Abstract
Background and Aim:
Avian pathogenic Escherichia coli cause extensive mortality in poultry flocks, leading to extensive economic losses. To date, in Algeria, little information has been available on virulence potential and antibiotics resistance of avian E. coli isolates. Therefore, the aim of this study was the characterization of virulence genes and antibiotic resistance profile of Algerian E. coli strains isolated from diseased broilers.
Materials and Methods:
In this study, 43 avian E. coli strains isolated from chicken colibacillosis lesions at different years were analyzed to determine their contents in 10 virulence factors by polymerase chain reaction, antimicrobial susceptibility to 22 antibiotics belonging to six different chemical classes and genomic diversity by pulsed-field gel electrophoresis (PFGE).
Results:
Mainly E. coli isolates (58.1%) carried two at six virulence genes and the most frequent virulence gene association detected were ompT (protectin), hlyF (hemolysin) with 55.8% (p<0.001), and iroN, sitA (iron acquisition/uptake systems), and iss (protectin) with 41.8% (p<0.001). Some strains were diagnosed as virulent according to their virulence gene profile. Indeed, 23.25% of the isolates harbored iroN, ompT, hlyF, iss, and sitA combination, 14% ompT, hlyF, and frzorf4 (sugar metabolism), and 11,6% iroN, hlyF, ompT, iss, iutA (iron acquisition/uptake systems), and frzorf4. The chicken embryo lethality assay performed on five isolates confirmed the potential virulence of these strains. All isolates submitted to PFGE analysis yielded different genetic profiles, which revealed their diversity. Overall, 97.2% of the isolates were resistant to at least one antibiotic and 53.5% demonstrated multi-antimicrobial resistance to three different antimicrobial classes. The highest resistance levels were against nalidixic acid (83.4%), amoxicillin and ampicillin (83.3%), ticarcillin (80.5%), pipemidic acid (75%), and triméthoprim-sulfamethoxazole (66.6%). For beta-lactam class, the main phenotype observed belonged to broad-spectrum beta-lactamases. However, extended-spectrum beta-lactamase associated with three at six virulence factors was also detected in 13 isolates. Two of them were attested virulent as demonstrated in the embryo lethality test which constitutes a real public threat.
Conclusion:
It would be imperative in avian production to discourage misuse while maintaining constant vigilance guidelines and regulations, to limit and rationalize antimicrobial use.
Keywords: antibiotic resistance, avian Escherichia coli, extended-spectrum beta-lactamase, virulence
Introduction
Poultry farming is undeniably the branch of animal production that has recorded a remarkable development in Algeria in recent years [1]. The poultry sector, which is 90% dominated by the private sector, has in less than a decade a significant leap with a considerable animal wealth of 240 million broilers and turkeys. However, the poultry industry still retains a dual character (industrial and artisanal models) [1]. In Algeria, as well as many other countries, colibacillosis is one of the most common bacterial infections in poultry and one of the main causes of mortality in chickens and turkeys, leading to significant losses in industrial poultry farming and carcass condemnations at slaughterhouse. The subgroup avian pathogenic Escherichia coli (APEC) is the etiologic agent of colibacillosis in chickens. Indeed, E. coli is a versatile species encompassing both commensals of the digestive tracts of many vertebrates, including humans, and pathogenic strains causing various intra- and extra-intestinal infections as a cause of airsacculitis, polyserositis, septicemia, poor growth performance, and carcass condemnation in affected flocks [2,3]. The disease-inducing potential of these isolates has been explained by the occurrence of specific virulence factors. Indeed, many virulence factors have been associated with APEC strains, although their role in the pathogenesis is not well known [4].
In animal production, antimicrobials are widely used as a growth promoter and in the treatment of infectious diseases. The use of antimicrobials in poultry production industries for the promotion of growth largely contributes to the high resistance to antimicrobial agents in normal flora of poultry and pathogenic microorganism [5].
Due to its ubiquity, E. coli has become one of the bacterial species that are commonly resistant to antibiotics and can transmit antibiotic-resistance genes from other Enterobacteriaceae species in the environment [6,7]. To date, little information has been available on characteristics of avian E. coli isolates in Algeria, especially their virulence factors content and antimicrobial resistance. Therefore, the aim of this study was the characterization of virulence genes and antibiotic resistance profile of Algerian E. coli strains isolated from diseased broilers.
Materials and Methods
Ethical approval
The tests on embryonated eggs do not require authorization from the ethics committee.
Isolation of E. coli strains
A total of 43 chicken visceral organs (liver, lungs, heart, and spleen) were collected randomly between 2006 and 2013 from diseased chicken broilers from different poultry farms located in various regions of Central Algeria (Provinces of Bouira, Bejaia, Tizi Ouzou, and Boumerdes).
All farms were all-in, all-out intensive systems either in cages or on litter floors. Samples of these organs were cultured in brain and heart infusion broth then subcultured on MacConkey agar plates and on desoxycholate agar. The plates were incubated under aerobic conditions at 37°C for 72 h. E. coli isolates identification was confirmed by detecting uidA gene by polymerase chain reaction (PCR) [8].
Virulence gene detection
Template DNA was prepared using the boiling method [9]. PCR reactions were performed according to published protocols [10,11]. The genes that were searched for were as follows: Adhesins (papC and felA), hemolysin (hlyF), protectins (iss and ompT), iron acquisition/uptake systems (iroN, iutA, and sitA), component of a T6SS (aec26), and sugar metabolism (frzorf4). The E. coli strains used as positive controls in PCR assays were as follows: BEN 2908 [12,13] for iutA, ompT, iss, sitA, aec26, and frzorf4, MT189 for felA, and BEN 2905 (J96) for papC.
Antimicrobial sensitivity testing
This test was performed by disk diffusion method on Mueller-Hinton agar using 22 antibiotic disk belonging to different antimicrobial classes including amoxicillin (30 µg), ampicillin (10 µg), amoxicillin/clavulanic acid (30 µg/disc), ticarcillin (30 µg), imipenem (10 µg/disc), aztreonam (30 µg/disc), cefazolin (30 µg), cefoxitin (10 µg), cefotaxime (30 µg), ceftazidime (30 µg), cefixime (30 µg), cefpirome (30 µg), kanamycin (30 µg), gentamicin (10 µg/disc), tetracycline (30 µg), sulfamethoxazole (1.25/23.75 µg), colistin (25 µg), nalidixic acid (30 µg), pipemidic acid (20 µg), ciprofloxacin (5 µg), ofloxacin (5 µg), and pefloxacin (5 µg). The presence of extended-spectrum beta-lactamases (ESBL) was detected by double-disc synergy method. Interpretation of the results was done according to CA-SFM procedures [14]. E. coli CIP7624 was used as quality control.
Pulsed-field gel electrophoresis (PFGE)
PFGE was performed using the restriction enzyme XbaI as described by Moulin-Schouleur et al. [15]. Agarose plugs were prepared from a bacterial culture grown in brain heart infusion broth to an optical density (OD) at 600 nm of 1.0. After incubation for 2 h at 37°C in a lysozyme solution (10 mM Tris-HCl, pH 9, 100 mM ethylenediaminetetraacetic acid (EDTA), 5 mg/ml lysozyme, 0.05% sarkosyl), they were then incubated overnight at 55°C (without shaking) in a lysis solution (10 mM Tris-HCl, pH9, 100 mM EDTA, 1 mg/ml proteinase K, 1% sodium dodecyl sulfate) and washed 3 times for 1 h each time in TE buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA).
For digestion, plugs were equilibrated in incubation buffer (Takara) containing 10 units XbaI restriction enzyme (Takara Bio Europe). PFGE was conducted in a CHEF-DRIII apparatus (Bio-Rad). Gels (1% agarose) were run at 14°C for 24 h in TBE buffer (4 mM Tris, 4 mM borate, 1 mM EDTA, pH 8.3) at 6 V/cm. PFGE was conducted in a CHEF-DRIII apparatus (Bio-Rad). The gels (1% agarose) were run at 14°C for 24 h in TBE buffer (Tris, 4 mM; borate, 4 mM; EDTA, 1 mM; pH 8.3) at 6 V/cm. The pulse times were increased from 10 to 30 s. As size markers, XbaI restriction fragments of Salmonella enterica serovar Braenderup H9812 were used. Cluster analysis using dice similarity indices was done in BioNumerics 6.6 software (at 0.5% tolerance and 0.5% optimization) (Applied Maths, Ghent, Belgium) to determine similarities and differences and to find or characterize the relationships among isolates.
Chicken embryo lethality test
The method followed for this assay was described by Nolan et al. [16] and adapted by Trotereau and Schouler [17]. Virulence of five E. coli isolates (three ESBL and two broad-spectrum β-lactamases) was tested by the inoculation of washed bacterial cultures into the allantoic cavity of 11-day-old specific-pathogen-free chicken embryos. About 1.5 ml of overnight cultures of isolates grown in lysogeny broth (LB) at 37°C with shaking (180 rpm) were briefly centrifuged and resuspended in 1.5 mL of sterile/apyrogenic Dulbecco’s phosphate-buffered saline (DPBS). After the measurement of the OD at 600 nm, the inoculum was adjusted at a concentration of 103 cfu/mL. The inoculation dose was confirmed by retrospective plating of serial dilutions onto LB agar plates. For inoculation, 100 µL of the diluted culture was administered into the allantoic cavity of 20 embryos per isolate and 10 eggs with 100 µL of sterile/apyrogenic DPBS. Embryos were candled once daily for 6 days post-challenge to monitor mortality. The data of survival were presented as Kaplan–Meier curves and analyzed using the log-rank test.
Statistical analysis
The data were analyzed using multiple correspondence analysis (MCA) and p-values were calculated using a Chi-square test to find any significant relationship. p<0.05 was considered statistically significant. The proportion comparison Rprop test was performed using the statistical program R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Identification of 43 isolates as E. coli was confirmed by the presence of the uidA gene in all isolates. PCR analysis for virulence factors of the 43 E. coli strains according to both [10,11] diagnostics showed that 55.8% of the isolates harbored hlyF and ompT genes; 41.8% iroN, iss, and sitA genes. To a lesser extent, iutA genes and frzorf4 fragment were detected in 13.9% of the isolates and felA gene on only 2.3% of the strains tested. In contrast, no isolate contained aec26 gene (Table-1).
Table-1.
Virulence genes detected in avian pathogenic Escherichia coli isolates.
| Virulence factor | Frequency | Percentage | p-value |
|---|---|---|---|
| iroN | 18/43 | 41.80 | 1.203×10−8*** |
| iutA | 6/43 | 13.90 | 4.482×10−15*** |
| ompT | 24/43 | 55.80 | 2.89×10−6*** |
| iss | 18/43 | 41.80 | 1.203×10−8*** |
| hlyF | 24/43 | 55.80 | 2.89×10−6*** |
| papC | 1/43 | 2.30 | 2.2×10−16*** |
| felA | 1/43 | 2.30 | 2.2×10−16*** |
| frzorf4 | 6/43 | 13.90 | 4.482×10−15*** |
| aec26 | 0 | 0 | 0 |
| sitA | 18/43 | 41.80 | 1.203×10−8*** |
| No factor | 18/43 | 41.80 | 1.203×10−8*** |
Very highly significant value. iutA (aerobactin siderophore receptor gene), hlyF (putative avian hemolysin), iss (episomal increased serum survival gene), iroN (salmochelin siderophore receptor gene), and ompT (episomal outer membrane protease gene), frzorf4 (sugar metabolism), aec26 (component of a T6SS), sitA (iron transport gene)
Otherwise, the strains had highly variable content of virulence genes. 11.6% of the isolates associated simultaneously up to six virulence factors and the association of iroN, ompT, hlyF, iss, and sitA factors dominated with 23.25% (p<0.001). The ompT, hlyF, and frzorf4 combination was present in 14% of the strains, followed by papC, felA, iroN, ompT, hlyF, and iss alone and associated with iutA in 2.3% of the strains studied. About 41.9% did not show any of the searched virulence factors.
APEC diagnosis
E. coli strains (37.2%) were considered as potentially highly virulent APEC, according to Johnson et al. [10], which classify an E. coli as pathogenic based on the presence of minimum four of five virulence genes carried by plasmids associated with highly pathogenic APEC. Insight, the embryos mortality rate exceeding 40% after 3 days, the lethality test on chicken embryo confirmed the pathogenicity of the most tested isolates comprising two (E48 and E88) ESBL profile (Figure-1).
Figure-1.
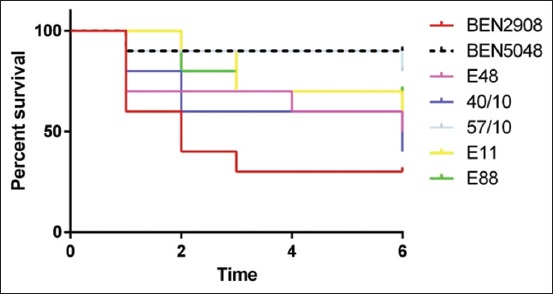
Chicken embryo test result on avian pathogenic Escherichia coli isolates. BEN 2908 APEC virulent strain; BEN 5048 negative control strains; E11, E48, and E88: ESBL profile; strains 40/10 and 57/10: broad-spectrum beta-lactamase profile.
In contrast, all isolate strains were considered non-typable according to Schouler et al. [11]. Eighteen isolates were considered as non-pathogenic strains regarding these both diagnostics. The most prevalent pattern with 23.25% of strains associate five virulence genes iroN, ompT, hlyF, iss, and sitA (Table-2). Regarding the search for virulence factors, high significant difference was found for the majority of genes proportions studied (p<2.2 e-16).
Table-2.
Virulence profile of avian pathogenic Escherichia coli isolates.
| Virulence profile | Virulence factor number | Frequency n=43 | Percentage | p-value |
|---|---|---|---|---|
| iroN ompT iss hlyF iutA sitA | 6 | 5 | 11.60 | 9.425×10−16*** |
| iroN ompT hlyF iss iutA | 5 | 1 | 2.30 | 2.2×10−16*** |
| iroN ompT hlyF iss sitA | 5 | 10 | 23.25 | 1.286×10−12*** |
| ompT hlyF iss | 3 | 1 | 2.30 | 2.2×10−16*** |
| ompT hlyF frzorf4 | 3 | 6 | 14.00 | 4.482×10−15*** |
| papC felA | 2 | 1 | 2.30 | 2.2×10−16*** |
| sitA | 1 | 1 | 2.30 | 2.2×10−16*** |
| None factor | 0 | 18 | 41.80 | 1.203×10−8*** |
Very highly significant value
Antibiotic resistance
Susceptibility study to 22 antibiotics belonging to seven chemical families of 36 isolates, from the collection of E. coli outcome colibacillosis lesions, revealed the sensitivity of a single isolate to all antibiotics. One isolate (2.7%) was resistant to a single molecule of antibiotic and 34 isolates (70.12%) to more than two antibiotics. In addition, a multidrug resistance observed on 53.5% of isolates covered more than 3 classes of antibiotics. In contrast, no resistance to colistin and imipenem was observed in studied isolates. The sensitivity and resistance profiles of the isolates tested are summarized in Table-3.
Table-3.
Susceptibility and resistance rates to antibiotics of avian pathogenic Escherichia coli isolates.
| Antibiotic | Number of isolates | Frequency and percentage of susceptibility (%) | Frequency and percentage of intermediate (%) | Frequency and percentage of resistance (%) | p-value |
|---|---|---|---|---|---|
| AMX | 36 | 5 (13.8) | 1 (2.8) | 30 (83.3) | 3.901×10−14*** |
| AMP | 36 | 5 (13.8) | 1 (2.8) | 30 (83.3) | 3.901×10−14*** |
| AMC | 36 | 10 (27.7) | 20 (55.6) | 6 (16.7) | 0.001503*** |
| TIC | 36 | 6 (16.7) | 1 (2.8) | 29 (80.5) | 5.23110−13*** |
| IPM | 36 | 38 (100) | 0 | 0 | <2.2×10−16*** |
| ATM | 35 | 20 (57.1) | 2 (5.8) | 13 (37.1) | 527×10−5*** |
| CZ | 34 | 9 (26.5) | 5 (14.7) | 20 (58.8) | 0.0003404*** |
| FOX | 35 | 27 (77.1) | 7 (20) | 1 (2.9) | 4.481×10−11*** |
| CTX | 36 | 21 (58.3) | 2 (5.6) | 13 (36.1) | 1.148×10−5*** |
| CAZ | 30 | 15 (50) | 2 (6.7) | 13 (43.3) | 0.0006426*** |
| CFM | 35 | 20 (57.1) | 1 (2.9) | 14 (40) | 5.403×10−6*** |
| CPO | 19 | 16 (84.2) | 1 (5.3) | 2 (10.5) | 5.829×10−8*** |
| K | 35 | 18 (51.4) | 2 (5.8) | 15 (42.8) | 9.142×10−5*** |
| GEN | 36 | 34 (94.4) | 1 (2.8) | 1 (2.8) | <2.2×10−16*** |
| TE | 35 | 15 (42,9) | 0 | 20 (57.1) | 8.931×10−7*** |
| CL | 35 | 35 (100) | 0 | 0 | <2.2×10−16*** |
| SXT | 36 | 11 (30.6) | 1 (2.8) | 24 (66.6) | 6.024×10−8*** |
| NA | 36 | 5 (13.8) | 1 (2.8) | 30 (83.4) | 3.901×10−14*** |
| PA | 36 | 4 (11.2) | 5 (13.8) | 27 (75) | 6.692×10−10*** |
| CIP | 35 | 14 (40) | 0 | 21 (60) | 4.129×10−7*** |
| OFX | 36 | 12 (33.4) | 1 (2.8) | 23 (63.8) | 2.7×10−7*** |
| PF | 35 | 15 (42.9) | 1 (2.8) | 19 (54.3) | 1.028×10−5*** |
The proportion comparison χ2 test performed for a value of α=5%;
Very highly significant value. AMX=Amoxicillin, AMP=Ampicillin, AMC=Amoxicillin+clavulanic acid, TIC=Ticarcillin, IPM=Imipenem, ATM=Aztreonam, CZ=Céfazolin, FOX=Cefoxitin, CTX=Cefotaxime, CAZ=Ceftazidime, CFM=Cefixime, CPO=Cefpirome, K=Kanamycin, GEN=Gentamicin, TE=Tetracycline, CL=Colistin, SXT=Triméthoprim+sulfamethoxazole, NA=Nalidixic acid, PA=Pipemidic acid, CIP=Ciprofloxacin, OFX=Ofloxacin, PF=Pefloxacin
Regarding beta-lactams, the results showed that the highest resistance rates were observed for aminopenicillins represented by ampicillin and amoxicillin and ticarcillin in the carboxypenicillin group with 83.3% and 80.5%, respectively. Average levels were noted with respect to other beta-lactams such as aztreonam (37.1%) and cefazolin (58.8%), cefotaxime (36.1%), cefixime (40%), and ceftazidime (43.3%), respectively, of the group of monobactams and cephalosporins of the first generation and third generation. The lowest resistance rates were recorded in the association amoxicillin-clavulanic acid (16.7%), the fourth-generation cephalosporins (cefpirome 10.5%) with the extreme value (2.9%) recorded for cefoxitin, the second-generation cephalosporin.
It should be noted that resistance to beta-lactams is associated with resistance to all other classes with the predominance of aminoglycoside with 14 isolates (38.8%) and beta-lactams-aminoglycoside-tetracycline-quinolones combination with 13 isolates (36.1%).
The analysis of the beta-lactam resistance profiles for all isolates according to CA-SFM [14] and Livermore et al. [18] showed that the main mechanism of resistance was the production of beta-lactamases. The majority of isolates (44.4%) carried broad-spectrum β-lactamases followed by the presence of ESBL on 36.1% of isolates and a single AmpC high-level cephalosporinase isolate.
Twenty-five strains (56.80%) carried at least one virulence factor and the profiles observed on the latter showed that the isolates were resistant to at least four antibiotics and also up to 16 antibiotics simultaneously.
The distribution of the isolates according to the presence of the virulence factors and the beta-lactam resistance profile (Table-4) showed that 56.30% of the broad-spectrum beta-lactamases profile was associated with the presence of five virulence genes iroN, ompT, iss, hlyF, and sitA and that the majority of ESBLs (46.2%) carried the ompT and hlyF genes and frzorf4 fragment.
Table-4.
Virulence profile associated with beta-lactam resistance mechanism.
| Mechanism virulence profile | BLSE (n=13) | Broad-spectrum beta-lactamase (n=16) | Cephalosporinase (n=1) |
|---|---|---|---|
| iroN ompT iss hlyF iutA sitA | 1 (7.7%) p1.509×10−5*** | 2 (12.4%) p3.612×10−6*** | 1 (100%) |
| iroN ompT iss hlyF sitA | 1 (7.7%) p1.509×10−5*** | 9 (56.3%) p0.0103** | - |
| ompT hlyF frzorf4 | 6 (46.2%) p0.007982*** | - | - |
| papC felA | - | 1 (6.25%) p7.07×10−7*** | - |
| sitA | - | 1 (6.25%) p7.07×10−7*** | - |
| None factor | 5 (38.4%) p0.002935** | 3 (18.8%) p1.566×10−5*** | - |
Very highly significant value;
highly significant value
The analysis of genetic profiles by PFGE showed the presence of five different genetic profiles, which reveals a diversity of the 43 avian E. coli analyzed (Figure-2). MCA analysis has shown that virulence determinants seem to have a phenotypical relationship with antibiotics resistance such revealed by MCA analysis (Figure-3).
Figure-2.
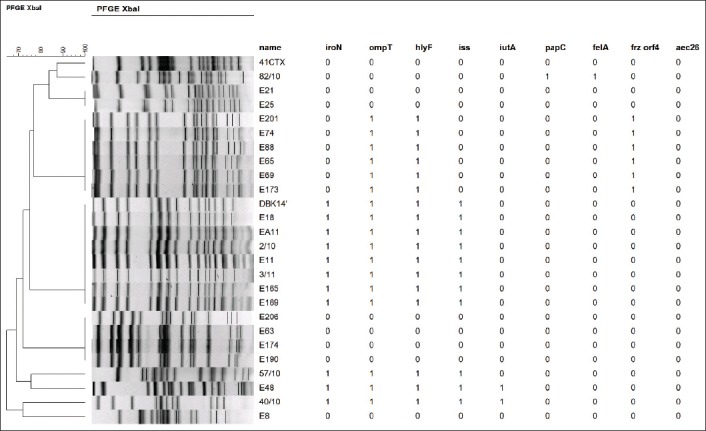
Molecular characterization and pulsed-field gel electrophoresis analysis of Escherichia coli isolates.
Figure-3.
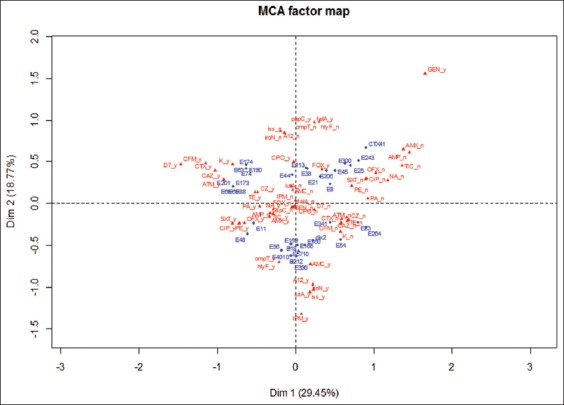
Graphical representation of the multiple correspondence analysis performed by the R software. Blue: Isolates; red: Virulence factors and antibiotics. The statistical data analysis by MCA has globally established a relationship between antibiotics resistance and virulence factors present in tested strains. The results of the ACM showed the first and second plans, respectively, expressing 29.45% and 18.77% of the total variability. The information contained on these plans is considered sufficient with 52.4% inertia value.
Discussion
Our results showed that E. coli strains studied are diverse with different pathogenicity patterns and some of them are considered as potentially highly virulent since they harbored four or five virulence genes [10,19]. However, certain strains isolated of lesions may not be pathogenic since none virulence factor has been detected with Johnson et al. [10] and Schouler et al. [11] APEC criteria.
APEC strains can be genetically very diverse and have a distinct repertoire of virulence genes [4]. It appears from the literature that virulence factors are not all present in the same isolate and there is not a single or a set of specific virulence genes systematically associated with APECs, hence, the difficulty diagnosis and implementation of treatment targeting all isolates [10,20]. Moreover, many authors report genetically diverse populations of E. coli in field cases of colibacillosis [21-24] and overall in APEC [25]. Furthermore, according to Collingwood et al. [26], colibacillosis in birds can result from infection with isolates of a pathotype other than APEC. Besides, most of the disease associated with E. coli in domestic poultry is as much a consequence of increased host susceptibility due to stress, immune suppression, coinfection, or poor welfare. This leads to more “opportunistic” infections rather than the result of infection with a specific pathotype.
In Algeria, the prevalence of virulence-associated factors still poorly is known. Among these rare studies, Lounis et al. [27] have reported, in opposition to our results, that the most prevalent genes in APEC were iutA (90.6%) followed by ompT (86.9%) and iss (85.8%). While Laarem et al. [28] detected the presence of 4 isolates (13.8%) of avian E. coli carried one of Shiga toxin E. coli-associated genes stx1, stx2, and ehxA alleles. Likewise, in a recent study in Algeria [29], a set of plasmidic virulence genes (iutA, fyuA, irp2, iroN, fimH, cvaC, traT, iss, sitA, ompT, hlyF, cvaA, etsA, etsB, eitA, and tsh) and chromosomal virulence genes (sitA fyuA, vat, and ibeA) associated with APEC have been detected, paradoxically, in fecal E. coli strains isolated from clinically healthy chickens.
An Egyptian studies have shown that among 91 non-repetitive E. coli isolates, 73 (80.2%) carried three or more of the APEC virulence genes iroN, ompT, iss, iutA, and hlyF [30] and according to Mohamed et al. [31], iss gene was found in 72.2% of the examined extraintestinal pathogenic E. coli (ExPEC) strains from diseased broiler chickens. More than 90% of the total APEC examined possessed iroN, ompT, hlyF, iss, and iutA, and 53.5% harbored plasmid pathogenicity islands. In Iran, eight different combination patterns of the virulence genes were detected among colibacillosis isolates [32]. In Zabol, as a border region of this country, 86.9% of isolates collected from chickens with colibacillosis were positive for iss gene [33]. For Paixão et al. [34], the iron uptake-related genes and the serum survival gene were more prevalent among APEC.
Despite the diversity of virulence gene profiles (iroN, ompT, hlyF, iss, and iutA and others) observed in suspected isolates of colibacillosis in South Africa [35], this study revealed, in agreement with our findings, that the iutA gene was not systematically present in the various samples.
It should be noted that papC, felA (2.3%), and aec26 genes are rarely found or inexistent in our strains set. Cunha et al. [36] also yielded a low prevalence of some genes that are frequently described in APEC, such as iss (37%), ompT, and hlyF (8% each). Consequently, the occurrence and frequency of these markers may vary according to the geographic origin and year of the isolation.
Moreover, this study does not exclude that the strains without virulence factor could have both variants of these genes [37] or an arsenal of virulence factors not detected which would be responsible for colibacillosis lesions. Further investigation should be undertaken for confirmation. It should be noted that these non APEC strain are resistant to at least one antibiotic and several are multidrug-resistant. Indeed, according to Moreno et al. [38] and Maciel et al. [39], commensal E. coli can also generate extra intestinal lesions influenced by antimicrobial resistance. On the other hand, these APEC characterization tests seem limited according to Dziva et al. [40] since far too often, avian isolates are considered as APEC according to exclusively on their PCR-detected genotypic profile. However, this is a misleading strategy since avian isolates should only be characterized as APEC if their virulence has been confirmed in animal models validated for avian colibacillosis [4].
Antimicrobial susceptibility tests were performed to characterize phenotypic features of isolates. Through the years, increased use of antibiotics has been observed in diverse activities in our country, including growth promote (although regulated since 2006), preventive, or treatment care. This extensive and uncontrolled use of antibiotics, combined with easiness of access, mostly for veterinary practice, led to the development of antibiotic resistance.
A high percentage of multidrug-resistant E. coli was detected in this study. It is known that E. coli strains isolated from poultry frequently show multi-resistance to more than one antimicrobial drug [41] which represents a global concern.
In Algeria, antibiotic resistance has been further studied than virulence contents of APEC. The most recent reports have shown that E. coli isolates in poultry products harbor high levels of resistance to tetracycline and sulfamethoxazole (96.6%), ciprofloxacin (72%), and amoxicillin (65.5%) [28]. Halfaoui et al. [42] have isolated pathogenic E. coli strains from broiler chicken with colibacillosis in the central of Algeria that presented a high level of resistance to tetracycline (94.12%), flumequine (91.5%), sulfamethoxazole-trimethoprim (88.89%), enrofloxacin (86.27%), nalidixic acid (85.62%), ampicillin (83.01%), and doxycycline (75.81%).
Another result from APEC [28] also showed that the highest rates of resistance were against tetracycline (97.4%). High levels of resistance were again observed in the same study for sulfisoxazole (94.9%), trimethoprim-sulfamethoxazole (92.3%), ampicillin (89.7%), and ofloxacin (84.6%). Colistin (2.8%) and gentamicin (35.9%) seemed to be among the most efficient antibiotics against APEC isolates. The moderate resistance of gentamicin may be due to its illicit use knowing that this antibiotic is prohibited in veterinary medicine in Algeria. While, it was observed an emergence of mcr-1-mediated colistin resistance in E. coli isolates from poultry in Algeria [43]. Curiously, none ESBL was revealed in these studies while these enzymes, especially CTX-M-1 type, were previously detected since 2015 [44].
Similar to our findings in Morocco [45] and Central Ethiopia [46], extremely high levels of resistance to amoxicillin (90.9% and 100%, respectively) and trimethoprim + sulfamethoxazole (82.2%) were recorded and low frequencies of resistances were noted for gentamicin (24.8%) and colistin (2%) [45], similarly, at Italian findings of Sgariglia et al. [25]. Only these past 2 years, several other publications across the globe have signalized rising levels of antibiotic resistance in APEC isolated from colibacillosis such as Nepal [47] and Senegal [48].
Our results have shown that virulence determinants seem to have a relationship with antibiotics resistance at least in its phenotypic aspect. According to Da Silva and Mendonça [49], the topic on the link on resistance/virulence is complex, considering the diversity of antimicrobial resistance genes, virulence factors, bacterial species, and hosts. Most reports on his topic correlate the epidemiology of specific resistance genes with virulence genetic traits. This is a first step toward understanding whether there is a connection between resistance and virulence [6]. It is conceivable that virulence genetic determinants, if located on the same genetic platform as antimicrobial resistance genes (plasmids, transposons, and integrons), may be comobilized under antimicrobial selective pressure [49] and that both clones and plasmid may be involved on the dissemination of multiresistant E. coli [48]. Therefore, although antibiotic resistance is not in itself a virulence factor, in certain situations, it is a key factor in the development of infection, and it may be considered a virulence like factor in specific ecological niches which antibiotic-resistant bacteria are able to colonize [50].
Conclusion
Our current study characterized the genetic contents of virulence and antimicrobial resistance in the E. coli strains isolated from broilers colibacillosis lesions in central of Algeria. Indeed, concerning APEC virulence characterization, this study remains a contribution and not definitive data for many other Algerian regions and does not represent the virulence gene content in the whole country.
However, the high frequency of antimicrobial resistance, associated with several virulence factors, in particular, the presence of ESBLs strains harboring until six virulence factors in these APEC strains, represents a potential public health problem that requires to maintain constant vigilance guidelines and regulations.
Authors’ Contributions
NM designed and performed the study and wrote the manuscript. NC, AT, CAA, and SB managed the analyses of the study and were involved in data analysis. CS direct and supervised the project. All authors read and approved the final manuscript.
Acknowledgments
This study was partly supported by Mouloud Mammeri University and ISP, INRA, Université de Tours. The authors did not receive any funding for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Kaci A. La filière avicole algérienne àl'ère de la libéralisation économique. Cah. Agric. 2015;24(3):151–160. [Google Scholar]
- 2.Dho-Moulin M, Fairbrother J.M. Avian pathogenic Escherichia coli (APEC) Vet. Res. 1999;30(2-3):299–316. [PubMed] [Google Scholar]
- 3.Clermont O, Olier M, Hoede C, Diancourt L, Brisse S, Keroudean M, Glodt J, Picard B, Oswald E, Denamur E. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infect. Genet. Evol. 2011;11(3):654–662. doi: 10.1016/j.meegid.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Mellata M. Human and avian extraintestinal pathogenic Escherichia coli:infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10(11):916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada K, Asai T. Role of antimicrobial selective pressure and secondary factors on antimicrobial resistance prevalence in Escherichia coli from food-producing animals in Japan. J. Biomed. Biotechnol. 2010;2010((June 2)):180682. doi: 10.1155/2010/180682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitout J.D. Extraintestinal pathogenic Escherichia coli:A combination of virulence with antibiotic resistance. Front. Microbiol. 2012;3((Jan 19)):9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitout J.D. Enterobacteriaceae that produce extended-spectrum beta-lactamases and AmpC beta-lactamases in the community:The tip of the iceberg? Curr. Pharm. Des. 2013;19(2):257–263. [PubMed] [Google Scholar]
- 8.Bej A.K, Steffan R.J, DiCesare J, Haff L, Atlas R.M. Detection of coliform bacteria in water by polymerase chain reaction and gene probes. Appl. Environ. Microbiol. 1990;56(2):307–314. doi: 10.1128/aem.56.2.307-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch E.F, Maniatis T. Molecular Cloning:A Laboratory Manual. 2nd ed. New York: Cold Spring Harbor Laboratory, Cold Spring Harbor; 1989. [Google Scholar]
- 10.Johnson T.J, Wannemuehler Y, Doetkott C, Johnson S.J, Rosenberger S.C, Nolan L.K. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J. Clin. Microbiol. 2008;46(12):3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schouler C, Schaeffer B, Brée A, Mora A, Dahbi G, Biet F, Oswald E, Mainil J, Blanco J, Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012;50(5):1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dho M, Lafont J.P. Escherichia coli colonization of the trachea in poultry:Comparison of virulent and avirulent strains in gnotoxenic chickens. Avian Dis. 1982;26(4):787–797. [PubMed] [Google Scholar]
- 13.Schouler C, Koffmann F, Amory C, Leroy-Sétrin S, Moulin-Schouleur M. Genomic subtraction for the identification of putative new virulence factors of an avian pathogenic Escherichia coli strain of O2 serogroup. Microbiology. 2004;150(Pt 9):2973–2984. doi: 10.1099/mic.0.27261-0. [DOI] [PubMed] [Google Scholar]
- 14.CA-SFM. Comitéde L'antibiogramme de la SociétéFrançaise de Microbiologie Recommandations. Paris, France: CA-SFM; 2015. [Google Scholar]
- 15.Moulin-Schouleur M, Schouler C, Tailliez P, Kao M.R, Brée A, Germon P, Oswald E, Mainil J, Blanco M, Blanco J. Common virulence factors and genetic relationships between O18:K1:H7Escherichia coli isolates of human and avian origin. J. Clin. Microbiol. 2006;44(10):3484–3482. doi: 10.1128/JCM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan L.K, Wooley R.E, Brown J, Spears K.R, Dickerson H.W, Dekich M. Comparison of a complement resistance test, a chicken embryo lethality test, and the chicken lethality test for determining virulence of avian Escherichia coli. Avian Dis. 1992;36(2):395–397. [PubMed] [Google Scholar]
- 17.Trotereau A, Schouler C. Use of a chicken embryo lethality assay to assess the efficacy of phage therapy. Methods Mol. Biol. 2019;1898(4):199–205. doi: 10.1007/978-1-4939-8940-9_17. [DOI] [PubMed] [Google Scholar]
- 18.Livermore D.M, Winstanley T.G, Shannon K.P. Interpretative reading:Recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. J. Antimicrob. Chemother. 2001;48(Suppl 1):87–102. doi: 10.1093/jac/48.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- 19.Vounba P, Kane Y, Ndiaye C, Arsenault J, Fairbrother J.M, Alambédji R.B. Foodborne molecular characterization of Escherichia coli isolated from chickens with colibacillosis in senegal. Pathog. Dis. 2018;15(8):517–525. doi: 10.1089/fpd.2017.2394. [DOI] [PubMed] [Google Scholar]
- 20.Guabiraba R, Schouler C. Avian colibacillosis:Still many black holes. FEMS Microbiol. Lett. 2015;362(15):fnv118. doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 21.La Ragione R.M, Woodward M.J. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res. Vet. Sci. 2002;73(1):27–35. doi: 10.1016/s0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 22.Ewers C, Janssen T, Wieler L.H. Avian pathogenic Escherichia coli (APEC) Berl. Münch. Tierärztl. Wochenschr. 2003;116(9-10):381–395. [PubMed] [Google Scholar]
- 23.Rodriguez-Siek K.E, Giddings C.W, Doetkott C, Johnson T.J, Fakhr M.K, Nolan L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151(Pt 6):2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- 24.Stacy A.K, Mitchell N.M, Maddux J.T, De la Cruz M.A, Durán L, Girón J.A, Curtiss R, Mellata M. Evaluation of the prevalence and production of Escherichia coli common pilus among avian pathogenic E. coli and its role in virulence. PLoS One. 2014;9(1):e86565. doi: 10.1371/journal.pone.0086565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sgariglia E, Mandolini N.A, Napoleoni M, Medici L, Fraticelli R, Conquista M, Gianfelici P, Staffolani M, Fisichella S, Capuccella M, Sargenti M, Perugini G. Antibiotic resistance pattern and virulence genesin avian pathogenic Escherichia coli (APEC) from different breeding systems. Vet. Ital. 2019;55(1):26–33. doi: 10.12834/VetIt.1617.8701.1. [DOI] [PubMed] [Google Scholar]
- 26.Collingwood C, Kemmett K, Williams N, Wigley P. Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Front. Vet. Sci. 2014;1((Oct 26)):5. doi: 10.3389/fvets.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lounis M, Ge Z, Yuehua L, Gao Y, Kaidi R, Oumouna M, Wang J, Oumouna K. Virulence traits of avian pathogenic (APEC) and fecal (AFEC)E. coli isolated from broiler chickens in Algeria. Trop. Anim. Health Prod. 2018;50(3):547–553. doi: 10.1007/s11250-017-1467-5. [DOI] [PubMed] [Google Scholar]
- 28.Laarem M, Barguigua A, Nayme K, Akila A, Zerouali K, El Mdaghri N, Timinouni M. Occurrence of plasmid-mediated quinolone resistance and virulence genes in avian Escherichia coli isolates from Algeria. J. Infect. Dev. Ctries. 2017;11(2):143–151. doi: 10.3855/jidc.8643. [DOI] [PubMed] [Google Scholar]
- 29.Messaili C, Messai Y, Bakour R. Virulence gene profiles, antimicrobial resistance and phylogenetic groups of fecal Escherichia coli strains isolated from broiler chickens in Algeria. Vet. Ital. 2019;55(1):35–46. doi: 10.12834/VetIt.799.3865.2. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A.M, Shimamoto T, Shimamoto T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 2013;303(8):475–483. doi: 10.1016/j.ijmm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed M.A, Shehata M.A, Rafeek E. Virulence genes content and antimicrobial resistance in Escherichia coli from broiler chickens. Vet. Med. Int. 2014;2014((Nov 24)) doi: 10.1155/2014/195189. Article ID 195189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asadi A, Saleh I.T.Z, Jamshidian M, Ghanbarpour R. ECOR phylotyping and determination of virulence genes in Escherichia coli isolates from pathological conditions of broiler chickens in poultry slaughter-houses of Southeast of Iran. Vet. Res. Forum. 2018;9(3):211–216. doi: 10.30466/vrf.2018.30827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonjar M.S.S, Salari S, Jahantigh M, Rashki A. Frequency of iss and irp2 genes by PCR method in Escherichia coli isolated from poultry with colibacillosis in comparison with healthy chicken in poultry farms of Zabol, South East of Iran. Pol. J. Vet. Sci. 2017;20(2):363–367. doi: 10.1515/pjvs-2017-0044. [DOI] [PubMed] [Google Scholar]
- 34.Paixão A.C, Ferreira A.C, Fontes M, Themudo P, Albuquerque T, Soares M.C, Fevereiro M, Martins L, Corrêa, de Sá M.I. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates. Poult. Sci. 2016;95(7):1646–1652. doi: 10.3382/ps/pew087. [DOI] [PubMed] [Google Scholar]
- 35.Van der Westhuizen W.A, Bragg R.R. Multiplex polymerase chain reaction for screening avian pathogenic Escherichia coli for virulence genes. Avian Pathol. 2012;41(1):33–40. doi: 10.1080/03079457.2011.631982. [DOI] [PubMed] [Google Scholar]
- 36.Cunha M.P.V, Saidenberg A.B, Moreno A.M, Ferreira A.J.P, Vieira M.A.M, Gomes T.A.T, Knöbl T. Pandemic extra-intestinal pathogenic Escherichia coli (ExPEC) clonal group O6-B2-ST73 as a cause of avian colibacillosis in Brazil. PLoS One. 2017;12(6):e0178970. doi: 10.1371/journal.pone.0178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delicato E.R, de Brito B.G, Gaziri L.C, Vidotto M.C. Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet. Microbiol. 2003;94(2):97–103. doi: 10.1016/s0378-1135(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 38.Moreno E, Prats G, Planells I, Planes A.M, Pérez T, Andreu A. Characterization of Escherichia coli isolates derived from phylogenetic groups A and B1 causing extraintestinal infection. Enferm. Infecc. Microbiol. Clin. 2006;24(8):483–489. doi: 10.1157/13092463. [DOI] [PubMed] [Google Scholar]
- 39.Maciel J.F, Matter L.B, Trindade M.M, Camillo G, Lovato M, de Ávila Botton S, Castagna de Vargas A. Virulence factors and antimicrobial susceptibility profile of extraintestinal Escherichia coli isolated from an avian colisepticemia outbreak. Microb. Pathog. 2017;103((Dec21)):119–122. doi: 10.1016/j.micpath.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Dziva F, Stevens M.P. Colibacillosis in poultry:Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37(4):355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 41.Nhung N.T, Chansiripornchai N, Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens:A review. Front. Vet. Sci. 2017;4(Aug 10):126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halfaoui Z, Menoueri N.M, Bendali L.M. Serogrouping and antibiotic resistance of Escherichia coli isolated from broiler chicken with colibacillosis in center of Algeria. Vet. World. 2017;10(7):830–835. doi: 10.14202/vetworld.2017.830-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabou S, Leulmi H, Rolain J.M. Emergence of mcr-1-mediated colistin resistance in Escherichia coli isolates from poultry in Algeria. J. Glob. Antimicrob. Resist. 2018;16:115–116. doi: 10.1016/j.jgar.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Meguenni N, Le Devendec L, Jouy E, Le Corvec M, Bounar-Kechih S, Bakour R, Kempf I. First description of an extended-spectrum cephalosporin- and fluoroquinolone resistant avian pathogenic Escherichia coli clone in Algeria. Avian Dis. 2015;59(1):20–23. doi: 10.1637/10804-022414-reg.1. [DOI] [PubMed] [Google Scholar]
- 45.Rahmatallah N, Nassik S, El Rhaffouli H, Amine I.L, El Houadfi M. Détection de souches multi-résistantes d'Escherichia coli d'origine aviaire dans la région de Rabat-Salé-Zemmour-Zaer. Rev. Mar. Sci. Agron. Vét. 2017;5(2):96–102. [Google Scholar]
- 46.Sarba E.J, Kelbesa K.A, Bayu M.D, Gebremedhin E.Z, Borena B.M, Teshale A. Identification and antimicrobial susceptibility profile of Escherichia coli isolated from backyard chicken in and around ambo, Central Ethiopia. BMC Vet. Res. 2019;15(1):85. doi: 10.1186/s12917-019-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subedi M, Luitel H, Devkota B, Bhattarai R.K, Phuyal S, Panthi P, Shrestha A, Chaudhary D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018;14(1):113. doi: 10.1186/s12917-018-1442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vounba P, Arsenault J, Bada-Alambédji R, Fairbrother J.M. Pathogenic potential and the role of clones and plasmids in beta-lactamase-producing E.coli from Achicken faeces in Vietnam. BMC Vet. Res. 2019;15(1):106. doi: 10.1186/s12917-019-1849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Da Silva G.J, Mendonça N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence. 2012;3(1):18–28. doi: 10.4161/viru.3.1.18382. [DOI] [PubMed] [Google Scholar]
- 50.Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence:A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013;26(2):185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


