Abstract
The HML2 (HERV-K) group constitutes the most recently acquired family of human endogenous retroviruses, with many proviruses less than one million years old. Many maintain intact open reading frames and provirus expression together with HML2 particle formation are observed in early stage human embryo development and are associated with pluripotency as well as inflammatory disease, cancers and HIV-1 infection. Here, we reconstruct the core structural protein (CA) of an HML2 retrovirus, assemble particles in vitro and employ single particle cryogenic electron microscopy (cryo-EM) to determine structures of four classes of CA Fullerene shell assemblies. These icosahedral and capsular assemblies reveal at high-resolution the molecular interactions that allow CA to form both pentamers and hexamers and show how invariant pentamers and structurally plastic hexamers associate to form the unique polyhedral structures found in retroviral cores.
Subject terms: Cryoelectron microscopy, Solution-state NMR, X-ray crystallography, Viral proteins
In retroviruses, the capsid protein (CA) forms a shell surrounding the viral core. Here the authors combine cryo-electron microscopy with NMR and X-ray crystallography to examine the CA structure from the human endogenous retrovirus HML2 (HERV-K) and determine the structures of four Fullerene CA closed shells that reveal the molecular basis of capsid assembly.
Introduction
The human genome contains a large number of endogenous retroviruses (ERVs) that provide a fossil record of human–pathogen interactions over millions of years1,2. In most instances, ERV open reading frames (ORFs) are degraded, containing stop codons, deletions and rearrangements. However, several members of the HML2 group of ERVs have proviruses less than one million years old3–6 with intact ORFs7 and HML2 particle formation is observed in early-stage human embryo development8 and is associated with pluripotency9, as well as inflammatory disease10–12, cancers13–16 and HIV-1 infection17–19. Moreover, viruses created by reconstruction of HML2 consensus sequences have been shown to produce infectious particles20,21.
Among the circulating exogenous retroviruses, the Gag polyprotein is processed into the matrix (MA), capsid (CA) and nucleocapsid (NC) proteins that form the structural layers within a mature retroviral particle. CA forms the capsid shell surrounding the viral core that protects and transports the viral genome and also interacts with host cell factors that are necessary for trafficking, nuclear entry and proviral integration22–24. Given these essential functions of the capsid, several structural studies have been undertaken that have provided the molecular details of the capsid’s individual hexameric and pentameric CA building blocks25–28. In addition, electron cryomicroscopy studies of whole retroviral cores and in vitro assemblies have provided insight into capsid assembly at low and medium resolution29–34. However, despite these advances the pleomorphic nature of retroviral capsids has so far confounded attempts to determine high-resolution structures of entire closed capsid shells containing both hexameric and pentameric subunits.
Here we express and purify CA (HML2 CArec) from a consensus HML2 Gag ERV20,21 and determine crystal and solution nuclear magnetic resonance (NMR) structures of the N-terminal (CArec-NTD) and C-terminal (CArec-NTD) domains, respectively. Further, we assemble particles from HML2 CArec in vitro and use single-particle cryogenic electron microscopy (cryo-EM) to determine the high-resolution structures of four different types of Fullerene shell assemblies. Our data reveal that the structures of the (CArec-NTD) and C-terminal (CArec-NTD) domains are largely conserved with those of CA from exogenous retroviruses. Analysis of the shell structures reveals the intra- and inter-molecular interactions that drives CA assembly into pentamers and hexamers and their association into shells that encapsidate the retroviral genome.
Results
In vitro assembly and cryo-EM structures of HML2 CA shells
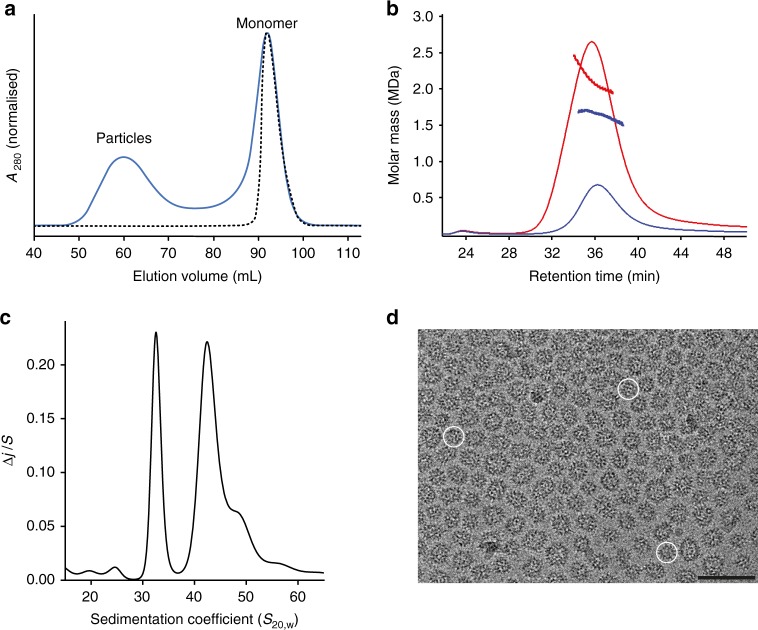
To assess if human ERVs have retained the capacity to assemble CA into shells, we synthesised a codon-optimised consensus HML2 CA coding sequence20,21 (Supplementary Fig. 1) and expressed the protein, HML2 CArec, in Escherichia coli. The addition of high salt (>1 M NaCl) to purified HML2 CArec induced the formation of high-molecular mass particles (Fig. 1a) and a strong concentration dependency of the size distribution (1.5–2.5 MDa) measured by size-exclusion chromatography (SEC) coupled to multi-angle laser light scattering (SEC-MALLS) demonstrated the presence of a heterogeneous mixture (Fig. 1b). Further resolution of the assembly reaction using sedimentation velocity revealed major 32S and 42S species together with a minor 50S component (Fig. 1c). Analysis by cryo-EM revealed a distribution of regular particles, comprising a large proportion of small 160 Å diameter spherical particles and additional larger capsular particles of up to 300 Å in the largest dimension (Fig. 1d).
Fig. 1. Hydrodynamic and EM analysis of HML2 CArec particles.
a Gel filtration chromatogram (A280) of an HML2 CArec assembly reaction (1.4 M NaCl; solid blue line) and the unassembled monomer (0.1 M NaCl; dashed black line) separated on a Superose 6 16/60 GL column. The peaks containing HML2 CArec monomer and early eluting assembled particles are labelled. b Solution molecular mass of HML2 CArec particles. The molar mass distribution of HML2 CArec particles analysed by SEC-MALLS employing a Superose 6 10/30 column equilibrated in 1 M NaCl. The chromatograms were recorded from the 90° detector of the photometer from samples of HML2 CArec assembly reactions carried out at 2 mg mL−1 (blue) and 10 mg mL−1 (red). The points, colour-coded the same as the chromatograms, are the molar mass measured at 1-s intervals throughout the elution of the chromatographic peaks and show the presence of heterogeneous species ranging from 1.5 to 3.0 MDa. c Continuous distribution function of sedimentation coefficients. The plot shows the 20–60S region derived from sedimentation velocity analysis of a HML2 CArec particle preparation. HML2 CArec particles with sedimentation coefficients of 32S, 42S and 50S are the major species. d Cryo-electron micrograph of a field of HML2 CArec particles showing a predominance of small (T = 1) particles (circled examples) and larger capsular particles, scale bar is 50 nm.
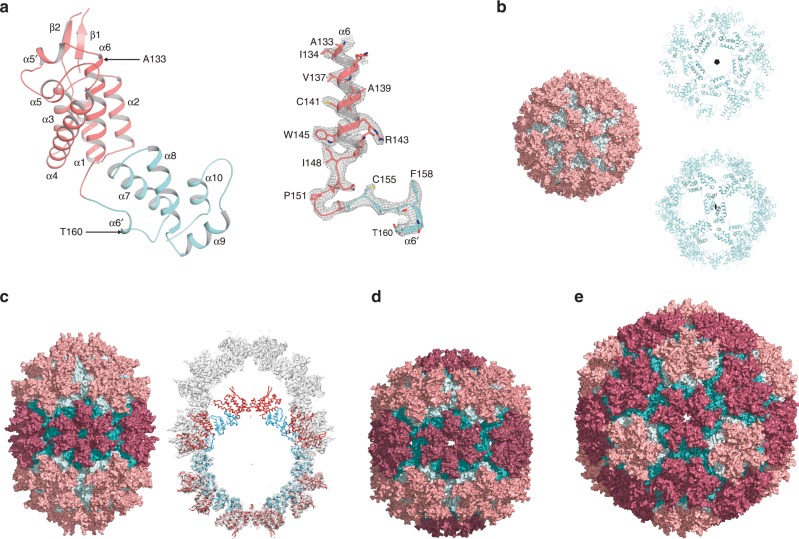
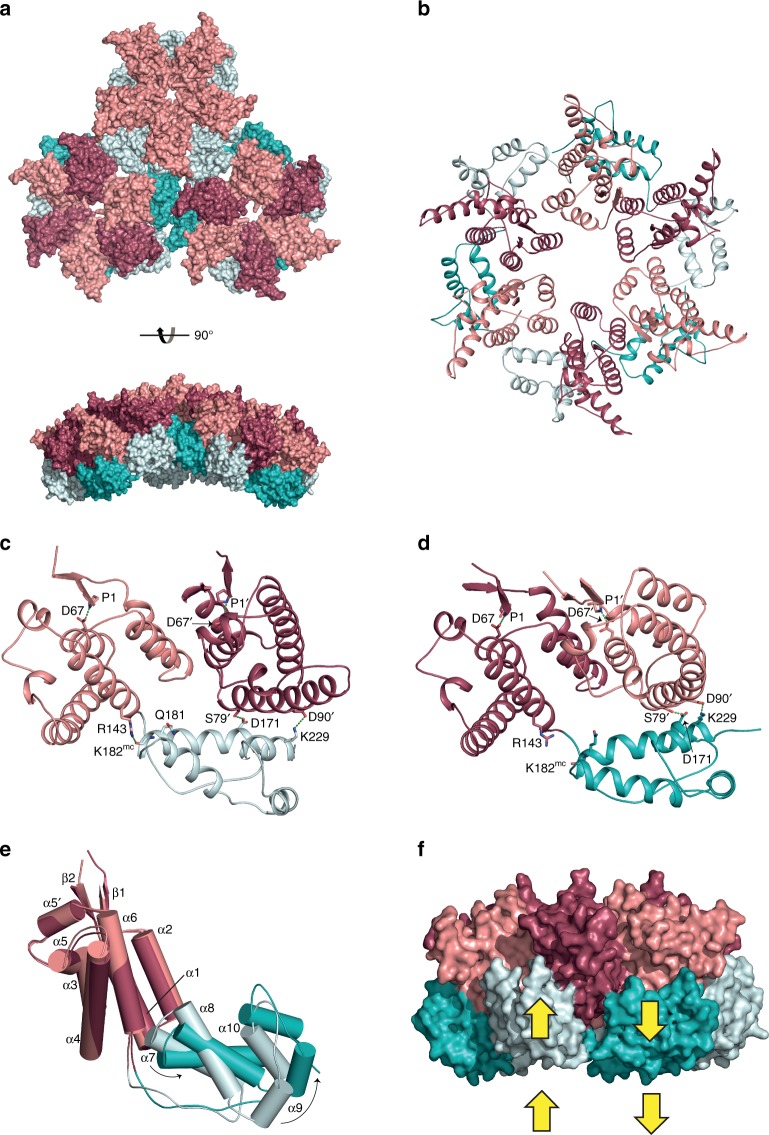
Cryo-EM combined with single-particle image reconstruction was employed to determine the structure of these HML2 CArec particles. After two- (2D) and three-dimensional (3D) classification of particle images, the structures of four types of particle were resolved to high-resolution (Fig. 2 and Supplementary Table 1). Details of classification, image processing and map resolution are presented in (Supplementary Figs. 2–5). The resolution of the maps varies from 2.7 to 4.3 Å, but show clear density for backbone and side chains and are of sufficient quality for de novo building of atomic models (Fig. 2a and Supplementary Fig. 6). The majority species comprises 12 HML2 CArec pentamers with all interfaces equivalent, obeying perfect T = 1 icosahedral symmetry (Fig. 2b). The structure is resolved at the highest resolution (2.7 Å), likely corresponding to the 32S species observed in sedimentation experiments. In addition, there are a smaller number of D5 and D6 symmetric capsular structures (Fig. 2c, d) and a T = 3 icosahedral particle (Fig. 2e) that together likely constitute the faster sedimenting 42–46S species. The D5 particle is an elongated or capsular expansion of the T = 1 particle with polar regions identical to T = 1, but containing five equatorially inserted distorted hexamers, consistent with local two-fold (dihedral) symmetry (Fig. 2c). The D6 particles are also capsular with fully six-fold symmetric hexamers at the poles each surrounded by a ring of six pentamers and with six equatorial hexamers with similar distortions to those observed in the D5 structure (Fig. 2d). The largest structure is a T = 3 particle (Fig. 2e), resolved to 4.3 Å and contains a T = 1 particle within the interior, although no fixed orientation between the shells is apparent (Supplementary Fig. 5a, b). The T = 3 shell is 300 Å in diameter and is comprised of 12 pentamers, each surrounded by five hexamers. Hexamers are located at icosahedral three-fold axes and interact across icosahedral two-fold axes. This combination of the four structures describe, at high resolution, the molecular interactions within CA pentamer and hexamer arrangements, as well as the inter-pentamer, pentamer–hexamer and inter-hexamer interactions that participate in the formation of closed Fullerene shell structures in vivo.
Fig. 2. Structure of HML2 CArec monomer and assembled particles.
a (Left) Cartoon representation of the HML2 CArec monomer from the T = 1 particle. The CArec-NTD, residues P1 to S153, and CArec-CTD, residues A154 to Q246, are coloured in pink and cyan, respectively. (Right) Experimental electron potential map (grey mesh) and model for the region indicated between α6 and α6′. Residues G152 to P156 in the linker region were built de novo into the density. b (Left) Surface representation of the T = 1 particle (60 monomers arranged as 12 pentamers) viewed along the five-fold symmetry axis with the NTDs and CTDs coloured as in a. (Right) The inner CTD “cage” viewed along the five-fold (upper) and the two-fold (lower). c (Left) D5 particle (90 monomers arranged as 12 pentamers and 5 hexamers) viewed along an equatorial pseudo-hexamer at the two-fold symmetry axis. Pentamer NTDs and CTDs are coloured as in a, and hexamer NTDs and CTDs are coloured in red and teal, respectively. (Right) Central slab of the D5 density map (grey surface) with the T = 1 particle aligned on the D5 polar pentamer. d D6 particle (108 monomers arranged as 12 pentamers and 8 hexamers) viewed along an equatorial pseudo-hexamer at the two-fold symmetry axis. Pentamers and hexamers are coloured as in c. e T = 3 particle (180 monomers arranged as 12 pentamers and 20 hexamers) viewed along an icosahedral two-fold symmetry axis. Pentamers and hexamers are coloured as in c.
To guide the structural interpretation of these particles at higher resolution, we determined the 1.8 Å crystal structure of the CArec N-terminal domain (CArec-NTD) (Supplementary Fig. 7a and Supplementary Table 2) and the solution NMR structure of CArec C-terminal domain (CArec-CTD) (Supplementary Fig. 7b, c and Supplementary Table 3). CArec-NTD has the same N-terminal β-hairpin and six-helix fold observed in exogenous orthoretroviral CA proteins. CArec-CTD comprises the same four-helix fold found in exogenous orthoretroviral CA-CTDs, but with an additional C-terminal distal helix (α11, residues A237-E248) that associates with the core of the CTD domain. Importantly, although α11 displays the sequential and medium-range inter-residue nuclear Overhauser effect (NOE) cross-peaks (Supplementary Fig. 7d) that define a helical conformation, the higher T2, lower T1 and lower heteronuclear 1H-15N NOE values observed in NMR relaxation experiments (Supplementary Fig. 8) together indicate it is more dynamic than the core domain (α7–α10). These high-resolution structures could be docked directly into the cryo-EM density and, with the additional building of the NTD–CTD linkers, the NTD–CTD interface and helix α8 at the CTD–CTD interface (Supplementary Fig. 6), refined well into each particle structure. Exceptions were the N-terminal β-hairpin that contains a large loop insertion and is more mobile than in the crystal structure, and the NTD–CTD linker region of D5 and D6 equatorial hexamers that displayed weaker density. In addition, the CTD α11 was not visible in these structures of mature particles, but given its proximity to the C terminus of CA, it may have a structural role in immature Gag assembly, similar to that observed for the SP1 spacer of HIV-135 and CAH helix of murine leukaemia virus (MLV) Gag32.
Structural superimposition with exogenous retroviral CA domains revealed the strongest similarity of CArec-NTD with the CA-NTD of the beta and alpharetroviruses Jaagsiekte sheep retrovirus (JSRV) and Rous sarcoma virus (RSV) (Z-scores 10.7 and 9.0) and the most distant relationship to the CA-NTD of the gammaretrovirus MLV (Supplementary Fig. 9a). Similarly, superimposition of HML2 CArec-CTD with orthoretroviral CA-CTDs (Supplementary Fig. 9b) revealed comparable Z-scores for all available structures (7.4–5.9), except for the MLV CA-CTD that aligned significantly more poorly (Z = 5.0). These data agree with phylogenetic schemes placing HML2 close to an alpha and betaretroviral ancestor36 and also reveal that extant beta or alpharetroviruses, although none currently infect humans, have maintained the structural features of CA endogenized into the human genome.
CA conformational switching in pentamers and hexamers
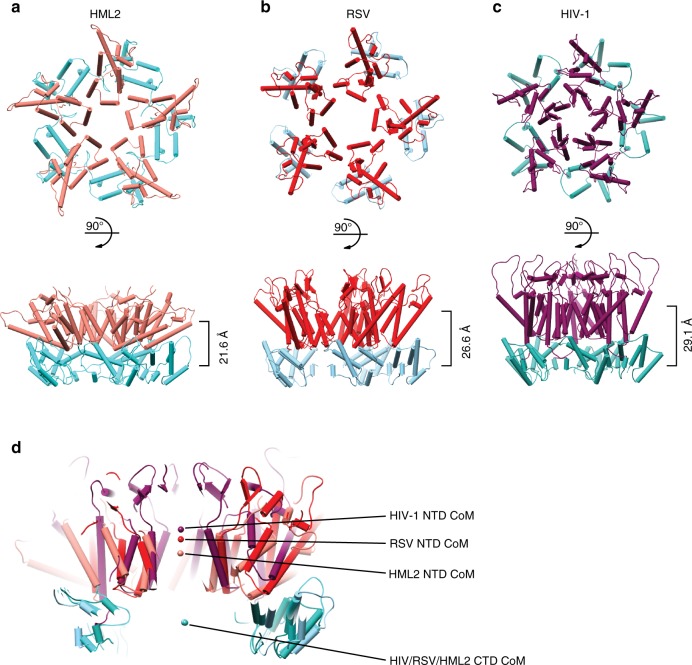
The T = 1 particle comprises 12 HML2 CArec pentamers. The NTDs of each pentamer are associated proximal to the five-fold axis and form a layer of structure at a radius of 86.3 Å (NTD centroid distance) from the particle centre. Beneath the NTDs, the CTDs form an “inner cage” layer (Fig. 2b) at a radius of 64.7 Å (CTD centroid distance), and more distal to the five-fold axis. The NTD layer does not contribute to inter-pentamer interactions and so the entire inter-pentamer interface is mediated through inner cage CTD–CTD interactions across icosahedral two-fold axes (Fig. 2b). Comparison of the HML2 CArec pentamer to CA pentamers studied at lower resolution for HIV29 and RSV33,37 shows a good alignment of CTDs, that they occupy similar radial positions and employ the same inner cage CTD–CTD interactions at icosahedral two-fold axes. However, the relationship between the NTD and CTD layers in the different retroviral genera varies substantially (Fig. 3). In HML2, the vertical displacement between the NTD and CTD, as judged by the centre of mass (CoM) of the two layers, is 21.6 Å. In the RSV pentamer, this increases to 26.6 Å, and in the HIV-1 pentamer, the layers are even further apart at 29.1 Å (Fig. 3a–d). Therefore, although the underlying CTD sub-lattice and individual domain structures (Supplementary Fig. 9) are conserved across retroviral genera, the differences in the NTD–CTD posture appears to be genera specific.
Fig. 3. Comparison of HIV-1, RSV and HML2 CA pentamers.
a–c Top and side view cartoon representations of CA pentamers with α-helices shown as cylinders, from a HML2 CArec, b RSV (built from PDB: 1EM9 and 3G1I docked into EMD 5772) and c HIV-1 (PDB: 5MCY). NTDs are coloured pink, red and purple and CTDs are coloured light cyan, light blue and cyan for HML2, RSV and HIV-1, respectively. Pentamers are aligned with respect to their CTDs. In the lower panels, the distance between the centre of mass (CoM) for each NTD and CTD ring is shown on the right. d Central section through CA pentamers aligned with respect to CTDs. The CoM for NTD and CTD rings is represented by the spheres, colour coded as in a–c and shows the coincident CoM for CTDs and the difference in CTD–NTD vertical displacement for each pentamer.
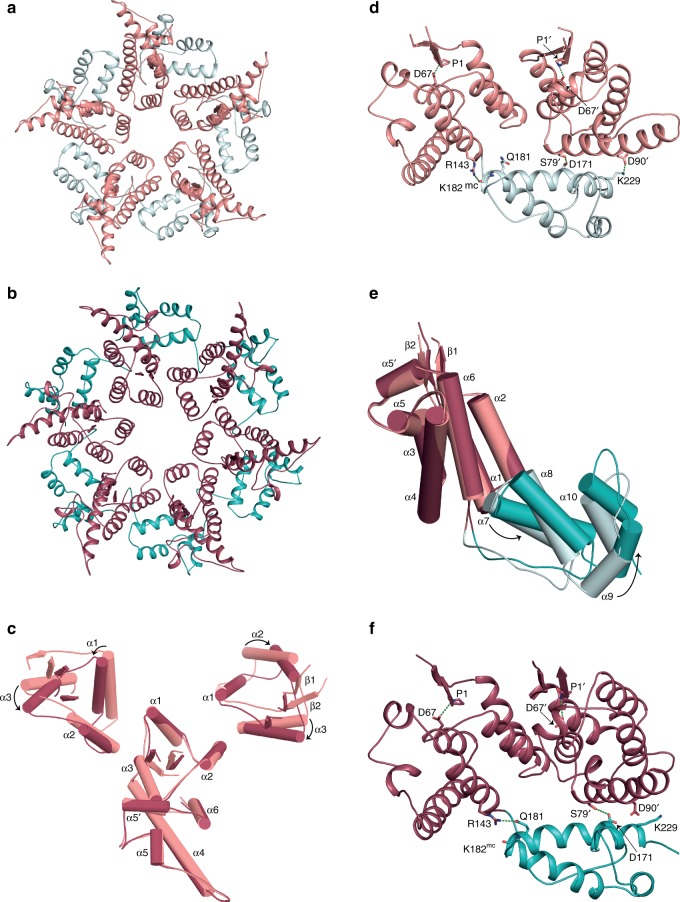
In the four shell types, regardless of the surrounding environment, the pentamers adopt an invariant configuration (root-mean-square deviation (RMSD) over 1082 Cα = 0.91 Å) and so a key question is how the CArec monomer is able to assemble into both pentamers and hexamers. In pentamers and hexamers, the packing of helices α1–α2–α3 around the centre of the ring mediates the inter-protomer NTD interface (Fig. 4a, b), and similar to HIV-126 and MLV25, the interface is largely polar, containing solvent facing accessible channels. However, a comparison of the pentamer (Fig. 4a) with the D6 polar hexamer (Fig. 4b), which obeys strict six-fold symmetry, shows that although helix α1–α2–α3 packing is maintained, in hexamers, the CArec monomers are positioned at a greater radius and are rotated away from the symmetry axis, relative to pentamers. As a result, the relative tilt angle between adjacent α1 helices is reduced and helix α2 comes closer to α3 of the neighbouring monomer allowing the additional monomer to be accommodated into the hexameric ring (Fig. 4c and Supplementary Movie 1).
Fig. 4. Intra-pentamer and intra-hexamer interactions in HML2 CArec particles.
a, b Cartoon representations of a the T = 1 pentamer and b the D6 polar hexamer viewed along the five-fold and six-fold symmetry axes, respectively. The NTDs and CTDs are coloured as in Fig. 2. c Alignment of the NTDs from a T = 1 pentamer (pink) and a D6 “polar” hexamer (red). The central NTD is aligned and the arrows indicate the relative displacement of helices α1, α2 and α3 in adjacent monomers at the intra-pentamer or intra-hexamer interface. d NTD–CTD interactions in the T = 1 pentamer. e NTD domain superposition showing the relative displacement of the CTD in the T = 1 pentamer (pink/cyan) and the D6 “polar” hexamer (red/teal). f NTD–CTD interactions in the D6 polar hexamer. In d, f residues making interactions are shown as sticks, with hydrogen bonds shown as dashes, the prime (′) notation indicates the adjacent NTD and “mc” indicates a main-chain interaction. The conserved N-terminal P1 to α3–D67 interaction is also shown in each NTD for orientation.
The transformation from pentamer to hexamer also involves remodelling of both the intra-monomer NTD–CTD interface and the CTD interaction with the NTD of the neighbouring monomer in either the pentameric or hexameric ring. In the pentamer, residue R143 located at the C terminus of α6 in the NTD makes a hydrogen bond to the main-chain carbonyl of K182, located at the C terminus of α7 in the CTD. This configuration facilitates formation of a specific hydrogen bonding arrangement between CTD residues D171 on α7 and K229 on α10 with S79′ and D90′ on α4 of the neighbouring monomer NTD (′ denoting a neighbouring monomer) (Fig. 4d) and defines the pentamer conformation. In the D6 hexamer, because of the expanded radius, for the CTD to maintain the interaction with the neighbouring NTD it is rotated by a further 15° and there is an accompanying 2 Å shift and tilt between α6 and α7 (Fig. 4e). This results in an alternative hydrogen-bonding configuration that defines the hexamer conformation where R143 now makes an intra-monomer hydrogen bond with the side chain of Q181 on the penultimate turn of α7, D171 is hydrogen bonded with S79′ but with a shifted geometry and the K229-D90′ interaction is absent (Fig. 4f). Therefore, by selecting one of these hydrogen-bonding configurations, CArec monomers can adapt to either the pentamer or hexamer conformation. Examination of the sequence conservation of residues making intra-monomer and inter-monomer NTD–CTD interactions (Fig. 5) reveals substantial sequence conservation within the betaretroviruses, extending to a limited extent in the alpharetroviruses. However, conservation of K229-D90′ is betaretrovirus exclusive and appears related to the genera-specific NTD–CTD posture (Fig. 3) that allows the close approach of the CTD to the C-terminal of helix α4 in the neighbouring NTD.
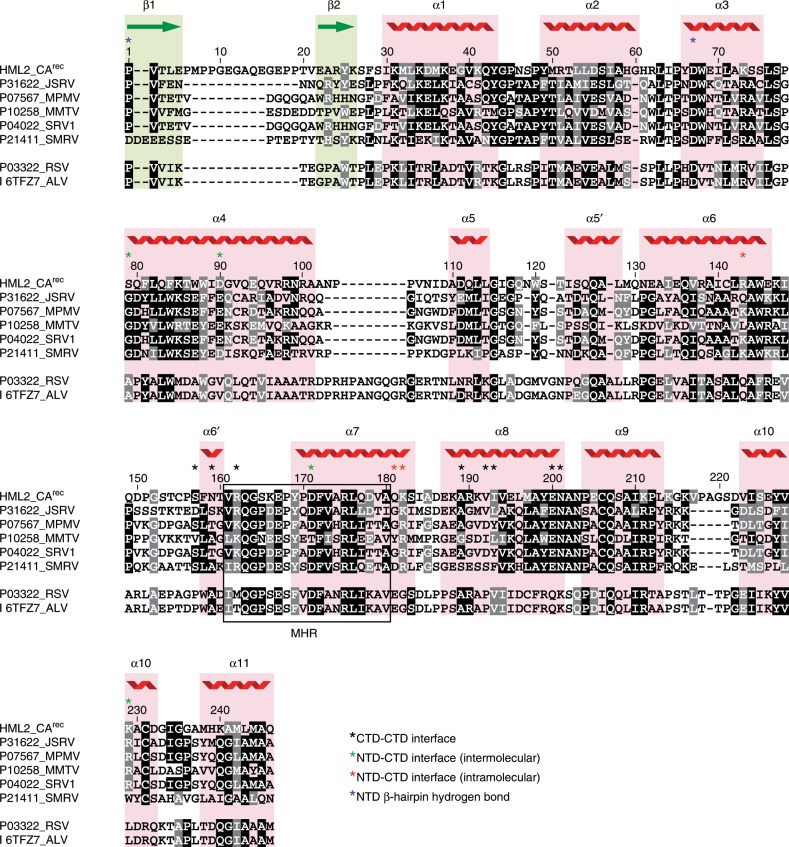
Fig. 5. Structure-based sequence alignment of HML2 CArec with alpha and betaretroviral CAs.
The alignment was performed domain-wise using PROMALS3D, numbering refers to the HML2 CArec sequence and Uniprot accession codes for each aligned sequence are shown on the left. The five betaretroviral: HML2 CArec, JSRV, MPMV, mouse mammary tumour virus (MMTV), Simain type D retrovirus (SRV1) and squirrel monkey retrovirus (SMRV), and two alpharetroviral: RSV and avian leukosis virus (ALV) CAs are grouped separately (upper and lower). The regions corresponding to HML2 CArec secondary structures are shaded red for α-helices and green for β-strands and also displayed pictorially above the sequences. Residues with ≥50% identity are highlighted in black, and ≥50% similarity in grey. The major homology region (MHR) identified in the CA of all the orthoretroviruses is boxed. Residues in HML2 CArec that make inter-molecular CTD–CTD, NTD–CTD or intramolecular NTD–CTD contacts and those that form the conserved NTD β-hairpin hydrogen bond are indicated with the coloured asterisks (black, green, red and blue, respectively).
In the T = 3 particle, there are no inter-pentamer interactions, instead each pentamer is surrounded by five hexamers. The hexamers contact both pentamers and hexamers through the CTD–CTD interactions with three alternating CArec protomers linking to T = 3 pentamers and the other three linking to T = 3 hexamers (Fig. 6a). These non-equivalent contacts are accommodated by relative shifts of adjacent CA monomers reducing the hexamer to three-fold symmetry (Fig. 6b), consistent with its location at the icosahedral three-fold axis. Compared to the symmetrical D6 polar hexamer, this creates pairs of monomers with a repacking of their NTD–NTD interfaces and associated shifts in the location of the CTD and its interaction with the NTD of the neighbouring monomer (Fig. 6c–e). When the CTD–CTD interaction is with a pentamer, the intra-hexamer CTD–NTD interaction has the same structural conformation and specific hydrogen bonding arrangement as the T = 1 pentamer conformation (Fig. 6c and Fig. 4d). Notably, all the pentamers in the T = 3 particle also maintain the same intra-pentamer hydrogen bonding arrangement as the T = 1 pentamer, even though each monomer now makes identical interactions with surrounding hexamers rather than pentamers. By contrast, when the CTD–CTD interaction is with a hexamer, the CTD shifts away from its NTD by 3.6 Å, the R143-mediated α6–α7 interaction is lost and the intra-hexamer CTD–NTD interaction has a structural conformation and hydrogen-bonding configuration that is comparable to that of the D6 hexamer (Fig. 6d, e). Thus, the same structural remodelling of inter-monomer interfaces that switches the pentamer to the hexamer conformation is exploited within the T = 3 hexamer to adapt to either the hexamer–pentamer or hexamer–hexamer environment.
Fig. 6. Variation of hexamer–pentamer and hexamer–hexamer interfaces in the T = 3 particle.
a A hexamer–pentamer–hexamer subassembly from the T = 3 particle shown in surface representation viewed from the exterior of the shell (upper) and from the side, highlighting the curvature (lower). NTDs and CTDs in the pentamer and those in the hexamer that interface with pentamers are coloured pink and cyan, respectively. NTDs and CTDs in the hexamer that interface with hexamers are coloured red and teal, respectively. b Cartoon representation of a hexamer from the T = 3 particle viewed down the icosahedral three-fold axis (colouring as in a). c, d NTD–CTD intra-hexamer interactions in the T = 3 hexamer. c A CA monomer, NTD (pink) and CTD (cyan) that interfaces with a neighbouring pentamer, and the adjacent NTD shown in red. d A CA monomer, NTD (red) and CTD (teal) that interfaces with a neighbouring hexamer, and the adjacent NTD is shown in pink. Residues making interactions are shown as sticks with hydrogen bonds shown as dashes, the prime (′) notation indicates the adjacent NTD and “mc” indicates a main-chain interaction. e NTD (pink/red) alignment of monomers in T = 3 hexamers showing the relative displacement of the CTD when the protomer interacts with either a neighbouring pentamer (cyan) or a neighbouring hexamer (teal). f Hexamer surface representation viewed perpendicular to the symmetry axis showing alternate CTDs displaced upwards (cyan, pentamer facing) and downwards (teal, hexamer facing). NTDs are coloured pink or red as in a.
This symmetry breaking of the T = 3 hexamer is also manifest at the level of whole particle geometry. Measurements of the radial distance of the centroid for T = 3 pentamers and hexamers show that pentamers are situated at a higher radius than hexamers, 143.9 and 136.5 Å, respectively. This difference in displacement is accommodated in T = 3 hexamers by the alternating shift-up of the CTDs (3.3 Å) to interact with adjacent pentamers and shift-down (3.3 Å) to interact with adjacent hexamers (Fig. 6f). Moreover, adaptation of hexamers to the local environment is also a feature of our other D5 and D6 capsular assemblies, where hexamers interact with each other in the equatorial belt. Again, the pentamer conformation remains invariant and the hexamer six-fold symmetry is broken by NTD domain movements, changes in the NTD–CTD linker conformation and in the intra-hexamer CTD–NTD hydrogen bonding configuration (Supplementary Fig. 10) and in the CTD radial position when interacting with either a neighbouring pentamer or hexamer. These observations regarding the plasticity of the hexamer structure are key when considering the pleotropic assemblies that constitute viral cores and provide an explanation of how CA accommodates the extremes of curvature that are encountered in these structures.
Building shells through CTD–CTD interactions
Given the importance of the CTD inner cage, we also analysed the CTD–CTD interactions that connect pentamers to pentamers, hexamers to pentamers and hexamers to hexamers in all of the Fullerene structures (Fig. 7). To undertake this analysis and as a basis for comparison, we first determined the NMR ensemble structure of the solution dimer and measured HML2 CArec-CTD self-association (KA = 3.6 × 103 M−1) (Supplementary Fig. 11 and Supplementary Table 4).
Fig. 7. Analysis of HML2 CArec CTD–CTD interactions.
a, b Structures of the CTD–CTD dimer interface in solution (lowest-energy NMR structure) (a) and in the T = 1 particle (b) are shown in cartoon representation. Residues involved in the interface are shown as sticks with hydrogen bonds shown as dashes. The boxed region is rotated 90° to show the hydrophobic interactions between helix α8 of each monomer, with the van der Waals radii shown as grey space fill. c Plot of the cross angles and displacements between α8 helices in all CTD–dimer pairs. The circles are colour-coded with respect to particle type, shown in the key. Groups 1, 2 and 3 are ringed and a schematic describing the nomenclature of POLAR, LONG and LAT is shown alongside. The class of interaction seen in each particle type and the group to which they belong is shown in the key below. d Panel of representative CTD dimers from the groups displayed in c. Top to bottom: NMR dimer (group 1); T = 1 pentamer (group 2); T = 3 pentamer on hexamer (group 2); T = 3 hexamer on hexamer (group 3). e alignment of CTD monomers (grey) showing relative displacement of the interacting CTD monomer in T = 1 (red; group 2) and T = 3 hexamer on hexamer (orange; group 3).
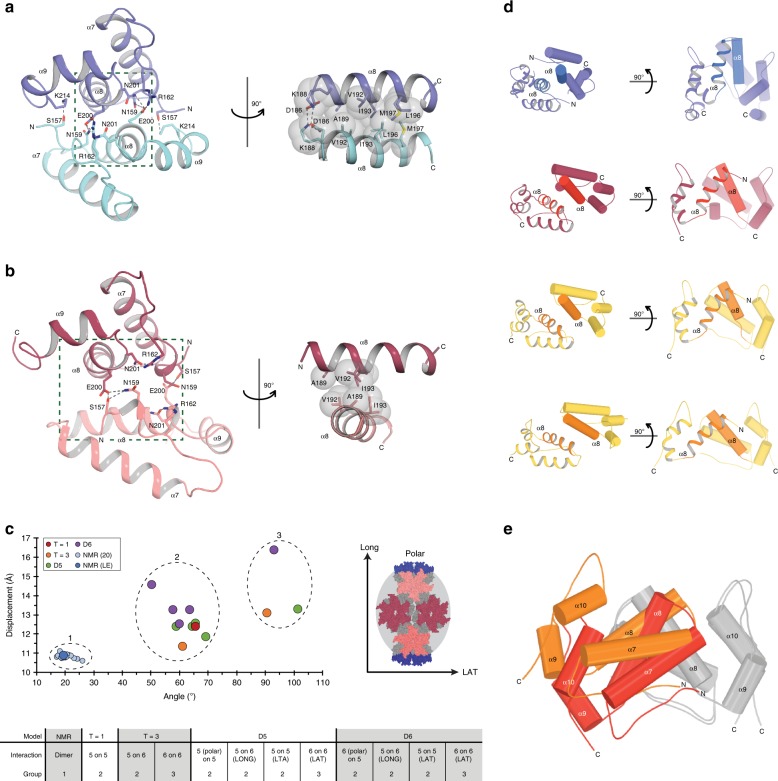
The solution structure CTD–CTD interface involves an outer network of polar interactions (residues S157-K214, N159-R162-E200′-N201′ and D186-K188) together with the packing of a core of hydrophobic side chains (residues: A189, V192, I193 and V194, L196, M197) that extend from opposing α8 helices (Fig. 7a). Residues at the CTD interface are largely conserved amongst the betaretroviruses (Fig. 5) and introduction of a I193A/L196A mutant, analogous to the W184A/M185A that disrupts in HIV CTD–CTD interactions26, abolishes self-association of HML2 CArec-CTD in solution (Supplementary Fig. 11e).
In this configuration, the α8 helices are oriented with a shallow relative crossing angle of 20° and a helix centroid displacement of 11 Å, allowing extensive hydrophobic contacts along their entire length. By comparison, at the unique type of CTD–CTD interface in the T = 1 particle (Fig. 7b) the α8 centroid displacement is maintained at 12 Å, but the crossing angle is significantly enlarged to 65°. This has the effect of twisting the interface relative to the solution state to meet the constraint imposed by icosahedral particle geometry, although remarkably, the hydrophobic interactions of A189, V192 and I193 are preserved, and although much of the polar network is lost, a new compensatory S157–E200–N159 interaction contact is present.
Parameterisation of all the CTD–CTD interactions observed in T = 1, T = 3, D5 and D6 shells in terms of the crossing angle and α8 centroid displacements provides a way to understand the detailed conformations that CTD–CTD dimers adopt within the particles. These data (Fig. 7c, d) show that the NMR solution ensemble populates one region, group 1, and all family members have α8 crossing angles near to 20° and displacements close to 11 Å. The CTD–CTD dimers in the particles fall into two other classes: group 2 that contains all CTD–CTD interactions at pentamer–pentamer and pentamer–hexamer interfaces and have 65° crossing angles and displacements between 11 and 15 Å, and group 3 that contains all the CTD–CTD interactions at hexamer–hexamer interfaces and have crossing angles near to 95° and displacements between 13 and 16 Å. T = 3 particles have two CTD–CTD configurations, a group 2 set from hexamer–pentamer interfaces with a similar configuration to that observed at T = 1 pentamer interfaces and a group 3 set with the larger crossing angle found at hexamer–hexamer interfaces (Fig. 7d, e). In the D5 and D6 particles, further configurations are possible depending on whether pentamers and hexamers are located at poles or are equatorial and interact either longitudinally or laterally (Fig. 7c). Nevertheless, all hexamer–pentamer interfaces still fall into group 2 and all hexamer–hexamer interfaces still fall into group 3, albeit in D6, the α8 displacement for the equatorial hexamer–hexamer interactions are larger than in D5, due to the lower curvature of the equatorial region.
Previously, a coarse-grained theoretical model for a larger HIV-1 fullerene cone-shaped capsid34 was constructed with a continuum of helix α8 crossing angles covering the range of groups 2 and 3 observed in HML2 CArec. Our data now demonstrate how discretisation of crossing angles between CTDs facilitates adaptation of CA to the different environments, particularly at the points of curvature that pentamers introduce into the structures.
Discussion
Our studies of HML2 CArec show that an ERV retains the ability to form capsid assemblies using the same architecture as exogenous viruses. The full diversity of interactions we observe in our structures reveals (1) plasticity of packing at the NTD–NTD interfaces, (2) alternative geometry and bonding configurations at the NTD–CTD interfaces and (3) the quantisation of the CTD–CTD association parameters. Plasticity at the NTD–NTD interface has been observed previously in crystal structures of bovine leukaemia virus CA28, where hexamers are distorted from perfect 6-fold geometry to allow the CTD–CTD interfaces to maintain a hexagonal lattice that would otherwise be dislocated by crystal packing forces. Further, flexibility has been observed in intact HIV-1 virion cores29 where small rotations at the interdomain linker and CTD–CTD interface are distributed over the whole core to accommodate differences in curvature of the Fullerene cone. Now our high-resolution structures enable us to classify and quantify these molecular interactions and provide a rule book for HML2 CArec pentamer and hexamer construction. Moreover, they define how switching of the intra-hexamer NTD–CTD configurations facilitates symmetry breaking of the hexamers to adapt to distinct local environments by positioning the CTD for interaction with the CTD of the adjacent pentamer or hexamer. This provides the basis for building structures with a wide range of shapes and sizes consistent with Fullerene geometry and more pleomorphic morphologies found in exogenous betaretroviruses, the highly similar alpharetroviruses and the more distant lentiviruses.
Methods
Details of constructs
A DNA sequence coding for the consensus HML2 CArec protein was synthesised codon optimised for E. coli expression (Geneart) (Supplementary Fig. 1). The sequence coding for the full-length (HML2 CArec; P1-Q246), amino terminal (P1-P151; CArec-NTD) and C-terminal domain (P156-Q246; CArec-CTD) were amplified by PCR and the products inserted into a pET22b expression vector (Novagen) between the NdeI and XhoI restriction sites in order to produce C-terminal hexa-histidine fusions. The I193A/L196A double mutation was introduced into CArec-CTD using the Quikchange II Site-directed Mutagenesis Kit (Agilent) according to the manufacturer’s instructions. The correct sequence of expression constructs was verified by automated DNA sequencing (Beckman Coulter Genomics), primer sequences used for PCR cloning and mutagenesis are presented in Supplementary Table 5. Proteins were expressed in the E. coli strain BL21 (DE3) by the addition of 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) to log-phase cultures followed by continued growth at 20 °C overnight. The bacteria were harvested and resuspended in 10 mL of lysis buffer per gram of cells (50 mM Tris, pH 8, 250 mM NaCl, 10 mM imidazole, 5 mM MgCl2, 0.5 mM TCEP (Tris(2-carboxyethyl)phosphine), 0.2% v/v Triton X-100). The cells were lysed by sonication and the clarified supernatant was injected onto a 5 mL HisTrap Column (GE Healthcare). Bound sample was washed with wash buffer (50 mM Tris, pH 8, 750 mM NaCl, 20 mM imidazole, 5 mM MgCl2, 0.5 mM TCEP, 0.2% v/v Triton X-100, 4 mM ATP) and His A buffer (20 mM Tris, pH 8, 250 mM NaCl, 10 mM imidazole, 0.5 mM TCEP) and eluted with His B buffer (20 mM Tris, pH 8, 250 mM NaCl, 500 mM imidazole, 0.5 mM TCEP). For HML2 CArec-CTD, the eluent was concentrated to ~3 mL and the protein further purified by SEC on a Superdex 75(26/60) column equilibrated in SEC buffer (20 mM Tris pH 8, 100 mM NaCl, 0.5 mM TCEP). For HML2 and CArec HML2 CArec-NTD the eluents from HisTrap column were diluted 25-fold in IEX A buffer (20 mM Tris pH 8, 0.5 mM TCEP) and applied a 6 mL Resource Q ion exchange column. Proteins were eluted using a 40 column-volume gradient into IEX B buffer (20 mM Tris pH 8, 1 M NaCl, 0.5 mM TCEP). Fractions containing HML2 CArec or HML2 CArec-NTD were concentrated to ~3 mL and further purified by SEC on a Superdex 200(26/60) column equilibrated in SEC buffer. All purified proteins were concentrated to 15–30 mg mL−1, flash frozen in liquid nitrogen and stored at −80 °C. Selenium was incorporated into the N-terminal domain CArec-NTD construct by replacement of methionine with seleno-methionine in defined culture medium and by inhibition of methionine biosynthesis just prior to IPTG induction38. For CArec-CTD NMR experiments, 15N and 13C-15N uniformly labelled protein was expressed in M9 minimal media with 15NH4Cl or 15NH4Cl and 13C6-glucose, as required, as sole nitrogen or nitrogen and carbon sources. To obtain triple-labelled, 2H-13C-15N samples, the M9 media containing 15NH4Cl and 13C6-glucose was prepared in 2H2O instead of H2O. Isotopically labelled and selenium incorporated samples were purified in the same way as unlabelled protein. Verification of N-terminal methionine processing, correct molecular mass, degree of selenium and isotopic label incorporation was obtained by electrospray ionisation mass spectrometry.
SEC-coupled multi-angle laser light scattering
SEC-MALLS was used to estimate the molar mass of HML2 CArec assemblies. Samples (100 µL) ranging from 2 to 11 mg mL−1 of HML2 CArec were applied to a Superose 6 10/300 GL column equilibrated in 20 mM Tris-HCl, 1 M NaCl, 0.5 mM TCEP, pH 8.0, at a flow rate of 0.3 mL min−1 at 25 °C. The scattered light intensity and the protein concentration of the column eluate were recorded using a DAWN-HELEOS laser photometer and OPTILAB-rEX differential refractometer respectively. The weight-averaged molecular mass of materials contained in chromatographic peaks were determined from the combined data from both detectors using the ASTRA software version 6.0.3 (Wyatt Technology Corp., Santa Barbara, CA, USA).
Cryo-EM sample preparation and data collection
HML2 CArec (16 mg mL−1) was adjusted with high salt buffer (20 mM Tris-HCl, 5 M NaCl, 0.5 mM TCEP, pH 8.0) to final salt and protein concentrations of 1.4 M and 10 mg mL−1, respectively. Samples were incubated at 4 °C for 1 h prior to plunge freezing. Quantifoil R2/2 200 mesh copper grids were prepared by glow discharge at 25 mA for 1 min in air (EMITECH). All grids were frozen using a Vitrobot mark III at 4 °C and 100% relative humidity. Two-microlitre sample was added to carbon side of grid and incubated for 30 s in the Vitrobot chamber before blotting for 0.5 s. Immediately after blotting, 2 µL of low salt buffer (20 mM Tris, pH 8, 100 mM NaCl) was applied, followed by a 5 s blot and plunge freezing into liquid ethane. Data from frozen-hydrated samples were collected on a Titan Krios (Thermo Fisher) in nanoprobe mode on a Falcon III detector operating in counting mode. Movies were collected at a magnification corresponding to a calibrated pixel size of 1.09 Å. Movies were recorded with an exposure time of 59.4 s, corresponding to 30 frames and a total dose of 30 e− Å−2.
Image processing
All movies were motion corrected on the fly using Scipion v1.139 with exposure weighting in MotionCor240. Initial parameters for the contrast transfer function correction were estimated from non-exposure-weighted sums in CTFFIND4.141. Micrographs with a quality of fit better than 7 Å resolution were selected for further analysis. A total of 1,498,437 particles from 10,935 dose-weighted micrographs were identified by template-based particle picking using RELION 2.142. Two cycles of reference-free 2D classification yielded 1,218,119 particles for further analysis. The particle classes were further subdivided based on particle diameter guided by known sizes for spherical capsular particles from prior negative stain studies: 715,082 (T = 1), 211,787 (D5 and D6) and 398 (T = 3).
For the reconstruction of the T = 1 particle, an ab initio model without symmetry was generated from a subset of particles (20,000) using cryoSPARC v.243. Two cycles of 3D classification were carried out to identify best particles for subsequent refinement. 3D auto-refinement in RELION 2.1 imposing I2 symmetry was performed on 121,357 particles belonging to the most populated, highest-resolution 3D classes, followed by 3D classification without alignment and auto-refinement of the highest resolution class. Particles and the map corresponding to the best class were then subject to manual refinement of all parameters using cisTEM44 with an initial high-resolution limit for refinement set to 10 Å and final limit of 5 Å. The final reconstruction, performed on 64,731 particles extracted into a 576 × 576 pixel box, has a resolution of 2.75 Å (0.143 Fourier shell correlation (FSC) threshold criterion45).
For the reconstruction of the D5 and D6 capsular particles, an ab initio model was generated without symmetry in cryoSPARC using a subset of particles. One cycle of 3D classification yielded two distinct species of capsular structures. Particles belonging to each species were separated into two stacks of 153,442 (D5) and 88,345 (D6) particles, respectively. Ab initio models were generated using cryoSPARC for each stack separately and further cycles of 3D classification in RELION, were performed both without symmetry as well as imposing D5 symmetry for the 153,442-particle stack and D6 symmetry for the 88,345-particle stack. For the D5 particle, a final stack of 93,221 particles was first refined in cryoSPARC using a homogeneous refinement procedure and then manually refined in cisTEM with an initial high-resolution limit set at 10 Å and final limit of 5 Å. Final refinements and reconstructions were performed on particles extracted into a 648 × 648 pixel box size. This produced a final map with resolution of 3.18 Å (0.143 FSC threshold criterion). For the D6 particle, a final stack of 16,723 particles was refined in an identical manner using cisTEM to produce a final map at 3.77 Å resolution (0.143 FSC threshold criterion).
For the remaining 398 particles from the original 2D classification showing T = 3 icosahedral symmetry, ab initio model generation in cryoSPARC and homogeneous refinement yielded a map of 4.66 Å resolution (0.143 FSC threshold criterion) from 359 particles. Manual refinement of this structure and corresponding particles in cisTEM extracted into a 648 × 648 pixel box size using a high-resolution limit for refinement set to 10 Å. In the last cycle of refinement, a spherical mask with a radius of 100 Å to remove the inner density further improved the map to 4.34 Å (0.143 FSC threshold criterion). Refinement of the inner layer was performed on the same stack of 359 particles, using a T = 1 map lowpass filtered to 40 Å with a mask around the T = 1 map to exclude the outer T = 3 density and refined imposing I2 symmetry. This produced a final map of a T = 1 particle to 5.24 Å (0.143 FSC threshold criterion).
Model building
Maps from the T = 1 and D5 particles were segmented using the Seggar tool, implemented in UCSF Chimera46 to extract density for a pentamer (T = 1) and hexamer (D5), respectively. The extracted map segments were then used for model building. Initially, the CArec-NTD and CArec-CTD structures were docked into density using rigid body refinement in chimera. The fits were then further optimised using the JiggleFit tools and missing sections of the model built into density in COOT47 with iterative rounds of real-space refinement in PHENIX48. The refined monomer structure from the pentamer and each unique interface of the hexamer were then used to construct appropriate asymmetric units for each capsid assembly. Whole particle structures were then further refined with NCS using PHENIX real-space refine to produce final refined models for each map. All models comprise residues 1–6 and 23–231, but with varying completeness in the region 149 to 156. The local resolution of maps was determined using ResMap49 and models were validated throughout refinement using MolProbity50 and quality of fit assessed using map vs. model FSC in PHENIX. Final structures were symmetry expanded in chimera to produce overall assemblies. Details of data collection and model refinement are presented in Supplementary Table 1.
Protein crystallisation and structure determination
HML2 CArec-NTD was crystallised using hanging drop vapour diffusion. Typically, a 40 mg mL−1 solution of HML2 CArec-NTD in 150 mM NaCl, 20 mM Tris-HCl and 0.5 mM TCEP, pH 8.0, was mixed with an equal volume of crystallisation solution containing 0.2 M sodium acetate trihydrate, 0.1 M Tris hydrochloride and 15% (w/v) PEG 4000 and suspended over a reservoir of the crystallisation solution. Drops were incubated at 18 °C, crystals appeared within 2–3 days and were transferred into fresh crystallisation solution supplemented with 20% (v/v) glycerol and flash frozen in liquid nitrogen. The crystals belonged to either the space group C2221 with three copies of HML2 CArec-NTD in the asymmetric unit (AU) or space group C2 with two copies of HML2 CArec-NTD in the AU. Seleno-methionine-derived protein was crystallised under the same conditions.
The structure of HML2 CArec-NTD was solved by a combination of single wavelength anomalous diffraction (SAD) and molecular replacement. Initially a 3.2 Å dataset was recorded from a C2221 orthorhombic crystal of the seleno-methionine substituted protein at a wavelength of 0.9791 Å and 100 K on beamline I03 at the Diamond Light Source (Didcot, UK). Data were processed using the HKL program package51 and 21 selenium atoms were located using SAD methods implemented in SHELX52. Best phases were calculated using only 9 selenium atoms together with non-crystallographic averaging and density modification in PHENIX, resulting in a Figure of Merit of 0.67 and a map of sufficient quality to unambiguously build a near complete model of a single protomer using Arp/Warp53. A further high-resolution 1.8 Å dataset was collected on a C2 monoclinic crystal at 100 K using Cu Kα radiation from an in-house X-ray source (Rigaku Micromax-007HF with a Raxis-IV detector). The monomer from the C2221 crystal was then employed as a molecular replacement search model with this C2 dataset. Molecular replacement was carried out using Phaser54 and located the two copies of HML2 CArec-NTD in the AU. The model was completed by iterative rounds of reciprocal space refinement in PHENIX and model building in COOT. TLS groups were determined using TLSMD55 and were included in the final round of refinement.
The final model comprises residues 1–6 and 23–151 (chain A) and residues 1–5 and 21–149 (chain B) and was refined to a Rwork/Rfree of 15.7/19.5%. The model quality was assessed using Molprobity and has good geometry with 98.8% of residues in the preferred region of the Ramachandran plot, only 1.2% in the additionally allowed region and no outliers. Details of crystal parameters phasing and data refinement statistics are presented in Supplementary Table 2.
NMR spectroscopy and structure determination
NMR experiments were recorded at 298 K on Bruker Avance 600-, 700-, 800- and 950-MHz spectrometers. 1H-15N, 1H-13C-15N and 2H-13C-15N-labelled CArec-CtD samples were prepared in buffer containing 20 mM Tris-HCl, 50 mM NaCl and 0.5 mM TCEP, pH 7.0. Protein concentration for the NMR experiments was ~2.3 mM. 1H, 13C and 15N resonance assignments for protein backbone were obtained from three-dimensional HNCA, HN(CO)CA, HNCO, HN(CA)CO and HNCACB, recorded on 2H-13C-15N-labelled samples, and from HN(CO)CACB, CBCA(CO)NH and HNCANNH experiments recorded on 13C-15N-labelled samples. Side chain resonances were assigned using 13C-15N-labelled samples and, for aliphatic proteins, 3D HBHA(CO)NH, CC(CO)NH, H(CCO)NH, (H)CCH-TOCSY and CCH-TOCSY spectra. Aromatic protons were instead assigned from the analysis of 1H-13C HSQC (heteronuclear single quantum coherence) and 3D 13C-edited NOESY-HSQC (NOE spectroscopy-HSQC) experiments tuned to aromatic carbons as well as 2D (HB)CB(CGCD)HD and 2D (HB)CB(CGCDCE)HE experiments. Inter-proton distance restraints for structural calculations were obtained from 3D 13C-edited NOESY-HSQC and 15N-edited NOESY-HSQC spectra recorded using a 100 ms mixing time from a fully protonated 13C-15N-labelled sample. The dimer interface of CArec-CTD was identified by inter-molecular distance restraints using F1-13C/15N-filtered, F3-13C-edited NOESY-HSQC spectra recorded with a 150 ms mixing time. The 3D-filtered spectra were obtained using an asymmetrically labelled dimer of CArec-CTD prepared by mixing equimolar unlabelled protein with uniformly 13C/15N-labelled protein (2 mM total protein concentration). Hydrogen bonds were identified on the basis of preliminary structure calculations and confirmed from analysis of CLEANEX-PM56 and HNCO-long range57 experiments. All spectral data were processed with NMRPipe58 and analysed with CARA59.
Backbone 15N relaxation measurements of T1 spin–lattice relaxation time, T2 spin–spin relaxation time and the steady-state heteronuclear 1H-15N NOE of HML2 CArec-CTD were recorded at 298 and 278 K on a 700 MHz spectrometer using 0.3 mM 15N-labelled samples. The time delays for T1 experiments were 10, 50, 100, 200, 400, 500, 750, 1000 and 1400 ms, and for T2 experiments were 8, 16, 32, 48, 64, 80, 96, 112, 128 and 160 ms. T1 and T2 relaxation data were obtained by fitting individual peak intensities using nonlinear spectral lineshape modelling to a single exponential using routines within NMRPipe. 1H-15N NOE values were calculated from peak intensity ratios obtained from spectra with and without 1H saturation prior to the 15N excitation pulse. The average errors were estimated at 3% for the T1 and T2 measurements and at 5% on the steady-state heteronuclear 1H-15N NOE values.
The solution structure of HML2 CArec-CTD was calculated using the program ARIA (Ambigious Restraints for Iterative Assignment v 2.3)60. Briefly, nine iterations of a simulated annealing protocol were performed where progressive NOE cross-peak assignment and conversion in the structure(s) calculation process were achieved based on NOE distance restraints and, for helices α7–α10, hydrogen bond and dihedral angle restraints as predicted by the program TALOS+61. The inter-molecular distance restraints defining the HML2 CArec-CTD homodimer interface were derived from inter-proton NOE correlations observed in the filtered NOESY experiments, while the corresponding NOEs were removed from the 3D 13C-NOESY-HSQC constraint list to avoid duplications. A set of 100 structures were calculated and the 20 lowest-energy structures of the set were taken to represent the converged ensemble and refined in an explicit water shell. The superimposition of the 20 water-refined structures is shown in Supplementary Fig. 11b. The quality of the calculated structure ensemble was assessed and validated with the Protein Structure Validation Suite-PSVS62 and Procheck-NMR63. For the final 20 lowest-energy NMR structures, no distance or torsional angle restraint was violated by more than 0.5 Å or 5°, respectively. The Ramachandran plot for the family of structures showed 88% of residues are in the most favoured region, 11% are in the additionally allowed region and only 1% are outliers. Details of the structure determination are summarised in Supplementary Table 3.
3D structural alignments and interface analysis
The European Bioinformatics Institute (EBI) protein structure comparison service (PDBeFold) (http://www.ebi.ac.uk/msd-srv/ssm/) was used to perform searches with HML2 CArec-NTD and HML2 CArec-CTD for structural homologues in the PDB. Orthoretroviral CA-NTDs and -CTDs comprised > 95 % of the top 50 hits. The fit qualities based on RMSD of Cα positions were ranked using the Z-score. Molecular interfaces were analysed using the EBI protein structure interface analysis service PDBePISA (http://www.ebi.ac.uk/msd-srv/prot_int/). Analysis of CTD–CTD crossing angle and displacements were performed as follows. Centroids of α8 helices were obtained by calculating the average x, y, z positions of all backbone atoms (N, CA, C) in the helix. Centroids for the “first half” of the helix and the “second half” of the helix were calculated and used as the start and end points of the vector. Angles between the vectors were obtained by calculating the dot product of the two vectors and dividing by the product of the vector magnitudes.
Structure-based sequence alignment
Structure-based sequence alignment of alpha and betaretroviral CA-NTDs and CA-CTDs was performed using the PROMALS3D64 server. Where necessary, alignments were adjusted manually based on the position of secondary structures observed in seed structures used for the individual CA-NTD and CA-CTD alignments.
Analytical ultracentrifugation
Sedimentation velocity experiments were performed in a Beckman Optima Xl-I analytical ultracentrifuge using conventional aluminium double sector centrepieces and sapphire windows. Solvent density and the protein partial specific volumes were determined from tabulated values65. Prior to centrifugation, samples were prepared by exhaustive dialysis against the buffer blank solution, 20 mM Tris-HCl, 2 M NaCl and 0.5 mM TCEP, pH 8.0. Centrifugation was performed at 20,000 r.p.m. (29,120 × g) and 293 K in an An-50-Ti rotor. Interference data were acquired at time intervals of 180 s at a sample concentration of 1.5 mg mL−1. Data recorded from moving boundaries were analysed using the size distribution function C(S) in the program SEDFIT66–68.
Sedimentation equilibrium experiments were performed in a Beckman Optima XL-I analytical ultracentrifuge using aluminium double sector centrepieces in an An-50 Ti rotor. Prior to centrifugation, samples were dialysed exhaustively against the buffer blank 20 mM Tris-HCl, pH 8, 150 mM NaCl and 0.5 mM TCEP. After centrifugation for 30 h at 22,000 r.p.m. (35,235 × g), interference data was collected at 2 hourly intervals until no further change in the profiles was observed. The rotor speed was then increased to 26,000 rpm (49,213 × g) and then 30,000 r.p.m. (65,520 × g) and the procedure repeated each time. Data were collected on samples of HML2 CArec-CTD and CArec-CTD (I193A/L196A) at varying protein concentration (50–200 µM) and at three speeds. The program SEDPHAT69,70 was used to determine weight-averaged molecular masses by nonlinear fitting of individual multi-speed equilibrium profiles to a single-species ideal solution model. Inspection of these data revealed that the HML2 CArec-CTD molecular mass showed significant concentration dependency and so global fitting to a monomer–dimer equilibrium model incorporating the data from the three speeds and three sample concentrations was applied to extract the dimerisation association constant (KA). Details of protein hydrodynamic parameters and sedimentation equilibrium data are presented in Supplementary Table 4.
To compare the self-association properties of HML2 CArec-CTD with the dimer interface mutant HML2 CArec-CTD (I193A/L196A), MSTAR analysis was performed on the equilibrium distributions recorded at 30,000 r.p.m. (65,520 × g) on 200 µM samples using the program SEDFIT-MSTAR71. The point average molecular weight (Mw*) showed the expected increase with increasing radius for CArec-CTD and yielded a weight-averaged molecular mass at the cell bottom (Mw,b) intermediate between monomer and dimer. For CArec-CTD (I193A/L196A), no radial dependency of Mw* was observed and the Mw,b obtained equated to monomer molecular mass.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We gratefully acknowledge the Francis Crick Structural Biology Science Technology platform, Diamond Light Source (Grant No. MX13775) and the MRC UK biomedical NMR facility for access. We thank members of the Rosenthal and Taylor Laboratory for advice and support. This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001143, FC001178), the UK Medical Research Council (FC001143, FC001178) and the Wellcome Trust (FC001143, FC001178); and by grants from the Wellcome Trust (108014/Z/15/Z) (IAT) and the UK Medical Research Council (U117574558, MC_PC_13051) (A.R.).
Author contributions
O.A., T.G., G.N., N.J.B., L.R., D.C.G., K.S., A.N. and I.A.T. performed experiments. O.A., T.G., G.N., N.J.B., D.C.G., L.R., K.S., A.N., A.R., J.P.S., I.A.T. and P.B.R. contributed to experimental design, data analysis and manuscript writing.
Data availability
The structural data that support the findings of this study have been deposited in the Protein Data Bank, BioMagResBank and EM Data Bank. The coordinates for HML2 CArec-NTD and HML2 CArec-CTD have the PDB accession numbers 6SA9 and 6SAI, respectively. Chemical shift assignments for HML2 CArec-CTD in the BioMagResBank have the accession number 34419. The coordinates for HML2 CArec T = 1, D5, D6 and T = 3 assemblies have PDB accession numbers 6SSJ, 6SSK, 6SSL and 6SSM and the EM maps have EMDB entry numbers EMD-10295 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10295], EMD-10296 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10296], EMD-10297 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10297] and EMD-10298 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10298], respectively. Other data are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Sébastien Alphonse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ian A. Taylor, Email: Ian.Taylor@crick.ac.uk
Peter B. Rosenthal, Email: Peter.Rosenthal@crick.ac.uk
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13786-y.
References
- 1.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WE. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019;17:355–370. doi: 10.1038/s41579-019-0189-2. [DOI] [PubMed] [Google Scholar]
- 3.Belshaw R, et al. Long-term reinfection of the human genome by endogenous retroviruses. Proc. Natl Acad. Sci. USA. 2004;101:4894–4899. doi: 10.1073/pnas.0307800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbulescu M, et al. Many human endogenous retrovirus K (HERV-K) proviruses are unique to humans. Curr. Biol. 1999;9:861–868. doi: 10.1016/S0960-9822(99)80390-X. [DOI] [PubMed] [Google Scholar]
- 5.Turner G, et al. Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr. Biol. 2001;11:1531–1535. doi: 10.1016/S0960-9822(01)00455-9. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JF, Coffin JM. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: implications for human and viral evolution. Proc. Natl Acad. Sci. USA. 2004;101:1668–1672. doi: 10.1073/pnas.0307885100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grow EJ, et al. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nature. 2015;522:221–225. doi: 10.1038/nature14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs NV, et al. Human endogenous retrovirus K (HML-2) RNA and protein expression is a marker for human embryonic and induced pluripotent stem cells. Retrovirology. 2013;10:115. doi: 10.1186/1742-4690-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynier F, et al. Increase in human endogenous retrovirus HERV-K (HML-2) viral load in active rheumatoid arthritis. Scand. J. Immunol. 2009;70:295–299. doi: 10.1111/j.1365-3083.2009.02271.x. [DOI] [PubMed] [Google Scholar]
- 11.Sicat J, Sutkowski N, Huber BT. Expression of human endogenous retrovirus HERV-K18 superantigen is elevated in juvenile rheumatoid arthritis. J. Rheumatol. 2005;32:1821–1831. [PubMed] [Google Scholar]
- 12.Ehlhardt S, et al. Human endogenous retrovirus HERV-K(HML-2) Rec expression and transcriptional activities in normal and rheumatoid arthritis synovia. J. Rheumatol. 2006;33:16–23. [PubMed] [Google Scholar]
- 13.Boller K, Janssen O, Schuldes H, Tonjes RR, Kurth R. Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. J. Virol. 1997;71:4581–4588. doi: 10.1128/jvi.71.6.4581-4588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contreras-Galindo R, et al. Human endogenous retrovirus K (HML-2) elements in the plasma of people with lymphoma and breast cancer. J. Virol. 2008;82:9329–9336. doi: 10.1128/JVI.00646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida T, et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008;8:15. [PMC free article] [PubMed] [Google Scholar]
- 16.Muster T, et al. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 2003;63:8735–8741. [PubMed] [Google Scholar]
- 17.Bhardwaj N, Maldarelli F, Mellors J, Coffin JM. HIV-1 infection leads to increased transcription of human endogenous retrovirus HERV-K (HML-2) proviruses in vivo but not to increased virion production. J. Virol. 2014;88:11108–11120. doi: 10.1128/JVI.01623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young, G. R. et al. HIV-1 infection of primary CD4(+) T cells regulates the expression of specific human endogenous retrovirus HERV-K (HML-2) elements. J. Virol.92, 10.1128/JVI.01507-17 (2018). [DOI] [PMC free article] [PubMed]
- 19.Garrison KE, et al. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 2007;3:e165. doi: 10.1371/journal.ppat.0030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YN, Bieniasz PD. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 2007;3:e10. doi: 10.1371/journal.ppat.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewannieux M, et al. Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 2006;16:1548–1556. doi: 10.1101/gr.5565706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K, et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matreyek KA, Yucel SS, Li X, Engelman A. Nucleoporin NUP153 phenylalanine-glycine motifs engage a common binding pocket within the HIV-1 capsid protein to mediate lentiviral infectivity. PLoS Pathog. 2013;9:e1003693. doi: 10.1371/journal.ppat.1003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaller T, et al. HIV-1 capsid-cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 2011;7:e1002439. doi: 10.1371/journal.ppat.1002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortuza GB, et al. High-resolution structure of a retroviral capsid hexameric amino-terminal domain. Nature. 2004;431:481–485. doi: 10.1038/nature02915. [DOI] [PubMed] [Google Scholar]
- 26.Pornillos O, et al. X-ray structures of the hexameric building block of the HIV capsid. Cell. 2009;137:1282–1292. doi: 10.1016/j.cell.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pornillos O, Ganser-Pornillos BK, Yeager M. Atomic-level modelling of the HIV capsid. Nature. 2011;469:424–427. doi: 10.1038/nature09640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obal G, et al. Structural virology. Conformational plasticity of a native retroviral capsid revealed by x-ray crystallography. Science. 2015;349:95–98. doi: 10.1126/science.aaa5182. [DOI] [PubMed] [Google Scholar]
- 29.Mattei S, Glass B, Hagen WJ, Krausslich HG, Briggs JA. The structure and flexibility of conical HIV-1 capsids determined within intact virions. Science. 2016;354:1434–1437. doi: 10.1126/science.aah4972. [DOI] [PubMed] [Google Scholar]
- 30.Byeon IJ, et al. Structural convergence between cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function. Cell. 2009;139:780–790. doi: 10.1016/j.cell.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schur FK, et al. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 A resolution. Nature. 2015;517:505–508. doi: 10.1038/nature13838. [DOI] [PubMed] [Google Scholar]
- 32.Qu K, et al. Structure and architecture of immature and mature murine leukemia virus capsids. Proc. Natl Acad. Sci. USA. 2018;115:E11751–E11760. doi: 10.1073/pnas.1811580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardone G, Purdy JG, Cheng N, Craven RC, Steven AC. Visualization of a missing link in retrovirus capsid assembly. Nature. 2009;457:694–698. doi: 10.1038/nature07724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G, et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013;497:643–646. doi: 10.1038/nature12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharat TA, et al. Cryo-electron microscopy of tubular arrays of HIV-1 Gag resolves structures essential for immature virus assembly. Proc. Natl Acad. Sci. USA. 2014;111:8233–8238. doi: 10.1073/pnas.1401455111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gifford RJ, et al. Nomenclature for endogenous retrovirus (ERV) loci. Retrovirology. 2018;15:59. doi: 10.1186/s12977-018-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keller PW, et al. A two-pronged structural analysis of retroviral maturation indicates that core formation proceeds by a disassembly–reassembly pathway rather than a displacive transition. J. Virol. 2013;87:13655–13664. doi: 10.1128/JVI.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doublie S. Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 1997;276:523–530. doi: 10.1016/S0076-6879(97)76075-0. [DOI] [PubMed] [Google Scholar]
- 39.de la Rosa-Trevin JM, et al. Scipion: a software framework toward integration, reproducibility and validation in 3D electron microscopy. J. Struct. Biol. 2016;195:93–99. doi: 10.1016/j.jsb.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohou A, Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Leiro R, Scheres SHW. A pipeline approach to single-particle processing in RELION. Acta Crystallogr. D. 2017;73:496–502. doi: 10.1107/S2059798316019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 44.Grant, T., Rohou, A., Grigorieff, N. cis-TEM, user-friendly software for single-particle image processing. Elife7, 10.7554/eLife.35383 (2018). [DOI] [PMC free article] [PubMed]
- 45.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Pettersen EF, et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 47.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 48.Zwart PH, et al. Automated structure solution with the PHENIX suite. Methods Mol. Biol. 2008;426:419–435. doi: 10.1007/978-1-60327-058-8_28. [DOI] [PubMed] [Google Scholar]
- 49.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat. Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovell SC, et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 51.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 52.Sheldrick GM. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D. 2010;66:479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris RJ, Perrakis A, Lamzin VS. ARP/wARP and automatic interpretation of protein electron density maps. Methods Enzymol. 2003;374:229–244. doi: 10.1016/S0076-6879(03)74011-7. [DOI] [PubMed] [Google Scholar]
- 54.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Painter J, Merritt EA. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D. 2006;62:439–450. doi: 10.1107/S0907444906005270. [DOI] [PubMed] [Google Scholar]
- 56.Hwang TL, van Zijl PC, Mori S. Accurate quantitation of water-amide proton exchange rates using the phase-modulated CLEAN chemical EXchange (CLEANEX-PM) approach with a Fast-HSQC (FHSQC) detection scheme. J. Biomol. NMR. 1998;11:221–226. doi: 10.1023/A:1008276004875. [DOI] [PubMed] [Google Scholar]
- 57.Cordier F, Grzesiek S. Direct observation of hydrogen bonds in proteins by interresidue 3hJNC‘ scalar couplings. J. Am. Chem. Soc. 1999;121:1601–1602. doi: 10.1021/ja983945d. [DOI] [PubMed] [Google Scholar]
- 58.Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 59.Bartels C, Xia TH, Billeter M, Guntert P, Wuthrich K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 60.Linge JP, Habeck M, Rieping W, Nilges M. ARIA: automated NOE assignment and NMR structure calculation. Bioinformatics. 2003;19:315–316. doi: 10.1093/bioinformatics/19.2.315. [DOI] [PubMed] [Google Scholar]
- 61.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhattacharya A, Tejero R, Montelione GT. Evaluating protein structures determined by structural genomics consortia. Proteins. 2007;66:778–795. doi: 10.1002/prot.21165. [DOI] [PubMed] [Google Scholar]
- 63.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 64.Pei J, Kim BH, Tang M, Grishin NV. PROMALS web server for accurate multiple protein sequence alignments. Nucleic Acids Res. 2007;35:W649–W652. doi: 10.1093/nar/gkm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laue, T. M., Shah, B. D., Ridgeway, T. M. & Pelletier S. L. in Analytical Ultracentrifugation in Biochemistry and Polymer Science (eds Harding, S. E., Rowe, A. J. & Horton J. C.) (The Royal Society of Chemistry, Cambridge, 1992).
- 66.Brown PH, Schuck P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys. J. 2006;90:4651–4661. doi: 10.1529/biophysj.106.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuck P, Perugini MA, Gonzales NR, Howlett GJ, Schubert D. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys. J. 2002;82:1096–1111. doi: 10.1016/S0006-3495(02)75469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vistica J, et al. Sedimentation equilibrium analysis of protein interactions with global implicit mass conservation constraints and systematic noise decomposition. Anal. Biochem. 2004;326:234–256. doi: 10.1016/j.ab.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 2003;320:104–124. doi: 10.1016/S0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- 71.Schuck P, et al. SEDFIT-MSTAR: molecular weight and molecular weight distribution analysis of polymers by sedimentation equilibrium in the ultracentrifuge. Analyst. 2014;139:79–92. doi: 10.1039/C3AN01507F. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The structural data that support the findings of this study have been deposited in the Protein Data Bank, BioMagResBank and EM Data Bank. The coordinates for HML2 CArec-NTD and HML2 CArec-CTD have the PDB accession numbers 6SA9 and 6SAI, respectively. Chemical shift assignments for HML2 CArec-CTD in the BioMagResBank have the accession number 34419. The coordinates for HML2 CArec T = 1, D5, D6 and T = 3 assemblies have PDB accession numbers 6SSJ, 6SSK, 6SSL and 6SSM and the EM maps have EMDB entry numbers EMD-10295 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10295], EMD-10296 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10296], EMD-10297 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10297] and EMD-10298 [https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10298], respectively. Other data are available from the corresponding authors upon reasonable request.