Abstract
Lipopolysaccharide (LPS) or repeated water avoidance stress (WAS) induces visceral allodynia and gut hyperpermeability via corticotropin-releasing factor (CRF) and proinflammatory cytokines, which is a rat irritable bowel syndrome (IBS) model. As butyrate is known to suppress the release of proinflammatory cytokine, we hypothesized that butyrate alleviates these colonic changes in IBS models. The visceral pain was assessed by electrophysiologically measuring the threshold of abdominal muscle contractions in response to colonic distention. Colonic permeability was determined by measuring the absorbance of Evans blue in colonic tissue. Colonic instillation of sodium butyrate (SB; 0.37−2.9 mg/kg) for 3 days inhibited LPS (1 mg/kg)-induced visceral allodynia and colonic hyperpermeability dose-dependently. Additionally, the visceral changes induced by repeated WAS (1 h for 3 days) or CRF (50 µg/kg) were also blocked by SB. These effects of SB in the LPS model were eliminated by compound C, an AMPK inhibitor, or GW9662, a PPAR-γ antagonist, NG-nitro-L-arginine methyl ester, a NO synthesis inhibitor, naloxone or sulpiride. SB attenuated visceral allodynia and colonic hyperpermeability in animal IBS models. These actions may be AMPK and PPAR-γ dependent and also mediated by the NO, opioid and central dopamine D2 pathways. Butyrate may be effective for the treatment of IBS.
Subject terms: Pharmacology, Stress and resilience, Physiology, Irritable bowel syndrome
Introduction
Stress-induced altered visceral sensorimotor function is known to be a significant contributor to the pathophysiology of irritable bowel syndrome (IBS)1. As the visceral changes by stress are eliminated by a corticotropin-releasing factor (CRF) antagonist, CRF may be a crucial molecule in IBS2.
Additionally, it has been recently recognized that the impaired gut barrier associated with abnormal immune response also plays a significant role in IBS1. Increased circulatory levels of proinflammatory cytokines and lipopolysaccharide (LPS) are detected in IBS3,4, and higher symptom severity is correlated with higher cytokine response induced by LPS in peripheral blood mononuclear cells4. We have shown previously that LPS injection induced visceral allodynia and colonic hyperpermeability in rats via the peripheral CRF, Toll-like receptor 4 (TLR4), interleukin (IL)-1 and IL-6 pathways5,6, which is thought to simulate the pathophysiology of IBS.
At the same time, repeated water avoidance stress (WAS), a well-known animal IBS model, or peripheral injection of CRF also induces these visceral changes via similar pathways to LPS6,7. In this context, CRF signalling activated by stress (LPS or repeated WAS) possibly induces these changes by modulating TLR4-cytokine signalling, and we considered that it is one of the important mechanisms of IBS6. Thus, suppression of the cytokine signalling may be effective for the treatment of this disease.
Butyrate is one of the short-chain fatty acids (SCFAs), which are the main metabolites produced by bacterial fermentation of dietary fiber and is a primary energy source for colonocytes. In addition, it regulates immune function, and exerts the suppressive effects of proinflammatory cytokines8,9. In this context, butyrate may be expected to improve visceral changes in these animal IBS models via the inhibition of cytokine signalling.
However, the effects of butyrate on visceral functions possibly related to the pathophysiology of IBS have been controversial so far. Rectal enema of butyrate decreases pain in response to rectal balloon distention in healthy human volunteers10. Moreover, butyrate reduced colonic paracellular permeability and enhanced the barrier in vitro11,12. In contrast, several researchers showed that the rectal instillation of butyrate aggravates visceral pain in non-stressed rats13–16, and none of the studies has shown that it exerts beneficial effects on visceral function in an animal IBS model.
In this study, we attempted to determine the effects of the colonic instillation of butyrate on visceral sensation and colonic permeability in rat IBS models, i.e. LPS, repeated WAS or CRF, to explore the possibility of therapeutic application of butyrate in IBS.
Methods
Animals
Adult male Sprague-Dawley rats (Charles River Laboratory, Atsugi, Japan) weighing about 300 g were used. The animals were group-housed (three to four rats per cage) in a regulated environment with illumination (12 h light/dark cycle) and temperature (23 °C−25 °C). Food (Solid rat chow, Oriental Yeast, Tokyo, Japan) and water were given ad libitum.
Chemicals
Sodium butyrate (SB; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) was dissolved in phosphate-buffered saline (PBS; 0.14 M NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.8 mM KH2PO4). LPS obtained from Escherichia coli with serotype 055:B5 (Sigma-Aldrich, St. Louis, MO, USA), a rat/human CRF (Peptide Institute, Inc., Asagi, Japan), NG-nitro-L-arginine methyl ester (L-NAME), naloxone hydrochloride and domperidone (Fujifilm Wako Pure Chemical) were dissolved in normal saline. Compound C (dorsomorphin; LC Laboratories, Inc., Woburn, MA, USA), GW9662 (Focus Biomolecules, Plymouth Meeting, PA, USA) and sulpiride (Fujifilm Wako Pure Chemical) were dissolved in dimethyl sulfoxide (Fujifilm Wako Pure Chemical). The doses and routes of administration of the chemicals were determined according to previous publications5,6,17–20.
Measuring visceral sensation
The conscious rats underwent colonic balloon distention to induce abdominal muscle contractions (visceromotor response, VMR), which were measured by an electromyogram (EMG). This method was previously validated as a quantitative measure of visceral nociception21. We evaluated the VMR threshold, defined as the volume (ml) of the distended balloon in the current study, and the experiments were performed as described previously6. The method of measurement was described briefly in the following.
Electrodes implantation and colonic distention balloon placement
Under isoflurane anesthesia, a small skin incision was made for the insertion of EMG electrodes (Teflon-coated stainless steel, 0.05 mm diameter) into the left side external oblique muscle in non-fasted rats. The electrodes were fixed to the muscle and the incised skin by cyanoacrylate instant adhesive. Then, the electrode leads were externalized directly through this closed incision without a subcutaneous (s.c.) tunnel and threaded through a urethane tube. Analgesics or antibiotics were not administered. A distention balloon (6-Fr disposable silicon balloon-urethral catheter, JU-SB0601; Terumo Corporation, Tokyo, Japan) was placed intra-anally with the distal end positioned 2 cm proximal to the anus.
Colonic distention and abdominal muscle contraction measurement
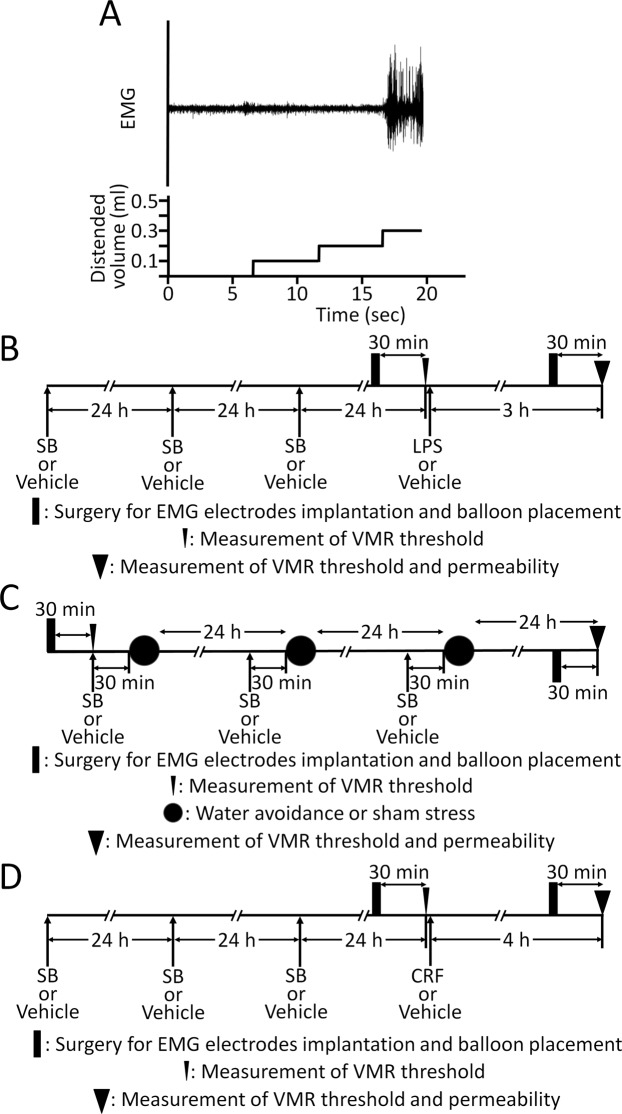
After the electrodes were fixed and the balloon was inserted, the rats were placed in Bollmann cages. The electrode leads were then connected to an EMG amplifier, and the signals were recorded by a PowerLab system (AD Instruments, Colorado Springs, CO, USA). Colonic distention was performed using the ascending method of limits paradigm with phasic distention by inflating the balloon by water using a syringe. The distention was increased progressively in 0.1 ml increments every 5 s until significant abdominal muscle contractions, i.e. VMR, were detected. The VMR threshold was defined as the distended balloon volume (ml) inducing VMR (Fig. 1A). The threshold was assessed twice (2-min interval), and the mean was calculated for each individual animal. The percentage change threshold was calculated as the threshold value after treatment divided by the basal threshold value and multiplied by 100.
Figure 1.
(A) Threshold of VMR determined by the distended balloon volume (ml) inducing apparent sustained abdominal muscle contractions. Demonstrable EMG recording is depicted. The threshold of VMR was 0.3 ml in this animal. (B) Schematic representation of the experimental protocol to explore the effects of SB on LPS-induced visceral changes. Colonic instillation of SB or the vehicle was performed for 3 consecutive days. The basal VMR threshold was measured 30 min after surgery for implanting EMG electrodes and placing the balloon, i.e. 24 h after the last enema. Then, LPS (1 mg/kg) or the vehicle was administered. Later, surgery and balloon placement were performed again, and the threshold was measured 3 h after injection followed by the measurement of colonic permeability. (C) Protocol for determining the effects of SB on repeated WAS-induced visceral changes. Thirty minutes after SB or the vehicle enema, the rats were subjected to either WAS or sham stress for 1 h. These treatments, i.e. enema and stress, were performed for 3 consecutive days. The basal threshold was measured before the initial treatments. The second threshold measurement was performed 24 h after the last stress session followed by the measurement of colonic permeability. (D) Effects of SB on CRF (50 µg/kg)-induced visceral changes were also determined. The measurements of the second threshold and colonic permeability were examined 4 h after injection of CRF or the vehicle.
Measurement of colonic permeability
Colonic permeability measurement was performed as described previously6. Briefly, the rats were anaesthetised by the administration of the mixture of medetomidine hydrochloride (Orion Pharma Ltd., Dhaka, Bangladesh; 0.15 mg/kg), midazolam (Sandoz, Tokyo, Japan; 2 mg/kg) and butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan; 2.5 mg/kg) intraperitoneally (i.p.) and underwent laparotomy. The colon was ligated at the junction with the cecum, and an open-tipped catheter (3-Fr, Atom, Tokyo, Japan) was inserted into the proximal colon through the hole made by a puncture using needle. The colon was gently flushed with PBS using the catheter in order to wash out all stools, and later, another ligation was added on the colon at approximately 4 cm from the proximal one. Then, 1 ml of 1.5% Evans blue in PBS was instilled into the colon segment through the catheter. After 15 min, the rats were euthanized by terminal exsanguination under deep isoflurane anesthesia. The colons were excised, washed with PBS and 6 mM N-acetyl-cysteine, and placed in 2 ml N,N-dimethylformamide for 12 h. Permeability was calculated by measuring the Evans blue concentration in the supernatant using a spectrophotometer at 610 nm.
Butyrate enema
The non-anesthetized rats placed in Bollmann cages underwent intra-anal insertion of a catheter (JU-SB0601; Terumo) with the distal end positioned 7 cm proximal to the anus. As the catheter was customized, the solution can diffuse from the distal end of the catheter. The rats received 0.5 ml SB solution (2, 6 and 16 mmol/l) once daily, i.e. at doses of 0.37, 1.1 and 2.9 mg/kg/day, for 3 consecutive days through the catheter. The controls were treated with the vehicle (PBS).
Stress protocol
Exposure to WAS was performed as described previously22. Briefly, rats were individually placed on a plastic platform (height, 8 cm; length, 6 cm; width, 6 cm) positioned in the middle of a plastic cage, which was filled with water up to 7 cm of the platform height. Control animals were individually placed in the same plastic cage, which was not filled with water (sham stress).
Experimental procedures
Six groups of five to eleven rats were used. After 24 h from the last colonic instillation of SB with different concentrations or PBS, the basal VMR threshold was assessed. Next, the electrodes and distention balloon were removed, and either the vehicle or LPS (1 mg/kg) was s.c. injected (Fig. 1B). The rats were returned to their home cages, and after 3 h, the second measurement of threshold was implemented followed by the measurement of colonic permeability5.
Next, four groups of five to six rats were used to evaluate the effects of SB on WAS model (Fig. 1C). First, the basal threshold was measured. Then SB or the vehicle enema followed by WAS or sham stress for 1 h was implemented for 3 consecutive days. The measurement of the second threshold followed by colonic permeability was performed 24 h after undergoing the last stress session7.
The effects of SB on CRF model were also tested using four groups of five to six rats. The second threshold was measured 4 h after injection of CRF (50 µg/kg, i.p.) or the vehicle followed by the measurement of colonic permeability (Fig. 1D)6.
Next, to evaluate the mechanisms of actions of SB, the effects of compound C (2 mg/kg s.c.), GW9662 (3 mg/kg s.c.), L-NAME (10 mg/kg i.p.), naloxone (1 mg/kg s.c.), sulpiride (200 mg/kg s.c.) or domperidone (10 mg/kg s.c.) were tested. The groups in these experiments consisted of five to six rats. These drugs were given together with SB or the vehicle enema.
Statistical analysis
Statistical analyses were performed using SYSTAT 13 software (Systat Software, Chicago, IL, USA). Data were presented as means ± SEM. Multiple comparisons were performed by one- or two-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test. Comparisons between the two groups were performed using Student’s t-test.
Ethical statement
For all studies, approval was obtained from the Research and Development and Animal Care Committees at Asahikawa Medical University (#17149, approved on August 2, 2017).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Results
SB blocked LPS-induced visceral allodynia and colonic hyperpermeability
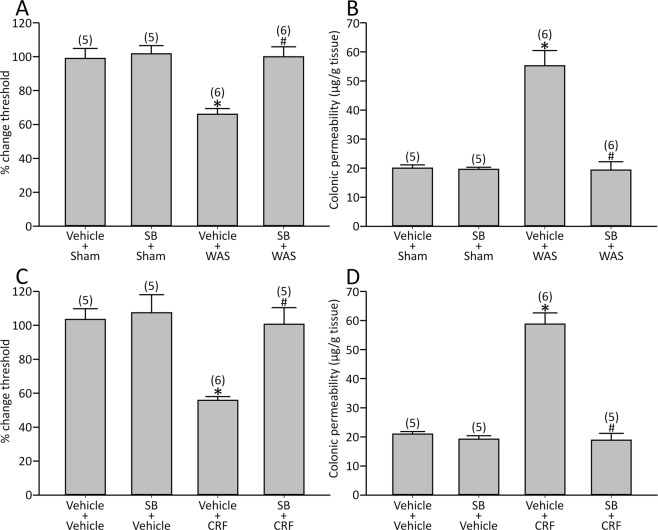
SB inhibited LPS-induced visceral allodynia in a dose-responsive manner [F(5,32) = 15.1, p < 0.05; Fig. 2A]. SB (2.9 mg/kg) fully reversed the threshold change by LPS. This dose of SB per se did not change the basal threshold of VMR (ml), i.e. before injection of LPS or the vehicle (0.62 ± 0.026 for SB, n = 10 vs. 0.63 ± 0.017 for vehicle, n = 18, p > 0.05).
Figure 2.
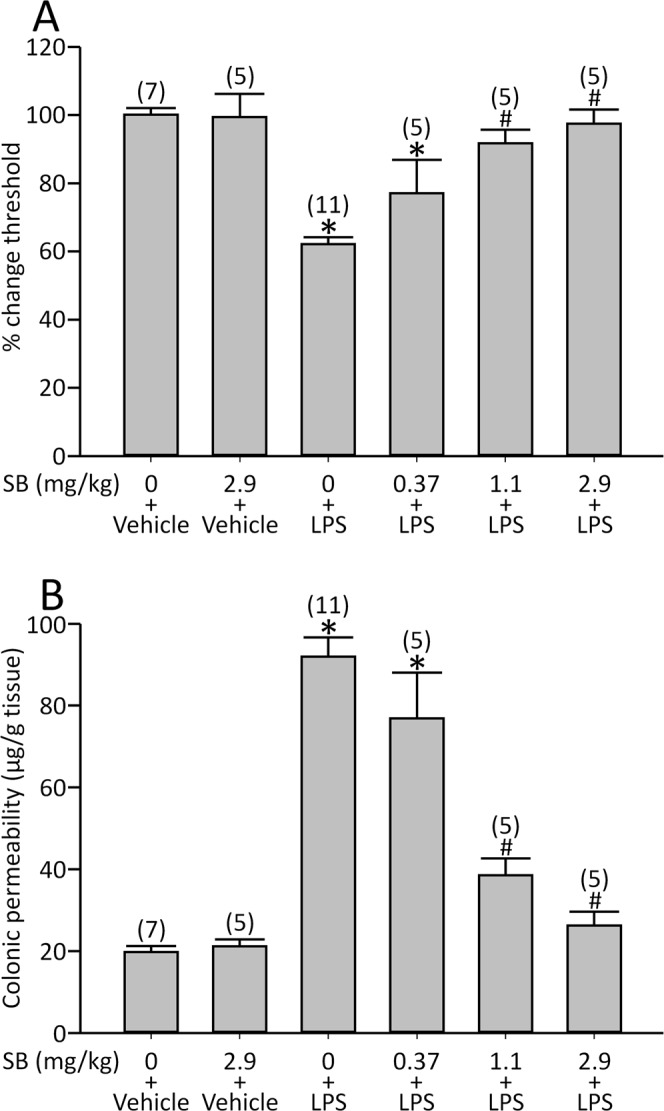
Effects of SB on LPS-induced visceral allodynia and colonic hyperpermeability. (A) LPS significantly reduced the threshold of VMR, and SB dose-dependently reversed this response. (B) LPS increased colonic permeability, which was also blocked by SB. *p < 0.05 vs. vehicle (SB 0) + vehicle, #p < 0.05 vs. vehicle (SB 0) + LPS by one-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5−11).
Additionally, SB inhibited LPS-induced colonic hyperpermeability dose-dependently [F(5,32) = 42.2, p < 0.05; Fig. 2B]. According to these results, we employed 2.9 mg/kg SB for the following experiments.
SB eliminated repeated WAS- or CRF-induced visceral allodynia and colonic hyperpermeability
Visceral changes induced by repeated WAS were abolished by SB [visceral sensation: effect of WAS: F(1,18) = 11.6, p < 0.05, effect of SB: F(1,18) = 12.8, p < 0.05 and interaction between WAS and SB: F(1,18) = 9.38, p < 0.05; colonic permeability: effect of WAS: F(1, 18) = 27.2, p < 0.05, effect of SB: F(1,18) = 29.2, p < 0.05 and interaction between WAS and SB: F(1,18) = 27.9, p < 0.05; Fig. 3A,B].
Figure 3.
Effect of SB on repeated WAS- or CRF-induced visceral changes. WAS induced visceral allodynia and colonic hyperpermeability, which were reversed by SB (A,B). SB also blocked CRF-induced visceral changes (C,D). Sham, sham stress. *p < 0.05 vs. vehicle + sham or vehicle + vehicle, #p < 0.05 vs. vehicle + WAS or vehicle + CRF by two-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5−6).
SB also blocked these CRF-induced visceral changes [visceral sensation: effect of CRF: F(1,17) = 12.9, p < 0.05, effect of SB: F(1,17) = 10.4, p < 0.05 and interaction between CRF and SB: F(1,17) = 7.29, p < 0.05; colonic permeability: effect of CRF: F(1,17) = 52.2, p < 0.05, effect of SB: F(1,17) = 64.6, p < 0.05 and interaction between CRF and SB: F(1,17) = 54.2, p < 0.05; Fig. 3C,D].
Compound C reversed the effects of SB on the LPS model
As SCFAs were reported to activate AMP-activated protein kinase (AMPK) signalling23, the role of AMPK on the effects of SB was determined. First, we evaluated the effects of compound C, an AMPK inhibitor, on the basal threshold, and the LPS-induced visceral allodynia and colonic hyperpermeability. Three s.c. injections of compound C altered neither the basal threshold (0.60 ± 0.020 ml for compound C, n = 10 vs. 0.60 ± 0.015 ml for vehicle, n = 10, p > 0.05) nor the visceral response induced by LPS [visceral sensation: effect of compound C: F(1,16) = 0.028, p > 0.05, effect of LPS: F(1,16) = 122.8, p < 0.05 and interaction between compound C and LPS: F(1,16) = 0.46, p > 0.05; colonic permeability: effect of compound C: F(1,16) = 0.017, p > 0.05, effect of LPS: F(1,16) = 37.9, p < 0.05 and interaction between compound C and LPS: F(1,16) = 0.025, p > 0.05].
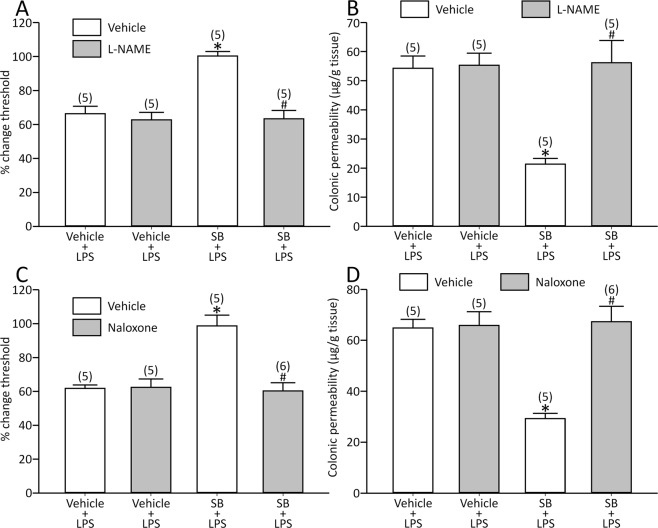
Then we performed separate series of experiments to explore the role of AMPK signalling on the effects of SB, and compound C reversed the effects of SB on the LPS model [visceral sensation: effect of compound C: F(1,17) = 12.3, p < 0.05, effect of SB: F(1,17) = 18.8, p < 0.05 and interaction between compound C and SB: F(1,17) = 19.0, p < 0.05; colonic permeability: effect of compound C: F(1,17) = 6.74, p < 0.05, effect of SB: F(1,17) = 33.0, p < 0.05 and interaction between compound C and SB: F(1,17) = 8.27, p < 0.05; Fig. 4A,B].
Figure 4.
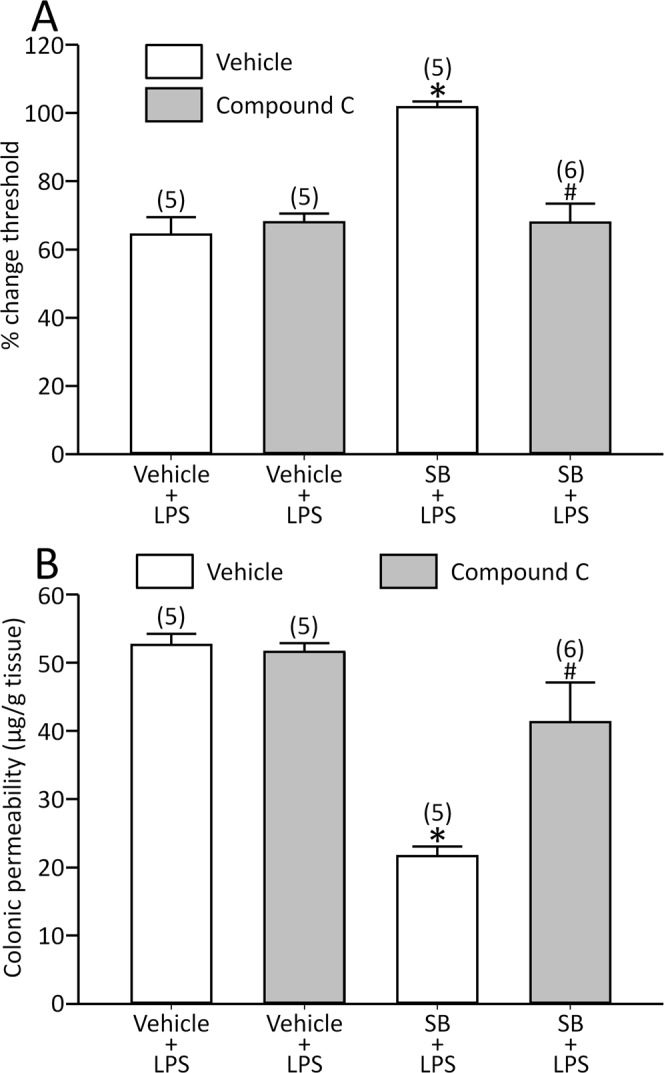
Compound C reversed the effects of SB on LPS-induced visceral allodynia (A) and colonic hyperpermeability (B). *p < 0.05 vs. vehicle + vehicle + LPS, #p < 0.05 vs. vehicle + SB + LPS by two-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5−6).
GW9662 eliminated the effects of SB on the LPS model
A butyrate-releasing derivative was shown to activate peroxisome proliferator-activated receptor-γ (PPAR-γ)24, and its role was explored. GW9662, a PPAR-γ antagonist, modified neither the basal threshold (0.60 ± 0.016 ml for GW9662, n = 12 vs. 0.60 ± 0.025 ml for vehicle, n = 10, p > 0.05) nor the response induced by LPS [visceral sensation: effect of GW9662: F(1,18) = 0.14, p > 0.05, effect of LPS: F(1,18) = 58.3, p < 0.05 and interaction between GW9662 and LPS: F(1,18) = 0.015, p > 0.05; colonic permeability: effect of GW9662: F(1,18) = 0.044, p > 0.05, effect of LPS: F(1,18) = 169.5, p < 0.05 and interaction between GW9662 and LPS: F(1,18) = 0.12, p > 0.05].
The drug abolished the effects of SB [visceral sensation: effect of GW9662: F(1,17) = 4.95, p < 0.05, effect of SB: F(1,17) = 6.82, p < 0.05 and interaction between GW9662 and SB: F(1,17) = 6.05, p < 0.05; colonic permeability: effect of GW9662: F(1,17) = 12.3, p < 0.05, effect of SB: F(1,17) = 9.78, p < 0.05 and interaction between GW9662 and SB: F(1,17) = 12.0, p < 0.05; Fig. 5A,B].
Figure 5.
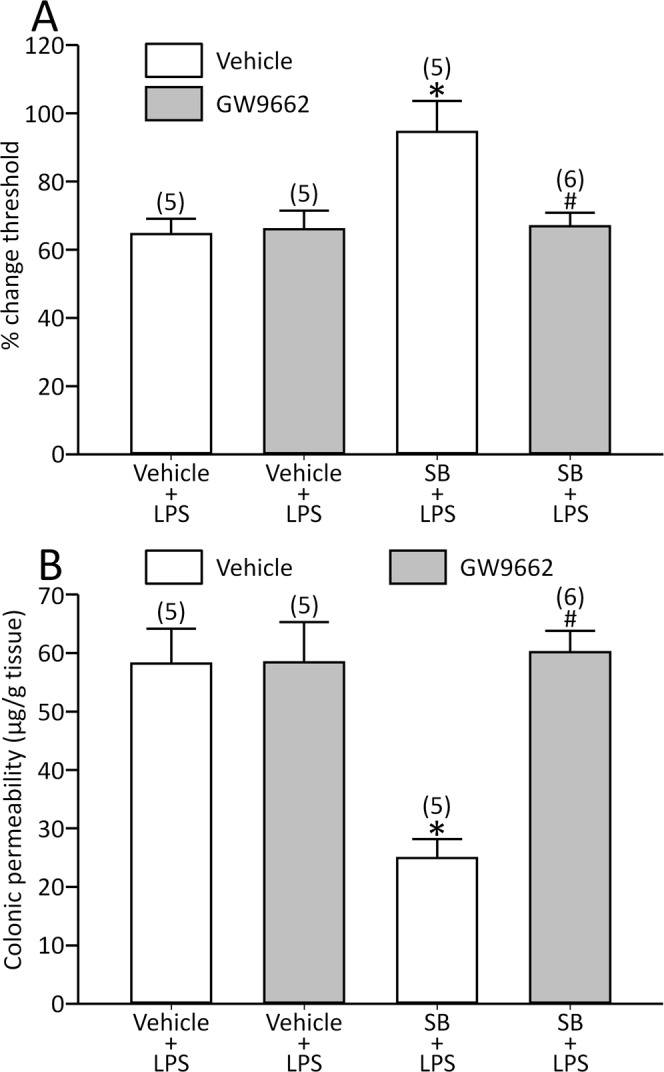
GW9662 blocked the effects of SB on LPS-induced visceral allodynia (A) and colonic hyperpermeability (B). *p < 0.05 vs. vehicle + vehicle + LPS, #p < 0.05 vs. vehicle + SB + LPS by two-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5−6).
L-NAME or naloxone reversed the effects of SB on the LPS model
Butyrate was shown to increase nitric oxide (NO) production from macrophages25, and NO is known to modulate pain response26,27. Therefore, we determined the role of NO. L-NAME, an NO synthesis inhibitor, did not alter either the basal threshold (0.60 ± 0.017 ml for L-NAME, n = 10 vs. 0.61 ± 0.016 ml for vehicle, n = 10, p > 0.05) or the response by LPS [visceral sensation: effect of L-NAME: F(1,16) = 0.24, p > 0.05, effect of LPS: F(1,16) = 88.7, p < 0.05 and interaction between L-NAME and LPS: F(1,16) = 0.152, p > 0.05; colonic permeability: effect of L-NAME: F(1,16) = 0.027, p > 0.05, effect of LPS: F(1,16) = 84.7, p < 0.05 and interaction between L-NAME and LPS: F(1,16) = 0.0001, p > 0.05].
At the same time, the drug fully reversed the effects of SB [visceral sensation: effect of L-NAME: F(1,16) = 22.9, p < 0.05, effect of SB: F(1,16) = 16.7, p < 0.05 and interaction between L-NAME and SB: F(1,16) = 15.5, p < 0.05; colonic permeability: effect of L-NAME: F(1,16) = 13.2, p < 0.05, effect of SB: F(1,16) = 10.6, p < 0.05 and interaction between L-NAME and SB: F(1,16) = 11.7, p < 0.05; Fig. 6A,B].
Figure 6.
L-NAME abolished the effects of SB on LPS-induced visceral changes (A,B). Naloxone also reversed the effects of SB (C,D). *p < 0.05 vs. vehicle + vehicle + LPS, #p < 0.05 vs. vehicle + SB + LPS by two-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5−6).
It is well known that opioid signalling is involved in visceral sensation28, and its role on the effects of butyrate was determined. Naloxone, an opioid receptor antagonist, altered neither the basal threshold (0.62 ± 0.013 ml for naloxone, n = 10 vs. 0.61 ± 0.018 ml for vehicle, n = 10, p > 0.05) nor the response by LPS [visceral sensation: effect of naloxone: F(1,16) = 0.059, p > 0.05, effect of LPS: F(1,16) = 99.6, p < 0.05 and interaction between naloxone and LPS: F(1,16) = 0.033, p > 0.05; colonic permeability: effect of naloxone: F(1,16) = 0.024, p > 0.05, effect of LPS: F(1,16) = 189.8, p < 0.05 and interaction between naloxone and LPS: F(1,16) = 0.03, p > 0.05].
Additionally, naloxone abolished the effects of SB [visceral sensation: effect of naloxone: F(1,17) = 15.2, p < 0.05, effect of SB: F(1,17) = 12.9, p < 0.05 and interaction between naloxone and SB: F(1,17) = 16.3, p < 0.05; colonic permeability: effect of naloxone: F(1,17) = 17.0, p < 0.05, effect of SB: F(1,17) = 13.0, p < 0.05 and interaction between naloxone and SB: F(1,17) = 15.3, p < 0.05; Fig. 6C,D].
Sulpiride abolished but domperidone did not alter the effects of SB on the LPS model
We have previously demonstrated that central dopamine signalling is an important modulator of visceral pain29, and its role on the effects of butyrate was determined. Sulpiride, a dopamine D2 receptor antagonist, did not modify the basal threshold (0.62 ± 0.015 ml for sulpiride, n = 10 vs. 0.62 ± 0.019 ml for vehicle, n = 10, p > 0.05) and the response by LPS [visceral sensation: effect of sulpiride: F(1,16) = 0.123, p > 0.05, effect of LPS: F(1,16) = 77.7, p < 0.05 and interaction between sulpiride and LPS: F(1,16) = 0.246, p > 0.05; colonic permeability: effect of sulpiride: F(1,16) = 1.83, p > 0.05, effect of LPS: F(1,16) = 190.9, p < 0.05 and interaction between sulpiride and LPS: F(1,16) = 1.93, p > 0.05].
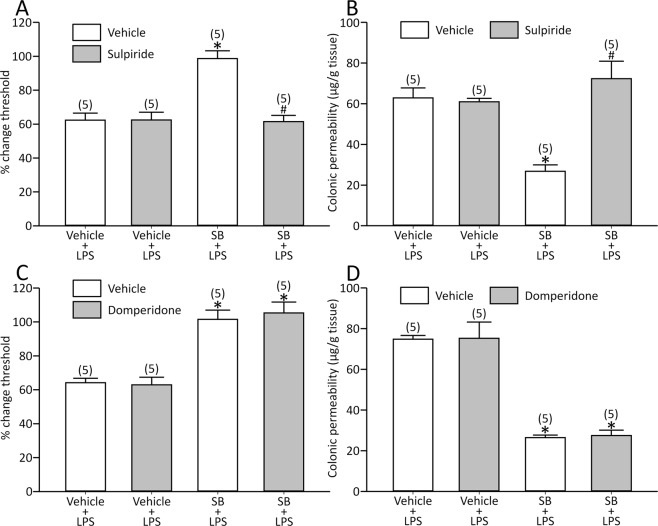
The drug reversed the effects of SB [visceral sensation: effect of sulpiride: F(1,16) = 18.8, p < 0.05, effect of SB: F(1,16) = 17.2, p < 0.05 and interaction between sulpiride and SB: F(1,16) = 19.0, p < 0.05; colonic permeability: effect of sulpiride: F(1,16) = 17.2, p < 0.05, effect of SB: F(1,16) = 5.52, p < 0.05 and interaction between sulpiride and SB: F(1,16) = 20.5, p < 0.05; Fig. 7A,B].
Figure 7.
Sulpiride eliminated the effects of SB on LPS-induced visceral changes (A and B), whereas domperidone did not modify the effects (C and D). *p < 0.05 vs. vehicle + vehicle + LPS, #p < 0.05 vs. vehicle + SB + LPS by two-way ANOVA followed by Tukey’s HSD test. Each column represents mean ± SEM. The number of rats examined is shown in parentheses (n = 5).
Domperidone, a peripheral dopamine D2 receptor antagonist, did not modify the basal threshold (0.64 ± 0.026 ml for domperidone, n = 10 vs. 0.63 ± 0.013 ml for vehicle, n = 10, p > 0.05) and the response by LPS [visceral sensation: effect of domperidone: F(1,16) = 0.256, p > 0.05, effect of LPS: F(1,16) = 108.8, p < 0.05 and interaction between domperidone and LPS: F(1,16) = 0.047, p > 0.05; colonic permeability: effect of domperidone: F(1,16) = 0.228, p > 0.05, effect of LPS: F(1,16) = 111.8, p < 0.05 and interaction between domperidone and LPS: F(1,16) = 0.047, p > 0.05].
At the same time, the drug did not modify the effects of SB [visceral sensation: effect of domperidone: F(1,16) = 0.067, p > 0.05, effect of SB: F(1,16) = 64.8, p < 0.05 and interaction between domperidone and SB: F(1,16) = 0.26, p > 0.05; colonic permeability: effect of domperidone: F(1,16) = 0.03, p > 0.05, effect of SB: F(1,16) = 124.8, p < 0.05 and interaction between domperidone and SB: F(1,16) = 0.005, p > 0.05; Fig. 7C,D].
Discussion
The effects of butyrate on visceral sensation are controversial. Bourdu et al.13 reported that SB enema induced visceral hypersensitivity in rats for the first time and advocated that this response by SB could be used for an experimental animal model of IBS. After that, this phenomenon has been well reconfirmed by several other researchers, and it is now recognized as one of the common rat IBS models14–16. In contrast, a few human studies showed that butyrate improved abdominal pain in patients with IBS30,31. Additionally, repeated WAS decreases butyrate-producing microbiota, and visceral hypersensitivity induced by stress is alleviated by the supplementation of butyrate-producing bacteria in rats32, which may be indirect evidence suggesting the ameliorative effect of butyrate in visceral pain.
In the study of Bourdu et al.13, the rats underwent 1 ml SB enema twice daily for 3 consecutive days, and the tested concentrations of SB solution ranged from 8 to 1000 mmol/l. Under this protocol, SB induced visceral allodynia in a dose-responsive manner. Meanwhile, butyrate concentrations in the cecal fluid and colonic contents of rats, pigs and monkeys were reported to be 3 to 7 mmol/l when diets contained little or no fermentable dietary fiber, and as high as 40 mmol/l when the diets provided ample fermentable fiber33. Given the evidence above, the concentrations of SB solution in that study seemed to be extremely high. Thus, we used the physiologic concentration of SB solution for enema, i.e. 2, 6 and 16 mmol/l. The doses of SB using these solutions were 0.37, 1.1 and 2.9 mg/kg/day, whereas the doses in that study ranged from 8.4 to 1048 mg/kg/day, which means that the highest tested dose in the current study equaled only about one third of the minimum tested dose by Bourdu et al.
The current study showed for the first time that SB abolished visceral allodynia in rat IBS models, which was completely different from the findings by the previous studies above. Our results strongly suggest that a high dose of SB may induce visceral hypersensitivity, and physiologic concentrations of SB may improve visceral pain. Intraluminal administration of physiologic doses of butyrate into the distal colon for 7 days decreases visceral pain and discomfort in response to colonic distention in healthy humans10,34, which may further support our results and the notion above.
At the same time, we also found that SB improved colonic barrier. Previous studies showed that butyrate in physiologic concentrations can enhance intestinal barrier function, but high-dose butyrate disrupts the barrier using Caco-2 cells in vitro35. These findings strongly suggest that an adequate dose of butyrate may improve visceral function.
We have recently demonstrated that LPS-, repeated WAS- or CRF-induced visceral allodynia and colonic hyperpermeability were mediated via peripheral CRF, TLR4 and the proinflammatory cytokine system5–7. The speculated mechanisms of the visceral changes in these IBS models are considered as follows6. Activating peripheral CRF receptors by stress stimulates TLR4 to alter tight junction (TJ) proteins36, thereby inducing colonic hyperpermeability. Impaired gut barrier induces bacterial translocation leading to increased LPS to trigger to release proinflammatory cytokines by activating TLR4, which induces visceral allodynia possibly through the activation of visceral afferent neurons37. At the same time, the cytokine also increases gut permeability via modifying TJ proteins38. Additionally, LPS not only stimulates TLR4 but also activates peripheral CRF receptors5. Thus, peripheral CRF and TLR4-cytokine signalling may develop a vicious cycle activating each other to induce these visceral changes.
In this scenario, visceral allodynia is considered to result from colonic hyperpermeability. In the current and our previous studies showed that visceral allodynia occurred associated with colonic hyperpermeability with no exception6,17,18,39. Moreover, Creekmore et al.40, demonstrated a positive correlation between the magnitude of visceral pain and paracellular permeability in repeated WAS model, and knockdown of occludin, one of the TJ proteins, induced intestinal hyperpermeability with visceral hypersensitivity. These results further support the notion above. Thus, SB may inhibit peripheral CRF-TLR4-cytokine signaling to improve colonic barrier followed by inhibition of visceral allodynia.
Actually, butyrate was reported to inhibit the expression of proinflammatory cytokines triggered by interferon-γ in RAW 264.7 cells41. Moreover, it was also shown that high-fat diet impaired gut barrier with increased serum level of LPS and upregulated the TLR4 gene and proinflammatory cytokines in the liver, which were improved by the intragastric administration of SB in mice42.
SCFAs are known to activate AMPK in the liver and muscles23. In addition, we have very recently shown that metformin, an AMPK activator, blocked the visceral changes in the same IBS models18. Thus, we hypothesized that the effects of butyrate are mediated via AMPK, and it actually happened, i.e. compound C reversed the effects of SB. Several in vitro studies proved that LPS-induced inflammatory response was inhibited by AMPK43,44. In this context, the effects of SB may result from the suppression of cytokine signalling via AMPK.
At the same time, it was reported that butyramide, a butyrate-releasing derivative exerts an anti-inflammatory effect via the upregulation of PPAR-γ in dextran sulphate sodium-induced murine colitis24. PPAR-γ inhibits the expression of various cytokines in macrophages45, and we have also confirmed previously that activating PPAR-γ by pioglitazone abolished the visceral changes in these IBS models17. Moreover, butyrate reduces colonic paracellular permeability by PPAR-γ activation in HT-29 cells11. In the current study, the effects of SB were reversed by PPAR-γ antagonist, suggesting that butyrate may inhibit cytokine signalling via PPAR-γ to exert the effects.
It is known that there exists a link between AMPK and PPAR-γ. It was shown that LPS increased the expression of TLR4 via the suppression of PPAR-γ in endothelial EA.hy926 cells, and the activation of AMPK prevented the increase of TLR4 protein via the rescue of the decreased PPAR-γ protein46, suggesting that PPAR-γ might be a downstream effector of AMPK. In contrast, several studies showed that PPAR-γ mediates the activation of AMPK47. In this context, both signalling can modulate each other. Although both signalling modulated the effects of SB in the current study, we did not determine which signalling was upstream. Further studies are needed to explore this issue.
We also showed that the effects of SB were reversed by L-NAME or sulpiride, but not by domperidone, suggesting that they were mediated via NO and central dopamine D2 signalling. These findings may support the result that the effects of SB were mediated via AMPK signalling, because the antinociceptive effect by metformin in the LPS model was mediated via NO and central dopamine D2 pathways, which were shown in our previous study18.
It has been demonstrated that NO pathway may be both pro-nociceptive and anti-nociceptive48. Based on the present study, we would raise a hypothesis that butyrate increases NO production from macrophages25, and NO inhibits proinflammatory cytokine genes in various immune cells49, thereby improving the IBS model.
Garrido-Gil et al.50 showed that central dopaminergic depletion increased the level of IL-1β in the colon, suggesting that brain dopamine reduces the vulnerability of gut inflammation. Additionally, butyrate possibly crosses the blood brain barrier51, and SB at a dose of 300 mg/kg i.p. protects dopamine neurons to improve the motor deficit in Parkinson’s disease model52, suggesting the possibility that SB act centrally to modulate dopamine signalling to exert the effects by suppressing cytokine.
However, we did not think that SB directly act on the brain in the current study for the following reasons. Butyrate is the main energy source for colonocytes23, and the majority of the luminal butyrate is consumed in the gut resulting in relatively low concentration of butyrate in portal vein. Then it is metabolized in liver and its concentration becomes lower in systemic circulation53. Moreover, the brain uptake of intravenous administration of butyrate was reported to be only less than 0.006% in baboons54. Therefore, the amount of brain uptake is considered to be negligible in the current experimental settings using physiologic concentration of SB enema. Butyrate is known to activate vagal afferents55, and activation of upper gut-innervating vagal afferents induces the release of dopamine from nigral neurons56. In this context, it is reasonable to think that SB may act peripherally and indirectly activate brain dopamine D2 receptor.
As it is known that opioid signalling is involved in altered visceral sensory function by stress28, the role of opioid receptor was determined. Opioid receptors are expressed in immune cells and modulate cytokine response57. We found that naloxone blocked the effects of SB. There is no direct report indicating that butyrate activates opioid signalling, but Pol et al.58 demonstrated that NO upregulated the µ-opioid receptor gene transcription in mice gut during intestinal inflammation. Moreover, NO stimulated the neuronal release of endogenous opioids to stimulate opioid receptors in the brain and the spinal cord26,27. These results suggest that butyrate may activate opioid receptors via NO to exert the effects. In this context, both peripheral and central opioid signalling may contribute to the effects of butyrate.
This study has several limitations. We did not show the direct evidence that SB inhibits the production of cytokines. Although the visceral changes observed in these IBS models were mediated via IL-1 or IL-65–7, the colonic mucosal levels of the cytokines were not elevated in the current experimental settings (data not shown). Therefore, we could not test the direct effect of SB on cytokine signalling. Since cytokines may act locally to visceral afferents or TJ inducing visceral changes, increased cytokines in the colonic mucosa were not prerequisite for these changes. In other words, activating local cytokine signaling and the elevated level of cytokines are different issues. Moreover, the sources of the cytokines responsible for the visceral changes were not determined. It is known that various cells other than macrophages or monocytes, such as fibroblasts, endothelial cells, neuronal cells and smooth muscle cells, secrete the cytokines59. Additionally, the role of opioid receptor subtypes on the effects of butyrate was not determined either. Before determining the precise mechanisms of actions by SB in a molecular or cellular level, we should clear the issue above.
In spite of these limitations, our results clearly showed that butyrate improved visceral changes in IBS models. Recently, several studies have been demonstrated that perturbations of the intestinal microbiota play a role in the pathophysiology of IBS. Although it is not definitely known that an altered microbiota is a cause or a consequence, it may be involved with the changes in intestinal motility, visceral sensation, mucosal barrier and the expression of pattern recognition receptors60. The microbiota generates and releases molecules that can signal to distant organs, which may induce these changes. Together with the evidence above, our results suggest that butyrate is one of the signalling molecules between the microbiota and host in the pathophysiology of IBS.
In conclusion, SB enema blocked visceral allodynia and colonic hyperpermeability in rat IBS models, which may be AMPK and PPAR-γ dependent, and mediated by the NO, central dopamine D2 and opioid pathways. Butyrate may be useful for the treatment of IBS.
Data availability
All data generated or analysed during this study this study are included in this published article.
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science KAKENHI, Grant-in-Aid for Scientific Research (C) [26460287 (TN) and 26460955 (TO)], Scientific Research on Innovative Areas [26120012 (KT)], and the research grant from the Akiyama Life Science Foundation (TN).
Author contributions
T.N. designed and performed the experiments, involved in data acquisition and analysis, and drafted the manuscript. S.M. and R.N. helped in data acquisition. K.T. and T.O. involved in study concept and design, and also methodology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr. Gastroenterol. Rep. 2009;11:270–277. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J. Gastroenterol. 2015;50:819–830. doi: 10.1007/s00535-015-1086-8. [DOI] [PubMed] [Google Scholar]
- 3.Dlugosz A, et al. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2015;27:1747–1754. doi: 10.1111/nmo.12670. [DOI] [PubMed] [Google Scholar]
- 4.Liebregts T, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J. Gastroenterol. 2017;52:72–80. doi: 10.1007/s00535-016-1208-y. [DOI] [PubMed] [Google Scholar]
- 6.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Altered colonic sensory and barrier functions by CRF: roles of TLR4 and IL-1. J. Endocrinol. 2018;239:241–252. doi: 10.1530/joe-18-0441. [DOI] [PubMed] [Google Scholar]
- 7.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Repeated water avoidance stress induces visceral hypersensitivity; role of IL-1, IL-6 and peripheral corticotropin-releasing factor. J. Gastroenterol. Hepatol. 2017;32:1958–1965. doi: 10.1111/jgh.13787. [DOI] [PubMed] [Google Scholar]
- 8.Gibson P, Rosella O. Interleukin 8 secretion by colonic crypt cells in vitro: response to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut. 1995;37:536–543. doi: 10.1136/gut.37.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa H, et al. Butyrate modulates gene and protein expression in human intestinal endothelial cells. Biochem. Biophys. Res. Commun. 2003;309:512–519. doi: 10.1016/j.bbrc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Vanhoutvin SA, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009;21:952–e976. doi: 10.1111/j.1365-2982.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M, Suzuki Y, Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem. Biophys. Res. Commun. 2002;293:827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourdu S, et al. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology. 2005;128:1996–2008. doi: 10.1053/j.gastro.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Long X, et al. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 2018;30:e13227. doi: 10.1111/nmo.13227. [DOI] [PubMed] [Google Scholar]
- 15.Matricon J, et al. Peripheral contribution of NGF and ASIC1a to colonic hypersensitivity in a rat model of irritable bowel syndrome. Neurogastroenterol. Motil. 2013;25:e740–754. doi: 10.1111/nmo.12199. [DOI] [PubMed] [Google Scholar]
- 16.Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466–1474. doi: 10.1136/gutjnl-2012-302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Pioglitazone improves visceral sensation and colonic permeability in a rat model of irritable bowel syndrome. J. Pharmacol. Sci. 2018;139:46–49. doi: 10.1016/j.jphs.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Nozu T, et al. Metformin inhibits visceral allodynia and increased gut permeability induced by stress in rats. J. Gastroenterol. Hepatol. 2019;34:186–193. doi: 10.1111/jgh.14367. [DOI] [PubMed] [Google Scholar]
- 19.Nozu T, et al. Lovastatin inhibits visceral allodynia and increased colonic permeability induced by lipopolysaccharide or repeated water avoidance stress in rats. Eur. J. Pharmacol. 2017;818:228–234. doi: 10.1016/j.ejphar.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Naruse T, Amano H, Koizumi Y. Possible involvement of dopamine D-1 and D-2 receptors in diazepam-induced hyperphagia in rats. Fundam. Clin. Pharmacol. 1991;5:677–693. doi: 10.1111/j.1472-8206.1991.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 21.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 22.Martínez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J. Pharmacol. Exp. Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- 23.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simeoli R, et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharmacol. 2017;174:1484–1496. doi: 10.1111/bph.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen LN, Lopes LC, Cordero RJ, Nosanchuk JD. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J. Antimicrob. Chemother. 2011;66:2573–2580. doi: 10.1093/jac/dkr358. [DOI] [PubMed] [Google Scholar]
- 26.Cahill, F. J., Ellenberger, E. A., Mueller, J. L., Tseng, L. F. & Quock, R. M. Antagonism of nitrous oxide antinociception in mice by intrathecally administered antisera to endogenous opioid peptides. J. Biomed. Sci., 7, 299–303, doi:25463 (2000). [DOI] [PubMed]
- 27.Chung E, et al. Dynorphin-mediated antinociceptive effects of L-arginine and SIN-1 (an NO donor) in mice. Brain Res. Bull. 2006;70:245–250. doi: 10.1016/j.brainresbull.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Larauche M, et al. Visceral analgesia induced by acute and repeated water avoidance stress in rats: sex difference in opioid involvement. Neurogastroenterol. Motil. 2012;24:1031–e1547. doi: 10.1111/j.1365-2982.2012.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okumura T, et al. Involvement of the dopaminergic system in the central orexin-induced antinociceptive action against colonic distension in conscious rats. Neurosci. Lett. 2015;605:34–38. doi: 10.1016/j.neulet.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Banasiewicz T, et al. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013;15:204–209. doi: 10.1111/j.1463-1318.2012.03152.x. [DOI] [PubMed] [Google Scholar]
- 31.Zaleski A, Banaszkiewicz A, Walkowiak J. Butyric acid in irritable bowel syndrome. Prz Gastroenterol. 2013;8:350–353. doi: 10.5114/pg.2013.39917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, J. et al. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol., 10.1111/jgh.14536 (2018). [DOI] [PMC free article] [PubMed]
- 33.Fitch MD, Fleming SE. Metabolism of short-chain fatty acids by rat colonic mucosa in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277:G31–40. doi: 10.1152/ajpgi.1999.277.1.G31. [DOI] [PubMed] [Google Scholar]
- 34.Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate- algesic or analgesic? Neurogastroenterol. Motil. 2011;23:975–979. doi: 10.1111/j.1365-2982.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, et al. Stress-Derived Corticotropin Releasing Factor Breaches Epithelial Endotoxin Tolerance. PLoS One. 2013;8:e65760. doi: 10.1371/journal.pone.0065760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nozu Tsukasa, Miyagishi Saori, Nozu Rintaro, Takakusaki Kaoru, Okumura Toshikatsu. Dehydroepiandrosterone sulfate improves visceral sensation and gut barrier in a rat model of irritable bowel syndrome. European Journal of Pharmacology. 2019;852:198–206. doi: 10.1016/j.ejphar.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Creekmore AL, Hong S, Zhu S, Xue J, Wiley JW. Chronic stress-associated visceral hyperalgesia correlates with severity of intestinal barrier dysfunction. Pain. 2018;159:1777–1789. doi: 10.1097/j.pain.0000000000001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharmacol. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Zhou D, et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017;23:60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai A, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem. Pharmacol. 2010;80:1708–1717. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 46.Sun H, et al. Interactions of TLR4 and PPARgamma, Dependent on AMPK Signalling Pathway Contribute to Anti-Inflammatory Effects of Vaccariae Hypaphorine in Endothelial Cells. Cell. Physiol. Biochem. 2017;42:1227–1239. doi: 10.1159/000478920. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, et al. Gentiopicroside Ameliorates Diabetic Peripheral Neuropathy by Modulating PPAR- Gamma/AMPK/ACC Signaling Pathway. Cell. Physiol. Biochem. 2018;50:585–596. doi: 10.1159/000494174. [DOI] [PubMed] [Google Scholar]
- 48.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011;25:243–254. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Kroncke KD, Fehsel K, Suschek C, Kolb-Bachofen V. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int. Immunopharmacol. 2001;1:1407–1420. doi: 10.1016/S1567-5769(01)00087-X. [DOI] [PubMed] [Google Scholar]
- 50.Garrido-Gil P, Rodriguez-Perez AI, Dominguez-Meijide A, Guerra MJ, Labandeira-Garcia JL. Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models. Mol. Neurobiol. 2018;55:7297–7316. doi: 10.1007/s12035-018-0937-8. [DOI] [PubMed] [Google Scholar]
- 51.Stilling RM, et al. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behav. Brain Res. 2015;291:306–314. doi: 10.1016/j.bbr.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 53.Hamer HM, et al. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim SW, et al. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. Nucl. Med. Biol. 2013;40:912–918. doi: 10.1016/j.nucmedbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G907–915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 56.Han W, et al. A Neural Circuit for Gut-Induced Reward. Cell. 2018;175:665–678 e623. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45:9–24. doi: 10.1007/s00726-011-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pol O, et al. The involvement of nitric oxide in the enhanced expression of mu-opioid receptors during intestinal inflammation in mice. Br. J. Pharmacol. 2005;145:758–766. doi: 10.1038/sj.bjp.0706227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang JM, An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study this study are included in this published article.