Abstract
Plant mitochondria are important energy-producing structure and ROS are generated as byproducts. APX is one enzyme of the AsA-GSH cycle to reduces H2O2 to water. We identified both PtomtAPX and PtosAPX are located in mitochondria of Populus tomentosa Carr. PtomtAPX is specifically targeted to mitochondria, while PtosAPX is dual targeted to both chloroplast and mitochondria. The expression of PtomtAPX in mitochondria was 60-fold that of PtosAPX by ELISA and qPCR analysis. Under high light stress, the expression levels of PtosAPX increased, while that of PtomtAPX only slightly changed. Compared to the WT, the antisense transgenic PtomtAPX cell lines showed slowed growth, smaller cells impaired mitochondria in MS medium under normal growth. RNA-seq results showed 3121 genes significantly altered expression in the antisense cells, and most of them are important for mitochondrial function, particularly in oxidative phosphorylation. Our findings demonstrates a mitochondrial location for one APX isoform, and provide valuable insight into the mechanism which ROS balance is modulated by AsA-GSH cycle in mitochondria.
Subject terms: Plant cell death, Plant molecular biology
Introduction
Reactive oxygen species (ROS) are generated as byproducts of normal cell metabolism in several organelles and their production is enhanced under stress conditions1. APX is one enzyme of the ascorbate-glutathione cycle (AsA-GSH cycle) in plants2,3. APX in Arabidposis are localized to the cytosol (cAPX, AT1G07890, AT3G09640, AT4G32320), chloroplast (thylakoid-bound APX [tAPX, AT1G77490] and stromal APX [sAPX, AT4G08390]), microbody (including the peroxisome and glyoxisome) (mAPX, AT4G35000, AT4G35970) by organelle-specific targeting peptides and transmembrane domains4–7 and to remove H2O2 in the organelles themselves2. In Arabidopsis, apx1(AT1G07890) knockout plants showed increased H2O2 levels, higher sensitivity to oxidative stress, and suppressed growth and development8–10. Arabidopsis apx3(AT4G35000) knockout mutants did not show suppressed growth under normal or stress conditions11. Arabidopsis double tapx(AT1G77490)/apx1 (AT1G07890) mutants showed late flowering, low protein oxidation during light stress and enhanced accumulation of anthocyanins12. The levels of ROS were increased and the germination was reduced in seeds of Arabidopsis APX6 knockout mutants13. Rice plants double silenced for cytosolic APXs showed normal growth and development and were able to survive under stress conditions14,15. Loss of function in OsAPX2(Os07 g0694700) showed semi-dwarf seedlings, yellow-green leaves and seed sterility16. Rice peroxisomal ascorbate peroxidase(OsAPX4; Os08g43560) knockdown showed early leaf senescence17. These results indicate that the APXs isoenzymes are indispensable for plant growth and development.
Jimenez et al. (1998) reported APX activity in the external side of the outer mitochondria membrane from pea18.Three different APX isoforms was showed in mitochondria of tomato (Lycopersicon esculentum) using native gel electrophoresis19. De Leonardis et al.20 reported high APX activity which maybe localized inside mitochondria using sonication-mediated disruption of potato mitochondria20. And the presence of more than one APX in mitochondria of both leaves and young green inflorescences of Chenopodium album was detected by native gel electrophoresis21. However, until now, no gene, cDNA, or protein sequence for the plant specifically mitochondrial isoform (mitAPX) has been described. Arabidopsis single or double chlAPX (sAPX and tAPX) mutants showed normal phenotype under normal growth conditions or under high light intensity stress growth conditions22–24. In addition, sAPX knockdown rice plants exhibit a normal phenotype and show normal biochemical and physiological performance under normal growth conditions25. These results suggest that sAPX is not important for H2O2 scavenging in chloroplasts and/or mitochondria of A. thaliana or rice.
In this study, we investigated there is a mitochondria-specific APX of P. tomentosa using green fluorescent protein (GFP) fusion experiments and immunoelectron microscopy. And PtosAPX is dual targeted to both chloroplast and mitochondria. The expression levels of PtomtAPX and PtosAPX were modulated by H2O2, NaCl, heat, drought, and cold. Compared to the WT, the antisense transgenic PtomtAPX cell lines showed slowed growth, smaller cells impaired mitochondria in MS medium under normal growth. The results indicated that PtomtAPX is specifically targeted to mitochondria and plays an important role in maintaining the redox balance in Populus tomentosa Carr.
Results
PtomtAPX is specifically targeted to mitochondria, and PtosAPX is targeted to both chloroplasts and mitochondria
The poplar database (the JGI Populus trichocarpav.1.1 genome browser; http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html; Tuskan et al. (2006)) was searched and 10 genes are predicted as APX proteins, and 2 genes(Protein Id: 209946, 798682) was speculated as putative mitochondrial/chloroplasts APX which has a mitochondria/chloroplast localization signal peptide. These homologous APX in P. tomentosa were cloned using primers specific for the Populus trichocarpa APX gene (Protein ID: 209946, 798682), respectivity. A 1,080 bp open reading frame (ORF) (homologous APX in Populus trichocarpa, Protein ID: 798682. Supplemental Fig. 1A) was isolated, sequenced, and named PtomtAPX, and a 1,086 bp ORF (homologous APX in Populus trichocarpa, Protein ID: 209946. Supplemental Fig. 1B) was isolated, sequenced, and named PtosAPX.
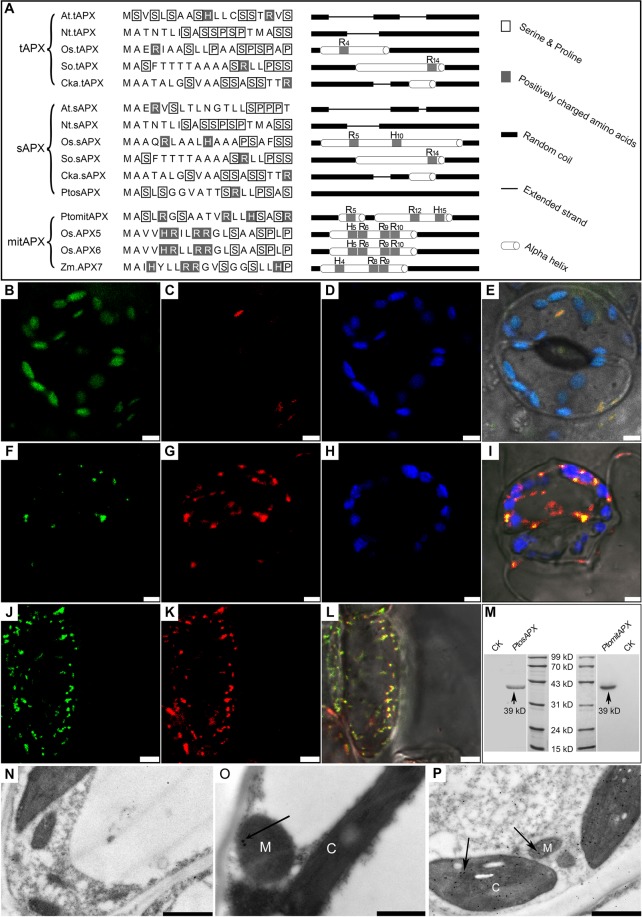
Sequence alignment revealed the presence of an N-terminal mitochondria/chloroplast-targeting peptide and two signatures of chloroplastic isoforms (KNIEEWP and ETKYTKDGPGAPGGQS) in PtosAPX and PtomtAPX, respectively (Fig. 1A and Supplemental Fig. 1). Phylogenetic analyses of APXs indicated that PtomtAPX and PtosAPX were chloroplastic and/or mitochondrial isoforms (Supplemental Fig. 2). Positively charged amino acid residues and amphipathic α-helix within the 19 N-terminal portion of the targeting peptide are important for the importation of proteins into mitochondria but not chloroplasts26. PtomtAPX had four positively charged residues and two amphiphilic α-helices in the targeting peptide, while PtosAPX had no positively charged residue and no amphiphilic α-helix (Fig. 1A). These results suggest that PtomtAPX is a mitochondrial isoform.
Figure 1.
Amino acid sequence and subcellular distribution of PtomtAPX and PtosAPX. (A) Targeting peptide alignment and secondary structure analyses. (B–E) Expression of the PtosAPX-GFP fusion protein in leaf epidermal cells of transgenic tobacco. (B) Fluorescence of PtosAPX-GFP fusion protein. (C) Mitochondria stained with MitoTracker Red. (D) Chloroplast autofluorescence. (E) Merged image. (F–L) Expression of the PtomtAPX-GFP fusion protein in leaf epidermal and root-tip cells of transgenic tobacco. (F,J) Fluorescence of PtomtAPX-GFP fusion protein. (G) and (K) Mitochondria stained with MitoTracker Red. (H) Chloroplast autofluorescence. (I,L) Merged images. Bars, 5 µm. (M) Western blotting analyses of total protein extracts from leaf cells of P. tomentosa using anti-PtomtAPX and anti-PtosAPX antibodies. (N) Negative control. (O) Immunoelectron microscopy of PtomtAPX. (P) Immunoelectron microscopy of PtosAPX. 1 µm (N–P). M, mitochondrion, C, chloroplast.
The subcellular localization of PtosAPX and PtomtAPX in P. tomentosa was determined by fusing their full-length coding sequences upstream of GFP under the control of the 35 S promoter (Fig. 1). The GFP signal of PtosAPX-GFP was detected not only in mitochondria co-stained with MitoTracker Red CMXRos (hereafter, CMXRos) but also in chloroplasts (red autofluorescence replaced by blue pseudocolor) in leaf epidermal cells. The GFP fluorescence of PtomtAPX-GFP was detected in mitochondria in leaf epidermal cells and root tips but not in chloroplasts.
Immunoelectron microscopy was performed to confirm the localization of PtosAPX and PtomtAPX. Immunogold labeling using rabbit anti-PtosAPX and anti-PtomtAPX antibodies showed that PtosAPX was localized to mitochondria and chloroplasts and PtomtAPX was localized to mitochondria (Fig. 1M–P). Taken together, these results suggest that PtomtAPX is localized to mitochondria in P. tomentosa, while PtosAPX is localized to both mitochondria and chloroplasts and shows greater similarity to a chloroplastic isoform.
PtomtAPX and PtosAPX expression in mitochondria
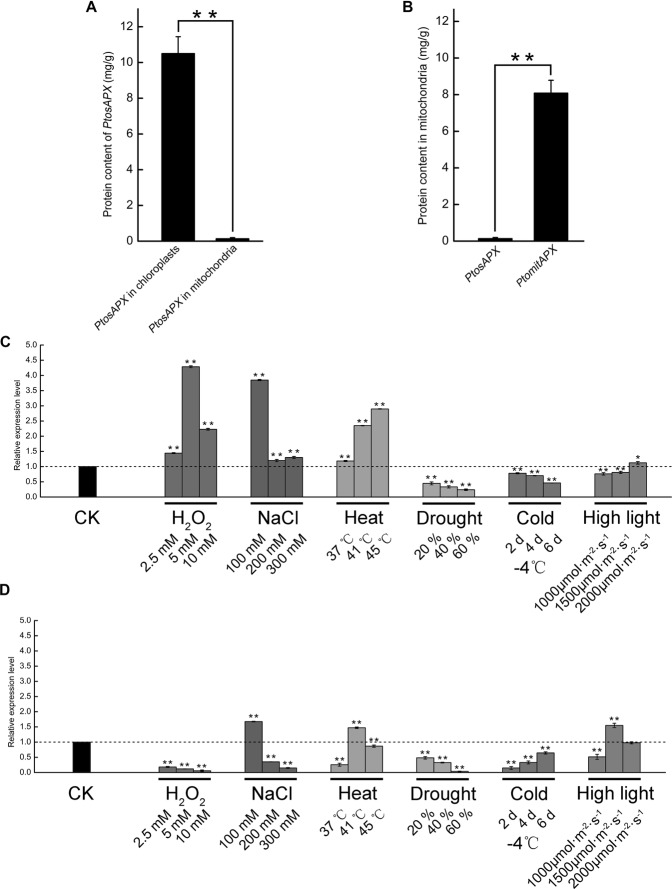
The presence of PtomtAPX and PtosAPX in mitochondria was investigated by enzyme-linked immunosorbent assay (ELISA). PtosAPX was detected in both mitochondria and chloroplasts. The PtosAPX level in chloroplasts was 78-fold that in mitochondria (Fig. 2A) and the PtomtAPX level in mitochondria was 60-fold that of PtosAPX (Fig. 2B). Therefore, PtomtAPX is the primary APX in mitochondria, while PtosAPX is likely to be a chloroplastic isoform.
Figure 2.
Expression profile of PtosAPX and PtomtAPX. (A) PtosAPX level in mitochondria and chloroplasts according to ELISA. (B) PtosAPX and PtomtAPX levels in mitochondria according to ELISA. (C–E) Expression of PtomtAPX and PtosAPX under abiotic stress according to qRT-PCR. (C) PtomtAPX. (D) PtosAPX. **Significantly different at P < 0.01 and *Significantly different at P < 0.05. Bars, standard deviations.
The expression levels of PtomtAPX and PtosAPX in the presence of H2O2, NaCl, heat, drought, cold, or high-intensity light were determined by qRT-PCR with normalization to the actin gene (Potri.001G309500). Under high-light stress, the expression levels of PtosAPX increased and decreased, respectively, while that of PtomtAPX only slightly changed (Fig. 2C,D). The expression levels of PtomtAPX and PtosAPX were modulated by H2O2, NaCl, heat, drought, and cold.
Enzymatic characteristics of PtomtAPX and PtosAPX
The expression constructs pET30a-PtomtAPX and pET30a-PtosAPX, which harbored the full-length cDNA minus the signal peptide, were expressed in Escherichia coli, resulting in production of recombinant PtomtAPX and PtosAPX, respectively. Sodium dodecyl sulfate (SDS)-PAGE showed that the molecular weight of purified recombinant PtomtAPX and PtosAPX were both 39 kDa. The reaction of PtomtAPX or PtosAPX with AsA and H2O2 followed Michaelis-Menten kinetics. At a fixed AsA concentration, the Km and Vmax values of PtomtAPX were 0.03 ± 0.00 mM and 1.54 ± 0.00 mM min–1 mg–1 for H2O2, respectively, while the Km and Vmax values of PtosAPX were 0.04 ± 0.01 mM and 1.64 ± 0.11 mmol∙min−1∙mg−1 for H2O2, respectively. At a fixed H2O2 concentration, the Km and Vmax values of PtomtAPX were 6.04 ± 0.06 mM and 3.00 ± 0.14 mM min–1 mg–1 for AsA, respectively, while the Km and Vmax values of PtosAPX were 4.30 ± 1.81 mM and 0.85 ± 0.25 mmol∙min−1∙mg−1 for AsA, respectively (Table 1). PtomtAPX and PtosAPX exhibited similar turnover rates (kcat), efficiencies (kcat/Km) and affinities (Km) for H2O2 and AsA. Therefore, PtomtAPX and PtosAPX are APX enzymes with similar activities.
Table 1.
Enzymatic properties of recombinant PtomtAPX and PtosAPX.
| APX isoforms | Substrate | Km (mM) | Vmax (mM min–1) | kcat (min–1) | kcat/Km (mM–1 min–1) |
|---|---|---|---|---|---|
| PtomtAPX | ASA | 6.04 ± 0.06 | 3.00 ± 0.14 | 928405.63 | 153709.54 |
| H2O2 | 0.03 ± 0.00 | 1.54 ± 0.00 | 475149.03 | 15838330.11 | |
| PtosAPX | ASA | 4.30 ± 1.81 | 0.85 ± 0.25 | 897148.78 | 208505.07 |
| H2O2 | 0.04 ± 0.01 | 1.64 ± 0.11 | 864716.73 | 20312959.95 |
Values are means ± SDs of three replicates.
Decreased PtomtAPX content leads to mitochondrial dysfunction and PCD
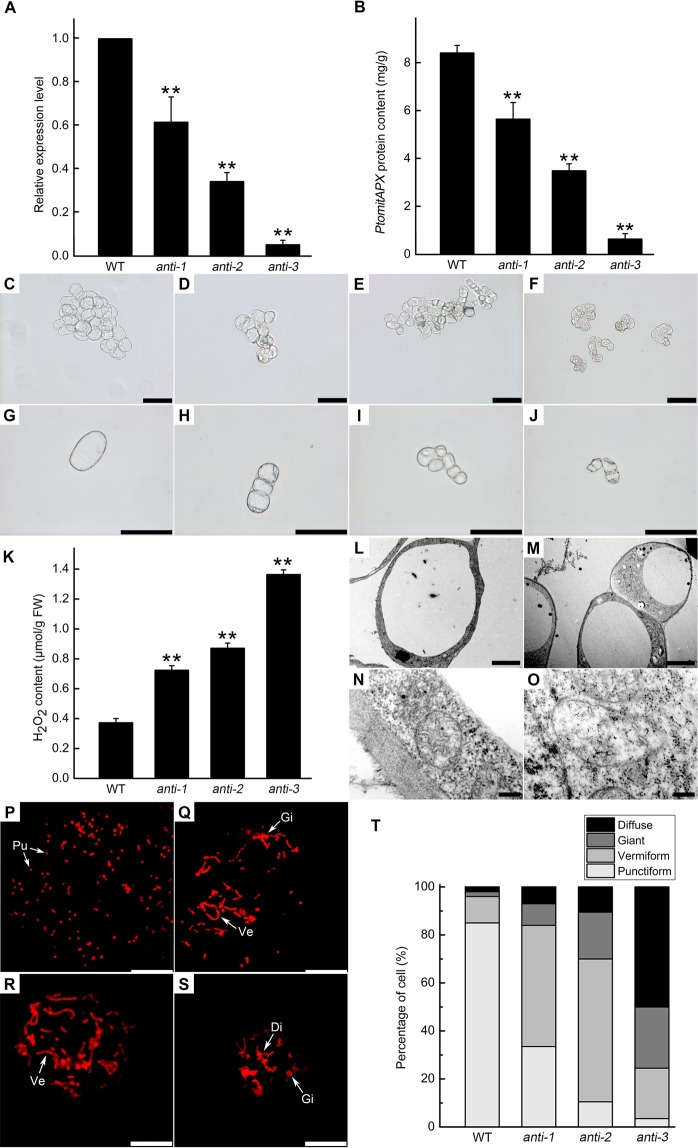
We used a pBI121-based construct to generate 45 antisense-PtomtAPX transgenic P. tomentosa suspension cell lines. The PtomtAPX transcript levels were 5–95% lower in 10 randomly selected PtomtAPX transgenic P. tomentosa cell lines compared to the WT. Three representative PtomtAPX transgenic cell lines with PtomtAPX transcript levels 61.63%, 34.29%, and 5.24% lower than the WT were named anti-1, anti-2, and anti-3, respectively (Fig. 3A). The PtomtAPX protein level was 67.18%, 41.55%, and 7.68% lower in anti-1, anti-2, and anti-3, respectively, than in the WT (Fig. 3B). Compared to the WT, the three transgenic PtomtAPX cell lines showed slowed growth and smaller cells in MS medium (Fig. 3C–J). Of the three cell lines, anti-3 showed the smallest cells and the most seriously reduced growth (around 70%) compared to the WT. Compared to the WT, the mitochondrial H2O2 content was increased in anti-1, anti-2, and anti-3, respectively (Fig. 3K and Supplemental Fig. 3).
Figure 3.
PtomtAPX expression, cell morphology and mitochondrial structure of PtomtAPX-antisense suspension cells. (A) qRT-PCR. (B) ELISA. **Significantly different at P < 0.01; bars represent standard deviations. (C–J) Cell morphology. (C,G) Morphology of WT; (D,H) anti-1; (E,I) anti-2, (F,J) anti-3. Bars, 100 μm. (K) Mitochondrial H2O2 content. (L–O) TEM of cell morphology and mitochondrial morphology. (L,N) WT; (M,O) PtomtAPX-antisense. Bars, 5 µm in (L,M) and 200 nm in (N,O). (P–T) MitoTracker Red fluorescence of mitochondria in suspension cells of the WT (P), anti-1 (Q), anti-2 (R), and anti-3 (S). Bars, 10 μm. (T) Frequencies of the types of mitochondria in 200 suspension cells. Pu, punctiform; Ve, vermiform; Gi, giant; Di, diffuse.
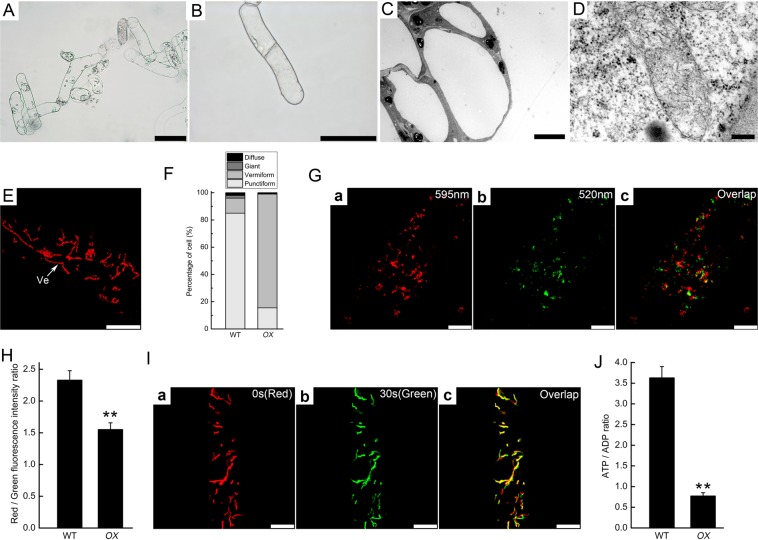
The structure of mitochondria in the PtomtAPX-antisense cells was assessed by transmission electron microscopy (TEM). WT cells exhibited a dense cytoplasm, clear structure, and a large vacuole occupying the majority of the cytoplasm (Fig. 3L). Numerous mitochondria with well-developed cristae and easily distinguishable outer and inner membranes were present in the WT cell lines (Fig. 3N). However, anti-1, anti-2, and anti-3 cells harbored a high frequency of abnormal mitochondria, which lacked cristae and internal structures, but retained a double membrane (Fig. 3M,O). According to CLSM, the proportion of mitochondria with an abnormal morphology was significantly increased in the transgenic cell lines, particularly in anti-3 (3.50% normal mitochondria compared to 85.00% in the WT) (Fig. 3P–T).
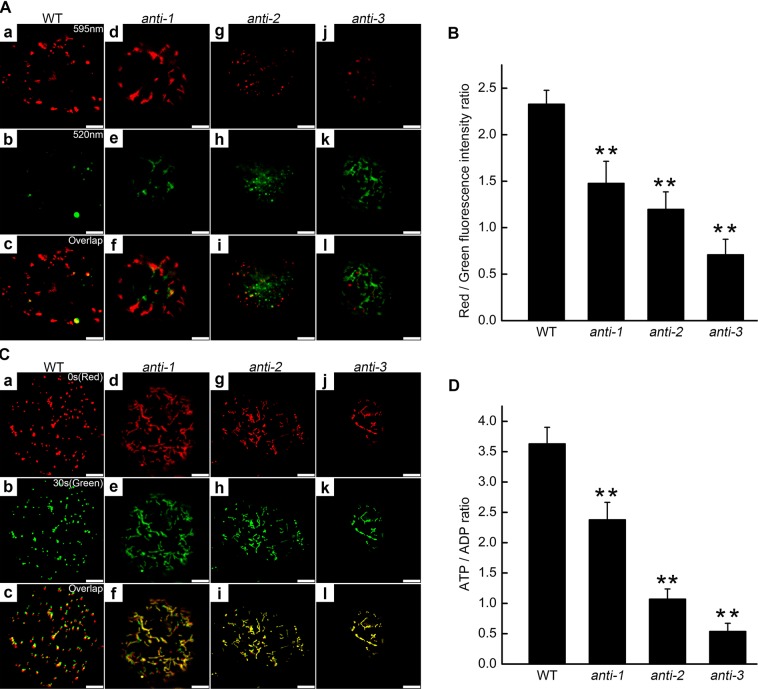
Changes in mitochondrial morphology may influence their function and decrease their ATP-generating capacity. To assess mitochondrial dysfunction, the mitochondrial membrane potential (Δψm) was determined using the fluorescent dye JC-1 and by calculating the red:green fluorescence ratio27.The function of mitochondria was impaired and the Δψm was markedly decreased in the PtomtAPX-antisense cell lines (Fig. 4A,B). Next, we visualized mitochondrial movement by CMXRos staining. Mitochondria were selected in time-lapse videos at 0 s (red images) and 30 s (green images) and the two images were overlaid; yellow mitochondria were considered non-motile. Mitochondria in WT cells showed motility, and few were yellow. By contrast, mitochondria in anti-1 and anti-2 cells showed reduced motility, while those in anti-3 cells showed little motility (Fig. 4C). Therefore, mitochondrial motility was markedly decreased in the PtomtAPX-antisense cells, suggesting impairment of mitochondrial function.
Figure 4.
Mitochondrial Δψm in PtomtAPX-antisense suspension cells. (A) Δψm of mitochondria of suspension cells (JC-1 staining). Mitochondria with a high Δψm are shown in red (595 nm channel) (a), (d), (g) and (j); depolarized mitochondria are shown in green (520 nm channel); (b), (e), (h) and (k); (c), (f), (i) and (l), merged images; bars, 10 μm. (B) JC-1 red: green fluorescence ratios based on 200 cells. (C) CMXRos (red) fluorescence micrograph of mitochondrial mobility; green, pseudo-color; yellow, colocalized puncta; bars, 10 μm. (D) ATP:ADP ratios. Bars, standard deviations. **Significantly different at P < 0.01.
Because ATP is produced in mitochondria by oxidative phosphorylation, and the decreased mitochondrial Δψm may be due to inhibition of electron transport chain (ETC) complexes, the ATP:ADP ratio and mitochondrial complex I, II, and III activities were determined. Compared to the WT, the ATP:ADP ratio was about 65.52%, 29.47%, 14.81%, and 21.27% in anti-1, anti-2, and anti-3 cells, respectively (Fig. 4D). Compared to the WT, complex I, II, and III activities were significantly decreased in anti-1, anti-2, and anti-3 cells, suggesting damage to ETC complexes (Table 2). Thus, energy production was decreased and ETC complexes were damaged in the PtomtAPX-antisense cells.
Table 2.
Activity of respiratory complexes.
| Group | nmol∙min−1∙mg−1 protein | ||
|---|---|---|---|
| Complex I | Complex II | Complex III | |
| WT | 93.59 ± 11.46 | 63.99 ± 8.36 | 99.28 ± 9.17 |
| anti-1 | 75.71 ± 8.14 | 48.72 ± 8.99 | 81.17 ± 6.59 |
| anti-2 | 58.60 ± 13.91 | 33.64 ± 7.09 | 52.75 ± 11.07 |
| anti-3 | 31.71 ± 11.18 | 17.12 ± 6.95 | 25.32 ± 9.58 |
| OX | 91.16 ± 10.80 | 62.43 ± 8.12 | 100.17 ± 10.48 |
Values are means ± SDs of three replicates.
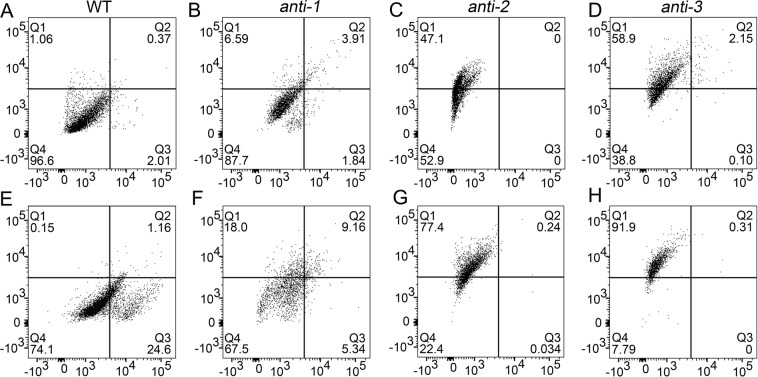
PCD is related to the overproduction of ROS in mitochondria22,28,29. Due to the reduced growth of antisense transgenic cell lines and plants, and the difficulty in obtaining the significant downregulation of the antisense plants, we suspected that deletion of PtomtAPX triggers PCD. We determined the degree of PCD in PtomtAPX-antisense suspension cell lines by two-channel flow cytometry (FCM). Normal (Q4, bottom left) and early apoptotic (Q3, bottom right) cells were stained by Hoechst dye, while apoptotic (Q1, top left) and advanced apoptotic (Q2, top right) cells were stained with propidium iodide (PI).
In the WT, most cells were normal (>96%) and few were apoptotic (<2%) or dead (<2%). In comparison, the proportion of apoptotic anti-1, anti-2, and anti-3 cells was increased by 12.30%, 47.10%, and 61.20%, respectively(Fig. 5A–D). Thus, the increased H2O2 content in the PtomtAPX-antisense cell lines resulted in a significant increase in PCD.
Figure 5.
PCD of PtomtAPX-antisense suspension cells. (A,D,G,J) PCD under untreated conditions. (B,E,H,K) PCD under H2O2 treatment (10 mM, 3 h). Frequencies were calculated based on 5,000 cells per treatment.
To further assess the relationship between H2O2 content and PCD, 10 mM exogenous H2O2 was added to the PtomtAPX-antisense cells to increase their H2O2 content. In the WT, adding 10 mM H2O2 increased the frequency of apoptotic cells by >25%, while the frequencies of apoptotic anti-1, anti-2, and anti-3 cells were increased by about 32.50%, 77.60%, and 92.20%, respectively(Fig. 5E–H). Treatment with 10 mM exogenous H2O2 resulted in the death of almost all anti-3 cells. Therefore, exogenous H2O2 increased the frequency of PCD in the PtomtAPX-antisense cell lines. Therefore, the H2O2 content is related to the degree of PCD.
The above results show that decreased PtomtAPX expression is correlated with increased PCD, which may explain both the reduced growth and difficulty generating, PtomtAPX-antisense P. tomentosa lines.
Mitochondrial dysfunction is due to an increased H2O2 content and oxidative damage
Non-enzymatic antioxidants such as ascorbate (AsA) and glutathione (GSH) can reduce the increased H2O2 level in mitochondria or the cytosol30. To determine the role of AsA and GSH in removing H2O2, we determined the mitochondrial AsA, DHA, GSH, and GSSG contents of the PtomtAPX-antisense cell lines. Compared to the WT, the AsA contents were 52.07%, 37.43%, and 15.70%, and the AsA:DHA ratios were 48.56%, 34.08%, and 14.52% in mitochondria of anti-1, anti-2, and anti-3 cells, respectively (Supplemental Fig. 4A). Compared to the WT, the GSH contents were 51.51%, 40.79%, and 27.37%, and the GSH:GSSG ratios were 43.40%, 36.01%, and 22.22%, in mitochondria of anti-1, anti-2, and anti-3 cells, respectively (Supplemental Fig. 4B). The greatest decreases in the AsA:DHA and GSH:GSSG ratios occurred in anti-3 cells. Thus, the transgenic cells are under oxidative stress and their H2O2 content could not be balanced by AsA and GSH.
An increased H2O2 content may lead to oxidative damage and reduce mitochondrial efficiency. Therefore, we evaluated mitochondrial lipid peroxidation by quantifying malondialdehyde (MDA) levels. Compared to the WT, the mitochondrial lipid peroxidation level was 1.74-, 2.55-, and 3.65-fold higher in anti-1, anti-2, and anti-3, respectively (Supplemental Fig. 4C). Moreover, the levels of oxidized proteins (with carbonyl groups) were increased in anti-1, anti-2, and anti-3, respectively, as determined by Western blotting (Supplemental Fig. 4D).
Therefore, the decreased PtomtAPX protein levels in the PtomtAPX-antisense cells resulted in increased H2O2 content, which could not be balanced by enzymatic or non-enzymatic antioxidant mechanisms due to the structural and functional impairment of mitochondria.
Overexpression of PtomtAPX decreases H2O2 content and slows growth
PtomtAPX-overexpressed cell lines were generated using a PtomtAPX overexpression construct under the control of the 35 S promoter (35Spro:PtomtAPX) (Fig. 6). qRT-PCR and ELISA showed that the PtomtAPX transcript and protein levels were increased 4-fold and 2.7-fold in the PtomtAPX-overexpressed cell lines compared to the WT, respectively.
Figure 6.
Growth reduction and mitochondrial dysfunction in PtomtAPX-overexpressed cells. (A,B) Morphology of PtomtAPX-overexpressed cells; bars, 100 μm. (C,D) Morphology of cells and mitochondria according to TEM; bars, 5 µm in (C) and 200 nm in (D). (E,F) MitoTracker Red fluorescence of mitochondria; bars, 10 μm; Frequencies were calculated based on 200 cells. Ve, vermiform. (G) Δψm of mitochondria (JC-1 staining). (H) JC-1 polymer (red):monomer (green) fluorescence ratios based on 200 cells. WT, WT cells; OX, PtomtAPX-overexpressed cells. (I) Mitochondrial mobility; bars, 10 μm. (J) ATP:ADP ratios. **Significantly different at P < 0.01. Bars, standard deviations.
For growth in MS medium, the PtomtAPX-overexpressed cell lines required 0.2 mg/L 6-BA. Compared to the WT, the PtomtAPX-overexpressed cell lines showed slowed growth and an abnormal morphology. The structure of mitochondria in OX cells was also abnormal by TEM and CLSM. The mitochondrial Δψm, motility, and energy production (ATP:ADP ratio) were decreased in the PtomtAPX-overexpressed plants, suggesting impairment of mitochondrial function. However, the complex I, II, and III activities were unchanged in OX (Table 2), suggesting that the respiratory chain complexes were not damaged.
The mitochondrial H2O2, AsA and GSH contents and AsA/DHA and GSH/GSSG ratios of the PtomtAPX-overexpression cells were not significantly different from those of the WT (Supplemental Figs. 4 and 5). The mitochondrial MDA level in PtomtAPX-overexpression cells was 41.02% of the WT (Supplemental Fig. 5). The oxidized protein level in PtomtAPX-overexpressed cells was slightly lower than that of the WT (Supplemental Fig. 4). These results suggest that oxidative damage was alleviated in the PtomtAPX-overexpression cells compared to the WT.
We suspected that the decreased H2O2 content in OX caused mitochondrial dysfunction, because H2O2 is an important signaling molecule. To further assess the relationship between H2O2 content and mitochondrial dysfunction, exogenous H2O2 was added to PtomtAPX-overexpressed cells. Addition of 10 or 100 mM exogenous H2O2 to PtomtAPX-overexpressed cells significantly alleviated mitochondrial damage and partially restored the mitochondrial membrane potential (Supplemental Fig. 5). Therefore, overexpression of PtomtAPX resulted in a decreased H2O2 content and mitochondrial dysfunction.
Gene expression in PtomtAPX-antisense and PtomtAPX-overexpressed cell lines
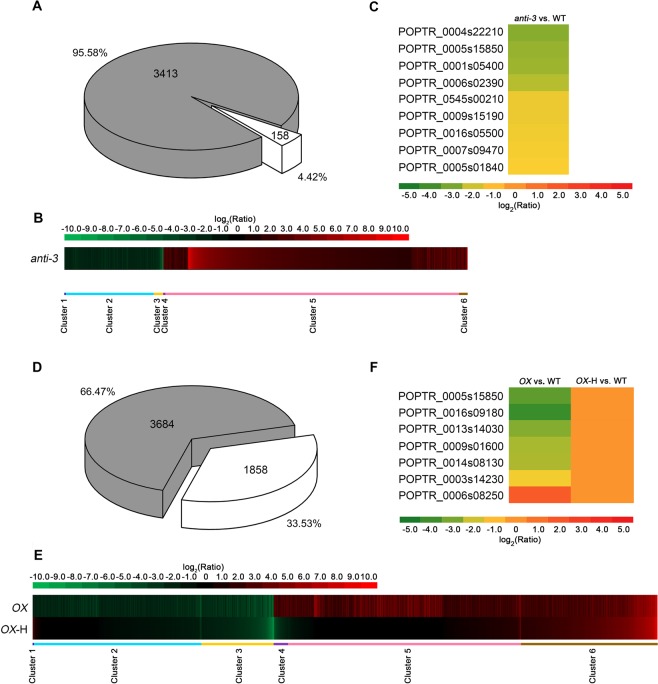
To further characterize the effects of PtomtAPX on mitochondria, we performed RNA-seq analyses of WT and PtomtAPX-antisense cells. Compared to the WT, 3,571 genes with significantly different expression levels (fold change >1.5 or < –1.5, and corrected P < 0.001) were detected in anti-3. (Fig. 7A and Supplemental Dataset 1). This indicates that oxidative damage is the cause of the significantly differential gene expression in anti-3.
Figure 7.
RNA-seq analyses of anti-3 and OX. (A) DEGs of anti-3 (anti-3 vs. WT) and their expression under AsA treatment (1 mM, 3 h) (corrected P-value < 0.001, fold change >1.5 or < –1.5; gray, genes recovered to normal expression level under AsA treatment). (B) Clustering of expression ratios (anti-3 vs. WT). Red, upregulation; green, downregulation. (C) Heat map of genes related to phenotypes of cells and mitochondria in anti-3, based on the RPKM values. (D) DEGs of OX (OX vs. WT) and their expression under H2O2 treatment (10 mM, 3 h) (corrected P-value < 0.001, fold change >1.5 or <–1.5; gray, genes recovered to normal expression level under H2O2 treatment). (E) Clustering displays of expression ratios (OX vs. WT). Red, upregulation; green, downregulation. (F) Heat map of genes related to phenotypes of cells and mitochondria in OX, based on RPKM values.
We clustered genes with similar expression patterns to further investigate the dynamic trend of the normalization of gene expression. The 3,571 genes were grouped into six clusters (Fig. 7B and Supplemental Dataset 1). Genes in cluster 1 (2 genes) and cluster 2 (781 genes) were downregulated, and the genes in cluster 4 (18 genes) and cluster 5 (2,612 genes) were upregulated in anti-3, and were wholly or partly normalized in anti-3-A, indicating that they are directly related to the abnormal morphology of cells and mitochondria. The expression levels of genes in cluster 3 (83 genes) which were downregulated in anti-3 and cluster 6 (75 genes) but upregulated in anti-3 were not normalized in anti-3-A (Fig. 7B and Supplemental Dataset 1).
Gene Ontology (GO) term enrichment analyses were performed to further characterize the function of the differentially expressed genes in anti-3 (Supplemental Dataset 2). The GO term “integral component of membrane” (P < 0.005) “UDP-glycosyltransferase activity” and “protein phosphorylation” (P < 0.005) showed enrichment (Supplemental Dataset 2). In particular, an OXPHOS-related gene (phosphatase family protein, POPTR_0007s09470) and inorganic pyrophosphatase (POPTR_0005s01840) were significantly downregulated in anti-3 (Fig. 7C). The activity of inorganic pyrophosphatase, which hydrolyzes inorganic pyrophosphate into two phosphates, is essential for many biosynthetic reactions and energy metabolism as well as for maintaining mitochondrial function. The deletion of inorganic pyrophosphatase compromises cell viability due to the loss of mitochondrial function31. The decreased expression of those genes in anti-3 was consistent with the damaged respiratory ETC in mitochondria of anti-3. The gene encoding phosphatidylinositol-4-phosphate 5-kinase (POPTR_0005s15850), which is involved in cytoskeletal rearrangement32, was also downregulated in anti-3 (Fig. 7C). A cytokinin riboside 5′-monophosphate phosphoribohydrolase gene (POPTR_0004s22210), the product of which activates cytokinins33, and a set of UDP-glycosyltransferases (POPTR_0001s05400, POPTR_0006s02390, POPTR_0545s00210 and POPTR_0009s15190), which are related to mitosis and strongly induced in dividing cells34, were downregulated in anti-3 (Fig. 7C). Cytokinins are master plant hormones that control cell division, senescence, and growth35. The decreased expression of those genes in anti-3 is consistent with the reduced proliferation of transgenic cells. In addition, a gene related to leaf senescence, alkaline α-galactosidase (POPTR_0016s05500)36, was downregulated in anti-3 (Fig. 7C). These genes are implicated in the altered growth and morphology of transgenic cells, which were smaller and had a slower growth rate than WT cells.
RNA-seq analyses of WT and PtomtAPX-overexpressed cells treated or not with 10 mM H2O2 were performed. Compared to the WT, 5,542 genes with significantly different expression levels (fold change >1.5 or <–1.5, and corrected P < 0.001) were detected in OX. The expression of 66.47% (3,684 genes) of those genes was normalized by 10 mM H2O2 (OX-H) (Fig. 7D and Supplemental Dataset 1). Thus, the decreased H2O2 content caused the significantly different gene expression levels in OX.
We also clustered genes with similar expression profiles to investigate the trends of these RNAs in OX compared to the WT. As shown in Fig. 7E, the 5,542 genes were grouped into six clusters (Supplemental Dataset 1). Genes in cluster 1 (1 genes) and cluster 2 (1,486 genes) were downregulated, and those in cluster 4 (127 genes) and cluster 5 (2,070 genes) were upregulated in OX and were normalized in OX-H, indicating that they are directly related to the abnormal morphology of cells and mitochondria. Genes in cluster 3 (643 genes), which were downregulated in OX, and cluster 6 (1,215 genes), which were upregulated in OX-H, were not normalized in OX-H (Fig. 7E and Supplemental Dataset 1). The GO term “hydrolase activity, acting on ester bonds” was significantly enriched (Supplemental Dataset 2). For example, two phosphatases (POPTR_0014s08130 and POPTR_0013s14030) were downregulated in OX and recovered to normal expression level in OX-H (Fig. 9F). Moreover, two cytokinin riboside 5′-monophosphate phosphoribohydrolases (POPTR_0016s09180 and POPTR_0009s01600), an UDP-glycosyltransferase (POPTR_0003s14230), and a phosphatidylinositol-4-phosphate 5-kinase (POPTR_0005s15850) were significantly differentially expressed in OX, and their expression was normalized in OX-H (Fig. 7F).The genes in cluster 5 were upregulated in OX and normalized in OX-H. For example, inorganic pyrophosphatase (POPTR_0006s08250) impairs mitochondrial function and was normalized in OX-H (Fig. 7F). These genes may be related to growth reduction, cell morphology and mitochondrial dysfunction due to their important roles in mitochondrial function, cytoskeleton, cytokinin activation, and cell division31–34. This is consistent with the need for the addition of 6-BA for the growth of PtomtAPX-overexpressed cells and the normalization of cellular morphology and growth rate, and mitochondrial function, by exogenous H2O2.
Non-enzymatic and enzymatic mechanisms maintain redox homeostasis in plant cells. H2O2 is scavenged principally by SOD, CAT, GPX, and PrxR, and the AsA-GSH cycle. None of these ROS-scavenging enzymes was significantly differentially expressed in anti-3 or OX compared to the WT, suggesting that the ROS pathway is not induced by the downregulation or upregulation of PtomtAPX.
APX mediates cross-compartment protection against the deleterious effects of H2O237. To determine whether APXs in other compartments can compensate for the loss of mitochondrial APX, we evaluated the expression levels of APXs in the chloroplasts, cytoplasm, and microbody. None of the APX genes was significantly differentially expressed in the PtomtAPX-antisense and PtomtAPX-overexpressed cell lines, suggesting that mitochondrial PtomtAPX is independent of APXs in other compartments and so provides little cross-compartment protection against H2O2.
Taken together, these results suggest that PtomtAPX expression is closely associated with H2O2 content and mitochondrial structure. Moreover, appropriate PtomtAPX expression is necessary to maintain a mitochondrial H2O2 level appropriate for mitochondrial structure and function in P. tomentosa.
Discussion
Plant mitochondria are major organelles of ROS production and are targets of ROS38,39. ROS-scavenging systems control the ROS balance in plant mitochondria. We identified two APXs, PtomtAPX and PtosAPX, in the mitochondria of P. tomentosa. PtomtAPX is specifically targeted to mitochondria and is the major APX involved in controlling mitochondrial H2O2 levels, and it influences mitochondrial structure and function and PCD in P. tomentosa. PtosAPX is targeted to both mitochondria and chloroplasts and is the minor role in controlling mitochondrial H2O2 levels.
Targeting peptides of mitochondrial and chloroplastic proteins (mTPs, also termed pre-sequences and cTPs, also termed transit peptides) has a similar amino acid composition and are indistinguishable by sequence analyses40,41. It is possible that mitochondrial isoforms have been misidentified as chloroplastic isoforms. For example, there were four chloroplastic APX in rice, but no mitochondrial isoform was confirmed2,5,6, indicating that mitochondrial isoform was mistaken for chloroplastic iosform. In this study, PtomtAPX and PtosAPX of P. tomentosa were also marked as chloroplastic isoforms (Supplemental Fig. 2). However, plant mTPs and cTPs exhibit quantitative and structural differences42.
Positively charged residues and amphipathic α-helix formed in 19 N-terminal amino acid are responsible for localization26,42,43. Our results indicate that chloroplastic APX has high serine and proline contents and a low arginine content. By contrast, PtomtAPX has three arginine residues and two amphipathic α-helices in its 19 N-terminal portion of targeting peptide (Fig. 1A). A rice chloroplastic APX isoform, OsAPX6, has been found in mitochondria of BY-2 tobacco but its presence in chloroplasts is unclear2,6,44. We found that the mTPs of OsAPX5 and OsAPX6 contained four positively charged amino acids (three of them are arginine residues) and an amphipathic α-helix, suggesting that they are targeted to mitochondria (Fig. 1A). Furthermore, three positively charged amino acids (two of them are arginine residues) and an amphipathic α-helix are present in Z. mays APX7 (Fig. 1A). Therefore, mitochondrial APX isoforms are present in various plant species, including model plants such as rice. The results of comparing the amino acid composition of targeting peptide of at least 100 proteins from diverse plant species showed increased intermediate amino acid led to the targeted of both chloroplasts and mitochondria44–48. Targeting of the same protein to two locations can result in co-regulation without a change in the genome. The presence of co-targeted enzymes of the AsA-GSH cycle may have a beneficial effect by restoring a normal level of ROS, which is important for the function of mitochondria and plastids.
Plant mitochondria in photosynthetic and non-photosynthetic organs have defense systems to prevent damage by H2O2 and to respond to environmental stresses49–51. Our results indicate that P. tomentosa has at least two mitochondrial APXs, PtomtAPX and PtosAPX, which have similar levels of activity. However, mitochondria had a low PtosAPX level, suggesting that PtosAPX is not an important player in ROS scavenging in mitochondria. The PtosAPX level in mitochondria was markedly lower than that of PtomtAPX, while the PtosAPX level in chloroplasts was higher than that in mitochondria, suggesting that PtomtAPX and PtosAPX are mitochondrial and chloroplastic isoforms, respectively. This is consistent with a previous report that dual-targeted proteins are more strongly targeted to one of the two organelles44.
The expression of PtomtAPX and PtosAPX differed significantly in the presence of various environmental stresses. In this study, the expression of PtosAPX and PtotAPX increased by high-light stress, whereas that of PtomtAPX was only slightly affected. The expression of chloroplastic APX(chlAPX) varies according to light intensity; e.g., high-intensity light decreases the expression of chlAPX in spinach leaves52. Exposure of Arabidopsis lacking tAPX to high-intensity light and MV stresses result in increased H2O2 accumulation and oxidation of proteins53. A mutant wheat line with decreased tAPX activity shows reduced photosynthetic activity and biomass accumulation in the presence of high-intensity light, suggesting that tAPX is essential for photosynthesis. Therefore, PtosAPX plays a role in the photosynthetic apparatus and PtomtAPX functions in the non-photosynthetic apparatus.
The major H2O2 detoxifying system in plant cells is the AsA-GSH cycle, in which APX is the key enzyme49,50. However, stromal/mitochondrial APX-knockdown lines, as well as single and double null mutants in chlAPX (sAPX and tAPX) in A. thaliana and rice, exhibit a normal phenotype and normal biochemical and physiological attributes under normal growth conditions, suggesting that chloroplast stromal/mitochondrial APX is not important for H2O2 scavenging in A. thaliana or rice23,24. In our research, the PtomtAPX-antisense cells under normal growth conditions had significantly higher H2O2 levels and an abnormal phenotype, including impaired mitochondrial structure and function and slowed growth. Transcriptomic analyses of anti-3 and WT revealed that the expression levels of genes encoding phosphatases and inorganic pyrophosphatases were significantly different, while genes related to cell growth (such as cytoskeleton, cytokinin, cell division, and senescence) were downregulated in anti-3. PtosAPX, was not upregulated in anti-3, indicating that PtosAPX cannot compensate for the function of PtomtAPX. Therefore, PtomtAPX, not PtosAPX, is the major APX in mitochondria.
Glutathione acts as a mobile pool of non-protein reduced sulfur, as an antioxidant, and in the detoxification of xenobiotics and heavy metals1,30. The GSH:GSSG ratio is an important indicator of the redox balance in plant cells. GR plays a central role in maintaining the GSH pool during stress1. The transcript level of GR, which plays a central role in maintaining the GSH pool, was not significantly increased in the PtomtAPX-antisense cells, and the GSH/GSSG ratio was significantly decreased, suggesting an insufficient glutathione pool to counteract the H2O2 level. Moreover, none of the APXs were significantly differentially expressed in anti-3 or OX according to RNA-seq analyses, indicating that cross-compartment protection among different organelles is not enough to protect mitochondria in the absence of PtomtAPX. Therefore, PtomtAPX is required to modulate H2O2 levels in mitochondria.
Over-accumulation of ROS triggers plant cell death. Excess ROS alter mitochondrial membrane permeability, decrease ΔΨm, reduce the electron density and number of cristae, and compromise inner and outer membranes; these effects trigger the release of cytochrome c, resulting in PCD. In plants, PCD is activated during various developmental processes and under diverse stress conditions54,55. Our results indicate that PCD was triggered by increased H2O2 levels and decreased PtomtAPX expression in the PtomtAPX-antisense cells. The addition of exogenous H2O2 to the PtomtAPX-antisense cells resulted in increased PCD. This is consistent with the slowed plant growth, small cells, and mitochondrial aggregation, swelling, loss of electron density and cristae, and decrease in ΔΨm in the PtomtAPX-antisense lines. Thus, downregulation of PtomtAPX expression in the PtomtAPX-antisense plants induced PCD and slowed growth. This may explain the difficulty generating PtomtAPX-antisense plants under normal growth conditions.
The decreased levels of PtomtAPX protein in the PtomtAPX-antisense cells resulted in higher H2O2 levels, which could not be counteracted by enzymatic or non-enzymatic mechanisms due to damaged and dysfunctional mitochondria. ROS can damage, by indiscriminate oxidation of macromolecules, various cellular compartments. In this study, excessive H2O2 levels impaired mitochondrial function in the transgenic lines. This is consistent with a previous report that high levels of ROS oxidize mitochondrial structural proteins, which reduces the efficiency of OXPHOS56. The damage to mitochondria was significantly alleviated by the addition of exogenous AsA.
The decreased H2O2 levels in the PtomtAPX-overexpressed cells resulted in decreased membrane potential and ATP production. However, mitochondrial lipids and structural proteins, specifically those related to OXPHOS, were not oxidized, suggesting that maintenance of ROS homeostasis is necessary for OXPHOS in the PtomtAPX-overexpressed cells. Moreover, RNA-seq showed that the expression of genes related to mitochondrial function, cytoskeleton, cytokinin activation, and cell division in OX cells was significantly recovered by the addition of exogenous H2O2. Thus, low H2O2 levels also impair mitochondrial function by an unknown mechanism.
ROS as signaling molecules regulate a number of processes during plant growth and development, such as cell elongation and differentiation, as well as the responses to a variety of environmental stimuli56,57. For example, in soybean, H2O2 levels increase from the tip of the hypocotyl (elongation zone) to the highly lignified base. In onion root fragments, H2O2 levels are high in cells undergoing elongation and lignification, and the former have lower total peroxidase activity58. Therefore, maintaining appropriate H2O2 levels is important for mitochondrial efficiency and cell growth in P. tomentosa, although the mechanism involved is unknown.
In conclusion, PtomtAPX is specifically targeted to mitochondria in which it is the primary APX, while PtosAPX is dual-targeted to mitochondria and chloroplasts. Expression of PtomtAPX in mitochondria is necessary for regulation of mitochondrial ROS levels and maintenance of mitochondrial function in P. tomentosa. Our findings provide valuable insight into the mechanism by which ROS balance is maintained and the function of the AsA-GSH cycle in mitochondria.
Materials and Methods
Plant materials and growth conditions
Suspension cells of P. tomentosa Carr. were grown in the dark at 25 °C. Transgenic suspension cells were obtained according to Li et al.59.
Molecular cloning and plasmid construction
Full-length PtomtAPX and PtosAPX cDNA were amplified using the primers PtomtAPX-F/R and PtosAPX-F/R, respectively, and sequenced. PtomtAPX and PtosAPX was amplified using G-PtomtAPX-F/R and G-PtosAPX-F/R and cloned into the pBI121-GFP vector to generate 35S:PtomtAPX-GFP and 35S:PtosAPX-GFP, respectively. Full-length PtomtAPX cDNA was amplified using OX-PtomtAPX-F/R and cloned into pBI121 to generate 35S:PtomtAPX (Supplemental Table 1). Full-length PtomtAPX cDNA was amplified using anti-PtomtAPX-F/R and cloned into pBI121. The amphiphilic α-helice is predicted using Bioedit 7.0.
Enzyme-activity assays
The ORF minus the targeting peptide of PtomtAPX and PtosAPX was amplified by PCR using P-PtomtAPX-F/R and P-PtostAPX-F/R, respectively, and inserted downstream of the pET30a plasmid T7 promoter (Novagen, Madison, WI). PtomtAPX and PtosAPX were expressed and purified according to Zhang et al.60. The activity of purified recombinant PtomtAPX using ASA and H2O2 as substrates was assayed as described previously59.
PtomtAPX and PtosAPX immunolocalization
Purified recombinant PtomtAPX or PtosAPX (5 mg) was used to immunize rabbits at 3-week intervals. The specificity of the anti-PtomtAPX and anti-PtosAPX antibodies was confirmed by hybridization with a membrane blotted with protein extracts from leaves of P. tomentosa. P. tomentosa leaves were fixed and subjected to immunolocalization as described previously61.
PtomtAPX-GFP and PtosAPX-GFP fusion targeting analyses
The GFP gene was amplified by PCR using the primers GFP-F/GFP-R. The product was inserted into pBI121(Clontech), which lacked the GUS-coding region to create the GFP(A)-pBI121 vector, the fusion construct of 35S:PtomtAPX-GFP, and 35S:PtomtAPX-GFP. Transgenic tobacco leaf epidermis and root-tip cells were visualized by CLSM (Leica SP8).
Enzyme-linked immunosorbent assays (ELISA)
An anti-PtomtAPX or anti-PtosAPX antibody (1:5,000 in PBS) was added to each well of a microtiter plate, followed by a goat anti-rabbit IgG HRP-conjugated secondary antibody (1:10,000 in PBS) (Supplemental Fig. 6). Protein from leaves or suspension cells was used for ELISA in accordance with the method of Zhang et al.61. Optical density values were determined using a Bio-Rad 680 Microplate Reader.
qRT-PCR
qRT-PCR was performed with the qRT-PtomtAPX-F/R or qRT-PtosAPX primers. The PCR conditions were 94 °C for 5 min; 40 cycles at 94 °C for 20 s, 58 °C for 20 s, 72 °C for 30 s, and 60 °C for 30 s; and 72 °C for 1 min. qRT-actin-F/R was used as normalization. Data were calculated using the 2−ΔΔCt method.
TEM
Suspension cells were fixed and embedded as described by Zhang et al.61.
CLSM
CLSM was performed using a Leica TCS-SP8 CLSM. The JC-1 signal was visualized by excitation at 488 nm and emission at 520–540 nm (green fluorescence) and 595–625 nm (red fluorescence). MitoTracker Red CMXRos (Invitrogen) was visualized by excitation at 578 nm and emission at 580–620 nm. Quantification was performed using Leica confocal software.
Staining
Suspension cells and root-tip cells of P. tomentosa were incubated in 0.3 μM CMXRos in slice buffer (0.3 mM sucrose, 5 mM TES, 5 mM MgCl2) at 30 °C in the dark for 10 min as described by Lord et al.46. Suspension cells and root-tip cells of P. tomentosa were incubated in 100 μM JC-1 for 20 min as described by Wang et al.62.
Isolation of mitochondria and chloroplast
Mitochondria or chloroplasts were isolated from leaves of P. tomentosa. The purity of the preparations was detected using cytochrome c oxidase for mitochondria, catalase for peroxisomes, alcohol dehydrogenase for cytosol, and alkaline pyrophosphatase for plastids (Supplemental Fig. 7) as described previously63. The chloroplast fractions were enriched using MinuteTM chloroplast isolation kit(Invent Biotechnologies).
Determination of H2O2 and MDA contents, ASA/DHA, GSH/GSSG, and ATP/ADP ratios, and ETC complex activity
H2O2 content was detected using Fluorimetric Hydrogen Peroxide Assay Kit (Sigma-Aldrich). MDA content was detected using Lipid Peroxidation (MDA) Assay Kit (Sigma-Aldrich). ADP and ATP levels were detected using a EnzyLight™ ATP assay (BioAssay Systems).The levels of AsA, GSSG and GSH were detected as described previously4,64.
Immunoblotting
The appearance of carbonyl groups in proteins was detected using an OxiSelect™ Protein Carbonyl Immunoblot Kit (Cell Biolabs, Inc.).
Flow cytometry
Suspension cells of P. tomentosa were incubation for 30 min in the dark with binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 1 mM MgCl2, 5 mM KCl, 2.5 mM CaCl2,1 μg/mL Hoechst and 1 μg/mL PI) and analyzed on a FACSCalibur flow cytometer (BD Biosciences).
RNA-Seq sample collection, Illumina sequencing, and data processing
Total RNA was isolated from WT, anti-3, and OX cells using TRIzol reagent according to the manufacturer’s protocol (Invitrogen). The samples were sequenced using an Illumina Genome Analyzer (HiSeq™ 2000; Illumina, San Diego, CA). The raw reads were data-filtered to obtain high-quality clean reads. The clean reads were mapped to the P. trichocarpa reference genome and reference genes using SOAPaligner/SOAP2. No more than two mismatches were allowed in the alignment. Gene expression levels were calculated as reads per kilobase per million reads (RPKM)65. The data are deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE121562) under accession number GSE121562.
Identification and analysis of differentially expressed genes
Differentially expressed genes (DEGs) among WT, anti-3, and OX cells were identified using the DEGseq R package (1.12.0)65 based on the normalized read counts. A corrected P-value < 0.001 and |log2ratio| > 1.5 were the thresholds for significantly different expression. GO enrichment analyses of the DEGs were performed using the GOseq R package66. GO terms with a P-value < 0.005 were considered significantly enriched in DEGs.
Accession numbers
Sequence data from this article can be found in the NCBI database under accession numbers MH910690 (PtomtAPX) and MH910610 (PtosAPX), AB022273 (Nt.tAPX), AB114856 (Os.tAPX), D77997 (So.tAPX), D83656 (Cka.tAPX), AB022274 (Nt.sAPX), AB114855 (Os.sAPX), D83669 (So.sAPX) and D88420 (Cka.sAPX).
Supplementary information
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (JC2015–01) and a grant from the Natural Science Foundation of China (31570582).
Author contributions
Di Liu and Hai Lu designed the experiments; Bin Yin, Jiaxue Zhang, Yadi Liu and Xiang Pan performed the experiments; Zhijing Zhao, Hui Li, Chong Zhang, Conghui Li, Xihua Du and Yinjun Li performed statistical analysis and prepared tables and figures. Bin Yin, Di Liu and Hai Lu wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bin Yin, Jiaxue Zhang, Yadi Liu and Xiang Pan.
Contributor Information
Di Liu, Email: liudi@bjfu.edu.cn.
Hai Lu, Email: luhai1974@bjfu.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-019-56148-w.
References
- 1.Considine MJ, Foyer CH. Redox regulation of plant development. Antioxid Redox Signal. 2014;21:1305–1326. doi: 10.1089/ars.2013.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum NA, et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res Int. 2016;23:19002–19029. doi: 10.1007/s11356-016-7309-6. [DOI] [PubMed] [Google Scholar]
- 3.Sofo A, Scopa A, Nuzzaci M, Vitti A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci. 2015;16:13561–13578. doi: 10.3390/ijms160613561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew O, Rudhe C, Glaser E, Whelan J. Characterization of the targeting signal of dual-targeted pea glutathione reductase. Plant Mol Biol. 2003;53:341–356. doi: 10.1023/B:PLAN.0000006939.87660.4f. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira FK, Menezes-Benavente L, Margis R, Margis-Pinheiro M. Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: inferences from the rice genome. J Mol Evol. 2004;59:761–770. doi: 10.1007/s00239-004-2666-z. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M. Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta. 2006;224:300–314. doi: 10.1007/s00425-005-0214-8. [DOI] [PubMed] [Google Scholar]
- 7.Najami N, et al. Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol Genet Genomics. 2008;279:171–182. doi: 10.1007/s00438-007-0305-2. [DOI] [PubMed] [Google Scholar]
- 8.Pnueli L, Liang H, Rozenberg M, Mittler R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003;34:187–203. doi: 10.1046/j.1365-313X.2003.01715.x. [DOI] [PubMed] [Google Scholar]
- 9.Davletova S, et al. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koussevitzky S, et al. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem. 2008;283:34197–34203. doi: 10.1074/jbc.M806337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narendra S, et al. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J Exp Bot. 2006;57:3033–3042. doi: 10.1093/jxb/erl060. [DOI] [PubMed] [Google Scholar]
- 12.Miller G, et al. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007;144:1777–1785. doi: 10.1104/pp.107.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, et al. ASCORBATE PEROXIDASE6 protects Arabidopsis desiccating and germinating seeds from stress and mediates cross talk between reactive oxygen species, abscisic acid, and auxin. Plant Physiol. 2014;166:370–83. doi: 10.1104/pp.114.245324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosa SB, et al. Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry. 2010;71:548–558. doi: 10.1016/j.phytochem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Bonifacio A, et al. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011;34:1705–1722. doi: 10.1111/j.1365-3040.2011.02366.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. Gene knockout study reveals that cytosolic ascorbate peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS One. 2013;8:e57472. doi: 10.1371/journal.pone.0057472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro CW, et al. Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Sci. 2017;263:55–65. doi: 10.1016/j.plantsci.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez A, Hernandez JA, Pastori G, del Rio LA, Sevilla F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittova V, et al. Comparison of mitochondrial ascorbate peroxidase in the cultivated tomato, Lycopersicon esculentum, and its wild, salt-tolerant relative, L. pennellii– a role for matrix isoforms in protection against oxidative damage. Plant Cell Environ. 2004;27:237–250. doi: 10.1046/j.1365-3040.2004.01150.x. [DOI] [Google Scholar]
- 20.De Leonardis S, Dipierro N, Dipierro S. Purification and characterization of an ascorbate peroxidase from potato tuber mitochondria. Plant Physiol Biochem. 2000;38:773–779. doi: 10.1016/S0981-9428(00)01188-8. [DOI] [Google Scholar]
- 21.Khanna-Chopra R, Jajoo A, Semwal VK. Chloroplasts and mitochondria have multiple heat tolerant isozymes of SOD and APX in leaf and inflorescence in Chenopodium album. Biochem Biophys Res Commun. 2011;412:522–525. doi: 10.1016/j.bbrc.2011.06.179. [DOI] [PubMed] [Google Scholar]
- 22.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Giacomelli L, Masi A, Ripoll DR, Lee MJ, van Wijk KJ. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol Biol. 2007;65:627–644. doi: 10.1007/s11103-007-9227-y. [DOI] [PubMed] [Google Scholar]
- 24.Kangasjärvi S, et al. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem J. 2008;412:275–285. doi: 10.1042/BJ20080030. [DOI] [PubMed] [Google Scholar]
- 25.Caverzan A, et al. The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci. 2014;214:74–87. doi: 10.1016/j.plantsci.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Ge C, Spånning E, Glaser E, Wieslander A. Import determinants of organelle-specific and dual targeting peptides of mitochondria and chloroplasts in Arabidopsis thaliana. Mol Plant. 2014;7:121–136. doi: 10.1093/mp/sst148. [DOI] [PubMed] [Google Scholar]
- 27.Krause M, Durner J. Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant Microbe Interact. 2004;17:131–139. doi: 10.1094/MPMI.2004.17.2.131. [DOI] [PubMed] [Google Scholar]
- 28.Hoeberichts FA, Woltering EJ. Multiple mediators of plant programmed cell death: interplay of conserved cell death mechanisms and plant-specific regulators. Bioessays. 2003;25:47–57. doi: 10.1002/bies.10175. [DOI] [PubMed] [Google Scholar]
- 29.Van Aken O, Van Breusegem F. Licensed to kill: mitochondria, chloroplasts, and cell death. Trends Plant Sci. 2015;20:754–766. doi: 10.1016/j.tplants.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Cheng MC, et al. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015;83:926–939. doi: 10.1111/tpj.12940. [DOI] [PubMed] [Google Scholar]
- 31.Guimier A, et al. Biallelic PPA2 Mutations Cause Sudden Unexpected Cardiac Arrest in Infancy. Am J Hum Genet. 2016;99:666–673. doi: 10.1016/j.ajhg.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/S0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 33.Mortier V, et al. Role of LONELY GUY genes in indeterminate nodulation on Medicago truncatula. New Phytol. 2014;202:582–593. doi: 10.1111/nph.12681. [DOI] [PubMed] [Google Scholar]
- 34.Woo HH, Orbach MJ, Hirsch AM, Hawes MC. Meristem-localized inducible expression of a UDP-glycosyltransferase gene is essential for growth and development in pea and alfalfa. Plant Cell. 1999;11:2303–2315. doi: 10.1105/tpc.11.12.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Ma XM, Kojima M, Sakakibara H, Hou BK. Glucosyltransferase UGT76C1 finely modulates cytokinin responses via cytokinin N-glucosylation in Arabidopsis thaliana. Plant Physiol Biochem. 2013;65:9–16. doi: 10.1016/j.plaphy.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Lee RH, Lin MC, Chen SC. A novel alkaline alpha-galactosidase gene is involved in rice leaf senescence. Plant Mol Biol. 2004;55:281–295. doi: 10.1007/s11103-004-0641-0. [DOI] [PubMed] [Google Scholar]
- 37.Henzler T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- 38.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Melser S, Lavie J, Bénard G. Mitochondrial degradation and energy metabolism. Biochim Biophys Acta. 2015;1853:2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Chotewutmontri P, Reddick LE, McWilliams DR, Campbell IM, Bruce BD. Differential transit peptide recognition during preprotein binding and translocation into flowering plant plastids. Plant Cell. 2012;24:3040–3059. doi: 10.1105/tpc.112.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira PF, Glaser E. Processing peptidases in mitochondria and chloroplasts. Biochim Biophys Acta. 2013;1833:360–370. doi: 10.1016/j.bbamcr.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Bhushan S, Kuhn C, Berglund AK, Roth C, Glaser E. The role of the N-terminal domain of chloroplast targeting peptides in organellar protein import and mis-sorting. FEBS Lett. 2006;580:3966–3972. doi: 10.1016/j.febslet.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Moberg P, et al. NMR solution structure of the mitochondrial F1beta presequence from Nicotiana plumbaginifolia. J Mol Biol. 2004;336:1129–1140. doi: 10.1016/j.jmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Carrie C, Law SR, Murcha MW, Whelan J. Acquisition, conservation, and loss of dual-targeted proteins in land plants. Plant Physiol. 2013;161:644–662. doi: 10.1104/pp.112.210997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters N, Small I. Dual targeting to mitochondria and chloroplasts. Biochim Biophys Acta. 2001;1541:54–63. doi: 10.1016/S0167-4889(01)00146-X. [DOI] [PubMed] [Google Scholar]
- 46.Carrie C, Small I. A reevaluation of dual-targeting of proteins to mitochondria and chloroplasts. Biochim Biophys Acta. 2013;1833:253–259. doi: 10.1016/j.bbamcr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 47.Berglund AK, et al. Dual targeting to mitochondria and chloroplasts: characterization of Thr–tRNA synthetase targeting peptide. Mol Plant. 2009;2:1298–1309. doi: 10.1093/mp/ssp048. [DOI] [PubMed] [Google Scholar]
- 48.Dinur-Mills M, Tal M, Pines O. Dual targeted mitochondrial proteins are characterized by lower MTS parameters and total net charge. PloS One. 2008;3:e2161. doi: 10.1371/journal.pone.0002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta. 2010;231:461–474. doi: 10.1007/s00425-009-1067-3. [DOI] [PubMed] [Google Scholar]
- 50.Sun H, Li L, Wang X, Wu S, Wang X. Ascorbate-glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J Plant Physiol. 2011;168:226–232. doi: 10.1016/j.jplph.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Fryer MJ, et al. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2003;33:691–705. doi: 10.1046/j.1365-313X.2003.01656.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 2000;123:223–234. doi: 10.1104/pp.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruta T, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- 54.Yao N, Eisfelder BJ, Marvin J, Greenberg JT. The mitochondrion–an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J. 2004;40:596–610. doi: 10.1111/j.1365-313X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- 55.Lord CE, Wertman JN, Lane S, Gunawardena AH. Do mitochondria play a role in remodelling lace plant leaves during programmed cell death? BMC Plant Biol. 2011;11:102. doi: 10.1186/1471-2229-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Ozyigit II, et al. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front Plant Sci. 2016;22:301. doi: 10.3389/fpls.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zabka A, Winnicki K, Polit JT, Maszewski J. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death(AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193–206. doi: 10.1016/j.plaphy.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, et al. Stable transformation of suspension-cultured Glycyrrhiza inflata batalin cells with Agrobacterium tumefaciens. Z Naturforsch C. 2012;67:603–610. doi: 10.1515/znc-2012-11-1211. [DOI] [PubMed] [Google Scholar]
- 60.Zhang XM, et al. NtCP56, a new cysteine protease in Nicotiana tabacum L., involved in pollen grain development. J Exp Bot. 2009;60:1569–1577. doi: 10.1093/jxb/erp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D, et al. The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell. 2014;26:2939–2961. doi: 10.1105/tpc.114.127282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Li Y, Xue H, Pritchard HW, Wang X. Reactive oxygen species-provoked mitochondria-dependent cell death during ageing of elm (Ulmus pumila L.) seeds. Plant J. 2015;81:438–452. doi: 10.1111/tpj.12737. [DOI] [PubMed] [Google Scholar]
- 63.Millar AH, Sweetlove LJ, Giegé P, Leaver CJ. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 2001;127:1711–1727. doi: 10.1104/pp.010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sultana A, et al. Catalases CAT1 and CAT3 are not key enzymes in alleviating gamma irradiation-induced DNA damage, H2O2 accumulation, or lipid peroxidation in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2013;77:1984–1987. doi: 10.1271/bbb.130336. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.