Characterization of quadruple mutants eliminating ATG1 reveals that prolonged fixed-carbon starvation induces an ATG1 kinase-independent autophagic route in Arabidopsis.
Abstract
Under nutrient and energy-limiting conditions, plants up-regulate sophisticated catabolic pathways such as autophagy to remobilize nutrients and restore energy homeostasis. Autophagic flux is tightly regulated under these circumstances through the AuTophaGy-related1 (ATG1) kinase complex, which relays upstream nutrient and energy signals to the downstream components that drive autophagy. Here, we investigated the role(s) of the Arabidopsis (Arabidopsis thaliana) ATG1 kinase during autophagy through an analysis of a quadruple mutant deficient in all four ATG1 isoforms. These isoforms appear to act redundantly, including the plant-specific, truncated ATG1t variant, and like other well-characterized atg mutants, homozygous atg1abct quadruple mutants display early leaf senescence and hypersensitivity to nitrogen and fixed-carbon starvations. Although ATG1 kinase is essential for up-regulating autophagy under nitrogen deprivation and short-term carbon starvation, it did not stimulate autophagy under prolonged carbon starvation. Instead, an ATG1-independent response arose requiring phosphatidylinositol-3-phosphate kinase (PI3K) and SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE1 (SnRK1), possibly through phosphorylation of the ATG6 subunit within the PI3K complex by the catalytic KIN10 subunit of SnRK1. Together, our data connect ATG1 kinase to autophagy and reveal that plants engage multiple pathways to activate autophagy during nutrient stress, which include the ATG1 route as well as an alternative route requiring SnRK1 and ATG6 signaling.
INTRODUCTION
Plants are often confronted with environmental challenges from which they cannot escape. Accordingly, they have developed sophisticated phenotypic plasticity and evolved a set of cellular and metabolic responses that promote survival under adverse conditions (Zhu, 2016). Energy availability is a key survival determinant for plants, and energy is obtained via both photosynthetic carbon assimilation during the day and continual respiratory oxidation of organic compounds by mitochondria (Smith and Stitt, 2007; Sweetlove et al., 2010; Araújo et al., 2011). Furthermore, when solar energy and carbohydrate limitations become acute, plants catabolize proteins and lipids to release amino acids and fatty acids, respectively, as alternative respiratory substrates (Araújo et al., 2011; Schertl and Braun, 2014; Hildebrandt et al., 2015; Fan et al., 2017).
Although the mechanisms for protein catabolism in plants under energetic stress are not fully understood, organelle-resident proteases and macroautophagy (hereafter referred to as autophagy) emerge as two important routes (van Wijk, 2015; Marshall and Vierstra, 2018b). For example, transcriptome analyses with Arabidopsis (Arabidopsis thaliana) and other plants revealed that the expression of many protease and autophagy genes are up-regulated by nutrient starvations (Contento et al., 2004; Buchanan-Wollaston et al., 2005; Avin-Wittenberg et al., 2015; Durgud et al., 2018; McLoughlin et al., 2018). In agreement, metabolomic profiling implied a direct connection between autophagy and alternative respiration in plants starved for fixed carbon, with defects in autophagy compromising the availability of amino acids and reduced-carbon metabolites followed by energy deprivation and premature senescence (Izumi et al., 2013; Avin-Wittenberg et al., 2015; Barros et al., 2017; Hirota et al., 2018).
Autophagic turnover is achieved by sequestration of proteins, protein complexes, lipid droplets, and even organelles into double membrane-bound vesicles called autophagosomes (Marshall and Vierstra, 2018b). These vesicles arise from cup-shaped phagophores projecting from the endoplasmic reticulum, which upon enclosure deliver cytoplasmic constituents to vacuoles (plants and fungi) or lysosomes (animals). After fusion with the tonoplast, the inner vesicle is released into the vacuole/lysosome as an autophagic body where the limiting membrane and cargo are then degraded by resident hydrolases (Galluzzi et al., 2017).
The AuTophaGy-related (ATG) proteins that drive this process, now in excess of 40 distinct protein groups, were first discovered in yeast (Saccharomyces cerevisiae), with orthologs subsequently identified in animals and plants (Feng et al., 2014; Marshall and Vierstra, 2018b). The set of autophagy components in plants includes (1) factors that initiate autophagy, including the four-subunit ATG1 kinase complex (ATG1/ATG13/ATG11/ATG101) whose activity responds to nutritional cues; (2) proteins that mediate the emergence of the phagophore, including the ATG9/ATG2/ATG18 complex that helps supply membrane during expansion; (3) components that remodel autophagic membranes, including the class III phosphatidylinositol-3-kinase (PI3K) complex composed of VACUOLAR PROTEIN SORTING34 (VPS34), VPS15, ATG6, and ATG14 or VPS38 subunits that generates a phosphatidylinositol-3-phosphate (PI3-P) signature; and (4) an enzymatic cascade related to ubiquitylation that decorates phagophores and autophagosomes with ATG8 attached to phosphatidylethanolamine (PE; Marshall and Vierstra, 2018b). Assembling the ATG8–PE adduct first involves generating a conjugate of ATG12 with ATG5, which in association with ATG16 then provides the ligase activity that attaches PE to ATG8. Once embedded in the emerging phagophore, lipidated ATG8 interacts with a plethora of factors that help drive phagophore expansion, autophagosome maturation, and fusion of autophagosomes with the vacuole as well as with receptors that tether appropriate cargo to the phagophore surface before enclosure (Marshall and Vierstra, 2018b; Marshall et al., 2019).
As a primary route for nutrient recycling, it is not surprising that autophagic flux is tightly coupled to intracellular energy and nutrient availabilities (Russell et al., 2014). In yeast and metazoans, these supplies are sensed by an intricate web of kinases, including the TARGET OF RAPAMYCIN (TOR) kinase and the AMP-ACTIVATED PROTEIN KINASE (AMPK, known as SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE1 [SnRK1] in plants; Dunlop and Tee, 2013; Russell et al., 2014), that converge on the ATG1 kinase to modify its activities through phosphorylation of ATG1 and its regulatory subunit ATG13. For example, AMPK directly phosphorylates the mammalian ATG1 ortholog UNCOORDINATED51-LIKE KINASE1 (ULK1) and mTOR under nutrient starvations to both activate ULK1 (Bach et al., 2011; Kim et al., 2011) and dampen the repressive effects of mTOR on autophagy (Inoki et al., 2003; Gwinn et al., 2008; Soto-Burgos and Bassham, 2017). In addition, the PI3K complex is a direct target of AMPK upon glucose withdrawal. AMPK stimulates PI3K activity by phosphorylating the VPS34 regulator Beclin1 (ATG6 ortholog in mammalian cells) to promote autophagy while simultaneously phosphorylating VPS34 to inhibit its nonautophagic functions (Kim et al., 2013a).
Although most core autophagy components are reasonably well defined in plants (reviewed in Wang et al., 2018 and Marshall and Vierstra, 2018b), how autophagy connects to energy and nutrient supplies is less well understood. Studies in Arabidopsis demonstrated that plants assemble an ATG1 kinase complex architecturally similar to those in mammals, including the ATG13 and ATG101 regulatory subunits and the ATG11 scaffold protein (Suttangkakul et al., 2011; Li et al., 2014). For ATG1, four isoforms are expressed, three of which (ATG1a, ATG1b, and ATG1c) possess both the N-terminal kinase and C-terminal regulatory domains. Unusually, the fourth variant, ATG1t, is missing the regulatory region. ATG1t isoforms truncated near identical positions are evident in a variety of angiosperms and gymnosperms (Suttangkakul et al., 2011), suggesting that ATG1t is not a pseudogene but fulfills a conserved, yet-to-be defined function within seed plants.
The phenotypic function(s) of the ATG1 kinase complex has recently been established through characterizations of Arabidopsis mutants null for the regulatory ATG13 and ATG11 subunits, which showed that the kinase is critical for the growth and survival of plants under nutrient-limiting conditions and for up-regulating autophagy under these conditions (Suttangkakul et al., 2011; Li et al., 2014). As in mammals and yeast, the activity of the complex appears to be controlled by the phosphorylation/dephosphorylation states of ATG1 and ATG13, possibly through the upstream TOR kinase (Suttangkakul et al., 2011). Phosphorylation of ATG1 during fixed-carbon starvation also appears to be driven by SnRK1 through its KIN10 catalytic subunit (Chen et al., 2017). Moreover, nutrient availability regulates the turnover of the ATG1 kinase complex, likely through phosphorylation. Both the ATG1 and ATG13 subunits rapidly disappear when Arabidopsis seedlings are starved of nitrogen and fixed carbon and reappear once favorable nutritional conditions are restored, thus providing a negative feedback mechanism to control autophagic flux (Suttangkakul et al., 2011).
Although the importance of the ATG1 kinase complex during plant autophagy has been confirmed (Suttangkakul et al., 2011; Li et al., 2014), its exact role(s) and targets remain unclear. Here, we generated a quadruple mutant eliminating all four Arabidopsis ATG1 isoforms and analyzed its autophagic response under nutrient limitations. Surprisingly, while we found that ATG1 and its ATG13 and ATG11 regulators are necessary for robust autophagy under nitrogen and short-term fixed-carbon starvations, as expected, they are not required for activating autophagy during prolonged fixed-carbon stress. Instead, an alternative route emerges that requires SnRK1 and PI3K complexes and is possibly regulated through the phosphorylation of ATG6 by the SnRK1 catalytic subunit KIN10. This discovery adds a second, presumably parallel regulatory pathway for initiating autophagy in plants under nutrient stress.
RESULTS
Prolonged Fixed-Carbon Stress Induces Autophagy in the atg11 and atg13ab Mutants
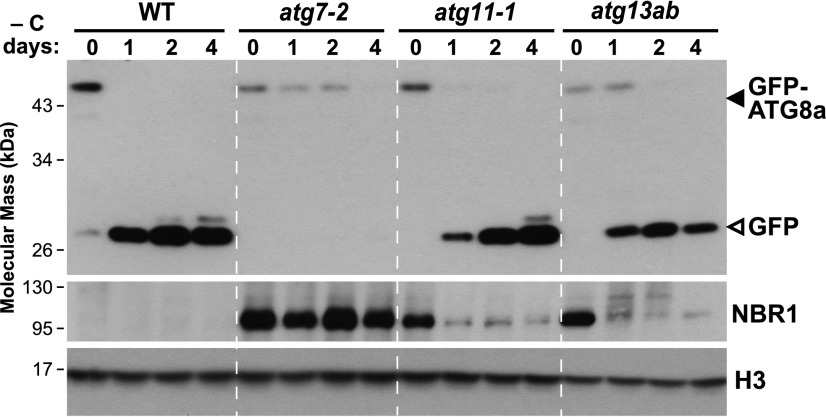
During our previous studies on Arabidopsis mutants null for the ATG11 and ATG13 subunits of the ATG1 kinase complex (atg11-1 and atg13a-1 atg13b-2 [designated as atg13ab]), we discovered that these mutant plants are more tolerant to fixed-carbon starvation induced by darkness than plants lacking core autophagy components, such as the atg7-2 mutant unable to lipidate ATG8 (Suttangkakul et al., 2011; Li et al., 2014). Presuming that this increased tolerance was generated by residual autophagy, we measured autophagic flux in the mutants by the release of free GFP from the GFP-ATG8 reporter that assesses autophagic transport to vacuoles. As demonstrated previously, ATG8 fused to GFP is rapidly degraded once delivered to vacuoles, while the released GFP moiety accumulates due to its relative stability once inside this compartment (Chung et al., 2010; Li et al., 2014; Marshall et al., 2015; Klionsky et al., 2016). As shown in Figure 1, when 1-week-old GFP-ATG8a seedlings grown under a long-day (LD) photoperiod (16 h of light/8 h of dark) without Suc were transferred to extended darkness for 24 h, free GFP readily accumulated in the wild-type background but not in the atg7-2 background. Surprisingly, the release of free GFP was still seen in similarly treated atg11-1 and atg13ab seedlings, indicating that autophagic turnover was retained in these mutants (Figure 1). This accumulation of free GFP was slower in atg11-1 as compared with wild-type or atg13ab seedlings, suggesting that ATG11 aids a rapid autophagic response to fixed-carbon starvation.
Figure 1.
Autophagy Activity in the atg11-1 and atg13ab Mutants under Fixed-Carbon Starvation Induced by Prolonged Darkness.
Wild-type, atg7-2, atg11-1, and atg13ab (atg13a-2 atg13b-1) seedlings were grown on MS solid medium without added Suc under an LD photoperiod for 1 week and then placed in darkness for the indicated times; total seedling extracts were then subjected to immunoblot analysis with anti-GFP and anti-NBR1 antibodies. The degradation of NBR1 and the release of free GFP (closed arrowhead) from the GFP-ATG8a reporter (open arrowhead) were used to indicate autophagic flux. Histone H3 was used to confirm nearly equal protein loading.
Residual autophagy in atg11-1 and atg13ab seedlings was further confirmed by assaying the levels of the autophagic receptor NEXT TO BRCA1 (NBR1)during prolonged dark treatment, which is degraded along with its cargo by autophagy (Svenning et al., 2011). As expected, NBR1 failed to accumulate in untreated wild-type seedlings but accumulated to high levels in atg7-2 seedlings and in wild-type seedlings treated with the vacuolar inhibitor concanamycin A (ConA), which stabilizes autophagic bodies inside vacuoles (Svenning et al., 2011; Supplemental Figure 1). However, a distinct accumulation pattern was seen for the atg11-1 and atg13ab seedlings. Whereas the hyperaccumulation of NBR1 was evident before darkness as in atg7-2 seedlings, extending the dark treatment led to a strong reduction in NBR1 levels in both mutants (Figure 1; Supplemental Figure 2), implying that an autophagic route independent of ATG11 and ATG13 was activated upon prolonged fixed-carbon starvation.
The Different Requirements of ATG11 and ATG13 during Nitrogen and Prolonged Fixed-Carbon Starvations
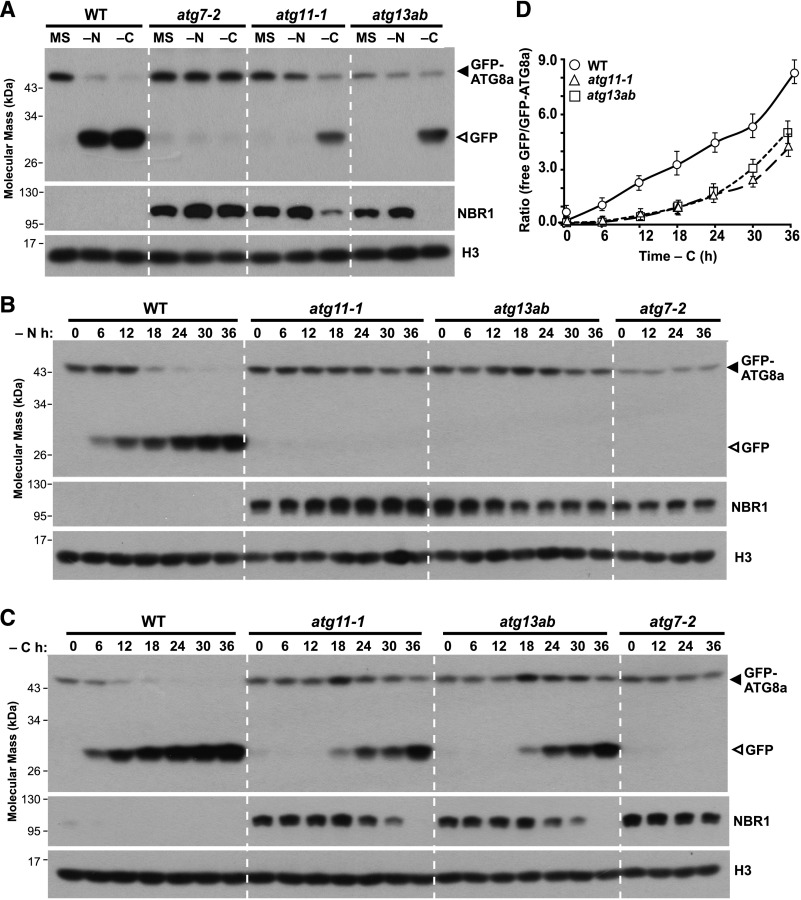
That autophagy occurred in the atg11-1 and atg13ab mutants upon fixed-carbon starvation differed from our previous observations for nitrogen starvation, which was substantially dampened in both lines similar to the strong atg7-2 mutant (Suttangkakul et al., 2011; Li et al., 2014). To compare these autophagic responses, GFP-ATG8a seedlings also harboring the mutations were first grown on solid Murashige and Skoog (MS) medium for 7 d under an LD photoperiod and then transferred to fresh MS liquid medium or medium missing nitrogen or Suc for 24 h. As shown in Figure 2A, free GFP was released from the GFP-ATG8a fusion in wild-type but not in atg7-2 seedlings under both nutrient stresses. By contrast, the accumulation of free GFP from GFP-ATG8a was completely abolished in the atg11-1 and atg13ab mutants starved for nitrogen but was readily detected when the mutants were starved for fixed carbon. Like GFP-ATG8a, the autophagic degradation of NBR1 was also easily observed in both mutants under fixed-carbon starvation.
Figure 2.
Fixed-Carbon Starvation but Not Nitrogen Starvation Induces Autophagy in the atg11-1 and atg13ab Mutants.
(A) Detection of autophagic degradation of GFP-ATG8a and NBR1. One-week-old wild-type, atg7-2, atg11-1, and atg13ab seedlings expressing the GFP-ATG8a reporter were grown on MS solid medium with 1% (w/v) Suc and then transferred to fresh MS liquid medium or medium missing either nitrogen (−N) or Suc (−C) for an additional 24 h. Total seedling extracts were immunoblotted with anti-GFP and anti-NBR1 antibodies. The GFP-ATG8a fusion and free GFP are indicated by closed and open arrowheads, respectively. Histone H3 was used to confirm nearly equal protein loading.
(B) and (C) Time-course analysis of NBR1 degradation and accumulation of free GFP released from GFP-ATG8a reporter during nitrogen or fixed-carbon starvation. One-week-old wild-type, atg7-2, atg11-1, and atg13ab seedlings expressing the GFP-ATG8a reporter were starved for the indicated times; total seedling extracts were then subjected to immunoblot analysis as in (A).
(D) Quantification of the free GFP:GFP-ATG8a ratio during fixed-carbon starvation by densitometric scans of the immunoblots shown in (C). Each data point represents the mean ± sd of three independent biological replicates.
To better compare the different autophagic responses to nitrogen and fixed-carbon starvations in atg11-1 and atg13ab mutants, we first incubated light-grown wild-type seedlings expressing GFP-ATG8a in liquid medium missing nitrogen or Suc for varying lengths of time to determine a condition in which autophagy could be induced to a similar extent. As shown in Figure 2A and Supplemental Figure 3, the impact of fixed-carbon starvation was more evident than that of nitrogen starvation; for example, the GFP:GFP-ATG8a ratio after nitrogen starvation for 30 h was similar to that after fixed-carbon starvation for 24 h. We then directly compared atg11-1 and atg13ab mutants expressing GFP-ATG8a within wild-type and atg7-2 seedlings. For nitrogen starvation, a continuous release of free GFP from GFP-ATG8a was evident in wild-type plants but was not observed in the atg7-2, atg11-1, or atg13ab plants even after 36 h (Figure 2B). Conversely, the accumulation of free GFP in response to fixed-carbon deprivation was reduced in atg11-1 and atg13ab backgrounds during the first 18 h but became robust by 24 h (Figure 2C).
This increased autophagic flux during prolonged fixed-carbon starvation become readily apparent when calculating the ratio of GFP to GFP-ATG8a (Marshall et al., 2015). Well-fed, untreated wild-type seedlings typically had a GFP:GFP-ATG8a ratio of ∼0.4, thus reflecting a baseline of autophagic flux. Consistent with fixed-carbon starvation activating bulk autophagy (Marshall and Vierstra, 2018b), this ratio rose dramatically to ∼3.4 and ∼8.2 after 18 and 30 h, respectively (Figure 2D). On the contrary, the GFP:GFP-ATG8a ratios were ∼0.1 for well-fed atg11-1 and atg13ab seedlings and increased only slightly to 0.8 after 18 h of fixed-carbon starvation. However, after 24 to 36 h of fixed-carbon starvation, the ratios for both mutants increased substantially (∼4.1 and ∼4.9 for atg11-1 and atg13ab seedlings after 36 h of starvation, respectively). Taken together, ATG11 and ATG13 appeared to be required for a rapid autophagic response to fixed-carbon deprivation but became dispensable during a long-term exposure. However, it should be noted that the GFP:GFP-ATG8a signal ratio for seedlings without fixed carbon for 36 h was weaker in atg11-1 and atg13ab lines as compared with the wild type despite being derived from the same transgene (Figures 2A and 2C), suggesting that the autophagic responses to prolonged fixed-carbon starvation were still less than normal.
As GFP in the vacuole was reported to be stabilized by darkness (Tamura et al., 2003), we closely examined the vacuolar activities in seedlings subjected to prolonged darkness to see whether their inhibition caused the accumulation of free GFP in atg11-1 and atg13ab lines. We first exposed wild-type seedlings expressing GFP-ATG8a to fixed-carbon starvation with or without the addition of ConA for varying lengths of time. During the first 30 h, the autophagic degradation of GFP-ATG8a and the free GFP:GFP-ATG8s ratios were more evident in seedlings without ConA (Supplemental Figures 4A and 4B), indicating that the vacuolar activities in seedlings treated with prolonged fixed-carbon starvation were higher than in seedlings with ConA. We further examined the autophagic degradation of NBR1 and GFP-ATG8a in atg11-1 and atg13ab lines subjected to nitrogen or fixed-carbon starvation with or without ConA. As shown in Supplemental Figure 4C, free GFP was undetectable after nitrogen starvation even with ConA addition but was readily seen after prolonged fixed-carbon starvation regardless of ConA treatment, suggesting that the autophagic activities in atg11-1 and atg13ab mutants were indeed induced by prolonged darkness.
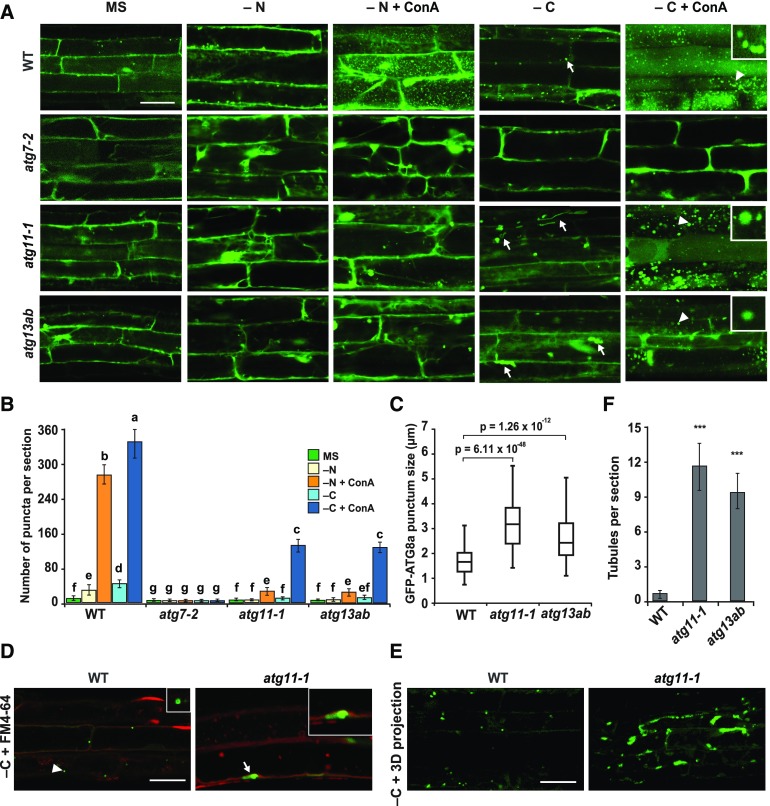
For further confirmation, we examined the autophagic vesicle formation directly in the atg11-1 and atg13ab mutants by confocal fluorescence microscopy of root cells expressing GFP-ATG8a. Whereas numerous autophagic bodies accumulated in the central vacuole of wild-type cells upon nitrogen or fixed-carbon starvation and ConA treatment, these puncta were not evident in similarly treated atg7-2 cells, as expected for a strong block in autophagy. Conversely, atg11-1 and atg13ab cells accumulated few GFP-ATG8a puncta when starved for nitrogen but accumulated substantially more when starved for fixed carbon, albeit at approximately threefold lower levels than those from the wild type (Figures 3A and 3B). The dampened accumulation of GFP-ATG8 puncta in root cells starved for nitrogen and supplemented with ConA was consistent with the results of the GFP-ATG8 cleavage assay (Figures 2B and 2C), which further suggests that the vacuolar inhibition by prolonged dark treatment is not the major reason underpinning the reduced levels of autophagy in the atg11-1 and atg13ab mutants.
Figure 3.
GFP-ATG8a-Containing Vesicles Are Delivered to the Vacuole upon Fixed-Carbon Starvation of atg11-1 and atg13ab Mutants.
(A) Deposition of autophagic bodies inside the central vacuole. Seedlings expressing GFP-ATG8a were grown for 6 d on MS solid medium with 1% (w/v) Suc and then transferred to fresh MS or nitrogen- (−N) or sucrose-deficient (−C) liquid medium with or without the addition of 1 μM ConA for 24 h before confocal fluorescence microscopic analysis of root cells. Lines tested include the wild type and the atg7-2, atg11-1, and atg13ab mutants. Abnormal tubular structures are indicated by white arrows. Bar = 10 μm.
(B) Numbers of puncta per section in the root cells of the wild-type, atg7-2, atg11-1, and atg13ab seedlings used in (A). The data represent average values calculated from three independent experiments; n = 45 sections per treatment per genotype. Letters indicate values that are statistically different from one another (P < 0.05) as determined using two-way ANOVA followed by multiple-comparisons Tukey’s test. Error bars represent sd.
(C) Size of autophagic bodies under sucrose-deficient conditions in (A). P values were determined by Student’s t test; n = 300 autophagic bodies per genotype randomly selected from at least 15 different regions within five sections from each of three independent experiments.
(D) and (E) Autophagosome-related tubular structures accumulate in the atg11-1 mutant. Six-day-old transgenic seedlings were exposed for 16 h to sucrose-deficient liquid medium (−C) and then stained by FM4-64 for 1 h to label tonoplast before observation (D). 3D projections shown in (E) were generated by using 30 slices collected in a total thickness of 30 μm. Consistent results were obtained from three independent experiments. Bars = 10 μm.
(F) Number of abnormal tubular structures per section seen by Z stack projection for roots exposed to the −C conditions used in (E). ***, P < 0.01, Student’s t test; n = 30 sections from three independent experiments per genotype. Error bars represent sd.
Interestingly, the autophagic bodies in atg11-1 and atg13ab roots starved for fixed carbon were significantly larger than those in wild-type roots (3.1 and 2.1 μm in average diameter for the atg11-1 and atg13ab lines, respectively, versus 1.7 μm for the wild type; Figures 3A and 3C). Similarly dilated autophagic bodies were also evident in cells from atg11-1 hypocotyls (Supplemental Figure 5). In addition, we saw the accumulation of extended tubules decorated with GFP-ATG8a (as confirmed from Z-stacked projections) in atg11-1 and atg13ab roots after fixed-carbon starvation (Figures 3A and 3D to 3F; Supplemental Movies 1 and 2). Simultaneous imaging with the endocytic membrane marker FM4-64 clearly showed that these tubules resided within the cytosol and thus were not abnormal autophagic bodies. Collectively, our observations implied that an ATG11- and ATG13-independent autophagic route exists that is active during prolonged fixed-carbon starvation but not during prolonged nitrogen starvation.
Reverse Genetic Analysis of ATG1
We considered two possible explanations for the partial recovery of autophagy in atg11-1 and atg13ab plants during prolonged fixed-carbon starvation. It could either reflect basal activity for ATG1 in the absence of ATG11 and ATG13 or an ATG1 kinase-independent mechanism for autophagy initiation. To assess these possibilities, we examined the role of the ATG1 subunit directly via a quadruple mutant assembled from the Arabidopsis T-DNA insertion collections that compromised the expression of all four ATG1 isoforms (Supplemental Figure 6). The atg1a-2 allele used here was previously described by Suttangkakul et al. (2011); its T-DNA interrupts the 11th exon, leading to a failure to express the full-length transcript. In our study, exonic T-DNA insertions were found for ATG1b and ATG1c, while an intronic insertion was found for ATG1t (Supplemental Figure 6A). RT-PCR analyses of homozygous mutant seedlings confirmed that the new mutations (atg1b-1, atg1c-1, and atg1t-1) all blocked transcription across the respective insertion sites and prevented synthesis of full-length mRNAs, strongly implying that these mutants also represent strong, if not null, alleles. However, the three lines did accumulate low levels of transcript from either upstream or downstream of the insertion sites, suggesting that the expression of shorter protein fragments was possible for each (Supplemental Figure 6B).
Assembly of the ATG12-ATG5 and ATG8-PE adducts is essential for ATG8-mediated autophagy. From analysis of the four single mutants and higher-order mutant combinations, including the atg1abct quadruple mutant (atg1a-2 atg1b-1 atg1c-1 atg1t-1), we found that ATG1, like ATG11 and ATG13 (Suttangkakul et al., 2011; Li et al., 2014), is not necessary for the synthesis of the ATG12-ATG5 adduct. Whereas the atg7-2 mutant blocked assembly of this 50-kD species and left only the free ATG5 protein present, none of the atg1 mutants blocked its accumulation (Supplemental Figure 7A). Similarly, we found that ATG1 is not necessary for the lipidation of ATG8. The ATG8-PE adduct was readily detected in atg1abc and atg1abct seedlings as in wild-type seedlings as a faster migrating species upon SDS-PAGE of membrane preparations in the presence of urea, which was eliminated upon treatment with phospholipase D (Supplemental Figure 7B). However, the atg1abc triple and atg1abct quadruple mutants, like the atg11-1 and atg13ab mutants (Suttangkakul et al., 2011; Supplemental Figure 7A), did have elevated levels of total ATG8 protein as compared with the wild type and various atg1 single and double mutants (Supplemental Figure 7A), implying that the turnover of ATG8 during its autophagic functions is compromised in the higher-order atg1 mutant lines.
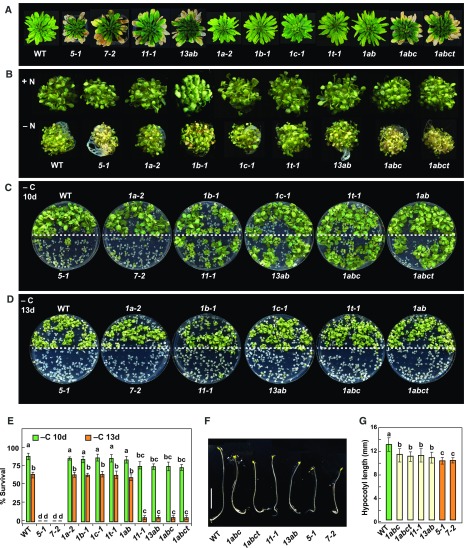
To define the importance of ATG1 in Arabidopsis development, we compared the growth of the atg1 mutant collection under nutrient-rich and nitrogen or fixed-carbon starvation conditions. Like other well-characterized atg mutants (Thompson et al., 2005; Phillips et al., 2008; Chung et al., 2010; Suttangkakul et al., 2011), the homozygous atg1abct quadruple mutant displayed accelerated leaf senescence under short-day (SD) conditions (8 h of light/16 h of dark) and a hypersensitivity to nitrogen limitation. These phenotypes were not evident in the atg1a-2, atg1b-1, atg1c-1, or atg1t-1 single mutants nor in the double mutant combinations, strongly suggesting that the four ATG1 isoforms are functionally redundant (Figures 4A and 4B). Similar to green atg11-1 and atg13ab seedlings, green atg1abct quadruple seedlings withstood the lack of Suc and extended darkness better than atg5-1 and atg7-2 seedlings but less so than wild-type seedlings (Figures 4C to 4E). Whereas most atg5-1 and atg7-2 seedlings died within 10 d, most of the atg1abc triple and atg1abct quadruple mutants died after 13 d. Again, the wild type and the atg1 single and atg1ab double mutants easily survived past 13 d of fixed-carbon deprivation.
Figure 4.
atg1 Mutants Display Phenotypes Characteristic of Autophagy Mutants.
(A) Accelerated senescence. atg mutants alongside the wild type were grown at 22°C on soil in an SD photoperiod for 10 weeks.
(B) Enhanced sensitivity to nitrogen starvation. Wild-type and atg mutants seedlings were grown for 1 week on MS liquid medium under continuous light conditions and then transferred to fresh MS medium (+N) or N-deficient (–N) medium for an additional 2 weeks.
(C) and (D) Enhanced sensitivity to fixed-carbon starvation. Wild-type and atg mutant seedlings were grown under an LD photoperiod on MS solid medium without added Suc (−C) for 2 weeks, transferred to darkness for 10 d (C) or 13 d (D), and then allowed to recover in LD conditions for 12 d.
(E) Survival of the fixed-carbon-deprived plants shown in (C) and (D). The data represent average values calculated from three independent experiments; n = 25 seedlings per genotype. Letters indicate values that are statistically different from one another (P < 0.05) as determined using two-way ANOVA followed by multiple-comparisons Tukey’s test. Error bars represent sd.
(F) and (G) Suppressed hypocotyl elongation. Wild-type and atg mutant seedlings were grown in continuous darkness on MS solid medium without Suc for 1 week. Representative photographs of the etiolated seedlings are shown in (F), and measurement of hypocotyl length performed using ImageJ is shown in (G). The data represent average values calculated from three independent experiments; n = 25 seedlings per genotype. Letters indicate values that are statistically different from one another (P < 0.05) as determined using one-way ANOVA followed by multiple-comparisons Tukey’s test. Error bars represent sd. Bar in (F) = 5 mm.
The phenotypic differences between the atg1 mutants and mutants with strongly compromised autophagy, such as atg7-2 and atg5-1, were also evident in seedlings kept in darkness from the onset of germination. Here, the lengths of etiolated hypocotyls from the atg11-1 and atg1abct lines were intermediate to those from the wild type and the atg5-1 and atg7-2 lines (Figures 4F and 4G). The suppressed elongation of the atg mutants presumably arose from impaired mobilization of nutrient reserves when autophagy was blocked (Avin-Wittenberg et al., 2015). The results from the fixed-carbon starvation and germination assays showed that eliminating ATG1 does not compromise autophagy as strongly as mutations disrupting components needed for ATG8/12 conjugation.
Both the unusual architecture of ATG1t and its widespread distribution among seed plants raised the possibility that this truncation has a unique role in autophagic regulation (Suttangkakul et al., 2011). Yeast two-hybrid (Y2H) assays showed that ATG1t, like ATG1a and ATG1b, interacts with its complex partner ATG13a when tested in the AD orientation (Supplemental Figure 8A). The DNA binding domain orientation self-activated the Y2H assay at least for ATG1b and ATG1c. ATG1a also interacts with ATG8 through a C-terminal ATG8-interacting motif (AIM), which allows it to bind to autophagic vesicles and eventually be consumed by autophagy (Li et al., 2014). Such binding was not evident for ATG1t, consistent with it missing the region that bears the AIM within ATG1a (Supplemental Figure 8A), suggesting that ATG1t does not independently bind autophagic surfaces and is not similarly regulated. Consistent with this possibility, a fusion of ATG1t to YFP was not found in vacuoles by confocal fluorescence microscopy upon exposing seedlings to nitrogen starvation and ConA (Supplemental Figure 8C).
As of yet, the functions of ATG1t are unclear despite its conservation within seed plants, with the likelihood that it has a minor role in regulating autophagy. For example, detailed phenotypic examination of YFP-ATG1t lines that might overexpress ATG1t, the single atg1t-1 mutant, and various combinations of atg1t-1 with mutations disrupting the other ATG1 isoforms (atg1a-1, atg1b-1, and atg1c-1), along with phenotypic comparisons of the atg1abc triple mutant with the atg1abct quadruple mutant, revealed no unusual phenotypes that could be attributed to ATG1t specifically (Figure 4; Supplemental Figure 8D). In fact, the atg1abc and atg1abct lines were phenotypically indistinguishable when either grown under nutrient-replete conditions or starved for nitrogen or fixed carbon (Figure 4).
Autophagy Activity in atg1 Mutants
We further assayed the impact of ATG1 during nitrogen or fixed-carbon starvation by the free GFP-release assay and confocal fluorescence microscopy of mutant lines expressing GFP-ATG8a. Like those seen for the atg11-1 and atg13ab mutants (Figures 2B and 2C), immunoblot assays for the autophagic degradation of NBR1 and GFP-ATG8a fusion in the atg1abc triple and atg1abct quadruple mutants showed that autophagic turnover was still induced by prolonged fixed-carbon starvation but not by nitrogen or short fixed-carbon starvation (Figure 5A; Supplemental Figure 9A). The degradation of NBR1 and the accumulation of free GFP were completely abolished in the atg1 triple and quadruple mutants upon nitrogen starvation regardless of ConA treatment but were still evident upon extended darkness and the lack of Suc (Figures 5A and 5C; Supplemental Figure 4D). Fluorescence microscopy detection of autophagic bodies provided similar results. Whereas few GFP-ATG8a-labeled puncta were seen in the central vacuole of atg1abc and atg1abct root cells pretreated with ConA and starved for nitrogen, they were significantly more abundant when the mutants were subjected to prolonged fixed-carbon starvation (Figures 5B and 5D). Interestingly, unlike atg11-1 and atg13ab mutants, which accumulated abnormally large GFP-ATG8a-decorated autophagic bodies and extended tubules, the autophagic bodies in the atg1 mutants appeared normal in size and accumulated fewer of these tubules (Supplemental Figures 9B and 9C). Together, our assays with the GFP-ATG8 reporter in the quadruple mutant were in support of an autophagic route independent of the ATG1 kinase.
Figure 5.
Prolonged Fixed-Carbon Starvation Induces Autophagy in the atg1 Mutants.
(A) Time-course analysis of NBR1 degradation and accumulation of free GFP released from GFP-ATG8a reporter during nitrogen (−N) or fixed-carbon (−C) starvation. One-week-old atg1abc and atg1abct seedlings expressing the GFP-ATG8a reporter were grown on MS solid medium with 1% (w/v) Suc and then were transferred to fresh medium missing either nitrogen or Suc for the indicated times. Total seedling extracts were then subjected to immunoblot analysis as in Figure 2A.
(B) Deposition of autophagic bodies inside the vacuole. Six-day-old seedlings expressing GFP-ATG8a were treated and observed as in Figure 3A. Bar = 10 μm.
(C) Quantification of the free GFP:GFP fusion ratio of the GFP-ATG8a reporter upon fixed-carbon starvation. Levels of free GFP and the GFP fusion were determined by densitometric scans of the immunoblots shown in (A). Each data point represents the mean ± sd of three independent biological replicates.
(D) Numbers of puncta per section in the root cells of the wild-type, atg1abc, and atg1abct seedlings used in (B). The data represent average values calculated from three independent experiments; n = 45 sections per treatment per genotype. Letters indicate values that are statistically different from one another (P < 0.05) as determined using two-way ANOVA followed by multiple-comparisons Tukey’s test. Error bars represent sd.
Ammonium Fails to Stimulate ATG1-Independent Autophagy
Prior studies with mammalian ULK1 (ATG1) revealed that ammonia, a by-product of amino acid catabolism that rises during glucose deprivation, induces a ULK1-independent autophagic route (Cheong et al., 2011). To assess whether the ATG1 kinase-independent route seen here in Arabidopsis was similarly impacted by ammonia, we first compared ammonia concentration in the wild type versus mutants compromised in the kinase. Analysis of 1-week-old seedlings grown under an LD photoperiod revealed no differences in ammonia content between the wild type and atg11-1, atg13ab, or atg1abct (Supplemental Table 1). Ammonia levels did rise slightly after a 24-h dark treatment of the wild type, but the mutants responded similarly.
To assess whether exogenous ammonia could stimulate autophagy, we transferred 1-week-old GFP-ATG8a seedlings to fresh MS liquid medium supplemented with 20 mM ammonium chloride (total NH4+ = 40 mM) and assayed for autophagy activity via the free GFP-release assay. Free GFP did not accumulate from the GFP-ATG8a reporter in either atg11-1 or wild-type seedlings even after a 24-h exposure (Supplemental Figure 10). Based on these results, we concluded that increased ammonia was not responsible for the autophagic response seen for the ATG1 kinase mutants after prolonged fixed-carbon starvation.
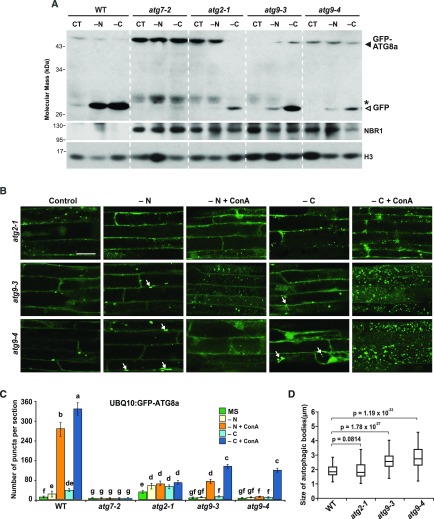
Prolonged Fixed-Carbon Starvation-Induced Autophagy Requires PI3K but Not ATG9
To uncover autophagic components that might be involved in this ATG1-independent route, we tested the response of other Arabidopsis atg mutants. Our first focus was on the ATG9/ATG2/ATG18 complex, given its role in membrane delivery and our data suggesting that autophagosome assembly is impaired in atg11-1 and atg13ab seedlings, as seen by the appearance of dilated autophagic bodies and tubules (Figures 3A, 3C, 3D, and 3F). However, both the free GFP-release assay and imaging of GFP-ATG8 by confocal fluorescence microscopy showed that homozygous atg2-1, atg9-3, and atg9-4 seedlings still accelerated autophagy under prolonged fixed-carbon starvation, albeit not to the same degree as in the wild type (Figure 6A; Supplemental Figure 11). The accumulation of free GFP was strongly dampened in the mutant seedlings under nitrogen starvation but evident upon prolonged fixed-carbon starvation (Figure 6A). Numerous fluorescent puncta were also seen in the vacuoles from atg9-3 and atg9-4 root cells upon fixed-carbon starvation and ConA treatment, but at approximately threefold lower levels than seen in wild-type cells (Figures 6B and 6C).
Figure 6.
Prolonged Fixed-Carbon Starvation Induces Autophagy in the atg2 and atg9 Mutants.
(A) Detection of autophagic degradation of NBR1 and GFP-ATG8a. One-week-old wild-type, atg2-1, atg9-3, and atg9-4 seedlings expressing the GFP-ATG8a reporter were grown on MS solid medium with 1% (w/v) Suc and then transferred to fresh MS liquid medium or medium missing either nitrogen (−N) or Suc (−C) for an additional 24 h; total seedling extracts were then subjected to immunoblot analysis as in Figure 1. CT, untreated controls.
(B) Deposition of autophagic bodies inside the vacuole of atg2 and atg9 mutants upon nitrogen or fixed-carbon starvation. atg2-1, atg9-3, and atg9-4 seedlings expressing GFP-ATG8a reporter were treated and visualized as in Figure 3A. Abnormal tubular structures are indicated by white arrows. Bar = 10 μm.
(C) Numbers of puncta per section in the root cells of the wild-type, atg7-2, atg2-1, atg9-3, and atg9-4 seedlings used in (B). The data represent average values calculated from three independent experiments; n = 45 sections per treatment per genotype. Letters indicate values that are statistically different from one another (P < 0.05) as determined using two-way ANOVA followed by multiple-comparisons Tukey’s test. Error bars represent sd.
(D) Size of autophagic bodies in the vacuoles of wild-type, atg2-1, atg9-3, and atg9-4 seedlings. P values were determined by Student's t test; n = 300 autophagic bodies per genotype randomly selected from at least 15 different sections with five sections from each of three independent experiments.
In agreement with Zhuang et al. (2017) and our own results from atg11-1 and atg13ab plants (Figure 3A), we often detected abnormal tubular structures decorated by GFP-ATG8a in atg9-3 and atg9-4 root cells under both nitrogen and fixed-carbon starvations (white arrows, Figure 6B). Autophagic bodies were similarly dilated (average diameters of 2.50 μm for atg9-3 and 2.72 μm for atg9-4 versus 1.81 μm for the wild type; Figure 6D). GFP-ATG8-labeled puncta were also frequently detected in the atg2-1 root cells regardless of treatment. However, these puncta were unusual in that they often failed to concentrate in the vacuole even after ConA treatment (Figures 6B and 6C), were not enlarged like those in the atg9-3 and atg9-4 backgrounds (1.79 μm diameter), and rarely appeared as tubules. Taken together, it appears that ATG9, like the ATG1 kinase complex, is important but not essential for the autophagy route induced upon prolonged fixed-carbon starvation, while the role(s) of ATG2 in such a route remains unclear.
We next examined the role(s) for the PI3K complex in activating autophagy during prolonged fixed-carbon starvation, given its ability to remodel autophagic membranes. Unfortunately, genetic investigations of this complex in Arabidopsis are hampered by the fact that all currently known homozygous mutants missing the catalytic VPS34 subunit and its VPS15 (Xu et al., 2011) and ATG6 cofactors are inviable (Fujiki et al., 2007; Qin et al., 2007; Harrison-Lowe and Olsen, 2008). For the fourth subunit (VPS38 or ATG14), there are mutants available for VPS38, but unfortunately, they display additional defects related to membrane trafficking (Lee et al., 2018; Liu et al., 2018).
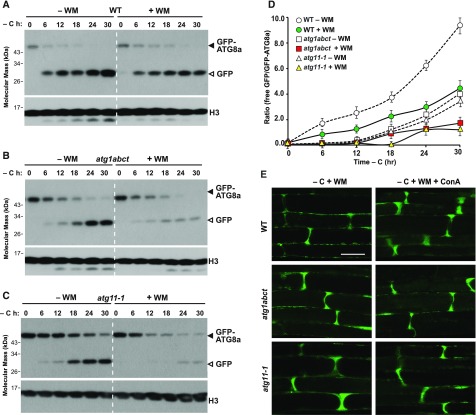
As an alternative, we used wortmannin (WM), a potent inhibitor of mammalian and plant PI3Ks (Matsuoka et al., 1995), which effectively blocks autophagy in Arabidopsis (Takatsuka et al., 2004; Zhuang et al., 2013; Shin et al., 2014). Upon incubating seedlings expressing GFP-ATG8a in darkness with sucrose-deficient liquid medium supplemented with 30 μM WM, we found that the drug slightly dampened the appearance of free GFP released from the GFP-ATG8a reporter in the wild type (Figure 7A). This inhibition became noticeably stronger in the atg1abct and atg11-1 mutant backgrounds, especially after prolonged fixed-carbon starvation, when little free GFP appeared (Figures 7B and 7C). However, the efficacy of WM was not restricted to fixed-carbon starvation but was also apparent in roots starved for nitrogen. As seen by confocal microscopy of GFP-ATG8a in atg1abct, atg11-1, and wild-type root cells, WM blocked the formation of autophagic bodies under both nitrogen and fixed-carbon starvations, with most of the fluorescence remaining diffuse within the cytosol in all three genotypes (Figure 7E; Supplemental Figure 12). Taken together, we concluded that formation and/or delivery of autophagosomes to vacuoles generally rely on PI3-P, presumably synthesized by the VPS34 kinase, and are not specific to prolonged fixed-carbon starvation.
Figure 7.
The PI3K Complex Is Required for the Autophagy Induced by Prolonged Fixed-Carbon Starvation.
(A) to (C) Effect of WM on the accumulation of free GFP released from GFP-ATG8a reporter during fixed-carbon starvation. One-week-old wild-type (A), atg1abct (B), and atg11-1 (C) seedlings expressing GFP-ATG8a were grown on MS solid medium with 1% (w/v) Suc and then transferred to sucrose-deficient liquid medium in darkness with or without the addition of 30 μM WM for the indicated times. Immunoblot analysis of total seedling extracts was performed as in Figure 2A. The GFP-ATG8a fusions and free GFP are indicated by closed and open arrowheads, respectively. Immunoblot detection of histone H3 was used to confirm nearly equal protein loading.
(D) Quantification of the free GFP:GFP fusion ratio of the GFP-ATG8a reporter upon fixed-carbon starvation with or without WM treatment. Levels of free GFP and the GFP fusion were determined by densitometric scans of the immunoblots shown in (A) to (C). Each data point represents the mean ± sd of three independent biological replicates.
(E) WM treatment blocks the formation of autophagic bodies upon fixed-carbon starvation. Six-day-old wild-type, atg1abct, and atg11-1 seedlings expressing GFP-ATG8a were exposed for 24 h to sucrose-deficient (–C) liquid medium and darkness with the addition of 1 μM ConA and 30 μM WM before confocal fluorescence microscopic analysis of root cells. Bar = 10 μm.
SnRK1 but Not TOR Is Essential for the Autophagy Induced by Extended Fixed-Carbon Starvation
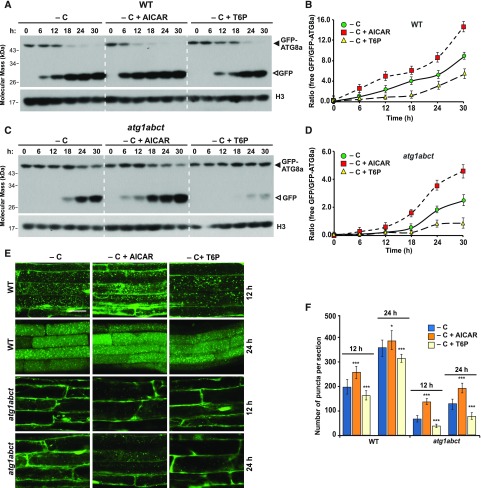
Previous studies with mammalian AMPK and yeast Snf1 demonstrated that both kinases activate autophagy in response to energy deprivation (Wang et al., 2001; Egan et al., 2011; Kim et al., 2011, 2013a). As SnRK1 is considered to be the Arabidopsis ortholog of these energy-sensing kinases, we examined whether it could direct the ATG1 kinase-independent route we observed during prolonged fixed-carbon starvation. As a first test, we studied the effects of 5-aminoimidazole-4-carboxamide ribonucleoside monophosphate (AICAR), which activates AMPK-like kinases and was reported to enhance autophagy in Arabidopsis (Paul et al., 2005; Soto-Burgos and Bassham, 2017), and trehalose-6-phosphate (T6P), which inhibits SnRK1 (Zhang et al., 2009). When atg1abct, atg11-1, and wild-type seedlings expressing GFP-ATG8a were grown for 7 d under an LD photoperiod and then transferred to darkness and sucrose-deficient liquid medium supplemented with 10 mM AICAR, autophagy was substantially accelerated, as seen by the free GFP-release assay (Figures 8A–8D; Supplemental Figures 13A and 13B). While the accumulation of free GFP in atg1abct seedlings at the early time points was not drastically affected by AICAR, this accumulation at later time points (24 and 30 h) was markedly increased (Figures 8C and 8D; Supplemental Figures 13A and 13C). AICAR also strongly increased the abundance of autophagic bodies, as seen by confocal fluorescence microscopy of roots treated for 12 or 24 h with this AMPK/SnRK activator (Figures 8E and 8F; Supplemental Figures 13C and 13D).
Figure 8.
SnRK1 Is Essential for the Autophagy Induced by Prolonged Fixed-Carbon Starvation.
(A) and (C) Immunoblot detection of the free GFP released during the autophagic degradation of GFP-ATG8a reporter in the wild type and the atg1abct mutant before and after exposure to AICAR or T6P. One-week-old seedlings expressing GFP-ATG8a were exposed to sucrose-deficient liquid medium and darkness with the addition of 10 mM AICAR or 0.1 mM T6P for the indicated times. Immunoblot analysis of total seedling extracts was performed as in Figure 2A.
(B) and (D) Quantification of the free GFP:GFP fusion ratio of the GFP-ATG8a reporter in (A) and (C), respectively. Levels of free GFP and the GFP fusion were determined by densitometric scans of the immunoblots. Each data point represents the mean ± sd of three independent biological replicates.
(E) The effect of AICAR and T6P treatments on the deposition of autophagic bodies inside the vacuole. Six-day-old seedlings expressing GFP-ATG8a were exposed for 12 or 24 h to sucrose-deficient liquid medium containing 1 μM ConA with the addition of 10 mM AICAR or 0.1 mM T6P added to the medium before confocal fluorescence microscopic analysis of root cells. Bar = 10 μm.
(F) Numbers of puncta per section in the root cells of the wild-type and atg1abct seedlings used in (E). ***, P < 0.01 and *, P < 0.05 indicate values that are statistically different from respective untreated controls as determined using Student’s t test; n = 30 sections from three independent experiments per genotype. Error bars represent sd.
Conversely, T6P had the opposite effect. Its use at 0.1 mM suppressed the release of free GFP (Figures 8A–8D; Supplemental Figures 13A and 13B) and dampened the accumulation of autophagic bodies during prolonged fixed-carbon starvation, which was strongly evident in the atg1abct background (Figures 8E and 8F; Supplemental Figures 13C and 13D). Collectively, these data suggest that SnRK1 acts as a positive regulator of autophagy during prolonged fixed-carbon starvation in Arabidopsis.
Recent studies showed that TOR kinase acts downstream of Arabidopsis SnRK1 in response to various abiotic stresses, including nutrient starvations (Soto-Burgos and Bassham, 2017). To examine the role of TOR in the SnRK1-activated route upon extended darkness, we studied the effect of the synthetic auxin 1-naphthaleneacetic acid (NAA), which activates TOR and was reported to inhibit autophagy in Arabidopsis (Pu et al., 2017). When 1-week-old wild-type seedlings expressing GFP-ATG8a were transferred to nitrogen-deficient medium supplemented with 20 nM NAA, autophagic flux was significantly reduced, as seen by the free GFP-release assay (Supplemental Figure 14A). However, such inhibition in autophagic activity was not observed in samples treated with prolonged fixed-carbon starvation (Supplemental Figure 14B). We also failed to detect significant changes by confocal fluorescence microscopy of roots treated for 24 h with NAA in the absence of Suc (Supplemental Figures 14D and 14E). Collectively, these data imply that TOR does not work with SnRK1 to induce autophagy during prolonged fixed-carbon stress.
KIN10 Phosphorylates ATG6 to Promote Autophagy during Prolonged Dark Treatment
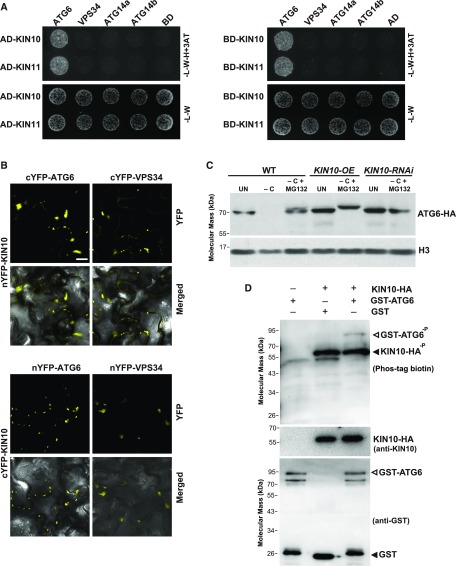
Because both PI3K and SnRK1 appear to be required for the autophagy route induced by prolonged fixed-carbon starvation (Figures 7 and 8), we speculated based on the mammalian AMPK model (Kim et al., 2013a) that the catalytic subunits of SnRK1, KIN10 and KIN11, activate the PI3K complex in the absence of fixed carbon to stimulate an ATG8-dependent autophagy route that bypasses the ATG1 kinase and ATG9. To explore this hypothesis, we first tested the interaction of Arabidopsis KIN10 and KIN11 with the PI3K subunits VPS34, ATG6, ATG14a, and ATG14b by Y2H assays. They revealed that KIN10 and KIN11 fused to the Gal4 DNA binding domain in either orientation bind to ATG6 but not to VPS34, ATG14a, or ATG14b (Figure 9A). The interaction between KIN10 and ATG6 was further confirmed by bimolecular fluorescence complementation (BiFC) assays using Nicotiana benthamiana leaves (Figure 9B; Supplemental Figure 15). When the nYFP-KIN10 or cYFP-KIN10 fusion was transiently coexpressed with reciprocally tagged ATG6, fluorescent BiFC signals were readily detected as cytoplasmic puncta. Interestingly, we also detected an interaction between KIN10 and VPS34 by BiFC (Figure 9B). As mammalian AMPK inhibits the activities of the PI3K complex that appears unrelated to autophagy by phosphorylating the VPS34 subunit (Kim et al., 2013a), this interaction might be relevant to a nonautophagic function of the PI3K complex during nutrient-rich conditions. By contrast, coexpression of KIN10 with ATG14a, ATG14b, or empty vector negative controls failed to reconstitute fluorescence under similar BiFC conditions (Supplemental Figure 15).
Figure 9.
SnRK1 Phosphorylates ATG6 during Fixed-Carbon Starvation.
(A) Y2H interactions of KIN10 or KIN11 with the subunits of the VPS34 complex. Full-length KIN10 or KIN11 expressed as N-terminal fusions to either the Gal4-activating (AD) or Gal4 DNA binding (BD) domains were coexpressed with complementary AD or BD fusions of ATG6, VPS34, ATG14a, and ATG14b on selection medium lacking Leu, Trp, and His and containing 10 μM 3-amino-1,2,4-triazole (-L-W-H+3AT; top panels), or on nonselection medium lacking Leu and Trp (-L-W; bottom panels).
(B) BiFC analysis testing the interaction of KIN10 with ATG6 and VPS34 in planta. N. benthamiana leaf epidermal cells were coinfiltrated with plasmids expressing the N- and C-terminal fragments of YFP fused to KIN10, ATG6, and VPS34. BiFC signals were detected by confocal fluorescence microscopy 36 h after infiltration. Bar = 10 μm.
(C) Prolonged fixed-carbon starvation and overexpression of KIN10 altered the SDS-PAGE mobility of ATG6. A plasmid encoding ATG6-HA was transiently expressed in protoplasts prepared from wild-type, KIN10-OE, or KIN10-RNAi plants for 16 h and then transferred to MS medium without Suc (−C) and incubated in darkness for 8 h with or without 50 μM MG132. Immunoblot analyses of protoplast extracts were performed with anti-HA antibodies. Immunoblot detection of histone H3 was used to confirm nearly equal protein loading. UN, protein samples extracted from protoplasts not exposed to fixed-carbon starvation.
(D) ATG6 is phosphorylated by KIN10 in vitro. Purified GST-ATG6 or GST was incubated with immunoprecipitated KIN10-HA in phosphorylation buffer followed by SDS-PAGE separation and immunoblotted with Phos-tag BTL-111, anti-KIN10, or anti-GST antibodies.
The interaction between KIN10 and ATG6 suggested that ATG6 is a KIN10 substrate and that ATG6 phosphorylation could promote autophagy. To examine this hypothesis, we tested for a possible electrophoretic mobility shift of the ATG6 protein when transiently expressed in protoplasts with KIN10 as an indirect assay of phosphorylation. When ATG6-hemagglutinin (HA) was synthesized in wild-type protoplasts, the abundance of ATG6-HA dropped dramatically after fixed-carbon starvation for 24 h (Figure 9C). This decrease was strongly inhibited by the proteasome-specific inhibitor MG132, as previously reported (Figure 9C; Qi et al., 2017), implying that ATG6 is a target of proteasomal degradation. Notably, a subtle but reproducible upshift in mobility during SDS-PAGE was observed for ATG6-HA when expressed in wild-type protoplasts under fixed-carbon stress and treated with MG132, which was not detected under nitrogen starvation (Figure 9C; Supplemental Figure 16A). This upshift under fixed-carbon starvation was more obvious and more rapid in similarly treated protoplasts derived from KIN10 overexpression (OE) plants, while no such upshift in ATG6-HA mobility was found in RNAi suppression lines for KIN10, implying that prolonged fixed-carbon starvation caused the modification and that KIN10 was involved (Figure 9C; Supplemental Figure 16B).
Since this increased SDS-PAGE mobility of ATG6 was consistent with its phosphorylation by KIN10 (Kim et al., 2013a; Russell et al., 2013), we further performed assays for protein phosphorylation in vitro to test whether ATG6 is a direct substrate of KIN10. KIN10 was enriched from transgenic seedlings overexpressing HA-tagged KIN10 by immunoprecipitation with anti-HA antibodies (Baena-González et al., 2007) and then used to phosphorylate ATG6 tagged with glutathione S-transferase (GST), which was then detected by Phos-tag biotin. The results showed that KIN10 phosphorylated ATG6 and itself but not GST (Figure 9D).
To confirm a role for KIN10 in regulating ATG6 during prolonged fixed-carbon starvation, we introgressed the KIN10-OE and KIN10-RNAi transgenes into the atg11-1 background expressing GFP-ATG8a. Upon incubating these lines in sucrose-deficient medium and darkness for varying lengths of time, we found that the levels of KIN10 paralleled autophagy activity induced by prolonged fixed-carbon starvation. As measured by the free GFP-release assay, the appearance of free GFP was slightly enhanced in KIN10-OE plants and strongly dampened in KIN10-RNAi plants (Figures 10A and 10B). The fluorescence signal from GFP-ATG8 in well-fed atg11-1 protoplasts appeared to be diffused throughout the cytosol but was concentrated into punctate structures upon fixed-carbon starvation (Figure 10C). Under identical conditions, the number of GFP-ATG8a-labeled foci significantly increased in the KIN10-OE atg11-1 protoplasts (KIN10-OE) and decreased in KIN10-RNAi atg11-1 protoplasts as compared with the atg11-1 parent (Figures 10C and 10E). For similarly treated atg11-1 protoplasts also expressing ATG6-HA, a higher number of GFP-ATG8a puncta was seen as compared with the protoplasts transiently expressing an empty vector (Figures 10D and 10E). This increase in GFP-ATG8a puncta was effectively blocked by introducing the KIN10-RNAi transgene, implying that KIN10 modifies the ATG6 subunit of the PI3K complex to promote autophagy during prolonged fixed-carbon starvation.
Figure 10.
KIN10 Is Required for the Autophagy Induced by Prolonged Fixed-Carbon Starvation in the atg11 Mutant.
(A) Immunoblot detection of the free GFP released during the autophagic degradation of GFP-ATG8a reporter during fixed-carbon starvation. One-week-old atg11-1, KIN10-OE atg11-1, and KIN10-RNAi atg11-1 seedlings expressing the GFP-ATG8a reporter were grown on MS solid medium with 1% (w/v) Suc and then transferred to sucrose-deficient liquid medium and darkness for the indicated times. Immunoblot analysis of total seedling extracts was performed as in Figure 1A. The GFP-ATG8a fusion and free GFP are indicated by closed and open arrowheads, respectively. Immunoblot detection of histone H3 was used to confirm nearly equal protein loading.
(B) Quantification of the free GFP:GFP fusion ratio of the GFP-ATG8a reporter used in (A). Levels of free GFP and the GFP fusion were determined by densitometric scans of the immunoblots. Each data point represents the mean ± sd of three independent biological replicates.
(C) to (E) Expression levels of KIN10 and ATG6 affect the formation of GFP-ATG8-positive puncta in the atg11-1 mutant. In (C), protoplasts were prepared from atg11-1, KIN10-OE atg11-1, and KIN10-RNAi atg11-1 plants expressing GFP-ATG8a. In (D), ATG6-HA or an empty vector control was transiently expressed in protoplasts prepared from atg11-1 and KIN10-RNAi atg11-1 plants. Protoplasts were then transferred to MS liquid medium without Suc (−C) in darkness for 24 h before confocal fluorescence microscopic observation. (E) represents the numbers of puncta per section in the protoplasts shown in (C) and (D). The data represent average values calculated from three independent experiments. Letters indicate values that are statistically different from one another (P < 0.05) as determined using one-way ANOVA followed by multiple-comparisons Tukey’s test; n = 90 protoplasts per treatment. UN, untreated protoplasts.
DISCUSSION
Based on cell biological and genetic studies with yeast and metazoans, the ATG1 kinase has emerged as a central autophagy regulator that responds to developmental and nutritional cues and works downstream of TOR (Papinski and Kraft, 2016; Zachari and Ganley, 2017). In our previous studies, the importance of this complex in plants was clarified from the analysis of Arabidopsis mutants lacking the accessory subunits ATG13 and ATG11, which promote plant survival when nutrients are limited and are required for proper delivery of autophagosomes to vacuoles (Suttangkakul et al., 2011; Li et al., 2014). Here, we further characterized the role(s) of this kinase through analysis of a quadruple mutant (atg1abct) deficient for all four ATG1 isoforms. Surprisingly, we found that while the autophagic response of the atg1abct mutant, like the atg11 and atg13ab mutants, is selectively impaired in its response to nitrogen and short-term fixed-carbon deprivations, its response to long-term fixed-carbon starvation continues unabated (Figures 2 and 3). These results support the crucial role of the ATG1 kinase for stimulating autophagy in response to nutrition stress as well as identify an ATG1 kinase complex-independent route that is up-regulated as fixed-carbon stress becomes more acute (Figure 5). Both routes require the core machinery needed for ATG8 lipidation and PI3-P synthesis, while the second route appears to rely, at least in part, on the modification of the PI3K subunit ATG6 by SnRK1 and its catalytic subunit KIN10 (Figures 9 and 10).
Several studies with mammalian cells have reported the existence of a ULK1 (ATG1)-independent autophagic route in response to (1) glucose withdrawal, (2) elevated levels of ammonia that accumulate during amino acid catabolism (Cheong et al., 2011), or (3) the deacetylation of VPS34 (Su et al., 2017). Our study showed that, although ammonia levels increased slightly in fixed-carbon-starved seedlings, an exogenous supply failed to induce autophagy in both the wild type and the atg11-1 mutant, implying that the ATG1 kinase-independent route is not activated by ammonia (Supplemental Figure 10; Supplemental Table 1). On the contrary, the catalytic KIN10 subunit of Arabidopsis SnRK1 is involved in the route induced by prolonged fixed-carbon starvation through phosphorylation of ATG6 (Figures 9C and 9D). As previously hypothesized by Carlsson and Simonsen (2015), our results are consistent with the ATG1/ULK1 and PI3K complexes acting synergistically to promote phagophore initiation and indicate that the activation of the PI3K complex alone is sufficient to orchestrate autophagosome formation during extended fixed-carbon stress. Why prolonged nitrogen stress does not elicit a similar backup route is unclear but is consistent with carbon and nitrogen stresses that have different impacts on autophagy (Marshall and Vierstra, 2018a).
At present, we do not know how the ATG1 kinase influences autophagic flux in response to nitrogen starvation and short-term fixed-carbon starvation. Studies with yeast and mammalian cells showed that it controls autophagosome formation through several mechanisms, including activating downstream substrates by phosphorylation, recruiting downstream regulators needed for phagophore nucleation, and tethering membranes/vesicles as the phagophore enlarges (Hurley and Young, 2017). Thus far, >30 proteins have been identified as the substrates of the mammalian ATG1 ortholog ULK1, including itself (Mercer et al., 2018) and the Beclin1 (ATG6) and ATG14L (ATG14-like) subunits of the PI3K complex, which are thought to raise PI3-P levels and subsequent autophagy by stimulating PI3K activity (Russell et al., 2013; Wold et al., 2016). ULK1 also phosphorylates AUTOPHAGY AND BECLIN 1 REGULATOR (AMBRA)-1 to prevent its binding to Beclin 1, thus promoting the translocation of PI3K complex to autophagy initiation sites (Di Bartolomeo, 2010), and ATG9 to regulate its trafficking (Young et al., 2006; Zhou et al., 2017).
Clearly, the identification of the downstream targets of the plant ATG1 kinase would help clarify the mechanisms that underlie how it controls autophagy flux in response to nitrogen starvation as well as other stimuli. Thus far, only ATG13 and ATG8 have been identified as possible substrates in Arabidopsis (Suttangkakul et al., 2011; Li et al., 2014). Counterparts of the PI3K complex (VPS34, VPS15, ATG6, and VPS38 or ATG14; Liu et al., 2018) and ATG9 (Hanaoka et al., 2002) have been identified in Arabidopsis, but it remains unknown whether they are regulatory substrates of ATG1. A consensus phosphorylation motif for ULK1-mediated phosphorylation was recently identified; it could aid the identification of substrates in vivo (Egan et al., 2015), but whether such a motif applies to plant ATG1 substrates awaits testing.
Another intriguing feature of the plant ATG1 kinase family is the presence of ATG1t. It contains just the kinase domain without the conserved C-terminal ATG8 binding AIM and the early autophagy targeting/tethering domain (Suttangkakul et al., 2011; Li et al., 2014), which are essential for binding ATG8 and membrane association (Hurley and Young, 2017). Our Y2H assays and confocal microscopy confirmed that ATG1t, unlike other members of the ATG1 family, does not interact with ATG8, likely due to the absence of an AIM, and showed that YFP-ATG1t is not deposited in vacuoles (Supplemental Figures 8A and 8C). Our phenotypic analysis of the mutants disrupting ATG1t alone or comparisons of atg1abc versus atg1abct plants revealed no differences in plants grown without or with nitrogen or fixed carbon, suggesting that ATG1t is not involved in bulk autophagy. Expression profiles available within Genevestigator (https://genevestigator.com) showed that ATG1t transcripts are significantly down-regulated by glucose, iron, cold stress, jasmonic acid application, and viral infection, suggesting that ATG1t could have a highly specific function within the autophagy system (Supplemental Figure 17).
Synthesis of PI3-P is an essential event in autophagy initiation and is driven by the PI3K complex. Studies with yeast and mammalian cells revealed that its activity is controlled by multiple mechanisms, including phosphorylation of Beclin1/ATG6 (Russell et al., 2014). Recent studies showed that Arabidopsis ATG6 associates with two autophagic adaptors, SH3 DOMAIN-CONTAINING PROTEIN (SH3P)-2 (Zhuang et al., 2013) and FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING (FREE)-1 (Gao et al., 2015), but the physiological consequences of these associations are unclear. Moreover, ATG6 seems to be degraded by the ubiquitin-26S proteasome system upon fixed-carbon starvation, which is mediated by the TNF RECEPTOR-ASSOCIATED FACTOR (TRAF)-1 proteins (Qi et al., 2017). In this study, we found that ATG6 is phosphorylated by KIN10 under these conditions, which might provide a mechanism to ensure that the PI3K complex remains active either via the canonical TOR/AMPK-ATG1 signaling system or in situations where the ATG1 kinase is insufficient or suppressed. As the ATG1 kinase complex is rapidly degraded upon nutrient starvations, presumably to down-regulate the process (Suttangkakul et al., 2011), this alternative route might extend autophagic clearance as stressful conditions, such as the lack of fixed carbon, become more acute. Currently, it remains unknown how KIN10-dependent phosphorylation of ATG6 could alone initiate ATG1 kinase complex-independent autophagy. However, it is noteworthy that we detected an interaction between KIN10 and VPS34 by BiFC (Figure 9B). Thus, it remains possible that the PI3K kinase complex simultaneously contributes to autophagy through its modification by KIN10.
Besides ATG1-independent autophagy, another surprising discovery was that the absence of the ATG11 and ATG13 subunits leads to the formation of abnormal autophagosome-related tubules upon fixed-carbon starvation (Figure 3A; Supplemental Movies 1 and 2). A similar phenomenon was seen when autophagy was induced by benzothiadiazole or DTT in root cells missing ATG9 (Zhuang et al., 2017). Phagophore emergence requires proper recycling of ATG9 and other ATG components to and from autophagosomal membrane stores (Carlsson and Simonsen, 2015). As studies with yeast Atg17/Atg11 showed that these Atg1 complex subunits are required for proper localization of Atg9 (Suzuki et al., 2007), it is plausible that the mislocalization of ATG9 in the atg11-1 (and also atg13ab) background interferes with phagophore expansion, similar to that seen in atg9 mutants, thus leading to these extended tubular structures.
Upon autophagy induction, yeast Atg1 coalesces Atg9-decorated vesicles to the phagophore assembly site, where Atg1-mediated phosphorylation of Atg9 further aids the recruitment of downstream components, including Atg18 and Atg8, to promote phagophore expansion (Papinski et al., 2014). Intriguingly, tubules were rarely detected in similarly treated atg1 triple or quadruple mutants in Arabidopsis (Figure 5B). Consequently, it is possible that ATG1 deficiency in Arabidopsis interferes with the recruitment of not only ATG9 but also of ATG18 and/or ATG8 that are required for phagophore expansion, thus developing autophagosome and autophagic bodies with more normal morphologies. Clearly, focused efforts are needed on the molecular mechanism(s) for how the plant ATG1 kinase controls ATG9 and other components participating in autophagosome elongation and maturation.
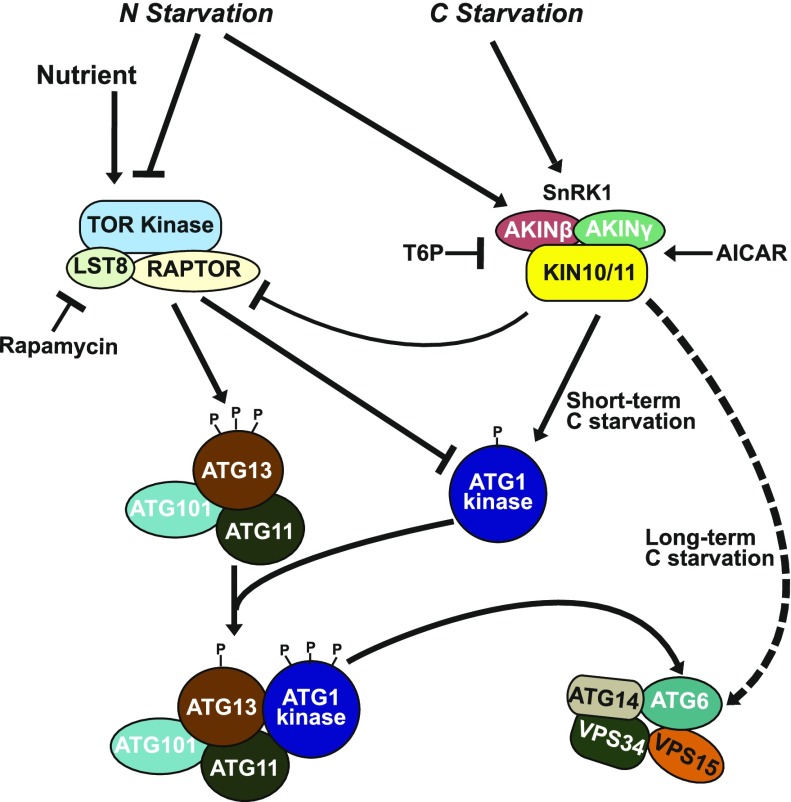
In summary, our study of mutants missing various components of the ATG1 kinase complex, including an atg1abct quadruple mutant, allowed us to identify two autophagic signaling routes in Arabidopsis that are responsive to fixed-carbon stress (Figure 11). Whereas the ATG1 kinase is required for autophagic activation in response to both nitrogen starvation and short-term fixed-carbon starvation, it is dispensable for that induced by prolonged fixed-carbon starvation. The second route appears to act through SnRK1, possibly through direct phosphorylation of the PI3K subunit ATG6 by the KIN10 catalytic subunit of SnRK1, to maintain robust autophagy in situations where the ATG1 kinase complex is either insufficient or down-regulated.
Figure 11.
Proposed Model for the Activation of Autophagy under Fixed-Carbon Starvation.
Under nutrient-rich conditions, TOR causes hypophosphorylation of the ATG1 subunit and hyperphosphorylation of the ATG13 subunit within the ATG1 kinase complex, preventing the association of ATG1 with ATG13, and subsequently dampens the kinase activity of ATG1. Under nitrogen and short-term fixed-carbon starvations, the phosphorylation statuses of ATG1 and ATG13 are reversed by SnRK1 and other kinases, allowing ATG1 and other subunits of the ATG1 kinase complex to assemble into an active complex, which in turn activates the PI3K complex to synthesize more PI3-P. Under prolonged fixed-carbon starvation, the PI3K complex is also activated via the phosphorylation of the PI3K subunit ATG6 by the active KIN10 subunit of SnRK1, which bypasses the requirement of the ATG1 kinase complex for autophagy initiation.
METHODS
Plant Materials, Growth Conditions, and Treatments
The Arabidopsis (Arabidopsis thaliana) atg1b-1 (GK-111E12), atg1c-1 (GK-821H10), and atg1t-1 (GK-335A12) T-DNA insertion mutants were obtained from TAIR (http://www.arabidopsis.org). The T-DNA insertion positions were verified by genomic PCR using a T-DNA left border-specific primer paired with gene-specific primers. See Supplemental Table 2 for detailed information on the primers used in this study. Each mutant was backcrossed three times to the wild-type Col-0 ecotype before phenotypic analysis. The atg1a-2 (Suttangkakul et al., 2011), atg1b-1, atg1c-1, and atg1t-1 alleles were crossed to generate the atg1abc triple and atg1abct quadruple mutants. The T-DNA mutants atg2-1 (Inoue et al., 2006), atg5-1 (Thompson et al., 2005), atg7-2 (Chung et al., 2010), atg9-3 (Shin et al., 2014), atg9-4 (Shin et al., 2014), atg11-1 (Li et al., 2014), atg13ab (atg13a-1 atg13-2; Suttangkakul et al., 2011), and nbr1-2 (Marshall et al., 2015) were described previously.
Transgenes expressing GFP-ATG8a under the control of the cauliflower mosaic virus 35S (Thompson et al., 2005) and Arabidopsis UBQ10 promoters (Kim et al., 2013b) were previously described. The 35S:GFP-ATG8a transgene in the atg7-2 (Chung et al., 2010), atg11-1 (Li et al., 2014), or atg13ab (Suttangkakul et al., 2011) backgrounds were reported previously, while the lines expressing the UBQ10:GFP-ATG8a transgene in the atg7-2, atg2-1, atg9-3, or atg9-4 backgrounds were as described (Shin et al., 2014). To determine the effect of ATG1 deletion on autophagosome formation, a plasmid harboring 35S:GFP-ATG8a (BASTA resistance; Thompson et al., 2005) was transformed into the atg1abc and atg1abct lines by the floral dip method (Clough and Bent, 1998). Homozygous atg1abc and atg1abct plants harboring the transgene were identified in the T3 generation by BASTA resistance and confocal fluorescence microscopy. A transgene expressing YFP-ATG1t driven by the cauliflower mosaic virus 35S promoter was created by recombining the full-length ATG1t cDNA in the TOPO entry vector into the destination vector pEarleyGate 104 (Earley et al., 2006). The sequencing-verified construct was introduced into Agrobacterium tumefaciens strain GV3101 and then transformed into the atg1t-1 mutant by the floral dip method. Homozygous atg1t-1 plants carrying 35S:YFP-ATG1t were identified in the T3 generation by antibiotic resistance and fluorescence confocal microscopy. The KIN10 overexpression (KIN10-OE) lines and KIN10-RNAi lines were described previously (Baena-González et al., 2007).
Unless otherwise noted, all Arabidopsis seeds were vapor-phase sterilized, soaked in water at 4°C for 2 d, and germinated on full-strength MS solid medium (4.3 g/L MS basal salts, 1% [w/v] Suc, 0.05% [w/v] MES [pH 5.7], and 0.7% [w/v] agar) at 20 to 22°C under an LD (16 h of light/8 h of dark) photoperiod. After 10 to 14 d, seedlings were transplanted to soil and grown at 21°C under an LD photoperiod using fluorescent light (at a light intensity of 120 μmol photons m−2 s−1). For senescence assays, plants were grown at 21°C under an SD (8 h of light/16 h of dark) photoperiod for 10 weeks. Nitrogen and fixed-carbon starvations were conducted according to Chung et al. (2010) and Phillips et al. (2008), respectively.
For the free GFP-release assays under nitrogen starvation, 1-week-old seedlings expressing GFP-ATG8a grown on MS solid medium supplemented with 1% (w/v) Suc were transferred to fresh MS liquid medium lacking nitrogen (MS basal salt micronutrient solution; Sigma-Aldrich, M0529) supplemented with 3 mM CaCl2, 1.5 mM MgSO4, 1.25 mM KH2PO4, 5 mM KCl, 1% (w/v) Suc, and 0.05% (w/v) MES (pH 5.7), and incubated under continuous light. For the free GFP-release assays under fixed-carbon starvation, 1-week-old seedlings were transferred to fresh MS liquid medium lacking Suc, covered with foil, and kept in darkness. For the free GFP-release assays after ammonia exposure, 1-week-old seedlings were transferred to fresh MS liquid medium supplemented with 20 mM ammonium nitrate (final concentration of ammonium nitrate is 40.6 mM). For chemical treatments, 1-week-old seedlings grown on solid MS medium were transferred to fresh MS medium supplemented with 1 μM ConA (Santa Cruz Biotechnology, sc-202111), 30 μM WM (Cell Signaling Technology, 9951S), 10 mM AICAR (Santa Cruz Biotechnology, sc-200659), 0.1 mM T6P (Santa Cruz Biotechnology, sc-216004), or 20 nM NAA (Sigma-Aldrich, N0640), with equivalent volumes of DMSO, ethanol, or water used as controls. Following treatment, tissues were harvested, frozen immediately with liquid nitrogen, and stored at −80°C before analysis.
Quantification of Ammonium
Ammonium content was determined according to Husted et al. (2000) with slight modification. Briefly, 100 μg of frozen tissue was homogenized and extracted with 1 mL of ice-cold 10 mM formic acid. The homogenate was washed with another 1 mL of ice-cold 10 mM formic acid and clarified by centrifugation at 16,000g (4°C) for 10 min. The supernatant (1 mL) was assayed for NH4+ colorimetrically at 660 nm using a Continuous Flow Analyzer system (Skalar SAN++, Skalar Analytical).
Protein-Protein Interaction Assays
Protein-protein interactions by Y2H were conducted using the ProQuest Two-Hybrid System (Thermo Scientific) as previously described (Suttangkakul et al., 2011). Full-length cDNAs in the entry vectors were recombined in-frame via Gateway LR Clonase (Thermo Fisher Scientific) reaction into either the GAL4 activation domain or GAL4 binding domain fragments in the pDEST22 or pDEST32 vector (Thermo Scientific), respectively. Pairwise combination of genes in pDEST22 and pDEST32 was cotransformed into yeast strain MaV203, with the empty vectors as controls. Protein-protein interactions were identified by growth after 2 d on medium lacking Leu, Trp, and His and containing 10 mM 3-amino-1,2,4-triazole, according to the manufacturer’s instructions (Thermo Fisher Scientific).
Protein-protein interactions in planta were assessed by BiFC (Citovsky et al., 2006). Full-length cDNAs of the indicated genes in entry vector were recombined in-frame into the N- or C-terminal half of EYFP in the pSITE-N-EYFP-C1 (ABRC stock number CD3-1648) or pSITE-C-EYFP-C1 vector (CD3-1649), respectively (Martin et al., 2009). Sequence-verified constructs were introduced into the A. tumefaciens strain GV3101 and then infiltrated into Nicotiana benthamiana leaves as described (Martin et al., 2009). Leaf sections were excised and examined by confocal fluorescence microscopy ∼36 to 48 h after infiltration.
RT-PCR Analyses
RNA extraction and RT-PCR analysis were performed according to Li et al. (2014). Briefly, total RNA was extracted from 100 mg of 2-week-old plate-grown seedlings using the RNeasy Plant Mini Kit (Qiagen) following the manufacturer’s instructions. One microgram of RNA was treated with DNase I (Thermo Fisher Scientific) and converted to cDNA using the SuperScript III first-strand synthesis system (Thermo Fisher Scientific) and oligo(dT)20 primers. cDNAs were generated by 35 cycles of PCR using Ex-Taq polymerase (TaKaRa) and the indicated primer pairs (Supplemental Figure 6A). UBC9 was used as an internal control (Li et al., 2014). Quantitative real-time PCR analyses of NBR1 and ATG1t transcripts were performed using the MyiQ5 two-color real-time PCR detection system and iQ SYBR Green supermix (Bio-Rad), with three technical replicates for each reaction. The relative transcript abundance of target genes was determined by the comparative threshold cycle method using the UBC9 gene as an internal reference (Li et al., 2014).
Protein Isolation and Immunoblot Analyses
Total protein was extracted from protoplasts and plate- or liquid-grown seedlings by homogenization in 3:1 (volume:fresh weight) SDS-PAGE sample buffer (125 mM Tris-HCl [pH 6.8], 5% [w/v] SDS, 20% [v/v] glycerol, and 10% [v/v] 2-mercaptoethanol). The homogenates were vortexed for 5 min, heated to 100°C, and clarified at 13,000g for 10 min. The supernatants were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, IPVH00010) for immunoblot analysis using peroxidase-labeled goat anti-mouse or goat anti-rabbit immunoglobulin for detection (Kirkegaard and Perry Laboratories). Antibodies against ATG1a (Suttangkakul et al., 2011), ATG5 and ATG8 (Thompson et al., 2005), and NBR1 (McLoughlin et al., 2018) were previously described. Antibodies against GFP, HA, histone H3, GST, and KIN10 were purchased from Roche Applied Science (catalog number 11814460001, lot number 14,442,000), Biodragon (catalog number B1021, lot number KJA7054531), AbCam (catalog number AB1791, lot number GR3197444-1), Biodragon (catalog number B1024, lot number KJA7054547), and Agrisera (catalog number AS10919, lot number 1510), respectively. Blots were developed using SuperSignal West Pico Chemiluminescent Substrate or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, PI34577 and PI34094), according to the manufacturer’s instructions. Densitometric quantification of protein level was performed using TotalLab software (Non-linear Dynamics), with at least three different exposures of the same blot examined to ensure that the exposure levels were within the linear range of the film.
In Vitro Phosphorylation Assay of ATG6
The in vitro protein phosphorylation assay was performed as described by Li et al. (2019). GST-ATG6 fusion proteins were expressed and purified from Escherichia coli (Rosetta) with GST resin. HA-tagged KIN10 was obtained by immunoprecipitation from HA-KIN10 transgenic plants exposed to darkness for 12 h. For in vitro phosphorylation assays, HA-KIN10 proteins were incubated with purified GST-ATG6 or GST in 30 μL of reaction buffer (10 mM Tris-HCl [pH 7.8], 0.5 mM DTT, 10 mM MgCl2, 2 mM MnCl2, and 50 mM ATP) for 1 h at room temperature. Samples were then resolved on a 10% (v/v) SDS-PAGE gel and probed with Phos-tag BTL-111 according to the manufacturer’s instructions (WAKO).
Transient Expression in Arabidopsis Protoplasts and Confocal Fluorescence Microscopy
Protoplasts were generated from leaf tissues of 4-week-old seedlings by the method of Wu et al. (2009) and suspended in sucrose-free medium (1.5 mM MES-KOH [pH 5.6], 150 mM NaCl, 125 mM CaCl2, 5 mM KCl, and 5 mM mannitol). The purified ATG6-HA plasmid (Qi et al., 2017) was introduced into the protoplasts by polyethylene glycol-mediated transformation; its expression was assayed by immunoblot analysis of cells harvested 18 h later.
Confocal fluorescence microscopy of stable transgenic lines expressing 35S:GFP-ATG8a, UBQ10:GFP-ATG8a, or 35S:YFP-ATG1t in various genetic backgrounds was conducted according to Suttangkakul et al. (2011) and Shin et al. (2014). Briefly, 6-d-old seedlings grown on MS solid agar medium containing 1% (w/v) Suc were transferred to MS medium lacking nitrogen or Suc with or without various chemical treatments. FM4-64 uptake experiments were conducted by incubating seedlings with the dye (12 μM) for 1 h (Zhuang et al., 2017) and then observing the cell fluorescence within the root elongation zone with a Zeiss 710-Meta laser scanning confocal microscope. GFP/FM4-64/chlorophyll and YFP were excited by a 488-nm and a 561-nm laser, respectively. The step size for Z-acquisition was 1 μm. Microscopy images were processed using the ZEN (Blue edition) imaging software (Zeiss) and converted to TIFF (for images) or AVI (for movies) format. GFP-ATG8a puncta were counted using the Adobe Photoshop CS6 measurement analysis option; diameters of GFP-ATG8a-positive puncta were measured using the ZEN profile option.
Statistical Analysis
Data reported in this study are means ± sd of three independent experiments unless otherwise indicated. P values were determined by two-tailed Student’s t test or one-way ANOVA followed by multiple comparisons using SPSS Statistics (SPSS) or two-way ANOVA followed by multiple-comparison Tukey’s test using R 3.6.0 (https://www.r-project.org/). See the Supplemental Data Set for detailed statistical results.
Accession Numbers
DNA and derived protein sequence data from this article are available in the Arabidopsis Genome Initiative database under the following accession numbers: ATG1a (AT3g61960), ATG1b (AT3G53930), ATG1c (AT2G37840), ATG1t (AT1G49180), ATG2 (AT3G19190), ATG5 (AT5G17290), ATG6 (AT3G61710), ATG7 (AT5G45900), ATG8a (AT4G21980), ATG9 (AT2G31260), ATG11 (AT4G30790), ATG13a (AT3G49590), ATG13b (AT3G18770), ATG14a (AT1G77890), ATG14b (AT4G08540), NBR1 (AT4G24690), VPS34 (AT1G60490), KIN10 (AT3G01090), and KIN11 (AT3G29160).
Supplemental Data
Supplemental Figure 1. Immunoblot detection of the NBR1 protein.
Supplemental Figure 2. NBR1 mRNA levels in seedlings during fixed-carbon starvation.
Supplemental Figure 3. Time-course analysis of autophagy response to both nitrogen and fixed-carbon starvations.
Supplemental Figure 4. The effect of prolonged fixed-carbon starvation on vacuolar activities.
Supplemental Figure 5. GFP-ATG8a-labeled puncta are delivered to the central vacuole of atg11-1 hypocotyl cells upon fixed-carbon starvation.
Supplemental Figure 6. Assembly of atg1 triple and quadruple mutants.
Supplemental Figure 7. Assembly of the ATG12–5 conjugate and the ATG8–PE adduct is normal in plants missing ATG1.
Supplemental Figure 8. Characterization of Arabidopsis ATG1t.
Supplemental Figure 9. Number of abnormal tubules and size of GFP-ATG8 puncta in atg1 mutants upon fixed-carbon starvation.
Supplemental Figure 10. Ammonium stress fails to induce autophagy.
Supplemental Figure 11. Detection of autophagic bodies in wild-type and atg7-2 seedlings expressing GFP-ATG8a driven by the UBQ10 promoter during nitrogen or fixed-carbon starvation.
Supplemental Figure 12. Wortmannin treatment blocks the formation of autophagic bodies upon nitrogen starvation.
Supplemental Figure 13. SnRK1 is essential for the autophagy induced by prolonged fixed-carbon starvation in the atg11-1 mutant.
Supplemental Figure 14. TOR is not essential for autophagy induced by prolonged fixed-carbon starvation in the atg1abct mutant.
Supplemental Figure 15. BiFC analysis of the interaction of KIN10 with ATG14a/ATG14b and the controls of BiFC analysis.
Supplemental Figure 16. The SDS-PAGE mobility of ATG6 in seedlings under nitrogen or fixed-carbon starvation.
Supplemental Figure 17. The transcript abundances of Arabidopsis ATG1 isoforms subjected to various biotic and abiotic stresses.
Supplemental Table 1. Ammonium contents of various atg mutants.
Supplemental Table 2. Oligonucleotide primers used in this study.
Supplemental Movie 1. The formation of GFP-ATG8a labeled autophagosomes in wild-type root cells after fixed-carbon starvation.
Supplemental Movie 2. The formation of GFP-ATG8a labeled abnormal tubular structures in the root cells of atg11-1 mutant after fixed-carbon starvation.
Supplemental Data Set. Detailed statistical analyses of the results in this study.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Weiming Hu, Richard S. Marshall, Guohui Zhu, Hua Qi, and Na Luo for technical help and reagents, Faqiang Feng for advice on statistical analysis, and Sarah J. Swanson for advice on confocal microscopic imaging. We thank the ABRC for providing all of the T-DNA seed pools. This work was supported by the National Natural Science Foundation of China (grant 31770356 to F.Q.L. and grant 31601830 to X.L.), the South China Agricultural University (Start-up grant 4600-K15409 to F.Q.L.), the Natural Science Foundation of Guangdong Province (grant 2018A0303130348 to X.H.), the China Scholars Fellowship Program (predoctoral fellowship to F.L.), the U.S. National Science Foundation Plant Genome Research Programs (grant IOS-1339325 to M.S.O. and R.D.V.), the U.S. National Institutes of Health National Institute of General Medical Sciences (grant R01-GM124452-01A1), and the U.S. National Science Foundation (grant IOS-1840687 to R.D.V.).
AUTHOR CONTRIBUTIONS
F.Q.L., X.H., and R.D.V. designed the research; F.Q.L., X.H., C.Y.Z., F.L., X.L., C.Y., and P.Z. performed the research; T.C., J.T., and C.G. contributed materials and technical information; F.Q.L., X.H., T.C., M.S.O., S.X., C.G., and R.D.V. analyzed the data; F.Q.L., X.H., and R.D.V. wrote the article with input from all authors.
References
- Araújo W.L., Tohge T., Ishizaki K., Leaver C.J., Fernie A.R. (2011). Protein degradation: An alternative respiratory substrate for stressed plants. Trends Plant Sci. 16: 489–498. [DOI] [PubMed] [Google Scholar]
- Avin-Wittenberg T., Bajdzienko K., Wittenberg G., Alseekh S., Tohge T., Bock R., Giavalisco P., Fernie A.R. (2015). Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell 27: 306–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M., Larance M., James D.E., Ramm G. (2011). The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem. J. 440: 283–291. [DOI] [PubMed] [Google Scholar]
- Baena-González E., Rolland F., Thevelein J.M., Sheen J. (2007). A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942. [DOI] [PubMed] [Google Scholar]
- Barros J.A.S., Cavalcanti J.H.F., Medeiros D.B., Nunes-Nesi A., Avin-Wittenberg T., Fernie A.R., Araújo W.L. (2017). Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana. Plant Physiol. 175: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., Leaver C.J. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 42: 567–585. [DOI] [PubMed] [Google Scholar]
- Carlsson S.R., Simonsen A. (2015). Membrane dynamics in autophagosome biogenesis. J. Cell Sci. 128: 193–205. [DOI] [PubMed] [Google Scholar]
- Chen L., Su Z.Z., Huang L., Xia F.N., Qi H., Xie L.J., Xiao S., Chen Q.F. (2017). The AMP-activated protein kinase KIN10 is involved in the regulation of autophagy in Arabidopsis. Front. Plant Sci. 8: 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H., Lindsten T., Wu J., Lu C., Thompson C.B. (2011). Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl. Acad. Sci. USA 108: 11121–11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T., Phillips A.R., Vierstra R.D. (2010). ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A and ATG12B loci. Plant J. 62: 483–493. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Lee L.Y., Vyas S., Glick E., Chen M.H., Vainstein A., Gafni Y., Gelvin S.B., Tzfira T. (2006). Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362: 1120–1131. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Contento A.L., Kim S.J., Bassham D.C. (2004). Transcriptome profiling of the response of Arabidopsis suspension culture cells to Suc starvation. Plant Physiol. 135: 2330–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolomeo S., et al. (2010). The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 191: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop E.A., Tee A.R. (2013). The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 41: 939–943. [DOI] [PubMed] [Google Scholar]
- Durgud M., et al. (2018). Molecular mechanisms preventing senescence in response to prolonged darkness in a desiccation-tolerant plant. Plant Physiol. 177: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K.W., Haag J.R., Pontes O., Opper K., Juehne T., Song K., Pikaard C.S. (2006). Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45: 616–629. [DOI] [PubMed] [Google Scholar]
- Egan D.F., et al. (2015). Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell 59: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan D.F., et al. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Yu L., Xu C. (2017). A central role for triacylglycerol in membrane lipid breakdown, fatty acid β-oxidation, and plant survival under extended darkness. Plant Physiol. 174: 1517–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., He D., Yao Z., Klionsky D.J. (2014). The machinery of macroautophagy. Cell Res. 24: 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Yoshimoto K., Ohsumi Y. (2007). An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol. 143: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., et al. (2017). Molecular definitions of autophagy and related processes. EMBO J. 36: 1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Zhuang X., Cui Y., Fu X., He Y., Zhao Q., Zeng Y., Shen J., Luo M., Jiang L. (2015). Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. USA 112: 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka H., Noda T., Shirano Y., Kato T., Hayashi H., Shibata D., Tabata S., Ohsumi Y. (2002). Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 129: 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Lowe N.J., Olsen L.J. (2008). Autophagy protein 6 (ATG6) is required for pollen germination in Arabidopsis thaliana. Autophagy 4: 339–348. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T.M., Nunes Nesi A., Araújo W.L., Braun H.P. (2015). Amino acid catabolism in plants. Mol. Plant 8: 1563–1579. [DOI] [PubMed] [Google Scholar]
- Hirota T., Izumi M., Wada S., Makino A., Ishida H. (2018). Vacuolar protein degradation via autophagy provides substrates to amino acid catabolic pathways as an adaptive response to sugar starvation in Arabidopsis thaliana. Plant Cell Physiol. 59: 1363–1376. [DOI] [PubMed] [Google Scholar]
- Hurley J.H., Young L.N. (2017). Mechanisms of autophagy initiation. Annu. Rev. Biochem. 86: 225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S., Hebbern C.A., Mattsson M., Schjoerring J.K. (2000). A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant. 109: 167–179. [Google Scholar]
- Inoki K., Zhu T., Guan K.L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Suzuki T., Hattori M., Yoshimoto K., Ohsumi Y., Moriyasu Y. (2006). AtATG genes, homologs of yeast autophagy genes, are involved in constitutive autophagy in Arabidopsis root tip cells. Plant Cell Physiol. 47: 1641–1652. [DOI] [PubMed] [Google Scholar]
- Izumi M., Hidema J., Makino A., Ishida H. (2013). Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol. 161: 1682–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim Y.C., Fang C., Russell R.C., Kim J.H., Fan W., Liu R., Zhong Q., Guan K.L. (2013a). Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152: 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K.L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee H., Lee H.N., Kim S.H., Shin K.D., Chung T. (2013b). Autophagy-related proteins are required for degradation of peroxisomes in Arabidopsis hypocotyls during seedling growth. Plant Cell 25: 4956–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy In Autophagy, Volume 12, pp. 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.N., Zarza X., Kim J.H., Yoon M.J., Kim S.H., Lee J.H., Paris N., Munnik T., Otegui M.S., Chung T. (2018). Vacuolar trafficking protein VPS38 is dispensable for autophagy. Plant Physiol. 176: 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Chung T., Vierstra R.D. (2014). AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 26: 788–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., et al. (2019). The plant ESCRT component FREE1 shuttles to the nucleus to attenuate abscisic acid signalling. Nat. Plants 5: 512–524. [DOI] [PubMed] [Google Scholar]
- Liu F., Hu W., Vierstra R.D. (2018). The Vacuolar Protein Sorting-38 subunit of the Arabidopsis phosphatidylinositol-3-kinase complex plays critical roles in autophagy, endosome sorting, and gravitropism. Front. Plant Sci. 9: 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.S., Hua Z., Mali S., McLoughlin F., Vierstra R.D. (2019). ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Cell 177: 766–781.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.S., Li F., Gemperline D.C., Book A.J., Vierstra R.D. (2015). Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in Arabidopsis. Mol. Cell 58: 1053–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.S., Vierstra R.D. (2018a). Proteasome storage granules protect proteasomes from autophagic degradation upon carbon starvation. eLife 7: e34532. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marshall R.S., Vierstra R.D. (2018b). Autophagy: The master of bulk and selective recycling. Annu. Rev. Plant Biol. 69: 173–208. [DOI] [PubMed] [Google Scholar]
- Martin K., Kopperud K., Chakrabarty R., Banerjee R., Brooks R., Goodin M.M. (2009). Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 59: 150–162. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Bassham D.C., Raikhel N.V., Nakamura K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130: 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F., Augustine R.C., Marshall R.S., Li F., Kirkpatrick L.D., Otegui M.S., Vierstra R.D. (2018). Maize multi-omics reveal roles for autophagic recycling in proteome remodelling and lipid turnover. Nat. Plants 4: 1056–1070. [DOI] [PubMed] [Google Scholar]
- Mercer T.J., Gubas A., Tooze S.A. (2018). A molecular perspective of mammalian autophagosome biogenesis. J. Biol. Chem. 293: 5386–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinski D., Kraft C. (2016). Regulation of autophagy by signaling through the Atg1/ULK1 complex. J. Mol. Biol. 428: 1725–1741. [DOI] [PubMed] [Google Scholar]
- Papinski D., et al. (2014). Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol. Cell 53: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.L., Sehnke P.C., Ferl R.J. (2005). Isoform-specific subcellular localization among 14-3-3 proteins in Arabidopsis seems to be driven by client interactions. Mol. Biol. Cell 16: 1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.R., Suttangkakul A., Vierstra R.D. (2008). The ATG12-conjugating enzyme ATG10 is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics 178: 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y., Luo X., Bassham D.C. (2017). TOR-dependent and -independent pathways regulate autophagy in Arabidopsis thaliana. Front. Plant Sci. 8: 1204–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Xia F.N., Xie L.J., Yu L.J., Chen Q.F., Zhuang X.H., Wang Q., Li F., Jiang L., Xie Q., Xiao S. (2017). TRAF family proteins regulate autophagy dynamics by modulating AUTOPHAGY PROTEIN6 stability in Arabidopsis. Plant Cell 29: 890–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Ma Z., Zhang L., Xing S., Hou X., Deng J., Liu J., Chen Z., Qu L.J., Gu H. (2007). Arabidopsis AtBECLIN 1/AtAtg6/AtVps30 is essential for pollen germination and plant development. Cell Res. 17: 249–263. [DOI] [PubMed] [Google Scholar]
- Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.Y., Kim J., Kim H., Neufeld T.P., Dillin A., Guan K.L. (2013). ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R.C., Yuan H.X., Guan K.L. (2014). Autophagy regulation by nutrient signaling. Cell Res. 24: 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertl P., Braun H.P. (2014). Respiratory electron transfer pathways in plant mitochondria. Front. Plant Sci. 5: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.D., Lee H.N., Chung T. (2014). A revised assay for monitoring autophagic flux in Arabidopsis thaliana reveals involvement of AUTOPHAGY-RELATED9 in autophagy. Mol. Cells 37: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.M., Stitt M. (2007). Coordination of carbon supply and plant growth. Plant Cell Environ. 30: 1126–1149. [DOI] [PubMed] [Google Scholar]
- Soto-Burgos J., Bassham D.C. (2017). SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One 12: e0182591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., et al. (2017). VPS34 acetylation controls its lipid kinase activity and the initiation of canonical and non-canonical autophagy. Mol. Cell 67: 907–921.e7. [DOI] [PubMed] [Google Scholar]
- Suttangkakul A., Li F., Chung T., Vierstra R.D. (2011). The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 23: 3761–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kubota Y., Sekito T., Ohsumi Y. (2007). Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 12: 209–218. [DOI] [PubMed] [Google Scholar]
- Svenning S., Lamark T., Krause K., Johansen T. (2011). Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy 7: 993–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove L.J., Beard K.F., Nunes-Nesi A., Fernie A.R., Ratcliffe R.G. (2010). Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 15: 462–470. [DOI] [PubMed] [Google Scholar]
- Takatsuka C., Inoue Y., Matsuoka K., Moriyasu Y. (2004). 3-Methyladenine inhibits autophagy in tobacco culture cells under sucrose starvation conditions. Plant Cell Physiol. 45: 265–274. [DOI] [PubMed] [Google Scholar]
- Tamura K., Shimada T., Ono E., Tanaka Y., Nagatani A., Higashi S.I., Watanabe M., Nishimura M., Hara-Nishimura I. (2003). Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 35: 545–555. [DOI] [PubMed] [Google Scholar]
- Thompson A.R., Doelling J.H., Suttangkakul A., Vierstra R.D. (2005). Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138: 2097–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk K.J. (2015). Protein maturation and proteolysis in plant plastids, mitochondria, and peroxisomes. Annu. Rev. Plant Biol. 66: 75–111. [DOI] [PubMed] [Google Scholar]
- Wang P., Mugume Y., Bassham D.C. (2018). New advances in autophagy in plants: Regulation, selectivity and function. Semin. Cell Dev. Biol. 80: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wilson W.A., Fujino M.A., Roach P.J. (2001). Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21: 5742–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M.S., Lim J., Lachance V., Deng Z., Yue Z. (2016). ULK1-mediated phosphorylation of ATG14 promotes autophagy and is impaired in Huntington’s disease models. Mol. Neurodegener. 11: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.H., Shen S.C., Lee L.Y., Lee S.H., Chan M.T., Lin C.S. (2009). Tape-Arabidopsis Sandwich: A simpler Arabidopsis protoplast isolation method. Plant Methods 5: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Gao X.Q., Zhao X.Y., Zhu D.Z., Zhou L.Z., Zhang X.S. (2011). Arabidopsis AtVPS15 is essential for pollen development and germination through modulating phosphatidylinositol 3-phosphate formation. Plant Mol. Biol. 77: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.R., Chan E.Y., Hu X.W., Köchl R., Crawshaw S.G., High S., Hailey D.W., Lippincott-Schwartz J., Tooze S.A. (2006). Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J. Cell Sci. 119: 3888–3900. [DOI] [PubMed] [Google Scholar]
- Zachari M., Ganley I.G. (2017). The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 61: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Primavesi L.F., Jhurreea D., Andralojc P.J., Mitchell R.A., Powers S.J., Schluepmann H., Delatte T., Wingler A., Paul M.J. (2009). Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 149: 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Ma K., Gao R., Mu C., Chen L., Liu Q., Luo Q., Feng D., Zhu Y., Chen Q. (2017). Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 27: 184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.K. (2016). Abiotic stress signaling and responses in plants. Cell 167: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Chung K.P., Cui Y., Lin W., Gao C., Kang B.H., Jiang L. (2017). ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc. Natl. Acad. Sci. USA 114: E426–E435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Wang H., Lam S.K., Gao C., Wang X., Cai Y., Jiang L. (2013). A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25: 4596–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]