Abstract
Background
Iron deficiency is one of the most common nutritional deficiencies, and has a number of physiological manifestations. Early, or non‐anaemic iron deficiency can result in fatigue and diminished exercise capacity. Oral iron preparations have a high incidence of intolerable side effects, and are ineffective in certain forms of iron deficiency. Consequently, intravenous iron preparations are increasingly used in the treatment of non‐anaemic iron deficiency. The newer, more stable iron preparations in particular purport to have a lower incidence of side effects, and are now used across a range of different patient populations.
Objectives
To assess the effects of intravenous iron therapy in the treatment of adults with non‐anaemic iron deficiency.
Search methods
On 18 October 2019 we electronically searched CENTRAL, MEDLINE, Embase, two further databases and two trials registries 2019. We handsearched the references of full‐text extracted studies, and contacted relevant study authors for additional data.
Selection criteria
We included randomised controlled trials that compared any intravenous iron preparation to placebo in adults. We excluded other forms of comparison such as oral iron versus placebo, intramuscular iron versus placebo, or intravenous iron studies where other iron preparations were used as the comparator. We also excluded studies involving erythropoietin therapy or obstetric populations.
Data collection and analysis
Two review authors screened references for eligibility, extracted data and assessed risk of bias. We resolved differences in opinion through discussion and consensus, and where necessary, involved a third review author to adjudicate disputes. We contacted study authors to request additional data where appropriate. The primary outcome measures were haemoglobin concentration at the end of follow‐up, and quality‐of‐life scores at end of follow‐up. Secondary outcome measures were serum ferritin, peak oxygen consumption (as measured by cardiopulmonary exercise testing), adverse effects (graded as mild to moderate and severe) and bacterial infection. We pooled data for continuous outcomes, which we then reported as mean differences (MDs) with 95% confidence intervals (CIs). We reported quality‐of‐life metrics as standardised mean difference (SMD), and then converted them back into a more familiar measure, the Piper Fatigue Scale. We analysed dichotomous outcomes as risk ratios (RRs). Given an expected degree of heterogeneity, we used a random‐effects model for all outcomes. We performed the analysis with the software package Review Manager 5.
Main results
This review includes 11 studies with 1074 participants. Outcome metrics for which data were available (haemoglobin concentration, quality‐of‐life scores, serum ferritin, peak oxygen consumption and mild to moderate adverse effects) were similar across the included studies. The incidence of severe adverse events across all studies was zero. None of the studies measured bacterial infection as a specific outcome metric.
Substantial heterogeneity influenced the results of the meta‐analysis, arising from differing patient populations, definitions of iron deficiency, iron preparations and dosing regimens, and time to end of follow‐up. Consequently, many outcomes are reported with small group sizes and wide confidence intervals, with a subsequent downgrading in the quality of evidence. The level of bias in many included studies was high, further reducing confidence in the robustness of the results.
We found that intravenous iron therapy may lead to a small increase in haemoglobin concentration of limited clinical significance compared to placebo (MD 3.04 g/L, 95% CI 0.65 to 5.42; I2 = 42%; 8 studies, 548 participants; low‐quality evidence). Quality‐of‐life scores (Piper Fatigue Scale MD 0.73, 95% CI 0.29 to 1.18; I2 = 0%; studies = 3) and peak oxygen consumption (MD 2.77 mL/kg/min, 95% CI −0.89 to 6.43; I2 = 36%; 2 studies, 32 participants) were associated with very low‐quality evidence, and we remain uncertain about the role of intravenous iron for these metrics. We were unable to present pooled estimates for the outcomes of serum ferritin at the end of follow‐up or mild to moderate adverse effects due to extreme statistical heterogeneity. Ultimately, despite the results of the meta‐analysis, the low‐ or very low‐quality evidence for all outcomes precludes any meaningful interpretation of results beyond suggesting that further research is needed. We performed a Trial Sequential Analysis for all major outcomes, none of which could be said to have reached a necessary effect size.
Authors' conclusions
Current evidence is insufficient to show benefit of intravenous iron preparations for the treatment of non‐anaemic iron deficiency across a variety of patient populations, beyond stating that it may result in a small, clinically insignificant increase in haemoglobin concentration. However, the certainty for even this outcome remains limited. Robust data for the effectiveness of intravenous iron for non‐anaemic iron deficiency is still lacking, and larger studies are required to assess the effect of this therapy on laboratory, patient‐centric, and adverse‐effect outcomes.
Keywords: Humans; Iron Deficiencies; Hemoglobins; Hemoglobins/metabolism; Infusions, Intravenous; Iron; Iron/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Intravenous iron for the treatment of non‐anaemic iron deficiency in adults
Background
Iron deficiency, when the body does not have enough of the mineral iron, is a common, nutritional deficiency. Iron is used by the body to make haemoglobin, a protein in red blood cells that enables them to carry oxygen around the body. Whilst iron deficiency is most commonly associated with a low level of haemoglobin in the blood (anaemia), early, or 'non‐anaemic' iron deficiency can also lead to symptoms such as tiredness and lack of energy. Non‐anaemic iron deficiency is often treated with oral iron, which is medicine taken by mouth, such as iron tablets. However, oral iron is likely to cause side effects, is not effective for certain types of iron deficiency, and takes time to work fully. In addition, newer iron preparations, such as intravenous iron, are more stable, have fewer side effects and have maximum benefit in a shorter time period.
Aim of the review
To review the evidence from randomised controlled trials (where people are allocated a treatment at random) on the safety and effects of intravenous iron in the treatment of early, or non‐anaemic iron deficiency.
Study characteristics
We found 11 studies with 1074 participants. A broad range of people were included in these studies, including people with heart failure, elite athletes, people with restless legs syndrome and otherwise fit and well women. We excluded studies that looked at children, pregnant women, and people being treated with erythropoietin (a hormone that stimulates the production of red blood cells).
Key results
Intravenous iron may lead to a small increase in the level of haemoglobin in the blood. We also assessed the effect of intravenous iron on quality of life, serum ferritin (iron stored in the body), peak exercise capacity, and milder side‐effects of iron administration but we were unable to determine whether or not intravenous iron was of benefit for these outcomes. This is because there were many differences between studies in the types of participants studied, the definition of iron deficiency used, the type of intravenous iron preparation prescribed and the length of the studies. We also tried to collect data on severe side effects and bacterial infection after iron infusion, but we were unable to find any studies that measured these effectively.
Certainty of the evidence
Because of the many differences between the relatively small number of studies included in this review, we are uncertain about the effect of intravenous iron in non‐anaemic iron deficiency beyond saying that it might cause an increase in haemoglobin concentration. Furthermore, the starting level of haemoglobin for people included in this review was considered 'normal' prior to their receiving treatment. Therefore, not only is this increase quite small, but the starting level of haemoglobin was considered adequate according to current guidance, and patients may not even notice an improvement in symptoms. We are not suggesting that intravenous iron is not of benefit for adults with non‐anaemic iron deficiency, rather that the current quality of evidence is not good enough to be certain about the effects of these drugs.
Conclusions
Overall, the evidence for intravenous iron for the treatment of non‐anaemic iron deficiency is of low or very low quality. Whilst intravenous iron might cause a small increase in haemoglobin concentration from an already normal level, we are uncertain about its effects in other outcomes that we examined as part of this review. Further research examining the effects of intravenous iron for the treatment of adults with non‐anaemic iron deficiency is required to help answer this research question.
The evidence is current to October 2019.
Summary of findings
Summary of findings for the main comparison. Intravenous iron compared to placebo for non‐anaemic, iron‐deficient adults.
| Intravenous iron compared to placebo for non‐anaemic iron deficient adults | ||||||
| Patient or population: non‐anaemic, iron‐deficient adults Setting: all healthcare settings (acute, subacute and community care) Intervention: intravenous iron Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with intravenous iron | |||||
| Haemoglobin concentration taken at the end of follow‐up | The mean haemoglobin concentration taken at the end of follow‐up was 131.93 g/L | MD 3.04 g/L higher (0.65 higher to 5.42 higher) | ‐ | 548 (8 RCTs) | ⊕⊕⊝⊝ Low1,2 | Intravenous iron may result in a slight, clinically insignificant increase in haemoglobin concentration taken at the end of follow‐up, but the evidence is limited. |
| Overall quality of life measured at the end of follow‐up | The mean overall quality of life measured at the end of follow‐up was 0 | Piper Fatigue Scale MD 0.73 lower (0.29 to 1.18 lower) | ‐ | 344 (3 RCTs) | ⊕⊝⊝⊝ Very low2,3,4 | We are uncertain about the effect of intravenous iron on overall quality of life measured at the end of follow‐up. |
| Serum ferritin concentration measured at the end of follow‐up | We were unable to perform meta‐analysis for this outcome due to substantial statistical heterogeneity (I2 = 100%). In all 7 studies a we observed a higher ferritin concentration in the intervention group relative to the control | 376 (7 RCTs) |
⊕⊝⊝⊝ Very low5,6 | We are uncertain about the effect of intravenous iron on serum ferritin at the end of follow‐up. | ||
| Bacterial infection | No studies measured this outcome | |||||
| Peak oxygen consumption taken at the end of follow‐up | The mean peak oxygen consumption taken at the end of follow‐up was 25.89 mL/kg/min | MD 2.77 mL/kg/min higher (0.89 lower to 6.43 higher) | ‐ | 32 (2 RCTs) | ⊕⊝⊝⊝ Very low4,7,8 | We are uncertain about the effect of intravenous iron on peak oxygen consumption taken at the end of follow‐up. |
| Serious adverse events | No studies reported any serious adverse events | |||||
| Mild adverse effects | Study population | RR 1.19 (0.97 to 1.45) | 440 (3 RCTs) | ⊕⊝⊝⊝ Very low4,7,9 | We are uncertain about the effect of intravenous iron on mild to moderate adverse effects taken at the end of follow‐up. | |
| 402 per 1,000 | 478 per 1,000 (390 to 583) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Downgraded one level for inconsistency: there was moderate statistical heterogeneity and multiple points of methodological heterogeneity, with the dose of iron administered and the time to end of follow‐up. 2Downgraded one level for imprecision: the confidence interval around the point prevalence estimate of effect is wide, and on Trial Sequential Analysis, the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power. 3Downgraded one level for risk of bias: none of the included studies were at low risk of bias, and we judged the largest included study to be at high risk of bias from multiple protocol deviations and no reporting of the per protocol analysis. 4Downgraded one level for inconsistency: despite mild or negligible statistical heterogeneity, we observed multiple points of methodological heterogeneity, with the dose of iron administered and the time to end of follow‐up. 5Downgraded two levels for inconsistency: there was substantial statistical heterogeneity in the pooled result (I2 = 100%) and multiple points of methodological heterogeneity, with dose of iron administered and time to end of follow‐up. 6Downgraded two levels for imprecision: the point prevalence estimates in each of the included studies are highly imprecise, as reflected by the large confidence interval of the total result. The generated effect size is considerably less than the required effect size calculated by Trial Sequential Analysis. 7Downgraded one level for risk of bias: one of the two included studies was at high risk of bias for participant blinding. The outcome in question could potentially be compromised by performance bias. 8Downgraded two levels for imprecision: the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power. There is considerable difference in the mean difference in the two included studies. 9Downgraded one level for imprecision: on Trial Sequential Analysis, the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power.
Background
Description of the condition
Elemental iron is ubiquitous in the biosphere and has been incorporated into the essential physiological processes of many organisms, including humans (Abbaspour 2014). Depletion of bodily iron stores has a number of manifestations, with profound physiological consequences. The most recognised complication of iron deficiency is anaemia, as the lack of iron results in failure of haemoglobin production (WHO 2001). However, anaemia is effectively the final stage of iron deficiency, and early forms of the disease can be of detriment to physical health and well‐being, with fatigue, diminished mental acuity, reductions in work capacity and productivity, and reduced exercise tolerance all being reported in the literature (Musallam 2018).
The identification and management of iron deficiency (particularly early, or non‐anaemic iron deficiency) is made challenging by different forms of the disease. Broadly speaking, iron deficiency takes the following two forms (Goodnough 2012).
Absolute iron deficiency: this refers to the absence of sufficient iron stores to maintain effective erythropoiesis (Goodnough 2011; Pasricha 2013). This is normally due to increased requirements in excess of stored iron, decreased intake of dietary iron, increased red cell loss or decreased absorption of dietary iron (Nelson 1994; Reveiz 2011). The condition is not associated with a derangement in iron regulatory or erythropoietic mechanisms.
Functional iron deficiency: this exists where, despite an apparently adequate store, iron cannot be effectively mobilised to participate in erythropoiesis (Pasricha 2010). Understanding the pathophysiology of functional iron deficiency was greatly enhanced by the discovery of the 25‐amino acid peptide, hepcidin (Beard 2001; Drakesmith 2012; Ganz 2003; Goodnough 2011; Jordan 2009; Krause 2000; Nemeth 2004a; Nemeth 2004b; Park 2001; Weiss 2005).
In a healthy person, simple indices of iron status such as ferritin (a storage form of iron predominantly found in the liver, which is detectable in serum as it leaks into the circulation) and transferrin saturation (percentage occupation of iron carrier molecules in the circulation) are sufficient to diagnose iron deficiency (Lim 2018). However, in the setting of inflammation, ferritin acts as an acute‐phase reactant and serum concentration increases, meaning interpretation of ferritin alone as a measure of iron deficiency becomes unreliable. Simultaneously, in response to the same inflammatory process that makes serum ferritin difficult to interpret, a 25‐amino acid protein is produced, known as hepcidin. The functions of hepcidin are two‐fold:
prevention of iron overload through limiting excessive iron absorption in the proximal small intestine and regulation of iron release from macrophages participating in recycling (Ganz 2003), and;
prevention of iron acquisition by pathogens as a component of innate immunity (Krause 2000).
Hepcidin impairs the function of the key iron regulatory protein, ferroportin, thereby preventing the transport of iron across basement membranes (Nemeth 2004a). This inhibits the uptake of iron from the gastrointestinal tract, the transport of stored iron out of the liver, and the reclamation of iron from circulating macrophages. Whilst serum levels of ferritin appear high, iron is unable to circulate or be delivered to the bone marrow, which in turn leads to iron‐restricted erythropoiesis (Weiss 2005). There is increasing evidence that ferritin is a key modulator of the inflammasome, and that this increased ferritin seen in inflammation may be an active player in innate immunity, as opposed to a consequence of cell damage and leakage (Kell 2014).
Estimation of the incidence of iron deficiency is difficult, as many clinicians will only think to perform iron studies after first making a diagnosis of anaemia. In the perioperative context, iron deficiency in one form or another is relatively common, affecting 35% to 37% of cardiac surgical patients (Miles 2018a; Rössler 2019), and an even higher proportion of colorectal surgical patients (Miles 2019a). In the context of heart failure, 50% of patients are affected by iron deficiency (von Haehling 2019), although an estimate of the relative proportions between anaemic and non‐anaemic is not possible for the reasons previously stated. In a recent audit of hospital inpatients with heart failure and anaemia, only 29% had an appropriate assessment of iron status, suggesting that the appropriate assessment of non‐anaemic patients is likely to be even poorer (Simon 2019). An overall estimate of non‐anaemic iron deficiency across every patient population, and the distribution between absolute and functional iron deficiency cannot be given at this time, although upcoming prospective work in a variety of populations will likely be useful in providing much needed demographic data.
Description of the intervention
In this systematic review, we have investigated intravenous iron therapy as an intervention for non‐anaemic, iron‐deficient adults. Current guidelines recommend oral iron as the first line of treatment for people who are iron‐deficient. However, oral iron therapy is associated with some issues relating to compliance and efficacy.
Oral iron may result in gastrointestinal side effects, meaning that adherence to therapy may be poor (Cancelo‐Hidalgo 2013; Gereklioglu 2016). Whilst recent research findings have suggested alternate or third daily dosing strategies may minimise side effects (Stoffel 2017), current guidelines still recommend daily dosing.
Use of oral therapy does not lead to rapid incorporation of iron into the body. This is especially true in functional iron deficiency, where inflammation prevents the transport of iron across the enterocyte due to the activity of the hepcidin‐ferroportin axis (Goodnough 2012; Nemeth 2004a; Nemeth 2004b; Nemeth 2009).
Correction of haemoglobin levels using oral therapy alone may be slow, sometimes requiring weeks of therapy until substantive gains are made, and gains may be attenuated by ongoing blood loss (Cançado 2011; Johnson‐Wimbley 2011). This is of particular relevance in the urgent surgery population, where emerging evidence is beginning to suggest a contribution of non‐anaemic iron deficiency to poor post‐operative outcome (Miles 2018a; Miles 2019a; Rössler 2019), and hence a more rapid correction of iron status may be desirable (Muñoz 2017).
These issues are avoided by giving iron through the intravenous route. By bypassing the hepcidin‐ferroportin axis, the treatment has an improved clinical effect in the setting of inflammation, and does not have the same gastrointestinal side effects of oral iron. Consequently, intravenous iron preparations are being used more widely for patients who, under previous guidelines, would not have received this therapy as first‐line treatment (Favrat 2014). Previously, parenteral iron preparations were highly labile and prone to the excessive release of free iron into the circulation, with an associated risk of side effects (Bailie 2012). However, the development of newer, high‐molecular‐weight and more stable preparations has markedly reduced the incidence of these events (Avni 2015). Consequently, administration of parenteral iron is becoming more widespread.
How the intervention might work
Iron therapy has been increasingly advocated in a variety of clinical scenarios including perioperative optimisation of haemoglobin (Clevenger 2015). Iron is a limiting factor to oxygen transport and storage when iron is insufficient for erythropoiesis (Ganz 2012). Iron deficiency may also affect adenosine triphosphate (ATP) production and increase the predominance of energy production towards anaerobic sources, such as anaerobic glycolysis (Hinton 2014; Melenovsky 2016).
Accordingly, even in the absence of anaemia, insufficient iron stores may have non‐haematological effects that are detrimental to health, well‐being and functional status (Musallam 2018). This hypothesis has been tested in people with heart failure, where insufficient iron stores are associated with impaired exercise performance, increased fatigue and reduced health‐related quality of life (Jankowska 2016; Klip 2013). In this setting, administration of iron therapy may improve symptoms.
As noted previously, oral iron therapy for the treatment of iron deficiency has several limitations related to efficacy and compliance, particularly when oral supplements are administered daily. The use of intravenous iron in scenarios where a rapid response is required (such as the individual undergoing urgent surgery), or where inflammation is present, is considered preferable. It has been hypothesised that the administration of large amounts of intravenous iron, and subsequent overload of the reticuloendothelial capacity for iron, leads to a transient and compensatory reduction in hepcidin expression, allowing replenishment of iron stores through export from the plasma (Cançado 2011).
Why it is important to do this review
It has been recognised for some time that iron deficiency is a staged process, and that anaemia, whilst the most recognisable manifestation of this pathology, effectively represents the end stage of the disease (Suominen 1998). Observational studies from different populations have highlighted the impact of the pathology on exercise performance and fatigue (Barberan‐Garcia 2015; Pratt 2016), as well as the benefits of correction (Favrat 2014). Recently, a number of perioperative guidelines and consensus statements have advocated for the correction of iron deficiency in people about to undergo major surgery (National Blood Authority 2012; Muñoz 2017).
Pathological organisms also rely on iron for key functions. It is increasingly recognised that certain regulatory processes in the body exist to reduce the availability of free iron in the circulation at times of inflammation and infection (Drakesmith 2012; Ganz 2003; Nemeth 2009). It has been postulated that administration of parenteral iron to bypass these regulatory mechanisms may lead to an increased risk of bacterial infection (Drakesmith 2012). Evidence for this effect is conflicting. A systematic review in hospital inpatients found a 33% increased risk of infection where parenteral iron was administered (Litton 2013), but this is not reflected in large, retrospective cohort analyses (Muñoz 2014), or other meta‐analyses examining the safety of newer, high‐molecular‐weight iron preparations (Avni 2015; Rognoni 2016). Evidence from developing countries suggests that population‐based interventions to address the high incidence of nutritional iron deficiency concomitantly increased the incidence of infectious diseases (Pasricha 2013).
Given these apparent conflicts, we perceived a need for further clarification of the role of iron therapy to treat non‐anaemic iron deficiency. There are several reasons to assess the effects of intravenous iron on the correction of non‐anaemic iron deficiency across patient groups:
the current definition of anaemia in the non‐pregnant adult is not dependent on the presence or absence of comorbidities and is based on historical expert consensus (Butcher 2017);
a haemoglobin concentration above the historical threshold for anaemia may still be clinically important, particularly for women (Blaudszun 2018; Favrat 2014; Miles 2019b);
intravenous iron therapy for people with anaemia is associated with an increase in haemoglobin in a wide variety of clinical scenarios;
in patients undergoing major surgery, a higher haemoglobin at the start of operation has been shown to be the only correctable factor protecting against allogeneic blood transfusion (Klein 2017);
iron depletion may have pronounced metabolic effects, even in the absence of anaemia, particularly with respect to fatigue and cognition (Favrat 2014); and
iron depletion (even in the absence of anaemia) worsens the functional capacity (and the effect of corrective interventions) of a variety of different patient populations (Barberan‐Garcia 2015; Pratt 2016).
There is yet to be a systematic review determining the aggregate effect of intravenous iron therapy in isolation on features of iron deficiency other than anaemia. This review has the potential to substantially guide practice in this evolving area, where intravenous iron therapy is increasingly being used for the management of non‐anaemic iron deficiency. A high‐quality summary of the evidence is required to adequately inform practice, and direct the development of future randomised controlled trials.
Objectives
To assess the effects of intravenous iron therapy in the treatment of adults with non‐anaemic iron deficiency.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) for inclusion in this review. We included RCTs irrespective of blinding, language of publication, publication status, date of publication, study setting or sample size. We did not include quasi‐randomised trials, cross‐over trials or other non‐RCT designs. We considered quasi‐randomised trials to be any controlled trial where the method of allocation was not truly random (i.e. allocation based on medical record number, date of birth, day of week, etc.). We considered cluster‐randomised trials for inclusion if the method of randomisation was truly random (i.e. random number sequence, coin flip, etc.). We excluded cross‐over trials as we felt this was an inappropriate design to assess this intervention.
Types of participants
We included all adults (18 years and above) with functional or absolute non‐anaemic iron deficiency. Non‐anaemia was defined as haemoglobin (Hb) greater than 130 g/L for men and greater than 120 g/L for non‐pregnant women. Studies that did not differentiate a Hb between men and women, and set a non‐anaemic definition of greater than 120 g/L for both sexes were also included.
In order to capture the broadest possible population, we reviewed a series of RCTs from the existing literature to define iron deficiency, and chose the least restrictive definition (Beck‐da‐Silva 2013). We defined iron deficiency as:
absolute iron deficiency: ferritin less than 100 µg/L;
functional iron deficiency: ferritin more than 100 µg/L and transferrin saturation (TSAT) less than 20%.
We excluded pregnant and puerperal women because of considerable differences in the definition of anaemia in pregnancy. We also excluded studies from paediatric populations.
We excluded participants who were treated with erythropoietin or other erythropoiesis‐stimulating agents (ESA) alone or in combination with iron.
Types of interventions
We considered any study comparing any formulation of intravenous iron with placebo. We considered all doses and preparations of intravenous iron. We excluded oral iron preparations from the review because the therapeutic benefit of oral iron is difficult to assess due to the presence of multiple confounding factors (principally poor compliance due to side effects, or malabsorption due to concomitant inflammation or duodenal pathology). We feared that this would introduce substantial and unquantifiable heterogeneity into the analysis, especially as the included studies would cover a wide range of patient populations, some of which, by definition, would be unable to take oral iron. In order to adequately assess the biological effect of iron loading across multiple patient groups, we necessarily excluded oral iron interventions.
Types of outcome measures
Primary outcomes
Haemoglobin concentration (g/L), measured at the end of follow‐up
Overall quality of life, taken at the end of follow‐up, as measured by a quantitative quality‐of‐life measurement scale
The outcomes chosen reflected key quantitative and qualitative endpoints for this intervention. Quality‐of‐life scoring systems have the ability to assess the clinical effects of iron replenishment separate from changes in haemoglobin concentration. There is considerable controversy in this area, particularly with respect to existing definitions of anaemia and evidence of continued haemoglobin response to iron replenishment despite haemoglobin concentration being apparently 'normal' (Butcher 2017; Favrat 2014). We used an assessment of both a laboratory parameter of response to iron therapy (haemoglobin concentration) and quality‐of‐life metrics to determine if observed improvements with iron therapy in previous studies are related to improvements in haemoglobin concentration or another, as yet undefined metric.
Secondary outcomes
Serum ferritin measured at the end of follow‐up
Peak oxygen consumption (VO2 peak or VO2 max), as measured by cardiopulmonary exercise testing taken at the end of follow‐up
Risk of bacterial infections. We included this outcome from studies where there was a clear definition of how a bacterial infection was detected and where measurement occurred equally in both groups.
Risk of serious adverse events at the end of follow‐up, defined as any event that would increase mortality; were life‐threatening; required inpatient hospitalisation or resulted in persistent or significant disability; or any important medical events that might jeopardise the participant or that required intervention to prevent them within 30 days of cessation of treatment (ICH‐GCP 1996).
Risk of mild adverse events at the end of follow‐up, defined as any event that did not meet the definition of a serious adverse event but that required treatment or resulted in patient discomfort. Examples include headache, rash or nausea. We included hypophosphataemia of any severity in this category.
Information size calculation
For all meta‐analyses performed, we used Trial Sequential Analysis software (Copenhagen Trial Unit 2016), in order to consider the adequacy of the power (Imberger 2015; Mascha 2015). We used a type 1 error risk of 5% and a type 2 error risk of 10%, the pooled standard deviation for continuous data and unweighted mean of the control event rate for categorical data, and the diversity calculated from the actual meta‐analysis.
Information size in meta‐analysis can be considered similar to an a priori power calculation for a planned RCT, powered to observe a particular magnitude of effect. We undertook hypothetical calculations for information size using G*Power v3.1 for each of the primary outcome measures. It should be noted that these calculations did not take into account inherent heterogeneity between studies.
Overall quality of life, taken at the end of follow‐up: Favrat 2014 described mental quality‐of‐life scores (SF‐12) taken at 56 days for intervention (47.3 ± 8.7) and control (45.1 ± 9.1). Based on this, an appropriately powered RCT to examine this effect size, with a type 1 error risk of 5% and a type 2 error risk of 10%, is 692.
Concentration of haemoglobin, taken at the end of follow‐up: Anker 2009 described haemoglobin concentration at 24 weeks for intervention (133 ± 1.0 g/L) and control (132 ± 1.0 g/L) groups. Based on this, an appropriately powered RCT to examine this effect size, with a type 1 error risk of 5% and a type 2 error risk of 10%, is 46.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Information Specialist searched the following databases on 18 October 2019:
Cochrane Central Register of Controlled Trials (which contains the Cochrane Injuries Trials Register; CENTRAL; 2019, Issue 10) in the Cochrane Library (Appendix 1);
MEDLINE Ovid (1946 to October 2019; Appendix 2);
Embase Ovid (1947 to October 2019; Appendix 3);
Web of Science: Science Citation Index Expanded (SCI‐EXPANDED; 1970 to October 2019; Appendix 4);
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to October 2019; Appendix 4);
Clinicaltrials.gov (www.clinicaltrials.gov; Appendix 5);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp; Appendix 6).
Searching other resources
We screened the reference lists of all included studies and previous review articles for potential additional studies.
Data collection and analysis
We conducted this review with adherence to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
We are aware that the wide range of intravenous iron preparations currently available implies that a network meta‐analysis could be considered more informative. However, the body of literature was not large enough to justify this approach. A future update of this review may uncover sufficient evidence to enable such an analysis.
Selection of studies
Two review authors (LFM and EL) identified studies for inclusion independently of each other. We resolved any disagreement between review authors through discussion, or, if required, through involvement of a third review author (DS). We listed excluded studies along with the reason for exclusion. We investigated all eligible articles as full text. Where information in studies was unclear or missing we contacted the authors of individual studies directly for clarification and information.
Data extraction and management
Independent of one another, two review authors (LFM and EL) extracted data into a specifically‐designed and pilot‐tested form for this review, which included the following.
Country of study participant recruitment
Year and language of publication
Year the study was conducted
Study design
Sample size
Inclusion and exclusion criteria
Study population characteristics and clinical settings
Iron therapy details, including dose, route, frequency and duration
Study‐specific outcomes
Outcomes included in this review
Information to assess the risk of bias
Details of prospective study registration
Details of ethical review committee approval
Sources of support and study funding
Assessment of risk of bias in included studies
We assessed included studies for risk of bias according to the criteria outlined in Table 8.5.d in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). The domains used to assess the risk of bias were: selection bias (random sequence generation and allocation concealment); blinding bias (blinding of participants and personnel and blinding of outcome assessment); attrition bias (amount, nature and handling of incomplete outcome data); reporting bias (selective reporting of outcome data); other bias (bias not covered elsewhere such as source of funding bias).
We categorised individual studies as being at low, high or unclear risk of bias overall according to the following criteria:
low risk of bias (plausible bias unlikely to seriously alter the results);
unclear risk of bias (plausible bias that raises some doubt about the results); or
high risk of bias (plausible bias that seriously weakens confidence in the results).
Measures of treatment effect
We used different treatment effects depending on the type of data. We calculated the mean difference (MD), with 95% confidence intervals (CIs), for continuous outcomes (e.g. iron store indices), and standardised mean difference (SMD) with 95% CIs for assessing treatment effect in quality of life (taking into account different scales used across studies). Subsequently, we transformed this into the quality‐of‐life measure used for the highest number of participants in the study, the Piper Fatigue Index. We used risk ratio (RR) with 95% CIs to measure treatment effects for dichotomous variables.
Unit of analysis issues
The unit of analysis was the individual participant with iron deficiency who was undergoing treatment. As we did not consider cross‐over trials, we did not encounter any unit of analysis issues.
Dealing with missing data
We contacted all authors of the included studies with the aim of obtaining missing information. Where no response was forthcoming, we took the percentage of missing data into account when analysing and interpreting the results. If appropriate, we estimated any such data from available information using the mean value from the relevant group for the required outcome. For continuous measures, where possible we obtained SDs from other measures, such as standard errors (SEs), CIs, and P values. Where we were unable to extract any meaningful data, and it was not possible to estimate data, we necessarily excluded these studies.
For dichotomous measures, we obtained proportions or percentages to estimate the number of events or participants assessed for that outcome.
Assessment of heterogeneity
Given a lack of common protocols used in research studies we expected a certain amount of clinical heterogeneity in the included studies. This was related to a number of factors but some potential sources of heterogeneity included variations in patient groups, different iron treatment regimens used and disparity in the quality of the study conduct. We used the Chi2 test to explore heterogeneity of included studies with a significant alpha level of 0.10. We also measured heterogeneity using the I2 statistic (Higgins 2003), to quantify inconsistencies and D2 to adjust information size calculations as part of Trial Sequential Analysis (Wetterslev 2009).
In addition to statistical assessments, we provided a descriptive assessment of heterogeneity as per the 'PICO' (population, intervention, comparison, outcome) model as part of the discussion. We expected heterogeneity in a number of areas, necessitating the use of a random‐effects model. Specific areas where heterogeneity was expected include the following.
Population: we expected marked differences in population, ranging from otherwise healthy people (Favrat 2014), to people with heart failure (Anker 2009).
Intervention: we expected different preparations and dosages of iron. Whilst most modern treatment regimens contain fairly standardised dosages of elemental iron, we did not know what the effects of more historical preparations or regimens would be.
Comparison: comparison was limited to placebo. We expected minimal heterogeneity.
Outcomes: there was some heterogeneity due to differences in quality‐of‐life scores that the studies used. We hoped that the use of SMD would ameliorate some of this.
Assessment of reporting biases
As there was fewer than 10 studies for all outcome measures, a formal assessment of publication bias using funnel plots, and subsequently Egger's test (Egger 1997) was not possible.
Data synthesis
If there were two or more studies with data for our defined outcomes, and data were sufficiently homogeneous, we performed a meta‐analysis. We used the software package Review Manager 5 (Review Manager 2014). We calculated the effect estimate using a random‐effects model. We pooled data using the Mantel‐Haenszel technique and subsequently assessed these outcomes using RRs. We pooled continuous variables using the inverse variance method, and reported results as mean ± SD.
We considered the estimate of heterogeneity in our interpretation of the results, including an assessment of how the quantity of heterogeneity, and its source, may have affected the reliability of our conclusions.
Where studies used difference scales for the assessment of continuous outcomes (i.e. quality‐of‐life score), we used SMD, with a subsequent transformation back into a single outcome measure. Due to the heterogeneity of scoring systems and population, we arbitrarily elected to present the data as the score used by the highest number of participants included in the meta‐analysis (Piper Fatigue Scale).
When studies used a lower score to indicate superiority (Favrat 2014; Grote 2009), we transformed it to a positive integer for statistical analysis by multiplying the mean effect by −1.
Subgroup analysis and investigation of heterogeneity
We performed the following three subgroup analyses if there were more than two studies included in each analysis. We performed the subgroup analyses for three categories of participant.
Underlying pathology: participants who received iron as part of a treatment regimen for heart failure. Given that a single article dealt with a post‐operative population, but this population was cardiac‐specific, we elected to classify this study as pertaining to heart failure (Johansson 2015).
Underlying pathology: participants who were not otherwise classified into a pathology‐specific category by the original protocol. These included athletes, participants with restless leg syndrome, and otherwise well participants.
Time to follow‐up: we stratified this to those participants who completed follow‐up at less than 10 weeks, as opposed to more than 10 weeks.
Sensitivity analysis
We planned to conduct a sensitivity analysis on the primary outcome for each of the main analyses, by excluding any studies that demonstrated high or unclear risk of bias in any of the five domains. We also planned to assess the impact of any study that had a large effect size on the results of the meta‐analysis, and assessed the effects of missing data (Dealing with missing data). However, given the low or very low quality of the evidence assessed, and the relatively small number of studies included in the review, we elected not to perform this analysis.
Trial Sequential Analysis
We performed Trial Sequential Analysis to preserve the risk of type 1 and type 2 errors at desired levels in the setting of sparse data and potential repeated testing (Wetterslev 2009). For all meta‐analyses performed, we used Trial Sequential Analysis (Copenhagen Trial Unit 2016), in order to consider the adequacy of the power and to adjust the 95% confidence intervals if the data were sparse (Imberger 2015; Mascha 2015). Preserving a type 1 error risk of 5% and a type 2 error risk of 10%, we constructed monitoring boundaries using the pooled SD for continuous data and the unweighted mean of the control event rate for categorical data, and the diversity calculated from the actual meta‐analysis.
Summary of findings and assessment of the certainty of the evidence
We have presented the results of this review for all comparisons in a 'Summary of findings' table. We included the following outcomes.
Mean difference in concentration of haemoglobin (g/L), taken at the end of follow‐up
Standardised mean difference in quality‐of‐life scores in health‐related quality of life. We 'back‐translated' these into the Piper Fatigue Score for presentation.
Mean difference in ferritin (µg/L), taken at the end of follow‐up
Mean difference in incidence of bacterial infection
Mean difference in peak oxygen consumption (VO2 peak or VO2 max), taken at the end of follow‐up
Risk of serious adverse events (anaphylaxis, circulatory collapse, hospitalisation)
Risk of mild adverse events (headache, dizziness, rash, hypophosphataemia)
We prepared the 'Summary of findings' table using GRADEpro GDT software (GRADEpro GDT). In accordance with the GRADE approach we undertook an assessment of the quality of evidence for each outcome. We examined the risk of bias within studies, directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias, and assessed the quality of evidence as either high, moderate, low or very low (Schünemann 2019).
In particular, we considered the appropriateness of extrapolating our results from all participants with iron deficiency to the perioperative setting and how the indirectness in this interpretation is likely to decrease the certainty in our results.
Results
Description of studies
Results of the search
The search conducted by the Cochrane Injuries Group Information Specialist yielded 2582 references. After de‐duplication and reviewing the reference lists of the included articles we screened the study reports for inclusion. Following primary screening, we selected 61 articles for full‐text screening, and ultimately included 11 studies in qualitative and quantitative analysis (Anker 2009; Burden 2015a; Charles‐Edwards 2019; Favrat 2014; Grote 2009; Johansson 2015; Krayenbuehl 2011; Okonko 2008; Van Veldhuisen 2017; Wong 2016; Woods 2014).
Included studies
Population
The 11 included studies provided 1074 participants. Of these studies, five examined a heart failure cohort (Anker 2009; Charles‐Edwards 2019; Okonko 2008; Van Veldhuisen 2017; Wong 2016), two examined elite athletes (Burden 2015a; Woods 2014), two examined otherwise well, pre‐menopausal women (Favrat 2014; Krayenbuehl 2011), one examined people with restless legs syndrome (Grote 2009), and one examined a post‐operative cardiac surgical cohort (Johansson 2015). All included studies were RCTs of intravenous iron preparations versus placebo. All studies were available through database searches as full manuscripts, with the exception of Wong 2016, which was a conference extract.
The studies used different definitions of iron deficiency depending on the study and population (Table 2).
1. Study populations and definitions of iron deficiency.
| Study | Population | Definition of iron deficiency |
| Anker 2009 | Heart failure | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Burden 2015a | Elite athletes | Ferritin < 30 mcg/L for women and < 40 mcg/L for men |
| Charles‐Edwards 2019 | Heart failure | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Favrat 2014 | Pre‐menopausal women | Ferritin < 15 mcg/L or < 50mcg/L where TSAT < 20% |
| Grote 2009 | Restless legs syndrome | Ferritin < 45 mcg/L |
| Johansson 2015 | Post‐cardiac surgery | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Krayenbuehl 2011 | Pre‐menopausal women | Ferritin < 50 mcg/L |
| Okonko 2008 | Heart failure | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Van Veldhuisen 2017 | Heart failure | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Wong 2016 | Heart failure | Ferritin < 100 mcg/L or 100‐200 mcg/L where TSAT < 20% |
| Woods 2014 | Elite athletes | Ferritin 30‐100 mcg/L |
Intervention
Studies used a variety of different treatment regimens for the administration of the study drug (Table 3). Five studies (Burden 2015a; Charles‐Edwards 2019; Favrat 2014; Johansson 2015; Wong 2016) used a single administration, whilst six (Anker 2009; Grote 2009; Krayenbuehl 2011; Okonko 2008; Van Veldhuisen 2017; Woods 2014), used repeat dosing at various points throughout the study. Ferric carboxymaltose was the preferred iron preparation in eight studies (Anker 2009; Burden 2015a; Charles‐Edwards 2019; Favrat 2014; Grote 2009; Van Veldhuisen 2017; Wong 2016; Woods 2014), iron sucrose in two studies (Krayenbuehl 2011; Okonko 2008), and iron isomaltoside in one (Johansson 2015). The total dose of administered iron where calculation was possible ranged between 300 mg (Woods 2014), up to 2500 mg (Van Veldhuisen 2017).
2. Iron preparations and dosing regimens.
| Study | Preparation | Dose | Frequency |
| Anker 2009 | Ferric carboxymaltose | 200 mg | Weekly until replete, then monthly |
| Burden 2015a | Ferric carboxymaltose | 500 mg | Single dose |
| Charles‐Edwards 2019 | Iron isomaltoside | 608 ± 204 mg | Single dose |
| Favrat 2014 | Ferric carboxymaltose | 1000 mg | Single dose |
| Grote 2009 | Ferric carboxymaltose | 200 mg | Five doses over two weeks |
| Johansson 2015 | Iron isomaltoside | 1000 mg | Single dose |
| Krayenbuehl 2011 | Iron sucrose | 200 mg | Four doses over two weeks |
| Okonko 2008 | Iron sucrose | 200 mg | Weekly until replete, then monthly |
| Van Veldhuisen 2017 | Ferric carboxymaltose | 500‐1000 mg | Six‐weekly until week 12 |
| Wong 2016 | Ferric carboxymaltose | 1000 mg | Single dose |
| Woods 2014 | Ferric carboxymaltose | 100 mg | Two‐weekly for four weeks |
Comparator
Most included studies used 0.9% sodium chloride as a placebo comparator. Two studies were open‐label interventions (Okonko 2008; Van Veldhuisen 2017). Studies used variable blinding to conceal the characteristic reddish‐brown colour of iron‐containing solutions. We discuss these in the 'Risk of bias' discussion for individual studies.
Outcome
Time to end of follow‐up differed substantially between included studies, and ranged from between 2 to 24 weeks (Table 4).
3. Duration of follow‐up after study drug administration.
| Study | End of follow‐up |
| Anker 2009 | Week 24 |
| Burden 2015a | Week 4 |
| Charles‐Edwards 2019 | Week 2 |
| Favrat 2014 | Week 8 |
| Grote 2009 | Week 11 |
| Johansson 2015 | Week 4 |
| Krayenbuehl 2011 | Week 12 |
| Okonko 2008 | Week 18 |
| Van Veldhuisen 2017 | Week 24 |
| Wong 2016 | Week 4 |
| Woods 2014 | Week 6 |
With respect to primary outcome, five studies aimed to improve various quality‐of‐life scoring systems (Anker 2009; Favrat 2014; Grote 2009; Krayenbuehl 2011; Wong 2016), three aimed to optimise Hb concentration or total Hb mass (Burden 2015a; Johansson 2015; Woods 2014), and two aimed to improve VO2 peak or other markers of exercise capacity (Okonko 2008; Van Veldhuisen 2017). Whilst Charles‐Edwards 2019 did have VO2 and quality of life available as outcome metrics, they did not present subgroup results for non‐anaemic participants.
For the majority of outcomes specified a priori, data were expressed as mean ± SD. Where data were expressed as mean ± SD for baseline participant characteristics, and were subsequently reported as change in variable from baseline, these outcomes were necessarily excluded (Krayenbuehl 2011). Where data were expressed graphically and not numerically, we contacted the study authors to request these data. Where we were unable to obtain the information, we excluded these outcomes too (Anker 2009; Favrat 2014).
Excluded studies
After selection for full‐text screening, we excluded 50 articles for ineligible patient population, ineligible study design, ineligible route of administration, not measuring outcomes of interest or ineligible comparator.
We identified some studies as containing information on the relevant population and outcomes that study authors could potentially extract so we could include them in the review. Where our attempts to contact the study authors were unsuccessful, and we could extract no further relevant data without the assistance of the study authors, we had to exclude these studies (Boomershine 2018; Filippatos 2013; Fontana 2014; Gybel‐Brask 2018; Trenkwalder 2017; Van Craenenbroeck 2013). The reasons for exclusion of full‐text articles is listed in the PRISMA flowchart for this review (Moher 2009; Figure 1), and in Characteristics of excluded studies.
1.
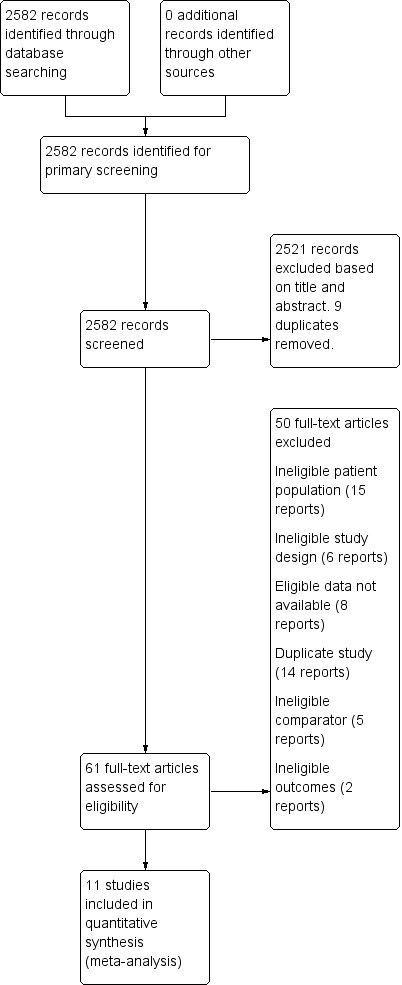
Flow diagram of studies included in the systematic review
Risk of bias in included studies
Graphical representations of risk of bias are shown in Figure 2 and Figure 3. The assessment of risk of bias is individually displayed for each included study in Figure 2 and proportionally ranked for each 'Risk of bias' indicator in Figure 3.
2.
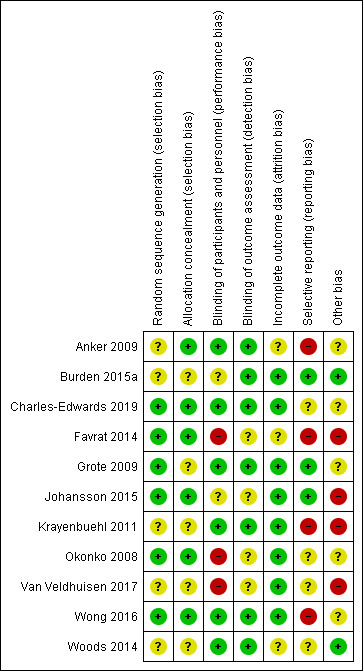
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.
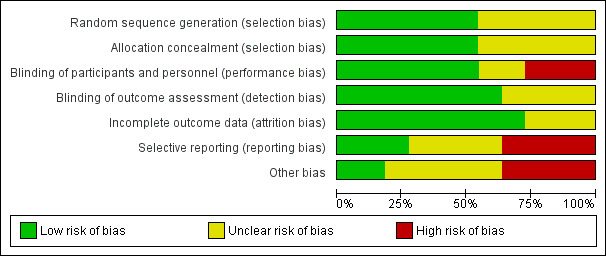
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
We did not consider any included studies at high risk of selection bias. However, we considered five of the included studies at unclear risk of selection bias due to poorly specified or unspecified randomisation schedules (Anker 2009; Burden 2015a; Krayenbuehl 2011; Van Veldhuisen 2017; Woods 2014), and five due to poorly specified or unspecified allocation concealment (Burden 2015a; Grote 2009; Krayenbuehl 2011; Van Veldhuisen 2017; Woods 2014). Four studies had unclear risk of bias for both selection bias domains (Burden 2015a; Krayenbuehl 2011; Van Veldhuisen 2017; Woods 2014). We judged the remaining studies to be at low risk of selection bias in both random sequence generation and allocation concealment domains (Charles‐Edwards 2019; Johansson 2015; Okonko 2008; Wong 2016).
Blinding
Performance bias
Due to the distinct colour of iron‐containing solutions, blinding of participants and personnel must necessarily involve the use of opaque syringes or administration bags and tubing to prevent performance bias. Three of the included studies were at high risk performance bias. Two of these were open‐label studies (Okonko 2008; Van Veldhuisen 2017), and one did not blind the study investigators and the use of opaque administration sets was unclear (Favrat 2014). We considered two studies at unclear risk of performance bias (Burden 2015a; Johansson 2015), as these studies stated that they were blinded, but did not specifically reference the techniques they used in the study. The remaining six studies specifically referred to the use of concealed study drug administration and we considered them at low risk of performance bias (Anker 2009; Charles‐Edwards 2019; Grote 2009; Krayenbuehl 2011; Wong 2016; Woods 2014).
Detection bias
We did not consider any of the included studies at high risk of detection bias. We considered four studies at unclear risk of detection bias as they did not explicitly state separation of outcome assessors from study drug administration in the manuscript (Favrat 2014; Johansson 2015; Okonko 2008; Van Veldhuisen 2017). We judged the remaining studies (Anker 2009; Burden 2015a; Charles‐Edwards 2019; Grote 2009; Krayenbuehl 2011; Wong 2016; Woods 2014) to be at low risk of detection bias.
Incomplete outcome data
We did not consider any studies at high risk of attrition bias. We considered outcome reporting to be complete with a low risk of bias in eight studies (Burden 2015a; Charles‐Edwards 2019; Grote 2009; Johansson 2015; Krayenbuehl 2011; Okonko 2008; Van Veldhuisen 2017; Wong 2016). We considered three studies at unclear risk of attrition bias because they only displayed relevant data in graphical form (Anker 2009; Favrat 2014; Woods 2014).
Selective reporting
We considered four studies at high risk of reporting bias due to failure to report baseline or follow‐up data for prespecified outcomes (Anker 2009; Favrat 2014; Krayenbuehl 2011; Wong 2016). Three studies reported all outcomes specified in the study methods and we considered them at low risk of reporting bias (Burden 2015a; Grote 2009; Johansson 2015). The risk of bias was unclear in four studies as there was inadequate information to determine if they had reported all relevant data, or they had reported data as specified in the protocol but in a form not amenable to extraction (Charles‐Edwards 2019; Okonko 2008; Van Veldhuisen 2017; Woods 2014).
Other potential sources of bias
Nine studies received some form of pharmaceutical company support. In four of these, a study author was a direct employee of the company in question, and we assessed the study as high risk of bias (Favrat 2014; Johansson 2015; Krayenbuehl 2011; Van Veldhuisen 2017). We assessed the risk of bias in the remaining five studies as unclear (Anker 2009; Charles‐Edwards 2019; Grote 2009; Okonko 2008; Wong 2016). Two studies had no pharmaceutical company involvement and we assessed them as low risk of bias (Burden 2015a; Woods 2014).
Effects of interventions
See: Table 1
The summary of findings for the full study population is displayed in Table 1.
Primary outcomes
Haemoglobin concentration
Eight studies reported haemoglobin concentration at the end of follow‐up (Analysis 1.1). Meta‐analysis suggested that the mean difference in haemoglobin concentration taken at the end of follow‐up was 3.04 g/L higher in the intervention group (95% CI 0.65 to 5.42; I2 = 42%; 8 studies, 548 participants). We rated the overall quality of evidence for this outcome as 'low' according to GRADE criteria. Consequently, whilst intravenous iron may result in a small (and likely clinically insignificant) increase in haemoglobin taken at the end of follow‐up, our confidence in the effect estimate is limited.
1.1. Analysis.
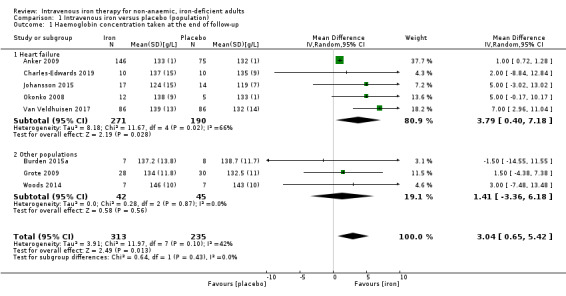
Comparison 1 Intravenous iron versus placebo (population), Outcome 1 Haemoglobin concentration taken at the end of follow‐up.
Risk of bias: none of the included studies were at low risk of bias, and two were unblinded. However, as loss to follow‐up was minimal, and the outcome is entirely objective, we did not downgrade the evidence level.
Inconsistency: we noted moderate statistical heterogeneity (I2 = 42%). There were multiple points of methodological heterogeneity, with the dose of iron administered and the time to end of follow‐up. We downgraded the evidence one level.
Indirectness: whilst the included studies examined multiple different populations, these were consistent with the study question. The intervention, comparator and outcome were consistent across the included studies. We did not downgrade the evidence level.
Imprecision: the confidence interval around the point prevalence estimate of effect is wide, and on Trial Sequential Analysis, the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power. We downgraded the evidence one level.
Quality of life
Three studies reported overall quality of life measured at the end of follow‐up (Analysis 1.2). Meta‐analysis suggested that the SMD of quality‐of‐life scores was 0.35 points higher in the intervention group at the end of follow‐up (95% CI 0.14 to 0.57 higher). We converted this back to the quality‐of‐life scoring system used by the largest number of participants in the included studies, Piper Fatigue Scale (Favrat 2014). Using this metric, intravenous iron had a mean difference in Piper Fatigue Scale 0.73 points lower (95% CI 0.29 to 1.18 points lower; I2 = 0%; 3 studies, 344 participants). We rated the overall quality of evidence for this conclusion as 'very low' according to GRADE criteria. Consequently, we are uncertain about the effect of intravenous iron on quality‐of‐life scoring taken at the end of follow‐up.
1.2. Analysis.
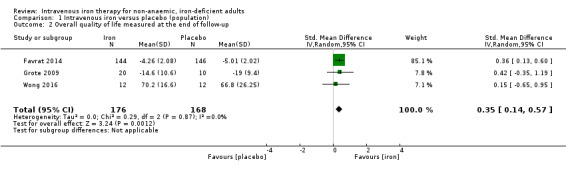
Comparison 1 Intravenous iron versus placebo (population), Outcome 2 Overall quality of life measured at the end of follow‐up.
Risk of bias: none of the included studies were at a low risk of bias, and we judged the largest included study to be at high risk of bias from multiple protocol deviations and no reporting of the per protocol analysis. We downgraded the evidence two levels.
Inconsistency: we saw negligible statistical heterogeneity as reflected by the I2 statistic value. There were multiple points of methodological heterogeneity, with the dose of iron administered and the time to end of follow‐up. Accordingly, we downgraded the evidence one level.
Indirectness: whilst the included studies examined multiple different populations, these were consistent with the study question. The intervention, comparator and outcome were consistent across the included studies. Accordingly, we did not downgrade the evidence level.
Imprecision: the generated effect size falls considerably short of a conservatively estimated effect size to deliver appropriate statistical power. We downgraded the evidence one level.
Secondary outcomes
Ferritin concentration
Seven studies reported ferritin concentration at the end of follow‐up. The pooled results of this meta‐analysis were typified by extreme statistical heterogeneity, and wide confidence intervals, with marked differences in means and SDs between the included studies. This is due to differences in definition of iron deficiency, differences in iron regimen and differences to time to end of follow‐up. Consequently, we have not presented a pooled analysis of ferritin concentration as part of this review. Nevertheless, in all seven studies, we observed a higher ferritin concentration in the intervention group relative to the control. We undertook a subgroup analysis for this outcome based on underlying pathology (Analysis 1.3; see also 'Exploration of heterogeneity' section below). We undertook a GRADE assessment of the quality of evidence despite not presenting a global pooled result for this outcome.
1.3. Analysis.
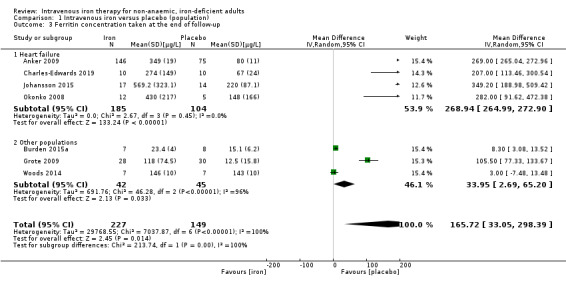
Comparison 1 Intravenous iron versus placebo (population), Outcome 3 Ferritin concentration taken at the end of follow‐up.
Risk of bias: none of the included studies were at low risk of bias, and one was unblinded. However, as loss to follow‐up is minimal and the outcome is entirely objective, we did not downgrade the quality of evidence.
Inconsistency: we observed substantial statistical heterogeneity in the pooled result (I2 = 100%). There were multiple points of methodological heterogeneity, with dose of iron administered and time to end of follow‐up. We downgraded the evidence two levels.
Indirectness: whilst the included studies examined multiple different populations, these were consistent with the study question. Intervention, comparator and outcome were consistent across the included studies. We did not downgrade the evidence level.
Imprecision: the point prevalence estimates in each of the included studies are highly imprecise, as reflected by the large confidence interval of the total result. The generated effect size is considerably less than the required effect size calculated by Trial Sequential Analysis. We downgraded the evidence two levels.
Peak oxygen consumption
Only two studies reported peak oxygen consumption (VO2 peak or VO2 max) measured at the end of follow‐up (Analysis 1.4). Meta‐analysis suggested that the mean peak oxygen consumption taken at the end of follow‐up in the intervention group was 2.77 mL/kg/min higher (95% CI 0.89 lower to 6.43 higher; I2 = 36%; 2 studies, 32 participants). We rated the overall quality of evidence for this conclusion as 'very low' according to GRADE criteria. Consequently, we are uncertain about the effect of intravenous iron on peak oxygen consumption taken at the end of follow‐up.
1.4. Analysis.
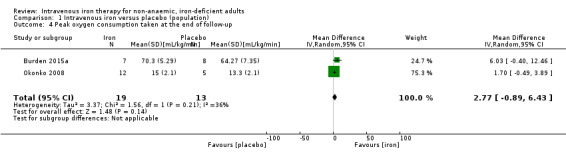
Comparison 1 Intravenous iron versus placebo (population), Outcome 4 Peak oxygen consumption taken at the end of follow‐up.
Risk of bias: one of the two included studies was at high risk of bias for participant blinding. The outcome in question could potentially be compromised by performance bias, and we downgraded the evidence one level.
Inconsistency: we observed minor statistical heterogeneity (I2 = 36%). There were multiple points of methodological heterogeneity, with the dose of iron administered and the time to end of follow‐up. Accordingly, we downgraded the evidence one level.
Indirectness: whilst the included studies examined multiple different populations, these were consistent with the study question. The intervention, comparator and outcome were consistent across the included studies. Accordingly, we did not downgrade the evidence level.
Imprecision: the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power. There is considerable difference in the mean point estimate of effect in the two included studies. We downgraded the evidence two levels.
Incidence of mild to moderate adverse effects
Four studies reported incidence of mild to moderate adverse effects (Analysis 1.5). These referred to essentially participant‐reported side effects such as headache, fatigue and nausea. No studies reported data on hypophosphataemia. We did not consider adverse events that were unrelated to study drug administration (i.e. admission to hospital for exacerbation of heart failure) to be an adverse effect of study drug infusion. For example, we considered all adverse events in Johansson 2015 to be unrelated to study drug administration, and so did not consider these data further. Meta‐analysis of the remaining three studies suggested that whilst intravenous iron was associated with a point prevalence increase in the risk of mild to moderate adverse events (RR 1.19, 95% CI 0.97 to 1.45; I2 = 0%; 3 studies, 440 participants), the test for subgroup differences did not reveal a statistically significant difference between the subgroups. We rated the overall quality of evidence for this conclusion as 'very low' according to GRADE criteria. Consequently, we are uncertain about the effect of intravenous iron on the incidence of mild to moderate adverse effects taken at the end of follow‐up.
1.5. Analysis.
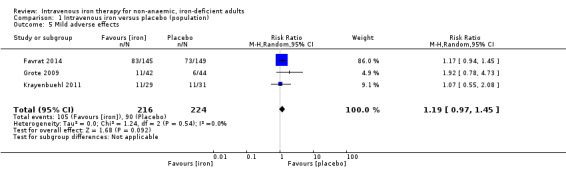
Comparison 1 Intravenous iron versus placebo (population), Outcome 5 Mild adverse effects.
Risk of bias: the largest included study was at high risk for participant blinding, and had multiple protocol deviations. Most side effects were qualitative, with no objective verification. We downgraded the evidence one level.
Inconsistency: we observed minimal statistical heterogeneity (I2 = 0%) but noted methodological heterogeneity regarding the dose of iron administered and the time to end of follow‐up. We downgraded the evidence one level.
Indirectness: whilst the included studies examined multiple different populations, these were consistent with the study question. Intervention, comparator and outcome were consistent across the included studies. We did not downgrade the evidence quality.
Imprecision: the generated effect size falls short of a conservatively estimated effect size to deliver appropriate statistical power. There is a relatively consistent difference in the mean difference in the three included studies. We downgraded the evidence one level.
Bacterial infection
Only one study (Anker 2009), recorded data on the incidence of bacterial infection. As we were not able to separate data for anaemic and non‐anaemic participants for this outcome for this study, we were not able to draw conclusions about these data.
Incidence of severe adverse effects
No studies reported any serious adverse events.
Exploration of heterogeneity using subgroup analysis
We specified a number of different subgroup analyses a priori (Subgroup analysis and investigation of heterogeneity). As specified in the protocol (Miles 2018b), we undertook subgroup analyses differentiating between underlying pathology and time to end of follow‐up (less than 10 weeks versus 10 weeks or more). We did not undertake a planned subgroup analysis based on type of iron deficiency because studies included in the functional iron deficiency group all used a combination definition of iron deficiency, including a patient population who, on the basis of the inclusion criteria specified a priori, would have met criteria for both functional and absolute iron deficiency. It was not possible to separate these data, nor were data presented on the relative proportions of absolute and functional iron deficiency in these studies.
Underlying pathology
A sufficient number of studies were available to perform analyses for haemoglobin concentration at the end of follow‐up (Analysis 1.1), and ferritin concentration at the end of follow‐up (Analysis 1.3).
Haemoglobin concentration at the end of follow‐up
Participants with heart failure demonstrated a modest improvement in haemoglobin concentration at the end of follow‐up (MD 3.79 g/L, 95% CI 0.40 to 7.18; I2 = 66%; 5 studies, 461 participants). Participants with other pathologies had a point prevalence difference suggesting a lower incrementation in haemoglobin concentration with lower heterogeneity (MD 1.41 g/L, 95% CI −3.36 to 6.18; I2 = 0%; 3 studies, 87 participants), although the confidence intervals for this metric crossed 0 and P = 0.43. There is no statistically significant difference between these two groups. As previously mentioned, substantial confounding of this result was present, and the studies that examined heart failure as a pathology (with the addition of Johansson 2015), were also the studies that used functional iron deficiency as a definition (Analysis 1.1; Table 5).
4. Subgroup analysis to examine heterogeneity in haemoglobin concentration meta‐analysis.
| Number of studies | Participants | Mean difference (g/L) | 95% confidence interval | z‐score | P value | |
| Study population | ||||||
| All populations | 8 | 548 | 3.04 | 0.65 to 5.42 | 2.38 | |
| Heart failure | 5 | 461 | 3.79 | 0.40 to 7.81 | 2.19 | 0.43 |
| Other populations | 3 | 87 | 1.41 | −3.36 to 6.18 | 0.58 | |
| Type of iron deficiency | ||||||
| Functional iron deficiency | 5 | 461 | 3.79 | 0.40 to 7.81 | 2.19 | 0.43 |
| Absolute iron deficiency | 3 | 87 | 1.41 | −3.36 to 6.18 | 0.58 |
Ferritin concentration at the end of follow‐up
Extreme statistical heterogeneity was demonstrated on the primary meta‐analysis for this outcome, preventing the publication of a pooled result. We hypothesised that because those studies that examined heart failure were more likely to include participants with functional iron deficiency, the higher starting ferritin in these participants would result in a higher ferritin level at the end of follow‐up, resulting in the aforementioned heterogeneity. To confirm this hypothesis, and following the published protocol for this review (Miles 2018b), we proceeded to perform separate analyses for those studies that examined people with heart failure (Anker 2009; Charles‐Edwards 2019; Johansson 2015; Okonko 2008), and those that examined 'other' populations (Burden 2015a; Grote 2009; Woods 2014).
In those participants with heart failure, the results of the meta‐analysis suggested that intravenous iron resulted in an increase in serum ferritin (MD 268.94 µg/L, 95% CI 264.99 to 272.90; I2 = 0%; 4 studies, 291 participants), relative to those participants with other pathologies (MD 59.94 µg/L, 95% CI −12.94 to 132.83; I2 = 96%; 3 studies, 87 participants). The difference between these two groups was statistically significant at P = 0.01. However, the wide confidence intervals, the persistence of extreme statistical heterogeneity in the other‐populations group, and very low‐quality evidence means that we remain uncertain of the exact effect of intravenous iron on serum ferritin in this latter subgroup. However, some narrative synthesis of this evidence is possible, and may partially highlight the origin of this heterogeneity. Burden 2015a randomised elite athletes to receive intravenous iron or placebo. Both groups were similar at baseline with a serum ferritin of 20.3 ± 7.2 µg/L in the intravenous iron group and 19.3 ± 6.9 µg/L in the control group. At 24 hours, the intravenous iron group demonstrated an increase in serum ferritin to 70.7 ± 10.0 µg/L, but this level had returned to baseline by the end of follow‐up at four weeks (23.4 ± 4.0 µg/L). In contrast, serum ferritin fell over the course of the study in the placebo group to 15.1 ± 6.2 µg/L. These results stand in contrast to the other two studies in this subgroup. Grote 2009 (a study conducted in patients with restless legs syndrome), observed a sustained increase in serum ferritin (from 20.1 ± 11.9 µg/L to 118.4 ± 75.4 µg/L) in participants in the intravenous iron arm at 11 weeks after dosing. Woods 2014 (again conducted in elite athletes) observed a similar sustained increase in the intravenous iron arm, with serum ferritin increasing from 62.8 ± 21.9 µg/L to 127.0 ± 66.3 µg/L over four weeks. We performed a sensitivity analysis excluding the results of Burden 2015a from the subgroup analysis, which resulted in a drop in statistical heterogeneity to 32%, suggesting that this failure to maintain sustained incrementation was the source of the initial result. One potential mechanism for this finding was the dosing strategy used by Burden 2015a (Table 3): in contrast to Grote 2009 and Woods 2014, Burden 2015a used a single dose of intravenous iron instead of multiple doses over the course of study. On the basis of the current available evidence, it is not possible to confirm the biological plausibility of this theory, and given the very low quality of the evidence as outlined above, we remain uncertain as to the exact effect of intravenous iron in facilitating a sustained increase in serum ferritin in patients with absolute iron deficiency (Analysis 1.3; Table 6).
5. Subgroup analysis to examine heterogeneity in ferritin concentration meta‐analysis.
| Number of studies | Participants | Mean difference (µg/L) | 95% confidence interval | z‐score | P value | |
| Type of iron deficiency | ||||||
| Functional iron deficiency | 3 | 271 | 269.06 | 265.10 to 273.02 | 133.18 | 0.02 |
| Absolute iron deficiency | 3 | 87 | 7.62 | 3.15 to 18.39 | 1.61 |
Time to follow‐up
A sufficient number of studies were available to perform an analysis for haemoglobin concentration at the end of follow‐up (Analysis 2.1). Sufficient studies were available to perform an analysis for ferritin concentration at the end of follow‐up, but due to the previously described issues with statistical heterogeneity, we did not perform it. We saw a similar point prevalence increase in haemoglobin concentration in participants who concluded follow‐up at less than 10 weeks (MD 2.90 g/L, 95% CI −2.16 to 7.96; I2 = 0%' 4 studies, 80 participants) and participants who completed their follow‐up in more than 10 weeks (MD 3.37 g/L, 95% CI −0.02 to 6.76; I2 = 72%; 4 studies, 468 participants).
2.1. Analysis.
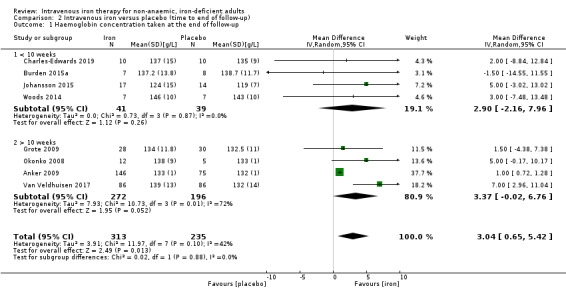
Comparison 2 Intravenous iron versus placebo (time to end of follow‐up), Outcome 1 Haemoglobin concentration taken at the end of follow‐up.
Trial Sequential Analysis
We performed Trial Sequential Analysis to preserve the risk of type 1 and type 2 errors at desired levels in the setting of sparse data and potential repeated testing (Wetterslev 2009). For all primary meta‐analyses performed, we used Trial Sequential Analysis in order to consider the adequacy of the power and to adjust the 95% confidence intervals if the data were sparse (Copenhagen Trial Unit 2016; Imberger 2015; Mascha 2015). Preserving a type 1 error risk of 5% and a type 2 error risk of 10%, we constructed monitoring boundaries using the pooled SD for continuous data and the unweighted mean of the control event rate for categorical data, and the diversity calculated from the actual meta‐analysis.
Using the assumptions described in our methods, the Trial Sequential Analyses showed that existing data are insufficient for all our outcomes to demonstrate a statistically significant result, despite our anticipated study power as per the calculated information size.
For the comparison of intravenous iron with placebo on haemoglobin concentration at the end of follow‐up, the estimated required information size was 1068. The meta‐analysis included 596 participants and was therefore underpowered given the assumptions we used. The adjusted 95% CI for haemoglobin concentration at the end of follow‐up was −0.8 to 7.2 g/L, demonstrating the increased uncertainty present due to sparse data and no statistically significant increase in the group receiving iron.
For the comparison of intravenous iron with placebo on quality‐of‐life scores, the estimated required information size was 1895. The meta‐analysis included 566 participants and was therefore underpowered given the assumptions we used. The adjusted 95% CI for participant‐centred outcomes was −3 to 8 points, demonstrating the increased uncertainty present due to sparse data.
For the comparison of intravenous iron with placebo on ferritin concentration at the end of follow‐up, the estimated required information size was 2282. The meta‐analysis included 367 participants and was therefore underpowered given the assumptions we used. The adjusted 95% CI for ferritin concentration at the end of follow up was −440 to 779 μmol/L, demonstrating the increased uncertainty present due to sparse data and no statistically significant increase in the group receiving iron.
For the comparison of intravenous iron with placebo on peak oxygen consumption at the end of follow‐up, the estimated required information size was 154. The meta‐analysis included 32 participants and was therefore underpowered given the assumptions we used. The adjusted 95% CI for peak oxygen consumption at the end of follow up was −6 to 12 mL/kg/min, demonstrating the increased uncertainty present due to sparse data.
For the comparison of intravenous iron with placebo on mild to moderate adverse effects, the estimated required information size was 32,302. The meta‐analysis included 566 participants and was therefore underpowered given the assumptions we used. The data were too sparse for adjusted confidence intervals to be meaningful.
Discussion
Summary of main results
This review included 11 studies that compared intravenous iron with placebo across a range of patient populations. There was considerable variability in the dosing regimen, iron preparation and duration of follow‐up between studies. The dose per week of iron varied from 100 mg to 1000 mg, although multiple studies used a single‐dose model (six studies), as repeated dosing (five studies) that varied between individual participants depending on the study.
Reporting of the primary outcome measures was variable, with eight studies reporting haemoglobin concentration at the end of follow‐up, and three studies reporting quality of life at the end of follow‐up. For the secondary outcome metrics, laboratory‐centred outcome metrics were relatively widely reported, specifically ferritin concentration (seven studies), peak oxygen consumption (two studies) and mild to moderate side‐effects (four studies).
Intravenous iron may cause a small but ultimately clinically unimportant increase in haemoglobin concentration, but we assessed the quality of this evidence as low. The quality of evidence for quality‐of‐life scores was very low, so we are uncertain about the effects of intravenous iron on this outcome metric. With respect to secondary outcomes, we judged it inappropriate to present a pooled estimate for ferritin due to the extreme statistical heterogeneity. We hypothesised that this was due to differences in starting ferritin between those participants with heart failure (the constituent subgroup studies including participants with both absolute and functional and absolute iron deficiency) and those from other populations (the constituent subgroup studies including only participants with absolute iron deficiency). We evaluated these differences in a subgroup analysis that partially confirmed this hypothesis. However, severe statistical heterogeneity persisted in the other population subgroups, suggesting that there are confounding factors that remain undetected in this analysis. Ultimately, the very low level of evidence assigned to this outcome, and our lack of confidence in a robust estimate of differences between the groups, prevents us from making concrete finding on the precise effect of intravenous iron on serum ferritin concentration. We are similarly uncertain regarding the effects of intravenous iron on peak oxygen consumption, or mild to moderate side effects, again because of the very low quality of the evidence. The incidence of bacterial infection and severe adverse effects across all included studies was zero.
Overall completeness and applicability of evidence
Whilst the 11 included studies covered a wide range of patient populations, and met the inclusion criteria that were specified a priori, we were ultimately prevented from reaching robust conclusions as to the role of intravenous iron in the treatment of non‐anaemic iron deficiency, despite biological plausibility. This was due to the often severe statistical and methodological heterogeneity evident in many of the pooled analyses. We encountered further difficulties when considering the reporting of outcome measures. Studies frequently reported outcomes in different ways, resulting in their exclusion from the meta‐analysis, despite our efforts to contact the relevant study authors.
The included studies were also affected by multiple methodological differences, related to the population studied, the definition of iron deficiency used, the preparation of intravenous iron, the dose and the frequency of the study drug. Further confounding was evident on subgroup analysis, with those studies that used a functional iron deficiency definition (Anker 2009; Charles‐Edwards 2019; Johansson 2015; Okonko 2008; Van Veldhuisen 2017), also including participants who met diagnostic criteria for absolute iron deficiency. However, while these considerations make drawing firm conclusions from this review difficult, it does provide important insights into the differing approaches used by various study authors, and the challenges faced by clinicians when attempting to apply this evidence to the individual patient. This is a problem that has been encountered by authors of best practice guidelines previously; for example, Muñoz 2017 cited three different definitions of iron deficiency as part of their consensus statement before finally defining non‐anaemic iron deficiency as a TSAT less than 20%, without any reference to ferritin, a definition not encountered in any study screened as part of this review, included or excluded. This is not to say that the authors of these guidelines were incorrect in applying this definition, but that, as shown by this review, there is currently limited evidence as to which form of iron deficiency, and which population would benefit most from receiving this treatment.
Quality of the evidence
We graded the overall quality of evidence for the various laboratory outcomes in the review as 'low' or 'very low' (Table 1). For the most part this was due to inconsistency, manifested by considerable statistical heterogeneity, and imprecision, manifested by failure to reach an appropriate sample size (as demonstrated by Trial Sequential Analysis). Differences in length of follow‐up and dosing regimen contributed to the statistical heterogeneity identified in the meta‐analysis. Despite apparent biological plausibility for certain outcomes, and point prevalence estimates and confidence intervals that, in and of themselves, would appear to be suggestive of an effect, the low quality of the evidence means that we are uncertain about the effect of intravenous iron on most of the outcomes presented in this review.
For participant‐centred and adverse effect outcome metrics, the subjective nature of the reported outcomes and the risk of performance bias in several studies was an additional factor in the substantial downgrading of evidence, together with the inconsistency and imprecision identified above.
The factors identified above substantially reduce our confidence in the body of evidence considered as part of this review.
Potential biases in the review process
A key review decision taken following the consideration of the study data was to alter the inclusion criteria around the definition of a non‐anaemic status. This may have resulted in underreporting of the indirectness of the included studies relative to the original research question, whereby the indirectness of the included studies would have been inadequately considered.
Even with the change in the study inclusion criteria outlined above, there remain a low number of studies included in this review, and according to the specifications outlined in our original protocol we are unable to make a judgement on publication bias (Miles 2018b). Given the extensive involvement of pharmaceutical companies in studies examining this research question, we are unable to exclude the influence of publication bias on our review.
Agreements and disagreements with other studies or reviews
We are aware of two other reviews that have examined similar research questions. In Burden 2015b, the authors focused on elite athletes alone, and assessed the effects of multiple different routes of administration of iron on exercise capacity. The authors concluded in their review that, "iron treatments improve the iron status and aerobic capacity of iron deficiency non‐anaemic endurance athletes". No included studies in this review examined the effect of intravenous iron (being limited to intramuscular and oral iron alone). The authors subsequently published an RCT on the effect of intravenous iron in non‐anaemic, iron‐deficient elite athletes, which we included in our review (Burden 2015a).
In Houston 2018, the authors examined the effect of oral, intramuscular and intravenous iron on fatigue and exercise capacity in adults. Whilst the review was limited to studies conducted in a primary care setting, it identified three studies (Burden 2015a; Favrat 2014; Krayenbuehl 2011), that utilised intravenous iron in this setting. Whilst the pooled results of all routes of administration led the authors to conclude that in non‐anaemic, iron‐deficient adults in a primary care setting, "iron supplementation is associated with reduced subjective measures of fatigue but not with objective improvements in physical capacity". This is broadly consistent with our own results for quality of life and peak oxygen consumption.
Authors' conclusions
Implications for practice.
Despite the recommendation for the use of intravenous iron in non‐anaemic, iron‐deficient adults in best practice guidelines (particularly in perioperative medicine (Muñoz 2017; National Blood Authority 2012), our review finds that the evidence underpinning these recommendations is limited. There is low‐quality evidence that intravenous iron may result in a small, clinically insignificant increase in haemoglobin concentration, but the evidence for our other outcomes of interest (ferritin concentration, quality‐of‐life scores, exercise capacity) is of very low quality so we are unable to draw conclusions about these outcomes. Methodological heterogeneity with respect to inconsistent definitions of iron deficiency and anaemia make it difficult to determine which patient populations are likely to benefit from this intervention, or if benefit can be expected at all.
Implications for research.
This review highlights the poor quality of evidence for intravenous iron therapy in non‐anaemic, iron‐deficient adults across a range of laboratory and patient‐centred outcome measures, and a range of different patient populations. Further good‐quality data from randomised controlled trials (RCTs) are required to determine the validity of existing best practice recommendations for the routine correction of non‐anaemic iron deficiency. However, prior to this occurring, it is necessary to more clearly define iron deficiency, and so determine which populations are likely to derive maximum benefit. Certainly, for patients with concomitant inflammation or medical comorbidities, the use of serum ferritin in isolation is potentially problematic, due to loss of discrimination for the detection of iron deficiency. In the context of treatment with intravenous iron, this point is important, as those patients with functional iron deficiency are less likely to respond to oral iron, and will theoretically derive increased benefit from parenteral therapy. With increased awareness of the important role of hepcidin in the pathogenesis of functional iron deficiency, it is likely that future definitions will include this, and other research tools, such as soluble transferrin receptor, as part of defining iron deficiency. Future RCTs should take note of this during planning so as to better contextualise their results in this evolving field.
Acknowledgements
Thanks to Cochrane Injuries, particularly Marialena Trivella (Statistics Editor) and Helen Wakeford (Managing Editor), for their comments through the editorial process. We would also like to thank Jane Falconer and Sarah Dawson, the Information Specialists responsible for the searches.
Special thanks to the peer reviewers Joanne Joseph, Jacquelyn Powers, Ewelina Rogozinska and Noemi Roy, whose comments helped improve this review.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to Cochrane Injuries. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Iron] this term only #2 MeSH descriptor: [Iron Compounds] this term only #3 MeSH descriptor: [Ferric Compounds] this term only #4 MeSH descriptor: [Ferrous Compounds] this term only #5 (iron or ferric* or ferrous) #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Injections, Intravenous] this term only #8 (intravenous* or IV or inject*) #9 #7 or #8 #10 #6 and #9 #11 (nonanemi* or nonanaemi* "non anemi*" or "non anaemi*" or NAID or IDNA) #12 ("no anemia" or "no anaemia" or "not anemic" or "not anaemic" or "without anemia" or "without anemic" or "without anaemia" or "without anaemic") #13 MeSH descriptor: [Iron] this term only and with qualifier(s): [Deficiency ‐ DF] #14 (iron depletion or iron deficien*) #15 MeSH descriptor: [Anemia, Iron‐Deficiency] this term only and with qualifier(s): [Prevention & control ‐ PC] #16 #11 or #12 or #13 or #14 or #15 #17#10 and #16 #18 MeSH descriptor: [Infant] explode all trees #19 MeSH descriptor: [Child] explode all trees #20 neonat* or newborn* or infant* or child* or schoolchild* #21 MeSH descriptor: [Pregnancy] explode all trees #22 pregnan* or postpartum #23 #18 or #19 or #20 or #21 or #22 #24 #17 not #23
Appendix 2. MEDLINE Ovid search strategy
1. Iron/ 2. Iron compounds/ or Ferric Compounds/ or Ferrous Compounds/ 3. (iron or ferric* or ferrous).ti,ab,kw,rn. 4. or/1‐3 5. Injections, Intravenous/ 6. (intravenous* or IV or inject*).tw. 7. or/5‐6 8. 4 and 7 9. (nonan?emi* or non an?emi* or NAID or IDNA).ab,ti. 10. ("no anemia" or "no anaemia" or "not anemic" or "not anaemic" or "without anemia" or "without anemic" or "without anaemia" or "without anaemic").ti,ab. 11. Iron/df 12. (iron depletion or iron deficien*).ti,ab,kf. 13. Anemia, Iron‐Deficiency/pc 14. or/9‐13 15. 8 and 14 16. randomi?ed.ab,ti. 17. randomized controlled trial.pt. 18. controlled clinical trial.pt. 19. placebo.ab. 20. clinical trials as topic.sh. 21. randomly.ab. 22. trial.ti. 23. 16 or 17 or 18 or 19 or 20 or 21 or 22 24. (animals not (humans and animals)).sh. 25. 23 not 24 26. 15 and 25 27. exp infant/ 28. exp child/ 29. (neonat*or newborn* or infant* or child* or schoolchild*).tw. 30. exp pregnancy/ 31. (pregnan* or postpartum).ti. 32. or/28‐31 33. 26 not 32
Appendix 3. Embase Ovid search strategy
1. iron therapy/ 2. iron derivative/ 3. ferric ion/ 4. ferrous ion/ 5. (iron or ferric* or ferrous).ti,ab. 6. or/1‐5 7. exp intravenous drug administration/ 8. (intravenous* or IV or inject*).tw. 9. or/7‐8 10. 6 and 9 11. iron deficiency anemia/ 12. iron deficiency/pc [Prevention] 13. (nonan?emi* or non an?emi* or NAID or IDNA).ab,ti. 14. ("no anemia" or "no anaemia" or "not anemic" or "not anaemic" or "without anemia" or "without anemic" or "without anaemia" or "without anaemic").ti,ab. 15. (iron depletion or iron deficien*).ti,ab. 16. or/11‐15 17. 10 and 16 18. exp Randomized Controlled Trial/ 19. exp controlled clinical trial/ 20. exp controlled study/ 21. comparative study/ 22. randomi?ed.ab,ti. 23. placebo.ab. 24. *Clinical Trial/ 25. exp major clinical study/ 26. randomly.ab. 27. (trial or study).ti. 28. 18 or 19 or 20 or 22 or 23 or 24 or 25 or 26 or 27 29. exp animal/ not (exp human/ and exp animal/) 30. 28 not 29 31. 17 and 30 32. exp infant/ 33. exp child/ 34. (neonat*or newborn* or infant* or child* or schoolchild*).tw. 35. exp pregnancy/ 36. exp postpartum hemorrhage/ 37. (pregnan* or postpartum).ti. 38. or/32‐37 39. 31 not 38
Appendix 4. Web of Science search strategy
#16 #14 Not #15 #15 TI= (mouse OR mice OR rat OR rats) #14 #12 NOT #13 #13 TS=(pregnan* OR postpartum OR neonat* OR newborn* OR infant* OR child* OR schoolchild*) #12 #11 AND #10 #11 TS=HUMAN #10 #9 AND #8 #9 TS=((clinical OR control* OR placebo OR random OR randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random) SAME (trial* or group* or study or studies or placebo or controlled)) #8 #5 AND #6 AND #7 #7 TS= (intravenous* OR IV OR inject*) #6 TS=(ferrous OR ferric OR iron) #5 #1 OR #2 OR #3 OR #4 #4 TS= ("no anemia" OR "no anaemia" OR "not anemic" OR "not anaemic" OR "without anemia" OR "without anemic" OR "without anaemia" OR "without anaemic") #3 TS=(non‐anemic OR non‐anaemic) #2 TS="iron depletion" #1 TS=("iron deficiencies" OR "iron deficiency" OR "iron deficient")
Appendix 5. ClinicalTrials.gov search strategy
Condition or disease = (non anaemic OR non anemic OR non anaemia OR non anemia OR no anemia OR no anaemia OR not anemic OR not anaemic OR without anemia OR without anemic OR without anaemia OR without anaemic) AND Other terms = iron AND (intravenous OR intravenous OR IV OR injection)
Appendix 6. WHO ICTRP search strategy
(non anaemic OR non anemic OR non anaemia OR non anemia OR no anemia OR no anaemia OR not anemic OR not anaemic OR without anemia OR without anemic OR without anaemia OR without anaemic) = condition AND iron = intervention
Data and analyses
Comparison 1. Intravenous iron versus placebo (population).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin concentration taken at the end of follow‐up | 8 | 548 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.65, 5.42] |
| 1.1 Heart failure | 5 | 461 | Mean Difference (IV, Random, 95% CI) | 3.79 [0.40, 7.18] |
| 1.2 Other populations | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 1.41 [‐3.36, 6.18] |
| 2 Overall quality of life measured at the end of follow‐up | 3 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.14, 0.57] |
| 3 Ferritin concentration taken at the end of follow‐up | 7 | 376 | Mean Difference (IV, Random, 95% CI) | 165.72 [33.05, 298.39] |
| 3.1 Heart failure | 4 | 289 | Mean Difference (IV, Random, 95% CI) | 268.94 [264.99, 272.90] |
| 3.2 Other populations | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 33.95 [2.69, 65.20] |
| 4 Peak oxygen consumption taken at the end of follow‐up | 2 | 32 | Mean Difference (IV, Random, 95% CI) | 2.77 [‐0.89, 6.43] |
| 5 Mild adverse effects | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.97, 1.45] |
Comparison 2. Intravenous iron versus placebo (time to end of follow‐up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin concentration taken at the end of follow‐up | 8 | 548 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.65, 5.42] |
| 1.1 < 10 weeks | 4 | 80 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐2.16, 7.96] |
| 1.2 > 10 weeks | 4 | 468 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐0.02, 6.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anker 2009.
| Methods | RCT; parallel‐group (phase III) | |
| Participants | Patients with chronic heart failure as determined by NYHA status or ejection fraction with iron deficiency determined by ferritin < 100 mcg/L or 100‐299 mcg/L if TSAT < 20% . Patients randomised at 75 sites in 11 countries | |
| Interventions | Participants randomised to receive intervention (ferric carboxymaltose, 200 mg) or equivalent volume of placebo (0.9% sodium chloride) in a 2:1 ratio. Dosing was weekly until theoretical repletion achieved (week 8 or week 12), then monthly until end of follow‐up (week 24). | |
| Outcomes | Primary end points were self‐reported patient global assessment and NYHA functional class. Secondary end points included 6MWT distance and HRQoL. We were unable to extract these data due to isolated graphical representation. We were able to extract ferritin, Hb concentration and TSAT at the end of follow‐up and these are included in the review. | |
| Study funding arrangements | Study was sponsored by Vifor Pharma | |
| Author conflicts of interest | Multiple study authors report speaking and advisory fees from multiple pharmaceutical companies, including the study sponsor. Multiple study authors report being employees of the company sponsoring the study. | |
| Sample size | 459 participants were randomised 2:1 to receive the intervention. Of these, a non‐anaemic subgroup that received the intervention (n = 146) or the control (n = 76) treatment was examined. | |
| Notes | Study was registered in a public clinical trials registry (NCT 00520780) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a central interactive voice‐response system, we randomly assigned eligible patients, in a 2:1 ratio, to receive either ferric carboxymaltose (provided by Vifor Pharma) or placebo (normal saline)" Comment: whilst a central interactive voice response system was used, no comment is made on how the sequence was actually determined. |
| Allocation concealment (selection bias) | Low risk | Central interactive voice‐response system used. Site‐specific investigators highly unlikely to have been able to influence this. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Because ferric carboxymaltose is a dark‐brown solution that is easily distinguishable from the saline placebo, study personnel responsible for the preparation and administration of the study drug (including at least one physician) were aware of the group assignments and therefore were not involved in any study assessments. To ensure that patients were unaware of the study drug they were receiving, black syringes were used to administer the study treatment and a curtain (or something similar) was used to shield the injection site from the patient’s view." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants were blinded using opaque syringe and a curtain to conceal the injection site. Hence, self‐reported outcomes can be considered blinded. For other outcomes, outcome assessors were not involved in study drug preparation or administration." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: whilst there was dropout for the study due to participant withdrawal and death, they performed an ITT analysis. Data for key outcome metrics (HRQoL, NYHA classification, 6MWT) only represented graphically, and we could not extract them. |
| Selective reporting (reporting bias) | High risk | Comment: whilst the study included a specific 'non‐anaemic' subgroup, data for all outcomes in this group were not reported for this manuscript. This includes EQ‐5D‐5L, self‐reported global assessment, NYHA functional class and 6MWT. Whilst these data have been reported in designated substudies for non‐anaemic patients, this is not amenable to extraction (Comin‐Colet 2013Filippatos 2013; Van Craenenbroeck 2013). |
| Other bias | Unclear risk | Comment: study authors published protocol ahead of time, and the study was listed on a clinical trials registry. However, the study was drug‐company sponsored, and no reference was made to the conditions of funding. |
Burden 2015a.
| Methods | RCT; parallel‐group (phase III) | |
| Participants | National and international standard endurance runners with iron deficiency as defined by ferritin < 30 mcg/L for women and < 40 mcg/L for men, and non‐anaemic status as defined by Hb > 120 g/L for women and men | |
| Interventions | Single dose of intervention (500 mg ferric carboxymaltose) or equivalent volume of placebo (0.9% sodium chloride) administered after baseline testing. End of follow‐up performed at 4 weeks | |
| Outcomes | Laboratory metrics (serum ferritin, serum iron, TSAT and Hb concentration), hepcidin and VO2 max were recorded. | |
| Study funding arrangements | Study was funded by the Biotechnology and Biological Sciences Research Council. No overt pharmaceutical company funding was identified. | |
| Author conflicts of interest | The study authors declare no competing financial interests. | |
| Sample size | 15 participants were randomised to receive the intervention (n = 7) or the control (n = 8) treatment. | |
| Notes | Study was apparently not registered with a public clinical trials registry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no reference is made to the method of sequence generation despite randomisation apparently having been performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no reference is made to allocation concealment in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Treadmill test 1 was performed before receiving either an intravenous iron injection (500‐ mg Ferinject; Vifor Pharma Ltd., Opfikon, Switzerland) or placebo injection (0.9% sterile saline solution) carried out in hospital conditions and under the supervision of a doctor." Comment: no reference is made to how the characteristic appearance of the intervention drug was concealed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher (N. P.) who performed the iron injection did not disclose to the physiology testers (R. B. and C. P.) which intervention the athlete had received until the end of the trial period." Comment: blinding of outcome assessment appears to have been adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data for all participants are fully reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes are fully reported. |
| Other bias | Low risk | Comment: there appears to be no commercial involvement and the study authors have no competing interests to declare. |
Charles‐Edwards 2019.
| Methods | RCT; parallel‐group (phase III). Single‐centre study | |
| Participants | Patients aged > 21 years with symptomatic congestive heart failure, exercise limitation and iron deficiency (ferritin < 100 mcg/L or 100‐300mcg/L with TSAT < 20%). Patients were enrolled into defined anaemic and non‐anaemic subgroups as per the WHO criteria. | |
| Interventions | Single dose of intervention (ferric carboxymaltose dosed according to the Ganzoni formula) or equivalent volume of placebo (0.9% sodium chloride) administered after baseline testing. End of follow‐up performed at 2 weeks | |
| Outcomes | Primary endpoint was skeletal muscle energetics as assessed by PCR half‐time. Secondary end points were skeletal muscle energetics as reflected by ADP and other phosphate compounds/pH, exercise capacity as measured by 6MWT and VO2 peak on cardiopulmonary exercise testing, symptoms as measured by NYHA classification, QoL as measured by KCCQ, Hb and iron status (ferritin, transferrin saturation, soluble transferrin receptor), pro‐BNP and LVEF. | |
| Study funding arrangements | Study was funded by the British Heart Foundation. No industry support was required. | |
| Author conflicts of interest | 2 of the study authors received speaking fees and research support from Pharmacosmos and Vifor Pharma Pty Ltd. | |
| Sample size | 20 participants in the non‐anaemic subgroup were randomised to receive the intervention (n = 10) or placebo (n = 10) | |
| Notes | Study was prospectively registered with a clinical trials registry (Eudra‐CT 2012‐005592‐13) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the end of the second visit, patients were randomly assigned within 2 strata (anemic or nonanemic) in permuted blocks of 4 to a single total‐repletion dose of IIM or placebo (normal saline)
in a 1:1 ratio. Randomization was performed by a clinical trials pharmacist using an automated web‐based system (Sealed Envelope Ltd)." Comment: random sequence generation appears adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "To achieve blinding, allocated therapy was dispensed by a clinical trials pharmacist to unblinded research nurses..." Comment: allocation concealment achieved through separation of pharmacist and researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "unblinded research nurses... prepared and administered the infusions using opaque intravenous bags and giving sets (Medipak). All other members of the research team vacated the infusion room before the allocated therapy was collected from pharmacy. A curtain shielded the infusion arm from the patient. The unblinded nurse was not involved in assessing end points." Comment: both researchers and participants appear adequately blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: researchers successfully separated from the allocation and administration processes. Participants blinded according to current best practice. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No subject was lost to follow‐up, and only 1 patient did not attend an end‐of‐study visit because of hospitalization. Overall, 4.2% of the analysed data were imputed." Comment: sensitivity analysis of imputed data was consistent with overall result trends. Lost participant was in the anaemic arm, and did not impact on the analysis of the non‐anaemic subgroup. |
| Selective reporting (reporting bias) | Unclear risk | Comment: specific QoL and exercise capacity data were reported for the overall cohort, but no subgroup analysis based on anaemic status was performed. |
| Other bias | Unclear risk | Comment: 2 of the study authors received speaking fees and research funding from pharmaceutical companies. |
Favrat 2014.
| Methods | RCT; parallel‐group (phase III). Undertaken in 21 centres across Europe | |
| Participants | Premenopausal, menstruating women > 18 years of age with symptomatic fatigue but normal or borderline Hb (> 115 g/L) at screening. Iron deficiency was defined as ferritin < 50 mcg/L with TSAT < 20%, or ferritin < 15 mcg/L. | |
| Interventions | Single dose of intervention (ferric carboxymaltose, 1000 mg) or an equivalent volume of placebo (250 mL 0.9% sodium chloride) | |
| Outcomes | Primary endpoint was proportion of women achieving ≥ 1 improvement in Piper Fatigue Score at end of follow‐up (day 56). Secondary endpoints included changes in Piper Fatigue Score, SF‐12 and computerised cognitive tests. Changes in Hb, ferritin and TSAT were also recorded. | |
| Study funding arrangements | Vifor Pharma Pty Ltd sponsored this study and supported the study design. Funding was provided for a clinical research organisation, statistical analysis and manuscript preparation. | |
| Author conflicts of interest | The study authors declare multiple conflicts of interest, having accepted honoraria from Vifor Pharma Pty Ltd and other organisations. | |
| Sample size | 290 participants were randomised to receive the intervention (n = 144) or the control (n = 146) treatment. | |
| Notes | Study registered in a public trials register (NCT 01110356) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation followed a computer‐generated list of random numbers that has been prepared by the clinical research organization using block randomisation with variable block length." |
| Allocation concealment (selection bias) | Low risk | Comment: on the basis of the randomisation method described, there is little interaction between the clinical research organisation and the researchers involved in the screening and enrolment of patients prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "investigators received a set of sealed envelopes that corresponded to a randomisation number and contained the identity of the study drug, and prepared and administered the study drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no comment is made on the blinding of outcome assessors, and the risk of bias is therefore unclear. In particular, there is no specific separation mentioned on separating investigators responsible for preparation of study drug and those responsible for assessing outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: consequences of protocol analysis not reported. Different metrics used when reporting haematinic data |
| Selective reporting (reporting bias) | High risk | Quote: "The most common major protocol deviations were ‘disallowed concurrent medications’ (15 patients in the placebo and 19 patients in the FCM group) and ‘selection criteria not met’ (5 patients in the placebo and 8 patients in the FCM group)." Comment: whilst a per protocol analysis was performed, this was not reported. In addition, haematinic data were reported differently in the participating characteristics and outcomes assessment, precluding extraction. Poor reporting of haematinic follow‐up data. First study author contacted for access to raw data ‐ no response. Data reported as mean (IQR) at baseline, and as mean (SD), or mean (range) at day 56. Poor reporting of haematinic follow‐up data |
| Other bias | High risk | Comment: study author (AM) an employee of supporting pharmaceutical company |
Grote 2009.
| Methods | RCT; parallel‐group (phase III). Undertaken in 3 centres in Sweden | |
| Participants | Patients aged 18‐70 years with restless legs syndrome and iron deficiency. Iron deficiency originally defined as serum ferritin < 30 mcg/L. Later revised to < 45 mcg/L. Hb was not considered as part of study inclusion criteria, but mean Hb at start of study was > 120 g/L. | |
| Interventions | 5 doses of 200 mg iron sucrose (1000 mg total) evenly spread over 2 weeks | |
| Outcomes | Primary outcome measure was IRLS score at end of follow‐up (week 11). Hb and ferritin data were also collected. Adverse event data were collected. | |
| Study funding arrangements | Study supported by Renapharma Pty Ltd, Uppsala, Sweden | |
| Author conflicts of interest | Authors deny any conflicts of interest | |
| Sample size | 86 participants were randomised to receive the intervention (n = 43) or the control (n = 43) treatment. | |
| Notes | Study registered in a public trials register (ISRCTN 82469428). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: no specific reference to type of sequence generation used, but stated to be "random and consecutive". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no clear statement on allocation concealment, and lack of information on sequence generation precludes a "low‐risk" judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Specific logistics were implemented to keep the study blinded to both patients and study personnel. Infusions were prepared by the local pharmacy, infusion bags and disposables were non‐transparent. Infusions and blood chemistry results were supervised by personnel otherwise not involved in the care of the patient." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Specific logistics were implemented to keep the study blinded to both patients and study personnel. Infusions were prepared by the local pharmacy, infusion bags and disposables were non‐transparent. Infusions and blood chemistry results were supervised by personnel otherwise not involved in the care of the patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participant exclusions were accounted for. An ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Comment: all stated outcomes were reported consistently through to end of follow‐up. |
| Other bias | Unclear risk | Comment: unspecified funding was received from Renapharma Pty. Ltd. |
Johansson 2015.
| Methods | RCT; parallel‐group (phase III). Undertaken in a single centre in Denmark | |
| Participants | Patients > 18 years scheduled to undergo elective CABG surgery who were not anaemic according to WHO criteria were included in the study. Data extracted by authors from primary study for iron‐deficient patients (defined as ferritin < 100 mcg/L or 100‐300 mcg/L where TSAT < 20%) | |
| Interventions | Single dose of study drug infused prior to surgery (iron isomaltoside, 20 mg/kg) or equivalent volume of placebo (0.9% sodium chloride) | |
| Outcomes | Primary outcome was a lower decrease in Hb after cardiac surgery. Secondary end points were requirement for allogeneic blood transfusion, iron‐related parameters and safety endpoints. | |
| Study funding arrangements | Study was funded by Pharmacosmos A/S. | |
| Author conflicts of interest | Single (senior) study author is an employee of Pharmacosmos A/S. No further conflicts of interest were declared. | |
| Sample size | 60 participants were randomised 1:1 to receive the intervention (n = 30) or the control (n = 30) treatment. | |
| Notes | Iron deficiency was not considered an inclusion criteria for this study, but we contacted study authors and extracted data for participants who were iron deficient as per the inclusion criteria for this review. Study was registered with a public trials register (NCT 01563367) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Permuted block randomisation with a block size of 4 was used to randomise the patients. The randomisation list was prepared centrally by a contract research organization, Max Neeman International Data Management Centre, using a validated computer program (Statistical Analysis Software [SAS] 9.1.3; SAS Institute Inc., Cary, NC) PROC PLAN procedure. An interactive web response system method was used to randomise the eligible patient to the treatment groups. When the patient data had been entered into the interactive web response system, a unique randomisation number was generated for the patient, identifying which treatment the patient was allocated to." |
| Allocation concealment (selection bias) | Low risk | Quote: "The screening and enrolment of the patients were performed by the investigator at the site, whereas the entering of the patient data into the interactive web response system generating the randomisation number was typically performed by the trial nurse or trial coordinator." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients in the iron isomaltoside 1000 group received iron isomaltoside 1000 as a single‐dose infusion of 1000 mg over 15 min with a maximum single dose of 20 mg/kg. Patients in the placebo group received saline (Natriumklo‐ rid 9 mg/ml; Fresenius Kabi, Copenhagen, Denmark) as a single‐dose infusion of 100 ml over 15 min." Comment: no comment is made in the article on attempts to conceal the characteristic colour of the iron infusion. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no discussion made on involvement of investigators with outcome assessment and administration of presumably unblinded study drug. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: data for NAID participants was provided in full by study authors on request. All outcomes were fully reported. |
| Selective reporting (reporting bias) | Low risk | Comment: Data for NAID participants was provided in full by study authors on request. All outcomes were fully reported. |
| Other bias | High risk | Comment: study was conceived and run by Pharmacosmos A/S. |
Krayenbuehl 2011.
| Methods | RCT; parallel‐group (phase III). Undertaken in 4 centres in a single country | |
| Participants | Premenopausal, menstruating women 18 years of age who presented with fatigue were evaluated for inclusion in the study. Inclusion criteria were serum ferritin concentration < 50 ng/mL, Hb concentration > 120 g/L, and adequate contraception for the study period. | |
| Interventions | 4 infusions containing study drug (200 mg iron sucrose) or an equivalent volume of placebo (0.9% sodium chloride) over 2 weeks | |
| Outcomes | Primary outcome was change in BFI score between baseline and end of follow‐up (week 12). Additional data on Hb, ferritin, TSAT and adverse events were collected. | |
| Study funding arrangements | "This study was funded by Vifor Pharma (Villars‐sur‐Glane, Switzerland). The sponsor of the study was involved in the study design and was responsible for data collection and storage. The study authors had full access to all data and were responsible for the analysis and interpretation of the data presented in this publication." | |
| Author conflicts of interest | 1 study author is a consulting expert for Vifor Pharma in the field of obstetrics and gynaecology. | |
| Sample size | 90 participants were randomised to receive the intervention (n = 43) or the control (n = 47) treatment. | |
| Notes | Study was registered with a public trials register (ISRCTN 78430425) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation schedule was generated by Cardinal Health Germany GmbH (Schorndorf, Germany). In total, 172 randomisation numbers were generated." Comment: no comment made on precise nature of randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Comment: no comment made on nature of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study medication was prepared and administered by a staff member other than the investigator. Both the infusion bag and the injection site were covered and nontransparent tubing was used, ensuring that the patient could not see the infusion solution at any time. The investigator was not present during the infusion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study medication was prepared and administered by a staff member other than the investigator. Both the infusion bag and the injection site were covered and nontransparent tubing was used, ensuring that the patient could not see the infusion solution at any time. The investigator was not present during the infusion." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all exclusions between enrolment and end of study accounted for and ITT/per protocol analyses performed |
| Selective reporting (reporting bias) | High risk | Comment: all outcomes reported. BFI baseline data not reported as median (IQR), but in fatigue form only. Data suggest that iron group may have had lower levels of fatigue at outset of study. P value for this difference not reported. |
| Other bias | High risk | Comment: extensive drug company funding for study. Drug company was sponsor and custodian of data. |
Okonko 2008.
| Methods | RCT; parallel‐group (phase III). Undertaken at 2 centres in a single country | |
| Participants | Participants aged > 21 years with symptomatic congestive heart failure, exercise limitation and iron deficiency (ferritin < 100 mcg/L or 100‐300mcg/L with TSAT < 20%). Hb was not a specific inclusion or exclusion criteria, but a specific non‐anaemic subgroup was examined (defined by Hb > 120 g/L). | |
| Interventions | Weekly infusions of study drug (200 mg iron sucrose) unless ferritin > 500 ng/mL, until ferritin > 500 ng/mL, and then monthly thereafter until follow‐up concluded. Participants in the control group did not receive any intervention. | |
| Outcomes | Primary end point was change in absolute VO2 peak from baseline to end of follow‐up (week 18). Secondary end points relevant to this review included exercise duration, Hb, ferritin and TSAT, NYHA functional class, patient global assessment, MLHFQ score and fatigue score | |
| Study funding arrangements | Study supported by a British Heart Foundation Grant. Study drug was supplied by Vifor Pharma Pty Ltd. | |
| Author conflicts of interest | Some study authors have accepted honoraria from Vifor Pharma Pty Ltd, attended advisory meetings, or spoken at symposia. | |
| Sample size | Participants were randomised in a 2:1 to receive the intervention (n = 12) or the control (n = 5) ‐ this refers to the non‐anaemic subgroup only. | |
| Notes | Study was registered with a public trials register (NCT 00125996). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation was within each center, using random permuted blocks of 6 within each Hb strata. Patients were stratified according to Hb levels ( 12.5 g/dl vs. 12.5 to 14.5 g/dl)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was concealed from the investigators involved in cardiopulmonary exercise testing and echocardiography." Comment: given computer‐generated randomisation within each hospital, allocation was likely concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: 1 group of participants did not receive any therapy, meaning there was no concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: CPET and TTE assessors were blinded, but not participants, therefore, potential for inadvertent unblinding exists. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all outcomes were fully reported for the overall cohort, and associated subgroups including the anaemic and non‐anaemic subgroups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes stated in the protocol reported in full. However, some outcomes relating to QoL scores (patient global assessment, MLHFQ and fatigue scores) were only presented graphically, making data extraction impossible. |
| Other bias | Unclear risk | Comment: multiple involved investigators received drug company funding |
Van Veldhuisen 2017.
| Methods | RCT; parallel‐group (phase III). Participants were enrolled from 28 sites across 9 countries. | |
| Participants | Participants were diagnosed with systolic heart failure, and were treated in an outpatient setting. Participants were required to have iron deficiency as determined by a serum ferritin < 100 ng/mL, or 100‐300 ng/mL where TSAT < 20%. Anaemic patients were not specifically excluded, but the mean Hb of the intervention and control groups was > 120 g/L. | |
| Interventions | Participants were randomised to receive IV iron (ferric carboxymaltose) using a bespoke dosing strategy based on Hb and body weight (500‐1000 mg) with repeat dosing at 6 and 12 weeks, or to placebo (equivalent dose of 0.9% sodium chloride). | |
| Outcomes | Primary endpoint was change in VO2 peak from baseline to week 24. Secondary endpoints includes effects on Hb, ferritin, TSAT and cardiac biomarkers, QoL and safety endpoints. | |
| Study funding arrangements | The study was sponsored by Vifor Pharma, Switzerland. The lead author is an Established Investigator of The Netherlands Heart Foundation. | |
| Author conflicts of interest | Multiple study authors received research funding, consultancy fees or honoraria from Vifor Pharma Pty Ltd. Two study authors are employees of Vifor Pharma Pty Ltd. | |
| Sample size | A total of 172 participants were studied and randomised 1:1 to receive the intervention (n = 86) or the control (n = 86) treatment. | |
| Notes | Study was registered with a public trials register (NCT 01394562). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information is given on the process of randomisation or sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is given on processes or means of ensuring allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: this was an open‐label study. Participants and site personnel were not blinded to the group allocation or the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: whilst VO2 peak was assessed at central laboratory, the clinicians performing the test itself were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: reasons for exclusions were clearly stated, and both full analysis set and per protocol analyses were performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: whilst there was complete outcome reporting, some data (notably TSAT and ferritin) were reported as medial only, with no IQR or other variability data included. |
| Other bias | High risk | Comment: the study was sponsored by Vifor Pharma Pty Ltd, and multiple authors accepted funding from Vifor Pharma Pty Ltd, or are employees of Vifor Pharma Pty Ltd. |
Wong 2016.
| Methods | RCT; parallel‐group (phase III). Single‐centre study | |
| Participants | Chronic heart failure patients being managed in an outpatient setting. Patients had stable disease, but were judged to be iron deficient as per a serum ferritin < 100 mcg/L or < 300 mcg/L where TSAT was < 20%. Anaemic participants were not excluded, and so only data from non‐anaemic participants were extracted in association with the study authors. | |
| Interventions | Single dose of intervention (ferric carboxymaltose, 1000 mg) or an equivalent volume of placebo (250 mL 0.9% sodium chloride) | |
| Outcomes | Primary outcome was change in mean KCCQ score at 2 weeks post‐intervention. Secondary end points were 6MWT distance, PHQ‐9, grip strength, NT‐proBNP, Hb, ferritin and TSAT | |
| Study funding arrangements | No information available on study funding arrangements | |
| Author conflicts of interest | No information available on study funding arrangements | |
| Sample size | 24 non‐anaemic participants were randomised 1:1 to receive the intervention (n = 12) or control (n = 12) treatment | |
| Notes | Study was retrospectively registered on a public trials register (ACTRN 12615000952549) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was generated randomly by computer software performed at the clinical trials pharmacy. Randomization was stratified by NYHA class (I vs II + III)." |
| Allocation concealment (selection bias) | Low risk | Comment: no suggestion that using the technique described that allocation would have been known by the researchers ahead of time. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Clinician was blinded as infusion was administered by a research nurse not involved in data collection/analysis. Subjects were blinded as infusion pump was set up behind a curtain to shield their view." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinician was blinded as infusion was administered by a research nurse not involved in data collection/analysis. Subjects were blinded as infusion pump was set up behind a curtain to shield their view." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[single participant] refused to attend follow‐up on day 7 and refused to perform 6 minute walk test. Data from the walk test and data from day 7 were excluded from analysis." Comment: single participant lost to follow‐up and accounted for in analysis |
| Selective reporting (reporting bias) | High risk | Comment: despite data on haematinic markers and PHQ‐9 being collected, formal results were not available in the data provided by researchers. However, reference is made in the conference abstract notes to there being no difference between groups with respect to these metrics. |
| Other bias | Unclear risk | Comment: no pharmaceutical company involvement implied, but conference abstract lacks an explicit statement indicating independent study. |
Woods 2014.
| Methods | RCT; parallel‐group (phase III). Study conducted in a single centre | |
| Participants | Elite athletes with non‐anaemic iron deficiency (as determined by Hb > 120 g/L and serum ferritin between 30‐100 mcg/L). Patients taking oral iron therapy were excluded from the study. | |
| Interventions | Patients randomised to receive dosing of IV iron (100 mg ferric carboxymaltose) or placebo (0.9% sodium chloride) every 2 weeks for 4 weeks (3 doses) | |
| Outcomes | Primary outcome was improvement in tHb mass at the end of follow‐up. Additional secondary outcome measures included VO2 peak, Brunel Mood Scale and BFI as well as Hb, ferritin and TSAT. We could not extract data on QoL scores due to absence of baseline data and presentation of data not amenable to extraction. VO2 peak data for baseline measurement was obtained after the first dose of iron was given and hence we excluded this metric. | |
| Study funding arrangements | Study funded by a University of Canberra grant for post‐doctoral fellowship research. The funder had no role in study design, data collection, data analysis, decision to publish or preparation of the manuscript. | |
| Author conflicts of interest | No conflicts of interest declared | |
| Sample size | 14 participants were randomised 1:1 to receive the intervention (n = 7) or control (n = 7) treatment | |
| Notes | Study was not registered in a public trials registry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no reference made to the randomisation technique, beyond a statement that it was undertaken |
| Allocation concealment (selection bias) | Unclear risk | Comment: given a random method was allegedly used, presumably the potential for allocation concealment existed. However, as study authors made no specific reference to methods used, the risk of bias is unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Throughout each injection, the participant’s arm was shielded using a screen with the injection solution prepared out of sight. In addition to all athletes, all research staff associated with the testing and training sessions were blinded to the treatment groups and were unable to access blood results, nor preside over the injections. Thus, only the medical staff administering the injections had access to grouping allocation information." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Throughout each injection, the participant’s arm was shielded using a screen with the injection solution prepared out of sight. In addition to all athletes, all research staff associated with the testing and training sessions were blinded to the treatment groups and were unable to access blood results, nor preside over the injections. Thus, only the medical staff administering the injections had access to grouping allocation information." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: all participants completed follow‐up with no missing data. However, as we could not extract some data due to the style of presentation, and the lack of baseline data, we cannot give a judgement of low risk. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all stated outcomes in the article were reported, but as the study was not prospectively registered and we did not see the, the risk of bias remains unclear. |
| Other bias | Low risk | Comment: only issue is the lack of information regarding prospective registration and lack of a protocol. This was addressed in previous domain. |
ADP: adenosine diphosphate; BFI: Brief Fatigue Inventory; BNP: B‐type natriuretic peptide; CABG: coronary artery bypass graft; CPET: cardiopulmonary exercise testing; EQ‐5D‐5L: Euroquol 5 dimension, 5 level quality‐of‐life measure; Hb: haemoglobin; HRQoL: health‐related quality of life; IQR: interquartile range; IRLS: International Restless Legs Syndrome Rating Scale; ITT: intention‐to‐treat; IV: intravenous; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEF: left ventricular ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaire; NAID: Non‐Anaemic Iron Deficiency; NYHA: New York Heart Association; PCR: polymerase chain reaction; QoL: quality of life; PHQ‐9: Patient Health Questionnaire (depression); RCT: randomised controlled trial; SD: standard deviation; SF‐12: 12‐item short form survey; tHb: total haemoglobin; TSAT: transferrin saturation; TTE: transthoracic echocardiogram; VO2 max: peak oxygen consumption; WHO: World Health Organization; 6MWT: 6‐metre walk test;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2011 | Ineligible participant population (unable to determine iron or Hb status) |
| Arutyunov 2009 | Ineligible comparator (comparison of 2 different preparations of IV iron) |
| Baribeault 2011 | Ineligible comparator (comparison of 2 different preparations of IV iron) |
| Bart 2018 | Ineligible participant population (study included iron‐replete participants) |
| Boerboom 2017 | Ineligible comparator (comparison of IV vs oral iron) |
| Boomershine 2018 | Eligible data not available |
| Bruinvels 2017 | Ineligible study design (observational study) |
| Cekic 2015 | Ineligible study design (observational study) |
| Cho 2016 | Ineligible study design (cross‐over trial) |
| Comin‐Colet 2013 | Eligible data not available |
| Deng 2017 | Ineligible participant population (participants received erythropoiesis‐stimulating agents) |
| Filippatos 2013 | Eligible data not available |
| Fishbane 2001 | Ineligible study design (participants received erythropoiesis‐stimulating agents) |
| Fontana 2014 | Eligible data not available |
| Froessler 2016 | Ineligible participant population (included majority anaemic participants) |
| Garvican‐Lewis 2018a | Ineligible participant population (study included iron‐replete participants) |
| Garvican‐Lewis 2018b | Ineligible participant population (study included iron replete‐participants) |
| Gutzwiller 2010 | Ineligible outcomes (no outcomes specified by review protocol reported) |
| Gutzwiller 2012 | Ineligible outcomes (no outcomes specified by review protocol reported) |
| Gybel‐Brask 2018 | Eligible data not available |
| Hsiao 2016 | Ineligible participant population (participants received erythropoiesis‐stimulating agents) |
| Huang 2015 | Ineligible participant population (participants received erythropoiesis‐stimulating agents); ineligible comparator (comparison of 2 different preparations of IV iron) |
| Kapoian 2006 | Ineligible participant population (participants received erythropoiesis‐stimulating agents) |
| Karakas 2019 | Eligible data not available |
| Kulnigg‐Dabsch 2013 | Ineligible participant population (participants were anaemic) |
| Louzada 2016 | Ineligible comparator (comparison of 2 different preparations of IV iron) |
| Oliver 2010 | Ineligible participant population (included majority anaemic participants) |
| Peeling 2007 | Ineligible comparator (IM iron) |
| Ponikowski 2015 | Ineligible participant population (included majority anaemic participants) |
| Reinisch 2014 | Ineligible study design (observational study) |
| Reinisch 2015 | Ineligible participant population (observational study) |
| Rita Gomes 2018 | Ineligible participant population (included majority anaemic participants) |
| Sloand 2004 | Ineligible participant population (participants received erythropoiesis‐stimulating agents) |
| Smith 2009 | Ineligible study design (cross‐over trial) |
| Trenkwalder 2017 | Eligible data not available |
| Van Craenenbroeck 2013 | Eligible data not available |
| Yeo 2018 | Ineligible participant population (included majority anaemic participants) |
Hb: haemoglobin; IM: intramuscular; IV: intravenous;
Differences between protocol and review
The original review protocol (Miles 2018b), specified that only those studies that included participants judged to be non‐anaemic as per World Health Organization criteria (WHO 2001), would be included in the review. Where a non‐gendered definition of non‐anaemia was used (haemoglobin (Hb) > 120 g/L), these studies would also be considered for inclusion. After initial full‐text extraction, it became apparent that multiple studies used alternative criteria to determine if a patient was anaemic, or did not consider haemoglobin concentration at all when including patients in the study, considering iron status alone. Were we to have proceeded with the a priori defined criteria as specified in the protocol, it would have limited our review to 10studies, two in which non‐anaemic status was consistent with WHO criteria (Charles‐Edwards 2019; Krayenbuehl 2011), two others where the study authors agreed to extract data for non‐anaemic participants (Johansson 2015; Wong 2016), four that used non‐WHO definitions of non‐anaemic states (Burden 2015a; Favrat 2014; Grote 2009; Woods 2014), and two that included a non‐WHO compliant, non‐anaemic subgroup from which we could extract data (Anker 2009; Okonko 2008). We elected to include an additional study for whom the mean Hb of the control and intervention groups was > 120 g/L (Van Veldhuisen 2017), but which may have included participants with anaemia at the lower margins of the confidence intervals for Hb distribution. We could have potentially cited this as evidence of indirectness in our review when considering our original research question. However, given the already low‐ or very low‐quality evidence, and the systemic and wide‐ranging nature of the problem, we elected not to.
Included studies did not provide effective discrimination between absolute and functional iron deficiency (Table 2), meaning that we could not effectively apply the a priori criteria we planned to use to differentiate between absolute and functional iron deficiency. As a result of this, we were unable to perform the planned subgroup analysis based on type of iron deficiency.
The study protocol made a priori reference to performing a subgroup analysis based on "time to end of follow‐up", where we would analyse short‐, medium‐ and long‐term time periods. Instead, in this review, we performed this analysis using less than 10 weeks, and equal to or more than 10 weeks as the discriminator. This was due to a lack of consensus within the literature as to the time course for repletion of iron stored in non‐anaemic iron deficiency after a dose of intravenous iron, and the relatively small number of included studies. We selected 10 weeks as the study that we used in the protocol to determine study power for the primary outcome metric (Favrat 2014), suggested that haemoglobin concentration probably plateaued at more than eight weeks after this initial dose. A more nuanced evaluation should be possible as more evidence comes to light, potentially as part of an update to this review.
The sole study that dealt with a perioperative population concerned participants with cardiac surgery (Johansson 2015). We classified this study as pertaining to 'heart failure' as opposed to 'other pathology'.
We were unable to undertake the sensitivity analysis that we specified a priori as no study demonstrated low risk of bias across the five domains, a relatively small number of studies were ultimately included, and the overall quality of evidence for the outcomes of interest were rated as low or very low.
Following expert advice, we translated the standardised mean difference of quality‐of‐life scores into the mean difference of the Piper Fatigue Index, the quality‐of‐life metric used for the most participants included in the analysis.
Contributions of authors
LFM drafted the initial protocol, performed the screening, data extraction and 'Risk of bias' assessment, performed the meta‐analysis and wrote the initial draft of the manuscript.
EL assisted in the revision of the protocol and performed the screening, data extraction and 'Risk of bias' assessment.
GI assisted in the revision of the protocol, performed the Trial Sequential Analysis and assisted in revision of the manuscript.
DS assisted in the revision of the protocol and in the revision of the manuscript.
Sources of support
Internal sources
Centre for Integrated Critical Care, University of Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
LFM: the institution of the author has received in‐kind and unrestricted financial support for two currently running studies from Vifor Pharma Pty Ltd. The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
EL: the institution of the author has received in‐kind support for a study from Vifor Pharma Pty Ltd (Litton 2016). The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
GI: none known
DS: the institution of the author has received in‐kind and unrestricted financial support for two currently running studies from Vifor Pharma Pty Ltd. The author has not personally received any honoraria or speaking fees from any company. No other conflicts of interest.
New
References
References to studies included in this review
Anker 2009 {published data only (unpublished sought but not used)}
- Anker SD, Comin CJ, Filippatos J, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. New England Journal of Medicine 2009;361:2436‐48. [DOI] [PubMed] [Google Scholar]
Burden 2015a {published data only}
- Burden RJ, Pollock N, Whyte GP, Richards T, Moore B, Busbridge M, et al. Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Medicine and Science in Sports and Exercise 2015;47(7):1399‐407. [DOI] [PubMed] [Google Scholar]
Charles‐Edwards 2019 {published data only}
- Charles‐Edwards G, Amarel N, Sleigh A, Ayis A, Catibog N, McDonagh T, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency ‐ FERRIC‐HF II randomized mechanistic trial. Circulation 2019;139:2386‐98. [DOI] [PubMed] [Google Scholar]
Favrat 2014 {published data only}
- Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, et al. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron‐deficient women‐‐PREFER a randomized, placebo‐controlled study. PloS One 2014;9(4):e94217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grote 2009 {published data only}
Johansson 2015 {published data only}
- Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer) reduces postoperative anaemia in preoperatively non‐anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double‐blind placebo‐controlled clinical trial (the PROTECT trial). Vox Sanguinis 2015;109(3):257‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krayenbuehl 2011 {published data only}
Okonko 2008 {published data only}
- Okonko DO, Grzeslo A, Witkowski T, Mandal Ak, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. Journal of the American College of Cardiology 2008; Vol. 51, issue 2:103‐12. [DOI] [PubMed]
Van Veldhuisen 2017 {published data only}
- Veldhuisen DJ, Ponikowski P, Meer P, Metra M, Bohm M, Doletsky A, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; Vol. 136:1374‐83. [DOI] [PMC free article] [PubMed]
Wong 2016 {published data only}
- Wong C, Ng A, Lau J, Kritharides L, Sindone A. Early responses to intravenous iron therapy in patients with chronic heart failure and iron deficiency. Heart, Lung & Circulation 2016;25 (Suppl 2):S107. [DOI] [PubMed] [Google Scholar]
Woods 2014 {published data only}
- Woods A, Garvican‐Lewis LA, Saunders PU, Lovell G, Hughes D, Fazakerley R, et al. Four weeks of IV iron supplementation reduces perceived fatigue and mood disturbance in distance runners. PLoS ONE 2014; Vol. 9, issue 9:e108042. [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Allen 2011 {published data only}
- Allen RP, Butcher A, Du W. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi‐centred, placebo‐controlled preliminary clinical trial. Sleep Medicine 2011;12(9):906‐13. [DOI] [PubMed] [Google Scholar]
Arutyunov 2009 {published data only}
- Arutyunov GP, Bylova NA, Ivleva AY, Kobalava ZD. The safety of intravenous (IV) ferric carboxymaltose versus IV iron sucrose in patients with chronic heart failure (CHF) and chronic kidney disease (CKD) with iron deficiency (ID). European Journal of Heart Failure 2009;21(6):ii71. [Google Scholar]
Baribeault 2011 {published data only}
- Baribeault D. Sodium ferric gluconate (SFG) in complex with sucrose for IV infusion: bioequivalence of a new generic product with the branded product in healthy volunteers. Current Medical Research and Opinion 2011;27(8):1653‐7. [DOI] [PubMed] [Google Scholar]
Bart 2018 {published data only}
- Bart NK, Hungerford SL, Curtis MK, Cheng H‐Y, Southern JL, Petousi N, et al. Effects of modest iron loading on iron indices in healthy individuals. Journal of Applied Physiology 2018;125:1710‐19. [DOI] [PubMed] [Google Scholar]
Boerboom 2017 {published data only}
- Boerboom A, Schijns W, Aarts E, Hunfeld M, Cense H, Vrouenraets B, et al. Optimization of iron supplementation after Roux‐en‐y gastric bypass. Obesity Surgery 2017;27(Suppl 1):200. [Google Scholar]
Boomershine 2018 {published data only}
- Boomershine CS, Koch TA, Morris D. A blinded, randomized, placebo‐controlled study to investigate the efficacy and safety of ferric carboxymaltose in iron‐deficient patients with fibromyalgia. Rheumatology & Therapy 2018;5(1):271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruinvels 2017 {published data only}
- Bruinvels G, Pedlar C, Burden R, Brown N, Butcher A, Chau M, et al. Ironwoman trial: the impact of intravenous iron on exercise performance in iron deficient, exercising women. American Journal of Hematology 2017;92(8):E388. [Google Scholar]
Cekic 2015 {published data only}
- Cekic C, Ipek S, Aslan F, Akpinar Z, Arabul M, Topal F, et al. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterology Research and Practice 2015;9(Suppl 1):S336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2016 {published data only}
- Cho YW, Allen RP, Earley CJ. Clinical efficacy of ferric carboxymaltose treatment in patients with restless legs syndrome. Sleep 2016;25:16‐23. [DOI] [PubMed] [Google Scholar]
Comin‐Colet 2013 {published data only (unpublished sought but not used)}
- Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Luscher TF, Mori C, et al. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. European Heart Journal 2013;34(1):30‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 2017 {published data only}
- Deng Y, Wu J, Jia Q. Efficacy of intravenous iron sucrose in hemodialysis patients with restless legs syndrome (RLS): a randomized, placebo‐controlled study. Medical Science Monitor 2017;23:1254‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippatos 2013 {published data only (unpublished sought but not used)}
- Filippatos G, Farmarkis D, Comin‐Colet JC, Dickstein K, Luscher TF, Willenheimer R, et al. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. European Journal of Heart Failure 2013;15:1267‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fishbane 2001 {published data only}
- Fishbane S, Shapiro W, Dutka P, Valenzuela OF, Faubert J. A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney International 2001;60(6):2406‐11. [DOI] [PubMed] [Google Scholar]
Fontana 2014 {published data only (unpublished sought but not used)}
- Fontana S, Juni P, Niederhauser C, Keller P. Lack of effectiveness of intravenous iron infusion in healthy blood donors with low ferritin: a double‐blind randomized controlled trial. Vox Sanguinis 2014;107(Suppl 1):98. [Google Scholar]
Froessler 2016 {published data only}
- Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Annals of Surgery 2016;264(1):41‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvican‐Lewis 2018a {published data only}
- Garvican‐Lewis LA, Vuong VL, Govus AD, Peeling P, Jung G, Nemeth E, et al. Intravenous iron does not augment the hemoglobin mass response to simulated hypoxia. Medicine and Science in Sports and Exercise 2018;50(8):1669‐78. [DOI] [PubMed] [Google Scholar]
Garvican‐Lewis 2018b {published data only}
- Garvican‐Lewis LA, Vuong VL, Govus AD, Schumacher YO, Hughes D, Lovell G, et al. Influence of combined iron supplementation and simulated hypoxia on the haematological module of the athlete biological passport. Drug Testing & Analysis 2018;10(4):731‐41. [DOI] [PubMed] [Google Scholar]
Gutzwiller 2010 {published data only}
- Gutzwiller FS, Blank PR, Schwenglenks M, Szucs TD. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure in the FAIR‐HF trial. Value in Health 2010;13(7):A353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutzwiller 2012 {published data only}
- Gutzwiller FS, Schwenkglenks M, Blank P R, Braunhofer P G, Mori C, Szucs T D, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR‐HF trial: an analysis for the UK. European Journal of Heart Failure 2012;14(7):782‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gybel‐Brask 2018 {published data only}
- Gybel‐Brask M, Seeberg J, Thomsen Ll, Johansson PI. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron‐deficient blood donors: a randomized double‐blind placebo‐controlled clinical trial. Transfusion 2018;58:974‐81. [DOI] [PubMed] [Google Scholar]
Hsiao 2016 {published data only}
- Hsiao P‐J, Chan J‐S, Wu K‐L, Chiang W‐F, Huang J‐S, Wu C‐C, et al. Comparison of short‐term efficacy of iron sucrose with those of ferric chloride in hemodialysis patients: an open‐label study. Journal of Research in Medical Sciences 2016;21(1):99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang L, Lee D, Kent A, Troster S, MacGinley R, Roberts MA, et al. Effects of intravenous iron carboxymaltose versus iron sucrose on fibroblast growth factor‐23 in patients receiving dialysis: a randomised controlled trial. Nephrology 2015;20(S3):45. [Google Scholar]
Kapoian 2006 {published data only}
- Kapoian T, Coyne DW, Rizkala AR. Safety of administration of IV iron to maintenance haemodialysis patients with elevated serum ferritin and low transferrin saturation: information from the DRIVE trial. Nephrology, Dialysis and Transplantation 2006;21(Suppl 4):iv164. [Google Scholar]
Karakas 2019 {published data only}
- Karakas M. Effect of ferric carboxymaltose on LVEF as assessed by cardiac MRI in patients with chronic heart failure and iron deficiency: a randomized, double‐blinded, placebo‐controlled, dual centre trial. European Journal of Heart Failure 2019;21(S1):175‐6. [Google Scholar]
Kulnigg‐Dabsch 2013 {published data only}
- Kulnigg‐Dabsch S, Schmid W, Howaldt S, Stein J, Mickisch O, Waldhör T, et al. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled ThromboVIT trial. Inflammatory Bowel Diseases 2013;19:1609‐16. [DOI] [PubMed] [Google Scholar]
Louzada 2016 {published data only}
- Louzada Ml, Hsia CC, Al‐Ani F, Ralley F, Xenocostas A, Martin J, et al. Randomized double‐blind safety comparison of intravenous iron dextran versus iron sucrose in an adult non‐hemodialysis outpatient population: a feasibility study. BMC Hematology 2016;16(Mar 11):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2010 {published data only}
- Oliver A, Sierra P, Noe L, Monllau V, Ortiz JC. Efficacy of post‐operative intravenous iron in elective major urological surgery. Transfusion Alternatives in Transfusion Medicine 2010;11(Suppl 2):33. [Google Scholar]
Peeling 2007 {published data only}
- Peeling P, Blee T, Goodman C, Dawson B, Claydon G, Beilby J, et al. Effect of iron injections on aerobic‐exercise performance of iron‐depleted female athletes. International Journal of Sport Nutrition and Exercise Metabolism 2007;17(3):221‐31. [DOI] [PubMed] [Google Scholar]
Ponikowski 2015 {published data only (unpublished sought but not used)}
- Ponikowski P, Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. European Heart Journal 2015;36:657‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinisch 2014 {published data only}
- Reinisch W. Intravenous iron need to maintain stable haemoglobin an open‐label, 1 year extension study of iron isomaltoside 1000 in subjects with inflammatory bowel disease. Journal of Crohn's and Colitis 2014;8(Suppl 1):S247. [Google Scholar]
Reinisch 2015 {published data only}
- Reinisch W, Altorjay I, Zsigmond F, Primas C, Vogelsang H, Novacek G, et al. A 1‐year trial of repeated high‐dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scandanavian Journal of Gastroenterology 2015;50(10):1226‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rita Gomes 2018 {published data only}
- Rita Gomes R, Rodrigues J, Aguiar F, Graca C, Torres A, Araujo I, et al. Iron supplementation in an acute heart failure unit. European Journal of Heart Failure 2018;20(Suppl 1):94. [Google Scholar]
Sloand 2004 {published data only}
- Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double‐blind, placebo‐controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. American Journal of Kidney Diseases 2004;43(4):663‐70. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith TG, Talbot NP, Privat C, Rivera‐Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. Journal of the American Medical Association 2009;302(13):1444‐50. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2017 {published data only}
- Trenkwalder C, Winkelmann J, Oertel W, Virgin G, Roubert B, Mezzacasa A. Ferric carboxymaltose in patients with restless legs syndrome and nonanemic iron deficiency: a randomized trial. Movement Disorders 2017;32(10):1478‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Craenenbroeck 2013 {published data only}
- Craenenbroeck EM, Conraads VM, Greenlaw N, Gaudesius G, Mori C, Ponikowski P, et al. The effect of intravenous ferric carboxymaltose on red cell distribution width: a subanalysis of the FAIR‐HF study. European Journal of Heart Failure 2013;15:756‐62. [DOI] [PubMed] [Google Scholar]
Yeo 2018 {published data only}
- Yeo TJ, Yeo PSD, Hadi FA, Cushway T, Lee KY, Yin FF, et al. Single‐dose intravenous iron in Southeast Asian heart failure patients: a pilot randomized placebo‐controlled study (PRACTICE‐ASIA‐HF). ESC Heart Failure 2018;5(2):344‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abbaspour 2014
- Abbaspour N, Hurrell R, Kelioshadi R. Review on iron and its importance for human health. Journal of Research in Medical Sciences: the Official Journal of Isfahan University of Medical Sciences 2014;19:164‐74. [PUBMED: 24778671] [PMC free article] [PubMed] [Google Scholar]
Avni 2015
- Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clinic Proceedings 2015;90:12‐23. [DOI] [PubMed] [Google Scholar]
Bailie 2012
- Bailie GR. Comparison of rates of reported adverse events associated with I.V. iron products in the United States. American Journal of Health‐System Pharmacy 2012;69(4):310‐20. [DOI] [PubMed] [Google Scholar]
Barberan‐Garcia 2015
- Barberan‐Garcia A, Rodríguez DA, Blanco I, Gea J, Torralba Y, Arbillaga‐Etxarri A, et al. Non‐anaemic iron deficiency impairs response to pulmonary rehabilitation in COPD. Respirology 2015;20:1089‐95. [DOI] [PubMed] [Google Scholar]
Beard 2001
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. Journal of Nutrition 2001;131:S568‐80. [DOI] [PubMed] [Google Scholar]
Beck‐da‐Silva 2013
- Beck‐da‐Silva L, Piardi D, Sodler S, Rohde LE, Pereira‐Barretto AC, Albuquerque D, et al. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. International Journal of Cardiology 2013;168:3439‐42. [DOI] [PubMed] [Google Scholar]
Blaudszun 2018
- Blaudszun G, Munting KE, Butchart A, Gerrard C, Klein AA. The association between borderline pre‐operative anaemia in women and outcome after cardiac surgery: a cohort study. Anaesthesia 2018;73(5):572‐8. [DOI] [PubMed] [Google Scholar]
Burden 2015b
- Burden RJ, Morton K, Richards T, Whyte GP, Pedlar CR. Is iron treatment beneficial in iron‐deficient but non‐anaemic (IDNA) endurance athletes? A systematic review and meta‐analysis. British Journal of Sports Medicine 2015;49:1389‐97. [DOI] [PubMed] [Google Scholar]
Butcher 2017
- Butcher A, Richards T, Stanworth SJ, Klein AA. Diagnostic criteria for pre‐operative anaemia–time to end sex discrimination. Anaesthesia 2017;72:811‐4. [DOI] [PubMed] [Google Scholar]
Cancelo‐Hidalgo 2013
- Cancelo‐Hidalgo MJ, Castelo‐Branco C, Palacios S, Haya‐Palazuelos J, Ciria‐Recasens M, Manasanch J, et al. Tolerability of different oral iron supplements: a systematic review. Current Medical Research and Opinion 2013;29(4):291‐303. [DOI] [PubMed] [Google Scholar]
Cançado 2011
- Cançado RD, Muñoz M. Intravenous iron therapy. Revista Brasileira de Hematologia e Hemoterapia 2011;33:461‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clevenger 2015
- Clevenger B, Mallett SV, Klein AA, Richards T. Patient blood management to reduce surgical risk. British Journal of Surgery 2015;102:1325‐37. [DOI] [PubMed] [Google Scholar]
Copenhagen Trial Unit 2016 [Computer program]
- Copenhagen Trial Unit, Centre for Clinical Intervention Research. Trial Sequential Analysis. Version 0.9.5.5 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2016.
Drakesmith 2012
- Drakesmith H, Prentice AM. Hepcidin and the iron‐infection axis. Science 2012;338:768‐72. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2003
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anaemia of inflammation. Blood 2003;102:783‐8. [DOI] [PubMed] [Google Scholar]
Ganz 2012
- Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harbor Perspectives in Medicine 2012;2:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gereklioglu 2016
- Gereklioglu C, Asma S, Korur A, Erdogan F, Kut A. Medication adherence to oral iron therapy in patients with iron deficiency anemia. Pakistani Journal of Medical Science 2016;32:604‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodnough 2011
- Goodnough LT, Nemeth E, Ganz T. Detection, evaluation and management of iron‐restricted erythropoesis. Network 2011;116:4754‐61. [DOI] [PubMed] [Google Scholar]
Goodnough 2012
- Goodnough LT. Iron deficiency syndromes and iron‐restricted erythropoesis. Transfusion 2012;52:1584‐92. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Higgins 2019b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hinton 2014
- Hinton PS. Iron and the endurance athlete. Applied Physiology, Nutrition and Metabolism 2014;39:1012‐8. [DOI] [PubMed] [Google Scholar]
Houston 2018
- Houston BL, Hurrie D, Graham J, Perija B, Rimmer E, Rabbani R, et al. Efficacy of iron supplementation on fatigue and physical capacity in nonanaemic iron‐deficient adults: a systematic review of randomised controlled trials. BMJ Open 2018;8(8):e019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation and ICH Guidelines. Parexel Barnett: Media, 1996. [Google Scholar]
Imberger 2015
- Imberger G, Gluud C, Boylan J, Wetterslev J. Systematic reviews of anesthesiologic interventions reported as statistically significant: problems with power, precision, and type 1 error protection. Anesthesia and Analgesia 2015;121:1611‐22. [DOI] [PubMed] [Google Scholar]
Jankowska 2016
- Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, Haehling S, Doehner W, et al. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. European Journal of Heart Failure 2016;18(7):786‐95. [DOI] [PubMed] [Google Scholar]
Johnson‐Wimbley 2011
- Johnson‐Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therapeutic Advances in Gastroenterology 2011;4:177‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2009
- Jordan JB, Poppe L, Haniu M, Arvedson T, Syed R, Li V, et al. Hepcidin revisited, disulphide connectivity, dynamics and structure. Journal of Biological Chemistry 2009;284:24155‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kell 2014
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker as it is mainly a leakage product from damaged cells. Metallomics 2014;6:748‐73. [DOI] [PubMed] [Google Scholar]
Klein 2017
- Klein AA, Collier T, Yeates J, Miles LF, Fletcher SN, Evans C. The ACTA PORT‐score for predicting perioperative risk of blood transfusion for adult cardiac surgery. British Journal of Anaesthesia 2017;119(3):394‐401. [DOI: 10.1093/bja/aex205] [DOI] [PubMed] [Google Scholar]
Klip 2013
- Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. American Heart Journal 2013;165:575‐82. [DOI] [PubMed] [Google Scholar]
Krause 2000
- Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz‐Knappe P, et al. LEAP‐1, a novel highly disulphide‐bonded peptide, exhibits antimicrobial activity. FESB Letters 2000;480:147‐50. [DOI] [PubMed] [Google Scholar]
Lim 2018
- Lim J, Miles LF, Litton E. Intravenous iron therapy in patients undergoing cardiovascular surgery: a narrative review. Journal of Cardiothoracic and Vascular Anesthesia 2018;32(3):1439‐51. [DOI] [PubMed] [Google Scholar]
Litton 2013
- Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta‐analysis of randomised clinical trials. BMJ Open 2013;347(August):f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litton 2016
- Litton E, Baker S, Erber WN, Farmer S, Ferrier J, French C, et al. Intravenous iron or placebo for anaemia in intensive care: the IRONMAN multicentre randomized blinded trial. Intensive Care Medicine 2016;42:1715‐22. [DOI] [PubMed] [Google Scholar]
Mascha 2015
- Mascha EJ. Alpha, beta, meta: guidelines for assessing power and type I error in meta‐analyses. Anesthesia and Analgesia 2015;121:1430‐3. [DOI] [PubMed] [Google Scholar]
Melenovsky 2016
- Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. European Journal of Heart Failure 2017;19:522‐30. [DOI] [PubMed] [Google Scholar]
Miles 2018a
- Miles LF, Kunz SA, Na LH, Braat S, Burbury K, Story DA. Postoperative outcome following cardiac surgery in non‐anaemic iron‐replete and iron‐deficient patients ‐ an exploratory study. Anaesthesia 2018;73(4):450‐8. [DOI] [PubMed] [Google Scholar]
Miles 2019a
- Miles LF, Sandhu RN, Grobler AC, Heritier S, Burgess A, Burbury KL, et al. Associations between non‐anaemic iron deficiency and outcomes following surgery for colorectal cancer: an exploratory study of outcomes relevant to prospective observational studies. Anaesthesia and Intensive Care 2019;47(2):152‐9. [DOI] [PubMed] [Google Scholar]
Miles 2019b
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Musallam 2018
- Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned?. Current Medical Research and Opinion 2018;34(1):81‐93. [DOI] [PubMed] [Google Scholar]
Muñoz 2014
- Muñoz M, Gõmez‐Ramírez S, Cuenca J, García‐Erce JA, Iglesias‐Aparicio D, Haman‐Alcober S, et al. Very‐short‐term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 2014;54:289‐99. [DOI] [PubMed] [Google Scholar]
Muñoz 2017
- Muñoz M, Acheson AG, Auerbach M, Besser M, Habler I, Kehlet H, et al. International consensus statement on the peri‐operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233‐47. [DOI] [PubMed] [Google Scholar]
National Blood Authority 2012
- National Blood Authority. Module 2: Perioperative. Patient Blood Management Guidelines 2012.
Nelson 1994
- Nelson M, Bakaliou F, Trivedi A. Iron‐deficiency anaemia and physical performance in adolescent girls from different ethnic backgrounds. British Journal of Nutrition 1994;72:427‐33. [DOI] [PubMed] [Google Scholar]
Nemeth 2004a
- Nemeth E, Rivera S, Gavatan V, Keller C, Taudorf S, Pedersen BK, et al. IL‐6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation 2004;113:1271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemeth 2004b
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090‐3. [DOI] [PubMed] [Google Scholar]
Nemeth 2009
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Hematologica 2009;122:78‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2001
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Journal of Biological Chemistry 2001;276:7806‐10. [DOI] [PubMed] [Google Scholar]
Pasricha 2010
- Pasricha S, Flecknoe‐Brown SC, Allen KJ, Gibsom PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Medical Journal of Australia 2010;193:525‐32. [DOI] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs B. Control of iron deficiency anaemia in low and middle income countries. Blood 2013;121:2607‐17. [DOI] [PubMed] [Google Scholar]
Pratt 2016
- Pratt JJ, Khan KS. Non‐anaemic iron deficiency ‐ a disease looking for recognition of diagnosis: a systematic review. European Journal of Haematology 2016;96(6):618‐28. [DOI: 10.1111/ejh.12645] [DOI] [PubMed] [Google Scholar]
Reveiz 2011
- Reveiz L, Gyte GM, Cuervo LG, Casabuenas A. Treatments for iron‐deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003094.pub3] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rognoni 2016
- Rognoni C, Venturini S, Meregaglia M, Marmifero M, Tarricone R. Efficacy and safety of ferric carboxymaltose and other formulations in iron‐deficient patients: a systematic review and network meta‐analysis of randomised controlled trials. Clinical Drug Investigation 2016;36:177‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rössler 2019
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Simon 2019
Stoffel 2017
- Stoffel N, Cercamondi CI, Brittenham G, Zeder C, Geurts‐Moespot AJ, Swinkels DW. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice‐daily split dosing in iron‐depleted women: two open‐label, randomised controlled trials. Lancet Haematology 2017;4(11):E524‐33. [DOI] [PubMed] [Google Scholar]
Suominen 1998
- Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor‐ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92:2934‐9. [PubMed] [Google Scholar]
von Haehling 2019
- Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC: Heart Failure 2019;7(1):36‐46. [DOI] [PubMed] [Google Scholar]
Weiss 2005
- Weiss G, Goodnough LT. Anaemia of Chronic Disease. New England Journal of Medicine 2005;352:1011‐32. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9(86):e1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization, United Nations University, UNICEF. Iron deficiency anemia: assessment, prevention and control. A guide for programme managers. WHO 2001; Vol. www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/, issue (accessed 24 July 2018).
References to other published versions of this review
Miles 2018b
- Miles LF, Litton E, Imberger G, Story D. Intravenous iron therapy for non‐anaemic iron deficient adults. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD013084] [DOI] [PMC free article] [PubMed] [Google Scholar]


