Abstract
Infectious disease, predominately chlamydiosis, contributes significantly to the decline in health of wild koala (Phascolarctos cinereus) populations in some regions of Australia. In this study, we describe the development and evaluation of a simple, sensitive, and specific loop‐mediated isothermal amplification (LAMP) assay for the detection of Chlamydia pecorum in koalas as a point‐of‐care diagnostic tool that can be used in any wildlife hospital and in the field on specialized instrumentation. A set of primers targeting a 188‐bp region of the C. pecorum genome was designed. 100% specificity of the LAMP assay was revealed by demonstrating no cross‐reactivity with 33 nontarget pathogens, and complete correlation with qPCR results for 43 clinical swabs collected opportunistically from wildlife hospitals. In sensitivity evaluations, the technique successfully detected serial dilutions of extracted C. pecorum DNA with a detection limit of 44 IFU/ml.
Keywords: Chlamydia pecorum, diagnostics, koala, loop‐mediated isothermal amplification, point‐of‐care
In this study, we describe the development and evaluation of a Chlamydia pecorum‐specific loop‐mediated isothermal amplification (LAMP) assay on the Genie ® III as a rapid point‐of care diagnostic test that can be easily incorporated into wildlife hospitals or koala field surveys. Furthermore, we present a simplistic one‐step lysis method for template preparation using koala conjunctival, urogenital, and rectal swabs. We have demonstrated the C. pecorum LAMP assay is highly sensitive and specific compared with real‐time PCR, with a level of detection of 44 C. pecorum IFU/ml.
![]()
1. INTRODUCTION
Spanning over 15 years, approximately 50% of koalas admitted to South East Queensland (SEQLD) wildlife hospitals have presented with chlamydiosis, frequently in combination with trauma and body wasting (Gonzalez‐Astudillo, Allavena, McKinnon, Larkin, & Henning, 2017). The obligate intracellular bacteria Chlamydia pecorum is the most pathogenic and prevalent bacteria affecting koalas (Devereaux, Polkinghorne, Meijer, & Timms, 2003; Jackson, White, Giffard, & Timms, 1999; Marsh, Kollipara, Timms, & Polkinghorne, 2011) and infects the ocular, urinary, and genital mucosae with acute and/or persistent exposure to infection resulting in blindness, cystitis, and infertility (Blanshard & Bodley, 2008; Wan et al., 2011). Not all infections manifest in overt disease, and approximately half the koalas in SE Queensland appear to be healthy carriers of C. pecorum and do not exhibit any signs of disease (Polkinghorne, Hanger, & Timms, 2013). Diagnosis of chlamydial disease in koalas has been by a combination of visual assessment of clinical signs of disease, ultrasonography of the koala urogenital tract, and, up until recently, a diagnostic point‐of‐care (POC) solid‐phase enzyme immunoassay for detection of Chlamydia lipopolysaccharide antigen (Clearview®); however, this product is no longer commercially available. In addition, Hanger et al. (2013) showed that Clearview was only 43.2% sensitive and 92% specific compared with C. pecorum detection via qPCR, indicating that a Clearview result lacked specificity and sensitivity. With production of Clearview® ceasing, there is currently no alternative POC method for detection of C. pecorum infection in koalas available to wildlife hospitals, and consequently, there has been an increasing reliance on external diagnostic laboratories for confirmation of infection via molecular detection incorporating nucleic acid amplification tests such as conventional and real‐time PCR (Hulse et al., 2018; Sachse et al., 2009).
Polymerase chain reaction (PCR) is considered the “gold standard” diagnostic assay of choice for detection of Chlamydia based on its reliability and high sensitivity and specificity. However, a key disadvantage of PCR‐based methods has been the restriction of its use to research and/or diagnostic laboratories to undertake sample processing and time‐consuming thermocycling protocols, which can delay diagnosis and subsequent treatment by 24–48 hr. This means that infection status of koalas is not available to veterinarians and koala carers until well after initial decisions on animal management have been determined, leading to delays for application of animal therapeutic treatment regimes.
Molecular diagnostic tools that can be performed at POC have been developed incorporating loop‐mediated isothermal amplification (LAMP), a newly developed, simple yet sophisticated, gene amplification method (Notomi, Mori, Tomita, & Kanda, 2015). The key advantage of LAMP is that it is a simple and rapid protocol, which means “on‐the‐spot” pathogen diagnosis is possible. It offers high sensitivity and specificity even when there is a decreased amount of target DNA available (Notomi et al., 2000). Using LAMP, the target sequence is amplified at a constant temperature of 60–65°C (no cycling required) using multiple sets of primers and a polymerase with high strand displacement activity in addition to a replication activity. Typically, four different primers are used to identify six distinct regions on the target gene, which adds highly to the specificity. An additional pair of "loop primers" further accelerates the reaction. Due to the specific nature of the action of these primers, the amount of DNA produced using LAMP is considerably higher than PCR‐based amplification, meaning results are available in less than an hour. As confirmation of amplification of the correct target, an anneal step, consisting of 98–80ºC at 0.01ºC/s resulting in an anneal temperature of the amplicon produced, is performed after the isothermal step. Each target will have its own signature anneal temperature which is determined by the GC content of the generated amplicon. Positive amplification followed by an anneal temperature at the expected temperature is confirmation of a true positive in a sample.
The Genie ® III (OptiGene Limited) is a compact, portable, battery‐operated device for isothermal amplification and real‐time fluorescence detection at the POC. LAMP assays performed on the Genie ® III provide a rapid diagnostic tool with high specificity, due to the multiple primer sets; high sensitivity; all with minimal sample template preparation, which allows for easy incorporation as a diagnostic tool within the field or wildlife hospital, as previously reported by Jelocnik et al. (2017). In the present study, we describe the development and validation of a C. pecorum‐specific LAMP assay on the Genie ® III using conjunctival, urogenital, and rectal koala swabs, aimed at POC pathogen detection by wildlife hospital veterinary clinicians and koala ecologists in the field.
2. MATERIALS AND METHODS
2.1. Sample template preparation
Duplicate conjunctiva, urogenital sinus (females) or penile urethra (males), and rectal samples were collected from koalas admitted to Currumbin Wildlife Hospital (Currumbin, Queensland) and Australia Zoo Wildlife Hospital (Beerwah, Queensland) using rayon‐tipped aluminum‐shafted swabs (Copan, Murrieta, California). Opportunistic sampling of koalas admitted to wildlife hospitals included animals exhibiting clinical signs of disease and symptoms unrelated to infectious disease (e.g., trauma, ill thrift, poor body condition).
Two crude DNA lysing methods to isolate DNA template from patient swabs were evaluated, as recommended by Optigene LAMP user guide for Mastermixes and assay optimization. The first method consisted of a two‐step lysis procedure, whereby 0.2 ml of nuclease free water was added to a swab in a 2‐ml microtube followed by vigorous mixing. A 50 µl aliquot was then heated to 95°C for 5 min to disrupt cell membranes and release cell contents into the buffer. A second method consisted of a crude one‐step lysis method for the LAMP assay by the addition of 0.2 ml alkaline solution (potassium hydroxide, KOH) to a swab in a 2‐ml microtube followed by vigorous mixing. The addition of KOH to the swab enables destruction of the cell wall of any bacteria or host cells present, releasing the cell contents, including bacterial DNA into the lysis buffer.
2.2. Bacterial strains and DNA preparation
Assay specificity was determined by testing DNA extracts from 33 bacterial pathogens, including DNA extracted from additional Chlamydia sp. isolates to assess LAMP assay cross‐reactivity and nontarget bacterial species identified as possible natural flora within the conjunctival and urogenital tract of koalas or close relatives of these bacterial species (See Table A1) (Blanshard, 1994; Blanshard & Bodley, 2008). Bacterial genomic DNA was extracted from isolates using the DNeasy Blood & Tissue Kit (QIAGEN Inc) according to manufacturer's instructions. In addition, DNA was isolated from quantified (inclusion‐forming units; IFU/ml) reference C. pecorum strain culture and used as a positive control for the development and optimization of the LAMP assay. Assay sensitivity and limit of detection were determined by constructing a standard curve in the range of 4.39 × 101–107 (44–43,900,000 IFU/mL) using genomic DNA extracted from C. pecorum culture purified and quantified as described in Caldwell, Kromhout, and Schachter (1981).
2.3. LAMP primer design
LAMP primers were designed from the Chlamydia pecorum P787, complete genome sequence (GenBank accession # CP004035) with the target primer sequences coding for the C. pecorum mreC gene, encoding for cell shape determining protein, with an amplicon length of 188 bp. Six oligonucleotide primers, consisting of the outer forward primer (F3), outer backward primer (B3), forward inner primer (FIP), backward inner primer (BIP), loop forward (LoopF), and loop backward (LoopB), were selected using licensed LAMP Designer 1.15 software (Premier Biosoft). Primer sequences were designed to specifically avoid cross homologies with other chlamydial species. Intraspecies specificity of the target sequence was confirmed using the Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/) and Geneious Prime 2019.1.3. Primers were synthesized by Integrated DNA Technologies. Table 1 provides information on the name and sequence of each oligonucleotide primer, and Figure 1 presents the nucleotide position and target gene location of LAMP primers within the C. pecorum genome generated from Geneious Prime 2019.1.3.
Table 1.
Primer sets designed for C. pecorum LAMP and qPCR
| Purpose | Primer name | Nucleotide sequence (5′–3′) | Source | GenBank accessiona |
|---|---|---|---|---|
| LAMP assay | F3 | TATCGTGATCCTGCACATTG | This study | CP004035 |
| B3 | CGCATTGCAATTACAGAAGG | This study | (6,925–7,112) | |
| FIP | TCCCGAAAGCACAGGAGAATTCGCTCGTGTTGGGTAGATG | This study | ||
| BIP | AAGGTGCTAGTTGGTCTTGTGGCTTCATGCCTTCATCCGTAA | This study | ||
| LoopF | TCTTTACTCCTTTGTCCTTCCC | This study | ||
| LoopB | AAGCAATCTCGTGTACGGTT | This study | ||
| qPCR | OmpB‐F | CCAAGCATAATCGTAACAA | Hulse et al., 2018 | U56924 (125–265) |
| OmpB‐R | CGAAGCAAGATTCTTGTC | Hulse et al., 2018 | ||
| OmpB‐Cy5 | Cy5‐ACTTGTTGGCAATTCTTCTCTTCACA | Hulse et al., 2018 | ||
| OmpA‐F | ATGAAAAAACTCTTAAAATCGG | Kollipara et al., 2013 | NC_015408 | |
| OmpA‐R | TTAGAATCTGCATTGAGCAG | Kollipara et al., 2013 | (56741–57916) |
Numbers in parentheses indicate nucleotide position.
Figure 1.

C. pecorum LAMP assay primer sequences and nucleotide positions in the target gene regions. Outer F3 and B3 primers are shown in red; inner FIP and BIP in green; and LoopF and LoopB in purple
2.4. LAMP reaction and amplification condition
To make a comparative analysis and establish specificity of the LAMP assay by comparing amplification results with qPCR, published primers for two independent qPCR assays that targeted C. pecorum OmpA and OmpB gene sequences (Table 1) were used on DNA extracted (MagJET genomic DNA kit, Thermo Fisher Scientific) and purified (KingFisher Flex, Thermo Fisher Scientific) from the duplicate conjunctiva, urogenital sinus, and rectal clinical swab samples used for development of the LAMP assay (Hulse et al., 2018; Kollipara et al., 2013). C. pecorum OmpA and OmpB qPCR were performed on a RotorGene‐Q real‐time PCR cycler (Qiagen) to validate LAMP assay results.
LAMP reaction was carried out using a total of 25 µl of the reaction mixture containing 5 µl of primer mix (Table 2), 15 µl of LAMP Isothermal Master Mix (Optigene), and 5 µl of sample. DNA lysis buffer was added as a template.
Table 2.
LAMP assay primer mix
| Primer (25 pmol/µl) | Volume (µl) | Concentration (µmol/L) |
|---|---|---|
| F3 | 2.5 | 0.2 |
| B3 | 2.5 | 0.2 |
| LoopF | 12.5 | 1.0 |
| LoopB | 12.5 | 1.0 |
| FIP | 25 | 2.0 |
| BIP | 25 | 2.0 |
| Nuclease free H2O | 170 |
The LAMP assay was run at 65°C for 30 min with a melting curve analysis step (annealing curve 98°–80°C ramping at 0.01°C/s) on the portable Genie ® III (OptiGene) real‐time fluorometer. A no template (water) control and DNA extracted from C. pecorum strain culture were used as negative and positive controls, respectively. Positive and negative results of samples were evaluated based on amplification plots, time to positivity, and melting curve and temperature of target amplicons analyzed automatically by the Genie III system.
3. RESULTS
3.1. Evaluation of sample template preparation
Comparison of sample template preparation revealed the heating method did not appear to sufficiently lyse the template, whereby no amplification of product occurred from samples confirmed positive via real‐time PCR results. Establishing that the first method of sample template preparation was unsuccessful, the second method of sample template preparation was applied for the remainder of the study. The addition of KOH to swab samples sufficiently released template DNA into the buffer prior to LAMP testing, confirmed by comparison with real‐time PCR results of the duplicate sample.
3.2. Chlamydia pecorum limit of detection—Sensitivity
Table 3 presents the time to amplification of the C. pecorum standard curve and corresponding melt temperature. Linear regression of the standard curve determined the LAMP assay level of detection, established at 44 C. pecorum IFU/ml and amplifying in less than 20 min. Figure 2 graphically presents the amplification data of the standard curve generated from Genie Explorer V2.0.5.0 (OptiGene Limited).
Table 3.
C. pecorum standard curve dilution series time to amplification and melt temperature
| C. pecorum concentration (IFU/ml) | Time to amplification | Melt (°C) |
|---|---|---|
| 4.39 × 107 | 05:30 | 84.75 |
| 4.39 × 106 | 07:30 | 84.59 |
| 4.39 × 105 | 08:00 | 84.68 |
| 4.39 × 104 | 08:15 | 84.55 |
| 4.39 × 103 | 10:15 | 84.42 |
| 4.39 × 102 | 13:00 | 84.71 |
| 4.39 × 101 | 18:00 | 84.77 |
| NTC | ‐ | ‐ |
Figure 2.
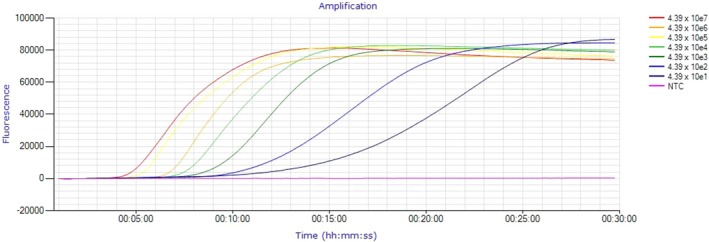
Amplification of C. pecorum standard curve
3.3. Comparative specificity of C. pecorum LAMP Assay
Specificity of the C. pecorum LAMP assay was demonstrated by amplification only occurring in DNA extracted from the C. pecorum isolate with no cross‐hybridization with nontarget bacterial DNA (Table A1). A total of 43 DNA extracts from clinical samples were tested with two C. pecorum qPCR assays, each targeting different genes, and compared with LAMP assay results from the corresponding duplicate clinical swab, whereby sample template was prepared by the addition of KOH to the swab. Table 4 presents 100% agreement between the three methods of detection with Table A2 demonstrating comparison with LAMP assay time to amplification with real‐time PCR amplification cycling threshold for both qPCR assays.
Table 4.
Comparison of results from C. pecorum real‐time PCR and LAMP assays using clinical swabs
| Assay | Koala conjunctival swabs | Koala urogenital swabs | Koala rectal swabs |
|---|---|---|---|
| Positive | Positive | Positive | |
| C. pecorum OmpA (real‐time PCR) | 12/24 | 13/16 | 1/3 |
| C. pecorum OmpB (real‐time PCR) | 12/24 | 13/16 | 1/3 |
| C. pecorum LAMP | 12/24 | 13/16 | 1/3 |
4. DISCUSSION
Rhodes et al. (2011) have suggested that chlamydial infection and disease, predominately attributed to C. pecorum, is a key agent of population decline of koalas in SEQLD. Previously, POC tests for identification of C. pecorum infections in koalas have relied on the Clearview® Chlamydia Antigen test, despite the poor sensitivity of the test as reported by Hanger et al. (2013). Thus, identification of C. pecorum infection in the koala is now predominately determined by nucleic acid amplification tests (e.g., Hulse et al., 2018). However, the use of these assays, which are highly sensitive and specific to the target pathogen, is generally restricted to research and diagnostic laboratories, a process that can be time‐consuming and present delays to the veterinary clinician in diagnosis and animal management decisions.
Among the vast diversity of available nucleic acid amplification protocols, the technique of loop‐mediated isothermal amplification can be considered a very promising application for POC systems for pathogen detection. The availability of a point‐of‐care LAMP assay, such as the one described and validated in this study, enables the clinician to provide a definitive diagnosis within an hour of clinical samples being collected and immediate application of appropriate therapeutic interventions.
In this study, we describe the design of a LAMP assay for the rapid detection of C. pecorum on a Genie ® III and the evaluation of its performance against koala clinical samples and nontarget pathogens as a POC application. This study has shown that the C. pecorum LAMP assay is 100% sensitive and specific in detecting C. pecorum infection from koala clinical swab samples, when compared to the real‐time PCR assays, with a level of detection at 44 C. pecorum IFU/ml. While it is uncommon for a diagnostic assay to be 100% sensitive and specific compared with the gold standard method, it is highly likely that if there was more than one sample type being evaluated, there would be lower correlation between tests. However, this study evaluated the standard sample type collected from koalas for molecular detection of Chlamydia sp. within wildlife hospitals.
LAMP has successfully been used to diagnose various pathogens and viruses in humans and animals (Dhama et al., 2014), and due to its simplicity, robustness, and low cost, LAMP can be used as a simple screening assay in the field or at the point of care by clinicians. While Jelocnik et al. (2017) presented a similar study whereby LAMP assays were developed targeting C. psittaci and C. pecorum and were designed to run on a Genie ® III as a diagnostic tool for both human and veterinary clinicians, this study has further developed the concept to establish a one‐step method of swab sample preparation prior to LAMP testing, hence eliminating the heating step outlined in this study and the method presented by Jelocnik et al. (2017) further minimizing the additional use of labor and equipment. This study has demonstrated that cell lysis via KOH rapidly releases sufficient quantity of template DNA from crude samples, while minimizing the presence of amplification inhibitors and therefore the likelihood of false negatives occurring.
The methodology outlined in this study has already been successfully incorporated as a point‐of‐care diagnostic tool within Currumbin Wildlife Hospital, Queensland, RSPCA Wildlife, Wacol, Queensland, and Moggill Koala Rehabilitation Centre, Queensland, and used in the field for “on the spot” diagnosis of captured wild koalas. Senior veterinarian of Currumbin Wildlife Hospital, Dr Michael Pyne reported that following 6 months of using the C. pecorum LAMP assay on the Genie III platform as part of the hospital's in‐clinic diagnostics, he is now able to rapidly and accurately assess hospitalized koalas for chlamydial infection and make quick decisions on treatment options in subclinical cases, which allows for faster release times when assessing koalas post‐treatment (M. Pyne, personal communication, 20 May 2019). In the field, rapid monitoring of the infectious status of koalas is important in an effort to better understand the prevalence and epidemiology of chlamydiosis, but this has currently not been possible due to current nucleic acid detection methods requiring 24–48 hr to perform in a diagnostic laboratory. The C. pecorum LAMP assay is a rapid test that can be performed in the field on freshly collected samples that require minimal processing and thereby be used to fast track conservation management decisions.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
Conceptualization: Ken Beagley and Lyndal Hulse; data curation, investigation, and formal analysis: Lyndal Hulse; funding acquisition: Stephen Johnston and Ken Beagley; methodology: Sean McDonald and Lyndal Hulse; writing—original draft preparation: Lyndal Hulse; writing—review and editing: Stephen Johnston, Ken Beagley, and Lyndal Hulse.
ETHICS STATEMENT
Samples were collected under the Department of Environment Scientific Purposes Permit WISP16751015 and University of Queensland Animal Ethics Permit AE02305.
ACKNOWLEDGMENTS
This study was funded by the following Queensland State Government Koala Research Grant; “The Pathology, Incidence, Treatment and Management of Chlamydiosis in the Male Koala” (Project Number KRG005, Department of Environment and Heritage Protection); and the Community Sustainability Action Koala Research Grant; “Development of a rapid point‐of‐care test for detecting infections in the koala” (Project Number CSAR17016, Queensland University of Technology). We gratefully acknowledge Patrick Blackall from the Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation, University of Queensland; Louise Harris, Veterinary Microbiology Department, Vetnostics, QML Pathology, Murarrie, Queensland; Olusola Martins Olaogun, Asia Pacific Centre for Animal Health, University of Melbourne; Emma Sweeney and Emily Bryon, School of Biomedical Sciences, Faculty of Health, Institute of Health Biomedical Innovation, Queensland University of Technology, Kelvin Grove, QLD, Australia; for donating the isolates for specificity testing.
APPENDIX 1.
Table A1.
Specificity of the C. pecorum LAMP assay against target and nontarget reference strains and isolates
| Strain/isolate | C. pecorum | |
|---|---|---|
| Time to amplification | Melt (°C) | |
| Mycoplasma gallisepticum ts−11 | ‐ | ‐ |
| Mycoplasma synoviae MS‐H | ‐ | ‐ |
| Ureplasma parvum serovar 3 S34425 P4JN | ‐ | ‐ |
| Ureplasma parvum serovar 6 UPS6 | ‐ | ‐ |
| Ureplasma parvum serovar 5 UPS5 | ‐ | ‐ |
| Ureplasma parvum serovar 10 UPS10 | ‐ | ‐ |
| Chlamydia pecorum (Mars Bar) | 05:30 | 84.74 |
| Chlamydia trachomatis serovar D | ‐ | ‐ |
| Chlamydia trachomatis serovar E | ‐ | ‐ |
| Chlamydia muridarum | ‐ | ‐ |
| Chlamydia caviae | ‐ | ‐ |
| Chlamydia pneumoniae A03 | ‐ | ‐ |
| Chlamydia pneumoniae (koala) | ‐ | ‐ |
| Bordetella bronchiseptica BR977 (pig) | ‐ | ‐ |
| Bordetella bronchiseptica BR976 (koala) | ‐ | ‐ |
| Bordetella bronchiseptica BR978 | ‐ | ‐ |
| Bordetella hinzii TC58T (=LMG 13501T) (chicken) | ‐ | ‐ |
| Bordetella avium 591−77T (=ATCC 35086T) (turkey) | ‐ | ‐ |
| Bordetella bronchiseptica TC42 (turkey) | ‐ | ‐ |
| Bordetella bronchiseptica TC6T (=ATCC 19395T) (dog) | ‐ | |
| Staphylococcus aureus BR256 (=ATCC 29213) | ‐ | ‐ |
| Escherichia coli BR316 (=ATCC 25922) | ‐ | ‐ |
| Pseudomonas aeruginosa BR317 (=ATCC 27853) | ‐ | ‐ |
| Staphylococcus pseudintermedius (dog) | ‐ | ‐ |
| Escherichia coli (dog) | ‐ | ‐ |
| Streptococcus gpG (dog) | ‐ | ‐ |
| Pseudomonas aeruginosa (dog) | ‐ | ‐ |
| Pseudomonas aeruginosa BR317 (=ATCC 27853) | ‐ | ‐ |
| Staphylococcus pseudintermedius (dog) | ‐ | ‐ |
| Klebsiella pneumoniae (human) | ‐ | ‐ |
| Klebsiella pneumoniae (horse) | ‐ | ‐ |
| Klebsiella pneumoniae (dog) | ‐ | ‐ |
| Klebsiella pneumoniae (koala) | ‐ | ‐ |
| Klebsiella oxytoca (human) | ‐ | ‐ |
| Klebsiella oxytoca (horse) | ‐ | ‐ |
Table A2.
Specificity of C. pecorum LAMP assay against real‐time PCR C. pecorum OmpA and OmpB target genes
| Sample number | Koala swab sample | C. pecorum LAMP | C. pecorum OmpA (real‐time PCR) | C. pecorum OmpB (real‐time PCR) | |
|---|---|---|---|---|---|
| Time to amplification | Melt temperature (°C) | Cycling threshold | Cycling threshold | ||
| 78,729 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Right conjunctival | 10:00 | 84.97 | 23.22 | 29.06 | |
| Urogenital | 10:30 | 85.40 | 22.06 | 23.98 | |
| 78,732 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Right conjunctival | 0:00 | 0.00 | 0 | 0 | |
| Urogenital | 15:00 | 85.15 | 26.01 | 32.71 | |
| 78,661 | Urogenital | 11:00 | 85.15 | 35.04 | 34.51 |
| Rectal | 13:45 | 84.96 | 28.46 | 33.78 | |
| 71,942 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Right conjunctival | 0:00 | 0.00 | 0 | 0 | |
| Urogenital | 0:00 | 0.00 | 0 | 0 | |
| 72,035 | Urogenital | 17:08 | 84.56 | 18.16 | 22.41 |
| 72,097 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| 78,861 | Pooled conjunctival | 0:00 | 0.00 | 0 | 0 |
| Urogenital | 10:45 | 85.07 | 25.23 | 33.09 | |
| 78,822 | Pooled conjunctival | 13:45 | 84.76 | 24.31 | 25.51 |
| Urogenital | 10:15 | 84.96 | 25.59 | 29.45 | |
| 79,193 | Pooled conjunctival | 0:00 | 0.00 | 0 | 0 |
| Urogenital | 0:00 | 0.00 | 0 | 0 | |
| Rectal | 0:00 | 0.00 | 0 | 0 | |
| 71,499 | Left conjunctival | 9:30 | 84.51 | 24.36 | 28.57 |
| Right conjunctival | 12:00 | 84.32 | 23.84 | 31.21 | |
| Urogenital | 16:30 | 84.61 | 31.43 | 30.31 | |
| 71,494 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Urogenital | 7:30 | 84.42 | 22.65 | 27.98 | |
| 71,586 | Left conjunctival | 9:45 | 84.66 | 20.61 | 27.95 |
| Right conjunctival | 14:00 | 84.77 | 22.59 | 27.31 | |
| Urogenital | 15:30 | 85.00 | 20.76 | 29.44 | |
| 71,257 | Left conjunctival | 14:15 | 84.86 | 18.79 | 22.40 |
| Right conjunctival | 7:15 | 84.97 | 17.98 | 20.66 | |
| Urogenital | 6:15 | 84.87 | 15.47 | 19.10 | |
| 71,472 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Right conjunctival | 0:00 | 0.00 | 0 | 0 | |
| Urogenital | 18:45 | 85.26 | 31.25 | 25.69 | |
| 71,719 | Left conjunctival | 16:30 | 84.36 | 27.95 | 23.82 |
| 71,720 | Left conjunctival | 0:00 | 0.00 | 0 | 0 |
| Right conjunctival | 17:03 | 84.70 | 30.55 | 32.40 | |
| Urogenital | 16:45 | 84.56 | 20.85 | 27.83 | |
| 78,931 | Left conjunctival | 17:30 | 84.56 | 26.05 | 27.99 |
| Right conjunctival | 12:45 | 84.17 | 24.29 | 27.00 | |
| Urogenital | 18:15 | 84.27 | 30.83 | 28.00 | |
| Rectal | 0:00 | 0.00 | 0 | 0 | |
| 78,858 | Urogenital | 0:00 | 0.00 | 0 | 0 |
Hulse LS, McDonald S, Johnston SD, Beagley KW. Rapid point‐of‐care diagnostics for the detection of Chlamydia pecorum in koalas (Phascolarctos cinereus) using loop‐mediated isothermal amplification without nucleic acid purification. MicrobiologyOpen. 2019;8:e916 10.1002/mbo3.916
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- Blanshard, W. , & Bodley, K. (2008). Koalas Vogelnest L., & Woods R. (Eds.), Medicine of Australian Mammals (pp. 227–327). Collingwood, Vic.: CSIRO Publishing. [Google Scholar]
- Caldwell, H. D. , Kromhout, J. , & Schachter, J. (1981). Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis . Infection and Immunity., 31, 1161–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux, L. N. , Polkinghorne, A. , Meijer, A. , & Timms, P. (2003). Molecular evidence for novel chlamydial infections in the koala (Phascolarctos cinereus). System and Applied Microbiology, 26(2), 245–253. 10.1078/072320203322346092 [DOI] [PubMed] [Google Scholar]
- Dhama, K. , Karthik, K. , Chakraborty, S. , Tiwari, R. , Kapoor, S. , Kumar, A. , & Thomas, P. (2014). Loop‐mediated Isothermal Amplification of DNA (LAMP): A New Diagnostic Tool Lights the World of Diagnosis of Animal and Human Pathogens: A Review. Pakistan Journal of Biological Sciences, 17(2), 151–166. 10.3923/pjbs.2014.151.166 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Astudillo, V. , Allavena, R. , McKinnon, A. , Larkin, R. , & Henning, J. (2017). Decline causes of Koalas in South East Queensland, Australia: A 17‐year retrospective study of mortality and morbidity. Scientific Reports, 7, 42587 10.1038/srep42587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger, J. , Loader, J. , Wan, C. , Beagley, K. W. , Timms, P. , & Polkinghorne, A. (2013). Comparison of antigen detection and quantitative PCR in the detection of chlamydial infection in koalas (Phascolarctos cinereus). The Veterinary Journal, 195(3), 391–393. 10.1016/j.tvjl.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Hulse, L. S. , Hickey, D. K. , Mitchell, C. M. , Ellis, W. , Beagley, K. , & Johnston, S. (2018). The Development and Application of Two Multiplex Real Time PCR Assays for the Detection and Speciation of Bacterial Pathogens in the Koala (Phascolarctos cinereus). Journal of Veterinary Diagnostic Investigation, 30(4), 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M. , White, N. , Giffard, P. , & Timms, P. (1999). Epizootiology of Chlamydia infections. Veterinary Microbiology, 65, 255–264. [DOI] [PubMed] [Google Scholar]
- Jelocnik, M. , Islam, M. M. , Madden, D. , Jenkins, C. , Branley, J. , Carver, S. , & Polkinghorne, A. (2017). Development and evaluation of rapid novel isothermal amplification assays for important veterinary pathogens: Chlamydia psittaci and Chlamydia pecorum . PeerJ, 5, e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollipara, A. , Polkinghorne, A. , Wan, C. , Kanyoka, P. , Hanger, J. , Loader, J. , … Timms, P. (2013). Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Veterinary Microbiology, 167(3–4), 513–522. [DOI] [PubMed] [Google Scholar]
- Marsh, J. , Kollipara, A. , Timms, P. , & Polkinghorne, A. (2011). Novel molecular markers of Chlamydia pecorum genetic diversity in the koala (Phascolarctos cinereus). BMC Microbiology, 11(1), 77–77. 10.1186/1471-2180-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi, T. , Mori, Y. , Tomita, N. , & Kanda, H. (2015). Loop‐mediated isothermal amplification (LAMP): Principle, features, and future prospects. Journal of Microbiology, 53(1), 1–5. 10.1007/s12275-015-4656-9 [DOI] [PubMed] [Google Scholar]
- Notomi, T. , Okayama, H. , Masubuchi, H. , Yonekawa, T. , Watanabe, K. , Amino, N. , & Hase, T. (2000). Loop‐mediated isothermal amplification of DNA. Nucleic Acids Research, 28(12), i–vii. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkinghorne, A. , Hanger, J. , & Timms, P. (2013). Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Veterinary Microbiology, 165(3–4), 214–223. 10.1016/j.vetmic.2013.02.026 [DOI] [PubMed] [Google Scholar]
- Rhodes, J. R. , Ng, C. F. , de Villiers, D. L. , Preece, H. J. , McAlpine, C. A. , & Possingham, H. P. (2011). Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biological Conservation, 144(3), 1081–1088. 10.1016/j.biocon.2010.12.027 [DOI] [Google Scholar]
- Sachse, K. , Vretou, E. , Livingstone, M. , Borel, N. , Pospischil, A. , & Longbottom, D. (2009). Recent developments in the laboratory diagnosis of chlamydial infections. Veterinary Microbiology, 135(1–2), 2–21. 10.1016/j.vetmic.2008.09.040 [DOI] [PubMed] [Google Scholar]
- Wan, C. , Loader, J. , Hanger, J. , Beagley, K. , Timms, P. , & Polkinghorne, A. (2011). Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Australian Veterinary Journal, 89(10), 409–412. 10.1111/j.1751-0813.2011.00827.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


