http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/14-5-reading-jackson a video presentation of this article
http://aasldpubs.onlinelibrary.wiley.com/hub/journal/10.1002/(ISSN)2046-2484/video/14-5-interview-jackson the interview with the author
Abbreviations
- BAR
balance of risk
- BMI
body mass index
- ICU
intensive care unit
- ld‐MaS
large droplet macrosteatosis
- LFT
liver function test
- MELD
Model for End‐Stage Liver Disease
- NMP
normothermic machine perfusion
- sd‐MaS
small droplet macrosteatosis
Key Points
Donor steatosis will likely become more prevalent as national obesity rates increase.
Donor livers with any amount of small droplet macrosteatosis (sd‐MaS) and mild (<30%) large droplet macrosteatosis (ld‐MaS) are, for the most part, considered safe for transplantation; however, livers with moderate (30%‐60%) or severe (>60%) ld‐MaS have been associated with inferior posttransplant outcomes.
Two main strategies have been proposed to minimize the risks of donor livers with moderate or severe ld‐MaS: careful recipient selection and normothermic machine perfusion (NMP), although the latter is currently considered experimental.
Liver transplantation is the preferred treatment for end‐stage liver disease. However, there are far fewer donor livers available for transplant than there are candidates in need of them. One strategy to mitigate this disparity is to use marginal livers, which are often discarded, but might be safely transplantable in certain clinical scenarios. Steatotic donor livers fall into this category and are likely to be increasingly more common given the ongoing obesity epidemic. As such, a better understanding of the effect of donor steatosis on posttransplant outcomes, and strategies to minimize the associated risk, is imperative to safely expand utilization of steatotic livers, thereby increasing the number of patients able to benefit from lifesaving liver transplantation.
Assessment and Types of Steatosis
The assessment of donor steatosis is often made in two stages (Fig. 1). First, the procuring surgeon makes a visual and tactile assessment of steatosis. The surgeon will assess the overall appearance of the liver. An enlarged liver with round edges might indicate the presence of steatosis. Next, liver color is examined, which appears yellow with significant steatosis. In addition, the degree of steatosis can be assessed by gently pinching the liver and examining the color after releasing. The firmness and friability of the liver is assessed, because steatotic livers are often friable. Biopsies are not routinely performed on donor livers without any of these characteristics; they are assumed to have an inconsequential degree of steatosis.
Figure 1.
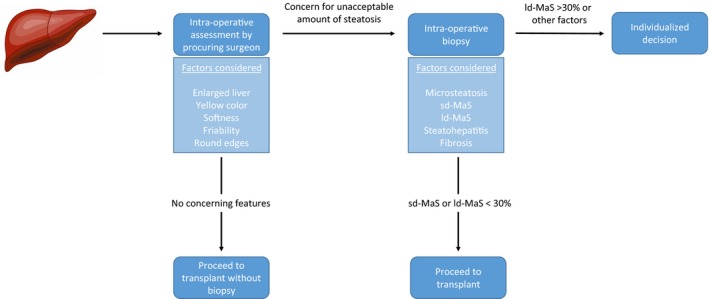
Assessment of suspected steatotic donor liver. The initial assessment of a donor liver is performed by the procuring surgeon. Tactile and visual characteristics indicative of steatosis (yellow color, exceptionally soft liver, friability, enlarged, round edges) will usually lead the surgeon to request a biopsy if an unacceptable amount of steatosis is suspected. In the absence of these characteristics, the liver is considered to be low risk, and transplantation can proceed. On biopsy, the percent sd‐MaS, ld‐MaS, and inflammation/fibrosis is assessed. Isolated sd‐MaS of any amount and mild (<30%) ld‐MaS are generally considered safe for transplantation. Conversely, moderate (30%‐60%) or severe (>60%) ld‐MaS, or other types of steatosis in the presence of other risk factors for poor outcome (inflammation, fibrosis) will lead to an individualized decision, although usually these livers are discarded.
Biopsies are often performed on livers that appear to have an unacceptable degree of steatosis, and the degree of steatosis is quantified by pathological review. Unfortunately, there is a fair amount of imprecision in the surgical transplant literature when describing steatotic donor livers. Most of these studies distinguish only between microsteatosis and macrosteatosis, whereas the pathological literature distinguishes between microsteatosis, sd‐MaS, and ld‐MaS.1 True microsteatosis is a rare condition where the liver is diffusely replaced by innumerable intracytoplasmic tiny vesicles and is seen only in the context of specific conditions (i.e., Reye’s syndrome, acute fatty liver of pregnancy). Donors with these conditions, and hence true microsteatosis, are rarely seriously considered as liver donors.
However, both sd‐MaS and ld‐MaS are commonly encountered in donor livers. sd‐MaS is often incorrectly referred to in the surgical transplant literature as “microsteatosis,” and it exists when one or a few smaller intracytoplasmic vacuoles are present but do not displace the nucleus from its central location. Conversely, ld‐MaS (commonly referred to as “macrosteatosis” in the surgical transplant literature) exists when one or more large vacuoles are present and displace the nucleus to an eccentric location. Both sd‐MaS and ld‐MaS are reported as the percent (0%‐100%) of hepatic parenchymal area that consists of these vacuoles, but ld‐MaS is further categorized as mild (<30%), moderate (30%‐60%), and severe (>60%) ld‐MaS (Fig. 2). Although beyond the scope of this review, accurate pathological interpretation is critical but can be highly variable depending on local expertise available at the time of organ recovery.
Figure 2.
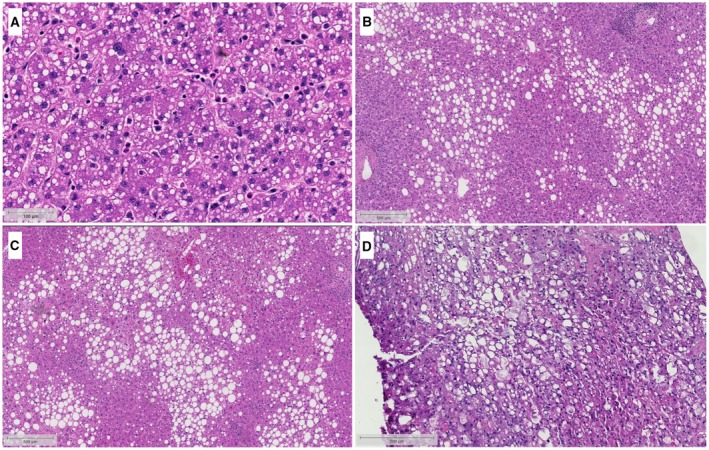
Distinguishing between sd‐MaS and ld‐MaS. Diffuse sd‐MaS (A) presents as small, intracytoplasmic vacuoles that do not displace the nucleus away from its normal central location. Due to the small vacuole size, sd‐MaS can be difficult to see at lower magnification, and so this image is shown at a higher magnification than the ld‐MaS figures (B‐D). On the other hand, ld‐MaS (B‐D) presents as a large vacuole that displaces the nucleus to an eccentric location. ld‐MaS is further categorized as mild (<30%, B), moderate (30%‐60%, C), and severe (>60%, D). Courtesy Johns Hopkins Hospital Department of Pathology.
Outcomes Using Steatotic Livers
Studies have not consistently shown a relationship between sd‐MaS or mild (<30%) ld‐MaS and unacceptable posttransplant outcomes; therefore, most surgeons will consider these low risk. However, many studies have shown an increased risk for poor posttransplant outcomes when using livers with moderate or severe ld‐MaS,2, 3, 4, 5 although some smaller, single‐center studies have shown no increase in risk. In a retrospective study of ld‐MaS using the United Network for Organ Sharing registry, receiving a donor liver with at least moderate ld‐MaS was associated with a 71% increased risk for graft failure compared with receiving a liver with <15% ld‐MaS.2 Moderate or severe ld‐MaS has also been associated with increased rates of primary nonfunction and early allograft dysfunction. Grafts with severe ld‐MaS are not commonly used.
Strategies to Minimize Risk for Steatotic Grafts
Recipient Selection
Despite an increased risk for poor posttransplant outcomes with moderate or severe ld‐MaS, several studies have shown that similar, if not equivalent, outcomes can be obtained between livers with and without ld‐MaS, with careful recipient selection. The following characteristics have been shown to be associated with similar or equivalent outcomes using livers with and without moderate or severe ld‐MaS: a careful donor/recipient matching algorithm,6 a recipient with a balance of risk (BAR) score ≤9 (low‐risk recipients),7 and a recipient Model for End‐Stage Liver Disease (MELD) score <24.8 These studies suggest that low‐risk recipients may tolerate donor moderate or severe ld‐MaS with similar, if not equivalent, outcomes to nonsteatotic livers. However, it is worth noting that two of these studies are single‐center, retrospective, and underpowered to detect smaller, but clinically important, effect sizes (Table 1).
Table 1.
Summary of the Literature Examining Careful Recipient Selection to Minimize Risks Associated With Steatotic Donor Livers
| Author | Year | N | % ld‐MaS | Outcome | Characteristics Associated With Acceptable Outcome |
|---|---|---|---|---|---|
| Chavin et al.6 | 2013 | 9 | >60% | Patient and graft survival | Donor/recipient selection algorithm: Recipient/donor pairs with fewer than two factors from each set of characteristics had equivalent outcomes with >60% ld‐MaS grafts and nonsteatotic grafts. |
| Donor risk factors: age >60 years, vasopressor use, ICU stay >48 hours, elevated LFTs, sodium ≥155 mEq/L, acute myocardial infarction, diabetes, BMI ≥ 40 | |||||
| Recipient risk factors: age >60 years, fulminant liver failure, prior transplant recipient, portal vein thrombosis, multiple abdominal surgeries, spontaneous bacterial peritonitis, MELD score >30, BMI ≥ 40 | |||||
| Dutkowski et al.7 | 2012 | 530 | ≥30% | Patient and graft survival | Although recipients of steatotic grafts with a BAR score ≤9 had a 1.33‐fold adjusted increase in risk for graft failure with a ≥30% ld‐MaS liver, 1‐year graft survival was still considered to be acceptable (82% versus 86%). |
| McCormack et al.11 | 2007 | 20 | >60% | Patient survival | Recipients with a MELD score ≤24 or in acute liver failure who received a >60% ld‐MaS graft had an equivalent 3‐year patient survival rate with nonsteatotic grafts (83% versus 84%). |
In each of these studies, there was a specific focus on recipient selection (either prospectively or retrospectively) to find a group of recipients who might have similar outcomes with either a steatotic or a nonsteatotic liver. Although several other studies have shown no, or minimal, differences in posttransplant outcomes when using livers with moderate or severe ld‐MaS, those studies have considered their entire transplant population, without specifically identifying a group of recipients in whom the risks of a steatotic liver were minimized.
Machine Perfusion
NMP, as well as other related techniques, seeks to minimize the ischemia/reperfusion injury that mediates much of the steatotic liver’s negative impact on outcomes. NMP maintains the liver’s normal physiological state by providing it with oxygen and nutrients at 37°C, through a series of pumps, an oxygenator, and a heat exchanger. In one trial, NMP reduced the incidence rate of early allograft dysfunction by 74% and was associated with 50% fewer discarded livers, compared with traditional static cold storage, albeit in nonsteatotic livers.9 A recently published trial used “defatting” protocols to enhance lipid metabolism of 10 livers discarded because of steatosis and showed a reduction of tissue triglycerides by 38% and macrovesicular steatosis by 40% with only 6 hours of NMP.10 Although its use is still considered experimental, NMP represents an exciting potential strategy to minimize the risk of steatotic livers.
Conclusion
Given national trends of obesity, donor steatosis is likely to become an increasingly common challenge facing the transplant community. Distinguishing between microsteatosis, sd‐MaS, and ld‐MaS is critical, because these have different effects on posttransplant outcomes. Although sd‐MaS and mild ld‐MaS have not been shown to impact posttransplant outcomes, the presence of ≥30% ld‐MaS has been associated with an increased risk for graft failure and primary nonfunction; however, these findings have not been replicated in all studies. Therefore, careful recipient selection or the use of NMP may be able to minimize the negative effects of ≥30% ld‐MaS on posttransplant outcomes.
This study was supported by grants F32DK113719 (to K.R.J.) and K23DK115908 (to J.G.‐W.) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflict of interest: Nothing to report.
References
- 1. Brunt EM. Pathology of fatty liver disease. Mod Pathol 2007;20(suppl 1):S40‐S48. [DOI] [PubMed] [Google Scholar]
- 2. Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: Incorporating macrosteatosis into high‐risk donor assessment. Liver Transpl 2010;16:874‐884. [DOI] [PubMed] [Google Scholar]
- 3. de Graaf EL, Kench J, Dilworth P, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol 2012;27:540‐546. [DOI] [PubMed] [Google Scholar]
- 4. Noujaim HM, de Ville de Goyet J, Montero EF, et al. Expanding postmortem donor pool using steatotic liver grafts: A new look. Transplantation 2009;87:919‐925. [DOI] [PubMed] [Google Scholar]
- 5. Deroose JP, Kazemier G, Zondervan P, et al. Hepatic steatosis is not always a contraindication for cadaveric liver transplantation. HPB (Oxford) 2011;13:417‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chavin KD, Taber DJ, Norcross M, et al. Safe use of highly steatotic livers by utilizing a donor/recipient clinical algorithm. Clin Transplant 2013;27:732‐741. [DOI] [PubMed] [Google Scholar]
- 7. Dutkowski P, Schlegel A, Slankamenac K, et al. The use of fatty liver grafts in modern allocation systems: Risk assessment by the balance of risk (BAR) score. Ann Surg 2012;256:861‐868; discussion 868‐869. [DOI] [PubMed] [Google Scholar]
- 8. McCormack L, Dutkowski P, El‐Badry AM, et al. Liver transplantation using fatty livers: Always feasible? J Hepatol 2011;54:1055‐1062. [DOI] [PubMed] [Google Scholar]
- 9. Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557:50‐56. [DOI] [PubMed] [Google Scholar]
- 10. Boteon YL, Attard J, Boteon AP, et al. Manipulation of lipid metabolism during normothermic machine perfusion: effect of defatting therapies on donor liver functional recovery. Liver Transpl 2019; 10.1002/lt.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCormack L, Petrowsky H, Jochum W, et al. Use of severely steatotic grafts in liver transplantation: A matched case‐control study. Ann Surg 2007;246:940‐948. [DOI] [PubMed] [Google Scholar]


