Abstract
Purpose
Long non-coding RNAs (lncRNAs) have been proved to act crucial parts in the progress of human tumor. However, the role of lncRNAs in drug resistance of tumor cells remains to be further elucidated. The present study aimed to explore whether lncRNA NCK-AS1 could affect the cisplatin (DDP) resistance in human osteosarcoma cell and the underlying molecular mechanism.
Methods
The expression of NCK1-AS1 and miR-137 in osteosarcoma cells was detected by qRT-PCR. CCK-8 assay, colony formation assay, Western blotting, wound healing assay and transwell assay were employed to assess the cell proliferation, migration and invasion. In addition, CCK-8 assay, flow cytometry, qRT-PCR and resistance gene activity analysis were performed to assess the DDP sensitivity of osteosarcoma cells. The interaction between NCK1-AS1 and miR-137 was identified using a dual-luciferase reporter gene assay and RNA immunoprecipitation (RIP) assay.
Results
The results revealed that NCK1-AS1 was significantly upregulated in osteosarcoma cells, as well as in DDP-resistant osteosarcoma cells. NCK1-AS1 silence inhibited the proliferation, migration and invasion of osteosarcoma cells, whereas enhanced the sensitivity of osteosarcoma cells to DDP. Furthermore, NCK1-AS1 directly interacted with miR-137 and overexpression of miR-137 suppressed the proliferation, migration and invasion of osteosarcoma cells. Most importantly, miR-137 overexpression enhanced the sensitivity of osteosarcoma cells to DDP, and high expression of NCK1-AS1 reversed the influences of miR-137 overexpression on DDP-resistant cells.
Conclusion
In short, NCK1-AS1 knockdown enhanced DDP sensitivity of osteosarcoma cells by regulating miR-137, which may be a novel potential target for anti-DDP resistance in human osteosarcoma.
Keywords: osteosarcoma, cisplatin, drug resistance, NCK1-AS1, miR-137
Introduction
Osteosarcoma is a primary malignant bone tumor characterized by the direct formation of immature bone or osteoid tissue by tumor cells, most commonly affecting children and young people.1,2 The long-term survival rate of osteosarcoma patients has been raised to 70% with the combination of surgery and chemotherapy,3 such as methotrexate, doxorubicin, and cisplatin (DDP) which is the most widely used platinum-based anticancer drug for solid tumors.4 However, the therapeutic efficacy of DDP on osteosarcoma is gradually declined owing to the emergence of DDP resistance.5 Therefore, a better understanding of the molecular mechanisms underlying DDP resistance in osteosarcoma is essential to improve the treatment and prognosis of osteosarcoma.
Long non-coding RNAs (lncRNAs) are a class of transcripts that are longer than 200 nucleotides without protein-coding capacity.6 Accumulating evidence demonstrates that lncRNAs play vital roles in malignant physiological or pathological processes in tumors, such as proliferation, invasion, metastasis, and apoptosis.7 Moreover, lncRNAs are regarded as important regulatory factors in cancer-related drug resistance.8 For instance, overexpression of LncRNA MEG3 enhanced cisplatin sensitivity by targeting miR-21-5p/SOX7 axis in non-small cell lung cancer.9 LncRNA HOTAIR promoted cisplatin resistance in gastric cancer via activating the PI3K/AKT/MRP1 genes by regulating miR-126.10 As a newly discovered lncRNA, NCK-AS1 has been recently found to promote proliferation and induce cell cycle progression in cervical cancer.11 In addition, knockdown of lncRNA NCK-AS1 increased the chemosensitivity to cisplatin in cervical cancer.12 However, the biological role of NCK1-AS1 in osteosarcoma remains unclear.
To date, the interaction between lncRNAs and microRNAs has attracted great attention.13 One way for lncRNAs to exert potential function was to directly interact with microRNAs (miRNAs) as sponges and regulate their expression.14 Another way is to serve as competing endogenous RNAs (ceRNAs) to separate miRNAs from mRNAs.9 microRNA-137 (miR-137), a novel tumor suppressor, has been found to be downregulated in several cancer including osteosarcoma,15 lung cancer16 and glioblastoma.17 It has been demonstrated that miR-137 acted as a tumor suppressor by targeting enhancer of zeste homolog 2 in osteosarcoma.18 Furthermore, miR-137 was proved to be downregulated in osteosarcoma and regulate cell proliferation and migration through targeting FXYD6.19 Yet, there has no evidence to confirm the role of miR-137 in DDP resistance in osteosarcoma.
In the present study, the expression of NCK-AS1 and miR-137 in osteosarcoma cells was measured and the functions of NCK-AS1 and miR-137 on osteosarcoma proliferation, migration and DDP resistance were investigated. More importantly, we demonstrated that NCK-AS1 could regulate cisplatin resistance via targeting miR-137 in osteosarcoma cells.
Materials And Methods
Cell Lines And Cell Culture
Osteosarcoma cell lines (MG63, KHOS and U2OS) and the normal osteoblastic cell line (hFOB) were obtained from the CCTCC (China Center for Type Culture Collection, Shanghai, China). The osteosarcoma cell lines and the hFOB cell line were maintained in DMEM (Invitrogen-Life Technologies Inc.) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin in an incubator with an atmosphere of 5% CO2 at 37 °C. To establish DDP-resistant osteosarcoma cells (MG63-cis, KHOS-cis and U2OS-cis), the cells were exposed to incremental doses of DDP (Sigma-Aldrich Co., USA). To maintain the DDP-resistant phenotype, 2 μM DDP was added to the medium of DDP-resistant osteosarcoma cells every day until the experiments were performed.
Cell Transfection
The plasmid vectors shRNA- NCK1-AS1, pcDNA- NCK1-AS1, and negative control (control shRNA and control pcDNA) were purchased from GenePharma Company (Shanghai, China). The miRNA-137-mimic and negative control miR-NC were synthesized by Invitrogen (Nanjing, China). The plasmid vectors and the mimics were transfected into osteosarcoma cells by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocols.
RNA Extraction And qRT-PCR
Total RNA was extracted from osteosarcoma cell lines with a TRIzol reagent (Invitrogen, USA) in accordance with the manufacturer’s protocol. The purity and concentration of RNA were determined by NanoDrop 3000 (ThermoScientific, MA) at 260 and 280 nm. Then, the RNA was converted to cDNA by PrimeScript RT Master Mix (Takara, Japan). PCR reaction was performed in triplicates on an ABI PRISM 7900 Fluorescent Quantitative PCR system (Applied Biosystems, USA) with the SYBR Premix ExTaq kit (Takara, Japan). Furthermore, GAPDH and U6 small nuclear RNA served as the internal control for the lncRNA and miRNA, respectively.
Cell Proliferation Assay
MG63 and MG63-cis cells were cultured in 96-well plates at 2×103 cells/well. Cell proliferation was measured by CCK-8 after transfection for 24, 48 and 72 h. The transfected cells were treated with 10 μL CCK-8 reagent (Dojindo, Japan) and incubated for 2 h in the dark. The OD value was measured at 450 nm with the microplate reader (BioTek Instruments, VT). Three experiments were performed independently.
Colony Formation Assay
After transfection, MG63 and MG63-cis cells were seeded into six-well plates at a density of 4x105 cells/well and cultured in DMEM with 10% FBS at 37°C for 2 weeks. When the visible clones appeared, cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Colonies were counted with a light microscope (Olympus Corp, Japan). The number of colonies which contains more than 50 cells was counted. All the experiments were repeated for three times.
Western Blot Analysis
Transfected cells were harvested, and protein concentrations were analyzed by a BCA protein assay kit (Beyotime, China). Equal amount of total protein (40 μg) was separated by 10% SDS-PAGE and transferred onto PVDF membranes. Subsequently, the membrane was blocked in 5% BSA at room temperature for 1 h and incubated at 4°C overnight with primary antibodies against CDK2(1:1000, ab235941), cyclinE1(1:2000, ab71535), p21 (1:1000, ab227443), MMP9 (1:1000, ab38898), MMP13 (1:3000, ab39012), Bcl-2 (1:2000, ab196495), Bax (1:1000, ab53154), cleaved caspase-3 (1µg/mL, ab2302), MRP1 (1:1000, ab233383), GST-π (1:3000, ab53943), ABCB1(1:1000, 13342S, Cell Signaling Technology). GAPDH was used as an internal control. After washing three times with PBST, the membrane was incubated with HRP-conjugated secondary antibodies (1:1000, Santa Cruz) for 2 h at room temperature. Finally, the protein bands on the membrane were visualized with Quantity One software (Bio-Rad Life Science, China) Image analysis were performed using Image J (NIH, USA) and GraphPad Prism 6 (GraphPad Software, USA).
Cytotoxicity Assay
The sensitivity of MG63 and MG63-cis cells to DDP treatment was detected by CCK-8 assay. Transfected cells were seeded into each well of 96-well plates. After cell attachment, cells were treated with different doses of DDP (0, 10, 20, 40, 60 and 80μM) for 48h. Then CCK-8 solution was added and incubated for 2 h at 37 °C. Half‐maximal inhibitory concentration (IC50) of DDP was estimated using GraphPad Prism 6.0 software. The OD value was measured at 450 nm with the microplate reader. Three experiments were performed independently.
Wound Healing Assay
Transfected cells were seeded into a six-well plate and cultured to grow to 90% confluency in DMEM with 10% FBS at 37 °C. The cell monolayers were wounded by a white pipette tip and washed three times with serum-free medium. After incubation for 24 h, the number of migrating cells was counted by an inverted microscopy. Five fields were randomly chosen to analyze in each well.
Transwell Assay
The transwell chambers (Corning Costar, Cambridge, MA) were first coated with 0.1mL of matrigel (Becton Dickinson, MA) at 37 °C for 1 h. After 72 h transfection, the cells were collected and suspended at a final concentration of 2×105 cells/mL in serum-free DMEM. Cell suspensions were then placed into the upper wells, and the medium with 5% fetal bovine serum was loaded in the lower chamber. After incubation for 24 h, the non-invaded cells on the upper face of the transwell membrane were wiped off with a cotton swab. Then the invaded cells on the lower face were fixed with 100% methanol, stained with hematoxylin and eosin and finally counted with a microscope. Five randomly chosen fields were counted for each group.
Cell Apoptosis
The cells apoptosis was detected by the FITC Annexin V/PI Apoptosis Detection Kit I (Ribobio, China) according to the manufacturer’s protocol. Briefly, the cells were washed with pre-cooled PBS and re-suspended with binding buffer after transfection for 72h. Then, the cells were incubated with Annexin V-FITC and propidium iodide for 20 min in darkness. A FACScan flow cytometer was applied for apoptosis analysis. Each experiment was carried out in triplicate.
Dual Luciferase Reporter Assay
The fragments of NCK1-AS1 3′ untranslated region (UTR) that included the putative binding sites of miRNA-137 were cloned into a pGL3 vector (Promega, WI) to create the NCK1-AS1 reporter (NCK1-AS1-WT). The mutants in NCK1-AS1 were constructed by mutating the miRNA-137 seed region binding site. Subsequently, miRNA-137 mimic and its negative control (mimic-NC) were co-transfected with constructed luciferase reporter vectors by using the Lipofectamine 2000 transfection reagent (Invitrogen). The luciferase activity was confirmed by dual Glo™ Luciferase Assay System (Promega) at 48 hrs post-transfection.
RIP Assay
RNA immunoprecipitation (RIP) assays were performed using an EZ-Magna RiP Kit (Millipore, USA) following the manufacturer’s instructions. MG63 and MG63-cis cells were lysed by the buffer of RIP lysis and incubated with magnetic beads labelled with an anti-AGO2 antibody or normal mouse IgG control (Abcam). The RNAs were isolated and the levels of NCK1-AS1 and miRNA-137 were measured by qRT-PCR.
Statistical Analysis
SPSS 23.0 software (SPSS Inc., USA) was used to analyze the data. All the experimental data were expressed as mean ± SD. One-way ANOVA and a two-tailed unpaired Student’s t-test were applied to compare the multiple groups and differences between two groups, respectively. P < 0.05 was considered to indicate statistical significance.
Results
NCK1-AS1 Was Upregulated In Parental And DDP-Resistant Osteosarcoma Cell Lines
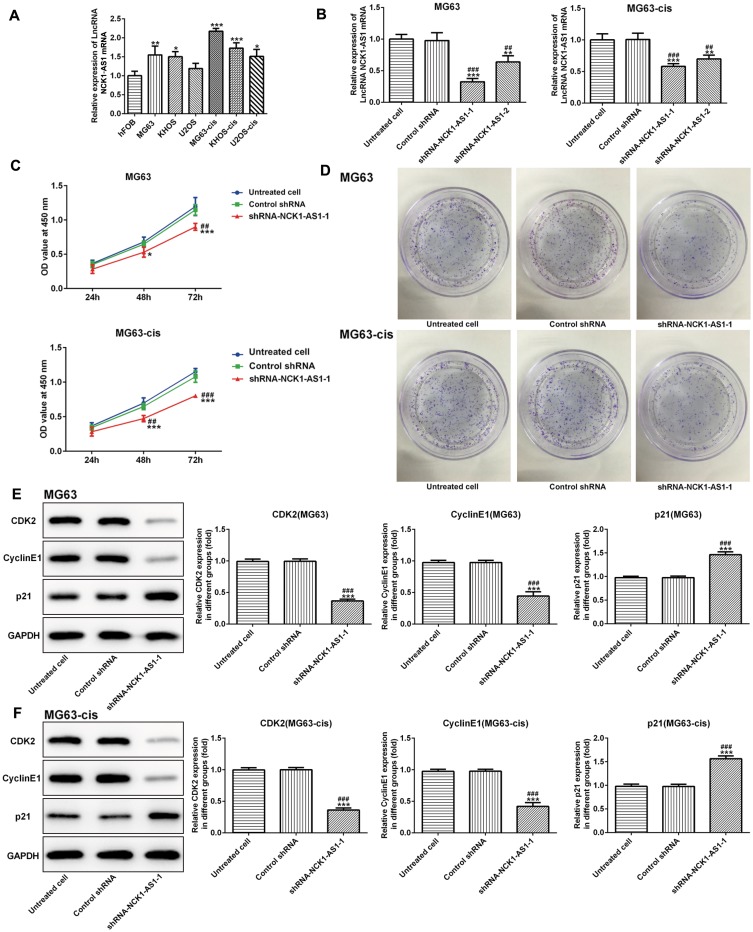
To elucidate the role of NCK1-AS1 in the development of osteosarcoma, the expression of NCK1-AS1 in osteosarcoma cell lines and the normal osteoblastic cell-line hFOB was measured. The result of qRT-PCR showed an obvious increase in NCK1-AS1 expression in both parental and DDP-resistant osteosarcoma cells, particularly in MG63 and MG63-cis cells (Figure 1A). Thus, MG63 and MG63-cis cells were chosen for subsequent experiments.
Figure 1.
NCK1-AS1 expression and effects of NCK1-AS1 silence on proliferation in parental and DDP-resistant osteosarcoma cells. (A) mRNA level of NCK1-AS1 in normal and osteosarcoma cells. (B) mRNA expression of NCK1-AS1 after transfection of with shRNA- NCK1-AS1 vectors in MG63 and MG63-cis cells. (C) Cell viability of shRNA- NCK1-AS1 transfected MG63 and MG63-cis cells was measured by CCK-8 assay. (D) Colony formation assay was carried out to evaluate the capacity of cell proliferation of parental and DDP-resistant MG63cells transfected with shRNA- NCK1-AS1. (E and F) Levels of CDK2, cyclinE1and p21 were detected by Western blot analysis. The data are shown as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Untreated cells; ##P < 0.01, ###P < 0.001 vs. Control shRNA group.
Knockdown Of NCK1-AS1 Attenuates Cell Proliferation, Invasion And Migration In MG63 And MG63-Cis Cells
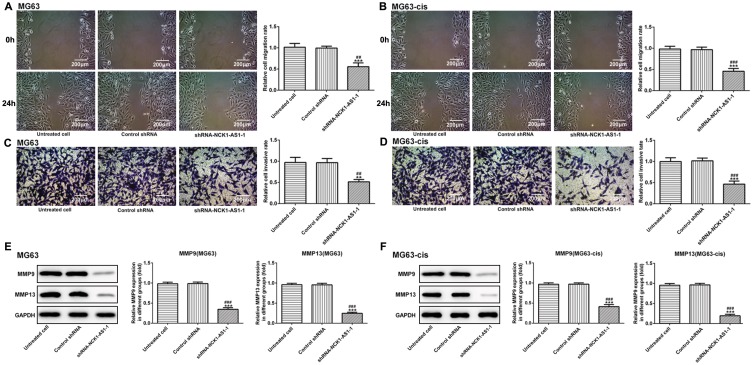
Next, we examined the influence of NCK1-AS1 on MG63 and MG63-cis cells by downregulating NCK1-AS1 expression with shRNA- NCK1-AS1 vectors. According to qRT-PCR, we verified that NCK1-AS1 expression was significantly reduced in the transfected DDP-resistant cells and their parental cells (Figure1B). The result of CCK-8 assay exhibited that cell growth was extremely inhibited after knockdown of NCK1-AS1 when compared with the vector control (Figure 1C). The result of colony formation assay showed a significant decrease of colony numbers in MG63/MG63-cis cells transfected with shRNA-NCK1-AS1 (Figure 1D). Consistently, we found the declining protein level of CDK2 and cyclinE1 while the level of p21 was elevated in two transfected cell lines compared with the negative control (Figure 1E and F). In addition, wound healing assay and transwell assay were employed to determine cell invasion and migration. As shown in Figure 2A–D, the capacities of invasion and migration were markedly inhibited due to NCK1-AS1silence. The data was further validated by a Western blotting assay that showed lower protein levels of MMP9 and MMP13 in MG63/MG63-cis cells transfected with shRNA- NCK1-AS1 compared to the negative control group (Figure 2E and F). These data indicated that NCK1-AS1 knockdown may exert its anticancer effects by suppressing the proliferation, invasion and migration of osteosarcoma cell.
Figure 2.
Knockdown of NCK1-AS1 inhibits the invasion and migration in MG63 and MG63-cis cells. Wound healing assay was used to measure migratory ability of MG63 (A) and MG63-cis (B) cells treated with NCK1-AS1 silence. Image magnification: 100×. The invasive capacity of MG63 (C) and MG63-cis (D) cells was determined by transwell assay. Image magnification: 100×. (E and F) Western blot analysis was employed to assess the protein levels of MMP9 and MMP13 in parental and DDP-resistant MG63cells. The data are shown as the means ± SD. **P < 0.01, ***P < 0.001 vs. Untreated cells; ##P < 0.01, ###P < 0.001 vs. Control shRNA group.
NCK1-AS1 Silence Enhances The Sensitivity Of DDP-Resistant MG63 Cells
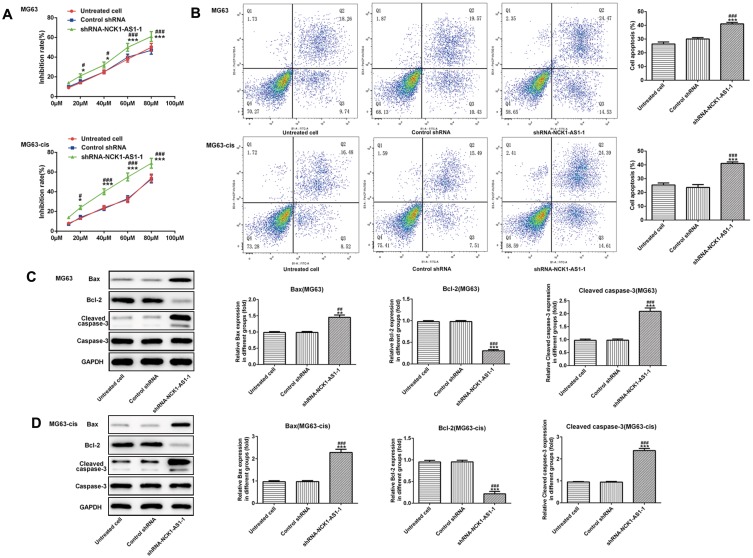
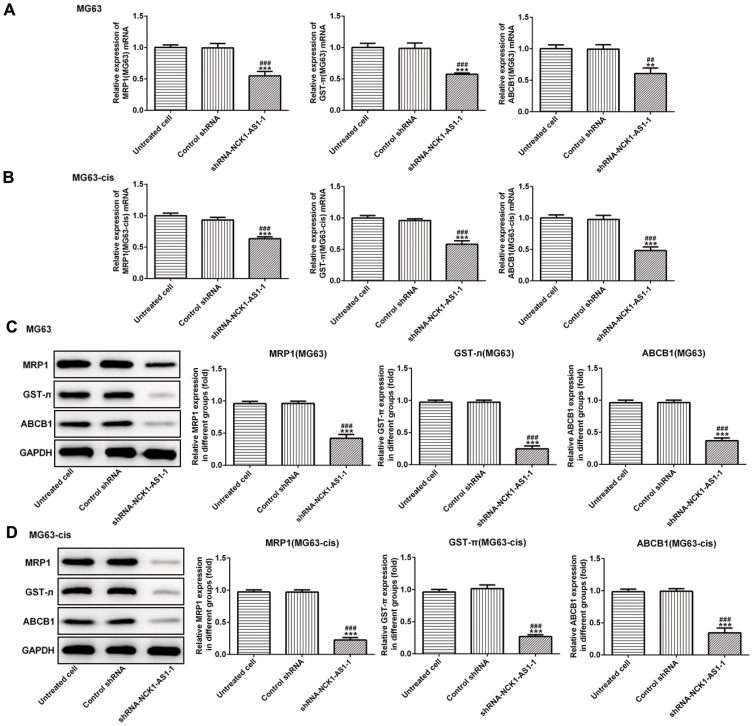
We continued to test the involvement of NCK1-AS1 in DDP resistance of MG63 cells. CCK-8 assay was used to examine cytotoxicity and IC50 in DDP-resistant MG63 cells. The results showed that exposed to cisplatin, cell viability in transfected DDP-resistant and parental MG63 cells was considerably decreased in a dose-dependent manner when transfected with shRNA-NCK1-AS1. In addition, higher IC50 values were exhibited in MG63 and MG63-cis cells with shRNA-NCK1-AS1 transfection in contrast to the control shRNA group (Figure 3A). Flow cytometric analysis showed that knockdown of NCK1-AS1 evidently promoted apoptosis rate in both MG63 and MG63-cis cells (Figure 3B). Meanwhile, the results from Western blotting assay showed the downregulated protein expression of Bcl-2 and upregulated levels of Bax and cleaved caspase 3 in the two cell lines after transfection with shRNA-NCK1-AS1 (Figure 3C and D). Furthermore, the expression levels of three drug resistance gene MRP1, GST-π and ABCB1 were also detected. As shown in Figure 4, the mRNA expression and protein levels of MRP1, GST-π and ABCB1 were significantly decreased in shRNA-NCK1-AS1 transfected MG63 cells. These results revealed that inhibition of NCK1-AS1 plays an important role in cisplatin sensitivity in DDP-resistant cells.
Figure 3.
Effects of NCK1-AS1 silence on the DDP sensitivity of MG63 and MG63-cis cells. (A) Cell viability of MG63 and MG63-cis cells transfected with shRNA- NCK1-AS1 was detected by CCK-8 assay in the present of different dose of DDP. (B) Flow cytometric analysis was performed to estimate MG63 and MG63-cis cell apoptosis after transfection with shRNA- NCK1-AS1. Levels of Bax, Bcl-2 and cleaved caspase 3 in MG63 (C) and MG63-cis (D) cells were measured by Western blot analysis. The data are shown as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Untreated cells; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Control shRNA group.
Figure 4.
The expression of drug resistance genes including MRP1, GST-π and ABCB1 was decreased in parental and DDP-resistant MG63 cells transfected with shRNA- NCK1-AS1 or control shRNA. The mRNA expression of MRP1, GST-π and ABCB1 in MG63 (A) and MG63-cis (B) cells was detected by qRT-PCR. The protein level of MRP1, GST-π and ABCB1 was determined by Western blots in MG63 (C) and MG63-cis (D) cells. The data are shown as the means ± SD. **P < 0.01, ***P < 0.001 vs. Untreated cells; ##P < 0.01, ###P < 0.001 vs. Control shRNA group.
NCK1-AS1 Targets miRNA-137 In MG63 Cells
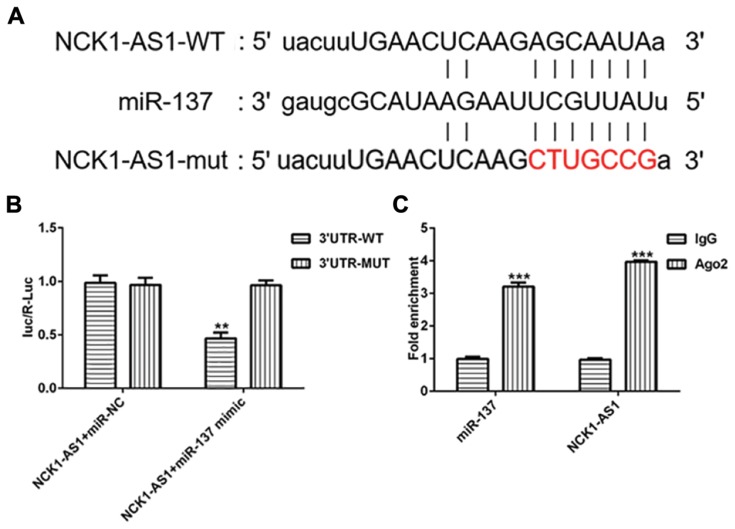
To further investigate the mechanism by which NCK1-AS1 inhibited DDP resistant of MG63 cells, the online database “starBase” (http://starbase.sysu.edu.cn) was used to search the target miRNAs that could bind to NCK1-AS1 and we found miRNA-137 harbored the complementary sequences with NCK1-AS1, suggesting that miRNA-137 could be a ceRNA for NCK1-AS1 (Figure 5A). To prove the putative, luciferase reporters was constructed, containing the wild-type and mutant sequences of NCK1-AS1. The luciferase activity of wild-type NCK1-AS1 reporter was notably inhibited in MG63 cells transfected with miRNA-137 mimic, while that of mutant reporter did not be obviously affected (Figure 5B). Furthermore, the RNA immunoprecipitation (RIP) assay was also performed to validate the interaction of miRNA-137 and NCK1-AS1. As presented in Figure 5C, compared with the IgG immunoprecipitates control, NCK1-AS1 and miRNA-137 from MG63 cells were enriched in Ago2 immunoprecipitates. These data demonstrated that miRNA-137 is the direct target of NCK1-AS1 in MG63 cells.
Figure 5.
NCK1-AS1 directly targets miRNA-137. (A) The binding sequence between NCK1-AS1 and miRNA-137 were predicted putatively. (B) The luciferase activity of wild-type or mutant NCK1-AS1 reporter was detected in MG63 cells transfected with shRNA- NCK1-AS1. (C) Immunoprecipitation (RIP) assay was performed to confirm the relationship between NCK1-AS1 and miRNA-137. The data are shown as the means ± SD. **P < 0.01, ***P < 0.001 vs. Untreated cells.
miRNA-137 Is Associated With The Proliferation, Invasion And Migration Of DDP-Resistant MG63 Cells
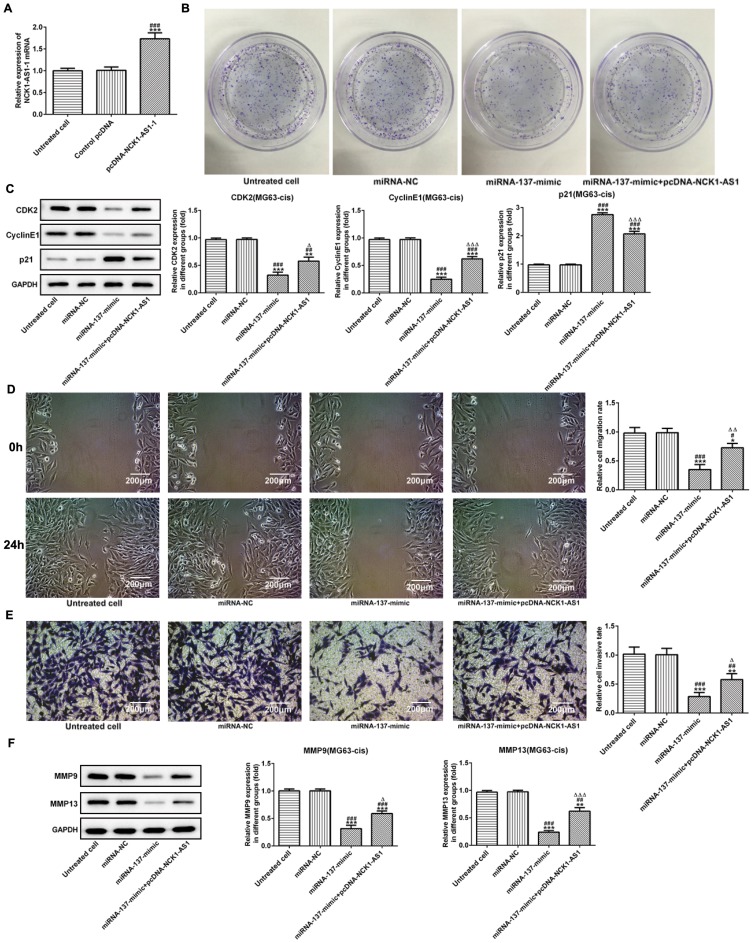
Based on the above findings, we explored the biological role of miRNA-137 in DDP-resistant MG63 cells. NCK1-AS1 expression was upregulated by transfecting overexpressed NCK1-AS1 vectors (pcDNA-NCK1-AS1) into the MG63-cis cells (Figure 6A). The colony formation assay showed that miRNA-137 mimic noticeably inhibited the cell growth while the multiplication capacity of MG63-cis cells was recovered when transfected with pcDNA-NCK1-AS1 compared with the miRNA-137 mimic group (Figure 6B). Regarding Western blot analysis, CDK2 and cyclinE1 had a significant reduction, and p21 decreased in miRNA-137 mimic transfected cells, while co-transfection with miRNA-137 mimic and pcDNA-NCK1-AS1 reversed the protein levels above (Figure 6C). More importantly, we found that overexpression of miRNA-137 extremely suppressed the abilities of invasion and migration of MG63-cis cells, but upregulation of NCK1-AS1 abolished the suppressive effect on the invasion and migration of miRNA-137 (Figure 6D and E). Additionally, data from the Western blotting assay revealed that transfection with miR‑137 mimic considerably reduced the protein levels of MMP9 and MMP13. By contrast, NCK1-AS1 overexpressed cells showed an increased tendency on both protein levels when compared with the miRNA-137 mimic treatment (Figure 6F). The results above suggested that miRNA-137 overexpression may inhibit the proliferation, invasion and migration, in DDP-resistant MG63 cells.
Figure 6.
Overexpression of miRNA-137 represses cell proliferation, invasion and migration of MG63-cis cells. (A) qRT-PCR was employed to measure the expression level of NCK1-AS1 in MG63-cis cells after transfection of pcDNA-NCK1-AS1. (B) The colony formation assay was applied in MG63-cis cells transfected with miRNA-137 mimic or/and pcDNA-NCK1-AS1. (C) The levels of CDK2, cyclinE1 and p21 were evaluated in MG63-cis cells transfected with miRNA-137 mimic or/and pcDNA-NCK1-AS1. The migratory and invasive capacity of MG63-cis cells were measured by wound healing assay (D) and transwell assay (E), respectively. Image magnification: 100×. (F) MMP9 and MMP13 level in MG63-cis cells was altered by transfection of miRNA-137 mimic or/and pcDNA-NCK1-AS1. The data are shown as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Untreated cells; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. miRNA-NC group; △P < 0.05, △△P < 0.01, △△△P < 0.001 vs. miRNA-137 mimic.
Overexpression Of miRNA-137 Regulates DDP Resistant Of MG63 Cells
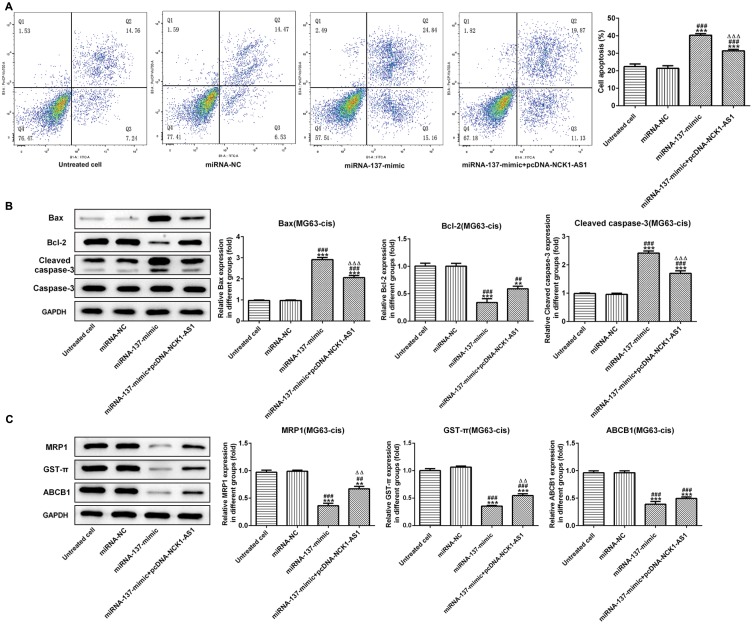
Finally, we further assessed the effect of miRNA-137 on cisplatin resistant in MG63-cis cells. As showed in Figure 7A, the result of flow cytometric analysis showed that miRNA-137 mimic facilitated the DDP-induced apoptosis while overexpressed NCK1-AS1 attenuated the apoptosis in DDP-resistant MG63 cells. A similar result of the effects that miRNA-137 exerts on the DDP-resistance was observed via Western blot assay (Figure 7B). Moreover, we discovered that the activities of MRP1, GST-π and ABCB1 were significantly inhibited in MG63-cis cells transfected with miRNA-137 mimic, while NCK1-AS1 overexpression elevated the miRNA-137 mimic-induced decreased levels of the three proteins (Figure 7C). Thus, we verified that miRNA-137 enhances the sensitivity of DDP-resistance MG63 cells and the miRNA-137-induced effects were reversed by overexpressing NCK1-AS1 in MG63-cis cells.
Figure 7.
Effects of miRNA-137 overexpression on DDP resistant of MG63-cis cells. (A) upregulation of miRNA-137 promoted cell apoptosis while overexpression of NCK1-AS1 inhibited the apoptosis of MG63-cis cells after transfection with miRNA-137 mimic or/and pcDNA-NCK1-AS1. (B) Western blot analysis was implemented to measure the levels of Bax, Bcl-2 and cleaved caspase 3 in MG63-cis cells. (C) Upregulation of miRNA-137 declined the level of MRP1, GST-π and ABCB1, whereas NCK1-AS1 overexpression increased the level of those proteins in MG63-cis cells. The data are shown as the means ± SD. **P < 0.01, ***P < 0.001 vs. Untreated cells; ##P < 0.01, ###P < 0.001 vs. miRNA-NC group; △△P < 0.01, △△△P < 0.001 vs. miRNA-137 mimic.
Discussion
DDP has been widely used in various cancers including osteosarcoma.20,21 However, the DDP chemoresistance hinders its remarkable long-term curative effect.22 Aberrant expression of lncRNAs was thought to be associated with the occurrence and malignance of cancers, and even tumor chemoresistance.23 For instance, Huang et al revealed that LncRNA NR2F1-AS1 modulated oxaliplatin (OXA) resistance by endogenous sponging miR-363 in hepatocellular carcinoma.24 LncRNA CCAT1 was revealed to regulate the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 signaling pathway.25 Hu et al found that downregulation of lncRNA NEAT1 in osteosarcoma contributed to the enhanced sensitivity to cisplatin and inhibition of tumor growth.26 The involvement of lncRNA in different stages of cancers showed extremely close relationship with drug resistance of cancers, so more profound understanding of molecular mechanism by which lncRNAs resist chemoresistance of tumors is pivotal.
In the current study, we investigated the role and the potential mechanism of lncRNA NCK-AS1 in DDP resistance of osteosarcoma cells. The results demonstrated that NCK1-AS1 expression was upregulated in both parental and DDP-resistant osteosarcoma cell lines. In addition, NCK1-AS1 silence inhibited cell proliferation by attenuating cell viability and abolishing the activities of proliferation-related proteins CDK2 and cyclinE1, repressed cell invasion and migration, and suppressed the protein expression of MMP9 and MMP13 in parental and DDP-resistant MG63 cells. Consistently, Li et al reported that LncRNA NCK1-AS1 was greatly upregulated in cervical cancer tissues and knockdown of NCK1-AS1 inhibited cell proliferation and affected the cell-cycle progression by NCK1-AS1/miR-6857/CDK1 pathway. However, the protein expressions look exactly alike in untreated and control shRNA group. We think it may result from the little effect of transfection with negative vectors on biological processes of cells. Thus, the expressions of proteins involved in cell proliferation, metastasis, apoptosis and drug resistance in untreated and control shRNA group are similar.
Multidrug resistant transporters MRP1 and ABCB1 belong to the ATP-binding cassette (ABC) transporter superfamily and GST-π is a member of glutathione-S-transferases (GSTs), a family of phase II detoxification enzymes.27 Both these two families play major roles in cisplatin-induced multidrug resistance.28 The current study reported that the silencing of NCK1-AS1 also improved the sensitivity of DDP-resistant MG63 cells by accelerating the apoptosis and abolishing the multidrug resistant transporters, including MRP1, ABCB1 and GST-π. Zhang et al found that the silencing of LncRNA NCK1-AS1 weakened the MSH2 activity and accelerated the DDP sensitivity by targeting miR-134-5p in cervical cancer, consistent with our findings. These data suggest the regulative effects of NCK1-AS1 on DDP resistance in DDP-resistance osteosarcoma cells.
Emerging reports have described that lncRNAs function as competing endogenous RNAs (ceRNAs) or endogenous miRNA sponges via combining with miRNAs, to regulate their biological activity.29,30 In the present study, we found miRNA-137 had a predicted sequence which can bind to the 3′UTR of NCK1-AS1 by bioinformatic analysis. Further, the luciferase reporter and RIP assays were performed to validate that miRNA-137 binds to NCK1-AS1 directly. miRNA-137 has been reported to be a novel tumor suppressor and modulates the drug sensitivity in different kinds of cancer.31,32 It was documented that miRNA-137 expression was downregulated in osteosarcoma cell and the upregulation of miR-137 attenuated the proliferation and metastasis by affecting FXYD6 or EZH2.18,19 Moreover, miR-137 overexpression in multiple myeloma cells enhanced the bortezomib and eprirubicin sensitivity and ameliorated chromosomal instability by targeting AURKA.33 In neuroblastoma, miRNA-137 was also found to negatively regulate constitutive androstane receptor and suppress the resistant to doxorubicin in parental and doxorubicin-resistant cells.34 Consistent with the previous findings, our studies demonstrated that miRNA-137 acted as a tumor suppressor by inhibiting the proliferation, invasion and migration of osteosarcoma cell. Additionally, overexpression of miRNA-137 repressed the resistant to DDP in osteosarcoma cells. Interestingly, overexpression of NCK1-AS1 reversed the miRNA-137-induced sensitivity in DDP-resistant MG63 cells, suggesting that NCK1-AS1 plays a suppressive role and enhanced the DDP sensitivity in DDP-resistance osteosarcoma cells by sponging miRNA-137. However, the downstream signaling pathway of NCK1-AS1/miR-137 involved in the DDP resistant need to be explored in the further study.
Conclusion
In summary, our study revealed that NCK1-AS1 acts as an oncogene and silence NCK1-AS1 improved the DDP sensitivity of DDP-resistant osteosarcoma cells. Further mechanism research demonstrated that NCK1-AS1 regulated the DDP resistant in osteosarcoma cells via competing with miRNA-137. Thus, NCK1-AS1/ miRNA-137 axis could be an effective modulatory pathway to withstand the DDP resistant in the chemotherapy of osteosarcoma.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Picci P. Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi: 10.1200/JCO.2014.59.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins M, Wilhelm M, Conyers R, et al. Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysis. J Clin Oncol. 2013;31(18):2303–2312. doi: 10.1200/JCO.2012.43.8598 [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031 [DOI] [PubMed] [Google Scholar]
- 5.Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6(1):1–18. doi: 10.1111/j.1476-5829.2007.00142.x [DOI] [PubMed] [Google Scholar]
- 6.Sang H, Liu H, Xiong P, Zhu M. Long non-coding RNA functions in lung cancer. Tumour Biol. 2015;36(6):4027–4037. doi: 10.1007/s13277-015-3449-4 [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi: 10.1016/j.bbagrm.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 8.Ren K, Li Y, Lu H, et al. Long noncoding RNA HOTAIR controls cell cycle by functioning as a competing endogenous RNA in esophageal squamous cell carcinoma. Transl Oncol. 2016;9(6):489–497. doi: 10.1016/j.tranon.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Chen D, Ma H, Li Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco Targets Ther. 2017;10:5137–5149. doi: 10.2147/OTT.S146423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016. doi: 10.1007/s13277-016-5448-5 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Jia Y, Cheng J, Liu G, Song F. LncRNA NCK1-AS1 promotes proliferation and induces cell cycle progression by crosstalk NCK1-AS1/miR-6857/CDK1 pathway. Cell Death Dis. 2018;9(2):198. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang WY, Liu Y-J, He Y, Chen P. Suppression of long noncoding RNA NCK1-AS1 increases chemosensitivity to cisplatin in cervical cancer. J Cell Physiol. 2019;234(4):4302–4313. doi: 10.1002/jcp.27198 [DOI] [PubMed] [Google Scholar]
- 13.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859(1):169–176. doi: 10.1016/j.bbagrm.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 14.Cui M, Xiao Z, Wang Y, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res. 2015;75(5):846–857. doi: 10.1158/0008-5472.CAN-14-1192 [DOI] [PubMed] [Google Scholar]
- 15.Li H, Cui J, Xu B, He S, Yang H, Liu L. Long non-coding RNA XIST serves an oncogenic role in osteosarcoma by sponging miR-137. Exp Ther Med. 2019;17(1):730–738. doi: 10.3892/etm.2018.7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Li Y, Shen H, et al. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013;587(1):73–81. doi: 10.1016/j.febslet.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Silber J, Lim DA, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, Wu Q, Liu X, Xiong Y, Li H. MicroRNA-137 acts as a tumor suppressor in osteosarcoma by targeting enhancer of zeste homolog 2. Exp Ther Med. 2017;13(6):3167–3174. doi: 10.3892/etm.2017.4435 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Li ZM, Zhang H-Y, Wang Y-X, Wang W-B. MicroRNA-137 is downregulated in human osteosarcoma and regulates cell proliferation and migration through targeting FXYD6. J Drug Target. 2016;24(2):102–110. doi: 10.3109/1061186X.2015.1057149 [DOI] [PubMed] [Google Scholar]
- 20.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JJ, Shaw AT. Resisting resistance: targeted therapies in lung cancer. Trends Cancer. 2016;2(7):350–364. doi: 10.1016/j.trecan.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384 [DOI] [PubMed] [Google Scholar]
- 23.Xiong G, Feng M, Yang G, et al. The underlying mechanisms of non-coding RNAs in the chemoresistance of pancreatic cancer. Cancer Lett. 2017;397:94–102. doi: 10.1016/j.canlet.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Chen J, Ding C-M, Jin X, Jia Z-M, Peng J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J Cell Mol Med. 2018;22(6):3238–3245. doi: 10.1111/jcmm.13605 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wang Q, Zhang W, Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16(8):795–801. doi: 10.1080/15384101.2017.1301334 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hu Y, Yang Q, Wang L, et al. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep. 2018;38(3):BSR20180375. doi: 10.1042/BSR20180375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Fan D. Multidrug resistance in gastric cancer: recent research advances and ongoing therapeutic challenges. Expert Rev Anticancer Ther. 2007;7(10):1369–1378. doi: 10.1586/14737140.7.10.1369 [DOI] [PubMed] [Google Scholar]
- 28.Asada N, Tsuchiya H, Ueda Y, Tomita K. Establishment and characterization of an acquired cisplatin-resistant subline in a human osteosarcoma cell line. Anticancer Res. 1998;18(3a):1765–1768. [PubMed] [Google Scholar]
- 29.Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi: 10.1016/j.cell.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B, Liu T, Wu T, Wang Z, Rao Z, Gao J. microRNA-137 functions as a tumor suppressor in human non-small cell lung cancer by targeting SLC22A18. Int J Biol Macromol. 2015;74:111–118. doi: 10.1016/j.ijbiomac.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Xiao J, Peng F, Yu C, et al. microRNA-137 modulates pancreatic cancer cells tumor growth, invasion and sensitivity to chemotherapy. Int J Clin Exp Pathol. 2014;7(11):7442–7450. [PMC free article] [PubMed] [Google Scholar]
- 33.Qin Y, Zhang S, Deng S, et al. Epigenetic silencing of miR-137 induces drug resistance and chromosomal instability by targeting AURKA in multiple myeloma. Leukemia. 2017;31(5):1123–1135. doi: 10.1038/leu.2016.325 [DOI] [PubMed] [Google Scholar]
- 34.Takwi AA, Wang Y-M, Wu J, Michaelis M, Cinatl J, Chen T. miR-137 regulates the constitutive androstane receptor and modulates doxorubicin sensitivity in parental and doxorubicin-resistant neuroblastoma cells. Oncogene. 2014;33(28):3717–3729. doi: 10.1038/onc.2013.330 [DOI] [PMC free article] [PubMed] [Google Scholar]