Abstract
Background and Objectives:
Cannabis use is common in people with and mood and anxiety disorders, and rates of problematic use are higher than in the general population. Given recent policy changes in favour of cannabis legalization, it is important to understand how cannabis and cannabinoids may impact people with these disorders. We aimed to assess the effects of cannabis on the onset and course of depression, bipolar disorder, anxiety disorders, and post-traumatic stress disorder (PTSD), and also to explore the therapeutic potential of cannabis and cannabinoids for these disorders.
Methods:
A systematic review of the literature was completed. The Pubmed® database from January 1990 to May 2018 was searched. We included longitudinal cohort studies, and also all studies using cannabis or a cannabinoid as an active intervention, regardless of study design.
Results:
47 studies were included: 32 reported on illness onset, 9 on illness course, and 6 on cannabinoid therapeutics. Cohort studies varied significantly in design and quality. The literature suggests that cannabis use is linked to onset and poorer clinical course in bipolar disorder and PTSD, but this finding is not as clear in depression and anxiety disorders. There have been few high-quality studies of cannabinoid pharmaceuticals in clinical settings.
Conclusions and Scientific Significance:
These conclusions are limited by a lack of well-controlled longitudinal studies. We suggest that future research be directed towards high-quality, prospective studies of cannabis in clinical populations with mood and anxiety disorders, in addition to controlled studies of cannabinoid constituents and pharmaceuticals in these populations.
1. INTRODUCTION
Known for its ability to induce euphoria and relaxation, cannabis (marijuana) has been one of the most commonly-used illicit substances worldwide for decades. Public perceptions and attitudes towards cannabis are shifting. Many international jurisdictions have moved towards decriminalization, and others including: 10 American states and Washington, D.C.; South Africa; and Canada have legalized recreational marijuana use 1,2. Furthermore, many more jurisdictions have legalized medical marijuana and there is an increasing trend towards including some mental health disorders, in particular PTSD, among the approved indications for medical marijuana use 3. It has therefore become increasingly important for health practitioners, consumers, and policymakers to understand the effects of cannabis and its implications for public health, particularly for vulnerable populations including youth and people with mental health and addictive disorders 4–6.
Cannabis is comprised of more than 400 compounds, and among these are over 140 known cannabinoids 7. Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best characterized exogenous cannabinoids, and are known for their psychoactive and anxiolytic properties respectively 8. These compounds interact with the endocannabinoid system, which has been shown to be important for both brain development, and synaptic transmission modulation involved in managing emotional states, stress responses, and cognition 9,10. It is not challenging to imagine how manipulation of this system, either with cannabis or specific exogenous cannabinoids, may have implications for the pathophysiology and clinical course of mental health disorders 11. Furthermore, some have wondered if there is a role for cannabinoid-based pharmaceuticals such as, nabilone (THC analogue), dronabinol ((−)-trans- THC)), and nabiximols (THC and CBD, 1:1 ratio), in the treatment of mental health disorders12.
Large epidemiological cross-sectional datasets demonstrate an association between cannabis and multiple mental health disorders 13–16. Possible explanations for these correlations include: perceived therapeutic benefits of cannabis, the presence of multiple common risk factors, or that cannabis contributes to the pathophysiology of mental illness 16. Researchers have increasingly relied on long-term prospective studies to help understand these complex relationships and clarify the time order of cannabis and any mental health effects. From this data, some have concluded there is already reasonable evidence to suggest cannabis use increases the odds of psychosis 17,18; however, its effects in mood and anxiety disorders are much less clear. Moreover, there is less information known about the therapeutic potential of cannabis and medical cannabinoids in psychiatric disorders, since few treatment studies have been conducted 19.
Other narrative and systematic reviews have investigated the particular effects of cannabis and medical cannabinoids in one or more of the mood, anxiety, or psychotic disorders 20–23. The aim of the present review was to conduct a more critical appraisal of this literature using rigorous methodology (PRISMA systematic review 24 with study quality ratings using the Newcastle-Ottawa Scale 25) to evaluate the quality of evidence of cannabis effects across mood and anxiety disorders specifically. We considered each of the following important clinical questions:
Does cannabis contribute to the onset of mood and anxiety disorders?
Does cannabis affect the course of mood and anxiety disorders?
Is there any evidence to suggest cannabis or cannabinoids have therapeutic potential in people with mood and anxiety disorders?
2. METHODS
2.1. Search Strategy
A literature search based on the PRISMA guidelines for systematic reviews 24 was conducted by SB and SY using the Pubmed® database to find studies that investigated the role of cannabis in the onset, progression, and/or treatment of mood and anxiety symptoms or disorders. Articles published or available online in the English language between 1990 through the end of May 2018 were considered. Search terms (found in the title or abstract) used to find relevant articles were: ‘cannabis’ OR ‘tetrahydrocannabinol’ OR ‘cannabidiol’ OR ‘marijuana’ OR ‘cannabinoid’ OR ‘nabilone’ OR ‘dronabinol’ OR ‘nabiximols’ AND ‘social anxiety disorder’ OR ‘generalized anxiety disorder’ OR ‘panic disorder’ OR ‘agoraphobia’ OR ‘PTSD’ OR ‘post-traumatic stress disorder’ OR ‘depression’ OR ‘bipolar disorder’ OR ‘mania’ OR ‘hypomania’ NOT ‘rodent’ OR ‘mouse’ OR ‘rat’.
2.2. Screening and Study Eligibility
Titles and abstracts were screened for relevance by two of the authors (SB and SY), and articles making it through this process were downloaded and evaluated for eligibility and full text review by SB. Any uncertainties were reviewed and reconciled by the senior author (TPG).
All experimental or observational studies investigating the role of cannabis or cannabinoid pharmaceuticals on the treatment of mood and anxiety disorders were included. This is a small body of literature that lacks large well-controlled RCTs so it was decided the scope should not be narrowed.
For observational studies investigating the role of cannabis on the onset or progression of mood and anxiety disorders, we limited our scope to cohort studies collecting relevant data at more than one time-point. To be included, studies needed to have a measure of cannabis use at baseline. The primary outcome was a mental health measure for mood or anxiety symptoms or diagnoses, excluding suicide. We did not limit studies by age, number, or type of participants.
We excluded from our review: a) reviews and meta-analyses; b) studies of mental health symptoms only as secondary outcomes in people with other medical conditions (i.e. fibromyalgia); c) studies that did not include a baseline measure of cannabis use; and d) cross-sectional study designs. In the case of multiple publications deriving from the same study population, we selected the articles reporting the largest or the most recent data.
2.3. Recorded Variables
We recorded the following variables from each article: author, publication year, journal, study design, number of participants, study population description, follow-up time, cannabis use measures, mental health measures, intervention type with comparator (if applicable), variables controlled for in analyses or design, and the relevant findings.
2.4. Quality Assessment
The quality of selected cohort studies was assessed using the Newcastle Ottawa Scale (NOS) for Cohort Studies 25. This scale assesses quality based on three main categories: selection (1 point for representativeness of study population, 1 point for selection of non-exposed cohort, 1 point for blind assessment or secure record for data acquisition, and 1 point for baseline mental health measure), comparability of groups (1 point for controlling for other substance use or substance use disorder; 1 point for other demographic factors), and determination of outcomes of interest (1 point >12 month follow-up, 1 point for adequacy of follow-up, and 1 point for independent blind assessment or record linkage). Treatment studies were reviewed and evaluated separately.
3. RESULTS
3.1. Flow of Included Studies
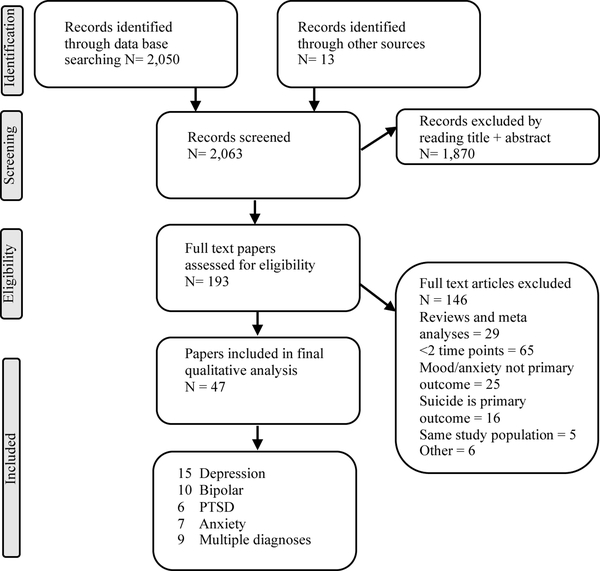
The searchers identified 2,063 hits of which 193 were considered potentially relevant, based on title and abstract screening. The full-text was then reviewed and a total of 47 studies were included as summarized in the PRISMA Flow Chart (Figure 1). Of these, 9 studies contained data for more than one diagnostic category of interest. Publication dates ranged from 1996 – 2018 and studies were conducted in a wide range of countries.
Figure 1.
PRISMA Flow Diagram
3.2. Bipolar Disorder
3.2.1. Bipolar Disorder Onset
Seven prospective studies met inclusion criteria (N=57,248) (Table 1). Most studies attempted to quantify cannabis use (CU) frequency. Primary outcomes included a new diagnosis of bipolar disorder (BD) type 1 or 2 (DSM III-R to DSM IV-R; ICD 10) or prevalence of hypomania or mania symptoms (HCL-32, CIDI). Follow-up times ranged from 3 to 20 years.
Table 1:
Cannabis and the onset of mood and anxiety disorders organized by symptom/disorder type
| Author, year | Participants | N | Follow up (yr) |
Cannabis Use |
Outcome | Adjustment Variables | Relevant Findings | NOS |
|---|---|---|---|---|---|---|---|---|
| MANIA SYMPTOMS AND BIPOLAR DISORDERS | ||||||||
| Marwaha et al. 2018 | Avon UK 1991 birth cohort | 3,370 | 5 | CU frequency (age 17) | Hypomania symptoms (HCL-32) (age 22) | Family adversity index, history of abuse, alcohol use, drug use, psychotic symptoms, depression history | Adolescent CU associated with f hypomania symptoms at age 22; dose dependent: >2x/wk (OR = 2.87 (1.68 – 4.91); any CU (OR=1.82 (1.45 – 2.28)) | 7 |
| Ratheesh et al. 2015 | Youth ↑risk BD (BAR) | 52 | 1 | CUD | BD-1, BD −2 (DSM IV-TR) | N/A | 25% with CUD and 17% without CUD developed BD (OR=1.7 (0.2–18.1). Outcome rate (4/52) too small to achieve significance | 5 |
| Tjissen et al. 2010 | Munich 1994 (age 14–17) represent. | 543 | 8.3 | Lifetime CU > 5 uses | Mania symptoms Munich-CIDI (DSM IV) | Age, sex, SES, family history of mood episodes, exposure to trauma, loss of a parent, alcohol use, personality style | Any CU associated with f mania symptoms (OR= 4.26 (1.42–12.76) p=0.010) | 9 |
| Henquet et al. 2006 | Dutch adult represent. | 4,815 | 3 | CU frequency | Mania, psychosis symptoms (CIDI) | Age, sex, ethnicity, education, marital status, neuroticism, lifetime drug use, last year alcohol use, baseline depression/ mania | Any CU associated with f mania symptoms after adjusting for covariates: daily CU (AOR 3.43 (1.42–8.26), monthly CU (AOR 2.23 (0.82–6.07) | 8 |
| DEPRESSION SYMPTOMS AND DEPRESSIVE DISORDERS | ||||||||
| Schoeler et al. 2018 | London males born in 1953 | 411 | 40 | CU frequency at age 14, 18, 32, 48 | Lifetime diagnosis of MDD (DSM-IV) | Alcohol, cigarette and other illicit drug use; psychiatric illness; childhood anxiety, conduct problems and behaviour and emotional problems | CU onset <18 yrs associated with f lifetime MDD (AOR= 2.41 (1.22–4.76) p= 001) and ↑ time to MDD for low risk users (HR=2.09 (1.16–3.74)) and high risk users (HR = 8.69 (2.07–36.5)) | 8 |
| Wilkinson et al. 2016 | US (1994) adolescents age 12–18 | 11,995 | 12 | 30-day CU frequency | Depression symptoms (CES- D) | Race, ethnicity, educational attainment of parents and respondent, age, sex | CU in earlier waves was not significantly associated with depressive symptoms in later waves | 7 |
| Womack et al. 2016 | Pittsburgh males from low SES families | 264 | 11 | Age 17, 20, 22: past year CU >2– 3x/week | Age 17; 20, 22: Depressive symptoms (BDI) | Caregiver BDI score, parent income, ethnicity, highest level of education age 22, youth antisocial behaviour, tobacco and alcohol use age 17, youth IQ, adult court records | Significant positive association between adolescent CU and mild depression at age 22 (B=0.493 SE=0.207, p<0.05). | 6 |
| Baggio et al. 2014 | Switzerland male conscripts | 5,223 | 1.25 | CU frequency; trajectory; CUD | Depression symptoms (WHO-MDI) | Age at first CU, language (German, English) | Only CUD was associated with increase in depression symptoms (B=0.087, P<0.001) | 4 |
| Otten & Engels 2013 | Netherlands adolescents +/− 5-HTTLP R allele | 306 | 4 | Lifetime CU; CU frequency in past month | Depression symptoms (DML) | Personality scores, alcohol use, tobacco use, parental education, parenting practices | CU associated with later depression symptoms in the presence of the short allele (B =0.34 (b=0.10), P<0.001), but not in its absence (B=−1.379 (b=−0.14), P=0.51) | 7 |
| Rasic et al. 2013 | Nova Scotia Grade 10 students | 1,582 | 2 | Past 30 day CU | Depression (CES- D >24 females; >22 males) | Alcohol use, other illicit drug use, living situation, school marks, age, gender | Adolescents with CU have f odds of depression (AC)R=1.10 (1.01–1.19), p<0.05); higher odds in those with heavier CU (AOR = 1.16(1.04–1.29), P<0.05) | 5 |
| Manrique-Garcia et al. 2012 | Sweden male conscripts (1969–1970) | 45,087 | 35 | Total number lifetime CU | Depression (ICD-8. 9. 10) | Personality disorder, IQ, disturbed behaviour in childhood, social adjustment, popularity, relationships, alcohol use, smoking, early adulthood SES, urbanicity | Heavy CU (> 50 uses) does not f risk of depression after adjusting for confounders (AHR= 0.9 (0.5–1.6)) | 9 |
| Marmorstein & Iacono 2011 | Minnesota Twin Family Study | 1,252 | 5 | CU frequency (age 17);’ CUD | Age 17. 20. 24: MDD Diagnosis (DSM III-R) | Gender; baseline MDD, AUD, nicotine dependence; psychosocial risks (not graduating high school by age 20, period of unemployment >6 months), crime | Adolescent CUD associated with f odds of later MDD (AOR=2.62 (1.22–5.65). Relationship partially mediated by psychosocial failure (AOR = 2.54 (1.40–4.60), p<0.05). | 9 |
| Harder et al. 2008 | Mid-Atlantic cohort (1985– 2001) | 1,494 | 7 | CUD before age 17 | MDE (DSM-IV) between ages 19– 24 | Demographics, SES, other drug use, childhood disturbances of psychological well being, parental monitoring, behavioural intervention status variables, pre exposure depression/anxiety | Early CUD not associated with MDE (OR=1.33 (0.76–2.23), p=0.32), when propensity scores used to adjust for confounders | 9 |
| Pederson 2008 | Norway 14 year olds (1992) | 2,902 | 13 | Past 12 month CU quantity at age 14. 16. 21. 27 | Depressed mood (Kandel and Davies score >9) | Parent SES, parental monitoring and support, parental substance use, pubertal development, student academics, school completion, conduct problems, alcohol intoxication, alcohol problems unemployment, daily smoking | No significant association found between early or late CU and later depression symptoms when adjusting for confounders (AOR = 0.9 (0.4–2.5)) | 7 |
| Georgiades & Boyle 2007 | Ontario birth cohorts (1966– 1979) | 3,294 | 18 | Past year CU frequency | 12 month prevalence MDD (CIDI-SF) | Family SES, family functioning, sex, grade failure, other medical condition, general health status, externalizing and internalizing symptom scales, tobacco use | CU in adulthood alone (AOR=2.58 (1.67– 3.99), p<0.001) or adolescence + adulthood (AOR=4.45 (2.05–9.66), p<0.001), associated with ↑ MDD diagnosis, but not adolescence alone (AOR=1.48 (0.65–3.40), p > 0.5) | 7 |
| Harder et al. 2006 | US 1979 birth cohort | 12,686 | 4 | Past year CU at age 19 | Depression (CES- Dscore>16) age 23 | Age, sex, aptitude, survey weight, general health limitations, region of residence, criminal activity, residence age 14, cigarette use, excessive alcohol use, hard drugs use | CU was not associated with depression when compared to non users weighted for other depression risk factors (AOR= 1.51 (0.64– 3.54)) | 7 |
| Patton et al. 2002 | Australia Victoria adolescents Age 15–21 | 1,601 | 7 | Highest CU frequency over a 6 month period | Depression and anxiety symptoms (CIS-R >12); | Teenage depression, anxiety, alcohol use, tobacco use, other illicit drugs, antisocial behaviour, parental separation, parental education, sex, age, rural vs urban residence, parental education | Weekly CU associated with j depression/anxiety symptoms in females (AOR=1.9 (1.1–3.3) p=0.01); but not males (AOR = 0.47 (0.17–1.3)) | 8 |
| Bovasso et al. 2001 | Baltimore sstudents (1980) | 1,920 | 15 | CUD(DSM III-R) | Depression symptoms (DIS DSMIII-R) | Demographic variables, stressful life events and chronic illnesses, baseline depression symptoms, mental health treatment, psychiatric disorder, substance abuse/dependence | CUD associated with j depression symptoms (OR=4.49 (1.51–13.26)) p<0.01) | 9 |
| ANXIETY SYMPTOMS AND DISORDERS | ||||||||
| Duperrouzel et al. 2018 | Miami adolescents | 250 | 1 | Past month CU frequency at baseline | Depression and anxiety symptoms (DASS-21) | Gender, alcohol use, nicotine use, and history of mood disorder | People with CU had a more gradual decline in anxiety symptoms over time (b=0.28, p=0.024) | 6 |
| Feingold et al. 2016 | US adult represent. | 34,653 | 3 | Past year CU frequency | AD (DSM IV- TR) | AUD, SUD, sex, race, education level, household income, marital status, age, region, other DSM diagnosis | Daily CU associated with later SAD after controlling for all confounders (AOR=1.98 (0.99–3.94)) | 8 |
| Bechtold et al. 2015 | Pittsburgh adolescent males | 506 | 22 | CU onset (early, late); CU chronicity | AD (DSM IV) | Past year substance use age 36, SES age 36, health insurance, health status, mental and physical health age 14 | CU groups did not differ in lifetime diagnoses of anxiety disorders | 7 |
| Zvolensky et al. 2008 | Oregon adolescents | 1,790 | 10 | Lifetime CU or CUD | DSM IV diagnosis panic attack or panic disorder | Life time history drug dependence, daily cigarette smoking status | CU not associated with developing panic attack (AOR 1.3 (0.55–3.2)); PD (AOR = 1.0 (0.34–3.2)) after adjusting for cigarette smoking | 7 |
|
PTSD | ||||||||
| Lee et al. 2018 | African American, Puerto Rican East Harlem students | 674 | 22 | No CU, chronic CU, moderate CU, early vs late quitters | PTSD symptoms at age 36 (PCL-S) | Gender, race/ethnicity, alcohol use, cigarette use, other illicit drug use, delinquency, low self control, depressive symptoms age 14, victimization, sexual assault age 19 | People with CU and exposed to trauma more likely to have PTSD symptoms: Chronic (AOR= 4.27 (1.28–14.20), p<0.05); Late quitters (AOR= 6.67 (1.62–27.44); p<0.01); Moderate users (AOR= 3.32 (1.0710.34); p< 0.05); but not early quitters (AOR = 1.75 (0.36–8.44)) | 7 |
| MULTIPLE SYMPTOMS OR DISORDERS | ||||||||
| Guttmann-ova et al. 2017 | Seattle youth | 808 | 20 | Age of CU, Regular CU (weekly), Duration of CU | Generalized and social anxiety; Depression symptom count (DIS-IV) | Adolescent tobacco and alcohol use, gender, ethnicity, childhood poverty, early environmental risk, baseline psychopathology | All CU groups, except adolescent limited regular users, had ↑ symptoms of GAD than non-users after controlling for all confounders. No significant association was found between CU and depression symptoms. | 8 |
| Danielsson et al. 2016 | Sweden adult represent. | 8,598 | 3 | Lifetime CU | Anxiety symptoms (SPRAS) Depression symptoms (MDI) | Substance use, sex, age, education, childhood adverse circumstances, ethnicity, place of upbringing | Baseline CU was not associated with later depression (RR=0.99 (0.82–1.17)) or anxiety (RR = 1.09 (0.98 – 1.20)) symptom scores after adjusting for all confounders | 7 |
| Scholes-Balog et al. 2016 | Australia grade 5 youth represent. | 927 | 12 | No CU; CU by age 12 or 19 | Depression and anxiety symptoms (K-10) | Alcohol, cigarette and other substance use, gender, parent education, school grades and antisocial behaviour (age 12) | CU (mean 1–2x/year) not significantly associated with depression and anxiety symptom scores | 6 |
| Gage et al. 2015 | Avon UK 1991 birth cohort | 1,791 | 2 | CU frequency (age 16) | AD or MDD (ICD 10)(age 18) | Alcohol and illicit drug use, family history depression, maternal education, urban living, childhood IQ, personality traits, victimization, conduct disorder, depression/ anxiety age 16 | CU frequency was not significantly associated with ↑ odds of AD (AOR = 0.96 (0.75–1.24) or MDD (AOR = 1.3 (0.98 – 1.72), p=0.065) by age 18 after adjusting for confounders | 7 |
| Feingold et al. 2015 | US adult represent. | 43,093 | 3 | CU frequency over last 12 months | BD-1, BD-2 MDD (DSM IV-TR) | Sex, age, education, income, marital status, urbanity, alcohol use, other substance use, other psychiatric disorder | Past year CU not associated with increased incidence of BD (AOR 1.17 (0.65–2.11) or MDD (AOR = 0.58 (0.22–1.51)) after adjusting for confounders | 8 |
| Degenhardt et al. 2013 | Victoria, Australia adolescents | 1,388 | 15 | CU frequency (age 16) | AD (ICD 10) (age 29) | Alcohol, nicotine, and illicit drug use, age, education level, nationality, adolescent anxiety/depression | Weekly CU in adolescence did not affect odds of MDD (OR = 1.2 (0.73–2.0), p = 0.6) or AD (OR = 1.4 (0.84–2.5)) by age 29 | 8 |
| Van Laar et al. 2007 | Holland adult represent (1996) | 5618 total | 3 | CU frequency | AD, MDD, dysthymia, BD (DSM III-R) | AUD/SUD, age, gender, education, urbanicity, employment, partner status, neuroticism, parental psych history, childhood trauma, life time psychiatric disorder | Any baseline CU associated with ↑ MDD (AOR = 1.68 (1.11–2.55), p < 0.05), but not dysthymia (AOR = 1.55 (0.67–3.58), p >0.05), BD (AOR = 5.38 (1.93–14.9) p< 0.01), or any AD (AOR = 1.27 (0.77–2.12) | 8 |
| Hayatbakhsh et al. 2007 | Australia 1981 birth cohort | 2,854 | 7 | CU frequency (age 21); Age first CU | Depression and anxiety symptoms (YASR) | Age 14 smoking/alcohol use, gender, maternal factors, family income | Frequent CU ↑ mood and anxiety symptoms at 21 years with early onset (OR=3.0 (1.8–5.2)) and late onset (OR = 2.3 (1.5–3.6) users | 8 |
| Fergusson et al. 1996 | New Zealand birth cohort (1977) | 1,265 | 2 | Any CU (age 14) | AD, MDD, or dysthymia age 16 (DSM III-R) | Substance use age 12; family social position, functioning, substance abuse; childhood behaviour problems, cognitive ability, truancy, plan to enter university, peer affiliations, conduct problems | CU by age 15 not associated with ↑ depressive disorder (AOR=1.4 (0.7–2.4)) or AD (AOR = 1.2 (0.5–2.8)) after adjusting for confounding variables | 8 |
Five studies examined large national data sets, and came to conflicting conclusions. In a Dutch national data set, it has been found that CU in an adult population (mean age 41.2) predicted both new diagnosis of bipolar disorder 26 and mania symptoms 27, in a dose-dependent manner even after adjusting for multiple confounding variables. Marwaha and colleagues found similar results in their analysis of a rich data set from a UK birth cohort comparing CU at age 17 and the presence of hypomania symptoms at age 22 28. This is in contrast to analysis of more recent US representative adult data showing that the association between CU and bipolar disorder onset was lost when other substance use disorders were accounted for in the statistical analysis 29.
One smaller study attempted to examine the role of CU in a more specific, and high-risk population. Ratheesh and colleagues 30 demonstrated that CU in a clinically high-risk population may increase odds of conversion to bipolar disorder, although there was a limited number of conversions and they were not able to reach statistical significance with this small sample.
3.2.2. Bipolar Disorder Course
Five prospective studies (N=4334) met inclusion criteria (Table 2). Analyses of a longitudinal data set of people with bipolar disorder from 14 European countries (N=3,684) showed that those who use cannabis have higher symptoms of mania and psychosis, but not depression, relative to non-using controls. Furthermore, discontinuing CU can improve outcomes to the level of non-users 31. Four smaller American and Australian studies also show altered disease course including lower remission 32, increased symptoms of mania 33,34, and rapid cycling35 in people with BD who are actively using cannabis.
Table 2:
Cannabis and the course of mood and anxiety disorders
| Author, year |
Participants | N | Follow up (yrs) |
Cannabis Use |
Outcome | Adjustment Variables | Relevant Findings | NOS |
|---|---|---|---|---|---|---|---|---|
|
BIPOLAR DISORDER | ||||||||
| Kim et al. 2015 | Australia adults with BD-1 | 239 | 2 | CU > 3 days/wk | Remission rate of mood episode (YMRS <12, HAM- D <8) | Sex, age, tobacco use, baseline mania and depression symptoms | CU associated with ↓ total (p=0.025) and ↓ depression (p=0.035) remission rates, but not with mania rates (p=0.051) using Man- Whitney U-test | 8 |
| Kvitland et al. 2015 | Norway BD-1 (first episode) | 101 | 1 | None, continued, or discontinued CU | Mood symptoms (YMRS, IDS-C), psychotic symptoms (PANSS) and GAF | Determined post hoc: Sex, age, pre morbid academic functioning, GAF, YMRS scores | Continued CU associated with mania symptoms (F=8.4, p 0.005, d= −0.3) and lower GAF (F=6.6, p 0.013, d=1.4), but not depression or psychotic symptoms | 7 |
| Zorrilla et al. 2014 | Europe adults with BD-I | 3,684 | 2 | None, continued, or discontinued CU | Remission (CGI-BP <3), recovery, relapse, functional impairment (multiple measures) | Age, sex, age at onset of BD, presence of psychosis, alcohol use, other substance use | Continued CU associated with ↓ recovery (χ2=14.85, P=0.001), ↓ Remission (χ=21.78, P<0.001), ↓recurrence (χ2=7.69, P=0.02) and ↑relapse (χ2=9.91, P = 0.007) | 9 |
| Baethge et al. 2008 | MA, USA Inpatients BD-1 (1st episode) | 166 | 4.7 (mean) | Regular CU, CUD | Affective symptoms or episodes (SCID- DSM IV) assessed every 3 months | Age, sex, years of total exposure, alcohol use | Mania symptoms associated with CU in same (RC=0.116 (0.053–0.178), p<0.001) or preceding (RC=0.11 (0.054–0.168, p<0.001) 3-month intervals, but not the following interval (RC=0.017 (0.003–0.037), p=0.09). Depression symptoms unrelated to CU. | 7 |
| Strakow- ski et al. 2007 | Cincinnati, USA BD-1 (1st episode) | 144 | 2.6 (mean) | CU frequency, CUD | Affective recovery, recurrence (YMRS, HAMD-17, SCID) | Age, sex, ethnicity, education, age at onset of CU | CU associated with ↑ time in manic episode (F=2.8, P=0.06) and rapid cycling | 7 |
| DEPRESSIVE DISORDERS | ||||||||
| Feingold et al. 2017 | US adults represent. with MDD (DSM-IV- TR) | 2,348 | 3 | CU frequency (any, daily); CUD | Recurrence, remission, suicidality, functional impairment | AUD, SUD, sex, race, education level, household income, marital status, age, region, other DSM diagnosis | Positive association with CU frequency and number of depression symptoms (B=0.62, SE=0.07, P=0.0019), but no difference found in remission, functional impairment, or suicidality between groups. | 8 |
|
ANXIETY DISORDERS | ||||||||
| Feingold et al. 2018 | US adult represent. with AD (DSM-IV- TR) | 4,007 | 3 | CU frequency (any, daily); CUD | Remission, suicide rate, functional impairment, QoL | AUD, SUD, sex, race, education level, household income, marital status, age, region, other DSM diagnosis | Non-users (65.9%) had ↑ remission rates compared to any CU (52.8%) and CUD (46.8%), but not statistically significant when adjusted for covariates. | 8 |
| Bricker et al. 2007 | US adult with PD, CU < weekly, in RCT for PD treatment | 232 | 1 | CU frequency at baseline, 3m, 6m, 9m, 12m | Anxiety and social phobia symptoms (fear questionnaire, ASI) | N/A (RCT) | Monthly CU did not moderate treatment effect of panic or social phobia measures | N/A |
| PTSD | ||||||||
| Wilkinson et al. 2015 | US male veterans, inpatients with PTSD and no other substance use | 2,276 | 0.33 | CU: None, stopped, continued, or started | PTSD symptoms (M PTSD-sf); violent behaviour score; suicidality | Sociodemographic factors, PTSD symptoms, employment, violent behaviour, incarceration history, length of stay, discharge status, year of admission | People who started or continued CU had higher measures of PTSD symptom severity (F=21.47, p<0.0001). Less days of CU was associated with ↓ symptoms (B=0.17, t=4.08, p<0.0001) and ↓ severity of violent behaviour (B=0.1, t= 2.79, P=0.0054) | 5 |
3.2.3. Cannabinoid Therapeutics in Bipolar Disorder
Only one case report (N=2) has investigated the potential therapeutic benefit of a cannabinoid in people with bipolar disorder (Table 3). CBD monotherapy was found to be an ineffective intervention in two female patients admitted to hospital with mania and psychotic features, who had previously responded to traditional mood-stabilizing medications 36
Table 3:
Cannabinoids in treatment studies
| Author, year |
Participants | Diagnosis | N | Design | Intervention | Comparator | Outcome Measurement | Relevant Findings |
|---|---|---|---|---|---|---|---|---|
| Zuardi et al. 2008 | Brazil Adult inpatients | BD-1 manic episode with psychotic features | 2 | Case report, 4 weeks | CBD 600–1200 mg PO daily, divided | Placebo, CBD + olanzapine | Mania symptoms (YMRS, BPRS) | No improvement identified with CBD monotherapy. CBD was safe and well tolerated. |
| Bergamaschi et al. 2011 | Undergrad. Students, treatment naive | SAD | 24 | Randomized double blind trail | CBD 600 mg PO x 1 dose | Placebo | Visual analogue mood scale; Public speaking Scale; Bodily symptoms scale;; skin conductance, blood pressure | Pretreatment of SAD patients with CBD significantly reduced anxiety, cognitive impairment, and discomfort in their speech performance; their measures were similar to healthy controls completing the same task |
| Jetley et al. 2015 | Canada male military personnel | PTSD | 10 | Randomized double blind cross over trial, 7 weeks each | Nabilone 0.5–3 mg PO nightly + treatment as usual | Placebo + treatment as usual | Nightmare severity (CAPS, PTSD dream rating scale), general well being (WBQ), CGI | Nabilone was significantly associated with improvement in PTSD nightmare severity (CAPS nightmare subscales, p=0.03), CGI-C (p=0.05), WBQ (p= −0.04) |
| Cameron et al. 2014 | Ontario male corrections inpatients | PTSD | 104 | Case series, open label, adjusted doses, retrospective chart review | Nabilone 0.5–6 mg PO nightly for any indication | N/A, within subject | Sleep hr/night, nights with nightmares/wk | Nabilone treatment associated with ↑ average sleep hr/night; (t= 13.7, p<0.001); ↓ nightmares/wk (t=17.9, p<0.001) 29.8% adverse events; 9.6% terminated trial |
| Roitman et al. 2014 | Israel outpatients, prior CU excluded | PTSD | 10 | Case series, open label, aadjusted doses, 3 weeks | THC 2.5 5 mg SL BID + treatment as usual | N/A, within subject | Symptom severity (CAPS, CGI), sleep quality, nightmare frequency | ↓ hyperarousal symptoms (p<0.02) jCGI-S (p<0.02) ↑ sleep quality (p<0.05) ↓ nightmare frequency (p<0.04) |
| Fraser 2009 | Ontario outpatients | PTSD with treatment resistant nightmares | 47 | Case series, open label, aadjusted doses | Nabilone 0.5 – 4 mg nightly + treatment as usual | N/A, within subject | Subjective rating nightmare intensity and hours of sleep in personal log | 72% experienced total cessation of nightmares, 13% satisfactory reduction in nightmares 28% mild-moderate side effects leading to discontinuation |
3.3. Depression
3.3.1. Depression Onset
We included 20 prospective studies that examined the effect of CU on developing depression symptoms [WHO-MDI, CES-D, CIS-R, Depressive Mood List, Kandel and Davies score, YASR], or receiving a new MDD or dysthymia diagnosis (DSM III-R – DSMV, ICD). Follow-up times ranged from 1 – 40 years. Studies varied significantly in CU measures and took into account one or more of the following: age of CU onset, duration of CU, frequency of CU, any lifetime CU, or presence of cannabis use disorder (CUD). Studies also varied in the adjustment variables selected and controlled for in the statistical analysis, and these are described in Table 1.
We identified six prospective studies demonstrating CU does not significantly affect the odds of a future depression diagnosis 29,37–41. Amongst these are two large national studies 29,37, as well as two small, but more robust analyses of rich data sets following cohorts from adolescence into early adulthood 38,39. Both Fergusson and colleagues (1996) and Gage and colleagues (2015) analyzed birth cohort data, but these results were limited to two years of follow-up in late adolescence only 40,41. In addition, there were 7 studies that demonstrated no significant effect of CU 42–47 or CUD (Bovasso, 2001) on future subclinical depression symptoms.
Three prospective studies suggest that CU is associated with increased risk of a future depression diagnosis 26,48–50 and one study that showed CUD was associated with a diagnosis of major depression 48. Amongst the CU studies, there was only one well-controlled representative trial with a large sample 26. Although Georgiades and Boyle 49 followed a large sample for several years, they did not control for other substance use, an important confounding variable. Schoeler and colleagues analyzed a small (N = 411), but detailed, data set of low SES males for 40 years. Not only did they find a significant positive association for CU and depression, they went on to report a stronger relationship in those with early onset CU and higher frequency of CU 50. With regards to subclinical depression symptoms, two studies in adolescent populations showed a positive prospective association with CU 51,52. Otten and Engels 53 also found a positive association between CU and depression symptom in adolescents, a relationship which they found to be partially mediated by the presence of a specific 5HT-R genotype (short allele of the 5-HTTLPR).
3.3.2. Depression Course
Only one large prospective cohort study using data from a US adult representative sample has investigated the role of CU on the course of unipolar depressive disorders54. They found that CU was positively associated with more depression symptoms after 3 years; however, groups did not differ significantly in rates of remission, functional impairment, or suicidality. CUD was significantly associated with anhedonia, weight change, sleep disturbance and psychomotor complaints.
3.3.3. Cannabinoid Therapeutics in Depression
No studies meeting criteria were identified.
3.4. Anxiety Disorders
3.4.1. Anxiety Onset
We included 12 prospective studies that examined the effect of CU on developing anxiety symptoms [Depression anxiety stress scale (DASS-21), Sheehan patient-rated anxiety scale (SPRAS), Kressler Psychological Distress Scale (K-10), Youth Adult Self Report Scale (YASR)], or receiving a new anxiety disorder diagnosis (generalized anxiety disorder (GAD), social anxiety disorder (SAD), panic disorder (PD) with or without agoraphobia, or specific phobia). Follow-up times ranged from 1 – 20 years. As with depression, studies of anxiety disorders and symptoms varied significantly in CU measures and took into account one or more of the following: age of CU onset, duration of CU, frequency of CU, any lifetime CU, or presence of cannabis use disorder (CUD). Studies also varied in the confounders selected and controlled for, and these are outlined in Table 1.
Nine cohort studies (N=20,288 participants) concluded there was no significant association between CU and developing a future anxiety disorder (AD) 26,38,40,41,55,56 or anxiety symptoms 44,45,57. Two studies considered the anxiety disorders as individual diagnoses rather than a collective: Zvolensky and colleagues did not find any association for CU and PD 55, and Van Laar and colleagues did not find a selective effect of CU on SAD, GAD, PD, or specific phobia 26. Only three of the studies were sufficient duration to follow a cohort from adolescence into adulthood 38,45,56. Although some of these studies initially found an increased odds of anxiety onset, the effect lost significance after adjusting for variables such as other substance use and early-life psychosocial factors.
Three studies (N=38,315) found CU increased the odds of developing an AD or anxiety symptoms 52,58,59. Analysis of a large US adult representative sample (N=34,653) showed increased odds of a new diagnosis of PD with agoraphobia and SAD in those with CU three years previously, but only the association for SAD was significant after accounting for additional confounding variables including other SUD 29. The remaining two studies, both with long follow-up times extending from adolescence into adulthood, demonstrated significantly increased odds of anxiety symptoms in those with CU 52,59.
3.4.2. Anxiety Disorder Course
Two studies met inclusion criteria (Table 2). Feingold and colleagues (2018)60 analyzed data from a US adult representative sample to investigate the role of CU on the course of anxiety disorders (64.3% specific phobia, 25% social anxiety, 18.4 % GAD, 19.3 % panic disorder) over three years. Outcomes included: remission, suicidality, functional impairment and health-related quality of life (QoL). There was a trend towards decreased remission rates in people with any CU or CUD, but none of the outcomes reached statistical significance after accounting for other variables in the statistical analysis.
Bricker and colleagues (2007) conducted an investigation to see if cannabis use would have a moderating effect on a combined medication and cognitive behavioural therapy intervention for people with PD. In their RCT (N=232), participants using cannabis did not have significantly different outcomes from those who were not using cannabis, regardless of the intervention, after one year 61.
3.4.3. Cannabinoid Therapeutics in Anxiety Disorders
Only one study (N=24) has investigated the potential therapeutic benefit of a cannabinoid in people with anxiety disorders (Table 3). Bergamaschi and colleagues 62 administered a one-time dose of CBD to undergraduate students with SAD before a public speaking trial. The group who received CBD had significant reductions in multiple measures of anxiety and discomfort, and were similar to healthy controls performing the same task.
3.5. Post-traumatic Stress Disorder (PTSD)
3.5.1. PTSD Onset
One prospective study (N=674) met inclusion criteria 63 (Table 1). Lee and colleagues (2018) conducted a longitudinal study following a population of students from Harlem New York from age 14–36, tracking CU patterns across the entire timeline. One third of the sample experienced a traumatic event. People with early-onset and continued CU were found to have increased odds of developing PTSD symptoms following trauma, compared to non-users and early quitters.
3.5.2. PTSD Course
The effect of CU on the course of PTSD symptoms has only been prospectively studied in a population of US veterans (N=2,276) who were admitted to specialized inpatient treatment facilities 64 (Table 2). Over a short time course (4 months) it was found that participants who started CU or continued CU had higher measures of PTSD symptom severity relative to non-users and those who quit CU during the study. The authors did not specifically report nightmare or sleep measures.
3.5.3. Cannabinoid Therapeutics in PTSD
There have been three studies using nabilone, a THC analogue, as a treatment in people diagnosed with PTSD (Table 3). In a small randomized placebo controlled trial of male Canadian military personnel (N=10), Jetley and colleagues found statistically significant improvements in nightmare severity in patients receiving nabilone 65. Other PTSD symptomology was not accounted for in the study design. Moreover, two open-label case series of nabilone in people with PTSD (N=104 and N=47 respectively 66,67) also showed benefit in sleep and nightmare related measures, but in both trials there were notable adverse event rates (9.6% and 28%) leading to discontinuation of nabilone. Only one open-label case series has been conducted for THC in a cannabis naïve population with PTSD (N=10) 68. After 3 weeks, there were improvements in hyperarousal symptoms, sleep quality, and nightmare frequency.
4.0. DISCUSSION
We completed a systematic review of the available evidence of cannabis and/or cannabinoids and their relationship to the onset, course, and treatment of mood and anxiety disorders.
4.1. Onset
Cannabis does not appear to be a clear independent risk factor for the onset of most mood and anxiety disorders and symptoms. The exception to this is PTSD: there is preliminary evidence that continued cannabis use may increase the odds of developing PTSD in people who have been exposed to trauma 63. This result has yet to be replicated in a broader population. In regards to anxiety disorders, our conclusion considers GAD, SAD, panic, agoraphobia, and specific phobia collectively. Few studies distinguished between individual anxiety disorders in their design or analyses; with significant effects noted for only GAD 59 and SAD 60. This differential effect among anxiety disorders may warrant further investigation. There is evidence showing cannabis use may increase subclinical mania symptoms 27,28,69, but this has unclear clinical significance and should be interpreted cautiously given this has not been a consistent finding for bipolar disorder 26,29.
4.2. Course
Cannabis appears to negatively affect multiple disease measures in bipolar disorder, but most notably the severity, persistence, and frequency of manic episodes and psychotic features. This finding supports the conclusions of a past systematic review and meta-analysis of the same topic 20. Cannabis use is also significantly associated with symptom severity of PTSD and depression, but it has not yet been demonstrated that CU affects relapse and remission rates, quality of life, or suicidality in these disorders. Although cannabis use appeared to affect remission rates in anxiety disorders as well 60, this effect was lost once adjusted for sociodemographic variables and other substance use disorders.
Compared to studies in the general population, there are far fewer studies investigating the role of cannabis on the clinical course of people with established mood and anxiety disorders. This is surprising given the obvious clinical relevance: not only are rates of cannabis use higher in people with mood and anxiety disorders 13–16, but depression and anxiety are two of the most frequently endorsed reasons for cannabis use in naturalistic cross-sectional data sets 23,70,71. Our results suggest that people with bipolar disorder, depression, and PTSD, should be cautioned about continued cannabis use despite the subjective therapeutic relief that is often reported. Furthermore, there may also be implications for public policy about medical marijuana. PTSD, for instance, is already an approved indication for medical marijuana in Canada and several US jurisdictions, which has the potential to minimize public perception of risk 3,72.
4.3. Therapeutics
The therapeutic potential of cannabinoids in mood and anxiety disorders has not been well-studied. Our work raises a potential utility of CBD in social anxiety disorder but larger, randomized trials are necessary to more clearly establish effectiveness and tolerability. We have also described preliminary data that suggests nabilone may be beneficial in treating nightmares related to PTSD; however, it still remains unclear how nabilone may impact other PTSD symptoms. This finding is in contrast to our previously stated result that cannabis use may have a negative impact on the overall course of PTSD. Several variables may contribute to this difference; most notably the potential for cannabinoids to have differential effects on different symptoms of an illness. ‘Cannabis use’ implies exposure to THC, CBD, and other cannabinoids in highly variable doses, whereas nabilone is a pure THC analogue.
There is additional literature, not included in this review, that considers the effects of cannabinoids such as dronabinol, nabiximols, and nabilone, on mood and anxiety symptoms as secondary outcomes in treatment studies of other primary diagnoses, mainly chronic pain conditions 19. There is some evidence that cannabinoids may have benefit for mood and anxiety symptoms in these populations, but these participants did not necessarily meet diagnostic criteria for mood or anxiety disorders. Further research is warranted in clinical populations with mental health diagnoses, especially for CBD which has been highlighted in preclinical work as a therapeutic agent of interest for anxiety disorders 73–75, but has not been well-studied in bipolar disorder and depression 76.
4.4. Strengths
One of the main strengths of this review is its clinical focus: we asked three important questions about mental health and cannabis use that could be of particular interest to public educators, policy makers, consumers, and mental health clinicians. Extending our search to all mood and anxiety disorders allowed us to highlight any differential roles for cannabis between disorders, and also to identify relative strengths and weaknesses in the available research for different conditions. Importantly, we decided to limit our scope to prospective research to help strengthen our understanding of the potential role of cannabis as a risk or prognostic factor for mood and anxiety disorders. Longitudinal studies can help establish temporality between two variables in naturalistic data sets and thus reduce the chance of reverse causality, which is a limitation of cross-sectional study designs.
4.5. Limitations
There are several limitations to this review. First and foremost is the limited number of studies that met inclusion criteria, especially for longitudinal studies of cannabis on the clinical course of mood and anxiety disorders, and for studies of cannabinoid therapeutics. Overall, the cannabis and psychosis research is more abundant and more extensively analyzed than it is for mood and anxiety disorders, allowing for relatively stronger conclusions that cannabis is both a risk factor 77,78 and negative prognostic factor 79 in primary psychotic disorders. Furthermore, preliminary randomized controlled trials have also suggested that CBD may have some therapeutic benefit for psychosis and requires further investigation 80,81.
There was significant heterogeneity in the populations, lengths of follow-up, and measured variables of the included studies in our review, which makes it challenging to make clear conclusions about the data. For instance, mean participant age, and age of cannabis use varied between studies, and it has been demonstrated in the psychosis literature that age of onset of cannabis use is relevant for mental health outcomes 82. Many of the large epidemiological studies, while robust and representative of a large adult populations, were not able to account for this important period of vulnerability 29,54,58,60. The duration of follow-up is also important: shorter and longer follow-ups may highlight immediate effects of intoxication and/or withdrawal compared to longer lasting effects of chronic cannabis use, respectively.
Despite our focus on longitudinal cohort studies, it is still not possible to draw conclusions about cannabis and causality for the onset and progression of mood and anxiety disorders. Although some studies attempted to strengthen their results by delineating a dose-response relationship, CU frequency and/or duration of use were often the only measures used to determine dose 26–28,38,40,52,59. The quantity of cannabis used over time, the method of consumption (edible, smoke, vapor), and the relative potency of cannabis and its constituents, is also important for determining level of exposure. THC and CBD content (% weight per volume), for instance, can vary considerably between strains, and they are known to have differential effects on mental health outcomes 83. This limitation makes it challenging to establish clear dose-response effects with cannabis in naturalistic study designs.
Mood and anxiety disorders and substance use often occur co-morbidly. It is likely that there is a complex network of overlapping and multidirectional relationships that explain the high rates of comorbidity, rather than individual theories alone like the self-medication or addiction vulnerability hypothesis (Lowe et al 2018). It is therefore very challenging within a naturalistic data set to parse direct relationships, such as the relationship between cannabis and the onset and course of mood and anxiety symptoms, given other confounding factors. It is evident from this review that co-morbid substance use and/or substance use disorders, as well as psychosocial factors, mediate and/or confound the relationship between cannabis and mental health. In several studies, initial significance was lost once these confounding variables were appropriately adjusted for in the analyses. We attempted to report all co-variates and adjusted odds ratios when possible, as this also contributes to a significant portion of the variability between studies.
4.6. Future Directions:
It is evident that we have much to learn and clarify about the relationship between cannabis and cannabinoids, and mental illness. Our work highlights a specific need for rigorous and well-designed studies, especially in clinical populations with mood and anxiety disorders, for whom it will be critical to understand the full impact of recreational cannabis use and therapeutic cannabinoids in this new age of medical cannabis, decriminalization, and legalization. For the general population, epidemiological studies of cannabis use must consider the full complexities of mental health and addiction, paying particular attention to adolescent cannabis use and its implications throughout adulthood. In addition, robust monitoring and data collection in countries with legalization, such as Canada, is encouraged until more definitive conclusions can be made.
5.0. ACKNOWLEDGEMENTS
This work was supported in part by a grant from the Astrid H. Flaska Fund/Scotiabank and NIDA grant R21-DA-043949 to Dr. George.
This work was supported in part by a grant from the Astrid H. Flaska Funds/Scotiabank and NIDA grant R21-DA-043949 to Dr. George.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Literature Cited:
- 1.Hasin DS. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology. 2018;43(1):195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler P. Cannabis is legal in Canada — here’s what you need to know. CBC News Canada; 2018. [Google Scholar]
- 3.Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL. Qualifying Conditions Of Medical Cannabis License Holders In The United States. Health affairs (Project Hope). 2019;38(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George TP, Hill KP, Vaccarino FJ. Cannabis Legalization and Psychiatric Disorders: Caveat “Hemp-tor”. The Canadian Journal of Psychiatry. 2018;63(7):447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall W, Lynskey M. Evaluating the public health impacts of legalizing recreational cannabis use in the United States. Addiction. 2016;111(10):1764–1773. [DOI] [PubMed] [Google Scholar]
- 6.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA. 2015;313(24):2474–2483. [DOI] [PubMed] [Google Scholar]
- 7.Palazzoli F, Citti C, Licata M, et al. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal 2018;150(Complete):25–32. [DOI] [PubMed] [Google Scholar]
- 8.Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The Pharmacologic and Clinical Effects of Medical Cannabis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2013;33(2):195–209. [DOI] [PubMed] [Google Scholar]
- 9.Yin A-q, Wang F, Zhang X. Integrating endocannabinoid signaling in the regulation of anxiety and depression. Acta Pharmacol Sin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nature Reviews Neuroscience. 2014;15:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarra-Lecue I, Pilar-Cuéllar F, Muguruza C, et al. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem Pharmacol 2018;157:97–107. [DOI] [PubMed] [Google Scholar]
- 12.Fraguas-Sánchez AI, Torres-Suárez AI. Medical Use of Cannabinoids. Drugs. 2018;78(16):1665–1703. [DOI] [PubMed] [Google Scholar]
- 13.Agosti V, Nunes E, Levin F. Rates of Psychiatric Comorbidity Among U.S. Residents with Lifetime Cannabis Dependence. The American Journal of Drug and Alcohol Abuse. 2002;28(4):643–652. [DOI] [PubMed] [Google Scholar]
- 14.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Cannabis use and cannabis use disorders among individuals with mental illness. Compr Psychiatry. 2013. [DOI] [PubMed] [Google Scholar]
- 15.Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies. BMC Psychiatry. 2014;14(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. [DOI] [PubMed] [Google Scholar]
- 17.Gage SH, Hickman M, Zammit S. Association Between Cannabis and Psychosis: Epidemiologic Evidence. Biol Psychiatry. 2016;79(7):549–556. [DOI] [PubMed] [Google Scholar]
- 18.Murray RM, Di Forti M. Cannabis and Psychosis: What Degree of Proof Do We Require? Biol Psychiatry. 2016;79(7):514–515. [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysisCannabinoids for Medical UseCannabinoids for Medical Use. JAMA. 2015;313(24):2456–2473. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: A systematic review and meta-analysis. J Affect Disord 2015;171(Complete):39–47. [DOI] [PubMed] [Google Scholar]
- 21.Steenkamp MM, Blessing EM, Galatzer‐Levy IR, Hollahan LC, Anderson WT. Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: A literature review. Depress Anxiety. 2017;34(3):207–216. [DOI] [PubMed] [Google Scholar]
- 22.Mammen G RS, Roerecke M, Bonato S, Lev-Ran S, Rehm J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79(4). [DOI] [PubMed] [Google Scholar]
- 23.Walsh Z, Gonzalez R, Crosby K,S Thiessen M, Carroll C, Bonn-Miller MO. Medical cannabis and mental health: A guided systematic review. Clin Psychol Rev 2017;51(Complete):15–29. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ: British Medical Journal. 2009;339(7716):332–336. [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 26.Van Laar M, Van Dorsselaer S, Monshouwer K, De Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102(8):1251–1260. [DOI] [PubMed] [Google Scholar]
- 27.Henquet C, Krabbendam L, de Graaf R, ten Have M, van Os J. Cannabis use and expression of mania in the general population. J Affect Disord 2006;95(1):103–110. [DOI] [PubMed] [Google Scholar]
- 28.Marwaha S, Winsper C, Bebbington P, Smith D. Cannabis Use and Hypomania in Young People: A Prospective Analysis. Schizophr Bull 2018;44(6):1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J Affect Disord 2015;172:211–218. [DOI] [PubMed] [Google Scholar]
- 30.Ratheesh A, Cotton SM, Betts JK, et al. Prospective progression from high-prevalence disorders to bipolar disorder: Exploring characteristics of pre-illness stages. J Affect Disord 2015;183:45–48. [DOI] [PubMed] [Google Scholar]
- 31.Zorrilla I, Aguado J, Haro JM, et al. Cannabis and bipolar disorder: does quitting cannabis use during manic/mixed episode improve clinical/functional outcomes? Acta Psychiatr Scand 2015;131(2):100–110. [DOI] [PubMed] [Google Scholar]
- 32.Kim S-W, Dodd S, Berk L, et al. Impact of Cannabis Use on Long-Term Remission in Bipolar I and Schizoaffective Disorder. Psychiatry Investig 2015;12(3):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kvitland LR, Melle I, Aminoff SR, et al. Continued cannabis use at one year follow up is associated with elevated mood and lower global functioning in bipolar I disorder. BMC Psychiatry. 2015;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baethge C, Hennen J, Khalsa H-MK, Salvatore P, Tohen M, Baldessarini RJ. Sequencing of substance use and affective morbidity in 166 first-episode bipolar I disorder patients. Bipolar Disorders. 2008;10(6):738–741. [DOI] [PubMed] [Google Scholar]
- 35.Strakowski SM, DelBello MP, Fleck DE, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry. 2007;64(1):57–64. [DOI] [PubMed] [Google Scholar]
- 36.Zuardi AW, Crippa JAS, Dursun SM, et al. Cannabidiol was ineffective for manic episode of bipolar affective disorder. Journal of Psychopharmacology. 2008;24(1):135–137. [DOI] [PubMed] [Google Scholar]
- 37.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Allebeck P. Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry. 2012;12(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degenhardt L, Coffey C, Romaniuk H, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108(1):124–133. [DOI] [PubMed] [Google Scholar]
- 39.Harder VS, Stuart EA, Anthony JC. Adolescent Cannabis Problems and Young Adult Depression: Male-Female Stratified Propensity Score Analyses. Am J Epidemiol 2008;168(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gage SH, Hickman M, Heron J, et al. Associations of Cannabis and Cigarette Use with Depression and Anxiety at Age 18: Findings from the Avon Longitudinal Study of Parents and Children. PLoS One. 2015;10(4):e0122896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fergusson D, Lynskey M, Horwood L. The short-term consequences of early onset cannabis use. J Abnorm Child Psychol 1996;24(4):499–512. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson AL, Halpern CT, Herring AH. Directions of the relationship between substance use and depressive symptoms from adolescence to young adulthood. Addict Behav 2016;60:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: testing for causal associations. Addiction. 2006;101(10):1463–1472. [DOI] [PubMed] [Google Scholar]
- 44.Danielsson A-K, Lundin A, Agardh E, Allebeck P, Forsell Y. Cannabis use, depression and anxiety: A 3-year prospective population-based study. J Affect Disord 2016;193:103–108. [DOI] [PubMed] [Google Scholar]
- 45.Scholes-Balog KE, Hemphill SA, Evans-Whipp TJ, Toumbourou JW, Patton GC. Developmental trajectories of adolescent cannabis use and their relationship to young adult social and behavioural adjustment: A longitudinal study of Australian youth. Addict Behav 2016;53:11–18. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen W. Does cannabis use lead to depression and suicidal behaviours? A population-based longitudinal study. Acta Psychiatr Scand 2008;118(5):395–403. [DOI] [PubMed] [Google Scholar]
- 47.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marmorstein NR, Iacono WG. Explaining associations between cannabis use disorders in adolescence and later major depression: A test of the psychosocial failure model. Addict Behav 2011;36(7):773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Georgiades K, Boyle MH. Adolescent tobacco and cannabis use: young adult outcomes from the Ontario Child Health Study. Journal of Child Psychology and Psychiatry. 2007;48(7):724–731. [DOI] [PubMed] [Google Scholar]
- 50.Schoeler T, Theobald D, Pingault J-B, Farrington DP, Coid JW, Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med 2018;48(13):2169–2176. [DOI] [PubMed] [Google Scholar]
- 51.Rasic D, Weerasinghe S, Asbridge M, Langille DB. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend 2013;129(1):49–53. [DOI] [PubMed] [Google Scholar]
- 52.Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and Anxiety and Depression in Young Adults: A Large Prospective Study. J Am Acad Child Adolesc Psychiatry. 2007;46(3):408–417. [DOI] [PubMed] [Google Scholar]
- 53.Otten R, Engels RCME. Testing bidirectional effects between cannabis use and depressive symptoms: moderation by the serotonin transporter gene. Addict Biol 2013;18(5):826–835. [DOI] [PubMed] [Google Scholar]
- 54.Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: A population based longitudinal study. Psychiatry Res 2017;251:225–234. [DOI] [PubMed] [Google Scholar]
- 55.Zvolensky MJ, Lewinsohn P, Bernstein A, et al. Prospective associations between cannabis use, abuse, and dependence and panic attacks and disorder. J Psychiatr Res 2008;42(12):1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bechtold J, Simpson T, White HR, Pardini D. Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol Addict Behav 2015;29(3):552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duperrouzel J, Hawes SW, Lopez-Quintero C, Pacheco-Colón I, Comer J, Gonzalez R. The association between adolescent cannabis use and anxiety: A parallel process analysis. Addict Behav 2018;78:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: Results from a population-based representative sample. Eur Neuropsychopharmacol 2016;26(3):493–505. [DOI] [PubMed] [Google Scholar]
- 59.Guttmannova K, Kosterman R, White HR, et al. The association between regular marijuana use and adult mental health outcomes. Drug Alcohol Depend 2017;179:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feingold D, Rehm J, Factor H, Redler A, Lev-Ran S. Clinical and functional outcomes of cannabis use among individuals with anxiety disorders: A 3-year population-based longitudinal study. Depress Anxiety. 2018;35(6):490–501. [DOI] [PubMed] [Google Scholar]
- 61.Bricker JB, Russo J, Stein MB, et al. Does occasional cannabis use impact anxiety and depression treatment outcomes?: results from a randomized effectiveness trial. Depress Anxiety. 2007;24(6):392–398. [DOI] [PubMed] [Google Scholar]
- 62.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacology. 2011;36:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JY, Brook JS, Finch SJ, Brook DW. Trajectories of cannabis use beginning in adolescence associated with symptoms of posttraumatic stress disorder in the mid-thirties. Subst Abus. 2018;39(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent in patients with posttraumatic stress disorder. The Journal of Clinical Psyhciatry. 2015;76(9):1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588. [DOI] [PubMed] [Google Scholar]
- 66.Cameron C, Watson D, Robinson J. Use of a Synthetic Cannabinoid in a Correctional Population for Posttraumatic Stress Disorder–Related Insomnia and Nightmares, Chronic Pain, Harm Reduction, and Other Indications: A Retrospective Evaluation. J Clin Psychopharmacol 2014;34(5):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraser GA. The Use of a Synthetic Cannabinoid in the Management of Treatment-Resistant Nightmares in Posttraumatic Stress Disorder (PTSD). CNS Neurosci Ther 2009;15(1):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, Open-Label, Pilot Study of Add-On Oral Δ9-Tetrahydrocannabinol in Chronic Post-Traumatic Stress Disorder. Clin Drug Investig 2014;34(8):587–591. [DOI] [PubMed] [Google Scholar]
- 69.Tijssen MJA, Van Os J, Wittchen HU, Lieb R, Beesdo K, Wichers M. Risk factors predicting onset and persistence of subthreshold expression of bipolar psychopathology among youth from the community. Acta Psychiatr Scand 2010;122(3):255–266. [DOI] [PubMed] [Google Scholar]
- 70.Cuttler C, Spradlin A, McLaughlin RJ. A naturalistic examination of the perceived effects of cannabis on negative affect. J Affect Disord 2018;235:198–205. [DOI] [PubMed] [Google Scholar]
- 71.Sexton M, Cuttler Carrie, Finnell John S., Mischley Laurie K. A cross sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis and Cannabinoid Research. 2016;1(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasin DS SA, Cerdá M, et al. US Adult Illicit Cannabis Use, Cannabis Use Disorder, and Medical Marijuana Laws1991–1992 to 2012–2013. JAMA Psychiatry. 2017;74(6):579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanessa PS, Alline CC. Evidences for the Anti-panic Actions of Cannabidiol. Curr Neuropharmacol 2017;15(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015;12(4):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loflin MJE, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Current Opinion in Psychology. 2017;14:78–83. [DOI] [PubMed] [Google Scholar]
- 76.Crippa JA, Guimarães FS, Campos AC, Zuardi AW. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front Immunol 2018;9(2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marconi A, Di Forti M, Murray RM, Lewis CM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull 2016;42(5):1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysisCannabis Use and Earlier Onset of Psychosis. Arch Gen Psychiatry. 2011;68(6):555–561. [DOI] [PubMed] [Google Scholar]
- 79.Schoeler T, Monk A, Sami MB, et al. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3(3):215–225. [DOI] [PubMed] [Google Scholar]
- 80.Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923–1932. [DOI] [PubMed] [Google Scholar]
- 81.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry. 2017;175(3):225–231. [DOI] [PubMed] [Google Scholar]
- 82.Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. J Am Acad Child Adolesc Psychiatry. 2017;56(3):214–225. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite Effects of Δ−9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology. 2009;35:764. [DOI] [PMC free article] [PubMed] [Google Scholar]