Abstract
Intestinal barrier dysfunction is hypothesized to be a contributing determinant of two prominent characteristics of aging: inflammation and decline in physical function. A relationship between microbial translocation (MT), or their biomarkers (lipopolysaccharide binding protein-1 [LBP-1], soluble cluster of differentiation [sCD]-14), and physical function has been reported in healthy older adults, rats, and invertebrates. However, it is not known whether the existence of comorbidities, or clinical interventions intended to reduce comorbidities through weight loss or exercise, alters this connection. We measured inflammation, MT, and physical function in 288 overweight/obese older patients with cardiometabolic disease and self-reported mobility limitations who were enrolled in a weight loss and lifestyle intervention study. At baseline, inflammatory cytokines and LBP-1 were positively correlated after adjustment for age, gender, and body mass index. A higher LBP-1 was significantly associated with poorer physical functional after covariate adjustment. Further, even when IL-6 levels were included in the models, 400-m walk time (p = 0.003), short physical performance battery (p = 0.07), and IL-8 (p < 0.001) remained positively associated with LBP-1. Lifestyle interventions improved body mass and some functional measures; however, MT and inflammation were unchanged. MT is reliably related to inflammation, and to poorer physical function in older adults with comorbid conditions. Intestinal barrier function did not appear to improve as a result of intervention assignment, suggesting alternative strategies are needed to target this pro-inflammatory pathway in aging.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00112-z) contains supplementary material, which is available to authorized users.
Keywords: Microbial translocation, Lipopolysaccharide-binding protein, Ageing, Physical function
Introduction
Decline in physical function is a characteristic of aging that can begin as early as the fifth decade of life (Hall et al. 2016). In addition to its importance in maintaining independence, physical function in the elderly can predict future cardiovascular disease, mobility disability, and mortality (Newman et al. 2006). Chronic, sterile, and low-grade inflammation indicated by circulating C-reactive protein (CRP), and interleukin 6 (IL-6) levels, is another characteristic of aging. This phenomenon has been termed inflammaging (Saffrey 2014). However, the basis of this increased inflammatory burden is unclear (Hollander and Tarnawski 1985; Franceschi et al. 2007). Possible sources of circulating inflammatory stimuli include self-debris, such as macromolecules that result from cellular breakdown, and live or dead bacterial products sourced from microbiota (Franceschi et al. 2007).
The gastrointestinal (GI) tract is the largest area of the human body that interacts with the environment. The human GI tract is estimated to contain > 1014 microorganisms (Thursby and Juge 2017). These microorganisms are essential for digestion and absorption and are part of the natural barrier against pathogens. Although the GI tract has constant contact with these microorganisms, significant bacterial invasion of the human body rarely happens as long as the GI barrier is intact. Microbial translocation (MT) is the passage of both viable and nonviable microbial products, such as lipopolysaccharide (LPS), across an anatomically intact intestinal barrier and is considered a normal process (D'Ettorre et al. 2012). The barrier is comprised of physical (mucus and the epithelial cells), biochemical (bile salts, enzymes, antibacterial proteins), immunological (IgA and immune cells), and microbial components (the microbiota) (Chassaing et al. 2014). Any defect in these aforementioned barrier elements can result in elevated immune activation and inflammation and/or infection. Intestinal barrier dysfunction is commonly observed in aging vertebrates and invertebrates, and is associated with multiple markers of aging, including systemic metabolic dysfunction (Rera et al. 2012; Ghosh et al. 2015; Kavanagh et al. 2016; Wilson et al. 2018). A current hypothesis is that decreasing intestinal barrier function with aging can cause increased MT into the systemic blood circulation that subsequently causes systemic inflammation (inflammaging) and significant clinical outcomes (e.g., metabolic syndrome, decreased physical function, and mortality).
In a cross-sectional study of 59 older healthy adults, MT burden, measured by LPS-binding protein (LBP), was inversely correlated with physical function, measured by short physical performance battery (SPPB) and grip strength. Correlations between LBP and physical function remained significant even after adjusting for each inflammatory biomarker (Stehle Jr. et al. 2012). Although this study shed light on the association between MT and physical function in the elderly, it was a small study in participants without common comorbidities that typify normal aging. It is not clear if the association remains in older adults with comorbidities and likely higher inflammatory burdens. We aimed to examine the relationship between MT and physical function in overweight or obese older adults who had either cardiovascular disease (CVD) or cardiometabolic dysfunction and evidence of self-reported limitations in mobility. Furthermore, it is unclear whether common behavioral interventions that improve mobility may do so through some effect on MT burden. With the known association between inflammation and physical performance (Brinkley et al. 2009), we hypothesized that increased MT may cause chronic inflammation and subsequent declines in physical function with aging.
Methods
Study design
This was a secondary analysis of a randomized clinical trial: The Cooperative Lifestyle Intervention Program (CLIP; NCT00119795). CLIP was a randomized controlled trial of 288 ambulatory, older (60–79 years), community-dwelling adults from three NC counties conducted between January 2005 and April 2010. The detailed study design and trial outcomes have been previously reported (Rejeski et al. 2011; Beavers et al. 2013; Beavers, Beavers et al. 2014).
Eligibility criteria
Subjects were enrolled if they were ambulatory, overweight or obese, community-dwelling older adults who had either CVD or cardiometabolic dysfunction and evidence of self-reported limitations in mobility. Subjects had to meet the criteria: (1) age 60 to 79 years; (2) having less than 60 min/week of moderate, structured PA; (3) having a body mass index (BMI), calculated as weight in kilograms divided by height in meters squared, greater than 28; (4) having evidence of a myocardial infarction, percutaneous transluminal angioplasty, chronic stable angina, or cardiovascular surgery in the past 6 months or an Adult Treatment Panel III diagnosis of the metabolic syndrome; (5) having a self-reported mobility limitation; and (6) willingness to sign an informed consent and a HIPPA authorization form.
Subjects were excluded if they had (1) a baseline BMI of 40 or higher; (2) bipolar disorder, depression, or schizophrenia; (3) unstable angina, symptomatic congestive heart failure, or exercise-induced complex ventricular arrhythmias; (4) resting blood pressure greater than 160/100 mmHg; (5) diagnosis of systemic diseases that precluded participants from safely participating in the interventions; (6) a fasting blood glucose level higher than 140 mg/dl (7.77 mmol/l), type 1 diabetes mellitus (DM), or type 2 DM with insulin therapy; (7) active treatment for cancer; (8) clinically significant visual or hearing impairment; (9) dementia, delirium, or impaired cognitive function; (10) participation in another medical intervention study; (11) having more than 21 alcoholic drinks per week; (12) inability to walk unassisted; and (13) inability to speak or read English.
Intervention arms
Successful aging (SA)
This group received scripted educational lessons on health tailored for older adults. The SA group met weekly for the first 2 months, monthly through month 6, and then bimonthly until the end of the study. Participants in this arm did not receive a progressive, supervised program of physical activity (PA) or diet for weight loss (WL); however, both PA and nutrition for aging were addressed as separate and distinct topics.
Physical activity
The goal of the PA intervention was to gradually increase or shape PA in a home-based environment to more than 30 min of moderately intense activity on most days of the week for a total of more than 150 min/week. Participants walked at a moderate intensity of “somewhat hard” as assessed by the Borg Rating of Perceived Exertion Scale (Borg 1982) and written self-monitoring logs were used to document. The first 6 months was considered as an intensive phase and the activities include counseling sessions: three 90-min group sessions and one 30-min individual session each month. Months 6–12 consisted of a transition phase that was followed by a maintenance phase out to 18 months. This report is restricted to data from the 6-month intensive phase of the intervention.
Weight loss and physical activity (WL + PA)
This group involved the PA plus dietary weight loss. The goal of WL was to reduce caloric intake to produce weight loss of 0.3 kg/week for the first 6 months for a total loss in mass of 7–10%. Participants were assigned a daily calorie intake goal based on baseline weight (1200–1500 kcal for participants < 250/114 lbs/kg, and 1500–1800 kcal for participants ≥ 250/114 lbs/kg). Participants were given food tracking booklets and at the end of each week, an average calorie intake was calculated. In addition to this weight loss intervention, PA sessions were given as described above.
Measurements
Participants were assessed pre-randomization and returned at 6 months. Demographics, medical history, and comorbidities were collected by self-report, and height and weight were measured at each visit. The 400-m walk test was used to assess mobility (Simonsick et al. 2001). Physical activity measured using the Lifecorder-EX accelerometer (New-Lifestyles Inc., Lees Summit, MO) was used to assess physical activity. Participants were asked to wear the accelerometer for 7 days (Rejeski et al. 2011). Short physical performance battery (SPPB) (Guralnik et al. 1994) which is composed of balance, gait speed, and chair speed was assessed at baseline and 6-month follow-up visit. Intensity levels 3–9 were classified as moderate to vigorous for this study of the elderly population. Physical activity classified as minutes of moderate to vigorous activity (AccelMVPA) and total physical activity (AccelPAEE) was analyzed.
Blood samples were collected in the early morning after a 12-h fast. The 6-month blood samples were collected after at least 24 h after the last acute bout of exercise. All blood was collected, processed, divided into aliquots, and stored in the Biological Specimen Repository at Wake Forest School of Medicine at − 80 °C. Adiponectin, high-sensitivity IL-6 (hsIL-6), IL6Sr, IL-8, and sTNFR1 were measured.
Biomarkers of microbial translocation (MT)
All analyses were performed by ELISA. We focused on the two plasma biomarkers: LBP-1 and soluble CD14 (sCD14). LBP-1 (Hycult Biotech, Netherlands) and sCD14 (R&D Systems, Minneapolis, MN) were measured in duplicate to improve accuracy of measures. LBP-1 is a soluble protein produced by hepatocytes and intestinal epithelial cells, which binds LPS and promotes immune responses (Muta and Takeshige 2001). sCD14 is a glycoprotein secreted from the liver as a result of toll-like innate immune receptor (TLR) family activation, allowing interaction with the antigen bound to LBP-1, and circulates to help bind bacterial products. sCD14 is a co-receptor for toll-like receptor 4. LBP-1 assay has an average coefficient of variation of 8.97% (standard error 1.58) and sCD14 has an average coefficient of variation of 11.8% (standard error 2.86).
Statistical analysis
Means and standard deviations (SDs) were presented for continuous baseline characteristics. Counts and percentages were presented for discrete baseline characteristics. Six-month mean changes and SDs in BMI, physical function, inflammatory markers, and MT markers were calculated across the three randomization groups and compared using analysis of variance.
Baseline associations of MT markers and inflammatory markers and physical function after controlling for age, gender, and BMI were calculated using linear regression models. The outcome measures are inflammatory markers and physical function and MT markers are the covariates of interest. The distributions of outcome measures were checked and transformations were performed if needed to satisfy the conditional normality assumption. Adiponectin, total activity energy expenditure, moderate to vigorous activity energy expenditure, IL-6, IL-8, and 400-m walk time were log-transformed. The associations relating MT markers to inflammatory markers and physical function were also performed using linear regression models, with additional adjustment for group randomization at 6 months. We also performed the sensitivity analysis for those measures with outliers, by removing outlying values that were defined as 3 SDs away from the mean value.
To study the effects of study interventions on 6-month change in markers of microbial translocation, analysis of covariance was used with adjustment of baseline outcome measure, age, gender, and BMI. The overall test for randomization groups with 2 degrees of freedom (df) was presented. Further, analysis of covariance was also used to examine the associations between baseline LBP-1 and 6-month changes in physical function after adjusting for baseline physical function, age, gender, BMI, and randomization groups.
Results
We analyzed data of 288 ambulatory, overweight or obese, community-dwelling participants aged 60–79 who had either CVD or cardiometabolic dysfunction and evidence of self-reported limitations in mobility. Baseline characteristics are demonstrated in Table 1. The mean age was 67, 67% were female, 82% were white, and the mean BMI was 32.9 kg/m2. Study outcomes relating to changes in body composition, physical function, and biomarker changes have been previously reported (Rejeski et al. 2011; Beavers et al. 2013 Beavers, Beavers et al. 2014). Collectively, these reports describe weight loss, improved IL-6 levels, and physical function with WT + PA and are trial outcomes relevant to the current report.
Table 1.
Summary statistics describing the clinical characteristics, physical function, inflammatory markers, and markers of microbial translocation for all study participants (n = 288) prior to randomization
| Variables | Baseline (n = 288) |
|---|---|
| Age (mean (SD)) | 67.0 (4.8) |
| Female (n (%)) | 193 (67) |
| White (n (%)) | 236 (82) |
| BMI (kg/m2 (SD)) | 32.9 (3.8) |
| 400-m walk time (seconds (SD)) | 353 (75) |
| AccelMVPA (minutes (SD)) | 115 (77) |
| AccelPAEE (minutes (SD)) | 1288 (597) |
| Adiponectin (ng/ml (SD)) | 7764 (5820) |
| IL-6 (pg/ml (SD)) | 2.7 (1.9) |
| Il-8 (pg/ml (SD)) | 3.9 (6.9) |
| IL_6sR (pg/ml (SD)) | 40,651 (12,127) |
| SPPB (SD) | 10 (1.5) |
| LBP (ng/ml (SD)) | 38 (13) |
| CD14 (ng/l (SD)) | 2.4 (1.0) |
Abbreviations: SD, standard deviation; BMI, body mass index; AccelMVPA, moderate to vigorous physical activity energy expenditure; AccelPAEE, total physical activity energy expenditure; IL, interleukin; SPPB, short physical performance battery; LBP-1, lipopolysaccharide-binding protein 1; sCD14, soluble cluster of differentiation 14
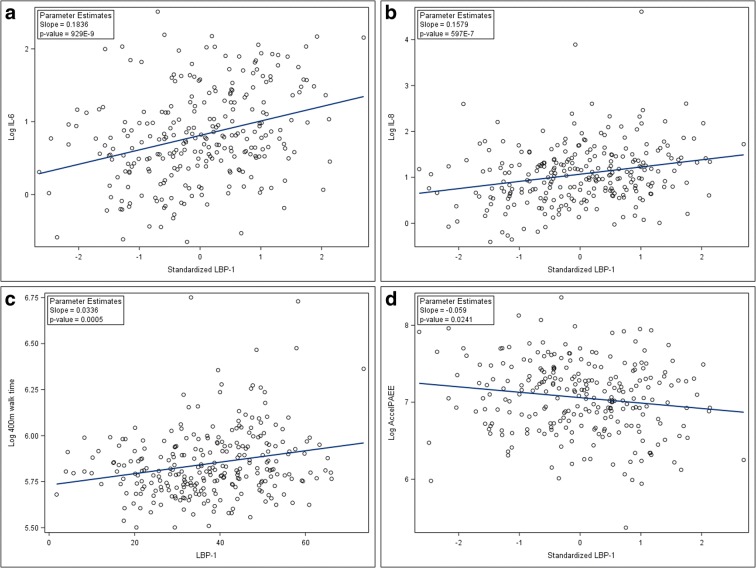
At baseline, the MT biomarker LBP-1 was associated with BMI (r = 0.143, p = 0.019) but not with age (p = 0.57). At baseline, serum LBP-1 level was associated with log-transformed IL-6 and log-transformed IL-8 both before and after controlling for age, gender, and BMI (Table 2; Fig. 1). Baseline serum LBP-1 levels were also associated with log-transformed total energy expenditure and SPPB, as well as log-transformed 400-m walk time. Serum sCD14 was associated with log-transformed IL-6 and log-transformed IL-8 after controlling for age, gender, and BMI (Table 2). In all analyses, LBP-1 associations were stronger than those with sCD14. The result for the sensitivity analysis after removing outliers was similar (Supplementary Table 1).
Table 2.
Baseline associations of MT markers with inflammatory markers and physical function after controlling for age, gender, and BMI
| LBP-1 (SD = 13.5) Standardized regression coefficient ± SE |
p value | SCD14 (SD = 0.97) Standardized regression coefficient ± SE |
p value | |
|---|---|---|---|---|
| Log adiponectin | − 0.05 ± 0.04 | 0.27 | 0.02 ± 0.04 | 0.64 |
| Log IL-6 | 0.18 ± 0.04 | < 0.001 | 0.08 ± 0.04 | 0.035 |
| Log IL-8 | 0.16 ± 0.04 | < 0.001 | 0.10 ± 0.04 | 0.012 |
| IL-6sR | 1412 ± 740 | 0.06 | 450 ± 738 | 0.54 |
| Log AccelMVPA | − 0.06 ± 0.05 | 0.18 | − 0.05 ± 0.05 | 0.28 |
| Log AccelPAEE | − 0.06 ± 0.03 | 0.024 | − 0.01 ± 0.03 | 0.81 |
| SPPB | − 0.17 ± 0.08 | 0.049 | − 0.13 ± 0.09 | 0.14 |
| Log 400-m walk time | 0.03 ± 0.01 | 0.001 | 0.01 ± 0.010 | 0.16 |
Abbreviations: SE, standard error
Fig. 1.
Scatterplots of baseline values showing unadjusted associations of raw LBP-1 concentrations and inflammation biomarkers: IL-6 (a) and IL-8 (b), and physical function measures; 400-m walk time (c), physical activity (d; total energy expenditure as measured by wearable accelerometers (AccelPAEE))
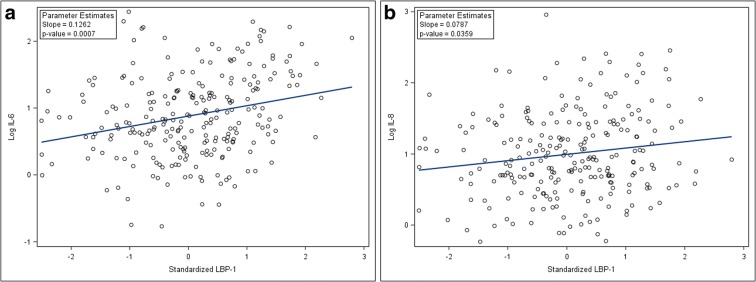
Despite the intervention arms of the trial being successful in achieving weight loss, improving physical function, and reducing IL-6 (Rejeski et al. 2011; Beavers et al. 2013), there were no significant differences in changes in MT markers by intervention groups after 6 months of study (Table 3). The analyses of MT and inflammatory markers and physical function at 6 months demonstrate that there remained a significant association between LBP-1 and log-transformed IL-6 and log-transformed IL-8 (Fig. 2). Also, serum sCD14 was associated with log-transformed serum IL-6 after controlling for age, gender, BMI, and randomization groups. However, there was no significant association with biomarkers of MT and physical function at 6 months (Table 4). The result for the sensitivity analysis after removing outliers was similar except that the association between sCD14 and log-transformed IL-8 became significant (p = 0.014; Supplementary Table 2). Further, baseline LBP-1 levels did not predict changes in physical function over the 6-month period.
Table 3.
Effects of study interventions on 6-month change in markers of microbial translocation
| Successful aging (N = 86) Raw mean (SD) |
Physical activity (N = 86) Raw mean (SD) |
Weight loss and physical activity (N = 95) Raw mean (SD) |
p value* | |
|---|---|---|---|---|
| 6-Month change in LBP-1 | 1.05 (11.50) | − 0.27 (11.38) | 0.93 (14.76) | 0.87 |
| 6-Month change in CD14 | 0.11 (1.05) | − 0.03 (1.00) | 0.04 (0.91) | 0.76 |
*p value (2df test) was calculated using the analysis of covariance after adjusting for baseline outcome measure, age, gender, and BMI
Fig. 2.
Scatterplots of unadjusted post-study (6-month) values showing the persistent associations of LBP-1 with inflammation: IL-6 (a), IL-8 (b)
Table 4.
Association between MT markers and inflammatory markers and physical function after controlling for age, gender, BMI, and randomization groups at 6 months (post-intervention)
| LBP-1 (SD = 13.5) Standardized regression coefficient ± SE |
p value | SCD14 (SD = 0.97) Standardized regression coefficient ± SE |
p value | |
|---|---|---|---|---|
| Log adiponectin | − 0.02 ± 0.04 | 0.68 | 0.02 ± 0.04 | 0.60 |
| Log IL-6 | 0.13 ± 0.04 | 0.001 | 0.08 ± 0.04 | 0.040 |
| Log IL-8 | 0.08 ± 0.04 | 0.046 | 0.08 ± 0.04 | 0.051 |
| IL-6sR | 1022 ± 789 | 0.20 | 626 ± 776 | 0.42 |
| Log AccelMVPA | 0.01 ± 0.05 | 0.78 | − 0.03 ± 0.05 | 0.51 |
| Log AccelPAEE | 0.02 ± 0.03 | 0.45 | − 0.01 ± 0.03 | 0.86 |
| SPPB | 0.03 ± 0.10 | 0.73 | 0.10 ± 0.10 | 0.30 |
| Log 400-m walk time | 0.01 ± 0.01 | 0.43 | 0.01 ± 0.01 | 0.47 |
Discussion
We demonstrate that there is a significant cross-sectional association between baseline biomarkers of MT (LBP-1, sCD14) and inflammatory markers including serum IL-6 and IL-8 in this study of 288 ambulatory, overweight or obese, community-dwelling participants of a clinical trial (The Cooperative Lifestyle Intervention Program, CLIP) aged 60–79 who had either CVD or cardiometabolic dysfunction and evidence of self-reported limitations in mobility. This is a novel description of the association between MT markers and physical function in a clinical population with comorbidities. Baseline LBP-1 was associated with poorer physical function, estimated by total energy expenditure, SPPB, and 400-m walk time, after controlling for age, gender, and BMI. sCD14 was also associated with 400-m walk time. This association has been reported in healthy elderly participants and we demonstrate that this biological relationship is indeed still present in less healthy adults, and MT remains associated with function even after adjustment for inflammatory biomarkers. In that prior, but smaller, study of 59 older adults (60–89 years), Stehle et al. demonstrated that LBP was inversely correlated with SPPB score and the correlation between LBP and SPPB remained significant even after adjusting for inflammatory biomarkers (Stehle Jr. et al. 2012). Our study demonstrated similar associations between markers of MT and physical function, but is novel as it is observed in a much larger number (n = 278) of older population with significant comorbidities. Chronic inflammation induced by MT that is evidenced by elevated inflammatory markers is known to be associated with low physical function in older adults with multiple comorbidities (Brinkley et al. 2009).
Our study indicated that diet and exercise do not reliably reduce biomarkers of MT. Two other studies have examined the results of health interventions in older adults with conflicting results (Ghosh et al. 2015; Rodriguez-Miguelez et al. 2015), where structured intensive exercise in a US-based population failed to change TLR4 muscle levels, whereas low-intensity exercise in a European-based population did improve muscle TLR4 levels. Muscle TLR4 is an endpoint that is elevated in older muscle and is presumed to be elevating in response to higher circulating LPS (Ghosh et al. 2015). Exercise interventions in aged rats and non-human primates replicated the positive effects of activity that were reported in a European study of older people (Oliveira et al. 2011; Kavanagh et al. 2016) and may reflect the generally healthier overall status of these European human and laboratory animal subjects. The lack of exercise and lifestyle effect of MT biomarkers in our US-based clinical population is consistent with that in the smaller US-based study reported by Ghosh et al. (2015).
Our study found that MT related to functional outcomes independent of IL-6, which might suggest a separate pathway exists in which TLR signaling in skeletal muscle leads to functional decline. Our prior non-human primate studies confirm MT mediates relationships between physical activity and insulin sensitivity, independent of IL-6 (Kavanagh et al. 2016). Thus, the GI barrier could be a new target for improving health in aging. The original reporting of this trial demonstrated that the weight loss plus exercise intervention was successful in reducing IL-6 concentrations (Beavers et al. 2013). This group also had reduced leptin concentrations, so IL-6 lowering was suggested to be mediated by the reductions in fat mass. Improvements in physical function and adiposity observed in the study did not alter gut barrier function and microbial translocation. Multiple observations now report that physical function and microbial translocation associate independently of IL-6; thus, this is a novel pathway to understand.
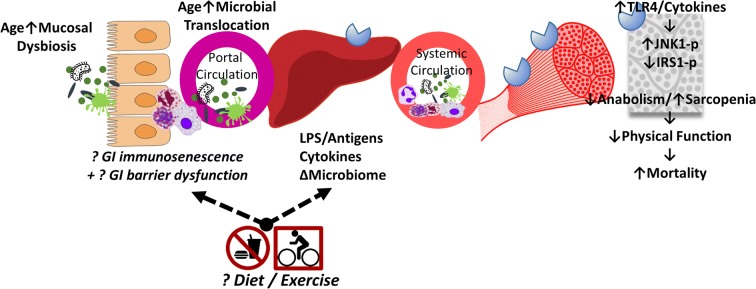
The exact reasons for why GI barrier dysfunction occurs in aging are unknown but at least in invertebrate models, the barrier loss is a transition marker that indicates imminent decline and death (Rera et al. 2012; Tricoire and Rera 2015; Dambroise et al. 2016). This “second phase of aging” is independent of chronological age and characterized by intestinal immunodeficiency which may be comparable with the older unhealthy adult, whereby inflammation is high and unresponsive to lifestyle interventions typically known to improve health outcomes. The gastrointestinal system is the largest immune organ in the human body with organized gut-associated lymphoid tissue, and lymphocytes in lamina propria and surface (Pabst et al. 2008). Relevant to the immune changes seen with age and the effects of lifestyle interventions, immune profiling of master cyclists (Duggal et al. 2018) reports that many cell populations were equivalent to those of younger adults. However, there were cell types that did not show differences after a lifetime of physical activity and included circulatory B cell populations, which have key roles in local mucosal immunity. We outline the interactions of elements in these pathways in Fig. 3, noting that the effect of diet and exercise may not alter the immunological competency at the mucosal surface.
Fig. 3.
Overall model that is supported by this, and prior studies. Unknown interactions are indicated by italics, question marks, and broken arrows. Aging leads to altered microbiome on the mucosal surface. It is unknown if the microbiome interferes with the barrier function (immune and physical components), or if the aging host’s reduction in gastrointestinal (GI) barrier function facilitates an altered surface microbiome, or some combination of both processes. Increased microbial translocation with aging is consistently observed, which travels via the portal circulation to incite inflammation in the liver via toll-like receptor (TLR) binding, and results in increased cytokines, and altered bacteria/bacterial products such as lipopolysaccharide (LPS)-binding protein. In the systemic circulation, inflammatory cytokines such as IL-6 are generally accepted to be elevated in aged adults. TLR4 and inflammatory signaling both cause reductions in insulin’s anabolic actions via c-Jun kinase (JNK1) activation, which inhibits insulin receptor signaling via insulin receptor substrate (IRS) 1 activation. Global inflammation and insulin resistance permit sarcopenia and reduced physical function, known to influence mortality risk. It is unknown if the benefits of dietary modification (restriction, fasting, or quality) or exercise are able to modulate the age-related changes at the primary steps within this pathway. To date, benefits of lifestyle interventions are seen in laboratory animal models and small clinical trials, but larger clinical trials fail to show these interventions can reliably reduce microbial translocation. Efforts to understand how the mucosal barrier and normal surface microbiome are needed to halt this microbial contribution to inflammaging
With aging, intestinal epithelial cells produce more pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β; they in turn increase intestinal epithelial tight junction permeability (Man et al. 2014). Further, the role of gut microbiome in human health recently has received much deserved attention. The collective genome size of gut microbiome is 150-fold (Wu et al. 2013) of the human genome, and the role in human health is beginning to be understood. Aging in general has not been associated with changes in the fecal microbiome that are generalizable (Jeffery et al. 2016; Anand et al. 2017), but the dysbiosis seen with aging is still an active area of investigation. Age-related differenecs in microbiome measured from the intestinal mucosal surface and in circulation suggest aging does change the microbiome that translocates across intestinal wall (Maffei et al. 2017; Buford et al. 2018; Wilson et al. 2018) While these niche microbiomes are different in older people, the microbial populations in the intestinal lumen, which are excreted, may be comparable (Lyra et al. 2012; Wilson et al. 2018). A different circulatory microbiome in aged people (Buford et al. 2018) is highly suggestive that alterations in the mucosal-associated microbiomes exist with aging, as described in animal models (Wilson et al. 2018). It is unknown if the immunological decline in the aging host dictates the composition of the mucosal microbiome and its passage across the intestinal barrier, or if the changed microbiome (different species, greater abundance) is able to evade or overwhelm the intestinal barrier. Microbes are metabolically active, and microbial metabolite profiles have been related to both positive and negative health span outcomes (Wang et al. 2011; Lewis et al. 2018). The microbiome composition, its local activity, and products all may have a biological effect on the competency of the intestinal barrier.
Our study has a few limitations. First, the study population was an elderly population who are overweight or obese with significant comorbidities and mobility limitation. Since there might be an association between comorbidities (e.g., cardiovascular disease) and MT, we cannot rule out any confounding effects of the participants’ comorbidities. Also, we used endogenous biomarkers of MT (LBP-1, sCD14) that are acute phase proteins. LPS is a direct marker of endotoxemia and has been measured for that purpose. However, the variability of LPS throughout the day and the need to collect the sample under LPS-free conditions make LPS a difficult marker to measure in human subjects to assess MT. For these reasons, LBP-1 was considered a preferred biomarker of MT burden.
The relationships of MT and specific physical functional outcomes are few. Our current study is the largest associative study performed to date and thus is significant in that the relationship between MT and physical function is present, independent of inflammatory biomarkers, and is poorly responsive to well-accepted health intervention strategies. Our study also focused on an unhealthy population, and it is known that obese and type II diabetes subjects have higher MT biomarker levels (Cani et al. 2007; Amar et al. 2008), and accompanying inflammatory outcomes are negatively correlated with muscle insulin sensitivity (Shi et al. 2006; Schaap et al. 2009; Liang et al. 2013; Kavanagh et al. 2016; Grosicki et al. 2018). In human myotubes, LPS can induce inflammatory response which in turn reduces the anabolic actions of insulin signaling (Liang et al. 2013) which is an important sarcopenia pathway. In a study of 178 elderly subjects, it was demonstrated that fecal microbial composition is correlated with measures of frailty and markers of inflammation (Claesson et al. 2012), and gut microbial diversity is inversely associated with frailty (Jackson et al. 2016) but causes and effects are undetermined. We believe that targeting intestinal immunocompetence to reduce MT would benefit insulin sensitivity, reduce inflammation, and overall be positive for muscular health in aging. Interventions known to aid the mucosal barrier function are few. Sevelamer reduces MT in dialysis patients but was not effective in HIV enteropathy (Sandler et al. 2014). Oral immunoglobulins have reported variable effectiveness (Asmuth et al. 2013; Wilson et al. 2018), and metformin has also reported to reduce MT (independent of its actions on the microbiome) (Moreno-Navarrete et al. 2011; Spruss et al. 2012). As ours and others’ studies show that diet and exercise are not universally beneficial for restoring intestinal barrier competency, strategies such as these should be pursued for reducing MT and inflammatory burden in older adults.
Electronic supplementary material
(DOCX 13 kb)
Acknowledgments
The authors gratefully acknowledge the mentorship and advice provided by Leanne Groban M.D., M.S., in the Department of Anesthesiology of Wake Forest School of Medicine.
Funding information
This study was funded in part by the Grants for Early Medical/Surgical Specialists’ Transition to Aging Research (GEMSSTAR, R03 AG050919) (SK), and the Claude D. Pepper Center Older Americans Independence Center (P30 AG21332) (SBK), Wake Forest School of Medicine, Winston-Salem, NC.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kylie Kavanagh and Sunghye Kim contributed equally to this work.
References
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- Anand R, Song Y, Garg S, Girotra M, Sinha A, Sivaraman A, Phillips L, Dutta SK. Effect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomes. Dig Dis Sci. 2017;62(4):1002–1008. doi: 10.1007/s10620-017-4449-6. [DOI] [PubMed] [Google Scholar]
- Asmuth DM, Ma ZM, Albanese A, Sandler NG, Devaraj S, Knight TH, Flynn NM, Yotter T, Garcia JC, Tsuchida E, Wu TT, Douek DC, Miller CJ. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS. 2013;27(14):2207–2217. doi: 10.1097/QAD.0b013e328362e54c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers KM, Ambrosius WT, Nicklas BJ, Rejeski WJ. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J Am Geriatr Soc. 2013;61(7):1089–1094. doi: 10.1111/jgs.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Nesbit BA, Ambrosius WT, Marsh AP, Nicklas BJ, Rejeski WJ. Effect of an 18-month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity (Silver Spring) 2014;22(2):325–331. doi: 10.1002/oby.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64(4):455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Carter CS, VanDerPol WJ, Chen D, Lefkowitz EJ, Eipers P, Morrow CD, Bamman MM. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40(3):257–268. doi: 10.1007/s11357-018-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. Mammalian gut immunity. Biom J. 2014;37(5):246–258. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Dambroise E, Monnier L, Ruisheng L, Aguilaniu H, Joly JS, Tricoire H, Rera M. Two phases of aging separated by the Smurf transition as a public path to death. Sci Rep. 2016;6:23523. doi: 10.1038/srep23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ettorre G, Douek D, Paiardini M, Ceccarelli G, Vullo V. Microbial translocation and infectious diseases: what is the link? Int J Microbiol. 2012;2012:356981. doi: 10.1155/2012/356981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal Niharika Arora, Pollock Ross D., Lazarus Norman R., Harridge Stephen, Lord Janet M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17(2):e12750. doi: 10.1111/acel.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Lertwattanarak R, Garduno Jde J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci. 2015;70(2):232–246. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosicki GJ, Fielding RA, Lustgarten MS. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcif Tissue Int. 2018;102(4):433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hall KS, Cohen HJ, Pieper CF, Fillenbaum GG, Kraus WE, Huffman KM, Cornish MA, Shiloh A, Flynn C, Sloane R, Newby LK, Morey MC (2016) Physical performance across the adult life span: correlates with age and physical activity. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed]
- Hollander D, Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology. 1985;31(3):133–137. doi: 10.1159/000212694. [DOI] [PubMed] [Google Scholar]
- Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O'Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery IB, Lynch DB, O'Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Brown RN, Davis AT, Uberseder B, Floyd E, Pfisterer B, Shively CA. Microbial translocation and skeletal muscle in young and old vervet monkeys. Age (Dordr) 2016;38(3):58. doi: 10.1007/s11357-016-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Rubinstein ND, Buffenstein R. A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence. Geroscience. 2018;40(2):105–121. doi: 10.1007/s11357-018-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Hussey SE, Sanchez-Avila A, Tantiwong P, Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013;8(5):e63983. doi: 10.1371/journal.pone.0063983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra A, Forssten S, Rolny P, Wettergren Y, Lahtinen SJ, Salli K, Cedgard L, Odin E, Gustavsson B, Ouwehand AC. Comparison of bacterial quantities in left and right colon biopsies and faeces. World J Gastroenterol. 2012;18(32):4404–4411. doi: 10.3748/wjg.v18.i32.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei VJ, Kim S, Blanchard ET, Luo M, Jazwinski SM, Taylor CM, Welsh DA. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci. 2017;72(11):1474–1482. doi: 10.1093/gerona/glx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol. 2014;289(1-2):112–118. doi: 10.1016/j.cellimm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, Fernandez-Real JM. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes. 2011;36(11):1442–1449. doi: 10.1038/ijo.2011.256. [DOI] [PubMed] [Google Scholar]
- Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem. 2001;268(16):4580–4589. doi: 10.1046/j.1432-1327.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, Guadagnini D, Carvalheira JB, Saad MJ. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60(3):784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pabst R, Russell MW, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008;29(5):206–208. doi: 10.1016/j.it.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Brubaker PH, Goff DC, Jr, Bearon LB, McClelland JW, Perri MG, Ambrosius WT. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–886. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109(52):21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Miguelez P, Fernandez-Gonzalo R, Collado PS, Almar M, Martinez-Florez S, de Paz JA, Gonzalez-Gallego J, Cuevas MJ. Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech Ageing Dev. 2015;150:12–19. doi: 10.1016/j.mad.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Saffrey MJ. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr) 2014;36(3):9603. doi: 10.1007/s11357-013-9603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL, Douek DC, Tressler R, Read SW, Wilson CC, Deeks SG, Lederman MM, Gandhi RT, Team ACTGA. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210(10):1549–1554. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Investig. 2012;92(7):1020–1032. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- Stehle JR, Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci. 2012;67(11):1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire H, Rera M. A new, discontinuous 2 phases of aging model: lessons from Drosophila melanogaster. PLoS One. 2015;10(11):e0141920. doi: 10.1371/journal.pone.0141920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson QN, Wells M, Davis AT, Sherrill C, Tsilimigras MCB, Jones RB, Fodor AA, Kavanagh K. Greater microbial translocation and vulnerability to metabolic disease in healthy aged female monkeys. Sci Rep. 2018;8(1):11373. doi: 10.1038/s41598-018-29473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Bushmanc FD, Lewis JD. Diet, the human gut microbiota, and IBD. Anaerobe. 2013;24:117–120. doi: 10.1016/j.anaerobe.2013.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)