = .054).
†A main effect of symmetry approached significance for step width SD (p = .064).
MSDCRP between the left shoulder and right hip showed a significant effect of arm swing (F(1, 14) = 8.13, p = .013, ); the MSDCRP between the right shoulder and left hip was also significantly affected by arm swing (F(1, 14) = 8.04, p = .013, ). The MSDCRP of both contralateral limb pairs showed significant increases from normal to active swing (p = .013). Neither limb pair was affected by the gait symmetry conditions.
Stability measures
See Tables 3 and 4 for a summary of the significant main effects for the stability measures and the descriptive statistics. Post-hoc tests showed that the active arm swing had significantly lower MLE compared to both normal (p = .002) and held (p = .001) swing conditions. Asymmetric walking had significantly higher MLE than symmetric (p < .001).
Table 4. Summary of stability related measures.
| Arm swing | ||||
|---|---|---|---|---|
| Symmetry | Held | Normal | Active | |
| MLE | Symmetric | 0.52 ± 0.03 | 0.51 ± 0.03 | 0.49 ± 0.04 |
| Asymmetric | 0.59 ± 0.06 | 0.56 ± 0.05 | 0.50 ± 0.03 | |
| Step width (cm) | Symmetric | 19.1 ± 3.6 | 19.2 ± 3.8 | 19.6 ± 4.4 |
| Asymmetric | 20.9 ± 3.6 | 20.8 ± 4.4 | 21.0 ± 4.4 | |
| Step width SD (cm) | Symmetric | 1.71 ± 0.45 | 1.80 ± 0.49 | 22.7 ± 0.68 |
| Asymmetric | 1.67 ± 0.40 | 1.74 ± 0.40 | 20.4 ± 0.54 | |
| Left step length (cm) | Symmetric | 62.9 ± 3.9 | 62.9 ± 3.4 | 69.2 ± 3.6 |
| Asymmetric | 54.6 ± 4.7 | 55.2 ± 3.3 | 60.6 ± 4.7 | |
| Left step length SD (cm) | Symmetric | 1.77 ± 0.37 | 16.0 ± 0.32 | 23.6 ± 0.54 |
| Asymmetric | 2.32 ± 0.77 | 23.0 ± 0.82 | 26.9 ± 1.1 | |
| Right step length (cm) | Symmetric | 63.8 ± 3.6 | 64.6 ± 3.0 | 69.9 ± 4.9 |
| Asymmetric | 64.1 ± 5.1 | 64.7 ± 3.7 | 69.3 ± 6.8 | |
| Right step length SD (cm) | Symmetric | 1.94 ± 0.58 | 1.73 ± 0.27 | 2.83 ± 0.92 |
| Asymmetric | 2.71 ± 0.64 | 2.81 ± 1.46 | 3.32 ± 0.84 | |
Step width variability was increased during active swing compared to both held (p = .001) and normal (p = .004). Step width increased during split-belt walking conditions (p = .001). Active swing resulted in increased step length compared to held and normal, respectively, for the right (p = .002, p = .001), and left (p < .001) sides. Right step length increased during split-belt walking (p < .001). Active swing also showed increased right step length variability compared to held (p = .002) and normal (p = .003). Asymmetric walking resulted in increased step length variability compared to symmetric for the left (p = .003) and right (p < .001).
Measures of asymmetry
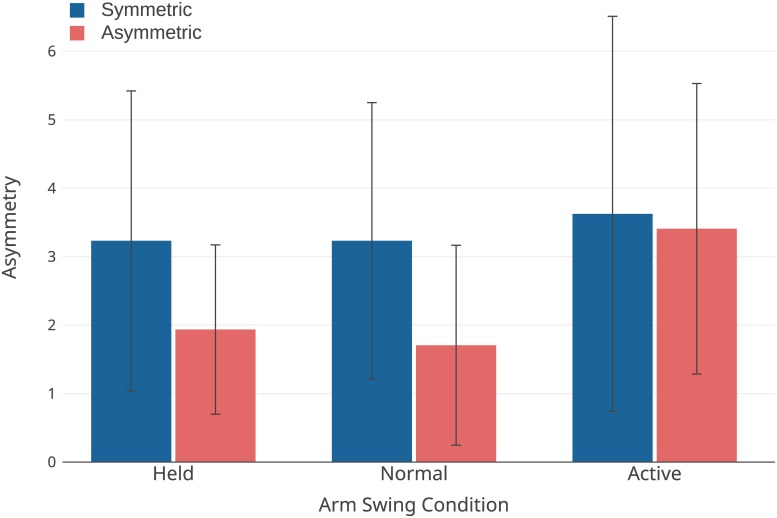
See Table 2 for a summary of the significant main effects for the measures of asymmetry. Given the Arms×Symmetry interaction effect in spatial step asymmetry, post-hoc tests showed a significant decrease between the held and active swing conditions only while in the asymmetric walking condition (p = .025), and the split-belt walking was significantly increased from symmetric gait (p < .001). Arm swing asymmetry was significantly decreased in the active swing conditions as compared to held (p = .016) and normal (p = .035) swing. Temporal asymmetry, shown in Fig 1, contained one outlier which was removed, and although it was not statistically significant, a trend towards significance was found for main effects of both arm swing (p = .064) and symmetry (p = .054).
Fig 1. Temporal step asymmetry.
Error bars are ±SD.
Discussion
In this study, we examined the influence of changes in arm swing amplitude and lower limb asymmetry on gait stability using several stability metrics. When compared to both normal and held arm swings, active arm swing resulted in reduced contralateral limb coordination and increases in gait variability. However, the active swing also showed improved local dynamic stability. As for gait asymmetry, split-belt walking resulted in increased gait variability in spatiotemporal measures and worsened local dynamic stability. Altogether, while the difference in local dynamic stability between active and normal swings matches our hypothesis that active swing would improve local stability, it conflicts with both the increased step width and length variability and the decreased arm-leg coordination. Regarding our hypothesis of asymmetric gait causing reductions in stability, both local dynamic stability and gait variability metrics show reduced stability during the split-belt walking conditions. Furthermore, the increased step variability, worsened local dynamic stability, and reduced coordinative stability during active arm swing, regardless of gait symmetry condition, does not support our hypothesis that active arm swing would mitigate decreases in stability caused by asymmetric walking.
On the basis of the apparently conflicting behavior between step variability and local dynamic stability, it is justified to revise the assumption that local dynamic stability and gait variability metrics are comparably related to stability, when defined as the likelihood of falling, and often referred to as global stability. While both types of measures have been linked to this definition of global stability in literature [17, 42, 43], the results here suggest a more nuanced application and interpretation of these metrics. The improved local dynamic stability at the cost of increased gait variability demonstrates the importance of local trunk stability as a movement outcome–the trunk does account for a large proportion of the body mass. However, the addition of gait variability as a measure of stability complements the trunk local dynamic stability by providing a direct measure of the base of support which may signal decreases in global stability and increased risk of falling before the trunk signals reflect decreases in stability. Dingwell et al. [44] found precisely this behaviour of a more locally stable trunk despite increased gait variability, and concluded that local dynamic stability and gait variability must be distinguished due to the fundamental differences of what they are classifying.
Therefore, the increased local dynamic stability of the trunk is in line with our hypothesis, increasing arm swing increases stability, if we clarify that stability here refers solely to the local stability of the trunk, which may change independently of the global stability. What remains unclear at this time is the cause of this increase in local trunk stability. We suggest two possible explanations, both of which may contribute to these independent changes in local trunk stability. Our initial hypothesis regarding increased arm swing, was primarily directed from a physics-based perspective, where increases in arm swing result in increases in angular momentum, thereby increasing the trunk’s resistance to change [45]. In this case, it is possible that the increased local stability from the greater angular momentum of the arms outweighs any decreases in arm swing coordination and increases in step variability. However, it is also possible that the more variable, or dynamic, nature of the base of support also contributed to a more locally stable trunk, but this would be difficult to confirm. A simulation study would be best suited to investigate this, however, it seems likely that replicating the behaviour seen here would require a model that actually demonstrates chaotic behaviour (i.e. the model can be mathematically represented as a dynamical system), as opposed to previously used passive dynamic walker models [43, 46].
With respect to the gait variability measures, we attribute the increase in gait variability between active swing and the other two conditions primarily to the decrease in inter-limb coordination. Decreased inter-limb coordination during active swing is also visible in a trend towards increased temporal step asymmetry when compared to normal swing. Large variability in the temporal asymmetry (Fig 1) contributed to inadequate power to detect an interaction effect; post-hoc power analysis using G*Power indicated more than 30 participants would be required to achieve sufficient power to confirm this interaction. The decrease in coordination during active arm swing is likely due to increased conscious control (i.e. dual tasking) of arm swing. Active arm swing acting as a dual-task could also contribute to the increased gait variability. This behaviour would be in line with the results of McFadyen et al. [47] who found changes in double support proportion and variation under the combined challenge of split-belt walking and a dual-task. However, while the negative effect of dual-tasking on stability is well established in impaired populations [48, 49], this negative effect is not as well supported in young adults studied here [48, 50], and the design of this study is not appropriate to resolve this question.
Whatever the cause of the increased gait variability during active arm swing, the physical challenge of maintaining stability must also increase with more variable steps as larger gait variability represents a continuous minor perturbation to gait. It remains to be seen if more moderate increases in arm swing amplitude could succeed, or if prior training with this level of swing amplitude could mitigate any affect of dual-tasking or coordination issues. Further studies which include a continuum of arm swing amplitudes would be also insightful as to the relationship between swing amplitude and stability, and whether there may be a transition point beyond which increased swing amplitude decreases stability.
Regarding the effects of the split-belt walking, the induced asymmetric gait reduced gait stability as expected by increasing step length and width variability as well as reducing the trunk local dynamic stability. The spatial step length asymmetry was dramatically increased by the split-belt walking also as expected, while the temporal step asymmetry and arm swing symmetry were unchanged. The results of temporal symmetry in the lower limbs during split-belt walking agree with past research by Malone et al. [51] which showed that both temporal and spatial asymmetry adapts to split-belt walking, and other studies showing that asymmetric gait requires more precise timing of gait patterns (i.e. temporal asymmetry should remain roughly the same or improve) [52, 53]. In our results, the trend towards improved temporal asymmetry in the held and normal swing conditions supports the previous results. The interaction between arm swing and split-belt walking for spatial asymmetry arose because the relative difference in step lengths between left and right sides for the held and active swing conditions remained the same—6cm—despite both step lengths increasing, which reduced the relative spatial asymmetry. Note that the spatial asymmetry calculated here differs from that used by Malone et al. [51] and does not necessarily conflict with previous results regarding spatial adaptation. The unchanged arm swing symmetry between symmetric and asymmetric gait is also in line with a previous study on upper and lower limb coordination during asymmetric gait which found that upper limb movements maintain symmetry and follow the rhythm of the fastest leg, even during increasingly asymmetric gait [53].
While it is possible that the decreased stability during the split-belt walking is due to the novelty, acting as a dual-task, previous research indicates that this is improbable [51, 52]. Indeed, Dietz et al. [54] saw adaptations to inter-limb coordination in 10-20 strides during split-belt walking across multiple split-belt speed ratios, and Reisman et al. [52] showed rapid changes to single limb characteristics, again across multiple speed ratios. Despite the adaptability of multiple aspects of gait (including some measures of gait stability), some studies suggest that not all characteristics of gait stability can adapt to long-term split-belt walking [31, 32]. A thorough investigation on gait stability in adults familiar with split-belt walking could confirm the likely destabilizing effects of asymmetric gait.
In addition, healthy older adults have been shown to have reduced adaptation to asymmetric gait than healthy young adults [55]. Similarly, people with PD have demonstrated worse adaptability than age-matched controls [56]. While not the only population suffering from asymmetric gait, people with PD are one example of those who demonstrate a pathological gait with clear changes in arm swing amplitude and symmetry, and gait asymmetry when compared to age-matched controls, while also showing decreased stability (i.e. increased risk of falls) [24, 57, 58]. Our results suggest that deficits in stability may be linked to the decreases in gait symmetry rather than decreases in arm swing symmetry. Future work involving both induced asymmetric gait and asymmetric arm swing and their effects on stability could clarify the relative contributions of each towards gait stability.
Limitations
A primary limitation of this study is that there were an insufficient number of participants to achieve adequate power to detect an interaction effect in the temporal step asymmetry. Also, it is important to note that populations with greater heterogeneity, such as people with Parkinson’s disease, would similarly require larger numbers of participants. Additionally, while the variables and metrics used in the present study were appropriate to resolve our hypotheses, the inclusion of other stability metrics, such as extrapolated center of mass, or analyses of angular momentum could offer complementary insights to the results of this study.
Conclusion
In conclusion, decreased arm swing was not negatively affected in any stability metric analyzed here, and although increased arm swing showed improved trunk local dynamic stability, simultaneous increases in gait variability metrics suggest that local dynamic stability should not be treated as precisely analogous to standard gait variability variables due to the fundamental differences of what and how they characterize stability. The larger gait variability also suggests that actively increasing arm swing amplitude requires an observable level of attentional demand in healthy young adults. Therefore, it would be interesting to repeat this protocol in populations with postural stability deficits, particularly in those with asymmetric gait pattern. As well, increases in lower, but not upper, limb asymmetry along with decreases in all stability metrics during split-belt walking indicate that the corresponding decreases in gait stability for asymmetric gaits are likely linked to the increased lower limb asymmetry–a finding relevant to pathological gaits with marked upper and lower limb asymmetries as well as decreased global stability.
Data Availability
All data analyzed in this paper, along with the code developed to analyze the data, are available on Zenodo at https://zenodo.org/record/3239464 (doi: 10.5281/zenodo.3239463).
Funding Statement
JN was supported in this project with funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant RGPIN-2016-04928 and NSERC Accelerator supplement RGPAS-493045-2016, http://www.nserc-crsng.gc.ca. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gutnik B, Mackie H, Hudson G, Standen C. How close to a pendulum is human upper limb movement during walking? HOMO—Journal of Comparative Human Biology. 2005;56(1):35–49. 10.1016/j.jchb.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 2. Kuhtz-Buschbeck JP, Jing B. Activity of upper limb muscles during human walking. Journal of Electromyography and Kinesiology. 2012;22(2):199–206. 10.1016/j.jelekin.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 3. Huang HJ, Ferris DP. Upper and lower limb muscle activation is bidirectionally and ipsilaterally coupled. Medicine and Science in Sports and Exercise. 2009;41(9):1778–1789. 10.1249/MSS.0b013e31819f75a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawashima N, Nozaki D, Abe MO, Nakazawa K. Shaping Appropriate Locomotive Motor Output Through Interlimb Neural Pathway Within Spinal Cord in Humans. Journal of Neurophysiology. 2008;99(6):2946–2955. 10.1152/jn.00020.2008 [DOI] [PubMed] [Google Scholar]
- 5. Ortega JD, Fehlman LA, Farley CT. Effects of aging and arm swing on the metabolic cost of stability in human walking. Journal of Biomechanics. 2008;41(16):3303–3308. 10.1016/j.jbiomech.2008.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang HS, Atkins LT, Jensen DB, James CR. Effects of constrained arm swing on vertical center of mass displacement during walking. Gait & Posture. 2015;42(4):430–434. 10.1016/j.gaitpost.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Bruijn SM, Meijer OG, Beek PJ, Dieën JHv. The effects of arm swing on human gait stability. Journal of Experimental Biology. 2010;213(23):3945–3952. 10.1242/jeb.045112 [DOI] [PubMed] [Google Scholar]
- 8. Punt M, Bruijn SM, Wittink H, van Dieën JH. Effect of arm swing strategy on local dynamic stability of human gait. Gait & Posture. 2015;41(2):504–509. 10.1016/j.gaitpost.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 9. Bruijn SM, Meijer OG, Beek PJ, Dieën JHv. Assessing the stability of human locomotion: a review of current measures. Journal of The Royal Society Interface. 2013;10(83):20120999 10.1098/rsif.2012.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arellano CJ, Kram R. The effects of step width and arm swing on energetic cost and lateral balance during running. Journal of Biomechanics. 2011;44(7):1291–1295. 10.1016/j.jbiomech.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 11. Nakakubo S, Doi T, Sawa R, Misu S, Tsutsumimoto K, Ono R. Does arm swing emphasized deliberately increase the trunk stability during walking in the elderly adults? Gait & Posture. 2014;40(4):516–520. 10.1016/j.gaitpost.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Mirelman A, Bernad-Elazari H, Nobel T, Thaler A, Peruzzi A, Plotnik M, et al. Effects of Aging on Arm Swing during Gait: The Role of Gait Speed and Dual Tasking. PLOS ONE. 2015;10(8):e0136043 10.1371/journal.pone.0136043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knutsson E. An Analysis of Parkinsonian Gait. Brain. 1972;95(3):475–486. 10.1093/brain/95.3.475 [DOI] [PubMed] [Google Scholar]
- 14. Zijlmans JCM, Poels PJE, Duysens J, Straaten Jvd, Thien T, Hof MAv, et al. Quantitative gait analysis in patients with vascular parkinsonism. Movement Disorders. 1996;11(5):501–508. 10.1002/mds.870110505 [DOI] [PubMed] [Google Scholar]
- 15.Carpinella I, Crenna P, Marzegan A, Rabuffetti M, Rizzone M, Lopiano L, et al. Effect of L-dopa and Subthalamic Nucleus stimulation on arm and leg swing during gait in Parkinson’s Disease. In: 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2007. p. 6664–6667. [DOI] [PubMed]
- 16. Siragy T, Nantel J. Quantifying Dynamic Balance in Young, Elderly and Parkinson’s Individuals: A Systematic Review. Frontiers in Aging Neuroscience. 2018;10 10.3389/fnagi.2018.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maki BE. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? Journal of the American Geriatrics Society. 1997;45(3):313–320. 10.1111/j.1532-5415.1997.tb00946.x [DOI] [PubMed] [Google Scholar]
- 18. Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait Variability in Community-Dwelling Older Adults. Journal of the American Geriatrics Society. 2001;49(12):1646–1650. 10.1046/j.1532-5415.2001.t01-1-49274.x [DOI] [PubMed] [Google Scholar]
- 19. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of Physical Medicine and Rehabilitation. 2001;82(8):1050–1056. 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 20. Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. European Journal of Neuroscience. 2006;24(6):1815–1820. 10.1111/j.1460-9568.2006.05033.x [DOI] [PubMed] [Google Scholar]
- 21. Wu Y, Li Y, Liu AM, Xiao F, Wang YZ, Hu F, et al. Effect of active arm swing to local dynamic stability during walking. Human Movement Science. 2016;45:102–109. 10.1016/j.humov.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 22. van Emmerik REA, Wagenaar RC. Effects of walking velocity on relative phase dynamics in the trunk in human walking. Journal of Biomechanics. 1996;29(9):1175–1184. 10.1016/0021-9290(95)00128-x [DOI] [PubMed] [Google Scholar]
- 23. Wagenaar RC, van Emmerik REA. Resonant frequencies of arms and legs identify different walking patterns. Journal of Biomechanics. 2000;33(7):853–861. 10.1016/s0021-9290(00)00020-8 [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Mahoney JM, Lewis MM, Guangwei Du, Piazza SJ, Cusumano JP. Both coordination and symmetry of arm swing are reduced in Parkinson’s disease. Gait & Posture. 2012;35(3):373–377. 10.1016/j.gaitpost.2011.10.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local Dynamic Stability Versus Kinematic Variability of Continuous Overground and Treadmill Walking. Journal of Biomechanical Engineering. 2000;123(1):27–32. 10.1115/1.1336798 [DOI] [PubMed] [Google Scholar]
- 26. van Schooten KS, Sloot LH, Bruijn SM, Kingma H, Meijer OG, Pijnappels M, et al. Sensitivity of trunk variability and stability measures to balance impairments induced by galvanic vestibular stimulation during gait. Gait & Posture. 2011;33(4):656–660. 10.1016/j.gaitpost.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 27. England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait & Posture. 2007;25(2):172–178. 10.1016/j.gaitpost.2006.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruijn SM, van Dieën JH, Meijer OG, Beek PJ. Is slow walking more stable? Journal of Biomechanics. 2009;42(10):1506–1512. 10.1016/j.jbiomech.2009.03.047 [DOI] [PubMed] [Google Scholar]
- 29. Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clinical Biomechanics. 2003;18(5):435–443. 10.1016/s0268-0033(03)00029-9 [DOI] [PubMed] [Google Scholar]
- 30. Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Experimental Brain Research. 2007;177(3):336–346. 10.1007/s00221-006-0676-3 [DOI] [PubMed] [Google Scholar]
- 31. Darter BJ, Labrecque BA, Perera RA. Dynamic stability during split-belt walking and the relationship with step length symmetry. Gait & Posture. 2018;62:86–91. 10.1016/j.gaitpost.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buurke TJW, Lamoth CJC, Vervoort D, Woude LHVvd, Otter Rd. Adaptive control of dynamic balance in human gait on a split-belt treadmill. Journal of Experimental Biology. 2018;221(13):jeb174896 10.1242/jeb.174896 [DOI] [PubMed] [Google Scholar]
- 33. Wilken JM, Rodriguez KM, Brawner M, Darter BJ. Reliability and minimal detectible change values for gait kinematics and kinetics in healthy adults. Gait & Posture. 2012;35(2):301–307. 10.1016/j.gaitpost.2011.09.105 [DOI] [PubMed] [Google Scholar]
- 34. Bezanson J, Edelman A, Karpinski S, Shah V. Julia: A Fresh Approach to Numerical Computing. SIAM Review. 2017;59(1):65–98. 10.1137/141000671 [DOI] [Google Scholar]
- 35. Lamb PF, Stöckl M. On the use of continuous relative phase: Review of current approaches and outline for a new standard. Clinical Biomechanics. 2014;29(5):484–493. 10.1016/j.clinbiomech.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 36. Fisher NI. Statistical Analysis of Circular Data. Cambridge University Press; 1993. [Google Scholar]
- 37. Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Experimental Brain Research. 2007;181(4):561–570. 10.1007/s00221-007-0955-7 [DOI] [PubMed] [Google Scholar]
- 38. Rosenstein MT, Collins JJ, De Luca CJ. A practical method for calculating largest Lyapunov exponents from small data sets. Physica D: Nonlinear Phenomena. 1993;65(1):117–134. 10.1016/0167-2789(93)90009-P [DOI] [Google Scholar]
- 39. Bruijn SM, van Dieën JH, Meijer OG, Beek PJ. Statistical precision and sensitivity of measures of dynamic gait stability. Journal of Neuroscience Methods. 2009;178(2):327–333. 10.1016/j.jneumeth.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 40. Bruijn SM, Kate WRTT, Faber GS, Meijer OG, Beek PJ, Dieën JHv. Estimating Dynamic Gait Stability Using Data from Non-aligned Inertial Sensors. Annals of Biomedical Engineering. 2010;38(8):2588–2593. 10.1007/s10439-010-0018-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang HG, Dingwell JB. Intra-session reliability of local dynamic stability of walking. Gait & Posture. 2006;24(3):386–390. 10.1016/j.gaitpost.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 42. Toebes MJP, Hoozemans MJM, Furrer R, Dekker J, van Dieën JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait & Posture. 2012;36(3):527–531. 10.1016/j.gaitpost.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 43. Su JLS, Dingwell JB. Dynamic Stability of Passive Dynamic Walking on an Irregular Surface. Journal of Biomechanical Engineering. 2007;129(6):802–810. 10.1115/1.2800760 [DOI] [PubMed] [Google Scholar]
- 44. Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos: An Interdisciplinary Journal of Nonlinear Science. 2000;10(4):848–863. 10.1063/1.1324008 [DOI] [PubMed] [Google Scholar]
- 45. Herr H, Popovic M. Angular momentum in human walking. Journal of Experimental Biology. 2008;211(4):467–481. 10.1242/jeb.008573 [DOI] [PubMed] [Google Scholar]
- 46. Bruijn SM, Bregman DJJ, Meijer OG, Beek PJ, van Dieën JH. Maximum Lyapunov exponents as predictors of global gait stability: A modelling approach. Medical Engineering & Physics. 2012;34(4):428–436. 10.1016/j.medengphy.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 47. McFadyen BJ, Hegeman J, Duysens J. Dual task effects for asymmetric stepping on a split-belt treadmill. Gait & Posture. 2009;30(3):340–344. 10.1016/j.gaitpost.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 48. Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement Disorders. 2006;21(7):950–957. 10.1002/mds.20848 [DOI] [PubMed] [Google Scholar]
- 49. Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. Journal of NeuroEngineering and Rehabilitation. 2011;8(1):2 10.1186/1743-0003-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait & Posture. 2007;26(1):113–119. 10.1016/j.gaitpost.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 51. Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? Journal of Neurophysiology. 2012;108(2):672–683. 10.1152/jn.00391.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reisman DS, Block HJ, Bastian AJ. Interlimb Coordination During Locomotion: What Can be Adapted and Stored? Journal of Neurophysiology. 2005;94(4):2403–2415. 10.1152/jn.00089.2005 [DOI] [PubMed] [Google Scholar]
- 53. MacLellan MJ, Qaderdan K, Koehestanie P, Duysens J, McFadyen BJ. Arm movements during split-belt walking reveal predominant patterns of interlimb coupling. Human Movement Science. 2013;32(1):79–90. 10.1016/j.humov.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 54. Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Experimental Brain Research. 1994;101(3):513–520. 10.1007/bf00227344 [DOI] [PubMed] [Google Scholar]
- 55. Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. Journal of Neurophysiology. 2012;108(4):1149–1157. 10.1152/jn.00018.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roemmich RT, Nocera JR, Stegemöller EL, Hassan A, Okun MS, Hass CJ. Locomotor adaptation and locomotor adaptive learning in Parkinson’s disease and normal aging. Clinical Neurophysiology. 2014;125(2):313–319. 10.1016/j.clinph.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Annals of Neurology. 2005;57(5):656–663. 10.1002/ana.20452 [DOI] [PubMed] [Google Scholar]
- 58. Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(6):721–725. 10.1136/jnnp.72.6.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed in this paper, along with the code developed to analyze the data, are available on Zenodo at https://zenodo.org/record/3239464 (doi: 10.5281/zenodo.3239463).