Abstract
Objective:
To determine if, similar to adults, children and adolescents demonstrate a threshold of total percent body fat (%BF) above which the slope of visceral adipose tissue (VAT) rises.
Methods:
This cross-sectional study included 557 youth, ages 8–18 years old, with a wide range of body mass index (BMI) values. Dual X-ray absorptiometry (DXA) was used to determine body composition (including VAT), and fasting blood was collected for measurement of lipids, glucose, insulin, and biomarkers. Segmented linear regression analysis identified the threshold for %BF unadjusted and adjusting for Tanner stage. Linear regression with robust variance estimation compared associations of risk factors and thresholds.
Results:
Thresholds of %BF were identified by sex (males=33%, females=38%), age (<12 years=34%, ≥12 years=30%), and race (White/non-Hispanic=31%, all other races/Hispanic=38%) above which the slope of VAT was significantly steeper (all p<0.001). The percentage of total body fat stored as VAT was higher above vs. below these thresholds (all p<0.001). Above threshold, but not below it, VAT was associated with triglycerides/HDL ratio, insulin, adiponectin, and blood pressure.
Conclusions:
The thresholds should be confirmed in longitudinal studies and may be useful in identifying youth at increased cardiometabolic risk in need of close clinical monitoring and/or intensive intervention to reduce excess adiposity.
Keywords: Visceral Adipose Tissue, Children, Adolescents
Introduction
Visceral adipose tissue (VAT) is a highly metabolically-active fat depot thought to be a primary driver of cardiometabolic risk factors such as hypertension, dyslipidemia, insulin resistance, inflammation, and oxidative stress in children and adolescents.1–3 In contrast, subcutaneous adipose tissue has been shown to be protective,4–6 suggesting that the ratio of these fat depots may in large part influence cardiometabolic health. In line with this theoretical construct, we previously reported sex-specific %BF thresholds in adults, above which there was a significant change in the slope of the relationship between VAT and total adiposity.7,8 Specifically, individuals above these thresholds had higher levels of VAT and a higher ratio of visceral/subcutaneous fat compared to those below the thresholds. Moreover, VAT was more highly correlated with cardiometabolic risk factors among individuals above vs. below the thresholds.8
A relevant hypothesis has suggested that subcutaneous fat depots may have a limited capacity for expansion such that additional fat accumulation exceeding a specific threshold may be associated with “spillover” into other ectopic depots including the visceral region.9–11 In this scenario, constraints on subcutaneous fat expandability in the presence of weight gain would be biologically disadvantageous and potentially accelerate the development of cardiometabolic risk factors. Though some studies have reported non-linear associations of BMI and waist circumference with VAT in children and adolescents,12,13 this “threshold hypothesis” has been incompletely investigated in the pediatric population, especially in youth with high levels of BMI consistent with severe obesity. However, the impetus to do so is supported by our previous observations showing that youth with VAT at or above the cohort mean had stronger associations of VAT with cardiometabolic risk factors compared to those below.14 The existence of such thresholds may have clinical significance in terms of screening and weight management practices, and may provide insight into appropriate weight loss goals for pediatric patients with adiposity levels above the cut-points.
Therefore, the purpose of this study was to determine if %BF thresholds exist in children and adolescents at which the slope of VAT significantly increases. Additionally, we sought to characterize the associations of VAT with cardiometabolic risk factors, adipokines, inflammation, and oxidative stress above and below the thresholds, if identified. We hypothesized that, similar to adults, children and adolescents would demonstrate %BF thresholds above which the slope of VAT would be steeper and levels of VAT much higher and more strongly associated with cardiometabolic risk factors.
Methods
Study Design and Participants
This cross-sectional study included children and adolescents ages 8 to <18 years old with a wide range of BMI values recruited from the greater Minneapolis and St. Paul, Minnesota area. For this analysis, data were combined from four different studies that utilized identical data collection methods and techniques: 1) a cross-sectional study evaluating cardiovascular health;15 2) baseline data from participants in a trial of topiramate for weight loss;16 3) baseline data from participants in an ongoing trial of exenatide for weight loss; and 4) baseline data from participants in an ongoing trial of financial incentives for weight loss. Participants were categorized according to BMI as having normal weight (<85th BMI percentile), overweight (85th percentile to <95th BMI percentile), class I obesity (≥95th BMI percentile to <120% of the 95th BMI percentile), class II severe obesity (≥120% of the 95th BMI percentile to <140% of the 95th BMI percentile), or class III severe obesity (≥140% of the 95th BMI percentile).17,18 The respective study protocols were approved by the University of Minnesota Institutional Review Board, and consent/assent was obtained from parents or guardians/participants.
Measurement of Clinical Variables
All testing was performed in the morning after participants had been fasting for a minimum of 12 hours. Pubertal development stage (Tanner stages 1–5) was determined by a pediatrician or a trained registered nurse. Height and weight were determined with participants wearing light clothes and without shoes using a wall-mounted stadiometer and an electronic scale, respectively. BMI was calculated as the body weight in kilograms divided by the height in meters squared. BMI-percentiles were determined using age- and gender-based definitions from the United States Centers for Disease Control and Prevention. Seated blood pressure was obtained after five minutes of quiet rest, on the right arm using an automatic sphygmomanometer and appropriately-fitted cuff. Three measurements were taken and the average of the final two was used. Blood was drawn for measurement of lipids, glucose, and insulin using standard procedures (analyzed by the Fairview Diagnostics Laboratories, Fairview-University Medical Center, Minneapolis, MN, USA, a Center for Disease Control and Prevention certified laboratory). On a subset of participants (N=336), high molecular weight (HMW) adiponectin (R&D Systems, Minneapolis, MN), C-reactive protein (CRP) (Alpco, Salem, NH), and oxidized LDL cholesterol (Mercodia, Uppsala, Sweden) were measured by ELISA in the University of Minnesota Cytokine Reference Laboratory (Clinical Laboratory Improvement Amendments licensed).
Body Composition and VAT Quantification
Total body composition was measured using DXA (iDXA, General Electric Medical Systems, Madison, WI, USA) and analyzed using its enCore™ software (platform version 16.2). Participants were scanned using standard imaging and positioning protocols while fasted >12 hours. Estimates of abdominal visceral and subcutaneous fat were obtained using the method described previously.19 Briefly, the DXA estimation method was developed by modeling the two similar regions of interest, DXA and computed tomography (CT) measured VAT volumes. In adults, the method showed strong association with CT for males (r2=0.949) and females (r2=0.957), respectively; and the 95% confidence interval mean difference was −96.0 to −16.3 cm3.19 The Bland-Altman bias was +67cm3 for females and +43 cm3 in males.19 Our group has demonstrated a high degree of correlation between single-slice measures of VAT assessed by CT and DXA in children and adolescents.20 DXA-derived visceral fat was strongly associated with cardiometabolic risk factors (to a level similar to that of CT-measured VAT), offering evidence supporting the clinical validity of the DXA method for quantify visceral fat in youth.20 All scans were reviewed by the same technician.
Statistical Analysis
Descriptive characteristics were calculated using the mean (SD) or N (%) within each BMI category for continuous and categorical variables, respectively. The relationship between %BF and VAT within sex and additionally by age group (8 to <12 years old, 12 to <18 years old) and race/ethnicity classification (White/non-Hispanic, all other races/Hispanic) was evaluated with segmented linear regression without additional covariates in the first model (model 1) and adjusted for Tanner stage in the second model (model 2) to identify a potential change in slope indicating a threshold/cut-point for a shift in VAT accumulation with increasing adiposity that may be associated with metabolic health status. Davies’ test was used to test if the slopes above and below the breakpoint were significantly different. The same analysis was performed using multiples of the 95th BMI percentile (instead of %BF) to explore whether thresholds could be identified with a more clinically-relevant metric. Percent VAT (%VAT) and the ratio of VAT to subcutaneous fat (VAT/SC ratio) in individuals above versus below threshold were compared using generalized estimated equations (GEE) with independence working correlation structure and robust variance estimation for confidence intervals and p-values. We present associations of %VAT with cardiometabolic risk factors as outcomes in individuals above vs. below threshold using an interaction of %VAT and an indicator for the given threshold, and their interaction. %VAT was used in the models with cardiometabolic risk factors to control for total fat mass differences without introducing multicollinearity between total fat mass and VAT mass in individuals above threshold. All analyses were conducted in R v3.5.1.
Results
Participant Characteristics
A total of 557 participants (mean age 13.8±2.7 years old; 260 (46.7%) males, 297 (53.3%) females) were included in this study. As expected, levels of body fat were higher and cardiometabolic risk factors were more adverse with increasing BMI category (Table 1).
Table 1.
Demographic, anthropometric, and clinical characteristics by BMI category.
Values presented are mean (sd) or N (%) where indicated.
| Covariate | Normal Weight | Overweight | Class I Obesity | Class II Obesity | Class III Obesity |
|---|---|---|---|---|---|
| (N=142) | (N=29) | (N=80) | (N=169) | (N=137) | |
| Male | 80 (56.3%) | 13 (44.8%) | 34 (42.5%) | 64 (37.9%) | 69 (50.4%) |
| Race: | |||||
| American Indian/Alaskan Native | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.2%) | 1 (0.7%) |
| Asian | 1 (0.7%) | 1 (3.4%) | 2 (2.5%) | 3 (1.8%) | 1 (0.7%) |
| African American/Black | 12 (8.5%) | 3 (10.3%) | 8 (10.0%) | 10 (5.9%) | 22 (16.1%) |
| Hawaiian/Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) |
| Multiple Races Selected | 6 (4.2%) | 2 (6.9%) | 9 (11.2%) | 14 (8.3%) | 19 (13.9%) |
| Other | 2 (1.4%) | 0 (0.0%) | 2 (2.5%) | 1 (0.6%) | 1 (0.7%) |
| Caucasian/White | 121 (85.2%) | 23 (79.3%) | 59 (73.8%) | 139 (82.2%) | 92 (67.2%) |
| Hispanic | 8 (5.6%)2 | 2 (6.9%) | 10 (12.5%) | 23 (13.6%)2 | 17 (12.4%)3 |
| White, Non-Hispanic | 116 (81.7%) | 21 (72.4%) | 51 (63.8%) | 123 (72.8%) | 79 (57.7%) |
| Tanner Stage: | |||||
| 1 | 54 (38.0%) | 5 (17.2%) | 22 (27.5%) | 12 (7.1%) | 4 (2.9%) |
| 2–4 | 67 (47.2%) | 16 (55.2%) | 41 (51.2%) | 68 (40.2%) | 67 (48.9%) |
| 5 | 12 (8.5%)9 | 7 (24.1%)1 | 14 (17.5%)3 | 89 (52.7%) | 61 (44.5%)5 |
| Age >12 Years | 81 (57.0%) | 20 (69.0%) | 45 (56.2%) | 144 (85.2%) | 126 (92.0%) |
| Age (years) | 12.5 (2.57) | 13.3 (2.61) | 12.6 (2.74) | 14.7 (2.45) | 14.8 (2.2) |
| Height (cm) | 153 (15.0) | 159 (12.3) | 156 (14.1) | 165 (11.3) | 169 (10.6) |
| Weight (kg) | 44.4 (13.4) | 61.5 (13.2) | 69.5 (18.3) | 97.4 (18.2) | 124 (23.3) |
| BMI (kg/m2) | 18.4 (2.47) | 24.0 (2.22) | 28.1 (3.77) | 35.3 (3.63) | 43.3 (5.29) |
| BMI Percentile | 48.0 (23.0) | 90.7 (3.13) | 97.5 (0.94) | 99.0 (0.27) | 99.6 (0.18) |
| Percentage of 95th BMI Percentile | 73.7 (6.74) | 92.9 (4.58) | 112 (5.82) | 130 (5.55) | 160 (16.5) |
| Trunk Fat (kg) | 3.92 (2.03) | 8.92 (3.56) | 13.4 (4.56) | 22.5 (5.22)5 | 32.1 (7.59)9 |
| Total Fat (kg) | 10.7 (4.11) | 20.0 (5.6) | 28.5 (8.53) | 43.9 (9.65)6 | 61.3 (12.8)11 |
| Total Tissue Fat (%) | 25.2 (5.95) | 34.5 (7.56) | 42.3 (5.31) | 47.2 (4.96)5 | 51.1 (4.15)9 |
| Visceral Fat Mass (g) | 80 (47) | 250 (238) | 486 (270)2 | 1041 (467)36 | 1729 (683)31 |
| Visceral Fat (%) | 0.84 (0.53)2 | 1.14 (0.81) | 1.66 (0.68)2 | 2.39 (0.9)38 | 2.8 (0.81)33 |
| VAT/SC Ratio | 0.01 (0.01)2 | 0.01 (0.01) | 0.02 (0.01)2 | 0.02 (0.01)38 | 0.03 (0.01)33 |
| Glucose (mg/dL) | 77.3 (9.0)3 | 79.9 (9.7) | 80.7 (8.94)1 | 78.7 (10.4)5 | 79.8 (8.52)4 |
| Insulin (mU/L) | 4.24 (2.79)3 | 7.73 (4.98)1 | 10.8 (6.18)3 | 18.2 (11.4)9 | 24.6 (14.5)6 |
| HDL Cholesterol (mg/dL) | 59.8 (14.4)3 | 51.0 (14.7) | 47.3 (11.1)1 | 43.8 (11.6)5 | 40.5 (8.48)4 |
| Triglycerides (mg/dL) | 71.2 (27.8)4 | 97.5 (41.8) | 115 (55.2)1 | 118 (59.7)5 | 122 (48.4)4 |
| Triglycerides/HDL Ratio | 1.29 (0.68)4 | 2.13 (1.2) | 2.7 (1.84)1 | 2.98 (1.9)5 | 3.24 (1.71)4 |
| LDL Cholesterol (mg/dL) | 80.5 (23.2)3 | 90.5 (23.6) | 95.7 (23.9)1 | 93.1 (30.0)6 | 94.2 (26.8)4 |
| Total Cholesterol (mg/dL) | 154 (26.7)3 | 161 (26.7) | 166 (28.0)1 | 160 (32.2)5 | 159 (31.3)4 |
| Oxidized LDL (U/l) | 41.6 (19.5)10 | 47.8 (22.7)1 | 56.0 (35.0)8 | 64.8 (42.5)111 | 59.8 (30.7)91 |
| HMW Adiponectin (μg/mL) | 5.5 (3.5)10 | 4.2 (2.6)1 | 3.4 (2.0)8 | 2.5 (1.3)111 | 2.3 (1.8)91 |
| CRP (mg/L) | 1.5 (3.2)10 | 4.4 (9.1)1 | 7.4 (10.7)8 | 7.6 (9.0)111 | 12.0 (9.9)91 |
| SBP (mmHg) | 106 (9.62) | 110 (9.11) | 114 (11.3) | 119 (10.5)1 | 126 (11.1)2 |
| SBP Percentile | 44.6 (24.7) | 51.3 (27.7) | 64.6 (25.6) | 68.4 (26.4)1 | 79.8 (20.8)2 |
| DBP (mmHg) | 56.7 (8.61) | 58.6 (6.85) | 58.8 (8.96) | 65.5 (8.31)1 | 69.3 (9.14)2 |
| DBP Percentile | 32.1 (21.1) | 32.6 (19.4) | 37.1 (22.3) | 49.9 (23.1)1 | 59.0 (24.7)2 |
Superscripts denote number missing observation, BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein; VAT/SC: visceral adipose tissue/subcutaneous fat
Identification of Visceral Fat Breakpoint
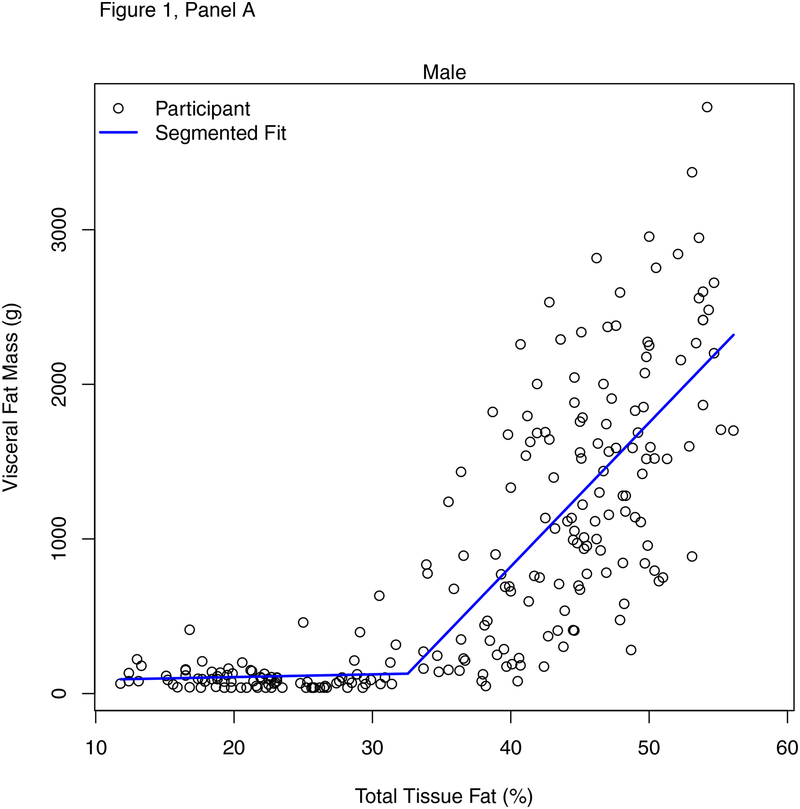
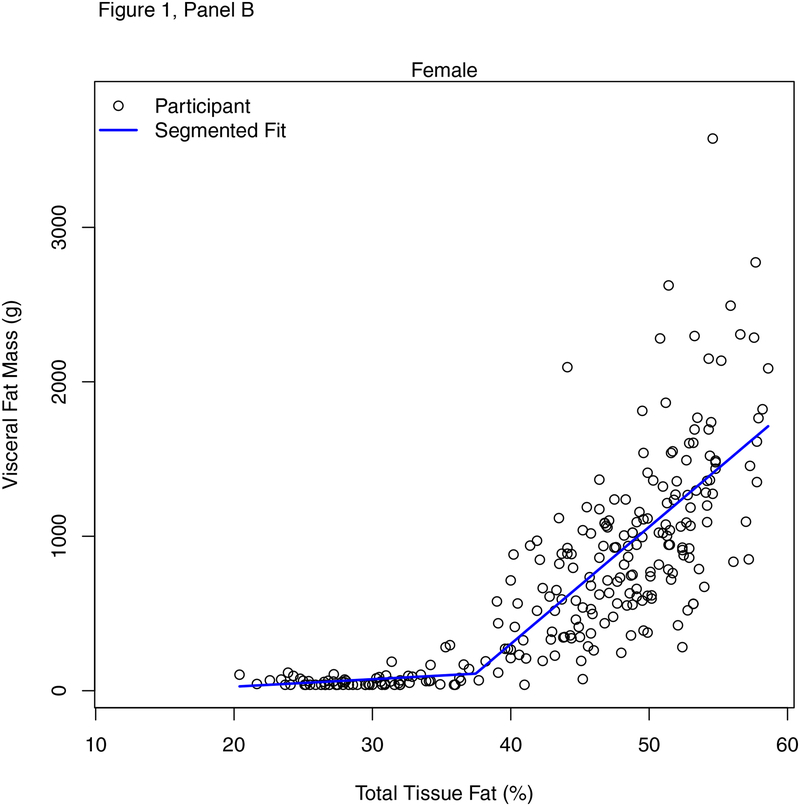
Figure 1 shows the relationship between %BF and VAT in males (panel A) and females (panel B). Table 2 shows results of the segmented regression analysis identifying the various breakpoints for %BF and multiple of the 95th BMI percentile on VAT. Thresholds of %BF were identified by sex (males = 32.6%, females = 37.5%), age (<12 years = 34.0%, ≥12 years = 29.6%), and race (white/non-Hispanic = 30.9%, all other races/Hispanic = 37.7%). The slopes of the relationship between %BF and VAT below and above the breakpoints were significantly different by sex, age grouping, and race/ethnicity with steeper slopes above vs. below the respective breakpoints. Among males, for every 1% unit-difference in %BF above threshold, VAT was higher by 93.1 grams. Among females, for every 1% unit-difference in %BF above threshold, VAT was higher by 75.8 grams. Breakpoints for multiples of the 95th BMI percentile were observed at approximately 0.91 in males (equating to values between the 87th to 90th BMI percentile), approximately 0.90 in females (equating to the values between the 87th and 90th BMI percentile), approximately 0.83 in younger children (equating to values between the 70th and 80th BMI percentile), and approximately 0.85 in older children (equating to values between the 79th and 86th BMI percentile). The slopes of the relationship between multiples of the 95th BMI percentile and VAT below and above the breakpoints were significantly different by sex, age grouping, and race/ethnicity with steeper slopes above vs. below the respective breakpoints.
Figure 1.
Sex-specific percent body fat thresholds for visceral fat mass in males (a) and females (b).
Table 2.
Segmented regression analysis results to identify the slope above and below the breakpoint of percent body fat (%BF) and multiple of the 95th BMI percentile based on outcome of visceral fat mass (g)
| %BF | Multiple of 95th BMI Percentile | ||||
|---|---|---|---|---|---|
| Group | Measure | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value |
| Males | Break Point (BP) | 32.6 (29.1,36.0) | - | 0.91 (0.81,1.01) | - |
| Slope Below BP | 1.8 (−19.1,22.6) | 0.869 | 67.1 (−1,299.8,1,433.9) | 0.923 | |
| Slope Above BP | 93.1 (78.0,108.2) | <0.001 | 2,677.1 (2,400.4,2,953.8) | <0.001 | |
| Slope Difference | 91.3 (65.6,117.0) | <0.001 | 2,610.1 (1,215.5,4,004.7) | <0.001 | |
| Females | Break Point (BP) | 37.5 (34.1,40.9) | - | 0.90 (0.76,1.04) | - |
| Slope Below BP | 4.8 (−17.3,27.0) | 0.667 | 375.8 (−634.9,1,386.4) | 0.465 | |
| Slope Above BP | 75.8 (64.7,86.9) | <0.001 | 1,894.5 (1,654.3,2,134.7) | <0.001 | |
| Slope Difference | 71.0 (46.2,95.7) | <0.001 | 1,518.8 (480.0,2,557.5) | 0.005 | |
| ≥12 Years Old | Break Point (BP) | 29.6 (25.4,33.8) | - | 0.85 (0.75,0.96) | - |
| Slope Below BP | −2.9 (−28.9,23.2) | 0.829 | 186.1 (−1,175.3,1,547.4) | 0.788 | |
| Slope Above BP | 65.6 (56.8,74.4) | <0.001 | 2,231.6 (2,018.0,2,445.2) | <0.001 | |
| Slope Difference | 68.5 (41.0,95.9) | <0.001 | 2,045.5 (667.6,3,423.5) | 0.003 | |
| <12 Years Old | Break Point (BP) | 34.0 (30.5,37.5) | - | 0.83 (0.72,0.94) | - |
| Slope Below BP | −1.9 (−14.1,10.2) | 0.753 | −3.7 (−874.0,866.7) | 0.993 | |
| Slope Above BP | 41.0 (33.6,48.5) | <0.001 | 1,131.3 (930.8,1,331.8) | <0.001 | |
| Slope Difference | 43.0 (28.7,57.2) | <0.001 | 1,135.0 (241.8,2,028.1) | 0.046 | |
| White, Non-Hispanic | Break Point (BP) | 30.9 (27.4,34.4) | - | 0.92 (0.84,1.00) | - |
| Slope Below BP | −1.6 (−21.3,18.0) | 0.870 | 222.0 (−590.3,1,034.2) | 0.591 | |
| Slope Above BP | 64.2 (55.1,73.3) | <0.001 | 2,554.3 (2,325.4,2,783.2) | <0.001 | |
| Slope Difference | 65.8 (44.2,87.4) | <0.001 | 2,332.4 (1,488.5,3,176.2) | <0.001 | |
| Non-White or Hispanic | Break Point (BP) | 37.7 (31.8,43.7) | - | 1.00 (0.75,1.25) | - |
| Slope Below BP | 10.3 (−20.5,41.1) | 0.509 | 411.9 (−1,220.1,2,043.9) | 0.619 | |
| Slope Above BP | 83.9 (63.3,104.4) | <0.001 | 2,184.4 (1,814.6,2,554.2) | <0.001 | |
| Slope Difference | 73.6 (36.5,110.6) | <0.001 | 1,772.5 (99.1,3,445.8) | 0.034 | |
Association of Visceral Fat with Cardiometabolic Risk Factors Above and Below the Threshold
Table 3 shows the relationships of %VAT with cardiometabolic risk factors above and below the various %BF thresholds for males and females. %VAT was significantly associated with most cardiometabolic risk factors among individuals above threshold, but was not associated with risk factors among individuals below threshold. With few exceptions, these relationships were consistent across the other subgroups (by age and race/ethnicity) using the %BF threshold and the multiple of the 95th BMI percentile thresholds (data not shown).
Table 3.
Cardiometabolic risk factor relationship with %VAT above and below the %BF threshold value for males and females using linear regression with robust variance estimation
| Below %BF Break Point | Above %BF Break Point | ||||
|---|---|---|---|---|---|
| Group | Outcome | Difference per VAT Percentage (95% CI) | P-value | Difference per VAT Percentage (95% CI) | P-value |
| Males | HDL Cholesterol (mg/dL) | 3.9 (−1.3, 9.0) | 0.142 | −5.6 (−7.2, −4.0) | <0.001 |
| Triglycerides (mg/dL) | 2.1 (−6.4, 10.7) | 0.628 | 20.4 (12.1, 28.6) | <0.001 | |
| Trig/HDL Ratio | 0.0 (−0.3, 0.3) | 0.909 | 0.9 (0.6, 1.2) | <0.001 | |
| Insulin (mU/L) | −0.1 (−1.1, 0.8) | 0.781 | 6.1 (4.3, 8.0) | <0.001 | |
| Oxidized LDL (U/l) | 4.3 (−3.1, 11.7) | 0.250 | −0.7 (−7.4, 6.0) | 0.835 | |
| HMW Adiponectin (μg/mL) | −0.66 (−1.46, 0.14) | 0.107 | −1.12 (−1.54, −0.71) | <0.001 | |
| CRP (mg/L) | 0.41 (−0.80, 1.62) | 0.506 | 2.53 (0.79, 4.28) | 0.004 | |
| SBP (mmHg) | 0.6 (−3.2, 4.4) | 0.767 | 6.5 (4.6, 8.4) | <0.001 | |
| DBP (mmHg) | 0.4 (−2.4, 3.2) | 0.783 | 4.4 (3.0, 5.8) | <0.001 | |
| Females | HDL Cholesterol (mg/dL) | −6.7 (−19.1, 5.8) | 0.293 | −2.2 (−3.9, −0.5) | 0.011 |
| Triglycerides (mg/dL) | 8.2 (−35.1, 51.5) | 0.711 | 9.8 (−0.1, 19.7) | 0.052 | |
| Trig/HDL Ratio | 0.2 (−0.8, 1.3) | 0.687 | 0.4 (0.1, 0.7) | 0.018 | |
| Insulin (mU/L) | −0.2 (−2.8, 2.3) | 0.871 | 5.6 (3.3, 7.9) | <0.001 | |
| Oxidized LDL (U/l) | 8.6 (−16.7, 33.8) | 0.507 | 5.9 (−2.1, 14.0) | 0.147 | |
| HMW Adiponectin (μg/mL) | 2.15 (−1.05, 5.34.0) | 0.188 | −0.75 (−1.11, −0.39) | <0.001 | |
| CRP (mg/L) | 0.84 (−1.74, 3.4) | 0.524 | 1.96 (−0.14, 4.06) | 0.067 | |
| SBP (mmHg) | −6.2 (−13.1, 0.7) | 0.078 | 2.9 (1.0, 4.8) | 0.003 | |
| DBP (mmHg) | −4.8 (−10.6, 1.0) | 0.105 | 1.8 (0.4, 3.3) | 0.012 | |
HDL: high density lipoprotein; LDL: low density lipoprotein; HMW: high molecular weight; CRP: C-reactive protein; SBP: systolic blood pressure; DBP: diastolic blood pressure
Discussion
In this study we identified breakpoints in %BF above which levels of VAT appeared to exponentially increase in children and adolescents. To our knowledge, this is one of the first reports of the existence of such thresholds in the pediatric population, yet it is important to note that similar thresholds have been reported in adults, with an almost identical cut-point in women vs. girls but a lower cut-point in men vs. boys.7,8 Initial observations of potential non-linear relationships of BMI12 and waist circumference13 with VAT in children and adolescents have been previously reported. Our results are in agreement with these studies and extend the findings within the context of a much larger sample with a wider range of BMI/adiposity levels and a higher proportion of participants within the severe obesity category. Based on these findings, we hypothesize that children and adolescents may experience different patterns of body fat deposition, depending upon age, sex, and race, when a certain threshold of total body adiposity is reached. Below these %BF thresholds, levels of VAT appeared to be extremely low, reflecting a healthier pattern of body fat distribution. Though speculative owing to the cross-sectional nature of our study, the threshold hypothesis is supported by evidence from over-feeding studies conducted in adults, which have demonstrated that increases in VAT are associated with dysfunctional subcutaneous adipose tissue expandability.9,21 Though not directly addressed in the current study and requiring further investigation, it seems reasonable to speculate that a possible physiological explanation for our observation is that the subcutaneous fat depot may have an upper limit of expansion, and when exceeded, additional fat storage is preferentially shunted to the visceral region.
The thresholds observed in our study, particularly the cut-points identified by BMI percentile, may be useful in the clinical setting to aid in risk stratification. Indeed, these thresholds may help identify children and adolescents most likely to experience steep increases in VAT levels with linear weight gain and consequently develop an adverse cardiometabolic risk factor profile. Interestingly, we found that all of the various breakpoints (by sex and age) fell within BMI percentile ranges below the current classification of obesity (≥95th BMI percentile). Although our findings in isolation do not lend to challenging the widely-accepted 95th BMI percentile cutoff for obesity, our results suggest that the inflection point associated with worsening of the cardiometabolic risk factor profile may fall below the obesity cut-point. Perhaps even youth below the 95th BMI percentile should be monitored closely for continued weight gain and development of cardiometabolic risk factors.
In the context of the current study, cardiometabolic risk factors were much more strongly associated with VAT among individuals above vs. below the respective thresholds, underscoring the primary role of visceral fat in driving cardiometabolic risk as early as the first two decades of life. These results may also explain why pediatric studies have been somewhat mixed in terms of the relative strength of associations of total body fat, subcutaneous fat, and VAT with cardiometabolic risk factors.1,3,14,20,22,23 In fact, the composition of a particular sample of participants, in terms of the percentages of individuals above and below threshold, may be the primary factor driving these relationships. The current data suggest that the range and distribution of adiposity level of the cohort studied may influence the relative strength of these relationships such that samples with higher mean %BF will demonstrate higher correlations of VAT with cardiometabolic risk factors since a higher percentage will be above the threshold.
The strengths of this study include the large sample size, inclusion of participants with a wide range of BMI values (normal weight to severe obesity), and the use of reliable and standardized methods for measuring body composition and cardiometabolic risk factors. Limitations include the relative lack of racial diversity, which somewhat hampered our ability to investigate differences in body fat distribution relationships across several race categories, the potential risk of sample bias owing to the combining of multiple study cohorts, and the fact that our measurement of visceral fat was based on DXA, rather than magnetic resonance imaging or CT - traditionally considered the gold-standard methods. Despite being labeled by some as only able to measure trunk fat, the iDXA technology can estimate visceral fat; however, the evidence base supporting its use is less-established as compared to magnetic resonance imaging or CT. However, it should be noted that the DXA-derived visceral fat method has been validated in adults and evidence points to its validity in children and adolescents.20 Finally, and perhaps most importantly, the study was cross-sectional in nature. Therefore, our findings should be viewed as hypothesis-generating and will need to be confirmed in future longitudinal studies that are designed to track temporal changes in body fat deposition patterns and associations with alterations in cardiometabolic risk and ideally clinical endpoints.
In conclusion, this study identified evidence of breakpoints in %BF above which levels of VAT appear to steeply rise in children and adolescents suggesting that VAT accumulation is not linear throughout the body fat and BMI continuum. Moreover, we identified a number of cardiometabolic risk factors that were elevated only among individuals above threshold, as well as observed that cardiometabolic risk factors were only associated with VAT above the breakpoints. Although these findings should be considered preliminary, the results raise the possibility of the existence of a total body fat threshold at which metabolic health diverges into the unhealthy realm primarily driven by an increase in relative VAT deposition favoring adipocyte dysfunction. We hypothesize that subcutaneous fat depots exhibit inherent biological limitations in their ability to expand (i.e., a ceiling effect) and when this threshold is exceeded, additional storage of fat begins to be preferentially stored in the visceral region ultimately leading to metabolic and cardiovascular decompensation. Additional studies will be needed to further investigate this hypothesis as well as characterize the pathophysiological mechanisms associated with altered patterns of fat deposition. Greater insight into this potentially important phenomenon may help guide clinical screening, monitoring, and prevention practices for obesity-associated co-morbidities and inform decisions about optimal timing and intensity of obesity treatments during childhood and adolescence.
What is already known about this subject?
Visceral adipose tissue (VAT) is a primary driver of increased cardiometabolic risk.
In adults, sex-specific thresholds of total percent body fat (%BF) have been identified above which levels of VAT steeply increase; whether similar thresholds exist in children and adolescents remains unclear.
What does your study add?
We identified sex-specific %BF thresholds at which VAT levels appear to steeply increase in children and adolescents.
Cardiometabolic risk factors, adipokines, and inflammation were more closely associated with VAT above vs. below the threshold, suggesting these cut-points may have clinical utility in identifying individuals at increased cardiometabolic risk and in need of more intensive intervention.
Acknowledgments:
The authors would like to thank the individuals who participated in the study. We are grateful for the expert study coordination provided by Ms. Annie Sheldon, Ms. Erin Hurley, Ms. Cameron Naughton, Mr. Neil Hultgren, Ms. Patti Laqua, and Ms. Kristin Garcia.
Grant Support: Funding for this project was provided by the National Heart, Lung, and Blood Institute/NIH (R01HL110957), the National Institute of Diabetes and Digestive and Kidney Diseases/NIH (R01DK105953, R01DK113631, and P30 DK050456), the National Center for Advancing Translational Sciences/NIH (UL1TR000114). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Kelly receives research support (drug/placebo) from Astra Zeneca Pharmaceuticals and serves as a consultant for WW, Novo Nordisk and Vivus Pharmaceuticals but does not accept personal or professional income for these activities. Dr. Ryder receives support from Boehringer Ingelheim Pharmaceuticals in the form of drug/placebo. Dr. Fox serves as a site principal investigator for a clinical trial sponsored by Novo Nordisk. The remaining authors report having no relevant disclosures.
References
- 1.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med 2008;162:453–61. [DOI] [PubMed] [Google Scholar]
- 2.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008;57:367–71. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Taksali SE, Dufour S, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 2005;90:3731–7. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009;32:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008;7:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch TA, Chow L, Dengel DR, et al. In adult twins, visceral fat accumulation depends more on exceeding sex-specific adiposity thresholds than on genetics. Metabolism 2015;64:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch TA, Steinberger J, Sinaiko AR, et al. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity (Silver Spring) 2015;23:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alligier M, Gabert L, Meugnier E, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab 2013;98:802–10. [DOI] [PubMed] [Google Scholar]
- 10.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans 2008;36:935–40. [DOI] [PubMed] [Google Scholar]
- 11.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta 2010;1801:338–49. [DOI] [PubMed] [Google Scholar]
- 12.Koren D, Marcus CL, Kim C, et al. Anthropometric predictors of visceral adiposity in normal-weight and obese adolescents. Pediatr Diabetes 2013;14:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubers M, Pourhassan M, Braun W, Geisler C, Muller MJ. Definition of new cut-offs of BMI and waist circumference based on body composition and insulin resistance: differences between children, adolescents and adults. Obes Sci Pract 2017;3:272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly AS, Dengel DR, Hodges J, et al. The relative contributions of the abdominal visceral and subcutaneous fat depots to cardiometabolic risk in youth. Clin Obes 2014;4:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fyfe-Johnson AL, Ryder JR, Alonso A, et al. Ideal Cardiovascular Health and Adiposity: Implications in Youth. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox CK, Kaizer AM, Rudser KD, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: A pilot randomized controlled trial. Obesity (Silver Spring) 2016;24:2553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
- 18.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr 2014;168:561–6. [DOI] [PubMed] [Google Scholar]
- 19.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes 2015;10(3):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannsen DL, Tchoukalova Y, Tam CS, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care 2014;37:2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res 2001;9:423–31. [DOI] [PubMed] [Google Scholar]
- 23.Hubers M, Geisler C, Plachta-Danielzik S, Muller MJ. Association between individual fat depots and cardio-metabolic traits in normal- and overweight children, adolescents and adults. Nutr Diabetes 2017;7:e267. [DOI] [PMC free article] [PubMed] [Google Scholar]