Supplemental Digital Content is available in the text.
Keywords: cognitive dysfunction, cognitive reserve, education, longitudinal studies, occupation, risk factors, stroke
Background and Purpose—
The theory of cognitive reserve (CR) was introduced to account for individual differences in the clinical manifestation of neuropathology. This study investigated whether CR has a modulating effect on cognitive impairment and recovery after stroke.
Methods—
This study is an interim analysis of the Korean Stroke Cohort for Functioning and Rehabilitation. A total of 7459 patients with first-ever stroke were included for analysis. Education, occupation, and composite CR scores derived from those 2 variables were used as CR proxies. Scores from the Korean version of the Mini-Mental State Examination analyzed for 30 months after stroke onset were analyzed.
Results—
Lower CR increased the risk of cognitive impairment after stroke. The odds ratio was 1.89 (95% CI, 1.64–2.19) in patients with secondary education and 2.42 (95% CI, 2.03–2.90) in patients with primary education compared with patients with higher education. The odds ratio was 1.48 (95% CI, 1.23–1.98) in patients with a skilled manual occupation and 2.01 (95% CI, 1.42–2.83) in patients with a nonskilled manual occupation compared with patients with a managerial or professional occupation. In the multilevel model analysis, the Korean version of the Mini-Mental State Examination total score increased during the first 3 months (1.93 points per month) and then plateaued (0.02 point per month). The slopes were moderated by the level of education, occupation, and composite CR score: the higher the level of education, occupation, or CR score, the faster the recovery. In the older adult group, the Korean version of the Mini-Mental State Examination scores showed a long-term decline that was moderated by education level.
Conclusions—
Education and occupation can buffer an individual against cognitive impairment caused by stroke and promote rapid cognitive recovery early after stroke. In addition, higher education minimizes long-term cognitive decline after stroke, especially in older patients.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03402451.
Cognitive impairment after stroke is a frequent and significant contributor to disability among patients with stroke.1,2 Furthermore, history of stroke is associated with an increased risk of dementia3 and could contribute to Alzheimer disease and subsequent dementia.4
The degree of cognitive decline or recovery after stroke varies depending on each individual’s premorbid capacity.1 Each individual has different educational, vocational, and socioeconomic experiences that affect the behavioral and clinical manifestations of neuropathological burdens. This idea has been well established as the theory of cognitive reserve (CR),5,6 which accounts for individual differences in clinical manifestations of Alzheimer disease. Several studies7–10 have applied CR variables, mostly education, to patients with stroke. Recent reviews and meta-analyses11,12 showed that a lower educational history is positively associated with cognitive decline and vascular cognitive impairment. However, those studies did not evaluate cognitive function in the early phase after stroke onset, or they did not evaluate cognitive function repetitively. Therefore, they provide only limited understanding about the long-term trajectory of cognitive change and the effects of CR on long-term cognitive function after stroke.
In this study, we investigated whether CR moderated the occurrence of cognitive impairment and recovery after stroke. We hypothesized that low CR could increase the risk of cognitive impairment after stroke and that high CR could promote faster cognitive recovery after stroke.
Methods
Our study data cannot be accessed publicly per the internal regulations of the Korean National Institute of Health because KOSCO (Korean Stroke Cohort for Functioning and Rehabilitation) is an ongoing project.
Study Design and Participants
This study is an interim analysis of the KOSCO, which is a 10-year longitudinal, multi-center, prospective cohort study of acute first-ever patients with stroke admitted to hospitals in 9 regional districts of Korea. KOSCO was designed to investigate the residual disabilities, activity limitations, and quality of life issues that arise in patients after suffering from their first-ever stroke. A total of 10 636 patients, aged 19 to 100 years, with a first-ever stroke (both ischemic and hemorrhagic) were recruited from August 2012 to May 2015. The criteria for inclusion were as follows: (1) first acute stroke (ischemic stroke or intracerebral hemorrhage) with a corresponding lesion or evidence of acute arterial occlusion on a computed tomography or magnetic resonance imaging; (2) age ≥19 years at stroke onset; and (3) onset of symptoms within 7 days before inclusion. Patients were excluded if they had a transient ischemic attack, history of stroke, traumatic intracerebral hemorrhage, or were not Korean. Written informed consent was obtained from all patients or, if the patient was unable to provide informed consent, from their legally authorized representatives before inclusion in the study. The study protocol was approved by the Ethics Committee of each participating hospital. A detailed protocol for this study has been published elsewhere.13
Procedure
In this study, we analyzed data from the first 8 waves (baseline, discharge, 3 months, 6 months, 12 months, 18 months, 24 months, 30 months) of the KOSCO study. Among the 10 636 patients registered in the KOSCO study, 7858 consented to long-term follow-up. All patients underwent comprehensive surveys and assessments, including motor function, language skills, and mood, as well as cognitive function testing at 7 days after stroke onset as a baseline, and they received the same assessments at every follow-up. Among the 7858 patients, 7639 with at least 1 Korean version of the Mini-Mental State Examination (K-MMSE) score available at any time point were screened for this cross-sectional analysis. Incomplete scores were not included in the analysis because the K-MMSE score was coded as missing if a patient could not complete the K-MMSE by themselves. Among the 7639 patients, 51 with premorbid medical conditions that could affect cognitive performance and 129 with no education or occupation information were also excluded. We performed a cross-sectional analysis of the final 7459 patients to examine the relative risk of low CR on cognitive impairment after stroke onset. Out of the 7459 patients, educational information was available for 7356 patients. Among them, 7014, 7120, 5354, 5014, 4778, 4642, 4585, and 4478 patients were followed up at 7 days, discharge, 3 months, 6 months, 12 months, 18 months, 24 months, and 30 months, respectively. Occupational information was available for 4669 patients out of the 7459 patients. Among them, 4444, 4525, 4196, 4042, 3695, 3575, 3539, and 3443 patients were followed up at 7 days, discharge, 3 months, 6 months, 12 months, 18 months, 24 months, and 30 months, respectively (Table I in the online-only Data Supplement). Composite CR scores were calculated for the 4566 patients with both educational and occupational information (Figure 1).
Figure 1.
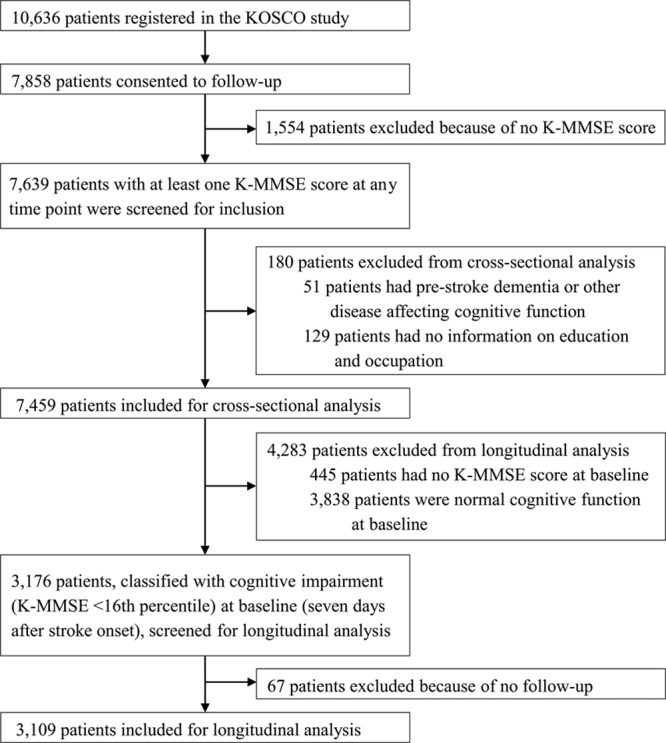
Flow chart of participants. K-MMSE indicates Korean version of the Mini-Mental State Examination; and KOSCO, Korean Stroke Cohort for Functioning and Rehabilitation.
Among the 7459 patients who were included in the cross-sectional analysis, we included 3176 who were classified as having cognitive impairment (K-MMSE <16th percentile) 7 days after stroke onset in a longitudinal analysis to observe the recovery pattern of their cognitive function. Sixty-seven patients were excluded because they did not have at least 1 more additional evaluation after the initial evaluation. If a patient missed an evaluation at any wave for personal reasons, we considered them to have skipped one wave, not dropped out, and they were evaluated at the next wave. The number of evaluations of the final 3109 patients varied from 2 to 8, with 496 assessed twice, 279 assessed 3×, 227 assessed 4×, 264 assessed 5×, 314 assessed 6×, 477 assessed 7×, and 1052 assessed 8×. Educational information was available for all of the 3109 patients in this analysis, and occupational information was available for 1669 patients. We calculated composite CR scores for the 1669 patients using both educational and occupational information (Figure 1).
CR Proxies
We used education and premorbid occupation as proxies for CR in our analysis. Education was classified based on the highest diploma that the patient acquired as follows: no formal education, primary education (primary school diploma), secondary education (middle or high school or 2-year college diploma), and higher education (university or graduate school diploma). Premorbid occupation was classified using the Korean Standard Classification of Occupations14: no occupation, nonskilled manual, skilled manual, and manager or professional. A composite CR score summarizing the information from the 2 CR variables was calculated based on the procedures described in a previous study,15 but we used a categorical principal component analysis instead of a factor analysis. categorical principal component analysis is appropriate for data reduction when variables are categorical. The single factor extracted (the composite CR score) accounted for 70.1% of the common variance in the 2 measures. The mean of the composite CR score was 0, and the SD was 1. A higher score indicates higher CR.
Outcomes
The K-MMSE16 was used as the outcome measure. The total possible score is 30 points. Cognitive impairment (total K-MMSE score <16th percentile) was defined based on age- and education-adjusted norms.17
Statistical Analysis
Descriptive information about the groups was compared using t tests or χ2 tests. We used ANCOVA to compare the severity of cognitive impairment among groups while controlling for background variables. Bonferroni correction was performed for post hoc analyses of the ANCOVA results. Logistic regression analyses were used to examine the relative risk of cognitive impairment at each CR level at every time point. Odds ratios with 95% CIs were calculated. SPSS version 19.0 software18 was used for all of those statistical analyses.
To analyze the longitudinal recovery patterns after stroke, we used a multilevel analysis to investigate whether cognitive function recovery patterns varied depending on CR. A multilevel model is appropriate for nested data in which observations are organized at >1 level.19 Observations in our data, K-MMSE scores, are nested within individuals. Because the clinical features of cognitive impairment in patients with stroke are characterized by rapid improvement at the initial stage and subsequent stable maintenance,1 an exponential function was applied first to see the natural course of recovery. We then reanalyzed the data using a linear regression function with 2 different slopes (piecewise regression), 1 for the initial active stage, and 1 for the later stable stage to clarify the clinical meanings of the coefficients because the coefficients of the exponential function are difficult to interpret directly. This procedure was expected to improve our understanding of the course of cognitive recovery in patients with stroke. In the piecewise regression model, we set the breakpoint to 3 months based on the graph derived from the exponential model. Because piecewise regression comprises 2 simple regression analyses, the coefficient values and t-ratios are interpreted the same as in a simple regression. Education and occupation were coded as dummy variables, and the composite CR score was used as a continuous variable. HLM ver.7 software19 was used for the statistical analysis of these data because it can be applied to data with different time points or intervals among subjects. All statistical analyses were adjusted for background variables: age, sex, initial National Institutes of Health Stroke Scale score, stroke subtype, and the stroke risk factors listed in Table II in the online-only Data Supplement. See the Methods in the online-only Data Supplement for a detailed description of this statistical analysis.
Results
Cross-Sectional Analysis
Because the demographic variables and several stroke risk factors differed significantly among the 4 education and occupation groups (Table 1 and Table II in the online-only Data Supplement), these background variables were included in the statistical analyses to control for unwanted effects.
Table 1.
Baseline Characteristics of the Patients Included in the Analysis
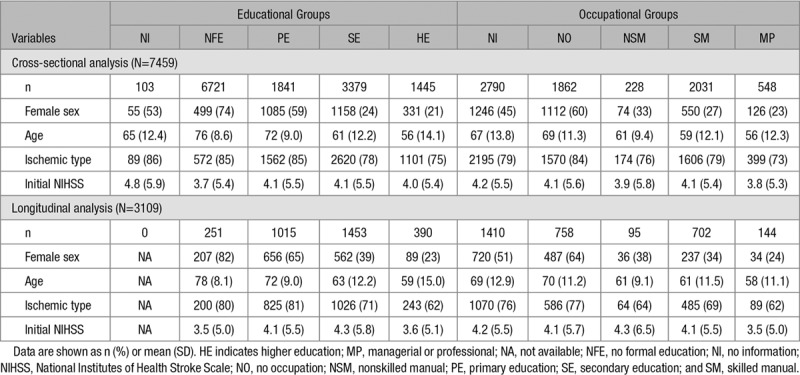
Before examining the relative risk of low CR on cognitive impairment, we compared the severity of cognitive deficits and frequency of cognitive impairments among the 4 education and 4 occupation groups at every time point (Tables III and IV in the online-only Data Supplement). In most comparisons, a lower level of education or occupation was associated with a lower K-MMSE score and higher frequency of cognitive impairment after stroke onset.
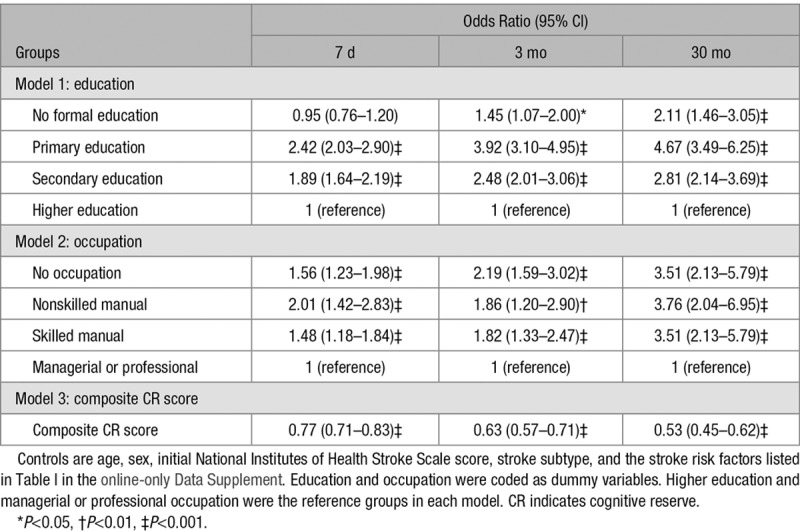
We applied logistic regression analyses to investigate whether low CR increased the risk of cognitive impairment after stroke. We used 3 independent models in the logistic regression with education, occupation, and composite CR score as the predictors (Table 2). Lower education, occupation, and composite CR score each increased the relative risk of cognitive impairment at every time point. At 7 days after stroke onset, the odds ratio was 1.89 (95% CI, 1.64–2.19) in patients with secondary education, 2.42 (95% CI, 2.03–2.90) in patients with primary education, and 0.95 (95% CI, 0.76–1.20) in patients with no education compared with patients with higher education (Table 2, Model 1). Compared with patients with a managerial or professional occupation, the odds ratio was 1.48 (95% CI, 1.23–1.98) in patients with a skilled manual occupation; 2.01 (95% CI, 1.42–2.83) in patients with a nonskilled manual occupation; and 1.56 (95% CI, 1.23–1.98) in patients with no occupation (Table 2, Model 2). The composite CR score was also significant in predicting cognitive impairment (Table 2, Model 3): the higher the composite CR score, the lower the risk (odds ratio, 0.77 [95% CI, 0.71–0.83]). In general, the odds ratios increased as the levels of education or occupation decreased, except for no formal education and no occupation, and as the follow-up evaluations progressed (See Table V in the online-only Data Supplement for logistic regression results at discharge, 6 months, 12 months, 18 months, and 24 months).
Table 2.
Results of Logistic Regression Analyses Predicting Cognitive Impairment at 7 Days, 3 Months, and 30 Months After Stroke Onset, Controlling for Background Variables
Longitudinal Analysis
In the multilevel analysis, the exponential function showed that the K-MMSE scores increased rapidly during the first 3 months and plateaued thereafter (Table VI in the online-only Data Supplement; Figure 2A-1 and 2B-1). Based on the graphs derived from the exponential model, we set the breakpoint in the piecewise regression function to 3 months. The first slope (<3 months) was steeper than the second slope (>3 months). In the whole group, the K-MMSE score increased 1.93 points per month (coefficient=1.93, P<0.001; Table VII, Model 1 in the online-only Data Supplement) during the first 3 months and 0.02 points per month (coefficient=0.02, P<0.001; Table VII, Model 1 in the online-only Data Supplement) thereafter. Comparison of the slopes among the 4 education (Table 3, Model 1) and 4 occupation (Table 3, Model 2) groups, controlling for background variables and initial K-MMSE score, showed that the first slope was significantly steeper in the groups with a higher level of education or occupation compared with the lowest education or occupation group (Figures 2A-2 and 2B-2). The K-MMSE score increased 2.79 points per month (coefficient=2.79, P<0.001; Table 3, Model 1) in the no-formal-education group (reference), 3.37 points per month in the group with primary education (2.79+0.58, P<0.001; Table 3, Model 1), 3.75 points per month in the group with secondary education (2.79+0.96, P<0.001; Table 3, Model 1), and 3.95 points per month (2.79+1.16, P<0.001; Table 3, Model 1) in the group with higher education. This pattern was similar in the occupation groups. The K-MMSE score increased 5.39 points per month (coefficient=5.39, P<0.001; Table 3, Model 2) in the no-occupation group (reference), 5.66 points per month in the group with nonskilled manual occupations (5.39+0.27, nonsignificant; Table 3, Model 2), 5.74 points per month in the group with skilled manual occupations (5.39+0.35, P<0.001; Table 3, Model 2), and 5.95 points per month (5.39+0.56, P<0.001; Table 3, Model 2) in the group with managerial or professional occupations. When we used composite CR score as the predictor, the result was consistent (Table 3, Model 3): a higher composite CR score increased the slope during the first 3 months (coefficient=0.29, P<0.001; Table 3, Model 3).
Figure 2.
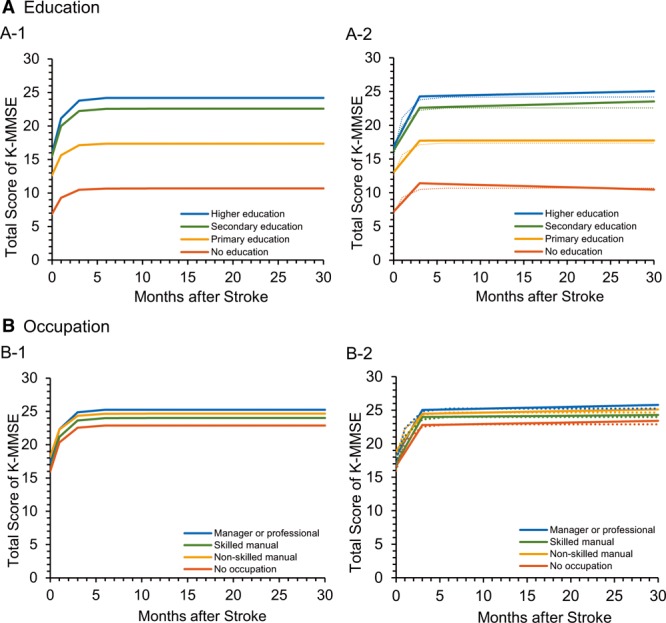
Cognitive changes after stroke onset for the 4 education groups and 4 occupation groups. A-1, Exponential model for the educational groups. A-2, Piecewise regression model for the educational groups. B-1, Exponential model for the occupational groups. B-2, Piecewise regression model for the occupational groups. K-MMSE indicates Korean version of the Mini-Mental State Examination.
Table 3.
Results of Multilevel Model Analyses Predicting Cognitive Changes During 30 Months After Stroke Onset, Controlling for Background Variables and the Initial Score on the Korean Version of the Mini-Mental State Examination
The second slope was almost flat (0.02 points per month for the whole group), but education level moderated the second slope as well: the higher the educational level, the steeper the slope. Occupation and composite CR score had no significant influence on the second slope. Because the long-term prognosis for stroke, implied by the second slope, is associated with the development of dementia, especially in older patients, we conducted a subgroup analysis by age group (Table VIII in the online-only Data Supplement). In the whole young adults group (age <65 years, n=1287), the K-MMSE score increased 2.34 points per month (coefficient=2.34, P<0.001; Table VIII, Model 1 in the online-only Data Supplement) during the first 3 months and 0.05 points per month (coefficient=0.05, P<0.001; Table VIII, Model 1 in the online-only Data Supplement) after the first 3 months. Education level affected only the first slope, and the second slope did not differ among education groups (Table VIII, Model 2 in the online-only Data Supplement; Figure 3A). In the whole older adults group (age ≥65 years, n=1822), the K-MMSE score increased 1.62 points per month (coefficient=1.62, P<0.001; Table VIII, Model 1 in the online-only Data Supplement) during the first 3 months, and the slope was moderated by education level (Table VIII, Model 2 in the online-only Data Supplement) as in the young adults group. However, the second slope did not increase as it did in the young adults group. Instead, the slope started decreasing by 0.01 points per month (coefficient=−0.01, nonsignificant; Table VIII, Model 1 in the online-only Data Supplement) after 3 months. In this group, the second slope was also moderated by education level: Patients with secondary education showed a net positive slope of 0.05 points per month (−0.03+0.08, P<0.001; Table VIII, Model 2, in the online-only Data Supplement) compared with the slope of patients with no education, who showed a decrease of −0.03 points per month (coefficient=−0.03, nonsignificant; Table VIII, Model 2 in the online-only Data Supplement; Figure 3B).
Figure 3.
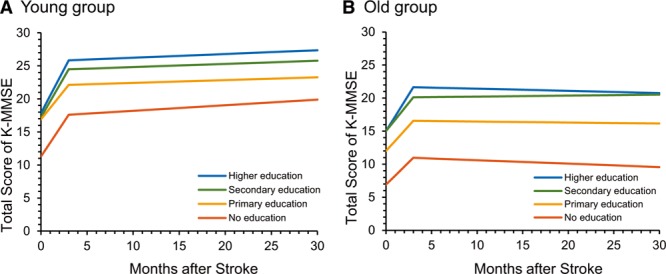
Cognitive changes after stroke onset for the 4 educational groups by age group. A, Piecewise regression model for the younger group (age <65 y). B, Piecewise regression model for the older group (age ≥65 y). K-MMSE indicates Korean version of the Mini-Mental State Examination.
Tables VIII through X in the online-only Data Supplement present more results from the age subgroup analysis with the 3 CR variables applied. Overall, the results are similar to the original results.
Discussion
The results of this study show that a lower premorbid education or occupation is associated with more severe cognitive dysfunction and an increased risk of cognitive impairment both immediately after stroke and over the subsequent 30 months. In addition, a higher education or occupation is associated with a more rapid recovery of cognitive function during the first 3 months after stroke.
Consistent with recent reviews reporting an increased risk of cognitive impairment after stroke in patients with low education,1,8,11,12 education in our study was an independent factor associated with cognitive impairment after stroke. In addition, our data show that higher education can predict rapid recovery after stroke onset during both the active and stable recovery phases, which has not been previously reported. In a study by Sachdev et al,7 education was a protective factor against cognitive decline after stroke or transient ischemic attack, but those authors compared cognitive function evaluated between 3 and 6 months with follow-up testing 14 months later. In a study by Ojala-Oksala,8 education was associated with cognitive decline, dementia, and successful survival, but cognitive function was assessed only once, at 3 months after the stroke. Therefore, previous studies could not examine the moderating role of CR on early recovery, which begins immediately after stroke onset, or on long-term changes.
The finding that the frequency of cognitive impairment in patients with no education was always lower than that in patients with primary education was inconsistent with our hypothesis. Because Korea does not have a long history of compulsory education, there are many older people who did not go to school but learned letters and knowledge through homeschooling. So their educational background might not be enough to reflect their cognitive capacity. Furthermore, the lower frequency rates of impairment found among patients with no education might be a result of the lower cutoff scores when adjusted for age and education. When we applied unadjusted cutoffs (23/24) to the K-MMSE, the frequency and odds ratios of the no education group were higher than those of the primary education group (Tables XI through XII in the online-only Data Supplement). Thus, the demographically adjusted norms might underdiagnose cognitive impairment in individuals with a low educational background.
Occupation was also associated with cognitive changes after stroke. Lower levels of occupation increased the risk of cognitive impairment, and higher levels of occupation increased the speed of cognitive recovery during the first 3 months after stroke. To the best of our knowledge, no similar findings have been reported in previous studies. Occupation has been used as a representative proxy for CR in previous studies of Alzheimer disease20,21 and was reportedly associated with incident dementia (relative risk, 2.20 [95% CI, 1.32–3.84]).20 Furthermore, a longitudinal study found that cognitive decline was predicted by being a farm worker (odds ratio, 2.37 [95% CI, 1.05–5.37]).21 In this study, we demonstrated the beneficial effects of occupation with respect to brain damage in patients with stroke as well.
However, researchers should be cautious when using occupation as a CR proxy because it might introduce selection bias. There were a lot of missing data for occupation, so we inspected the patterns. About 56% of the no education group did not provide job information, and 76% of women did not provide job information or reported that they had no job. Many people in the no education group, and women might have lower levels of occupation or no job and be unwilling to reveal their inferior social status. Therefore, occupation might not be a good proxy for CR because it cannot be applied equally to individuals with various cultural and socioeconomic backgrounds.
The favorable effects of education and occupation on cognitive function can be explained by CR theory. CR is defined as the capacity of a brain to delay or minimize the clinical manifestation of brain pathology.6 Although the exact mechanisms remain uncertain, enriched environments and life experiences, such as education or occupation, are believed to empower the brain to endure neuropathological processes in 2 ways. At the anatomic level, as suggested by Satz,22 individual differences in the number of synapses and brain volume confer differences in the capacity to endure neuropathological processes. At the functional level, Stern6 proposed a more active model in which CR is related to the ability to effectively recruit brain networks. Thus, the patients with stroke in this study who had high levels of education or occupation might have had more synapses, larger brains, or more robust brain networks that effectively resisted brain damage.
The long-term maintenance or deterioration of cognitive function recovered after a stroke is another issue among stroke survivors because cumulative vascular burden is associated with an increased risk of dementia.23 However, no one has yet investigated how well cognitive function recovered after stroke is maintained in the long term or how CR affects the maintenance process, partly because the concept of CR was developed in the context of Alzheimer disease,6,22 a degenerative disease.
Our additional subgroup analysis by age groups showed that the second slope (recovery after 3 months) continued to increase in the young adult group, which means that the recovered function was well maintained over the long term. This result is consistent with a recent study showing that global cognition improved over a 10-year period following the first stroke in young (aged 18–65 at stroke onset) stroke survivors.24 However, in the older adults group, the second slope decreased slightly, which is consistent with previous studies conducted in older adults that showed an ongoing cognitive decline after stroke, such as the REGARD (Reasons for Geographic and Racial Differences in Stroke)2 and the ELSA (English Longitudinal Study of Ageing)25 studies. Importantly, the decline shown by the second slope differed by educational level: the secondary education group showed a significantly increased second slope compared with the no education group, which showed a decreasing slope. These results suggest that lower education could increase the risk of cognitive impairment after stroke onset and ultimately be associated with long-term cognitive decline in older patients. Because cognitive decline in the elderly increases the risk of developing dementia, careful attention should be paid to changes in cognitive function in older patients with stroke with lower education.
We calculated a composite CR score for each patient to examine the combined effect of the 2 CR variables. Those results were consistent with the findings for the individual variables: the lower the CR score, the higher the risk of cognitive impairment, and the higher the CR score, the faster the cognitive recovery after stroke. These repetitive findings make our results more robust.
We performed one further analysis to control for recurrence because recurrent stroke is an important predictor of cognitive decline (Tables XIII through XV in the online-only Data Supplement). We had the same overall results as in the original analysis, but some of the coefficients were not statistically significant because of the small sample size.
Our study has several strengths. First, we observed repeated findings with 3 CR variables. Second, we tried to avoid over-diagnosing cognitive impairment in low-educated patients by applying demographically adjusted norms. The use of adjusted norms for a short screening test can be questioned because age and lower education are known to be risk factors for cognitive decline. We reanalyzed the data using a single cutoff score (23/24) of the K-MMSE, and the overall results stayed the same or even better compared to the original analysis (Tables XI through XII and XVI in the online-only Data Supplement). Third, our data show cognitive changes in both the active and chronic phases. These results were possible because we had 8 time points ranging from 7 days after stroke onset to 30 months, whereas previous studies2,7,8,25 could not show both the acute and chronic phases of recovery.
This study also has several limitations. First, there could have been selection bias with respect to the participants included in the analysis for occupation because lower educated participants, women, and older adults did not provide job information or reported that they had no premorbid job. Second, the K-MMSE detects cognitive impairment or dementia less sensitively than a comprehensive neuropsychological evaluation. The actual prevalence of cognitive impairment might be higher than estimated in our study. These results should be replicated in a future study with a more comprehensive neuropsychological assessment. Third, the cognitive function of patients with stroke might be related to motor functions or emotional status, ApoE genotype, physical exercise, diet, and brain volume, but we were unable to control for those variables because those data were unavailable. A more detailed analysis in a future study could reveal complex relationships among those factors. Fourth, education and premorbid occupation were used as proxies of CR in our analysis, but other proxies should be considered in a further study because early life intelligence and participation in leisure activities are reportedly associated with cognitive impairment after stroke.26,27 Fifth, we did not account for individual differences in cognitive trajectories, but it is also important to cluster individuals according to their recovery trajectories and examine the CR variables in those groups.
Conclusions
Our findings suggest that an individual’s enriched life experiences, including education and occupation, provide a buffer against cognitive impairment caused by stroke and promote rapid cognitive recovery after stroke. In addition, higher education minimizes long-term cognitive decline after stroke, especially in older patients. Within the frame of CR, the finding that education and occupation are active agents in moderating the brain’s response to stroke provides some insight into preserving cognitive decline after stroke by offering structured cognitive intervention to vulnerable patients.
Acknowledgments
Y.-H. Kim contributed to concept formation and design of the study protocol and interpretation of data. Dr Shin suggested the analytical strategy, performed data analysis, and drafted the article. All other authors contributed to the study design and data acquisition. All authors revised and approved the article.
Sources of Funding
This study was supported by a research program funded by the Korea Centers for Disease Control and Prevention (2019E320200) and a National Research Foundation of Korea Grant funded by the Korean government (Ministry of Science, ICT and Future Planning, NRF-2017R1A2A1A05000730).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.026829.
References
- 1.Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, et al. Post-stroke dementia–a comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savva GM, Stephan BC Alzheimer’s Society Vascular Dementia Systematic Review Group. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 4.Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta. 2016;1862:878–886. doi: 10.1016/j.bbadis.2015.12.023. doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satz P, Cole MA, Hardy DJ, Rassovsky Y. Brain and cognitive reserve: mediator(s) and construct validity, a critique. J Clin Exp Neuropsychol. 2011;33:121–130. doi: 10.1080/13803395.2010.493151. doi: 10.1080/13803395.2010.493151. [DOI] [PubMed] [Google Scholar]
- 6.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 7.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology. 2004;63:1618–1623. doi: 10.1212/01.wnl.0000142964.83484.de. doi: 10.1212/01.wnl.0000142964.83484.de. [DOI] [PubMed] [Google Scholar]
- 8.Ojala-Oksala J, Jokinen H, Kopsi V, Lehtonen K, Luukkonen L, Paukkunen A, et al. Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke. 2012;43:2931–2935. doi: 10.1161/STROKEAHA.112.667618. doi: 10.1161/STROKEAHA.112.667618. [DOI] [PubMed] [Google Scholar]
- 9.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Frequency and risk factors of vascular cognitive impairment three months after ischemic stroke in China: the Chongqing stroke study. Neuroepidemiology. 2005;24:87–95. doi: 10.1159/000081055. doi: 10.1159/000081055. [DOI] [PubMed] [Google Scholar]
- 10.Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol. 2001;8:621–627. doi: 10.1046/j.1468-1331.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Kessels RP, Eikelboom WS, Schaapsmeerders P, Maaijwee NA, Arntz RM, van Dijk EJ, et al. Effect of formal education on vascular cognitive impairment after stroke: a meta-analysis and study in young-stroke patients. J Int Neuropsychol Soc. 2017;23:223–238. doi: 10.1017/S1355617716001016. doi: 10.1017/S1355617716001016. [DOI] [PubMed] [Google Scholar]
- 12.Nunnari D, Bramanti P, Marino S. Cognitive reserve in stroke and traumatic brain injury patients. Neurol Sci. 2014;35:1513–1518. doi: 10.1007/s10072-014-1897-z. doi: 10.1007/s10072-014-1897-z. [DOI] [PubMed] [Google Scholar]
- 13.Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, et al. Korean Stroke Cohort for functioning and rehabilitation (KOSCO): study rationale and protocol of a multi-centre prospective cohort study. BMC Neurol. 2015;15:42. doi: 10.1186/s12883-015-0293-5. doi: 10.1186/s12883-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Korea. Korean Standard Classification of Occupations. Daejeon: Statistics Korea; 2007. [Google Scholar]
- 15.Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang Y, Na DL, Hahn S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308.. [Google Scholar]
- 17.Kang Y. A normative study of the Korean mini-mental state examination (k-mmse) in the elderly. Korean J Psychol. 2006;25:1–12.. [Google Scholar]
- 18.IBM Corp. IBM SPSS Statistics for Windows: Release 19. Armonk, NY: IBM Corp; 2010. [Google Scholar]
- 19.Raudenbush SW, Bryk AS, Congdon R. Hlm 7 for Windows [Computer Software] Lincolnwood, IL: Scientific Software International; 2011. [Google Scholar]
- 20.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 21.Alvarado BE, Zunzunegui MV, Del Ser T, Béland F. Cognitive decline is related to education and occupation in a Spanish elderly cohort. Aging Clin Exp Res. 2002;14:132–142. doi: 10.1007/BF03324426. [DOI] [PubMed] [Google Scholar]
- 22.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295.. [Google Scholar]
- 23.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 24.Elgh E, Hu X. Dynamic trajectory of long-term cognitive improvement up to 10 years in young community-dwelling stroke survivors: a Cohort Study. Front Neurol. 2019;10:97. doi: 10.3389/fneur.2019.00097. doi: 10.3389/fneur.2019.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng F, Yan L, Zhong B, Yang Z, Xie W. Progression of cognitive decline before and after incident stroke. Neurology. 2019;93:e21–e28. doi: 10.1212/WNL.0000000000007716. [DOI] [PubMed] [Google Scholar]
- 26.Verghese J, Cuiling Wang, Katz MJ, Sanders A, Lipton RB. Leisure activities and risk of vascular cognitive impairment in older adults. J Geriatr Psychiatry Neurol. 2009;22:110–118. doi: 10.1177/0891988709332938. doi: 10.1177/0891988709332938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makin SD, Doubal FN, Shuler K, Chappell FM, Staals J, Dennis MS, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J. 2018;3:145–156. doi: 10.1177/2396987317750517. doi: 10.1177/2396987317750517. [DOI] [PMC free article] [PubMed] [Google Scholar]


