Supplemental Digital Content is available in the text.
Key Words: leukemia, cancer, epidemiology, child health, systematic review, meta-analysis
Objective:
Current evidence regarding the association between paternal smoking before conception or during pregnancy and the risk of childhood acute lymphoblastic leukemia (ALL) are inconsistent. We aimed to systematically summarize the current evidence regarding this potential association.
Methods:
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE), we systematically retrieved PubMed, Embase, Web of Science, and Scopus, screened relevant literature, and assessed the methodologic quality of the included studies. We calculated the pooled estimates using random-effects models. We assessed statistical heterogeneity by I2 values and χ2 tests for the Cochrane Q statistic. We further investigate the dose-response relation using 2-stage nonlinear models.
Results:
A total of 17 case-control studies were identified, and the synthesized risk ratios (RRs) for smoking before conception (RR=1.15, 95% confidence interval: 1.04-1.27) and during pregnancy (RR=1.20, 95% confidence interval: 1.12-1.28) were both statistically significant. Moreover, the dose-response analysis showed a positive association as well.
Conclusion:
Current evidence from observational studies suggests the association between paternal smoking before conception or during pregnancy and the increased risk of childhood ALL, which needs to be confirmed in prospective studies.
Childhood leukemia is the most common cancer among children and adolescents younger than 18 years of age in the United States,1 which accounts for 29% of all childhood cancers,2 and acute lymphoblastic leukemia (ALL) is the most commonly observed subtype which accounts for ∼80% of childhood leukemia.1 Currently, the 5-year survival rate of childhood ALL is promising which is approaching 90%.1 Although the survival rate of childhood ALL looks favorable, the disease remains an intractable public health problem due to its increasing incidence. For example, McNally and Eden3 observed a significant increase in the incidence of childhood ALL at about 1% per year in well-developed countries. Therefore, there is a great need to study the etiology of childhood ALL, which can provide evidence for early intervention and prevention.
However, there is no consistent evidence to suggest that a single environmental or nutritional factor causes the disease, and some risk factors remain theoretical.4 In 2004, the International Agency for Research on Cancer (IARC) reported that there was a borderline association between parental smoking and the risk of childhood leukemia.5 In a recent systematic review and meta-analysis, Klimentopoulou et al6 reported that maternal smoking during pregnancy was not associated with the risk of childhood ALL. Momen et al7 also reported that maternal smoking was not associated with childhood ALL. These indicate that the etiologic association between prebirth parental smoking and childhood ALL may be attributed to paternal smoking. However, currently available epidemiologic studies investigating the effect of prebirth paternal smoking to the risk of childhood ALL are inconsistent.8–11
Presently, many potential parents choose to smoke. According to the data from the Centers for Disease Control and Prevention (CDC), ∼17.6% of adult males in the United States between the ages of 25 and 44 years choose to smoke12; this age range also corresponds to the period during which most of them choose to give birth or rear offspring. Prebirth paternal smoking is known to be associated with many childhood adverse health events, including but not limited to obesity, impaired lung function, asthma, and headache.13–15 Given these adverse effects, if paternal smoking before conception or during pregnancy increases the risk of childhood ALL, it will be more justifiable for us to boost tobacco control or smoking cessation program by informing and educating potential fathers of the hazards of smoking. Therefore, we performed this systematic review and meta-analysis of currently available epidemiology studies.
METHODS
Search Strategy
A systematic literature search of 4 electronic databases (PubMed, Embase, Web of Science, and Scopus) was performed on March 1, 2019, using keywords and controlled vocabularies that were related to “smoking,” “child,” and “leukemia” (Supplementary A, Supplemental Digital Content 1, http://links.lww.com/JPHO/A333). We also hand-searched the reference lists of previous systematic reviews of similar topics to obtain more eligible studies.
Study Identification
We followed the instructions of the Meta-analysis of Observational Studies in Epidemiology (MOOSE)16 and established restrictive selection criteria before study identification. In title/abstract screening, we considered studies that met the following criteria: (1) observational epidemiology study (a case-control, cohort, cross-sectional, nested case-control, and case-cohort study); (2) the exposure of interest was paternal smoking before conception or during pregnancy; (3) the outcome of interest was the risk of childhood ALL; and (4) written in English. In the full-text review process, we read the articles that were identified in the title/abstract screening process to determine whether they met the additional criteria: (1) full-text written in English; (2) reported the disease subtype (should be childhood ALL); (3) reported the exposure time window (before conception vs. during pregnancy); and (4) reported effect measure for paternal smoking or raw data that could be used to calculate unadjusted effect measure. The title/abstract screening and the full-text review were performed independently by reviewer pairs. Any discrepancy between the reviewers was resolved by discussion. Studies selected from the full-text review were included for systematic review and meta-analysis, and we performed data extraction and quality assessment for them independently.
The process of study identification and selection is presented in Figure 1 which includes essential details according to the requirement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).17
FIGURE 1.
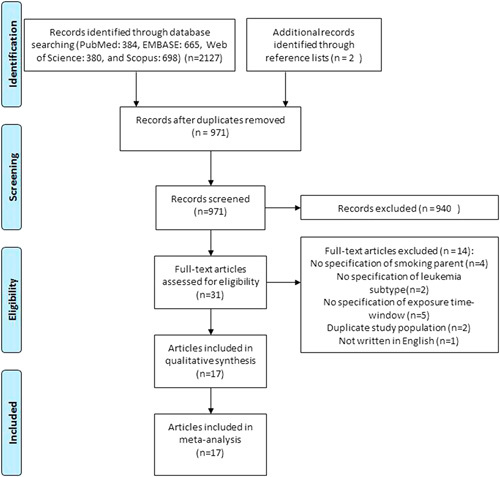
Flow chart for study identification and selection.
Data Extraction and Quality Assessment
After reading the selected articles, reviewers recorded information about the study characteristics, participants, exposure/outcome measurement, and measures of association; particularly, risk ratio (RR) was treated as the proximate measure of odds ratio due to the fact that childhood ALL was rare among the population. Moreover, we referred to The Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies in meta-analyses18 to evaluate the quality of included studies regarding aspects of representativeness, measurement reliability, and statistical adjustment (Supplementary B, Supplemental Digital Content 2, http://links.lww.com/JPHO/A334). This process was also performed in an independent manner by reviewers, and discrepancies were solved by discussion.
Synthesis Methods
For qualitative synthesis, we summarized the study characteristics of each individual study; for studies treating smoking as an ordinal variable, we first summarized the RRs of each exposure level qualitatively (Supplementary C, Supplemental Digital Content 3, http://links.lww.com/JPHO/A335) before the subsequent quantitative synthesis. In the overall meta-analysis, we pooled the measures of the association from studies treating smoking as a dichotomous variable by a random-effects model,19 and we weighted the RRs according to the SEs of ln(RR). A sensitivity meta-analysis was performed by including studies adjusting for certain covariates (child age at diagnosis, sex, race/ethnicity, parental age at birth, socioeconomic status, and maternal alcohol consumption during pregnancy) and studies with good quality in exposure measurement. In the dose-response analysis, we first synthesized studies reporting RRs of ordinal exposure by using RRs of the highest smoking consumption in a random-effects model. Then, we utilized a 2-stage restricted cubic spline model with 3 knots20 to investigate the dose-response association between frequency of paternal smoking and risk of childhood ALL. We used a relatively conservative approach to determine the dose in the restricted cubic spline model; particularly, the dose was determined by using the middle point of a range (eg, if the smoking was 10 to 20 cigarettes/d, the dose=15 cigarettes/d) or the lower bound (eg, for smoking>20 cigarettes/d, the dose=20 cigarettes/d) if the range was not reported.
To investigate statistical heterogeneity, we calculated the Cochrane Q statistic and I2. The former was a weighted sum of squares following the χ2 distribution;21 I2 was an indicator for inconsistency across studies, and it was interpreted as the proportion of variation between different studies among the total variation observed.22 Substantial statistical heterogeneity existed if the P-value derived from the Cochrane Q statistic was <0.05,21 and we also used “I2>50%” as the evidence of substantial statistical heterogeneity.21,22
To investigate publication bias, we visually inspected the funnel plots and performed Egger tests for studies included in the overall meta-analysis.23 The Egger test indicated a publication bias if P-value <0.05. Trim and fill were utilized to obtain an adjusted RR if publication bias existed.24
The statistical analyses were all performed with stratification based on time window (smoking before conception vs. during pregnancy), and they were performed in STATA 13.0 (StataCorp, LLP, College Station, TX).
RESULTS
Included Studies
We retrieved 2127 articles from the electronic databases, and we identified 2 additional articles from reference lists. After deduplication, we read the titles and abstracts of 971 articles and excluded 940 of them. In the full-text review, we read the full-text of 31 articles and excluded 14 of them. Finally, 17 studies8–11,25–37 were included in the systematic review and meta-analysis. The reasons for exclusion are shown in Figure 1.
Study Characteristics
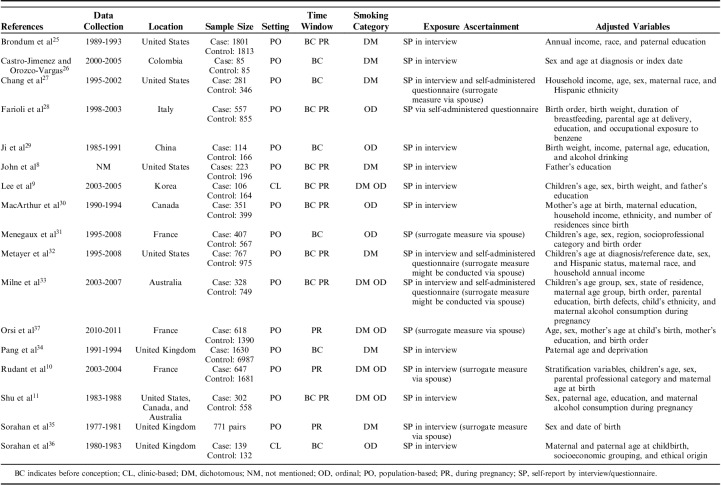
The study characteristics are summarized in Table 1. The included studies encompassed a wide time span (1977-2011), and they were conducted in various geographic locales. They yielded a total of 9127 childhood ALL cases. Among them, 8 studies8,9,11,25,28,30,32,33 reported effect measures for paternal smoking before conception and during pregnancy, 3 studies10,35,37 only reported effect measures for smoking during pregnancy, and 6 studies26,27,29,31,34,36 only reported effect measures for smoking before conception. Seven studies8,25–27,32,34,35 reported RRs of dichotomous exposure (smoking vs. nonsmoking), 5 studies28–31,36 reported RRs of ordinal exposure, and 5 studies9–11,33,37 reported RRs on both scales. The self-report approach was utilized to collect information about paternal smoking, and 7 studies10,27,31–33,35,37 collected paternal smoking data via surrogate measurement based on answers from the mothers. In terms of the study setting, most studies were population-based, and only 2 studies9,36 were clinic-based.
TABLE 1.
Study Characteristics
The included studies had some methodologic strengths. For example, all of the studies ascertained childhood ALL cases by robust approach (eg, medical record, diagnosis report, and cancer registry); in addition, they all used match method when selecting controls, which benefited statistical efficiency. However, they also had some methodologic limitations. First, the studies measured paternal smoking via answers from mothers were less reliable than direct measurement, which might lead to misclassification. Second, 1 study26 only had 85 cases, which could introduce imprecision to effect measure. Third, 2 of the included studies9,36 were clinic-based; of them, the study populations were systematically different from the community populations with respect to exposure status and general health status, which introduced some methodologic heterogeneity. Moreover, these studies were conducted in different time periods and locations, and these temporal and socioeconomic heterogeneities could not be adjusted by the random-effects model.
Overall Meta-Analysis
In overall meta-analysis for RRs of dichotomous exposure, 8 studies8,11,25–27,32–34 were included to investigate the association between paternal smoking before conception and childhood ALL risk, and 9 studies8–11,25,32,33,35,37 were synthesized for smoking during pregnancy. The synthesized RR for smoking before conception (Fig. 2) was 1.15 (95% confidence interval [CI]: 1.04-1.27), and all individual studies showed point estimates >1. For smoking during pregnancy (Fig. 2), all of the included studies had point estimates >1, and a positive and statistically significant synthesized effect measure (RR=1.20, 95% CI: 1.12-1.28) was obtained as well. In addition, we did not observe substantial statistical heterogeneity in either of these subgroups stratified by time window (before conception: I2=16.8%, PCochrane=0.298; during pregnancy: I2=0.0%, PCochrane=0.476).
FIGURE 2.
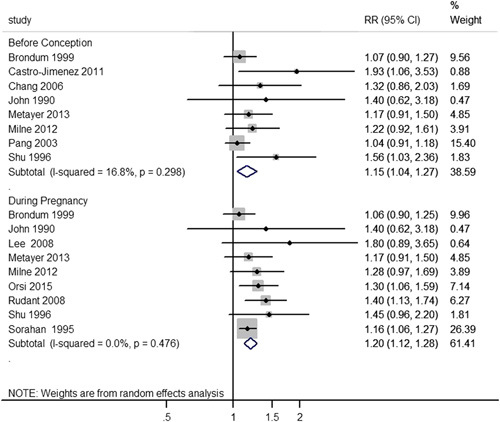
Overall meta-analysis stratified by the exposure time window. CI indicates confidence interval; RR, risk ratio.
Sensitivity Analysis
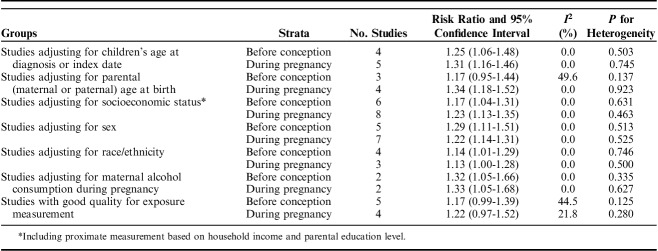
Table 2 presents the results of sensitivity analysis. By synthesizing studies adjusting for age at diagnosis, socioeconomic status, sex, race/ethnicity, and maternal alcohol consumption during pregnancy, we observed that the synthesized RRs were all statistically significant without substantial statistical heterogeneity in both exposure time windows. For studies adjusting for parental age at birth, the synthesized RR was only statistically significant for smoking during pregnancy; however, the CIs of synthesized RRs of the 2 exposure time windows were overlapped to a great extent, which indicated a statistical homogeneity between these pooled RRs. By synthesizing studies with good quality in exposure measurement, we observed that the synthesized RRs of the 2 exposure time windows were statistically homogeneous and marginally significant without substantial statistical heterogeneity.
TABLE 2.
Sensitivity Analysis
Dose-Response Analysis
For smoking before conception (Fig. 3), a total of 8 studies9,11,28–31,33,36 were included in the random-effects model by using RRs of the highest level of exposure; the synthesized RR was marginally significant (RR=1.33, 95% CI: 0.99-1.80) with substantial statistical heterogeneity (I2=58.8%, PCochrane=0.018). The 2-stage nonlinear curve (Fig. 4A) showed that the effect measure increased gradually as daily smoking consumption increased; however, it was not significant until the daily consumption reached 16 cigarettes/d, and the synthesized RR reached 2.0 at about 35 cigarettes/d. For smoking during pregnancy (Fig. 3), 5 studies10,28,30,33,37 were synthesized in the random-effects model by the same approach as mentioned before; the synthesized RR was statistically significant (RR=1.32, 95% CI: 1.09-1.60) with substantial statistical heterogeneity (I2=50.9%, PCochrane=0.086). The 2-stage nonlinear curve (Fig. 4B) showed a similar pattern as compared with Figure 4A; a significant effect measure was observed after 11 cigarettes/d, and it reached 1.4 at 20 cigarettes/d.
FIGURE 3.
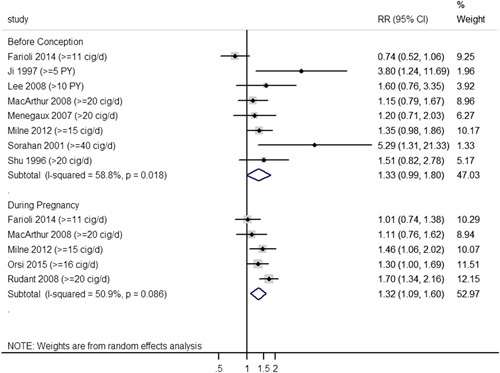
Meta-analysis by synthesizing RRs of the highest smoking consumption. CI indicates confidence interval; cig/d, cigarette per day; PY, pack-year; RR, risk ratio.
FIGURE 4.
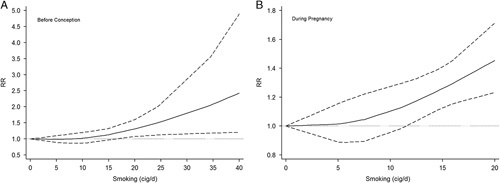
A, Dose-response curve by restricted cubic spline model (smoking before conception). The solid line is the fitted line, dash lines are the lines for 95% confidence interval, and dot line is the reference line. B, Dose-response curve by restricted cubic spline model (smoking during pregnancy). The solid line is the fitted line, dash lines are the lines for 95% confidence interval, and dot line is the reference line. cig/d indicates cigarette per day; RR, risk ratio.
Publication Bias
On the basis of the visual inspection of funnel plots and the results of Egger tests (Fig. 5), we observed evidence for publication bias for both groups. After trim and fill adjustment, the synthesized RR was 1.11 (95% CI: 0.96-1.28) for smoking before conception, and it was 1.19 (95% CI: 1.11-1.27) for smoking during pregnancy.
FIGURE 5.
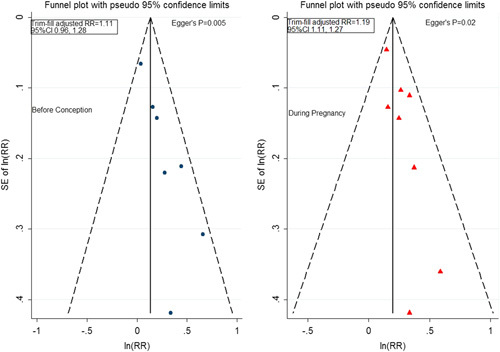
Funnel plots, Egger tests, and trim/fill adjusted effect measures stratified by time windows. CI indicates confidence interval; RR, risk ratio.
DISCUSSION
According to the results, paternal smoking before conception and during pregnancy were both associated with an increased risk of childhood ALL. This association was further examined and confirmed by sensitivity analysis and trim and fill adjustment as the estimate did not change too much after these adjustments. We also observed a monotonic nonlinear dose-response relation between paternal smoking and risk of childhood ALL in both time windows, and the risk was shown to be significant when daily smoking consumption was higher than a certain threshold of usage.
A recent systematic review and meta-analysis of this topic was updated in 2011. Liu et al38 came to the same conclusion as ours, and they reported that paternal smoking was positively associated with the risk of childhood ALL regardless of the exposure time windows. However, Liu et al38 did not utilize the linear or nonlinear model to investigate dose-response relation; instead, they utilized RRs of the highest smoking consumption in the meta-analysis when smoking was reported as an ordinal variable. Moreover, they did not conduct trim and fill to adjust for publication bias in meta-analysis. These issues were carefully considered and addressed in our study, which made our outcome more robust.
The pathogenesis mechanism of childhood ALL is not fully understood, but it may be related to sperm DNA damage and oxidative stress.39,40 Jenkins et al39 reported smoking was associated with increased risk of the variance in sperm DNA methylation patterns, and it might explain the relationship between paternal smoking and the risk to child health. One of the major carcinogenic polycyclic aromatic hydrocarbons in tobacco smoke is benzo[a]pyrene, which can form DNA adducts in sperm cells and cause subsequent damage.41 According to the 2-hit hypothesis42 of neoplasm, carcinogenesis is more likely to be initiated during the early life cycle if the zygote with impaired germ-line DNA grows up.43 Thus, the impaired sperm cell DNA, after zygote formation, may increase the risk of childhood ALL among offspring. Benzene is another major carcinogen in tobacco smoke. A previous systematic review reported that exposure to excessive benzene during pregnancy could increase the risk of childhood ALL; Zhou et al44 reported a positive association (odds ratio=1.25, 95% CI: 1.09-1.45) between benzene and risk of childhood ALL by synthesizing data from 28 case-control studies. In addition, smoking can diminish the levels of antioxidants and cause excessive oxidative stress in germ-line cells, which can also increase the risk of gene mutation45–47; for example, Fraga et al46 reported that seminal alpha-tocopherol levels were decreased in smokers by ∼30% as compared with nonsmokers. In addition to smoking, Donkin and Barrès48 demonstrated that environmental factors may alter the phenotype of the next generation through remodeling of the epigenetic blueprint of spermatozoa.
The human fetal liver serves as the primary organ for hematopoiesis during part of the embryonic period,49 which suggests that pathogenesis in fetal liver can be associated with subsequent risk of hematopoietic malignancies. Ning et al50 found a positive dose-response relationship between benzene levels in tobacco smoke and the frequency of micronucleus-containing polychromatic erythrocytes in blood from mouse fetal livers, indicating its potential harm to the hematopoietic system; also, DeMarini51 reported that the offspring of environmental tobacco smoke-exposed female mice exhibited increased levels of micronuclei in liver tissues and peripheral blood, and they also found that such exposure could increase the risk of sister chromatid exchanges in mouse fetal livers.
At the genetic level, overexpression or down-regulation of important fetal genes can be associated with the risk of childhood ALL. For example, Amson et al52 reported that human pim-1 proto-oncogene (PIM) was overexpressed in fetal liver and hematopoietic malignancies but not in normal human tissues; this suggested the possibility that carcinogens in tobacco smoke might increase the risk of subsequent hematopoietic malignancies by affecting the structure of PIM or influencing regulatory genes of PIM. By inspecting umbilical cord blood of infants whose mothers were exposed to passive smoking during pregnancy, Votavova et al53 detected the down-regulation of several genes that were associated with cellular defense responses and cellular immunity (GNLY, CD160, CD40, PRDM1, and SOCS3); this indicated that paternal smoking during pregnancy might affect the immune surveillance of the offspring, which could increase the risk of childhood ALL.
Our study has several strengths. First, we searched articles in 4 electronic databases, which generated a broad scope. Second, we utilized a 2-stage nonlinear dose-response model to investigate dose-response relation, which has never been done in previous systematic review and meta-analysis. However, our study still has some limitations. First, leukemia carcinogenesis can be related to the synergistic effect of both parents smoking. However, none of the included studies adjusted for maternal smoking status in the multivariable model. Second, all of the included studies used case-control design, which could not examine the temporality; in addition, in case-control studies, recall bias was likely to be introduced when researchers used a self-report method to collect smoking data. Given that the cases’ parents were more likely to recall smoking status correctly than the controls’ parents, a differential measurement error was likely to be introduced in the original studies, which could bias the estimate. Third, smoking is a time-dependent variable, but case-control studies cannot measure the smoking prospectively and longitudinally as compared with cohort studies, which makes it difficult for us to consider the influence of smoking variation during pregnancy to the effective measures. More importantly, paternal smoking during pregnancy might not be the direct source of passive smoking or environmental tobacco smoke; for example, fathers and mothers might be at different geographic sites when fathers consumed tobacco, which could influence the actual exposure levels to mothers. Fourth, the approach used to determine the dose in 2-stage nonlinear model was conservative, as we did not know the distribution of each exposure level; thus, choosing the middle point or using the lower bound as a surrogate might lead to bias and imprecision to the pooled estimate. Fifth, only 2 of the included studies were conducted in Asia, and this makes our conclusion less generalizable to Asian population. Last, articles that are not in English were excluded, which may lead to relevant articles were omitted.
In conclusion, we observe that paternal smoking before conception or during pregnancy is associated with an increased risk of childhood ALL; the dose-response analysis also confirms this relation and suggests the possibility of carcinogenesis threshold for paternal smoking. More large sample size cohort studies are needed in the future in which multiple measurements of smoking should be conducted. Our study has some public health implications; particularly, our results support the etiologic role of paternal smoking to childhood ALL, and this can make it justifiable for health practitioners to initiate health education or smoking cessation programs for potential fathers.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jpho-online.com.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.McNally R, Eden T. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br J Haematol. 2004;127:243–263. [DOI] [PubMed] [Google Scholar]
- 4.Eden T. Aetiology of childhood leukaemia. Cancer Treat Rev. 2010;36:286–297. [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 6.Klimentopoulou A, Antonopoulos CN, Papadopoulou C, et al. Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatr Blood Cancer. 2012;58:344–351. [DOI] [PubMed] [Google Scholar]
- 7.Momen NC, Olsen J, Gissler M, et al. Exposure to maternal smoking during pregnancy and risk of childhood cancer: a study using the Danish national registers. Cancer Causes Control. 2016;27:341–349. [DOI] [PubMed] [Google Scholar]
- 8.John EM, Savitz DA, Sandler DP. Prenatal exposure to parents smoking and childhood-cancer. Am J Epidemiol. 1991;133:123–132. [DOI] [PubMed] [Google Scholar]
- 9.Lee KM, Ward MH, Han S, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009;33:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudant J, Menegaux F, Leverger G, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE). Cancer Causes Control. 2008;19:1277–1290. [DOI] [PubMed] [Google Scholar]
- 11.Shu XO, Ross JA, Pendergrass TW, et al. Parental alcohol consumption, cigarette smoking, and risk of infant leukemia: a childrens cancer group study. J Natl Cancer Inst. 1996;88:24–31. [DOI] [PubMed] [Google Scholar]
- 12.Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am J Clin Nutr. 2011;94:164–171. [DOI] [PubMed] [Google Scholar]
- 14.Gilliland FD, Berhane K, McConnell R, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology—a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev Esp Nutr Hum Diet. 2016;20:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Can Med Assoc J. 2007;176:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 25.Brondum J, Shu XO, Steinbuch M, et al. Parental cigarette smoking and the risk of acute leukemia in children. Cancer. 1999;85:1380–1388. [PubMed] [Google Scholar]
- 26.Angel Castro-Jimenez M, Carlos Orozco-Vargas L. Parental exposure to carcinogens and risk for childhood acute lymphoblastic leukemia, Colombia, 2000-2005. Prev Chronic Dis. 2011;8:A106. [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JS, Selvin S, Metayer C, et al. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163:1091–1100. [DOI] [PubMed] [Google Scholar]
- 28.Farioli A, Legittimo P, Mattioli S, et al. Tobacco smoke and risk of childhood acute lymphoblastic leukemia: findings from the SETIL case-control study. Cancer Causess Control. 2014;25:683–692. [DOI] [PubMed] [Google Scholar]
- 29.Ji BT, Shu XO, Linet MS, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89:238–244. [DOI] [PubMed] [Google Scholar]
- 30.MacArthur AC, McBride ML, Spinelli JJ, et al. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control. 2008;19:283–295. [DOI] [PubMed] [Google Scholar]
- 31.Menegaux F, Ripert M, Hemon D, et al. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: a French population-based case-control study. Paediatr Perinat Epidemiol. 2007;21:293–299. [DOI] [PubMed] [Google Scholar]
- 32.Metayer C, Zhang L, Wiemels JL, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22:1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milne E, Greenop KR, Scott RJ, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175:43–53. [DOI] [PubMed] [Google Scholar]
- 34.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer. 2003;88:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorahan T, Lancashire R, Prior P, et al. Childhood cancer and parental use of alcohol and tobacco. Ann Epidemiol. 1995;5:354–359. [DOI] [PubMed] [Google Scholar]
- 36.Sorahan T, McKinney PA, Mann JR, et al. Childhood cancer and parental use of tobacco: findings from the inter-regional epidemiological study of childhood cancer (IRESCC). Br J Cancer. 2001;84:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orsi L, Rudant J, Ajrouche R, et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: the ESTELLE study. Cancer Causes Control. 2015;26:1003–1017. [DOI] [PubMed] [Google Scholar]
- 38.Liu R, Zhang L, McHale CM, et al. Paternal smoking and risk of childhood acute lymphoblastic leukemia: systematic review and meta-analysis. J Oncol. 2011;2011:854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins TG, James ER, Alonso DF, et al. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology. 2017;5:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai J, Wang Z, Qiao Z. The hazardous effects of tobacco smoking on male fertility. Asian J Androl. 2015;17:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, Wang YX, Sun L, et al. Urinary metabolites of polycyclic aromatic hydrocarbons, sperm DNA damage and spermatozoa apoptosis. J Hazard Mater. 2017;329:241–248. [DOI] [PubMed] [Google Scholar]
- 42.Greaves M. Childhood leukaemia. BMJ. 2002;324:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashley D. The two “hit” and multiple “hit” theories of carcinogenesis. Br J Cancer. 1969;23:313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Zhang S, Li Z, et al. Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: a meta-analysis of epidemiologic studies. PLoS One. 2014;9:e11046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81–85. [DOI] [PubMed] [Google Scholar]
- 46.Fraga CG, Motchnik PA, Wyrobek AJ, et al. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351:199–203. [DOI] [PubMed] [Google Scholar]
- 47.Saleh RA, Agarwal A, Sharma RK, et al. Effect of cigarette smoking on level of seminal oxidative stress in fertile men: a prospective study. Fertil Steril. 2002;78:491–499. [DOI] [PubMed] [Google Scholar]
- 48.Donkin I, Barrès R. Sperm epigenetics and influence of environmental factors. Mol Metab. 2018;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14:493–504. [DOI] [PubMed] [Google Scholar]
- 50.Ning HS, Kado NY, Kuzmicky PA, et al. Benzene-induced micronuclei formation IN mouse fetal liver blood, peripheral-blood, and maternal bone-marrow cells. Environ Mol Mutagen. 1991;18:1–5. [DOI] [PubMed] [Google Scholar]
- 51.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–474. [DOI] [PubMed] [Google Scholar]
- 52.Amson R, Sigaux F, Przedborski S, et al. The human protooncogene product P33PIM is expressed during fetal hematopoiesis and in diverse leukemias. Proc Natl Acad Sci USA. 1989;86:8857–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Votavova H, Merkerova MD, Krejcik Z, et al. Deregulation of gene expression induced by environmental tobacco smoke exposure in pregnancy. Nicotine Tob Res. 2012;14:1073–1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jpho-online.com.