Abstract
Semi-quantitative immunohistochemistry (IHC) is a powerful method for investigating protein expression and localization within tissues that involves using software, such as the freely available Fiji (ImageJ), to conduct deconvolution and downstream analysis. Currently, there is lack of an integrated protocol that includes a detailed procedure on how to measure or compare protein expression. Publications that use semi-quantitative methods to ascertain protein expression often don’t provide enough details in their methods section, which makes it difficult for the reader to reproduce their data. The current protocol provides an example and detailed steps of conducting semi-quantitative analysis of IHC images using Fiji software.
Keywords: Immunohistochemistry , Semi-quantification , Fiji ImageJ , DAB staining , Hematoxylin
Background
Semi-quantitative immunohistochemistry (IHC) has been widely used for investigating protein expression and localization within tissues (Matkowskyj et al., 2000; Cregger et al., 2006; Taylor and Levenson, 2006 ; Braun et al., 2013; Fedchenko and Reifenrath, 2014 ; Bauman et al., 2016; Pike et al., 2017; Lu et al., 2018; Crowe et al., 2019), and various software and methods have been used in semi-quantiative IHC to conduct deconvolution and downstream analysis. Some methods require advanced coding/mathematical experience or the use of subscription-based software that may not be feasible for every scientist ( Matkowskyj et al., 2000 ; Shu et al., 2016 ; Guirado et al., 2018 ). Fiji (ImageJ) is a freely available software (version 1.2; WS Rasband, National Institute of Health, Bethesda, MD). Publications using ImageJ for image quantification are often directed towards the creation of specific plugins for ImageJ or lack a step-by-step protocol ( Varghese et al., 2014; Chen Y, 2017; Guirado et al., 2018). However, there is no integrated protocol that includes a detailed but simple procedure of conducting such steps, including the use of ImageJ software. There are many open online forums where users post their questions about using Fiji ImageJ for image quantification purposes. This is often due to publications not providing sufficiently detailed methods sections, which makes it difficult for the reader to reproduce the data. It is also time-consuming for researchers who are not initially familiar with ImageJ software to figure out how to use this software. The current protocol provides an example for conducting semi-quantitative analysis of IHC images using ImageJ.
Equipment
Computer Specifications:
A 64-bit operating system that has Windows 7 or greater, Mac OS X 10.11 or greater, or Linux with kernel supporting GLIBC 2.14 and GLIBCXX 3.4.15 (typically kernels 2.6.39)
NVIDIA graphics card (GeForce, Quadro, or Tesla) with CUDA capabilities 2.0 or greater, see https://developer.nvidia.com/cuda-gpus for more details
Need up-to-date NVIDIA drivers (minimum version of 369)
Any computer with Java-based operating system and Excel available
Software
Free ImageJ Fiji software (Johannes Schindelin, Albert Cardona, Mark Longair, Benjamin Schmid, and others, https://imagej.net/Fiji/Downloads ), version 1.2 (no specific plugin was used)
Procedure
-
Staining of tissue using immunohistochemistry procedure
Reference to the procedure used for the staining of cells for the IHC protocol can be found in a previously published manuscript ( Crowe et al. , 2019 ).
-
Brief protocol for immunohistochemistry staining:
Cut FFPE tissue blocks into 4 µm sections and mount on positively charged slides.
Deparaffinize and rehydrate tissue.
Retrieve antigen in buffer of choice (citrate buffer at pH 6 was used for this protocol).
Block antigen with normal goat serum and incubate with primary antibodies ( i.e. , anti-OATP1B1 for this protocol).
Incubate with horseradish peroxidase (HRP)-conjugated secondary antibody and visualize by 3,3’-diaminobenzidine (DAB) to detect the protein of interest (OATP1B1 in the current protocol).
Stain nuclei with hematoxylin.
Hematoxylin and eosin (H&E) staining is not necessary for this protocol, but is useful when looking at the pathology of the tissue.
-
Image exporting and saving
The microscope should be set up correctly (i.e., white balanced) before acquiring new data for the ImageJ default values to work. Export and save the raw immunohistochemistry (IHC) immunohistochemistry images as a .tiff file format. Tiff format for images is preferred to prevent the loss of raw data and associated metadata.
-
Using Fiji for deconvolution of the IHC image.
Download and open Fiji software.
Click the “File” option and click “Open”. The IHC image will open up on the computer screen.
Click on the IHC image to make the image active.
Click the “Image” option and select “Color” > “Color Deconvolution.”
A new pop-up Color Deconvolution window will show up. For IHC images stained with 3,3’-diaminobenzidine (DAB) and hematoxylin (H), select the “H DAB” vector option. Leave “Show Matrices” and “Hide Legend” unchecked and click “Okay.” ( Figure 1 ).
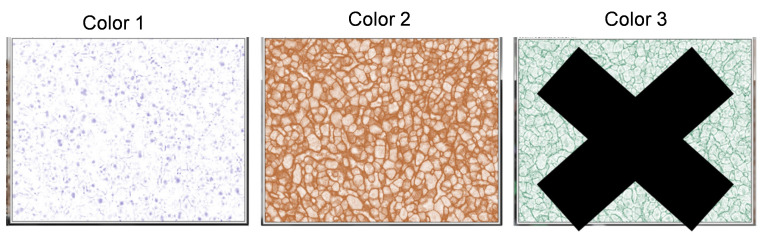
After selecting the H DAB option, three different images will pop up on the computer screen. Color 1 window represents only the Hematoxylin staining (blue/purple) and Color 2 window represents only the DAB staining (brown) ( Figure 2 ).
Exit out of the Color 3 window, as this will not be needed for the image analysis.
-
Thresholding the DAB-stained IHC image
Click on the DAB Color 2 image to activate it. DAB staining represents your primary antibody of interest. In this case, liver tissue was incubated with an OATP1B1 primary antibody, and the OATP1B1 expression is detected by DAB.
-
Go to “Image” and select “Adjust” and “Threshold”. After selecting the threshold, the brown image is converted into a black and white binary mask image, where all the values above the threshold are converted and represented in white. The background is black.
Note: A shortcut to threshold the image is achieved by pressing “Ctrl” + “Shift” + “T”.
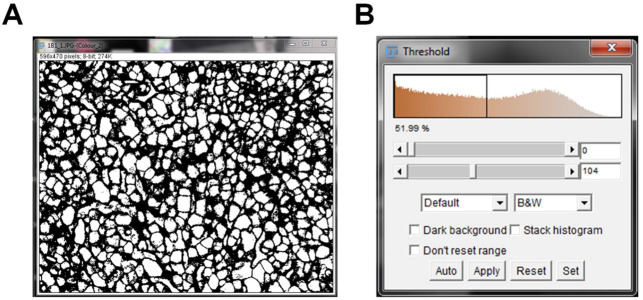
A new threshold window will pop up. The top bar indicates your minimum threshold value and the bottom bar indicates your maximum threshold value ( Figure 3 ).
Leave the minimum threshold value set at zero.
Adjust the maximum threshold value so that the background signal is removed, without removing the true DAB signal ( Figure 4A ). This is an arbitrary value, since it is set by the user. The maximum threshold value should be tested for at least five images to obtain an average maximum threshold value. Once the maximum threshold value is chosen, this will be set for all future IHC images. The measured threshold will only remain relevant provided that the microscope acquisition settings (color balance, exposure time, magnification, light source, camera vendor, etc.) remain constant throughout future experiments.
Once the maximum threshold image is set, click “Apply” on the threshold window. After clicking apply, the minimum and maximum threshold values will be 255 ( Figure 4B ).
-
Quantifying the DAB signal area in the IHC image
Go to “Analyze” and select “Set Measurements”.
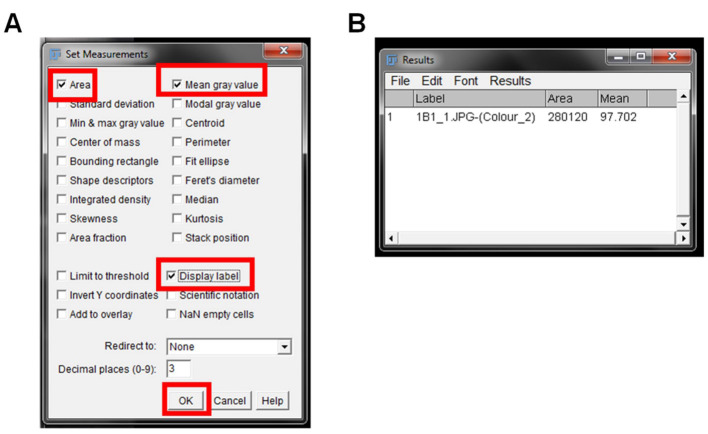
A “Set Measurement” pop up window will open. Select the “Area”, “Mean grey value”, and “Display Label” boxes, and leave all other boxes unchecked. “Area” will give the size of the IHC image. “Mean grey value” represents the quantified signal, and “Display Label” gives the information on the image name being quantified ( Figure 5A ).
Select “Okay” in the Set Measurement window. These options only need to be set once for the first image, and will be remembered for all other future images measured.
-
Go to “Analyze” and select “Measure”.
Note: A shortcut for measuring the signal area is “CTL + M”.
A “Results” window will pop up giving the name of the image (Label), size of the image (Area), and the average pixel intensity of the threshholded image as an indirect way to measure staining area (Mean) ( Figure 5B ).
Export results for later by clicking File>Save, as to avoid copy/paste errors.
Exit out of the Color 2 DAB-stained image.
-
Measuring the size of the nucleus
Click on the Hematoxylin Color 1 image to activate it.
Select the “Straight line” tool on the Fiji panel.
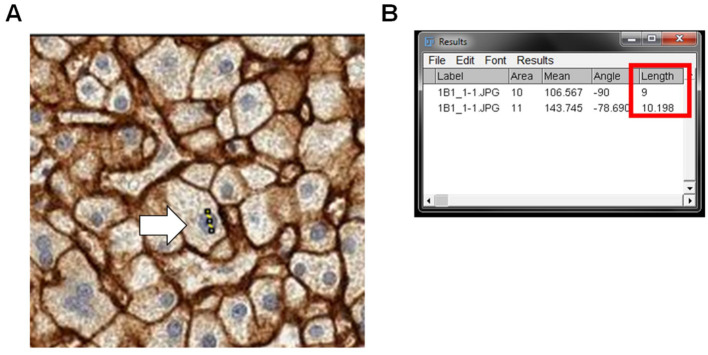
Measure the length of a nucleus by drawing a line across the nucleus with the Straight line tool ( Figure 6A ).
Go to “Analyze” and select “Measure”.
A Results window will pop up with the diameter of the nucleus (“Length”) ( Figure 6B ).
Measure ~10 different nuclei, using Steps F1–F5 in a representative IHC image, and take the average of these lengths to determine the average size of the nuclei. This serves as the average size of the nuclei for all IHC images from hereon. It should be done across multiple images if images are significantly different and represent multiple conditions.
-
Thresholding the hematoxylin-stained IHC image
Click on the Hematoxylin Color 1 image to activate it.
Go to “Image”, and select “Adjust” and “Threshold”. After selecting threshold, the blue/purple image is converted to a black and white image.
Set the minimum threshold value to zero.
Adjust the maximum threshold value so that the background signal is removed, without removing the true hematoxylin/nucleus signal. This is an arbitrary value, since the user sets it. The maximum threshold value should be tested for at least five images to get an average maximum threshold value. Once the maximum threshold value is chosen, this will be set for all future IHC images.
-
Once the minimum and maximum threshold values for the image are set, click “Apply” on the threshold window. After clicking apply, the minimum and maximum threshold values will be 255.
Of note, automatic thresholds are preferred, since manual thresholds must be adequately tested and kept constant for all other images in the experiment.
-
Quantifying the hematoxylin/nucleus signal in the IHC image
Select the Color 1 Hematoxylin image to activate it again.
Go to “Process” and select “Binary” > “Watershed”. This action will split the nuclei that are joined together into multiple nuclei.
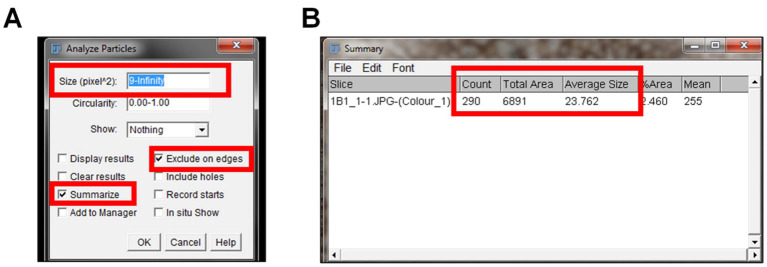
Go to “Analyze” and select “Analyze Particles”.
-
An “Analyze Particles” window will pop up giving multiple options ( Figure 7A ).
Size (pixel^2): Set the size of the nuclei to the average nuclei size measured in Step F6 to infinity ( i.e. , 6–Infinity).
Circularity: Leave it set at 0.00–1.00.
Show: Leave it set to “Nothing”.
Select “Summarize” and “Exclude on Edges” in the window. Summarize will give a summary of the particle’s measurements. Exclude on the edges means that nuclei on the outer edge of the image will not be included in the measurement.
-
Select “Okay” on the Analyze Particles window, and a Summary window with the output will pop up ( Figure 7B ). The output data includes:
Count indicates the number of nuclei in the IHC image.
Total area indicates the total area of the nuclei in the image.
Average area indicates the average size of the nuclei in the IHC image.
Copy the results in the Summary window to the same excel file for later analysis.
Exit out of the Color 1 Hematoxylin/Nuclei stained image.
-
Semi-quantitative analysis of the IHC image
Open the excel file containing the DAB and hematoxylin results outputted from the Fiji software.
For each image, divide the Mean grey intensity value from Step E5 by the number of nuclei measured in Step H5. This value represents the area of DAB stain normalized to the number of nuclei.
Take the average normalized area of DAB of for all IHC images for each sample/treatment, etc . to obtain an average and standard deviation values.
Figure 1. Color Deconvolution Window.
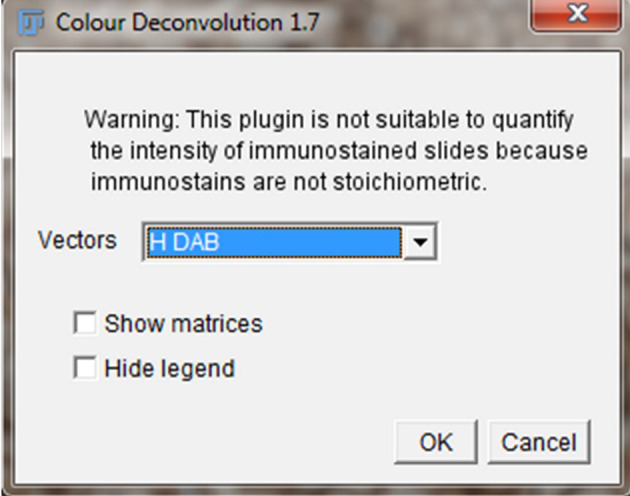
The Color Deconvolution Window will be used to separate the staining of the IHC image. The H DAB vector separates the IHC image into DAB staining (brown staining) for the protein of interest and Hematoxylin (H) staining for the nucleus.
Figure 2. Deconvolution of IHC image.
Separation of the IHC image into hematoxylin staining for the nuclei (Color 1) and DAB staining for OATP1B1 protein expression (Color 2) in hepatocytes within human liver tissue. Color 3 panel is for another staining, if applicable. For DAB and hematoxylin staining alone, the Color 3 panel can be eliminated. In DAB and hematoxylin staining, as in current studies, a warning “X” is shown so that users understand that the amount of antibody staining ( i.e. , DAB staining) in this case cannot be mathematically quantified, as it is not stoichiometric.
Figure 3. IHC image pre-threshold.
IHC image converted to black and white pixels prior to thresholding the image (A). The threshold pop up window pre-threshold indicates the baseline threshold values (B).
Figure 4. IHC image post-threshold.
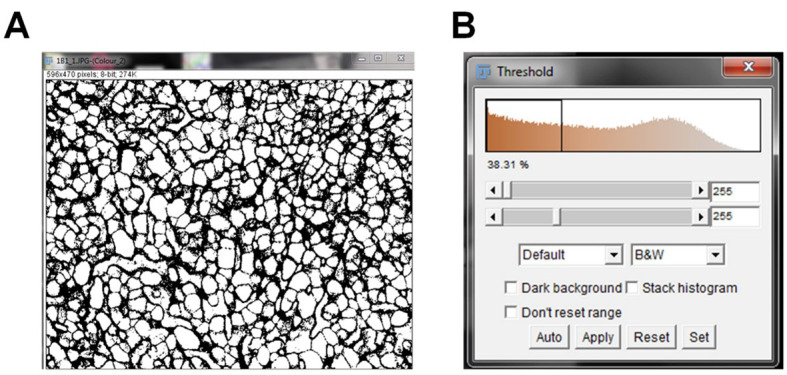
The background for the DAB staining in the IHC image was removed by adjusting the maximum threshold value (A). The minimum and maximum threshold values were set and applied (B).
Figure 5. Measurement of DAB staining.
The Set Measurements window to choose the options for output of each IHC image is shown. The suggested options for output are highlighted with red boxes (A). The Results output window is shown in (B), and contains the name of the image (Label), area of the image (Area), and mean grey value intensity (Mean).
Figure 6. Measurement of nuclei size.
The raw IHC image stained with DAB for OATP1B1 staining and hematoxylin for nuclei staining is used to measure the size of the nuclei. Using the straight-line tool, a line is drawn over the nucleus (A, white arrow pointing to yellow line). The distance of the line is measured by going to Analyze > Measure in the ImageJ Fiji toolbox panel. The Length (highlighted in red box) represents the diameter of the nucleus (B).
Figure 7. Quantification of nuclei in IHC image.
To measure the number of nuclei in each IHC image, the Analyze Particle window pops up after selecting Analyze > Analyze Particles (A). The size of the particle measured is set to the average diameter of nuclei to infinity. The options to summarize and exclude on the edges are selected prior to clicking okay. The summarize option leads to the summarized output of the count, total area, and the average size of the nuclei particles in the IHC image (B). Exclude on the eges indicates that no nuclei particles will be included in the analysis.
Data analysis
Expression of OATP1B1 and OATP1B3 were compared in genotyped human liver tissue stained with OATP1B1 or OATP1B3 DAB staining and hematoxylin. Using 79 genotyped human liver IHC samples, there was no significant difference between the genotypes for OATP1B1 (c.521 TC) polymorphism using a Student’s t -test ( Crowe et al. , 2019 ).
Application of this protocol to other studies should be done with caution, as it may not work for others if their microscope or staining protocols differ slightly, even if they were to imagestain the same protein with the same antibody. In addition, this protocol is not intended to replace the ImageJ manual, which is a great resource for users, and can be found at https://www.researchgate.net/publication/260261544_Analyzing_fluorescence_microscopy_images_with_ImageJ or https://petebankhead.gitbooks.io/imagej-intro/content/chapters/rois/rois.html .
Acknowledgments
This research was supported by NIH R01 GM094268 [W. Y]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Alexandra Crowe is an American Foundation of Pharmaceutical Education pre-doctoral Fellow. We thank Drs. Wei Zheng, Kar-Ming Fung, Feng Yin, and Erin Rubin for providing the FFPE tissues for this research.
Competing interests
No competing financial interests for this study.
Ethics
Use of human tissues was approved by the Institutional Review Board at the University of Oklahoma Health Sciences Center. A total of 79 formalin-fixed, paraffin-embedded (FFPE) archived human liver (42 from surgical resection and 37 from liver biopsy) and normal kidney tissue blocks were obtained from OUHSC Stephenson Cancer Center Biospecimen Acquisition Core and the Bank from the Department of Pathology at the University of Oklahoma Health Sciences Center.
The authors have noticed some errors in the original protocol that were now amended and clarified in the current protocol. The authors apologize for any inconvenience caused.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Bauman T. M. , Ricke E. A. , Drew S. A. , Huang W. and Ricke W. A. ( 2016 . ). Quantitation of protein expression and co-localization using multiplexed immuno-histochemical staining and Multispectral Imaging . J Vis Exp (110). doi: 10.3791/53837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braun M. , Kirsten R. , Rupp N. J. , Moch H. , Fend F. , Wernert N. , Kristiansen G. and Perner S. ( 2013 . ). Quantification of protein expression in cells and cellular subcompartments on immunohistochemical sections using a computer supported image analysis system . Histol Histopathol 28 ( 5 ): 605 - 610 . [DOI] [PubMed] [Google Scholar]
- 3. Chen Y. , Qi Y. , and Xu C. B. ( 2017 . ) A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software . Int J Clin Exp Med 10 ( 10 ): 14904 - 14910 . [Google Scholar]
- 4. Cregger M. , Berger A. J. and Rimm D. L. ( 2006 . ). Immunohistochemistry and quantitative analysis of protein expression . Arch Pathol Lab Med 130 ( 7 ): 1026 - 1030 . [DOI] [PubMed] [Google Scholar]
- 5. Crowe A. , Zheng W. , Miller J. , Pahwa S. , Alam K. , Fung K. M. , Rubin E. , Yin F. , Ding K. and Yue W. ( 2019 . ). Characterization of plasma membrane localization and phosphorylation status of organic anion transporting polypeptide(OATP) 1B1 c.521 T>C Nonsynonymous single-nucleotide polymorphism . Pharm Res 36 ( 7 ): 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fedchenko N. and Reifenrath J. ( 2014 . ). Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue- a review . Diagn Pathol 9 : 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guirado R. , Carceller H. , Castillo-Gomez E. , Castren E. and Nacher J. ( 2018 . ). Automated analysis of images for molecular quantification in immunohistochemistry . Heliyon 4 ( 6 ): e00669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen K. , Krusenstjerna-Hafstrom R. , Lohse J. , Petersen K. H. and Derand H. ( 2017 . ). A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: analytical performance measuring HER 2. Mod Pathol 30 ( 2 ): 180 - 193 . [DOI] [PubMed] [Google Scholar]
- 9. Kokolakis G. , Panagis L. , Stathopoulos E. , Giannikaki E. , Tosca A. and Kruger-Krasagakis S. ( 2008 . ). From the protein to the graph: how to quantify immunohistochemistry staining of the skin using digital imaging . J Immunol Methods 331 ( 1-2 ): 140 - 146 . [DOI] [PubMed] [Google Scholar]
- 10. Li H. , Spagnol G. , Naslavsky N. , Caplan S. and Sorgen P. L. ( 2014 . ). TC-PTP directly interacts with connexin43 to regulate gap junction intercellular communication . J Cell Sci 15 ): 3269 - 3279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu Z. , Liu Y. , Xu J. , Yin H. , Yuan H. , Gu J. , Chen Y. H. , Shi L. , Chen D. and Xie B. ( 2018 . ). Immunohistochemical quantification of expression of a tight junction protein, claudin-7, in human lung cancer samples using digital image analysis method . Comput Methods Programs Biomed 155 : 179 - 187 . [DOI] [PubMed] [Google Scholar]
- 12. Matkowskyj K. A. , Schonfeld D. and Benya R. V. ( 2000 . ). Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab . J Histochem Cytochem 48 ( 2 ): 303 - 312 . [DOI] [PubMed] [Google Scholar]
- 13. Pike J. A. , Styles I. B. , Rappoport J. Z. and Heath J. K. ( 2017 . ). Quantifying receptor trafficking and colocalization with confocal microscopy . Methods 115 : 42 - 54 . [DOI] [PubMed] [Google Scholar]
- 14. Shu J. , Dolman G. E. , Duan J. , Qiu G. and Ilyas M. ( 2016 . ). Statistical colour models: an automated digital image analysis method for quantification of histological biomarkers . Biomed Eng Online 15 : 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sysel A. M. , Valli V. E. , Nagle R. B. and Bauer J. A. ( 2013 . ). Immunohistochemical quantification of the vitamin B12 transport protein(TCII), cell surface receptor(TCII-R) and Ki-67 in human tumor xenografts . Anticancer Res 33 ( 10 ): 4203 - 4212 . [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor C. R. and Levenson R. M. ( 2006 . ). Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II . Histopathology 49 ( 4 ): 411 - 424 . [DOI] [PubMed] [Google Scholar]
- 17. Varghese F. , Bukhari A. B. , Malhotra R. and De A. ( 2014 . ). IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples . PLoS One 9 ( 5 ): e96801 . [DOI] [PMC free article] [PubMed] [Google Scholar]