Abstract
To better understand the disruptions of transcriptional regulations and gene expression in lung cancers, we constructed a multi-omics catalogue of the responses of lung cancer cells to a series of chemical compounds. We generated and analyzed 3,240 RNA-seq and 3,393 ATAC-seq libraries obtained from 23 cell lines treated with 95 well-annotated compounds. To demonstrate the power of the created multi-omics resource, we attempted to identify drugs that could induce the designated changes alone or in combination. The basal multi-omics information was first integrated into co-expression modules. Among these modules, we identified a stress response module that may be a promising drug intervention target, as new combinations of compounds that could be used to regulate this module and the consequent phenotypic appearance of cancer cells have been identified. We believe that the multi-omics profiles generated in this study and the strategy used to stratify them will lead to more rational and efficient development of anticancer drugs.
Subject terms: Epigenomics, Transcriptomics, Cancer genomics, Target identification
Introduction
Cancer clinical sequencing analyses at a varying scales identify the genotypes of cancers, including the driver mutations in lung adenocarcinoma cells1,2. These mutations are subjected to further analysis as potential targets for molecular targeting drugs. For example, EGFR tyrosine kinase inhibitors (e.g., gefitinib and erlotinib) and ALK inhibitors (e.g., crizotinib) are administered to patients with EGFR mutations and ALK fusions, respectively3–5. Suitable therapeutic approaches based on the genomic mutation patterns of each cancer have been successful. Nevertheless, approximately 30% of patients with lung adenocarcinoma do not benefit from this approach because appropriate molecular targeting drugs are not available6.
To alleviate shortages in the repertoire of chemical compounds that may serve as “seeds” for eventual drug development, current approaches utilize “high-throughput screening” which is becoming more expensive. Even when starting with millions of compounds, eventual success in obtaining a desirable drug cannot always be guaranteed. To overcome this drawback, the intensive utilization of the accumulated knowledge of genomes, epigenomes, and the transcriptional activities of cells and their molecular networks is currently underway; for example, the Encyclopedia of DNA Elements (ENCODE) database has been used for such studies7.
Indeed, omics information for cell lines, which are frequently used for cancer research, has been rapidly accumulated8–10. By making use of panels of cancer cell lines, numerous studies have revealed the details of the associations among genotypes and phenotypes. For example, the Cancer Cell Line Encyclopedia (CCLE) and other large projects have generated data on the genotypes and cellular responses to pharmacological intervention of hundreds of cancer cell lines10–12. Furthermore, target screening systems were applied to enrich the informational resources for the panels, particularly when novel genetic vulnerabilities in the cancer cells were investigated13–16. These high-throughput screens provide useful information about how the products of individual genes or their combinations could efficiently kill cancer cells and could therefore be targeted by drugs. Moreover, the Connectivity Map (CMAP)17 project compiled a large collection of gene expression profiles of cell lines treated with drugs, which predicted the modes of actions of drugs through comparisons of the similarities among transcriptional profiles18. In particular, large L1000 CMAP datasets comprised more than one million profiles19.
Nevertheless, in these large-scale drug screening projects, the systematic integration of different layers of omics information, including epigenome status and perturbations, even when epigenome targeting drugs have been considered, have not been fully implemented. Indeed, the highly diverse phenotypes of cancer cells, which vary according to the originating patient characteristics and tumor stages, may be mediated by the diverse activities of transcriptomes and epigenomes20. Therefore, without understanding these variations, it may be impossible to develop effective anti-cancer drugs.
This study attempted to construct a basic resource for multi-omics information about cell lines and their drug perturbations. We first determined the multi-omics features of representative lung adenocarcinoma cell lines8,21,22. The resulting dataset, which we generated using whole genome sequencing, comprised the representative patterns of the driver mutations in lung adenocarcinoma, such as EGFR and KRAS point mutations, ALK and RET fusions, and the amplifications of genes such as ERRB2 and MET genes1,2,23,24. Genomic mutational information was further associated with epigenomic and transcriptomic patterns, which were measured using ChIP-seq analysis of eight chromatin marks, bisulfite sequencing of DNA methylation and RNA-seq and TSS-seq analysis25,26.
The catalogue was further expanded to include information about how the transcriptomic and epigenomic activities of cells were perturbed in response to small compounds. To enhance the data output, we developed a new system to facilitate RNA-seq and ATAC-seq.27. To extract biological interpretations from the substantial amount of created data, we further integrated the datasets of the transcriptional regulatory signatures, consisting of the genomic and metabolomic signatures and their drug-perturbation patterns. Here, by systematically exploiting in-depth multilayered cellular omics information, we generated a resource for the development of more rational and efficient drug development strategies.
Results and Discussion
Overview of the study design
The strategic scheme used in this study is illustrated in Supplementary Fig. S1. Briefly, we induced perturbations in lung cancer cell lines using drugs and observed their consequences on multi-omics variables by using RNA-seq and ATAC-seq for transcriptome and epigenome analysis, respectively (Fig. 1). We simultaneously examined cell survival after drug treatment and evaluated the associations of drug sensitivities with the omics findings using conventional methods (Fig. 2). The collected multi-omics information was integrated into coregulated transcriptome modules (Figs. 3 and 4). We further characterized the modules and found that it was possible to control certain modules based on their landscape profiles (Fig. 5). Finally, we further utilized this approach to propose the module-based stratification of lung cancers for rational drug administration by targeting cell line-specific vulnerable modules (Fig. 6).
Figure 1.
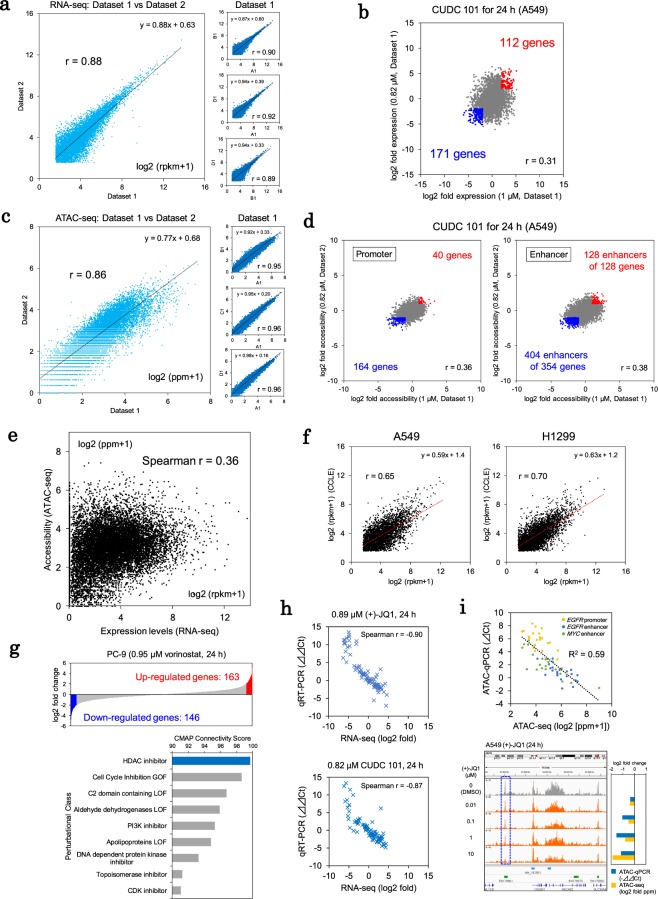
High-throughput transcriptome and epigenome perturbations. (a) Reproducibility of the RNA-seq data. The gene expression levels of the A549 cells (DMSO control) were compared. Left panel: the reproducibility among the different plates was evaluated. Right panel: the datasets within the same plates were compared. The Pearson correlation coefficients are shown in the inset. (b) Expression changes in A549 cells treated with CUDC 101. The fold changes in expression were calculated and compared between dataset 1 and dataset 2 for similar treatment conditions (≥4-fold and ≤1/4-fold changes are shown in red and blue, respectively). The Pearson correlation coefficients are shown in the inset. (c) Reproducibility of the ATAC-seq data. The signal intensities reflecting the open chromatin status are shown for the promoter regions of genes in the A549 cells in a manner similar to that shown in (a). (d) Quantification of the changes in the open chromatin status in A549 cells treated with CUDC 101. The fold changes in the intensities of the ATAC-seq signals were compared between the datasets in a manner similar to that shown in (b) (≥2 and ≤1/2-fold changes are shown in red and blue, respectively). (e) Association between the expression levels (RNA-seq) and chromatin accessibility of the promoter regions (ATAC-seq). (f) Comparison of transcriptome profiles between the in-house, high-throughput RNA-seq and public CCLE RNA-seq data. Two examples from among the 15 cell lines are shown. The Pearson correlation coefficients are shown in the inset. (g) Comparison of the transcriptome mode of action according to the CMAP signatures. The up/downregulated genes in PC-9 cells treated with vorinostat were determined as part of the signature of vorinostat (genes with ≥4 and ≤1/4-fold changes under vorinostat treatment, upper panel). The connectivity scores of the vorinostat signatures are shown for each CMAP perturbational class (lower panel). (h) qPCR validation of the RNA-seq data (see Supplementary Methods). The Spearman correlation coefficients are shown in the inset. (i) qPCR validation analysis of the ATAC-seq data (see Supplementary Methods). The intensities in terms of the open chromatin status were validated for three regulatory regions (upper panel). A549 cell ATAC-seq tags obtained during treatment with (+)-JQ1 were represented in the Integrative Genomics Viewer (IGV) (autoscale) (lower panel). The graph indicates the fold changes in the intensities of the ATAC-seq peaks (blue dashed box) (lower right).
Figure 2.
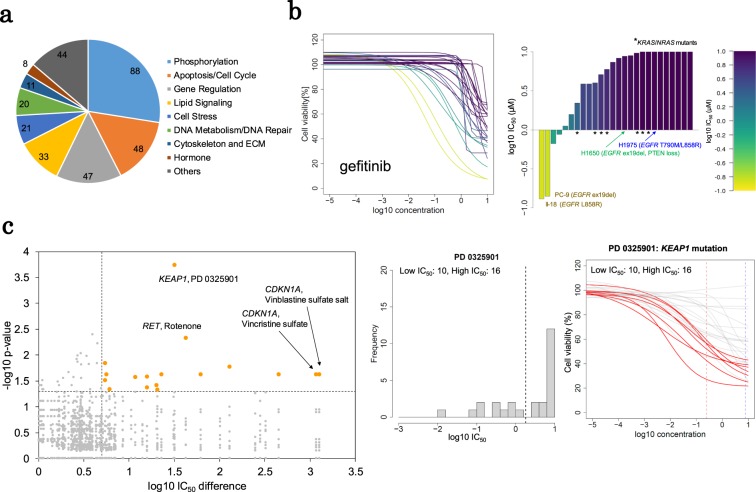
Molecular profiles predicted the drug sensitivities. (a) The classifications of 319 compounds tested for their effects on cell viability. (b) Differences in the sensitivity to gefitinib. The 8-point dose-response curve of gefitinib is shown (left). The log10 IC50 values of gefitinib are also shown in the bar graph (right). Each bar represents a cell line. The y axis indicates the IC50 values (µM) with log10 transformation. The mutational status of EGFR, KRAS and NRAS are shown in the inset. The colors are graded from yellow to purple depending on the sensitivity, and the color bar is shown in the margin. (c) Association between the mutations and drug sensitivity. In the left plot, the associated gene-compound pairs are shown. Orange dots represent significant pairs. The distribution of the IC50 values of PD 0325901 is shown in the middle graph. According to the IC50 values, the cell lines were divided into two groups (dashed line). The dose-response curve of PD 0325901 is shown in the right panel. The curves representing the KEAP1 mutant cells are red.
Figure 3.
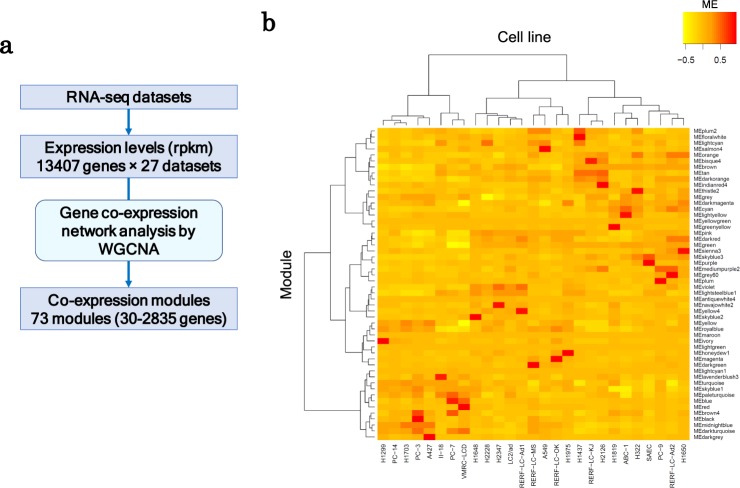
Transcriptional trends of lung adenocarcinoma based on gene co-expression networks. (a) A simple workflow of the WGCNA analysis. (b) The heat map of the module activities in the lung cancer cell lines. The eigengenes of the modules are represented as the module activities. The hierarchical clustering was conducted using Ward’s method with Pearson correlation. For the selected modules, the clustering analysis is provided in Fig. 6a.
Figure 4.
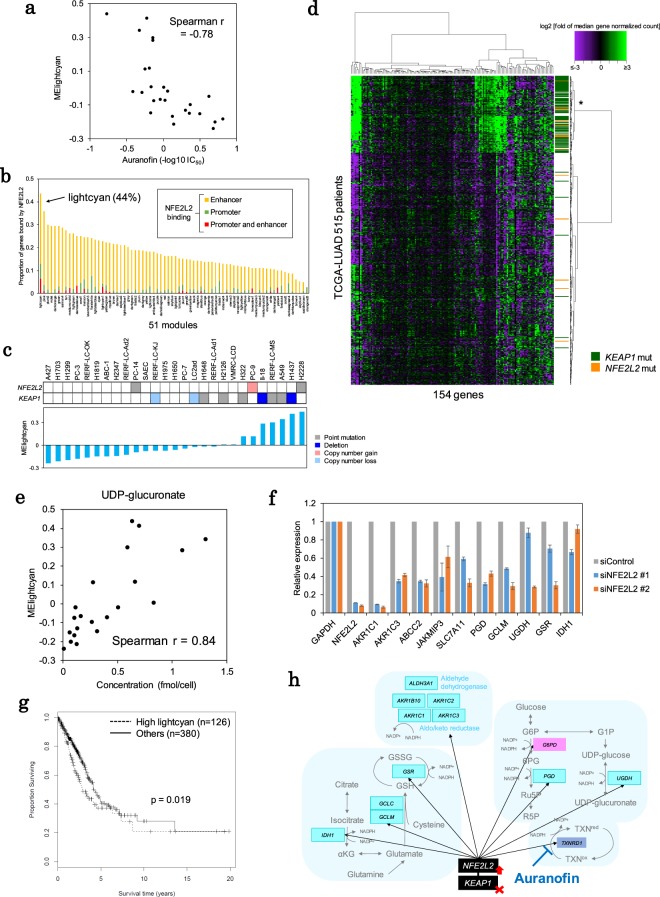
Antioxidative stress modules. (a) Sensitivity to auranofin was associated with activity of the “lightcyan (putative redox)” module. The IC50 values were compared with the module eigengenes of the module. The Spearman correlation coefficient is shown in the inset. (b) NFE2L2 targets were enriched within the “lightcyan (putative redox)” module members. The proportion of genes with NFE2L2 binding sites in the promoter or enhancer is shown for each module. (c) The association between KEAP1-NFE2L2 mutations and “lightcyan (putative redox)” module activity. The mutational patterns of KEAP1 and NFE2L2 are shown in the upper panel. The module activity is shown in the lower panel. The cell lines are ordered according to the ME for the module. (d) Expression patterns of the “lightcyan (putative redox)” module members in 515 TCGA-LUAD cases. The median fold changes in the expression levels are shown in the heat map. The mutational statuses of the KEAP1 and NFE2L2 genes are shown in the side bar in green and orange, respectively. The color key is shown in the margin. (e) Correlation between the amounts of UDP-glucuronate and “lightcyan (putative redox)” module activity. The Spearman correlation coefficient is shown in the inset. (f) NFE2L2 knockdown downregulated “lightcyan (putative redox)” module members. (g) Kaplan-Meier analysis of TCGA-LUAD cases based on the activity of the “lightcyan (putative redox)” module. Overall survival time was compared between cases with high module activity (asterisk in d) and other cases. (h) The putative redox module. Representative member genes in the “lightcyan (putative redox)” module are shown along the associated metabolic pathways.
Figure 5.
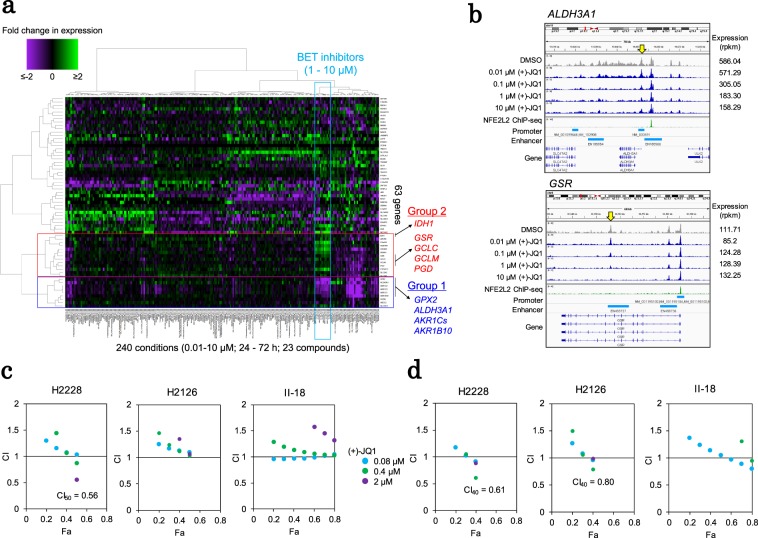
Epigenetically targeted inhibitors affect the KEAP1-NFE2L2-centered modules. (a) Transcriptome perturbation of the “lightcyan (putative redox)” module in A549 cells. Fold changes in expression associated with drug treatment (dataset-1) are shown for 63 core “lightcyan” module genes in the heat map. The color key is shown in the margin. The genes downregulated (group 1) or upregulated (group 2) by BET inhibitors are shown and are framed in red and blue, respectively. (b) Epigenomic perturbation by (+)-JQ1 treatment. The ATAC-seq patterns of ALDH3A1 (upper) and GSR (lower) were visualized using IGV (autoscale). Yellow arrows indicate the ATAC peaks with dose-dependent changes. (c) The combination indexes (CI) for dual stimulation with auranofin and (+)-JQ1 in the H2228, H2126 and II-18 cell lines. The CI-Fa (fraction affected) plot is shown. The method used for CI calculation is described in the Methods section and in Supplementary Fig. S11. The color legend is shown in the margin. (d) The CI-Fa plots of dual stimulation with (+)-JQ1 and vorinostat. The color legend is shown in c.
Figure 6.
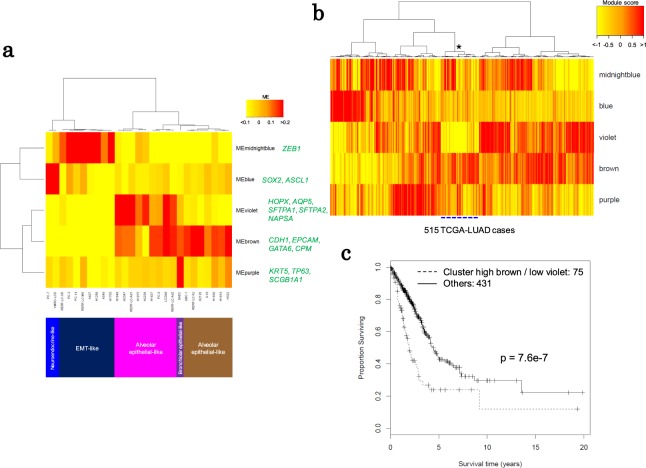
Module-based combinations for the stratification of lung cancer cell lines. (a) The lung cancer cell lines were clustered according to the module eigengenes of the five lineage-associated modules. Hierarchical clustering was performed using Ward’s method with Pearson correlation. The representative markers in the module are green. (b) TCGA-LUAD cases were classified according to the module scores of the five modules. The asterisk represents the cluster (75 cases are indicated by a dashed blue line) used in c. Hierarchical clustering of the module scores was performed using Ward’s method with Pearson correlation. (c) Kaplan-Meier analysis of the TCGA-LUAD cases divided into two groups according to the clustering of the module scores.
High-throughput transcriptome and epigenome sequencing using drug-treated cell lines
To expedite data generation for the RNA-seq and ATAC-seq analyses, we developed and employed high-throughput RNA-seq and ATAC-seq procedures (Supplementary Fig. S2). First, for RNA-seq, we utilized the Fluidigm C1 single-cell library preparation system. After cell lysis, the extracted RNA was transferred to a separate reaction chamber in the C1 system, in which subsequencing reactions were performed. We found that more cost-effective library preparation was possible using the C1 microreaction chamber without degrading the quality of the sequencing templates. A similar procedure was developed and performed for ATAC-seq (see below).
We selected 95 compounds representing well-annotated approved drugs and various molecular-targeting drugs, including epigenetic drugs and approved receptor tyrosine kinase inhibitors. We collected detailed information for four concentrations of 23 compounds after 24 h, 48 h, and 72 h. Therefore, our dataset comprised five cell lines treated with four concentrations each of 23 compounds at three time points (dataset 1) and 23 cell lines treated with a single concentration of 95 compounds at one time point (dataset 2) (Tables 1 and 2). Using dataset 1, we deeply examined the transcriptomic and epigenomic changes caused by the representative drugs, including kinase inhibitors, cytotoxic anti-cancer drugs and epigenetic targeting drugs. For the larger-scale screening of transcriptomic and epigenomic perturbation, we also analyzed dataset 2, which contained a larger number of compounds and cell lines. A full list of the compounds and the detailed experimental conditions are summarized in Supplementary Table S1.
Table 1.
General analyses of the high-throughput RNA-seq.
| Dataset | Cell line | Total data points* (% passed QC) |
Average numbers per data point | ||
|---|---|---|---|---|---|
| Mapped reads | %mapped | %intron in mapped | |||
|
Dataset-1 (4 conc. × 3 time points) |
A549 | 249 (87%) | 1,455,347 | 68% | 8% |
| H1299 | 253 (89%) | 1,275,695 | 69% | 7% | |
| H1648 | 251 (88%) | 1,319,718 | 68% | 7% | |
| H2347 | 245 (86%) | 1,414,144 | 70% | 9% | |
| II-18 | 231 (81%) | 1,433,442 | 72% | 10% | |
|
Dataset-2 (1 conc. × 1 time point) |
23 cells | 2011 (91%) | 1,295,897 | 72% | 9% |
*≥0.5 million total reads; spike in control within 2 sd; <15% intron reads.
conc.: concentration, sd: standard deviation.
Table 2.
General analyses of the high-throughput ATAC-seq.
| Total data points* (% passed QC) |
Average numbers per data point | |||||
|---|---|---|---|---|---|---|
| Mapped reads | %mapped | %chrM in mapped | MACS peaks | |||
|
Dataset-1 (4 conc. × 3 time points) |
A549 | 251 (87%) | 11,286,126 | 79% | 17% | 35,355 |
| H1299 | 269 (93%) | 5,821,704 | 69% | 65% | 17,184 | |
| H1648 | 264 (92%) | 5,943,835 | 71% | 58% | 18,571 | |
| H2347 | 276 (96%) | 4,752,301 | 70% | 64% | 26,166 | |
| II-18 | 256 (89%) | 5,838,263 | 70% | 64% | 18641 | |
|
Dataset-2 (1 conc. × 1 time point) |
23 cells | 2077 (94%) | 8,158,509 | 71% | 57% | 17,734 |
*≥10,000 MACS peaks.
conc.: concentration.
Evaluation of the RNA-seq and ATAC-seq datasets
We obtained 3,240 data points for the RNA-seq analysis. The RNA-seq data for each compound comprised an average of 1,356,520 and 1,794,226 sequencing reads in dataset 1 and dataset 2, respectively (Table 1). For quality control and initial evaluation purposes, the mapped reads were required to comprise at least 0.5 million reads containing 15% or fewer intron-mapped reads with an average reads per kilobase million (rpkm) value within ±2 standard deviations (sd) of that of the spike-in control for all wells. After the initial filtration, we further compared the expression abundances (rpkm values) of the protein-coding genes within the datasets to validate the reproducibility of the expression profiles using genes with an rpkm value ≥2 (Figs. 1a and S3). We observed a reasonably high positive correlation between the RNA-seq datasets (r = 0.90, concordance correlation coefficient (ccc) = 0.90 within each plate; r = 0.88, ccc = 0.88 between plates for A549 cells). The data was also reasonably correlated with standard RNA-seq data (Illumina TruSeq) that was previously obtained8 (r = 0.76 and r = 0.73) (Supplementary Fig. S3c). We also found that the gene expression changes were precisely represented in individual cases. An example of the changes induced by an HDAC inhibitor (CUDC 101) is shown in Fig. 1b. We observed that fold expression changes were moderately correlated between the RNA-seq datasets (r = 0.31, ccc = 0.31). The limited fraction of the genes showed significant changes even under the treatment with epigenetic targeting drugs (see also Supplementary Fig. S4).
For the ATAC-seq analysis, we selected ATAC-seq datasets with >10,000 peaks detected using MACS228, yielding 3,393 ATAC-seq datasets with an average of 8,158,509 sequencing reads. We calculated the tag densities (ppm values) for the promoter and enhancer regions to determine the chromatin accessibility. Similar to the RNA-seq analysis, we evaluated the reproducibility of the ATAC-seq analysis. We found that the tag densities of the promoters were correlated between dataset-1 and 2 under the control condition (r = 0.96, ccc = 0.95 within each plate, r = 0.86, ccc = 0.85 between plates of A549 cells) (Figs. 1c and S3). We examined the changes in open chromatin in drug-treated cells (r = 0.36, ccc = 0.32 for promoters; r = 0.38, ccc = 0.35 for enhancers between the datasets under the CUDC 101 treatment) (Fig. 1d). For the RNA-seq and ATAC-seq data, we also conducted saturation analysis of the sequencing depths to investigate whether the sequencing depths were adequate for high-throughput monitoring of drug perturbation. We confirmed that >1 M reads were enough to analyze the expression patterns in the high-throughput RNA-seq. For the ATAC-seq, the open chromatin patterns and the numbers of the detected promoters and enhancers were saturated at >2 M reads (Supplementary Fig. S5).
When we compared the RNA-seq data and the ATAC-seq data for the promoter regions, we found that they were moderately correlated (Spearman r = 0.36) (Fig. 1e). The inconsistencies between the transcriptome and epigenome data may indicate that the promoter regions likely included open chromatin regions that lacked enhancer activity under any particular condition. Additionally, other regulatory factors may have affected transcription such as the open chromatin status of the enhancer regions.
For the validation studies, we compared the RNA-seq profiles from this study with the CCLE data (Fig. 1f)10. To confirm the mode of action, as measured by our procedures, we also evaluated whether the transcriptome signatures represented in our datasets were consistent with the L1000 CMAP signatures19. Using the CMAP dataset as a reference, we confirmed that the transcriptome signatures of vorinostat were highly concordant with those of HDAC inhibitors in the CMAP dataset (Fig. 1g). These results suggest that our RNA-seq data accurately represented the transcriptome mode of action for a particular drug. By conducting qPCR validation, we investigated whether the changes in transcriptome and epigenome status during the drug treatments were accurately calculated by the sequencing and post sequencing analyses. The results of the qPCR experiments using the RNA-seq and ATAC-seq libraries were generally highly consistent with the results of the RNA-seq and ATAC-seq analyses (Fig. 1h,i; also see Supplementary Fig. S6). Based on this validation, we considered that the substantial collected datasets could sufficiently represent the cellular changes reflected in the multi-omics profiles in response to the given chemical compounds.
Multi-omics and the phenotypes of the lung adenocarcinoma cell lines
To measure the effects of the drugs on cell survival rates, we used 320 compounds, including those used for the multi-omics perturbation analysis. We measured the viability of each drug-treated cell line (Fig. 2a and Supplementary Table S2). This high-throughput screening assay provided a high average Z’ factor of 0.80 (range: 0.59–0.90). Consistent with previous studies10,11, we could evaluate the expected correlations between the genomic mutations/multi-omics statuses and the sensitivity of the cells to a series of drugs, including the EGFR inhibitors (Fig. 2b), multi tyrosine kinase inhibitors (i.e., crizotinib and vandetanib) and epigenetically targeted drugs (i.e., BET inhibitors) (Supplementary Fig. S7).
Based on the analysis of 26 representative cancer-related genes, including oncogenes, tumor suppressor genes, chromatin remodeling factors, and oncogenic fusion genes8, we divided the cell lines into two groups according to the cellular IC50 values and then compared the multi-omics statuses of the 26 genes in the two groups. This analysis identified 17 combinations associated with cell survival and genome and expression signatures (left panel, Fig. 2c). Genomic KEAP1 mutations were significantly enriched in cells sensitive to the MEK inhibitor PD0325901 (middle and right panels, Fig. 2c). In cancer cells, oncogenic MAPK signaling is aberrantly upregulated and may upregulate NFE2L2 transcription29. KEAP1 generally regulates the transcription factor NFE2L2 via a ubiquitin proteasome pathway30. When KEAP1 is mutated and NFE2L2 signaling is stabilized, cancer cells may be more addicted to MAPK oncogenic and KEAP1-NFE2L2 oxidation reduction (redox) signals and may become sensitive to MEK inhibitors. The identified KEAP1-centered module was subjected to further detailed analyses, as described in the following section (see below).
We used RNA-seq and ATAC-seq to identify the modes of action of the drugs (Fig. 1). For more detailed characterization of the drugs, we should consider the complex multi-omics status of each patient, which has not been sufficiently considered; this would strongly affect the transcriptional responses to drugs. Below, we describe transcriptome module-based target selection for the rational administration of drugs to lung cancer patients who harbor diverse genomic, epigenomic, and transcriptomic characteristics. We confirmed that potentially informative influences of drug treatments were unrecognized when assessed according to the cell survival rates.
Gene co-expression network analysis to identify gene expression modules
To integrate the omics responses with the cellular responses, which remained latent occasionally, we first evaluated the multi-omics profiles according to the transcriptional regulatory modules. Initially, based on the RNA-seq data for all cell lines included in the datasets of untreated conditions, we employed the weighted gene co-expression network analysis (WGCNA) method31. We identified 73 co-expression modules within the transcriptional signatures (Fig. 3a, Supplementary Fig. S8 and Supplementary Table S3). We expected that individual genes with similar expression patterns, which were likely regulated by shared mechanisms, would be modulated. Thus, a bird’s eye-view analysis of the responses to drug perturbation would be possible. We selected 51 modules for further analyses after excluding 22 modules that were likely included mainly due to cell line-specific copy number aberrations. WGCNA analysis offers a major advantage because the value of the “module eigengene” (ME) may be used as an indicator to represent the activity of the corresponding module (Fig. 3b). The ME is calculated based on the first principal component of the expression patterns of the gene members included in the module.
The antioxidative stress response module was associated with KEAP1-NFE2L2 mutations
We particularly focused on the “lightcyan” module, which may be controlled by a specific repertoire of drugs (Fig. 4a, see below). The “lightcyan” module comprised 158 genes, which were significantly associated with redox-related pathways enriched in “GO:0016616 oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor” (Bonferroni p = 0.0013) (Supplementary Table S4). The “lightcyan” module included genes that respond to oxidative stressors such as GSR and IDH132 (Supplementary Table S3).
To identify the transcription factor(s) controlling this module, we analyzed the ENCODE database7 to determine whether transcription factor bindings was enriched within the proximal regions of the 158 member genes. We found that NFE2L2 may represent an associated transcription factor. The binding of NFE2L2 to the promoter or enhancer regions of 67 genes (44% of the “lightcyan” module members) was indicated by the ChIP-seq data from A549 cells in the ENCODE dataset (Fig. 4b). In lung adenocarcinoma cells, either the activation of NFE2L2 or the loss of its negative regulator KEAP1 frequently occur. We found that cell lines harboring KEAP1 or NFE2L2 mutations showed significantly increased module activity as represented by their module eigengene (p = 0.0001, Wilcoxon rank sum test) (Fig. 4c). For clinical specimens, we also retrieved and analyzed the RNA-seq and whole exome sequencing datasets from the TCGA1. We analyzed the 515 clinical samples of lung adenocarcinoma (TCGA-LUAD) and found that the activity of the “lightcyan” module was strongly correlated with the mutational status of KEAP1 and NFE2L2 (Fig. 4d).
We then analyzed the functional relevance of this module. NFE2L2 is a transcription factor that is activated by oxidative stress and controls the expression of genes that encode proteins that mediate downstream redox reactions, thereby providing robust activity against oxidative stress in cancer cells33. It was also essential to analyze metabolites for understanding these redox reactions. We measured abundances of 113 metabolites by metabolome profiling (see Methods). When we analyzed the metabolic profiles of the cell lines, we found that seven metabolites were significantly correlated with “lightcyan” module activity (Spearman r >0.65, p < 0.002). UDP-glucuronate (Spearman r = 0.84, Fig. 4e) serves as a substrate of UDP-glucose 6-dehydrogenase, which is encoded by UGDH, a member of the “lightcyan” module and a transcriptional target of NFE2L2. Further evidence that supports the conclusion that other metabolites are associated with this NFE2L2-centered module is presented in Supplementary Table S5. For modules which were associated with metabolic profiles, such as the “lightcyan” module, the genes encoding enzymes could be identified as the key genes of the modules. For these cases, we found that metabolites are, indeed, good indicators representing functional and biological relevance of the modules.
Finally, we conducted an NFE2L2 knockdown experiment in the A549 cell line. We directly confirmed the downregulation of several core members of the “lightcyan” module (Fig. 4f). The lines of evidence collectively indicated that the “lightcyan” module was an NFE2L2-centered module, and the activation of the “lightcyan” module was represented by the transcriptome signatures of KEAP1- and NFE2L2-mutant cancers.
To evaluate the clinical effects of controlling this module, we reanalyzed the TCGA datasets. Among a group of cancers (128 cases, 25%) with high levels of “lightcyan” module activity, significantly poor prognosis was observed (p = 0.019) (Fig. 4g). We further analyzed other cancer types and identified the same transcriptional dysregulation in cancers such as lung squamous cell carcinoma and esophageal carcinoma (198 and 35 cases; 39% and 19%, respectively) (Supplementary Fig. S9), indicating the general importance of this module in cancer. Therefore, effective drug intervention strategies may have broad clinical relevance.
Drug interventions in the “lightcyan” module using auranofin
The activity of the “lightcyan” module was significantly correlated with sensitivity to auranofin treatment (r = 0.86, Spearman correlation coefficient) (Fig. 4a). Specifically, cell lines with increased expression levels of “lightcyan” module genes were resistant to auranofin. We sought to determine whether auranofin might be able to regulate the “lightcyan” module. Auranofin is used to treat rheumatoid arthritis. Auranofin is an inhibitor of thioredoxin reductase (encoded by TXNRD1/2)32. This drug is used as an approved drug to treat rheumatoid arthritis.
Figure 4h illustrates the association of antioxidative stress signaling with the “lightcyan” module. Auranofin may be associated with this module by regulating certain target genes of NFE2L2. However, in cell lines with increased “lightcyan” activity, other antioxidative stress genes and auranofin targets were also dysregulated, and these genes were not regulated by auranofin. Therefore, treatment solely with auranofin was insufficient for regulation of this module.
BED inhibitors partially regulated the antioxidative stress response module
Then, we asked whether any additional compounds or combinations thereof could collectively regulate the dysregulated “lightcyan” module as a whole, since it could not be regulated by auranofin. Specifically, we determined whether any compounds altered the transcription of the remaining two submodules of the “lightcyan” module (Fig. 5a). Group 1 included genes downregulated by BET inhibitors, such as GPX2 and ALDH3A1. On the other hand, genes upregulated by BET inhibitors were also detected in a different group, designated as group 2, which included IDH1 and GSR.
We analyzed the transcriptome and epigenome perturbation datasets of the compounds with desirable profiles and found that the BET inhibitors (+)-JQ1 and GSK1210151A may be candidates that could downregulate the expression of group 1 genes (Fig. 5a). BET inhibitors belong to a class of so-called epigenome drugs that prevent the association between bromodomain proteins and acetylated histones and affect transcription. Our multi-omics catalog data showed that the expression levels of the ALDH3A1, AKR1C3, and GPX2 genes were downregulated by BET inhibitors, which decreased the chromatin accessibility of their promoters or enhancers in a concentration-dependent manner (Figs. 5b and S10). We further investigated whether sensitivity to auranofin could be changed through transcriptional perturbations induced by BET inhibitors. We treated the cell lines with BET inhibitors combined with auranofin. In H2228 cells, which showed one of the highest activity levels in terms of the “lightcyan” module, auranofin and (+)-JQ1 showed synergistic activity (CI50 = 0.56) (Fig. 5c).
Despite the successful combined use of auranofin and (+)-JQ1, we found that the combination did not exert significant synergistic effects on some cell lines, such as H2126, II-18 and other cell lines (Figs. 5c and S11). We found that the combination of these compounds did not regulate the expression of group 2 genes (Fig. 5a). We additionally examined whether there were any other epigenetic drugs that could specifically control the expression of group 2 genes based on the observed omics information. As a candidate in place of auranofin, we used vorinostat with (+)-JQ1 treatment to more widely and moderately modulate the “lightcyan” module (Supplementary Fig. S12), and we observed a slight synergistic effect for (+)-JQ1 and vorinostat in some cells (CI40 = 0.61 in H2228 cells and CI40 = 0.80 in H2126 cells) with high activity in the “lightcyan” module (Fig. 5d).
To further elucidate the possible mode of action, we scrutinized the differences in the epigenome status between genes controlled and uncontrolled by BET inhibitors. For example, NFE2L2 binding sites reside in the enhancer region of ALDH3A1, which is one of the controlled genes. In this case, the chromatin structures represented in the ATAC-seq data were closed, and transcriptional activity was decreased by the BET inhibitors. In contrast, in GSR, the strong binding of NFE2L2 was localized to the promoter region, and the BET inhibitor did not close the associated chromatin structure, although a previously unknown enhancer located in the gene body (Fig. 5b) was inaccessible. These findings suggest that the regulatory mechanisms of NFE2L2 binding may be promoter- and enhancer-specific. In another example, OSGIN1 was significantly upregulated in response to BET inhibitors (Figs. 5a and S10). OSGIN1 is an oxidative stress response gene that is associated with the regulation of apoptosis. A regulatory region resides 25 kb upstream of this gene. We found that the chromatin structure was closed in response to treatment with BET inhibitors. When we analyzed the ENCODE ChIP-seq data, we found that this region overlapped with the binding site of the CTCF/cohesin complex34. The BET inhibitors may have changed the accessibility of the insulator regions, which may have affected the chromatin structure and transcriptional activity. We suggest that improving the understanding of the transcriptomic and epigenomic characteristics might help us to completely regulate the module.
Finally, we named the “lightcyan” module the “redox” module which was associated with KEAP1-NFE2L2 dysregulation, metabolic features of antioxidative pathways and auraofin sensitivity and could be partly regulated by epigenetic inhibitors.
Possible cell type-specific vulnerable modules
Overall, the above results indicate that the possible vulnerable modules, which should serve as the most effective targets for drug interventions, may differ depending on the cell type. Even when the putative redox module is not fully regulated in some cell lines, other modules may be targeted by other drug combinations. To further address this issue, we reorganized and characterized each of the lung cancer cell lines according to the patterns of the modules. We found that certain modules were associated with the lineages and differentiated phenotypes of the cells. We identified five modules that included representative markers of the cellular lineages and differentiated phenotypes. For example, the “brown” module included genes encoding epithelial markers such as E-cadherin (CDH1), EPCAM, GATA6 and CPM35. The “violet” module included the alveolar type-I and -II cell markers HOPX and AQP5 and the surfactant protein A genes (SFTPA1, SFTPA2, and SFTA2)36. The “violet” module also contained genes associated with the inflammatory response.
In contrast, the “midnightblue” module, which showed a significantly negative correlation with the “brown” module (r = −0.89, p = 4.0e-10, Pearson correlation coefficient) included the epithelial-mesenchymal transition regulator ZEB137. The “purple” and “blue” modules included bronchiolar markers of basal and neuroendocrine cells38,39. Based on the activity patterns of these characteristic modules, we clustered the cell lines depending on their omics signatures (Fig. 6a).
Unlike classifications based on representative surface markers and genomic mutations, this module-based classification should reflect system-level cancerous aberrations. Indeed, when we mapped the activities of the modules to the clinical samples, the clinical samples of lung cancers exhibited diverse module characteristics (Fig. 6b). For example, the patterns of module activity were occasionally associated with the degree of malignancy, as indicated by the presence of poor prognosis (Fig. 6c). Moreover, in vivo cancers frequently showed remarkably diverse variations, although their driver mutations were the same. Distinct therapeutic strategies may be required depending on the unique multi-omic and phenotypic characteristics of different cancer types. To develop a new drug for each of these cancer subtypes, appropriate cell lines should be selected as models. It follows that the reclassification of cancers according to their gene expression modulations, in addition to the used of the current simple classification scheme based on their genomic mutational patterns, should provide clues regarding the mechanisms of oncogenesis and tumor progression that could be pharmacologically targeted.
Methods
Cell lines
Human lung cancer cell lines were cultured and harvested as previously described8. They are described in our database DBKERO (https://kero.hgc.jp/)22. For high-throughput RNA-seq and ATAC-seq, cells (1,500–12,000 cells per well) were seeded and treated with compounds in 96-well culture plates and then cultured for 24–72 h.
Chemical compounds
Cells were treated with 320 compounds in the viability test. For this purpose, we selected 305 compounds from the Library of Pharmacologically Active Compounds (LOPAC, Sigma-Aldrich), 10 epigenetically targeted drugs and 4 tyrosine kinase inhibitors.
We designed high-throughput RNA-seq and ATAC-seq analyses that utilized drug treatment in a 96-well plate format. Two types of drug plates were prepared: dataset 1 included 24 compounds (23 compounds +a DMSO control) at four concentration points, and dataset 2 included 96 compounds (95 compounds +a DMSO control) at a single concentration and time point. For the RNA-seq and ATAC-seq analyses, 23 and 95 compounds, respectively, were selected from among the same compounds used for the viability test. According to their functional categories and growth inhibition rates, the compound showing some changes on cellular phenotypes were mainly selected. Some compounds were additionally selected from the FDA-approved Drug Library (Selleck Chemicals and Prestwick Chemical). The complete list is shown in Supplementary Table S1.
High-throughput RNA-seq
The culture medium was discarded from a 96-well culture plate kept on ice. The cells were washed using cold PBS. Total RNA was extracted using TRIzol (Invitrogen). Briefly, 100 μl TRIzol was added to each well, and the cells were lysed. We prepared a new 1.5 ml tube with 400 μl TRIzol and transfered the lysed cells into the tube. For RNA separation, 100 μl chloroform was added and mixed well, and the sample was incubated at room temperature for 10 min. After the incubation, the sample was centrifuged (12000 rpm, 15 min, 4 °C), and the supernatant was transfered to a new 1.5 ml tube. Isopropanol precipitation was performed for RNA purification. The RNA sample was eluted in 10 μl nuclease-free water.
To perform the automatic reverse transcription and amplification reactions in microchambers, we used the C1 Single-Cell Auto Prep System (Fluidigm) with a modified script (provided at https://kero.hgc.jp/). The detailed procedure is shown in the Supplementary Methods. Briefly, reverse transcription and cDNA amplification were performed using the C1 Integrated Fluidic Circuit (C1 Single-Cell Auto Prep IFC for Open App, Fluidigm) with the C1 system. The purified cDNAs were used for library construction with the Nextera XT DNA Sample Preparation Kit (Illumina). The library was sequenced with a 35-base single-end run using the HiSeq. 2500 System (Illumina).
High-throughput ATAC-seq
We developed a 96-well format ATAC-seq procedure according to the original ATAC-seq protocol27 with some modifications. The detailed procedure is shown in the Supplementary Methods. Briefly, the cells were dissociated using 0.25% Trypsin-EDTA (25200–056, Gibco) or AccutaseTM (A11105-01, Gibco) and pipetted into 8-channel tubes. For cell lysis, 50 μl lysis buffer (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2, and 0.1% NP-40) was used. For the ATAC reaction, 10 μl of 2 × TD buffer, 1 μl of Tn5 transposase and 4 μl of nuclease-free water were added, and the sample was incubated at 37 °C for 30 min. The transposed DNAs were purified using the ZR-96 DNA Clean & Concentrator-5 (Zymo Research) and eluted with 25 μl of nuclease-free water. PCR amplification was performed using 19.7 μl of transposed DNAs according to the original ATAC-seq protocol27, and then 10 μl of the sample in each of the 96 wells was mixed and purified. The libraries were sequenced with a 35-base single-end run using the HiSeq. 2500 platform (Illumina).
ATAC-seq under basal conditions
To evaluate the open chromatin patterns of lung cancer cell lines under basal conditions, approximately 5 × 104 cells were prepared and treated according to the ATAC-seq protocol previously described27.
Determination of the promoter and enhancer regions
To determine the promoter regions, we selected the representative transcriptional start sites (TSSs) according to the start positions of the RefSeq transcripts of each gene using TSS-seq data (accession number DRA005903)21. We determined 13,431 promoter regions located within ±1.5 kb from representative TSSs.
To define the enhancer regions, H3K27ac and H3K4me1 ChIP-seq data from 27 cell lines were used (accession numbers DRA001860 and DRA002311)8. The MACS240 peaks from the 27 cell lines were merged and the promoter regions were removed using bedtools41. We identified 312,226 and 535,538 regions according to the H3K27ac and H3K4me1 ChIP-seq peaks, respectively. All regions were merged using bedtools, and 515,599 regions were identified as enhancer regions. We used 56,961 enhancer regions that overlapped the ATAC-seq peaks under basal conditions. We assigned 32,111 enhancer regions to genes (±100 kb from TSSs).
Analysis of the high-throughput RNA-seq and ATAC-seq data
RNA-seq tags were mapped to the reference genome (UCSC hg19) using ELAND software (Illumina). The tags were mapped to the sequences obtained from the three RNA spike in controls. The rpkm values were calculated for 22,657 genes and the spike-in controls. For the quality control of the RNA-seq data, three criteria were used: 1) >0.5 million mapped reads, 2) <15% intron mapped reads, and 3) rpkm values for the spike-in control that were within ±2 sd of the average for each plate. For the analysis of reproducibility, we used protein-coding genes with rpkm values ≥2 in both datasets. The ATAC-seq tags were mapped to the reference genome (UCSC hg19) using ELAND software (Illumina). The peaks were detected using MACS2 with the default parameters. ATAC-seq data with >10,000 MACS peaks were used for further analysis. The ppm values were calculated for the promoter and enhancer regions. As ELAND software is a somewhat older software, we compared the mapping results from ELAND with those from other tools and confirmed that there were no significant differences among the results of the mapping tools for the analysis of the high-throughput RNA-seq and ATAC-seq data (Supplementary Fig. S3d,g).
CCLE RNA-seq data
For the lung adenocarcinoma cell lines A549 and H1299, RNA-seq data (CCLE_RNAseq_081117.rpkm.gct) were downloaded from the CCLE download page (https://portals.broadinstitute.org/ccle/data)10. The rpkm values were log2-tranformed after +1. For the comparison of the datasets obtained in this study, we used genes with rpkm values ≥2 in both datasets.
qPCR validation of RNA-seq and ATAC-seq data
For the cDNAs that were used in the high-throughput RNA-seq analysis, qPCR validation was performed using THUNDERBIRD SYBR qPCR Mix (QPS-201, TOYOBO). We performed one assay for each measurement. The detailed procedure is shown in the Supplementary Methods. The primer sequences are listed in Supplementary Table S6.
We also performed qPCR analyses of the five promoters and eight enhancers for the validation of the high-throughput ATAC-seq data. We performed one qPCR assay for each measurement. The ATAC DNAs, which were subjected to sequence analysis, were used. The detailed procedure is shown in the Supplementary Methods. The primer sequences are listed in Supplementary Table S7.
Cell viability test
Viability tests of cells treated with 320 drugs were performed using a 384-well plate high-throughput system. Each well of a 384-well plate contained 7.5 μl of culture medium. DMSO control wells were prepared with or without cells to serve as positive and negative controls, respectively. For high-throughput stimulation, 2.5 μl of a chemical compound was added to each well of the plates. For each well, 450–3,000 cells (15 μl of a cell suspension) were seeded and incubated for 72 h at 37 °C in an atmosphere containing 5% CO2. After incubation, 25 μl of the reagent provided with the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega) was added. A Multidrop Combi (Thermo Fisher Scientific) and a HORNET-NX (Wako) automatically dispensed the assay components. The cell viability rates were calculated according to the luminescence, which was measured using an EnVision Plate Reader (PerkinElmer). The assays were repeated twice (N = 2). The data were fitted to the logistic equation using XLfit (IDBS) as follows:
The values represented the variables as follows: y: % growth inhibition; x: concentration of the compound; A: minimum value of y; B: maximum value of y; C: EC50 value; D: Hill slope42.
Analysis of the association between the basal genomic status and drug sensitivity
The 26 cell lines were divided into two groups according to the IC50 values of each compound to produce the maximum degree of separation in terms of the IC50 values between the groups (Fig. 2c). The IC50 values were adjusted to 10 μM when the growth inhibition rate did not reach 50% at a 10 μM concentration. We focused on the mutational status of 26 cancer-related genes8 and used Fisher’s exact test to analyze the association between the mutational status and the cell groups defined by the IC50 value. An aberrant status was defined according to the following: for oncogenes, mutants were defined if the cell lines harbored genomic mutations and the expression was >5 rpkm; for tumor-suppressor genes, mutants were defined if they harbored mutations or showed low expression levels that were <5 rpkm. For other cancer-related genes, such as those encoding chromatin factors and fusion genes, mutants were defined if they showed genomic mutations. For CDKN2A, the DNA methylation status of the p16INK4A alternative promoter was considered. Finally, we extracted the gene-compound pairs with a >5-fold IC50 differences between the two cell groups and a significant enrichment of the mutational status of the gene in either group (p < 0.05, Fisher’s exact test).
CMAP analysis
Using the high-throughput RNA-seq data, genes with >4 or <0.25-fold changes in terms of their expression levels were extracted as parts of the signatures of the transcriptome mode of action. We used an RNA-seq dataset from PC-9 cells treated with vorinostat (0.95 μM for 24 h). Of the 4,798 genes expressed in either stimulated or DMSO control cells (rpkm value >10 for RNA-seq; ppm >5 ppm from the promoter region for ATAC-seq), 163 upregulated and 146 downregulated genes were extracted and designated a part of the signature of vorinostat. To verify the signature, we accessed CMAP datasets19 using CLUE (https://clue.io/). We used the CLUE L1000 Query to evaluate the similarities between the signature of our dataset to that of the CMAP datasets.
Weighted gene co-expression network
For the extraction of the gene co-expression modules, we used RNA-seq data (accession numbers DRA001846 and DRA002311) from 27 cultured cell lines (26 lung cancer cell lines and small airway epithelial cells) incubated under basal conditions. We extracted 73 modules using the WGCNA method31. The detailed procedure is shown in the Supplementary Methods. The complete list of the 73 module members is included in Supplementary Table S3. GO enrichment analysis of each module was performed using the WGCNA GOenrichmentAnalysis function. The top 10 GO terms ordered according to the Bonferroni corrected p-values (Bonferroni p < 0.1) are listed in Supplementary Table S4.
Analysis of the NFE2L2 binding patterns using ENCODE data
To evaluate the NFE2L2 binding patterns, NFE2L2 ChIP-seq data (ENCSR584GHV and NCSR949BZP) were obtained from the ENCODE project7. The fastq files (ENCFF002EAK and ENCFF002ECM) were mapped to UCSC hg19 using Bowtie2 (v2.0.6)43. The sorted bam files were created using SAMtools44. The peaks were detected using MACS2 (v2.1.0.20150420).
Metabolome analysis
For the metabolome analyses, capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) was performed using an Agilent CE-TOFMS system45. The raw data were analyzed using MasterHands (ver. 2.17.0.10)46. For further analysis, the average concentrations (fmol/cell, n = 2) were used for each cell line. A not detected (N.D.) value was omitted if the compound was detected using the other measurement. Concentrations were defined as zero if the metabolites were not detected by either measurement.
To evaluate the association with the transcriptome modules, Spearman correlation coefficients for the ME values of the modules and the concentrations of the metabolites were calculated. We detected 113 metabolites in >10 cell lines.
siRNA knockdown experiments
A549 cells were seeded (1500 cells/well in 96-well plates) and incubated for 24 h at 37 °C. NFE2L2 siRNAs (Silencer Select NFE2L2 s9491 and s9493, Applied Biosystems) and negative control siRNAs (Silencer Select Negative Control #1 siRNA, Applied Biosystems) were used for the transfection of the A549 cells. Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) and the siRNAs were diluted in serum-free MEM (M7278, Sigma-Aldrich). For the preparation of the RNA-lipid complexes, diluted Lipofectamine and siRNAs were mixed and incubated for 5 min, and 10 μl of siRNA-lipid reagents was added to the cells.
To confirm the knockdown efficiency and determine the changes in the expression levels of the downstream genes, qRT-PCR was conducted (n = 3). For qRT-PCR, cDNAs were synthesized using the SuperPrep Cell Lysis & RT Kit for qPCR (SCQ-101, TOYOBO), and qPCR was performed using the THUNDERBIRD SYBR qPCR Mix (QPS-201, TOYOBO). The PCR primers are listed in Supplementary Table S8.
Analysis of TCGA data
For The data from TCGA for lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC) and esophageal carcinoma (ESCA), RNA-seq v2 data was acquired using TCGA-Assembler v2.0.647 (the data checked on 2019/09/10). The clinical information was acquired from cBioPortal (downloaded on 2019/09/13). The somatic mutation data were downloaded from the NCI Genomic Data Commons (https://gdc.cancer.gov/) (downloaded on 2019/09/13-14). To generate the heat maps of the RNA-seq v2 data, the normalized counts were adjusted to 1 if the count was <1. The adjusted expression levels were normalized to the median expression levels in all patients and log2-transformed. Hierarchical clustering with Ward’s method was performed according to the Euclidean distance using the expression levels of the genes in the given modules.
To extract the core putative redox module member genes (Fig. 5a) that exhibited significant co-expression patterns in the clinical samples, we performed the Wilcoxon rank-sum test to compare the expression levels of TCGA-LUAD cases with high module activity (Fig. 4d) with those of other cases. We extracted 63 differentially co-expressed genes among the high module activity cases (Bonferroni p < 0.05).
To associate the TCGA transcriptome data with the modules, the “module score” for each TCGA-LUAD case was calculated for each module as follows: (1) the expression values (normalized counts) were log2-transformed after adding 1; (2) the log2-transformed values of 10 representative genes with the highest correlation with the ME were averaged for each case; (3) z-score normalization was applied. Kaplan-Meier analysis and the log-rank test were performed using the survival package in R.
Treatment with a combination of auranofin and (+)-JQ1
For the combination treatment using auranofin and (+)-JQ1, the assay plate was designed as shown in Supplementary Fig. S11. The cell viability rates were calculated as described above. The combination index (CI) was calculated according to the Chou-Talalay method48.
Treatment with a combination of vorinostat and (+)-JQ1
For the combination treatment using vorinostat and (+)-JQ1, the CI was calculated in a manner similar to that shown in Supplementary Fig. S11. By using three cell lines with high activity in the putative redox module, a cell viability test was performed twice, and the CI was determined according to the average cell viability rates.
Supplementary information
Acknowledgements
We are grateful to S. Tominaga, M. Kimura, Y. Kuze, T. Horiuchi, K. Imamura, M. Kombu, K. Abe, Y. Ishikawa, S. Shimazu and H. Wakaguri for technical assistance. This work was supported by JSPS KAKENHI grant numbers 16H06279 and 17H06306. This work was also supported by Research on Development of New Drugs and Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and Development (AMED) Grants JP19ck0106255 (to K.T. and Y.S.).
Author contributions
A.S., K.O., M.S. and Y.S. performed the sequencing analysis. A.S. performed the computational analysis of the multi-omics datasets. K.M. conducted the cell viability tests. K.O. performed the validation experiments. H.E. and T.S. performed the metabolome analysis. T.K. evaluated the biological significance of the identified expression aberrations. S.S., T.K., Y.S. and K.T. conceived and supervised the study. A.S., K.O. and Y.S. wrote the manuscript.
Data availability
All the raw sequencing data used for the high-throughput RNA-seq and ATAC-seq analyses have been registered in DDBJ under the accession numbers DRA006875 - DRA006902 (RNA-seq) and DRA006903 - DRA006930 (ATAC-seq). The omics sequencing data for the cell lines were previously published8,49. The datasets in this paper are provided in our database, DBKERO (https://kero.hgc.jp/)22.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ayako Suzuki and Keiichi Onodera.
Supplementary information
is available for this paper at 10.1038/s41598-019-55692-9.
References
- 1. The Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki A, et al. Identification and Characterization of Cancer Mutations in Japanese Lung Adenocarcinoma without Sequencing of Normal Tissue Counterparts. PLoS One. 2013;8:e73484. doi: 10.1371/journal.pone.0073484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straughan DM, Azoury SC, Shukla V. Anaplastic Lymphoma Kinase Inhibitors in Non-Small Cell Lung Cancer. Curr. Drug Targets. 2016;17:739–45. doi: 10.2174/1573399811666150615144336. [DOI] [PubMed] [Google Scholar]
- 4.Lynch TJ, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non–Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, et al. EGFR mutations in lung, cancer: Correlation with clinical response to gefitinib therapy. Science (80-.). 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Kohno T, et al. RET fusion gene: Translation to personalized lung cancer therapy. Cancer Sci. 2013;104:1396–1400. doi: 10.1111/cas.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki A, et al. Aberrant transcriptional regulations in cancers: Genome, transcriptome and epigenome analysis of lung adenocarcinoma cell lines. Nucleic Acids Res. 2014;42:13557–13572. doi: 10.1093/nar/gku885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg KCG, et al. Multi-omics of 34 colorectal cancer cell lines - a resource for biomedical studies. Mol. Cancer. 2017;16:116. doi: 10.1186/s12943-017-0691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghandi Mahmoud, Huang Franklin W., Jané-Valbuena Judit, Kryukov Gregory V., Lo Christopher C., McDonald E. Robert, Barretina Jordi, Gelfand Ellen T., Bielski Craig M., Li Haoxin, Hu Kevin, Andreev-Drakhlin Alexander Y., Kim Jaegil, Hess Julian M., Haas Brian J., Aguet François, Weir Barbara A., Rothberg Michael V., Paolella Brenton R., Lawrence Michael S., Akbani Rehan, Lu Yiling, Tiv Hong L., Gokhale Prafulla C., de Weck Antoine, Mansour Ali Amin, Oh Coyin, Shih Juliann, Hadi Kevin, Rosen Yanay, Bistline Jonathan, Venkatesan Kavitha, Reddy Anupama, Sonkin Dmitriy, Liu Manway, Lehar Joseph, Korn Joshua M., Porter Dale A., Jones Michael D., Golji Javad, Caponigro Giordano, Taylor Jordan E., Dunning Caitlin M., Creech Amanda L., Warren Allison C., McFarland James M., Zamanighomi Mahdi, Kauffmann Audrey, Stransky Nicolas, Imielinski Marcin, Maruvka Yosef E., Cherniack Andrew D., Tsherniak Aviad, Vazquez Francisca, Jaffe Jacob D., Lane Andrew A., Weinstock David M., Johannessen Cory M., Morrissey Michael P., Stegmeier Frank, Schlegel Robert, Hahn William C., Getz Gad, Mills Gordon B., Boehm Jesse S., Golub Todd R., Garraway Levi A., Sellers William R. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature. 2019;569(7757):503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffat J, et al. A Lentiviral RNAi Library for Human and Mouse Genes Applied to an Arrayed Viral High-Content Screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Luo B, et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl. Acad. Sci. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han K, et al. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 2017;35:463–474. doi: 10.1038/nbt.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsherniak A, et al. Defining a Cancer Dependency Map. Cell. 2017;170:564–576.e16. doi: 10.1016/j.cell.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb J, et al. The connectivity map: Using gene-expression signatures to connect small molecules, genes, and disease. Science (80-.). 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 18.Iorio F, et al. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl. Acad. Sci. 2010;107:14621–14626. doi: 10.1073/pnas.1000138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171:1437–1452.e17. doi: 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corces, M. R. et al. The chromatin accessibility landscape of primary human cancers. Science (80-.). 362 (2018). [DOI] [PMC free article] [PubMed]
- 21.Suzuki A, et al. DBTSS as an integrative platform for transcriptome, epigenome and genome sequence variation data. Nucleic Acids Res. 2015;43:D87–D91. doi: 10.1093/nar/gku1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, et al. DBTSS/DBKERO for integrated analysis of transcriptional regulation. Nucleic Acids Res. 2018;46:D229–D238. doi: 10.1093/nar/gkx1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imielinski M, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo JS, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22:2109–2119. doi: 10.1101/gr.145144.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchihara K, et al. Massive transcriptional start site analysis of human genes in hypoxia cells. Nucleic Acids Res. 2009;37:2249–2263. doi: 10.1093/nar/gkp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–110. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sporn MB, Liby KT. NRF2 and cancer: The Good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 33.Menegon S, Columbano A, Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Ong CT, Corces VG. CTCF: An architectural protein bridging genome topology and function. Nat. Rev. Genet. 2014;15:234–246. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotoh S, et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports. 2014;3:394–403. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotton DN, Morrisey EE. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosari F, et al. ASCL1 and RET expression defines a clinically relevant subgroup of lung adenocarcinoma characterized by neuroendocrine differentiation. Oncogene. 2014;33:3776–3783. doi: 10.1038/onc.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 2012;7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinlan AR, Hall IM. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallahi-Sichani M, Honarnejad S, Heiser LM, Gray JW, Sorger PK. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 2013;9:708–714. doi: 10.1038/nchembio.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–9. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soga T, et al. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal. Chem. 2009;81:6165–6174. doi: 10.1021/ac900675k. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6:78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Qiu P, Ji Y. TCGA-Assembler: open-source software for retrieving and processing TCGA data. Nat. Methods. 2014;11:599–600. doi: 10.1038/nmeth.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 49.Sereewattanawoot, S. et al. Identification of potential regulatory mutations using multi-omics analysis and haplotyping of lung adenocarcinoma cell lines. Sci. Rep. 8 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw sequencing data used for the high-throughput RNA-seq and ATAC-seq analyses have been registered in DDBJ under the accession numbers DRA006875 - DRA006902 (RNA-seq) and DRA006903 - DRA006930 (ATAC-seq). The omics sequencing data for the cell lines were previously published8,49. The datasets in this paper are provided in our database, DBKERO (https://kero.hgc.jp/)22.