Abstract
Introduction
Clinical decision support tools capable of predicting which patients are at highest risk for venous thromboembolism (VTE) can assist in guiding surveillance and prophylaxis decisions. The Trauma Embolic Scoring System (TESS) has been shown to model VTE risk in civilian trauma patients. No such support tools have yet been described in combat casualties, who have a high incidence of VTE. The purpose of this study was to evaluate the utility of TESS in predicting VTE in military trauma patients.
Methods
A retrospective cohort study of 549 combat casualties from October 2010 to November 2012 admitted to a military treatment facility in the USA was performed. TESS scores were calculated through data obtained from the Department of Defense Trauma Registry and chart reviews. Univariate analysis and multivariate logistic regression were performed to evaluate risk factors for VTE. Receiver operating characteristic (ROC) curve analysis of TESS in military trauma patients was also performed.
Results
The incidence of VTE was 21.7% (119/549). The median TESS for patients without VTE was 8 (IQR 4–9), and the median TESS for those with VTE was 10 (IQR 9–11). On multivariate analysis, Injury Severity Score (ISS) (OR 1.03, p=0.007), ventilator days (OR 1.05, p=0.02), and administration of tranexamic acid (TXA) (OR 1.89, p=0.03) were found to be independent risk factors for development of VTE. On ROC analysis, an optimal high-risk cut-off value for TESS was ≥7 with a sensitivity of 0.92 and a specificity of 0.53 (area under the curve 0.76, 95% CI 0.72 to 0.80, p<0.0001).
Conclusions
When used to predict VTE in military trauma, TESS shows moderate discrimination and is well calibrated. An optimal high-risk cut-off value of ≥7 demonstrates high sensitivity in predicting VTE. In addition to ISS and ventilator days, TXA administration is an independent risk factor for VTE development.
Level of evidence
Level III.
Introduction
Venous thromboembolism (VTE) is a frequent cause of morbidity in trauma patients. Pulmonary embolism (PE) represents the third leading cause of in-hospital deaths in trauma patients and is a leading cause of readmission.1 The incidence of VTE has varied in civilian trauma literature with recent studies reporting rates as low as 0.36% to as high as 9.1%.2–4 However, the VTE incidence in military combat casualties has been shown to be even higher, ranging from 2.2% to 28%.5–10 This disparity in VTE rates is likely related to the differences between military and civilian trauma patients. Combat casualties frequently have additional risk factors for VTE not often encountered in civilian trauma patients including multiple and/or above-knee amputations as well as prolonged immobilization at altitude during long distance aeromedical evacuation.5 Consistent with the Eastern Association for the Surgery of Trauma and CHEST guidelines,11 12 the current Department of Defense Joint Trauma System (JTS) Clinical Practice Guidelines regarding VTE prevention recommend initiation of chemoprophylaxis in trauma patients once ongoing bleeding and coagulopathy have been corrected.13 The JTS guidelines currently do not recommend screening duplex ultrasound in asymptomatic patients. Inferior vena cava filter (IVCF) placement can be considered in high-risk trauma patients who are not candidates for VTE chemoprophylaxis. However, the definition of ‘high risk’ is reliant on clinical judgement.
A clinical tool that can predict those at highest risk for developing VTE can assist in guiding surveillance and prophylaxis decisions. The Trauma Embolic Scoring System (TESS) has been shown to model VTE risk in civilian trauma patients.14 Described by Rogers et al, TESS is determined from five clinical variables—age, Injury Severity Score (ISS), body mass index (BMI), ventilator days, and presence of a lower extremity fracture (table 1). A TESS score of 0–2 is not considered to be at risk for VTE, a score of 3–6 is considered low risk, and a score of 7–14 is considered moderate to high risk.
Table 1.
Components of the Trauma Embolic Scoring System (TESS) and their associated scores (adapted from Rogers et al14
| Predictor | TESS score |
| Age (years) | |
| 18–29 | 0 |
| 30–64 | 1 |
| ≥65 | 2 |
| Injury Severity Score | |
| 1–9 | 0 |
| 10–16 | 3 |
| 17–25 | 3 |
| >25 | 5 |
| Pre-existing obesity | |
| No pre-existing obesity | 0 |
| Pre-existing obesity | 1 |
| Ventilation days | |
| No ventilation days | 0 |
| Ventilator days | 4 |
| Lower extremity fracture | |
| No lower extremity fracture | 0 |
| Lower extremity fracture | 2 |
A model predicting which patient demographics and injury patterns most closely associated with VTE has yet to be described in a military population. Therefore, the purpose of this study was to apply TESS retrospectively in a military trauma population to determine its discrimination and calibration in predicting VTE. We hypothesized that given the heterogeneity between military and civilian trauma populations, TESS would not predict VTE as accurately in a military trauma cohort. In addition, we sought to determine independent risk factors for VTE in this cohort of military trauma patients.
Methods
This retrospective study of all combat casualties injured in Iraq or Afghanistan and arriving to our facility during a period from October 1, 2010 to November 30, 2012 was approved by the Walter Reed National Military Medical Center Institutional Review Board. Data were obtained from the Department of Defense Trauma Registry (DoDTR) for combat casualties admitted to our facility during the study time period. Additional clinical information was obtained from a chart review of each patient. The DoDTR collects and maintains information regarding the demographics, diagnoses, and interventions of combatant and non-combatant casualties from the point of injury until definitive care in the USA at our role IV military treatment facility (MTF). Patients were excluded if they were not active duty US service members or if they had incomplete data. A TESS score was calculated based on available data for age, ISS, ventilator days, and presence of a lower extremity fracture. Given the absence of height and weight data prior to injury, a simulated BMI was determined based on the distribution of BMI in a similar cohort of combat casualty patients for which height and weight data were collected. We tested and verified the assumption that the BMI values in the known data set came from a normal distribution using the Shapiro-Wilk test (p=0.014). A BMI value was then randomly assigned to each patient in the cohort used in this study by generating a new distribution of BMI values with the same mean and SD as the patient cohort with known BMIs. The BMI distribution from which BMI values were simulated had a mean of 25.03 and an SD of 3.36 (range 17.7–35.9). Forty-four patients (8.0%) in this study cohort had an assigned BMI >30, consistent with the cohort from which BMI was simulated.
Although exact timing of the start of VTE chemoprophylaxis administration was not available, patients were started on chemoprophylaxis at the earliest possible time point in accordance with JTS guidelines.13 This took place prior to arrival in the continental United States (CONUS) once the risk of bleeding had been mitigated. The primary outcome of interest was the diagnosis of VTE—deep venous thrombosis (DVT) or PE. PE was diagnosed on spiral CT scan, and DVT was diagnosed on CT scan or by duplex ultrasonography of the extremities. Patients were stratified and compared based on the presence or absence of VTE, which was diagnosed either before or after arrival to our MTF.
For univariate analysis, statistical differences between continuous variables were determined by Student’s t-test for parametrically distributed data and by the Wilcoxon rank-sum test for non-parametrically distributed data. Differences in categorical variables were determined by a continuity-adjusted χ2 test or a Fisher’s exact test as appropriate. To evaluate independent clinical risk factors associated with VTE in our cohort, variables found to be statistically significant in univariate analysis were used to create a binary logistic regression model. A p value <0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve analysis was performed to assess the ability of TESS to discriminate between those who were diagnosed with VTE and those who were not (non-VTE group). Area under the curve (AUC) was determined, and the optimal cut-off value was found by calculating the maximum Youden’s index (sensitivity + specificity – 1).15 A logistic regression model was created to estimate the risk of VTE by TESS score, and calibration of this model was assessed by a Hosmer-Lemeshow goodness-of-fit test.16 For this test, a p value <0.05 was suggestive of imperfect calibration.17 Statistical analysis was performed using SAS V.9.4 (SAS Institute).
Results
A total of 560 patients were admitted to our MTF during the designated study period. Nine patients were excluded because they were not US service members. Two patients were excluded because of a lack of a complete data set available for them. This left 549 patients remaining for analysis.
Demographic data for our population, which include stratification by presence or absence of VTE, are displayed in table 2. One hundred and nineteen patients were diagnosed with VTE in our cohort, making the overall incidence of VTE at 21.7%. Sixty-two of these patients were diagnosed with VTE prior to arrival at our MTF (52.1% of VTE), whereas 57 patients were diagnosed at our facility (47.9% of VTE). Sixty-three patients were diagnosed with DVT—50 lower extremity (79.4%) and 13 upper extremity (20.6%). Sixty-three patients were diagnosed with PE and seven patients were diagnosed with both DVT and PE. Sixty-nine of 119 (60.0%) were diagnosed with VTE based on clinical suspicion, whereas 42 (35.3%) were found incidentally. Eight patients (6.7%) had VTE events where it was unclear from the records whether or not they were symptomatic or asymptomatic. VTE events were diagnosed an average of 9.7±9.9 days after injury (range 0–45 days) with 42 being diagnosed within 3 or less days from the date of injury (35.3%).
Table 2.
Demographic data with breakdown by VTE diagnosis and univariate analysis
| Total (n=549) | No VTE (n=430) | VTE (n=119) | P value | |
| TESS | 9 (5–11) | 8 (4–9) | 10 (9–11) | <0.0001* |
| Age (years) | 23 (21–27) | 23 (21–26) | 23 (21–27) | 0.81 |
| Male, n (%) | 546 (99.5) | 428 (99.5) | 118 (99.1) | 0.52 |
| ISS | 21 (12–29) | 17 (10–27) | 29 (21–38) | <0.0001* |
| Simulated BMI (kg/m2) | 24.9 (22.9–27.1) | 25.0 (23.0–27.1) | 24.5 (22.6–26.9) | 0.32 |
| Mortality, n (%) | 6 (1.1) | 5 (1.2) | 1 (0.8) | 0.76 |
| Blast injury, n (%) | 441 (80.3) | 332 (77.2) | 109 (91.6) | 0.0002* |
| Penetrating mechanism, n (%) | 486 (88.5) | 375 (87.2) | 111 (93.3) | 0.09 |
| Lower extremity fracture, n (%) | 350 (63.8) | 253 (58.8) | 97 (81.5) | <0.0001* |
| Lower extremity amputation, n (%) | 222 (40.4) | 144 (33.5) | 78 (65.6) | <0.0001* |
| Ventilator days | 3 (0–7) | 2 (0–5) | 7 (5–10) | <0.0001* |
| Received blood transfusion, n (%) | 422 (76.9) | 309 (71.9) | 113 (95.0) | <0.0001* |
| Blood products administered (units) | 17 (2–47) | 10 (0–35) | 50 (28–86) | <0.0001* |
| Received massive transfusion within 24 hours, n (%) | 192 (35.0) | 116 (27.0) | 76 (63.9) | <0.0001* |
| Administered TXA, n (%) | 224 (40.8) | 142 (33.0) | 82 (68.9) | <0.0001* |
| Administered recombinant factor VII, n (%) | 19 (3.5) | 12 (2.8) | 7 (5.9) | 0.15 |
| Repair or ligation of vascular injury, n (%) | 159 (29.0) | 112 (26.1) | 47 (39.5) | 0.006* |
| Repair or ligation of venous injury, n (%) | 71 (12.9) | 48 (11.2) | 23 (19.3) | 0.03* |
| Pelvic fracture, n (%) | 72 (13.1) | 45 (10.5) | 27 (22.7) | 0.0008* |
| Head Abbreviated Injury Scale 3+, n (%) | 115 (21.0) | 89 (20.7) | 26 (21.9) | 0.88 |
| Spinal cord injury, n (%) | 23 (4.2) | 17 (4.0) | 6 (5.0) | 0.61 |
n (%) shown for categorical variables.
Median (IQR) shown for continuous variables.
*P<0.05.
BMI, body mass index; ISS, Injury Severity Score; TESS, Trauma Embolic Scoring System; TXA, tranexamic acid; VTE, venous thromboembolism.
Six patients (1.1%) did not survive after transfer to the role IV level of care in CONUS. Of these six casualties, one had been diagnosed with VTE prior to arrival to our facility whereas the other five had not (p=0.76). A higher proportion of patients with VTE experienced blast injury compared with those without VTE (91.6% vs. 77.2%, p=0.0002).
When assessing the components of the TESS score, there was not a significant difference in age (p=0.81) or simulated BMI (p=0.32) between the VTE group and the non-VTE group. There was higher median ISS (29 vs. 17, p<0.0001), median ventilator days (7 vs. 2, p<0.0001), and incidence of lower extremity fracture (81.5% vs. 58.8%, p<0.0001) in the patients who were diagnosed with VTE as compared with the non-VTE group. Of note, the group of patients who had a lower extremity fracture includes those who had a lower extremity amputation.
Regarding blood product administration, patients diagnosed with VTE received a higher median total of blood product units (50 vs. 10, p<0.0001) and were more likely to have received both a massive transfusion within 24 hours of injury (63.9% vs. 27.0%, p<0.0001) as well as any blood product (95.0% vs. 71.9%, p<0.0001). More patients with a VTE received tranexamic acid (TXA; 68.9% vs. 33.0%, p<0.0001). There was not a significant difference in recombinant factor VII administration between the two groups (5.9% vs. 2.8%, p=0.15). Regarding other VTE risk factors, patients with a VTE diagnosis were more likely to have sustained a pelvic fracture (22.7% vs. 10.5%, p=0.0008) and have undergone repair or ligation of a venous injury (19.3% vs. 11.2%, p=0.03). There was also a higher incidence of lower extremity amputation (65.6% vs. 33.5%, p<0.0001) in patients diagnosed with VTE. No significant difference in the incidence of spinal cord injury (5.0% vs. 4.0%, p=0.61) or head injury with Abbreviated Injury Scale (AIS) score of 3 or higher (21.9% vs. 20.9%, p=0.88) was found between the two groups.
On univariate analysis, there was a statistically significant increase in the following variables for patients with VTE: ISS, blast injury, lower extremity fracture, lower extremity amputation, ventilator days, blood products administered, massive transfusion within 24 hours, TXA administration, repair or ligation of a venous injury, and pelvic fracture. When these variables were included in a multivariate binary logistic regression model, only ISS (OR 1.03, p=0.007), ventilator days (OR 1.05, p=0.02), and TXA administration (OR 1.89, p=0.03) were independent risk factors for VTE development (table 3).
Table 3.
Independent risk factors for development of VTE on multivariate logistic regression
| Variable | OR (95% CI) | P value |
| ISS | 1.03 (1.01 to 1.06) | 0.007 |
| Ventilator days | 1.05 (1.01 to 1.10) | 0.02 |
| TXA | 1.89 (1.07 to 3.33) | 0.03 |
ISS, Injury Severity Score; TXA, tranexamic acid; VTE, venous thromboembolism.
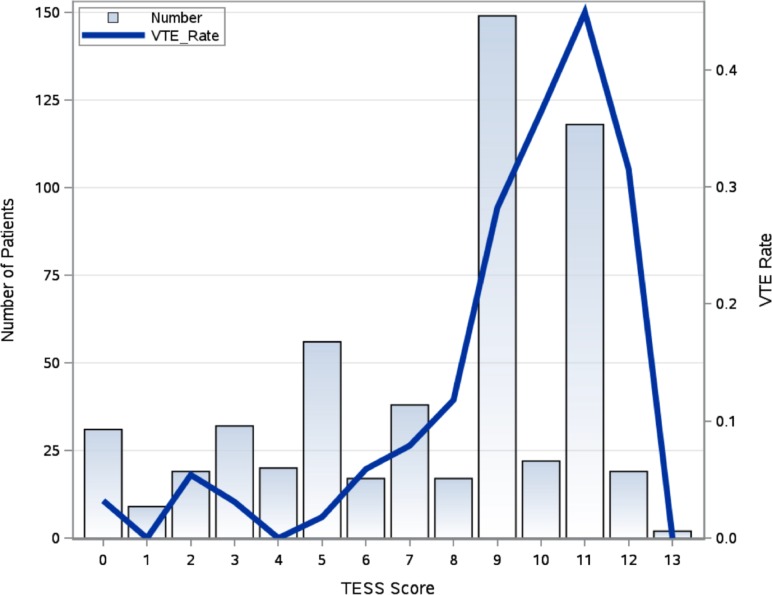
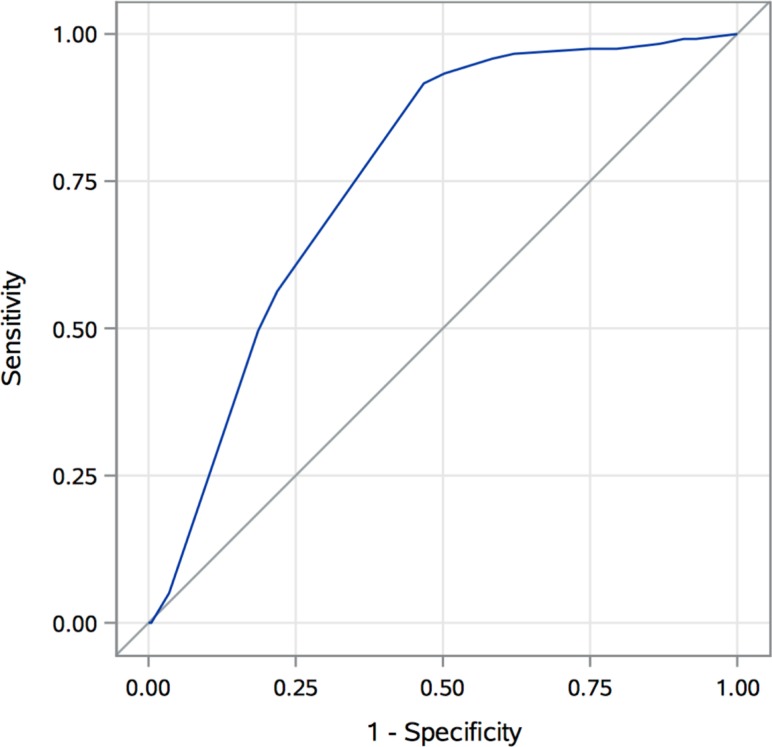
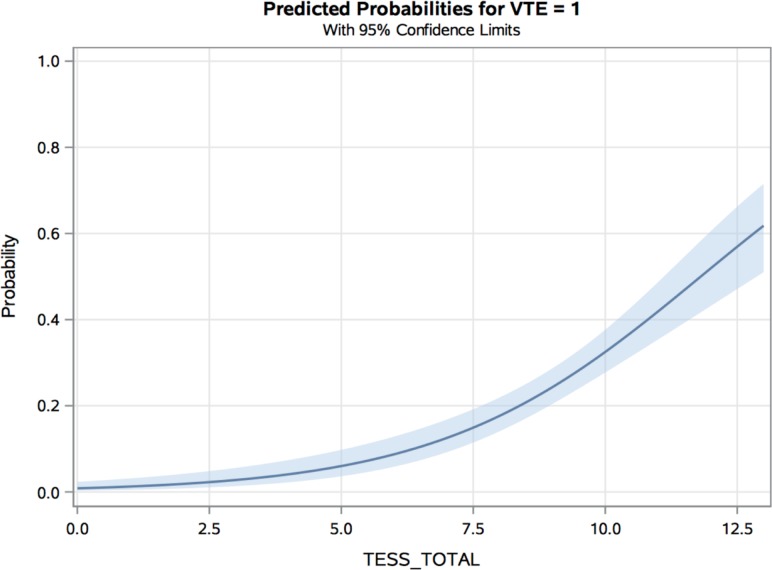
The median TESS for the entire cohort was 9, with an IQR of 5–11. The median TESS score for patients with VTE was 10, and the median TESS score for the non-VTE group was 8 (p<0.0001). Figure 1 is a histogram demonstrating the number of patients at each total TESS score integer (range 0–13) with a superimposed line graph displaying the observed VTE rate by total TESS score. Patients with a TESS score of 11 had the highest VTE rate of 45%. On ROC curve analysis (figure 2), TESS was found to have an AUC of 0.76 (95% CI 0.72 to 0.80, p<0.0001) in predicting the occurrence of VTE in this cohort of military trauma patients. By calculating the maximum Youden’s index (sensitivity + specificity – 1), an optimal cut-off value was found to be a total TESS score greater than or equal to 7. This had a sensitivity of 0.92, specificity of 0.53, negative predictive value of 0.97, and positive predictive value of 0.31. A logistic regression model was created to estimate the risk of VTE by TESS score based on this military trauma cohort (figure 3) and was found to be well calibrated, with a Hosmer-Lemeshow p value=0.32.
Figure 1.
Histogram of total Trauma Embolic Scoring System (TESS) score in a military trauma cohort with observed venous thromboembolism (VTE) rate by TESS score.
Figure 2.
Receiver operating characteristic curve demonstrating performance of Trauma Embolic Scoring System (TESS) in predicting venous thromboembolism (VTE) in a military trauma cohort. Area under the curve 0.76 (95% CI 0.72 to 0.80, p<0.0001).
Figure 3.
Logistic regression model demonstrating risk of venous thromboembolism (VTE) in military trauma by Trauma Embolic Scoring System (TESS) score with 95% CIs, Hosmer-Lemeshow p=0.32.
Discussion
This study demonstrates that for military trauma patients, TESS represents a useful clinical decision-making tool in predicting VTE. TESS shows moderate discrimination and good calibration in modeling VTE risk in our military cohort. With an optimal cut-off value of a TESS score greater than or equal to 7, both sensitivity and negative predictive value are greater than 90% in our cohort. These performance statistics corroborate the utility of TESS in determining which military trauma patients are at high risk of VTE and who may potentially benefit from screening imaging or IVCF placement in the setting of a contraindication to chemoprophylaxis. When compared with the initial description of TESS by Rogers et al,14 the sensitivity of 92% in this study exceeds the sensitivities of 87.5% in the local cohort and 77.4% in the National Trauma Data Bank cohort with a cut-off value of greater than or equal to 5. However, TESS is not as specific in military trauma patients—52% in our population compared with 77.5% and 75.6%, respectively. Therefore, the AUC was lower for TESS in our military trauma cohort than the AUC of 0.89 found in the original TESS article. TESS is thus useful for ruling out military patients at high risk for VTE but not for ruling in the diagnosis.
We initially hypothesized that TESS would not apply particularly well to military trauma because of the difference between combat and civilian casualties. The two populations are undoubtedly heterogeneous. Our cohort was younger with a median age of 23 as opposed to 40 in the cohort used to develop TESS. The cohort in this present study was also more severely injured with a median ISS of 21 compared with 9. Additionally, the VTE rate was over 20 times higher in our military cohort—21.7% versus 0.85%. Military patients who undergo prolonged aeromedical evacuation may also incur an increased risk of VTE from this prolonged period of immobility.
In spite of these differences between military and civilian populations, we demonstrated on multivariate analysis in this study that ISS and ventilator days, both clinical variables accounted for in the TESS score, were the only independent risk factors associated with VTE outside of TXA administration. This is consistent with other studies evaluating risk factors associated with VTE in both civilian and military trauma. In civilian patients, Knudson et al found the following risk factors associated with VTE: greater than 3 ventilator days, head AIS score greater than or equal to 3, lower extremity fracture with AIS score greater than or equal to 3, age greater than 40, venous injury, and major operative procedure.3 In other published studies of military trauma in Iraq and Afghanistan, the investigators found that ISS and ventilator days were independent VTE risk factors in addition to packed red blood cells and fresh frozen plasma transfused, bilateral lower extremity amputation, multiple extremity injuries, and pelvic fracture.6–8 Thus, although military and civilian trauma patients are vastly different populations, they may share significant risk factors for development of VTE in ISS and need for mechanical ventilation.
One of the advantages of TESS is its simplicity in calculation as it contains only five clinical variables. This is less cumbersome than Greenfield risk assessment profile (RAP), which has also been shown to model VTE risk in trauma patients but contains 15 clinical variables.18–20 Given limitations in the retrospective data available from our patients, we were not able to calculate a RAP score and compare its performance to TESS.
Current CHEST guidelines do not recommend screening venous duplex in asymptomatic patients.12 Interestingly, however, Allen et al showed that patients at high risk for VTE on RAP who underwent screening venous duplex ultrasound once weekly and were then treated appropriately if DVT was diagnosed may be at decreased risk for PE development.21 A similar strategy might be able to be employed in military trauma patients using TESS if a high-risk score of greater than or equal to 7 is used as a criterion for screening. Military patients with a high-risk TESS score for whom VTE chemoprophylaxis is contraindicated might also be candidates for prophylactic IVCF placement, which has been shown to be associated with reduction in PE and fatal PE in a meta-analysis of trauma patients.22
Developing a model predicting VTE in military trauma patients based on clinical variables alone that performs better than TESS would be difficult to achieve. The sensitivity and negative predictive value of TESS in military trauma are already greater than 90%. Additionally, no clinical variables outside of TXA administration were found to be significant predictors of VTE on multivariate analysis that are not already accounted for by TESS. Dente et al demonstrated that including patient biomarker data in addition to clinical data in machine learning algorithms allowed for the development of highly accurate models predictive of bacteremia and pneumonia in combat casualties.23 The inclusion of biomarker data in model development might be a direction for future research in VTE clinical decision support tools for trauma patients. An ongoing project at our institution identifying biomarker profiles associated with VTE has preliminarily showed high specificity. Thus, with the addition of laboratory data, a model predicting VTE could potentially achieve higher specificity while maintaining high sensitivity.
Although not a randomized study, an additional important finding was that TXA administration was an independent risk factor for the development of VTE on multivariate analysis. This is similar to what was found in a recent retrospective cohort study of casualties in Afghanistan from 2010 to 2014, where Howard et al showed that TXA administration may be associated with an increased risk of VTE.24 The authors also did not find a mortality benefit from TXA administration. They commented, however, that their study was likely underpowered for determining a true mortality difference. Johnston et al also recently demonstrated that TXA administration was an independent risk factor for VTE in military trauma patients.25 These findings of a potential increased VTE risk associated with TXA administration in trauma patients run contrary to the CRASH-2 trial, which showed no increase in vascular occlusive complications with TXA use.26 Likewise, although the MATTERs study showed higher rates of VTE on univariate analysis in patients who received TXA, a multivariate regression did not find TXA to be a risk factor for VTE.27 It should be pointed out that their overall VTE rate was much lower—nearly 10 times lower than in our study. It should also be noted that patients who receive TXA are generally more severely injured and thus intrinsically at higher risk for development of VTE. Current JTS Tactical Combat Casualty Care guidelines recommend TXA administration if a casualty is anticipated to need significant blood transfusion without viscoelastic evidence of fibrinolysis.28 Given the potential adverse thrombotic effects associated with TXA use, more consideration is needed regarding optimal use of TXA in combat casualties.
Several limitations deserve mentioning. Our study is limited by the fact that this was a retrospective cohort analysis. Although a chart review was performed to supplement data that were not available in the DoDTR database, we were unable to obtain height or weight data for every patient prior to injury. Thus, BMI was simulated based on another cohort of combat casualties for whom these data were available, likely introducing bias into our study. This bias is most likely minimal, however, as only 8% of service members from which BMI was simulated had a BMI >30. Additionally, only 1 point in the TESS score is allocated to those with a BMI >30, thus minimizing the significance of obesity in the total TESS score. Another potential limitation is that we pooled DVT and PE diagnoses into a single entity of VTE. Rogers et al similarly pooled DVT and PE when developing TESS.14 As Lundy et al described, primary pulmonary thrombosis without DVT may be a sequela of chest trauma and blast injury resulting in direct pulmonary artery (PA) thrombosis as opposed to PA occlusion from venous embolic disease.9 It is unclear if TESS would predict risk as well for these patients with primary pulmonary thrombus as it would for those who develop a DVT or PE that resulted from a DVT. Lastly, there is inherent survival bias present as all patients included in this study survived evacuation to the CONUS.
Footnotes
Contributors: JSO, MJB, EAE, and CJR conceptualized the research project and proposed the study design. PFW, SS, and JDC participated in data collection and assembly of data, data analysis, and interpretation. PFW conducted the literature search and drafted the original article. All authors critically reviewed the original article and contributed to revisions and final approval of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Walter Reed National Military Medical Center Institutional Review Board
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Roger FB. Venous thromboembolism in trauma patients: a review. Surgery 2001;130:12 10.1067/msy.2001.114558 [DOI] [PubMed] [Google Scholar]
- 2.Karcutskie CA, Meizoso JP, Ray JJ, Horkan D, Ruiz XD, Schulman CI, Namias N, Proctor KG, et al. Association of mechanism of injury with risk for venous thromboembolism after trauma. JAMA Surg 2017;152:40 10.1001/jamasurg.2016.3116 [DOI] [PubMed] [Google Scholar]
- 3.Knudson M, Ikossi D, Khaw L, Morabito D. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of surgeons national trauma data bank. Transactions Meet Am Surg Assoc 2004;240:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg 2011;254:632. [DOI] [PubMed] [Google Scholar]
- 5.Fang R, Rodriguez CJ. Venous thromboembolism among military combat casualties. Curr Trauma Rep 2016;2:48–53. 10.1007/s40719-016-0037-z [DOI] [Google Scholar]
- 6.Hannon M, Tadlock MD, Melcer T, Walker J, Bandle J, Nieses K, Galarneau M, et al. Venous thromboembolism after traumatic amputation: an analysis of 366 combat casualties. Am J Surg 2016;212:230–4. 10.1016/j.amjsurg.2016.01.031 [DOI] [PubMed] [Google Scholar]
- 7.Gillern SM, Sheppard FR, Evans KN, Graybill JC, Gage FA, Forsberg JA, Dunne JR, Tadaki DK, Elster EA, et al. Incidence of pulmonary embolus in combat casualties with extremity amputations and fractures. J Trauma 2011;71:613 10.1097/TA.0b013e3182282574 [DOI] [PubMed] [Google Scholar]
- 8.Holley AB, Petteys S, Mitchell JD, Holley PR, Collen JF. Thromboprophylaxis and VTe rates in soldiers wounded in operation enduring freedom and operation Iraqi freedom. Chest 2013;144:973 10.1378/chest.12-2879 [DOI] [PubMed] [Google Scholar]
- 9.Lundy JB, Oh JS, Chung KK, Ritter JL, Gibb I, Nordmann GR, Sonka BJ, Tai NRM, Aden JK, Rasmussen TE, et al. Frequency and relevance of acute peritraumatic pulmonary thrombus diagnosed by computed tomographic imaging in combat casualties. J Trauma Acute Care 2013;75:S215–20. 10.1097/TA.0b013e318299da66 [DOI] [PubMed] [Google Scholar]
- 10.Hutchison TN, Krueger CA, Berry JS, Aden JK, Cohn SM, White CE, et al. Venous thromboembolism during combat operations: a 10-y review. J Surg Res 2014;187:625–30. 10.1016/j.jss.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 11.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the East practice management guidelines work group. J Trauma 2002;53:164 10.1097/00005373-200207000-00032 [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel . Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:7S–47. 10.1378/chest.1412S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabo D. The Prevention of Deep Venous Thrombosis - Inferior Vena Cava Filter. Joint Trauma System Clinical Practice Guideline 2016;1:11. [Google Scholar]
- 14.Rogers FB, Shackford SR, Horst MA, Miller JA, Wu D, Bradburn E, Rogers A, Krasne M. Determining venous thromboembolic risk assessment for patients with trauma: the trauma embolic scoring system. J Trauma Acute Care Surg 2012;73:511–5. [DOI] [PubMed] [Google Scholar]
- 15.Bangdiwala SI, Haedo AS, Natal ML, Villaveces A. The agreement chart as an alternative to the receiver-operating characteristic curve for diagnostic tests. J Clin Epidemiol 2008;61:866–74. 10.1016/j.jclinepi.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Sauaia A. The anatomy of an article: methods and results. J Trauma Acute Care 2017;83:550. [DOI] [PubMed] [Google Scholar]
- 17.Ho KM, Rao S, Rittenhouse KJ, Rogers FB. Use of the trauma embolic scoring system (Tess) to predict symptomatic deep vein thrombosis and fatal and non-fatal pulmonary embolism in severely injured patients. Anaesth Intensive Care 2014;42:714 10.1177/0310057X1404200605 [DOI] [PubMed] [Google Scholar]
- 18.Greenfield LJ, Proctor MC, Rodriguez JL, Luchette FA, Cipolle MD, Cho J, et al. Posttrauma thromboembolism prophylaxis. J Trauma 1997;42:103 10.1097/00005373-199701000-00017 [DOI] [PubMed] [Google Scholar]
- 19.Gearhart MM, Luchette FA, Proctor MC, Lutomski DM, Witsken C, James L, Davis K, Johannigman JA, Hurst JM, Frame SB, et al. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery 2000;128:640 10.1067/msy.2000.108224 [DOI] [PubMed] [Google Scholar]
- 20.Hegsted D, Gritsiouk Y, Schlesinger P, Gardiner S, Gubler KD. Utility of the risk assessment profile for risk stratification of venous thrombotic events for trauma patients. Am J Surg 2013;205:517–20. 20-discussion 10.1016/j.amjsurg.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 21.Allen CJ, Murray CR, Meizoso JP, Ginzburg E, Schulman CI, Lineen EB, Namias N, Proctor KG, et al. Surveillance and early management of deep vein thrombosis decreases rate of pulmonary embolism in high-risk trauma patients. J Am Coll Surg 2016;222:72 10.1016/j.jamcollsurg.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 22.Haut ER. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta-analysis. Jama Surg 2014;149:202. [DOI] [PubMed] [Google Scholar]
- 23.Dente CJ, Bradley M, Schobel S, Gaucher B, Buchman T, Kirk AD, Elster E. Towards precision medicine: accurate predictive modeling of infectious complications in combat casualties. J Trauma Acute Care Surg 2017;83:609–16. [DOI] [PubMed] [Google Scholar]
- 24.Howard JT, Stockinger ZT, Cap AP, Bailey JA, Gross KR. Military use of tranexamic acid in combat trauma: does it matter? J Trauma Acute Care 2017;83:588. [DOI] [PubMed] [Google Scholar]
- 25.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolic events. Jama Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trial collaborators, C.-2 Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet 2010;376:23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 27.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (matters) study. Arch Surg 2012;147:113–9. 10.1001/archsurg.2011.287 [DOI] [PubMed] [Google Scholar]
- 28.Joint Trauma System TCCC updates: tactical combat casualty care guidelines for medical personnel: 3 June 2015. Journal of special operations medicine : a peer reviewed journal for SOF medical professionals 2015;15:129–47. [PubMed] [Google Scholar]