http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/14-5-reading-bojko a video presentation of this article
https://www.wileyhealthlearning.com/Activity/6952106/disclaimerspopup.aspx questions and earn CME
Abbreviations
- BCAA
branched‐chain amino acid
- IGF‐1
insulin‐like growth factor 1
- IL‐6
interleukin‐6
- mTOR
mammalian target of rapamycin
- PA
physical activity
- REE
resting energy expenditure
- TCA
tricarboxylic acid
- UPP
ubiquitin‐proteasome pathway
Sarcopenia is one of the most common complications in advanced liver disease, affecting 30% to 70% of patients with cirrhosis.1 This condition is of significant concern in this population because sarcopenia has been associated with higher mortality, increased hospital admissions, worse post–liver transplant outcomes, decreased quality of life, and increased risk for other complications associated with cirrhosis (Table 1).1 Sarcopenia in cirrhosis is multifactorial and is not completely explained by simple malnutrition (Table 2). It is difficult to treat, and there are currently no proven effective therapies to prevent or reverse sarcopenia.2 Although it is inarguable that barriers to adequate calorie and protein intake exist, such as anorexia and nausea, sarcopenia is also associated with complex metabolic and hormonal changes that are not adequately treated by nutrition and physical activity (PA) alone. A better understanding of the etiology of sarcopenia in cirrhosis may produce additional targeted therapies which may improve patient outcomes when used in combination with PA and nutrition optimization techniques (Table 3).
Table 1.
Negative Complications Associated With Sarcopenia1
|
Table 2.
|
Table 3.
| Current nutrition and PA recommendations |
| High‐calorie/protein diet |
| Late‐evening or overnight snacks (high protein) |
| PA (strength training) |
| BCAA supplementation |
| Potential future hormonal and metabolic targeted therapies |
| l‐Leucine and/or l‐citrulline supplementation |
| Testosterone supplementation (men) |
| Myostatin antagonists |
| mTOR signaling upregulators |
| Mitochondrial antioxidants |
Mechanisms of Sarcopenia in Cirrhosis
Sarcopenia in patients with cirrhosis is defined by loss of muscle mass and decreased functional capacity, as well as greater risk for morbidity and mortality.3 Although the pathogenesis of sarcopenia in cirrhosis is poorly understood, a number of proposed mechanisms have been described previously which represent an imbalance between muscle breakdown and formation.1, 4 Sarcopenia in cirrhosis appears to be affected by alterations in protein turnover, energy disposal, and hormonal and metabolic changes which lead to muscle depletion1, 4 (Fig. 1).
Figure 1.
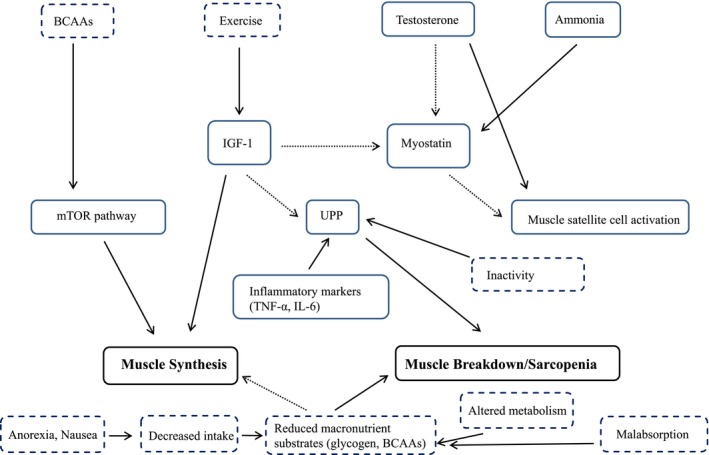
Overview of the mechanisms of muscle synthesis and breakdown in liver cirrhosis. Solid arrows indicate upregulators/promoters, dashed arrows indicate downregulators/inhibitors, solid boxes represent hormonal/chemical/inflammatory factors, and dashed boxes represent nutritional/metabolic/PA factors.
Altered Carbohydrate and Lipid Metabolism
Metabolic changes and alterations in protein turnover are major factors in muscle depletion in sarcopenia of chronic disease. Carbohydrates are used less for energy because of the decreased ability of hepatocytes to synthesize, store, and break down glycogen, resulting in increased ketogenesis and amino acid consumption.5 Increased mobilization of amino acids for gluconeogenesis and energy favors a more rapid transition from carbohydrate metabolism to ketogenesis, such as during the span of an overnight fast, resulting in risk for skeletal muscle loss and reduced fat stores.5, 6 One proposed intervention for sarcopenia is a high‐protein late‐night or overnight snack to prevent this shift in metabolism. Decreased hepatic glycogen stores in cirrhosis promote the use of fat and protein for gluconeogenesis and also result in lower circulating branched‐chain amino acids (BCAAs). BCAA supplementation has been proposed to address increased protein catabolism by providing additional energy substrate, as well as stimulation of muscle protein synthesis via activation of the mammalian target of rapamycin (mTOR) pathway; however, more randomized control trials are needed to determine efficacy.3, 4, 6, 7
Inhibition of Muscle Growth
Imbalance in muscle formation and degradation is mediated by multiple factors including hyperammonemia, increased myostatin, and decreased growth hormones. Of these, hyperammonemia, due to decreased hepatocellular clearance of ammonia from amino acid metabolism, appears to be the most well‐documented factor in cirrhosis to contribute to sarcopenia.1, 4, 8 Hyperammonemia upregulates myostatin production, which inhibits muscle growth by decreasing satellite cell proliferation and differentiation. Myostatin is typically suppressed by testosterone and insulin‐like growth factor 1 (IGF‐1); therefore, decreased levels of these growth hormones observed in cirrhosis also likely contributes to elevated myostatin expression in these patients.4, 9 Low levels of IGF‐1 may also reduce mTOR activation of muscle protein synthesis, further contributing to sarcopenia.9 Physical activity, specifically resistance training, has been proposed as an intervention in patients with sarcopenia of cirrhosis to prevent muscle breakdown and maintain physical function through its role in upregulating IGF‐1. This upregulation of IGF‐1 could result in downregulation of myostatin and subsequent increase in circulating growth hormones; however, more evidence is needed to support this recommendation for this condition. Another mechanism by which hyperammonemia contributes to sarcopenia is through the liver‐muscle axis; ammonia accumulates in skeletal muscle and prevents production of α‐ketoglutarate, a major substrate for the tricarboxylic acid (TCA) cycle. Due to reduced flux of the TCA cycle, mitochondrial function may be impaired, resulting in lower concentrations of adenosine triphosphate, which may translate to reduced protein synthesis.1, 10
Inflammation
Inflammation of chronic disease and circulating cytokines lead to inappropriate muscle autophagy. Research in sarcopenia of aging indicates increased muscle autophagy through the ubiquitin‐proteasome pathway (UPP), which is upregulated by increased levels of inflammatory cytokines such as tumor necrosis factor α (TNF‐α) and interleukin‐6 (IL‐6).4, 5 Cirrhosis, a known proinflammatory condition, may also contribute to sarcopenia because of increased resting energy expenditure (REE) partially driven by inflammatory mediators leading to increased whole‐body protein turnover.1, 4 Inactivity also stimulates the UPP pathway, providing further rationale for PA interventions.4
Malnutrition
Changes in nutrient ingestion, absorption, and utilization contribute to malnutrition in this population. Abdominal ascites, a major complication of cirrhosis, often causes abdominal pain and pressure, reduced appetite, and increased nausea. Elevated inflammatory markers such as TNF‐α may also exacerbate nausea and anorexia.4 Pancreatic insufficiency and decreased bile flow in a subset of this population may lead to increased fat malabsorption and decreased absorption of fat‐soluble vitamins.6 Malnutrition may also contribute to higher REE, increasing total energy and protein needs and further exacerbating the altered macronutrient metabolism and increased whole‐body protein turnover observed in cirrhosis. High‐calorie/high‐protein diets, oral nutrition supplements, and enteral or parenteral nutrition support (when appropriate) are often recommended to mediate sarcopenia in cirrhosis through increased nutrient intake; however, nutrition interventions alone cannot fully address the many other factors that contribute to sarcopenia in this population.6, 11
Conclusion
Sarcopenia may be considered one of the most common and significant complications of liver cirrhosis, and has been associated with adverse outcomes and increased morbidity and mortality. Current interventions are focused mainly on nutrition and PA, and have been inadequate on their own to prevent or reverse sarcopenia. A comprehensive understanding of many interconnected mechanisms is needed to develop a therapeutic approach that addresses increased energy and protein requirements, hormonal abnormalities, altered metabolic pathways, and nutritional deficiencies contributing to sarcopenia in cirrhosis.
Potential conflict of interest: Nothing to report.
References
- 1. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dasarathy S. Cause and management of muscle wasting in chronic liver disease. Curr Opin Gastroenterol 2016;32:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montano‐Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol 2014;20:8061‐8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sinclair M, Gow PJ, Grossman M, et al. Review article: sarcopenia in cirrhosis—aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 2016;43:765‐777. [DOI] [PubMed] [Google Scholar]
- 5. Sevastianos VA, Dourakis SP. Malnutrition and sarcopenia in advanced liver disease. J Nutr Food Sci 2016;6:487. [Google Scholar]
- 6. Tsiaousi ET, Hatzitolios AI, Trygonis SK, et al. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol 2008;23:527‐533. [DOI] [PubMed] [Google Scholar]
- 7. Tsien C, Davuluri G, Singh D, et al. Metabolic and molecular responses to leucine enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015;61:2018‐2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF‐κB‐mediated mechanism. Proc Natl Acad Sci USA 2013;110:18162‐18167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frost RA, Lang CH. Multifaceted role of insulin‐like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol Metab Clin N Am 2012;42:297‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobsen EB, Hamberg O, Quistorff B, et al. Reduced mitochondrial adenosine triphosphate synthesis in skeletal muscle in patients with Child‐Pugh Class B and Class C cirrhosis. Hepatology 2001;34:7‐12. [DOI] [PubMed] [Google Scholar]
- 11. Merli M, Berzigotti A, Zelber‐Sagi S, et al. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]


