Abstract
Adjustment of cerebral blood flow (CBF) to the increased oxygen and nutrient demands of active brain regions via neurovascular coupling (NVC) has an essential role in maintenance of healthy cognitive function. In advanced age, cerebromicrovascular oxidative stress and endothelial dysfunction impair neurovascular coupling, contributing to age-related cognitive decline. Recently we developed a resveratrol (3,4′,5-trihydroxystilbene)-containing fusogenic liposome (FL-RSV)-based molecular delivery system that can effectively target cultured cerebromicrovascular endothelial cells, attenuating age-related oxidative stress. To assess the cerebromicrovascular protective effects of FL-RSV in vivo, aged (24-month-old) C57BL/6 mice were treated with FL-RSV for four days. To demonstrate effective cellular uptake of FL-RSV, accumulation of the lipophilic tracer dyes in cells of the neurovascular unit was confirmed using two-photon imaging (through a chronic cranial window). NVC was assessed by measuring CBF responses (laser speckle contrast imaging) evoked by contralateral whisker stimulation. We found that NVC responses were significantly impaired in aged mice. Treatment with FL-RSV significantly improved NVC responses by increasing NO-mediated vasodilation. These findings are paralleled by the protective effects of FL-RSV on endothelium-dependent relaxation in the aorta. Thus, treatment with FL-RSV rescues endothelial function and NVC responses in aged mice. We propose that resveratrol containing fusogenic liposomes could also be used for combined delivery of various anti-geronic factors, including proteins, small molecules, DNA vectors and mRNAs targeting key pathways involved in microvascular aging and neurovascular dysfunction for the prevention/treatment of age-related cerebromicrovascular pathologies and development of vascular cognitive impairment (VCI) in aging.
Keywords: Fusogenic liposomes, Resveratrol, Oxidative stress, Aging, Cerebral circulation, Vascular cognitive impairment, Endothelial dysfunction, Functional hyperemia, Endothelium
Introduction
Normal functioning of the brain requires tight coordination between neuronal activity and cerebral blood flow, to ensure an adequate supply of oxygen and nutrients as well as effective washout of harmful metabolites (Tarantini et al. 2017a). During periods of intense neuronal activity, there is a requirement for prompt adjustment of oxygen and glucose delivery to the increased demands of active neurons through rapid adaptive increases in regional blood flow. This is ensured by a homeostatic mechanism known as neurovascular coupling. The resulting functional hyperemia is essential to maintain an optimal humoral microenvironment around firing neurons and thereby enabling their normal functioning.
There is a scientific consensus that microvascular contributions to cognitive impairment in elderly patients are critical (Tarantini et al. 2017a). Importantly, neurovascular coupling responses are impaired both in older adults (Zaletel et al. 2005; Topcuoglu et al. 2009; Stefanova et al. 2013; Fabiani et al. 2013) and in preclinical animal models of aging (Toth et al. 2014; Park et al. 2007), which contributes to the age-related cognitive decline (Tarantini et al. 2017a; Sorond et al. 2013; Sorond et al. 2011; Tarantini et al. 2015). Recent studies demonstrate that the age-related molecular and cellular mechanisms that contribute to neurovascular un-coupling include increased mitochondrial oxidative stress and mitochondrial dysfunction in endothelial cells and impairment of endothelial NO-mediated vasodilation (Tarantini et al. 2018; Tarantini et al. 2019). Preclinical studies demonstrate that pharmacological interventions that attenuate endothelial oxidative stress and improve NO mediation have the potential to increase neurovascular coupling responses and cerebral blood flow and improve cognitive function in rodent models of aging (Tarantini et al. 2018; Tarantini et al. 2019). Inspired by these findings, our long-term goal is to develop innovative therapeutic interventions to rescue cerebromicrovascular endothelial function and restore functional hyperemia in elderly patients to delay cognitive impairment (Toth et al. 2014). In order to achieve that goal, we have developed an innovative fusogenic liposome-based method for effective delivery of anti-geronic factors to cerebromicrovascular endothelial cells (Csiszar et al. 2010, 2014; Hersch et al. 2016; Kleusch et al. 2012; Kolasinac et al. 2018; Kube et al. 2017). The cellular uptake of conventionally pharmaceutically applied liposomes is mediated by either clathrin-dependent or clathrin-independent endocytosis, limiting the uptake efficiency to a small percentage. Our innovative liposomal carrier system delivers its cargo by protein-independent fusion with the cell membrane, allowing a delivery more efficient and less affected of lysosomal cargo degradation in contrast to aforementioned liposomes. Using such fusogenic liposomes (FLs) we successfully delivered the anti-geronic compound resveratrol with significant neurovascular protective effects (Baur et al. 2006; Lagouge et al. 2006; Smith et al. 2009) as shown in Fig. 1.
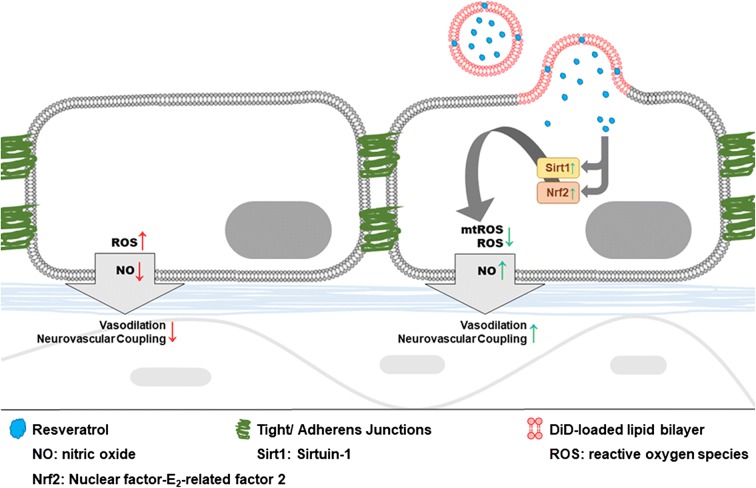
Fig. 1.
Proposed model for neurovascular protection by enhanced delivery of resveratrol to cells of the neurovascular unit in aged mice using fusogenic liposomes. The scheme depicts the enhanced membrane fusion between resveratrol-containing fusogenic liposomes and aged cerebromicrovascular endothelial cells (CMVECs) and the complex anti-aging neurovascular protective effects of increased cellular resveratrol levels. Note that synergistic interaction of the neutral lipid DOPE, the positively charged lipid DOTAP, and the aromatic resveratrol and DiD, results in an effective fusogenic mixture that readily delivers resveratrol to vascular cells (Csiszar et al. 2014). Resveratrol is highly lipophilic and is deeply located in the lipid bilayer. Only 10% of resveratrol molecules is found in the aqueous phase (Brittes et al. 2010). The model predicts that increased delivery of resveratrol encapsulated in fusogenic liposomes exerts anti-aging protective effects in cells of the neurovascular unit by activating sirtuins and Nrf2 and thereby attenuating age-related mitochondrial production of ROS (mtROS) and restoring bioavailability of vasodilator NO. We propose that resveratrol containing fusogenic liposomes could be used to deliver a complex cocktail of anti-geronic factors, which include proteins, small molecules, DNA vectors and miRNAs targeting key pathways involved in microvascular aging and neurovascular dysfunction
There is strong preclinical evidence that treatment with high doses of resveratrol exerts significant vasoprotective effects in aged mice and mice with accelerated vascular aging (Ungvari et al. 2007a; Pearson et al. 2008; Zhang et al. 2009). Resveratrol, a plant-derived polyphenolic stilbene, was shown to attenuate mitochondrial oxidative stress, improve mitochondrial function and increase NO bioavailability in cultured endothelial cells (Csiszar et al. 2014; Csiszar et al. 2009; Csiszar et al. 2012; Toth et al. 2015a; Ungvari et al. 2010; Ungvari et al. 2009; Ungvari et al. 2007b; Ungvari et al. 2007c). Treatment with resveratrol can further increase the activation of the nuclear factor (erythroid-derived 2) factor 2 (Nrf2) in vitro and in rodent models (Csiszar et al. 2014; Ungvari et al. 2010). Nrf2 is an inducible nuclear transcription factor that adjusts the redox balance in the cell and positively regulates expression of antioxidant enzymes like mitochondria and NADPH oxidases (Kovac et al. 2015). Furthermore, resveratrol promotes allosteric activation of NAD-dependent deacetylase sirtuin-1 (SIRT-1), a member of the sirtuin family of regulatory enzymes involved in life span extension mechanisms (Hubbard et al. 2013; Howitz et al. 2003). Genetic depletion of SIRT-1 in rodent cells was shown to diminish the beneficial antioxidative effects of resveratrol in multiple tissues (Price et al. 2012). Previous studies by us and other laboratories provide proof-of-concept that treatment of aged rodents with resveratrol exerts protective effects on the cerebral microcirculation (Toth et al. 2014; Oomen et al. 2009). Despite significant advances in our understanding of the endothelial protective and anti-geronic effects of resveratrol, its low aqueous solubility, relatively low bioavailability, rapid metabolism in the liver and rapid systemic elimination are barriers to its clinical application. Previously we confirmed that the uptake of resveratrol delivered by fusogenic liposomes (FL-RSV) to endothelial cells is substantially enhanced (Csiszar et al. 2014). Specifically, we demonstrated treatment of primary cerebromicrovascular endothelial cells isolated from aged rats with FL-RSV results in rapid and effective cellular uptake of resveratrol-containing liposomes, increasing cellular resveratrol levels and attenuating cellular production of reactive oxygen species (Csiszar et al. 2014).
The present study was designed to test successful targeting of the cerebral microcirculation by resveratrol-containing fusogenic liposomes and to assess the cerebromicrovascular and endothelial protective effects of in vivo treatment with FL-RSV. To achieve this goal, aged (24-month-old) C57BL/6 mice were treated with FL-RSV for four days. To demonstrate effective cellular uptake of FL-RSV, the animals were equipped with chronic cranial window, and accumulation of the lipophilic tracer dyes, DiD or DiO respectively, in cells of the neurovascular unit was monitored using two-photon imaging. Neurovascular coupling was assessed by measuring cerebral blood flow (CBF) responses (laser speckle contrast imaging) evoked by contralateral whisker stimulation. Endothelial function was also assessed in isolated aorta ring preparations.
Methods
Preparation of fusogenic liposomes
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-3-trimethylammonium-propane, chloride salt (DOTAP) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA) while the fluorescent lipophilic tracers 1,1′-dioctadecyl-3,3,3′,3’-Tetramethylindotricarbocyanine iodide (DiD) and 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) were purchased from ThermoFisher Scientific (Waltham, MA, USA). The method of liposomal preparation was modified from previously described protocols (Csiszar et al. 2014). For the preparation of fusogenic liposomes (FL), the lipids DOPE and DOTAP (25 mg/ml in chloroform) were mixed with DiD or DiO 1/1/0.05 or 1/1/0.1 (mol/mol), respectively. The lipophilic fluorophores DiD and DiO differed in fluorescence excitation and emission, yet showed similar physicochemical properties. Resveratrol (RSV; Sigma-Aldrich, St.Louis, MO, USA) was disolved in ethanol and added to the lipid solution in a ratio of 2/3 (w/w). The solvents were evaporated in vacuum, and the lipid film was hydrated using 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Sigma-Aldrich, St. Louis, MO, USA) to a final lipid concentration of 2 mg/ml at pH 7.4. After brief vortexing, lipid film hydration was followed by sonication in an ultrasonic bath (Sonocool, Bandelin electronic GmbH, Berlin, Germany) at 5 °C for 20 min. Those with higher fluorophore content were chosen for intravital imaging and histology.
Animals, treatment with FL-RSV
Young (3 months, n = 10) and aged (24 months, n = 20) male C57BL/6 mice were purchased from the aging colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). Animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center under a controlled photoperiod (12 h light; 12 h dark) with unlimited access to water and were fed a standard AIN-93G diet (ad libitum). Mice in the aged cohort were assigned to two groups (n = 10 each group).
Mice of each group were retro-orbitally injected for four days with 2 mg/kg/day of RSV encapsulated in fusogenic liposomes (FL-RSV) diluted in phosphate-buffered saline (PBS). PBS and empty fusogenic liposomes (FL) were used as vehicle control. Four hours after the last injection, mice of the aged cohort were used for experiments determining relative change of cerebral blood flow in the barrel cortex. Subsequently, all mice were transcardially perfused with PBS and decapitated, then dissected. The thoracic aortae of aged mice were removed directly after decapitation for the aortic ring assay, and brains of all mice were removed and fresh-frozen for measurement of the cortex fluorescence intensity. All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Chronic cranial window preparation, two-photon imaging
A group of C57BL/6 mice was equipped with a chronic cranial window for in vivo imaging according to the published protocols of Mostany and Portera-Cailliau (Mostany and Portera-Cailliau 2008a; Mostany and Portera-Cailliau 2008b). Briefly, mice were anesthetized with 2% isoflurane from a Surgivet Classic T3 vaporizer (Smiths Medical, Minneapolis, MN, USA) and the depth of anesthesia was monitored during the whole process by inspection of the toe pinch reflex. The subject was placed on a warming blanket-covered bench, and the head was fixed in a stereotaxic frame. Eye ointment was applied to both eyes to prevent them from drying out. After local disinfection, hair and then skin were removed from the skull, and the bone surface was cleared. A ~3 mm diameter circle was drawn with a pneumatic dental drill (Foredom, Blackstone Industries, Bethel, CT) until the bone had become thin enough to perform craniotomy. A glass coverslip (Thomas Scientific, Swedesboro, NJ, USA) previously soaked in 70% ethanol was rinsed in PBS and applied on the dura mater. The window was stabilized with liquid instant adhesive and secured by Jet Set-4 dental acrylic resin (Lang Dental, Wheeling, IL, USA). After the surgery, the animals were allowed at least ten days to stabilize before intravital microscopy.
For intravital microscopy, a FluoView 1000 MPE (Olympus, Tokyo, Japan) two-photon microscope coupled with a MaiTai HP DeepSee-OL 690 nm–1040 nm (Spectra-Physics, San Jose, CA, USA) laser and a XLPLN25XWMP 25× water immersion objective (Olympus, Tokyo, Japan) was used. Subjects were illuminated with 800–910 nm laser light adjusted to fluorophores. Mice were anesthetized with isoflurane (2% for initiation and then 1.5% for maintenance) then fixed in a stereotaxic frame. FL-RSV(DiD), or –(DiO) was administered 30 min before the microscopy. 100 μl FITC- labeled dextran (500 kDa) (Sigma-Aldrich, St. Louis, MO, USA) or Texas red- labeled dextran (70 kDa) (Thermo Fischer Scientific, Waltham, MA, USA) tracer was also injected along with FL-RSV(DiD), or –(DiO) retro-orbitally. The animals were imaged before and after injection. First, meningeal vessels were detected and the imaging depth was set to them. Cerebral vessels were examined ~0–200 μm deep. To detect the movement of FL-RSV 150 μm × 150 μm (x, y) region of interests (ROIs) were recorded for 30 s repeatedly, for several days in indicated time points (at least 3 ROIs per animal). For 3D volume scanning 508 μm × 508 μm × 50 μm (x, y, z) ROI was recorded. The images were analyzed using ImageJ 1.52i version (National Institutes of Health). Imported images were assembled to time-stacks or z-stack (maximum projection), and were cropped for presentation.
Measurement of neurovascular coupling responses
Mice in each group were anesthetized with isoflurane (4% induction and 1% maintenance), endotracheally intubated and ventilated (MousVent G500; Kent Scientific Co, Torrington, CT) as described previously (Tarantini et al. 2018; Tarantini et al. 2017b; Ungvari et al. 2017). A thermostatic heating pad (Kent Scientific Co, Torrington, CT) was used to maintain rectal temperature at 37 °C (Toth et al. 2014). End-tidal CO2 was controlled between 3.2% and 3.7% to keep blood gas values within the physiological range, as described previously (Tarantini et al. 2015; Toth et al. 2015b). Cannulation of the right femoral artery was performed for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT) (Toth et al. 2014). The blood pressure was within the physiological range throughout the experiments (90–110 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), the scalp and periosteum were pulled aside and the skull was gently thinned using a dental drill while cooled with dripping buffer. Recent advances in our understanding of cellular aging processes (Kim et al. 2018; Lee et al. 2018; Masser et al. 2018; Nacarelli et al. 2018; Reglodi et al. 2018; Sarker and Franks 2018) and mechanisms underlying vascular aging strongly suggest that age-related decline in neurovascular function (Tarantini et al. 2017b; Ungvari et al. 2017; Tarantini et al. 2017c; Fulop et al. 2018) and cognitive performance can be reversed. To assess neurovascular coupling responses a laser speckle contrast imager (Perimed, Järfälla, Sweden) was placed 10 cm above the thinned skull, and to achieve the highest CBF response the right whiskers were stimulated for 30 s at 10 Hz from side to side as described (Tarantini et al. 2017c). Differential perfusion maps of the brain surface were captured. Changes in cerebral blood flow (CBF) were monitored above the left barrel cortex in six trials in each group, separated by 5–10 min intervals. To assess the role of NO mediation, CBF responses to whisker stimulation were repeated 20 min after administration of the nitric oxide synthase inhibitor Nω-Nitro-L-arginine methyl ester (L-NAME, Sigma-Aldrich, St. Luis, MO). For data evaluation, the relative change in the CBF signal was compared between the baseline of the region of interest (ROI) and during stimulation. To rule out an unspecific increase of cerebral blood flow, the difference between the relative change for both lateral ROI‘s was used for further evaluation to determine specific changes for the contralateral somatosensory cortex. In each study, the experimenter was blinded to the treatment of the animals.
Assessment of endothelial function in the aorta
To assess the specific effect of FL-RSV treatment on endothelial function, endothelium-dependent vasorelaxation was assessed in isolated aorta ring preparations as described previously (Pearson et al. 2008). In brief, aortas were cut into ring segments 1.5 mm in length and mounted in myographs chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37 °C; gassed with 95% air and 5% CO2).. After an equilibration period of 1 h during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), they were pre-contracted with 10−6 M phenylephrine until reaching a plateau phase, and subsequent relaxation in response to increasing doses of acetylcholine was measured.
Demonstration of effective endothelial targeting by fusogenic liposomes in the aorta using confocal microscopy
The segmented aorta of a young C57BL/6 mouse treated with 10 mg/kg FL-RSV(DiD) was isolated four hours after a single dose injection, embedded in cryogel and frozen at −80 °C, then sectioned in 50 μm slices and transferred to glass. After counterstaining with 4′,6-diamidine-2′-phenylindole (DAPI) and mounting with ProLong™ diamond antifade mountant (ThermoFisher Scientific, Massachusetts, USA), sections were imaged using an SP8 confocal microscope (Leica, Hessen, Germany) and a water immersion objective (20x HC PL APO 0.75). DAPI and DiD were stimulated with a 405 nm UV laser and a white light laser system with a notch filter at 633 nm. Images were acquired at 8 bits.
Demonstration of effective targeting of the brain by fusogenic liposomes: Fluorescence intensity measurement in cortical samples
A longitudinal section of the fresh frozen brain of young C57BL/6 mice after four-day treatment was homogenized using an ultrasonic cell disruptor homogenizer in 1% Triton X-100 containing PBS (w/w; 1:10). The homogenized sample was centrifuged for 20 min at 4 °C and 15.000 rpm. The supernatant was divided into three wells of 200 μL in a flat black 96-well-plate, and fluorescence intensity was measured using a Spark® plate reader (Tecan Life Sciences, Maennedorf, Switzerland). For data evaluation, the blank intensity of 1% Triton X-100 in PBS was removed from the intensity of other samples.
Statistical analysis
Statistical analysis was carried out by one-way ANOVA followed by Tukey’s post-hoc test. A p value of less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
Effective uptake of FL-RSV into cells of the neurovascular unit and aortic endothelial cells
Our delivery system is schematically presented in Fig. 1. To confirm effective cellular uptake of fusogenic liposomes, we demonstrated the accumulation of the lipophilic tracer dyes DiD and DiO in cells of the neurovascular unit using two-photon microscopy (Figs. 2 and 3). As shown in Fig. 2c, d and 3d, after retro-orbital administration of FL-RSV a significant increase in DiD or DiO-stained cells in the neurovascular unit (both in endothelial and paravascular astrocyte-like and pericyte-like cells) was evident. For semi-quantitative assessment of cellular liposomal uptake, we compared background-corrected DiD fluorescence in the brain tissue after administration of empty fusogenic liposomes (FL) and FL-RSV. Previously we showed that the fusion efficiency of this lipid mixture could be modulated by incorporation of RSV in vitro (Csiszar et al. 2014). We hypothesize that the large delocalized π electron systems of resveratrol promotes destabilization of the lipid bilayer in the cell membrane, thereby significantly increasing uptake of liposomes via membrane fusion with endothelial cells in vitro (Csiszar et al. 2014). As expected on the basis of the in vitro studies (Csiszar et al. 2014), cellular incorporation of DiD upon in vivo administration of empty fusogenic liposomes was low, whereas cellular incorporation of DiD upon administration of FL-RSV was significantly increased (Fig. 2e).
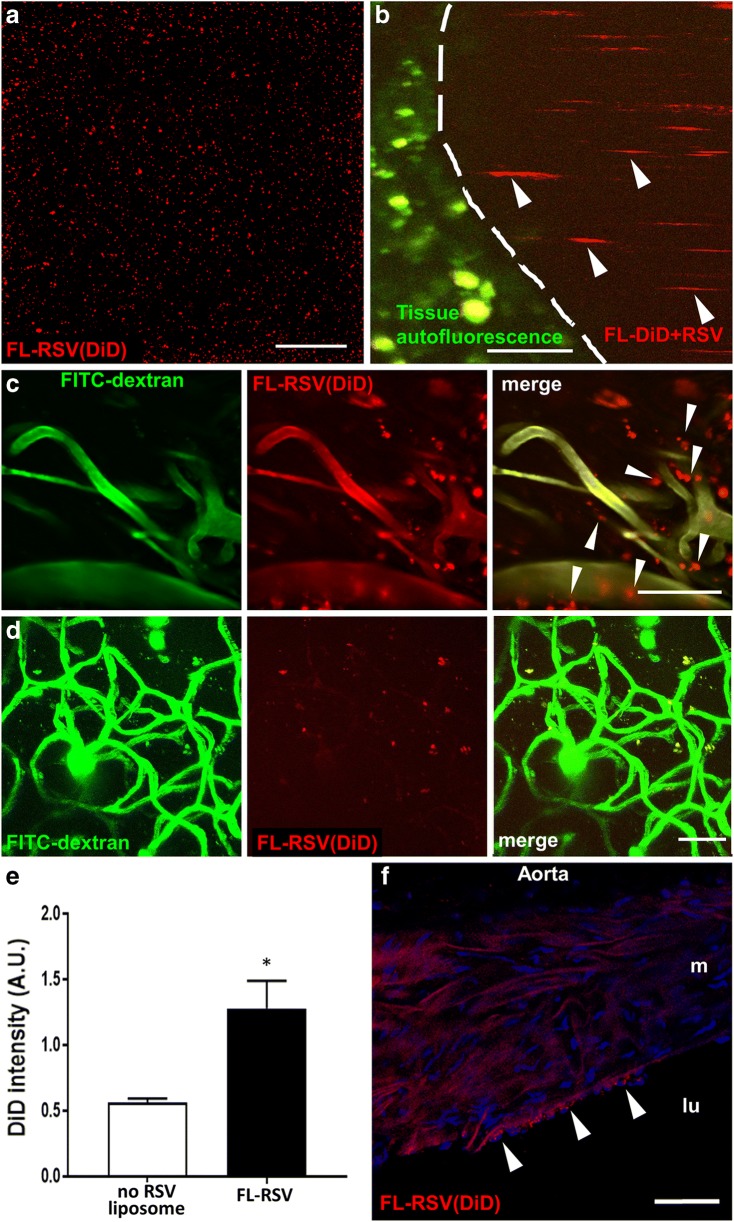
Fig. 2.
Fusogenic liposomes effectively deliver their content to cells of the neurovascular unit as well as endothelial cells of large arteries. Panel A: Resveratrol-containing fusogenic liposomes labeled with DiD [FL-RSV(DiD)] in suspension, visualized by two-photon microscopy. Scale bar: 100 μm. Panel B: Circulating FL-RSV(DiD) observed in the cerebral circulation with two-photon microscopy after retro-orbital injection. Note that imaging FL-RSV(DiD) can be used to assess cerebral blood flow along with the microvascular network. Arrows point to the moving FL-RSV(DiD) particles in the vessel lumen. Tissue autofluorescence (green) is shown for orientation; the dashed line indicates the vessel wall on the time-stack. Scale bar: 20 μm. Panel C: Real-time liposomal delivery of resveratrol was monitored by detecting the incorporation of the fluorescent tracer DiD (red) in cells of the neurovascular unit using two-photon microscopy. After retro-orbital injection of FITC-dextran and FL-RSV(DiD) the cerebral vasculature was imaged by two-photon microscopy. Vessels lumens (FITC-dextran; green) and fusogenic liposomes (DiD, red) are visible on the time stack images. Note the early (within 4 h) incorporation of DiD delivered by the fusogenic liposomes in the lipid membranes of cells of the neurovascular unit (arrows). Scale bar: 50 μm. Panel D: Early (within 4 h) accumulation of DiD delivered by the fusogenic liposomes in the lipid membranes of cells of the neurovascular unit. Z-stack image was recorded from the upper 0–100 μm depth of the cortex. Scale bar: 50 μm. Panel E: Comparison of the incorporation of DiD-labeled resveratrol containing fusogenic liposomes [FL-RSV] and DiD-labeled liposomes that did not contain resveratrol [FL] into the cerebral microcirculation. Note the significant increase in the intensity of incorporated tracer dye DiD in brain homogenates upon administration of FL-RSV(DiD) as compared to empty fusogenic liposomes and PBS treatment (see Methods). Data are mean ± S.E.M., *P < 0.05 vs. empty fusogenic liposomes (FL). Panel F: Liposomal delivery of resveratrol to arterial endothelial cells was visualized by detecting the fluorescent tracer DiD in frozen sections of the aorta. Note the increased DiD signal in the plasma membrane of the inner luminal endothelial cell layer upon delivery of resveratrol incorporated in positively charged liposomes (arrows; lu: lumen; m: media)
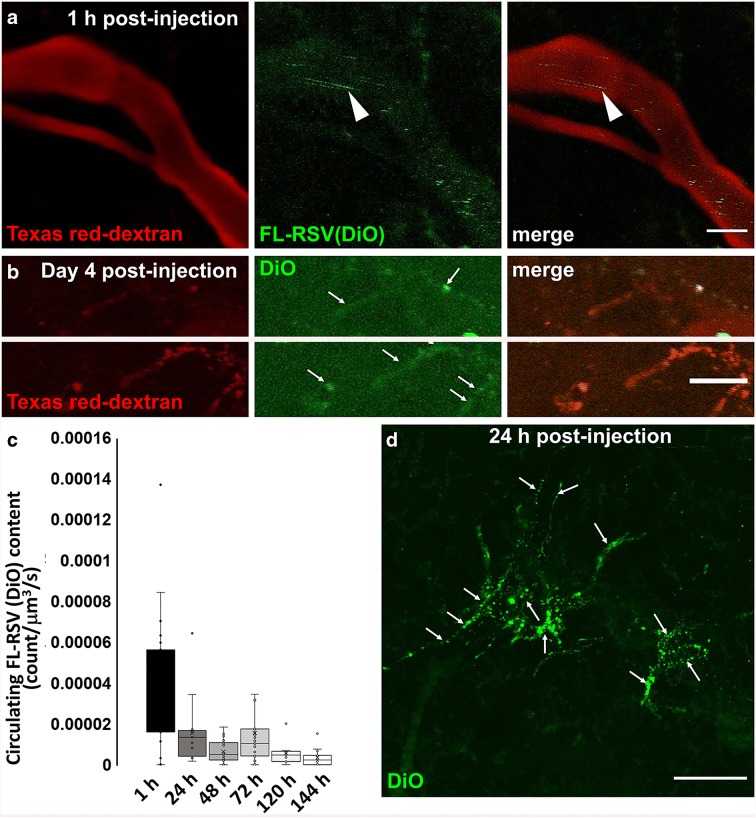
Fig. 3.
Long time tracking of fusogenic liposomes in the cerebral microcirculation. Panel A: Circulating fusogenic liposomes labeled with DiO [FL-RSV(DiO)] observed in the cerebral microcirculation with two-photon microscopy after retro-orbital injection of FL-RSV(DiO) and Texas red-dextran (to visualize vessel lumens). Arrow: moving FL-RSV(DiO) particles in the vessel lumen. Scale bar: 10 μm. Panel B: DiO labeled cells in the cerebral microcirculation. Four days after injection of a single dose of fusogenic liposomes, circulating FL-RSV(DiO) were virtually absent in the microvasculature. DiO labeling in the cells of the neurovascular unit was evident (arrows). Scale bar: 20 μm. Panel C: Time course of uptake of circulating FL-RSV(DiO). Shown is the relative count of circulating DiO-labeled FL-RSV particles in the cerebral microcirculation at the indicated time points post-retro-orbital injection. Calculated quantities of intraluminal circulating FL-RSV(DiO) particles are expressed as count/μm3/s. FL-RSV(DiO) are eliminated from the cerebral microcirculation within 24 h, suggesting daily dosing for efficacy testing of resveratrol incorporated in FL-RSV. Data (n ≥ 9 measurements) are presented as a box plot. *P < 0.05 vs. 1 h. Panel D: Intensive DiO staining is evident in the cells of the neurovascular unit at 24 h post-retro-orbital injection of FL-RSV(DiO). Arrows: DiO staining in the wall of cerebral microvessels. Scale bar: 50 μm
To demonstrate effective endothelial uptake of FL-RSV, we also assessed DiD incorporation in the endothelial layer of the aorta. Figure 2f shows that increased DiD fluorescence was localized to the luminal cellular layer of the aorta of FL-RSV treated mice, indicating effective delivery of resveratrol to endothelial cells.
To determine the liposomal clearance from the cerebral circulation, FL-RSV particles were monitored 6d post injection. As Fig. 3c shows, the number of circulating liposomes substantially decreased after one day consistent with the effective uptake of the liposomes from the systemic circulation. After several days of tracking DiO-labeled cells appeared in and around of the mouse microcirculation (Fig. 3d).
Treatment with FL-RSV rescues neurovascular coupling responses in aged mice by restoring NO mediation
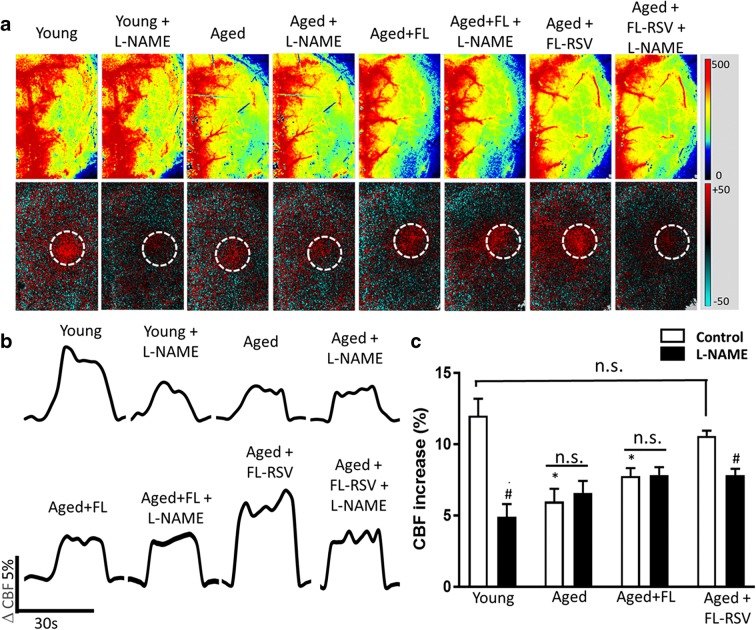
CBF responses in the whisker barrel cortex elicited by contralateral whisker stimulation were significantly decreased in aged mice compared to young animals indicating impaired neurovascular coupling in aging (representative laser speckle contrast images and CBF tracings are shown in Fig. 4a and b, summary data are shown in Fig. 4c) (Park et al. 2007; Tarantini et al. 2018; Tarantini et al. 2019). We found that four-day treatment with FL-RSV significantly increased CBF responses induced by contralateral whisker stimulation in aged mice, restoring neurovascular coupling to levels observed in young mice (Fig. 4c). In young animals administration of the NO synthase inhibitor L-NAME significantly decreased neurovascular coupling responses, eliminating the differences between the age groups (Fig. 4c). In aged animals treated with the liposomal controls, administration of L-NAME was without effect (Fig. 4c). In contrast, in FL-RSV treated aged mice L-NAME significantly decreased CBF responses elicited by whisker stimulation (Fig. 4c), suggesting that FL-RSV treatment restored the NO mediation of neurovascular coupling in aged animals.
Fig. 4.
Treatment with resveratrol encapsulated in novel fusogenic liposomes rescues endothelium-dependent neurovascular coupling responses in aged mice. Panel A: Representative pseudocolour laser speckle flowmetry maps of baseline CBF (upper row) and CBF changes in the whisker barrel field (circle) relative to baseline (differential images are shown in the bottom row) during contralateral whisker stimulation (30 s, 5 Hz) in untreated young (3 month old) and aged (24 month old) mice and aged mice treated with fusogenic liposomes (FL) and resveratrol containing fusogenic liposomes (FL-RSV), before and after administration of the NO synthase inhibitor L-NAME. Color bar represents CBF as percent change from baseline. Panel B shows the time-course of CBF changes in the whisker barrel cortex (see circle in Panel A) after the start of contralateral whisker stimulation (horizontal bars). Summary data are shown in panel C. Data are mean ± S.E.M. (n = 6–8 in each group), *P < 0.05 vs. Young; #P < 0.05 vs. Aged+FL-RSV. (one-way ANOVA with post-hoc Tukey’s tests)
Treatment with FL-RSV improves endothelial function in aged aortas
To further ascertain the endothelial protective effects of FL-RSV, endothelium-dependent vasodilator responses were tested in aorta ring preparations. In young vessels administration of acetylcholine resulted in significant relaxation, whereas these responses were significantly attenuated in vessels derived from aged mice (Fig. 5). Treatment of aged mice with FL-RSV significantly improved acetylcholine-induced vasorelaxation, restoring responses to the level observed in vessels of young mice. To assess the role of endothelium-derived NO, L-NAME was applied. L-NAME significantly inhibited acetylcholine-induced responses, eliminating the differences between the three groups. These finding suggests that FL-RSV significantly improves endothelial function by restoring endothelial NO mediation in aged vessels (data not shown).
Fig. 5.
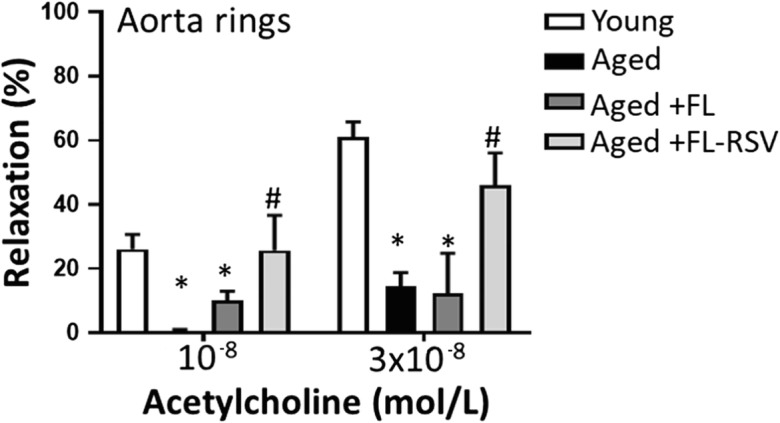
Treatment with resveratrol encapsulated in fusogenic liposomes rescues endothelium-dependent relaxation in aged mouse aortas. Acetylcholine-induced, L-NAME sensitive relaxation was measured in aorta ring preparations isolated from untreated young (3-month-old) and aged (24-month-old) mice and aged mice treated with fusogenic liposomes (FL) and resveratrol-containing fusogenic liposomes (FL-RSV). Data are mean ± S.E.M. (n = 5–8 for each data point).*P < 0.05 vs. Young; # P < 0.05 vs. Aged. (one-way ANOVA with posthoc Tukey’s test)
Discussion
The key finding of his study is that treatment with FL-RSV effectively targets the neurovascular unit and rescues endothelium-dependent neurovascular coupling responses in a mouse model of aging that recapitulates cerebromicrovascular dysfunction and cognitive deficits manifested in elderly patients.
Our previous studies demonstrated that resveratrol present within fusogenic liposomes significantly enhances their fusion to endothelial cell membranes and thus can facilitate the uptake of resveratrol into cultured cells (Csiszar et al. 2014). Here, we demonstrate for the first time that this lipophilic delivery system is also an effective approach to deliver resveratrol in vivo to the cells of the neurovascular unit, including vascular endothelial cells.
During the past three decades various liposomal carrier systems have been developed in pharmaceutical research, which enabled remarkable progress in the development of drugs with difficult-to-formulate active ingredients. While resveratrol is historically has been used as a dietary supplement, its tissue distribution after oral absorption is low, and high efficacies in vitro cannot be sufficiently translated to in vivo applications. Also, systemic use has been proven difficult, the lipophilic structure of resveratrol forbids a solubility >0.05 g/l in water (Robinson et al. 2015). Applying resveratrol in our newly developed liposomal carrier system FL-RSV facilitates the application of higher systemic doses if necessary. In our experiments, FL-RSV contained 0.3 g/l to apply a dose of 2 mg/kg. Using FL-RSV, we could show that the pharmacological effect of resveratrol in the cerebral microcirculation was comparable to previous data of orally applied resveratrol (Toth et al. 2014), yet the treatment period and dose could be reduced for comparable rescue of neurovascular responses.
Age-related impairment of neurovascular coupling responses manifests in older adults (Zaletel et al. 2005; Topcuoglu et al. 2009; Stefanova et al. 2013) and has been causally linked to cognitive decline (Sorond et al. 2013; Sorond et al. 2011). Our previous research has confirmed that advanced aging in mice is also associated with significant neurovascular dysfunction, characterized by diminished CBF changes in response to increased neuronal activation (Tarantini et al. 2017a; Toth et al. 2014; Tarantini et al. 2018; Tarantini et al. 2019). Here, we demonstrate for the first time that age-related impairment of neurovascular coupling responses is rescued by delivery of resveratrol using our innovative fusogenic liposome-based delivery system. Rescue of a critical homeostatic mechanism that matches nutrient and oxygen delivery to the increased needs of active neuronal tissue is expected to have beneficial effects on cognitive function in aging (Oomen et al. 2009; Zhao et al. 2013; Liu et al. 2012). This should be experimentally tested in future studies.
Endothelium-derived NO contributes importantly to neurovascular coupling responses (Tarantini et al. 2017a; Toth et al. 2014; Tarantini et al. 2015; Tarantini et al. 2018; Tarantini et al. 2019; Toth et al. 2015c). We find that administration of FL-RSV to aged animals restores NO mediation of neurovascular coupling responses, supporting the concept that potent endothelial protective effects of resveratrol play a key role in its anti-aging action (Ungvari et al. 2007a; Pearson et al. 2008; Ungvari et al. 2007c; Bernier et al. 2016; Csiszar et al. 2008; Csiszar et al. 2006; Zhang et al. 2010) The available evidence suggests that the mechanism(s) by which advanced age impairs cerebromicrovascular endothelial function involves an increased breakdown of NO by elevated levels of ROS (Toth et al. 2014; Tarantini et al. 2018; Tarantini et al. 2019). Restoration of endothelial function in aged peripheral arteries has also been reported using structurally different mitochondria-targeted antioxidants, including MitoQ, MitoTEMPO and SS-31 (Tarantini et al. 2018; Tarantini et al. 2019; Gioscia-Ryan et al. 2014; Kiss et al. 2019), suggesting that ROS production by dysfunctional mitochondria critically contributes to age-related endothelial oxidative stress. In that regard it is important that, resveratrol was shown to effectively inhibit mitochondrial ROS production in endothelial cells (Ungvari et al. 2009; Ungvari et al. 2007b). On the basis of the observation that administration of FL-RSV significantly inhibits oxidative stress in aged CMVECs in vitro (Csiszar et al. 2014), we posit that in aged mice fusogenic liposome-mediated delivery of resveratrol to the aged neurovascular unit restores NVC responses and endothelial function by preventing ROS-mediated scavenging of NO in the endothelial cells. The mechanisms by which resveratrol, in part via sirtuin activation, may attenuate mtROS production in aging are likely multifaceted. Age-related loss of efficiency in mitochondrial electron transport likely increases electron leak and mtROS production, which could be reversed by resveratrol- and sirtuin-induced up-regulation of mitochondrial electron transport chain subunits (Tarantini et al. 2019; Csiszar et al. 2009; Kiss et al. 2019; Dai et al. 2012; Ungvari et al. 2008). In addition, there is preclinical evidence that resveratrol also activates the antioxidative transcription factor Nrf2 in vascular cells, up-regulating antioxidant enzymes and promoting glutathione metabolism (Csiszar et al. 2014; Csiszar et al. 2012; Ungvari et al. 2010). It is likely that activation of Nrf2-dependent pathways (Fulop et al. 2018) contribute to the endothelial and neurovascular protective effects of FL-RSV treatment as well (Ungvari et al. 2010).
Our findings have important translational relevance that extends beyond resveratrol-mediated neurovascular protection. Our studies provide direct evidence that the cerebral microcirculation and specifically the neurovascular unit can be successfully targeted with systemically applied fusogenic liposomes. Our recent studies demonstrate that fusogenic liposomes are also ideal molecular carrier systems for effective delivery of other cargo as well, e.g. proteins and nucleic acids (Kube et al. 2017; Hoffmann et al. 2019). We propose that resveratrol containing fusogenic liposomes could be used to deliver a complex cocktail of anti-geronic factors, which include proteins, small molecules (Nacarelli et al. 2018; Moore et al. 2017; An et al. 2017; Urfer et al. 2017; Perrott et al. 2017; Grimmig et al. 2017), DNA vectors and miRNAs targeting key pathways involved in microvascular aging and neurovascular dysfunction (Ungvari et al. 2018).
Acknowledgements
This collaborative research project was conducted within the framework of the International Geroscience Training Program of the Oklahoma Center for Geroscience. This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, PB, ZU), the National Institute on Aging (R01-AG047879; R01-AG038747; R01-AG055395), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (GM104938, to AY), the Presbyterian Health Foundation (to ZU, AC, AY), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tabea Wiedenhoeft, Stefano Tarantini, Ádám Nyúl-Tóth and Andriy Yabluchanskiy contributed equally to this work.
References
- An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, Miller RA, Rabinovitch PS, Cox TC, Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017;39:457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittes J, Lucio M, Nunes C, Lima JL, Reis S. Effects of resveratrol on membrane biophysical properties: relevance for its pharmacological effects. Chem Phys Lipids. 2010;163:747–754. doi: 10.1016/j.chemphyslip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Phys. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Hersch N, Dieluweit S, Biehl R, Merkel R, Hoffmann B. Novel Fusogenic liposomes for fluorescent cell labeling and membrane modification. Bioconjug Chem. 2010;21:537–543. doi: 10.1021/bc900470y. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z (2014) Resveratrol encapsulated in novel Fusogenic liposomes activates Nrf2 and attenuates oxidative stress in Cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed]
- Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage. 2013 [DOI] [PMC free article] [PubMed]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmig B, Kim SH, Nash K, Bickford PC, Douglas Shytle R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience. 2017;39:19–32. doi: 10.1007/s11357-017-9958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch N, Wolters B, Ungvari Z, Gautam T, Deshpande D, Merkel R, Csiszar A, Hoffmann B. Biotin-conjugated fusogenic liposomes for high-quality cell purification. J Biomater Appl. 2016;30:846–856. doi: 10.1177/0885328215603026. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Hersch N, Merkel R, Csiszar A, Hoffmann B. Changing the way of entrance: highly efficient transfer of mRNA and siRNA via Fusogenic Nano-carriers. J Biomed Nanotechnol. 2019;15:170–183. doi: 10.1166/jbn.2019.2663. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wyckoff J, Morris AT, Succop A, Avery A, Duncan GE, Jazwinski SM. DNA methylation associated with healthy aging of elderly twins. Geroscience. 2018;40:469–484. doi: 10.1007/s11357-018-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z (2019) Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment GeroScience. in press [DOI] [PMC free article] [PubMed]
- Kleusch C, Hersch N, Hoffmann B, Merkel R, Csiszar A. Fluorescent lipids: functional parts of fusogenic liposomes and tools for cell membrane labeling and visualization. Molecules. 2012;17:1055–1073. doi: 10.3390/molecules17011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinac R, Kleusch C, Braun T, Merkel R, Csiszar A (2018) Deciphering the functional composition of Fusogenic liposomes. Int J Mol Sci 19 [DOI] [PMC free article] [PubMed]
- Kovac S, Angelova PR, Holmstrom KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube S, Hersch N, Naumovska E, Gensch T, Hendriks J, Franzen A, Landvogt L, Siebrasse JP, Kubitscheck U, Hoffmann B, Merkel R, Csiszar A. Fusogenic liposomes as Nanocarriers for the delivery of intracellular proteins. Langmuir. 2017;33:1051–1059. doi: 10.1021/acs.langmuir.6b04304. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40:163–176. doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GS, Zhang ZS, Yang B, He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sci. 2012;91:872–877. doi: 10.1016/j.lfs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- Masser DR, Hadad N, Porter H, Stout MB, Unnikrishnan A, Stanford DR, Freeman WM. Analysis of DNA modifications in aging research. Geroscience. 2018;40:11–29. doi: 10.1007/s11357-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Bowley B, Shultz P, Calderazzo S, Shobin E, Killiany RJ, Rosene DL, Moss MB. Chronic curcumin treatment improves spatial working memory but not recognition memory in middle-aged rhesus monkeys. Geroscience. 2017;39:571–584. doi: 10.1007/s11357-017-9998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostany R, Portera-Cailliau C (2008a) A craniotomy surgery procedure for chronic brain imaging. J Vis Exp [DOI] [PMC free article] [PubMed]
- Mostany R, Portera-Cailliau C (2008b) A method for 2-photon imaging of blood flow in the neocortex through a cranial window. J Vis Exp [DOI] [PMC free article] [PubMed]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience. 2018;40:243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrott KM, Wiley CD, Desprez PY, Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Mock C, Liang D. Pre-formulation studies of resveratrol. Drug Dev Ind Pharm. 2015;41:1464–1469. doi: 10.3109/03639045.2014.958753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker MR, Franks SF. Efficacy of curcumin for age-associated cognitive decline: a narrative review of preclinical and clinical studies. Geroscience. 2018;40:73–95. doi: 10.1007/s11357-018-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging. 2013;34:2277–2286. doi: 10.1016/j.neurobiolaging.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges E, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer's disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett. 2009;452:17–22. doi: 10.1016/j.neulet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]
- Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/Nitrative stress. Am J Physiol Heart Circ Physiol. 2010;299:H985–H994. doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602. doi: 10.1016/j.bbrc.2013.05.025. [DOI] [PubMed] [Google Scholar]