Abstract
Background:
Variants disruptive to CHD8 are among the most common mutations revealed by exome sequencing in autism spectrum disorder (ASD). Recent work indicates CHD8 plays a role in the regulation of other ASD risk genes. However, it is unclear whether or not a possible shared genetic ontology extends to the phenotype.
Methods:
This study (N = 143; 42.7% female) investigated clinical and behavioral features of individuals ascertained for the presence of a known disruptive ASD-risk mutation that is either (a) CHD8 (n = 15), (b) a gene targeted by CHD8 (Target, n = 22), or (c) a gene without confirmed evidence of being targeted by CHD8 (Other Gene, n = 106).
Results:
Results indicated shared features between CHD8 and Target groups that included less severe adaptive deficits in communication skills, similar functional language, more social motivation challenges in those with ASD, larger head circumference, higher weight, and lower seizure prevalence relative to the Other Gene group.
Conclusions:
These similarities suggest broader genetic ontology accounts for aspects of phenotypic heterogeneity. Improved understanding of the relationships between related disruptive gene events may lead us to improved understanding of shared mechanisms and lead to more focused treatments for individuals with known genetic mutations.
Keywords: CHD8, autism spectrum disorder, genetic subtypes, precision medicine, gene regulation, neurodevelopmental disorder
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by social communication deficits and the presence of repetitive or restricted behaviors. Although ASD is genetically heterogeneous, rare variants in the form of inherited and de novo copy number variants (CNVs) and largely de novo single-nucleotide variants (SNVs) are estimated to contribute 5-10% to the underlying genetic etiology of autism with no single event accounting for more than ~1% of all cases (1-9). Bridging underlying genetic etiology with ASD symptoms is complex, considering that the ASD phenotype is similarly variable, ranging from severe to mild neurocognitive symptoms. Efforts to identify subtypes based on the behavioral and neural phenotypes have been unsuccessful (10), and the rarity of specific genetic events has rendered subtyping at the CNV or single gene mutation level challenging and exceedingly resource-intensive. A promising avenue of research instead targets biological pathways shared by multiple ASD-associated risk genes as a means to better explain individual differences in ASD.
One such potential pathway involves the chromodomain helicase DNA-binding protein 8 gene (CHD8; located at 14q11.2) (8; 11). CHD8 is a chromatin modifier (12), and disruptive variants to CHD8 are among the most common mutations revealed by sequencing efforts in ASD cohorts (8; 13; 14). Moreover, thousands of CHD8 binding sites have been identified throughout the genome using chromatin immunoprecipitation followed by sequencing (ChIP-seq) in primary human and mouse brain tissue as well as in vitro models (13-15) enriched for ASD risk genes (13). A specific human phenotypic profile has been described for CHD8 mutation carriers, including ASD diagnosis or related symptoms, macrocephaly and brain overgrowth, intellectual disability (ID), specific facial features, gastrointestinal problems, and sleep disturbances (11; 13). However, to date, no study has evaluated the extent to which humans with mutations to genes modified by CHD8 show a similar phenotypic profile.
Given the high rate of ASD among individuals with a mutation to CHD8 (11) and the regulatory effects of CHD8 on other ASD risk genes (i.e., target genes) (13; 14), the aim of the current study was to evaluate whether mutations to CHD8 and target genes together constitute an etiological subtype of ASD. Among a sample of 143 individuals, we compared and contrasted the phenotypic presentations of individuals with an ASD-associated risk variant that either directly disrupts CHD8, disrupts a conserved CHD8 target gene, or disrupts a gene not identified as a conserved CHD8 target. Specifically, assuming a converging biological pathway, we hypothesized that those individuals with disruptive mutations to CHD8 or CHD8 targets would exhibit a unique phenotype from individuals with disruptive mutations to genes not directly targeted by CHD8.
Methods and Materials
Participants
Participants included 143 individuals (ages 44 months to 28.3 years; 42.7% female, 17 Non-White) with disruptive mutations to ASD associated risk genes. Consistent with a genetics-first approach, recruitment was not contingent on specific clinical diagnoses (e.g., ASD or no ASD). Participants were ascertained following identification of a disruptive genetic variant through clinical genetic testing (n = 80) or genetic testing conducted during participation in a research study where participant re-contact was possible (n = 63). There were no differences in ascertainment method between gene groups, p = .11. Written and informed consent and/or assent were obtained for all participants and approved by the local ethical review board.
For all participants, presence of a disruptive variant was confirmed through review of the clinical genetic testing lab report or through targeted or exome sequencing conducted as part of the referring study (16). Gene and variant information for participants are listed in Supplemental Table 2. The majority of genetic mutations were found to be de novo (n = 126); however, four were inherited (one from Target group; three from Other Gene group), and 13 were of unknown inheritance due to one or both parents not completing genetic testing (one from Target group; twelve from Other Gene group). Supplemental analyses were conducted to assess whether inheritance status influenced our results (SI Materials I).
Participants were assigned to one of three mutually exclusive groups. The CHD8 group (n = 15) included individuals with a disruptive mutation to CHD8. The Target group (n = 22) included participants with a disruptive mutation to an ASD risk gene previously identified as a conserved CHD8 bound target. Target group designation required positive evidence of CHD8-binding sites and co-expression with CHD8 (per (14)) as well as expression in the human brain (per (13)), as specified in Supplemental Table 3. Target group genes included ARID1B, CTNNB1, PTEN, SETBP1, TBL1XR1, and TRIP12. The Other ASD-Associated Gene group (“Other Gene” group; n = 106) included participants with a disruptive mutation to an ASD associated gene (16) that has not been identified as a conserved CHD8 target (i.e., a lack of evidence or lack of consensus between (13; 14)): ADNP, ANK2, ASH1L, CAPN8, CHD1, CHD2, DSCAM, DYRK1A, FOXP1, GRIN2B, KDM6B, LARP4B, LZTR1, MED13L, MYH10, NCKAP1, POGZ, SCN2A, SETD2, STXBP1, SUV420H1, SYNCRIP, TBR1, WDFY3, WDR33. The groups did not vary significantly based on age, p = .31, or sex of participants, p = .38 (see Table 1).
Table 1. Clinical and behavioral characterization.
For categorical variables, prevalence is reported as the total number in each group with the condition. Adaptive deficit was defined as having an ABC score at or below 70 on the Vineland-II. Abbreviations: prev. = prevalence; ASD = autism spectrum disorder; SD = standard deviation; ADOS-2 = Autism Diagnostic Observation Schedule, II; SA = Social Affect subscale; RRB = Restricted and Repetitive Behaviors subscale; CSS = calibrated severity score; SRS-2 = Social Responsiveness Scale, II; IQ = intelligence quotient; VABS-II = Vineland Adaptive Behavior Scales, II; HC = head circumference.
| CHD8 | Targets | Other Gene | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Group difference | p | |||||||||
| n | 15 | 22 | 106 | ||||||||
| Female : Male | 6:9 | 12:10 | 43:63 | χ2(2) = 1.96 | 0.375 | ||||||
| Mean age in months (SD) | 138.67 (67.7) | 108.05 (47.8) | 128.25 (65.7) | χ2(2) = 2.33 | 0.311 | ||||||
| Age range in months | 56-260 | 44-221 | 47-340 | ||||||||
| % | prev. | n | % | prev. | n | % | prev. | n | Group difference | p | |
| Ascertainment | |||||||||||
| Research study | 66.7% | 10 | 15 | 31.8% | 7 | 22 | 43.4% | 46 | 106 | χ2(2) = 4.51 | 0.105 |
| Clinical report | 33.3% | 5 | 15 | 68.2% | 15 | 22 | 56.6% | 60 | 106 | ||
| ASD Symptoms | |||||||||||
| ASD diagnosis | 100.0% | 15 | 15 | 70.6% | 12 | 17 | 77.2% | 71 | 92 | χ2(2) = 4.90 | 0.086 |
| ADOS-II Module | |||||||||||
| Module 1 | 21.4% | 3 | 14 | 35.3% | 6 | 17 | 50.0% | 43 | 86 | χ2(6) = 24.89 | < 0.001 |
| Module 2 | 42.9% | 6 | 14 | 58.8% | 10 | 17 | 12.8% | 11 | 86 | ||
| Module 3 | 35.7% | 5 | 14 | 0.0% | 0 | 17 | 33.7% | 29 | 86 | ||
| Module 4 | 0.0% | 0 | 14 | 5.9% | 1 | 17 | 3.5% | 3 | 86 | ||
| Cognitive and Adaptive Functioning | |||||||||||
| ID diagnosis | 53.3% | 8 | 15 | 86.4% | 19 | 22 | 75.7% | 78 | 103 | χ2(2) = 5.04 | 0.081 |
| Adaptive deficit | 60.0% | 9 | 15 | 77.3% | 17 | 22 | 86.0% | 86 | 100 | χ2(2) = 5.42 | 0.066 |
| Medical problems | |||||||||||
| Sleep issues | 100.0% | 15 | 15 | 81.8% | 18 | 22 | 90.3% | 93 | 103 | χ2 (2) = 4.52 | 0.103 |
| Seizure activity | 13.3% | 2 | 15 | 18.2% | 4 | 22 | 44.0% | 44 | 100 | χ2 (2) = 9.98 | 0.007 |
| Gastrointestinal | 80.0% | 12 | 15 | 77.3% | 17 | 22 | 81.0% | 81 | 100 | χ2 (2) = 0.16 | 0.925 |
| CHD8 | Targets | Other Gene | |||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Group difference | p | |
| ASD Symptoms | |||||||||||
| ADOS-2 CSS | 7.93 | 1.69 | 14 | 5.88 | 2.34 | 17 | 6.47 | 2.38 | 86 | χ2 (2) = 6.06 | 0.048 |
| ADOS-2 SA CSS | 7.36 | 2.06 | 14 | 5.76 | 2.36 | 17 | 6.44 | 2.54 | 86 | χ2 (2) = 3.54 | 0.170 |
| ADOS-2 RRB CSS | 9.07 | 1.21 | 14 | 6.82 | 2.43 | 17 | 7.07 | 2.39 | 86 | χ2 (2) = 12.3 | 0.002 |
| SRS-2 Total T-scores | 82.27 | 9.12 | 15 | 76.21 | 11.98 | 19 | 76.31 | 11.2 | 98 | F (2, 129) = 1.92 | 0.150 |
| Social Motivation | 76.13 | 12.57 | 15 | 64.95 | 11.21 | 19 | 64.84 | 12.68 | 98 | F (2, 129) = 5.44 | 0.005 |
| Awareness | 76.07 | 8.12 | 15 | 73.53 | 8.64 | 19 | 73.45 | 10.66 | 98 | F (2, 129) = 0.44 | 0.646 |
| Cognitive | 78.67 | 11.17 | 15 | 73.47 | 11.24 | 19 | 75.17 | 9.74 | 98 | F (2, 129) = 1.15 | 0.321 |
| Communication | 79.47 | 8.21 | 15 | 75.84 | 13.7 | 19 | 76.43 | 11.49 | 98 | F (2, 129) = 0.51 | 0.600 |
| Mannerisms | 81.47 | 10.84 | 15 | 76.74 | 16.79 | 19 | 74.71 | 13.58 | 98 | F (2, 129) = 1.61 | 0.204 |
| Cognitive and Adaptive Functioning | |||||||||||
| Full-scale IQ | 62.0 | 26.39 | 12 | 49.88 | 17.98 | 17 | 50.49 | 27.51 | 90 | χ2(2) = 2.93 | 0.231 |
| Verbal IQ | 63.75 | 28.99 | 12 | 55.59 | 24.4 | 17 | 50.3 | 28.34 | 80 | χ2(2) = 3.89 | 0.143 |
| Nonverbal IQ | 60.64 | 28.55 | 14 | 47.76 | 17.63 | 17 | 51.64 | 28.63 | 80 | χ2(2) = 1.86 | 0.395 |
| VABS-II Composite | 63.4 | 20.02 | 15 | 61.68 | 11.21 | 22 | 54.81 | 15.06 | 100 | F (2, 134) = 3.43 | 0.035 |
| Communication | 68.67 | 22.36 | 15 | 65.5 | 14.64 | 22 | 55.33 | 16.48 | 100 | F (2, 134) = 6.34 | 0.002 |
| Socialization | 63 | 18.28 | 15 | 64.68 | 13.98 | 22 | 58.7 | 14.08 | 100 | F (2, 134) = 1.85 | 0.161 |
| Daily Living | 64.53 | 19.76 | 15 | 62.27 | 10.93 | 22 | 56.33 | 16.87 | 100 | F (2, 134) = 2.44 | 0.091 |
| Physical features | |||||||||||
| HC (z-score) | 1.88 | 1.61 | 12 | 0.64 | 2.59 | 17 | −0.49 | 2.14 | 81 | F (2, 107) = 7.29 | 0.001 |
| Height (z-score) | 1.93 | 1.62 | 12 | 0.02 | 1.62 | 15 | −0.41 | 1.3 | 79 | F (2, 103) = 14.97 | <.0001 |
| Weight (z-score) | 1.13 | 0.81 | 11 | 0.57 | 1.17 | 15 | −0.24 | 1.62 | 75 | F (2, 98) = 5.13 | 0.008 |
| Behavior problems | |||||||||||
| CBCL Internalizing | 61.21 | 7.07 | 14 | 57.41 | 8.43 | 17 | 59.26 | 11.06 | 84 | F (2, 102) = 0.53 | 0.592 |
| CBCL Externalizing | 51.21 | 9.29 | 14 | 54.24 | 9.58 | 17 | 57.69 | 11.18 | 84 | F (2, 102) = 4.04 | 0.02 |
Measures
Clinical testing included direct assessment using standardized procedures with the clinician naive to the specific genetic mutation until final stages of data analysis. The assessment battery included measures of cognition, adaptive abilities, ASD symptoms, medical diagnoses, and physical measurements.
Clinical Assessment of ASD symptoms.
Research reliable clinicians administered the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2), using the module appropriate for the individual based on language ability (17). The Autism Diagnostic Interview-Revised (ADI-R) (18) was administered in order to support ASD diagnostic decisions. In order to compare group differences in language, the module of the ADOS was noted (Module 1 for those with no speech or single words, Module 2 for those with phrase speech, Modules 3 and 4 for those with fluent speech). ADOS-2 calibrated severity scores (CSS) were calculated for the total score as well as for the social affect and restricted and repetitive behavior domains. CSS range from 1-10 with scores of 8-10 in the high range, scores of 5-7 in the moderate range, scores 3-4 in the low range, and scores 1-2 in the minimal to no evidence range. Neurodevelopmental diagnoses (e.g., ASD, ID, Global Developmental Delay [GDD]) were assigned by licensed clinical psychologists per the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) (19) using information from testing results; clinical observations; and developmental, medical, and psychiatric history. Parents also completed the Social Responsiveness Scale, Second Edition (SRS-2) (20) , from which standardized T-scores were generated (mean = 50; SD = 10) for the total score and five subscale scores (i.e., Social Awareness, Social Cognition, Social Communication, Social Motivation, and Autistic Mannerism).
Cognitive, Language, and Adaptive Functioning.
Participants ages 4 through 17 years were administered the Differential Abilities Scales, Second Edition (DAS-II) (21). Participants ages 18 years and older were administered the Wechsler Abbreviated Scales of Intelligence, Second Edition (WASI-II) (22). For both instruments, standardized deviation scores (mean = 100; SD = 15) were generated except when the participant’s level of functioning did not allow for calculation of a deviation score (i.e., performance below the floor); in those cases, ratio scores were calculated by dividing mental age (defined as normative group-referenced age equivalencies) by the participant’s chronological age. In nine cases, valid test administration could not be conducted due to functioning level below the floor of the test. When this occurred, the floor deviation score was used to estimate an IQ value for the participant (i.e., DAS-II scores of 31 for full-scale IQ or 30 for verbal and nonverbal IQ). To assess adaptive functioning, parents were administered the Survey Interview form of the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) (23). An overall score, the Adaptive Behavior Composite (ABC), as well as domain scores in Communication, Daily Living Skills, and Socialization were generated (mean = 100; SD = 15). The Achenbach System of Empirically Based Assessment was used to assess internalizing and externalizing behavior challenges, specifically, using the Child Behavior Checklist (24) or Adult Behavior Checklist (25), where appropriate.
Medical Diagnoses.
The medical history was collected via structured interview adapted from the Simons Simplex Collection (SSC) (26) and involved characterization of co-morbid medical issues. Review of past medical records and past psychological and educational testing was also conducted to confirm parent report of diagnoses. Standardized medical exams were conducted by a licensed medical geneticist and included assessment of dysmorphic features, physical exam, and review of systems. Continuous measurements of occipital frontal head circumference, body height, and body weight were transformed to age- and sex-standardized z-scores. Standard scores for head circumference were calculated using population norms based upon White children (27), given that ethnicity-specific growth charts did not have publicly available norms (i.e., Hispanic children, n = 7 in our study), were not appropriate for mixed race/ethnicity (n = 7), and specific race designation (e.g., South Asian vs. Chinese) were not available (n = 3). Standard scores for height and weight were generated using norms from the National Health and Nutrition Examination Survey (28) of a representative sample of children in the United States. Dichotomous variables were derived to indicate whether individuals had medical problems related to the following: gastrointestinal issues, seizure activity, and sleep disturbances.
Statistical Analyses
Analyses were conducted in SPSS version 19 (IBM, Corp. Chicago IL, USA) with a focus on targeting effects of genetic group using a series of ANOVAs. Kruskal-Wallis and Mann-Whitney tests were used when distributions violated the assumption of normality for the following variables: age of participants, IQ, CSS. Binary logistic regression was used to compare groups for dichotomous variables (e.g., medical complaints) with one exception: 100% of the CHD8 group had an ASD diagnosis, thus pairwise chi-square were conducted. Chi-square was used to compare groups on language ability. Omnibus results, means, and standard deviations are presented for variables in Table 1. Bonferroni correction was applied by SPSS for all pairwise comparisons to account for conducting three comparisons in each analysis following ANOVA; p values reported in Table 1 are adjusted and should be interpreted with p < .05.
Results
Autism Symptoms
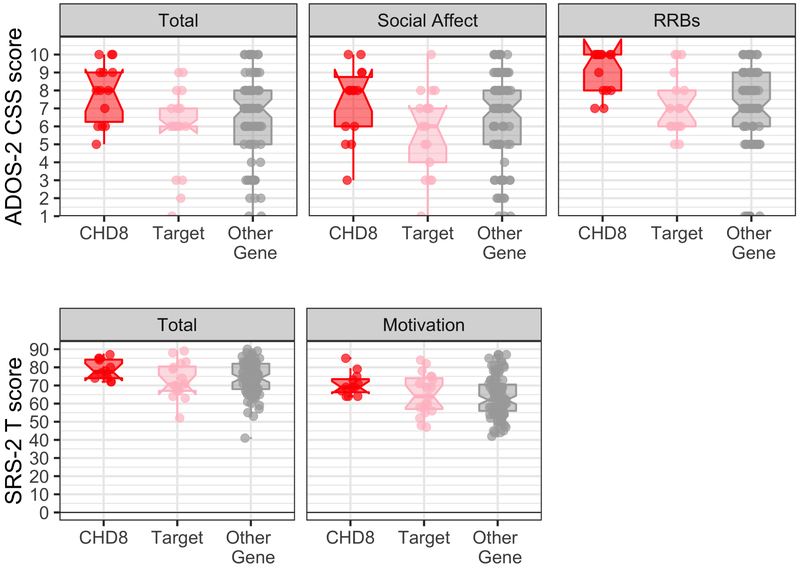
Consistent with the CHD8 phenotype (11), all children with CHD8 mutations in this cohort were diagnosed with ASD. Importantly, the prevalence rate of ASD was higher in the CHD8 group relative to both the Target group (70.6%, χ2 (1) = 5.23, p = .022) and the Other Gene group (78%; χ2 (1) = 4.26, p = .039). No differences were found between prevalence of ASD between the Target and Other Gene groups (p = .56). Group differences in ADOS-2 CSS (Figure 1) indicated increased severity of ASD symptoms in the CHD8 group relative to both other groups, p’s < .037. A similar pattern was found on the repetitive and restricted behaviors subscale but not the social affect subscale. Results were consistent when only those individuals with ASD were included in the analyses. The lack of social differences between groups was consistent with caregiver report on the SRS-2, with the exception of the social motivation subscale. As illustrated in Figure 1, results indicated that the CHD8 group exhibited more problems with social motivation than both the Other Gene group (p =.004) and the Target group, (p = .032). There were no differences between the Target and Other Gene groups (p = 1.0). However, within the subset of participants with an ASD diagnosis, the Target group had similarly elevated social motivation problems as the CHD8 group, p = .090, unlike the Other Gene group, p = .005.
Figure 1. Autism symptoms and social responsiveness problems between groups.
Autism symptoms (top row) are measured via ADOS-2 calibrated severity scores and illustrated for total symptoms as well as for social affect and restricted and repetitive behaviors. The CHD8 group demonstrated significantly more restricted and repetitive behavior compared to the other groups. Social responsiveness problems (bottom row) are illustrated for the SRS-2 overall T-scores and Social Motivation subscale. Error bars represent standard error of the mean. Horizontal bars reflect significant group differences with Bonferroni correction applied. The CHD8 group had significantly higher scores on this subscale compared to the Other Gene group, whereas they did not differ significantly from the Target group.
Cognitive, Language, and Adaptive Functioning
Groups had comparable rates of ID, and there were no group differences in cognition across any of the continuous measures, χ2(2) < 3.89, p’s > .09. However, normality tests indicated a bimodal distribution present for the Other Gene, p’s <.0001, but not CHD8 or Target groups, p’s > .15, (see SI Figure 1).
Groups differed on language ability based on the module of the ADOS-2 administered, χ2(6) = 24.89, p < .001. There was no difference between the CHD8 group and Target group, χ2(3) = 7.78, p = .051, and both the CHD8 group and Target group differed from the Other Gene group, χ2(3) = 9.04, p = .029 and χ2(3) = 21.34, p < .001, respectively.
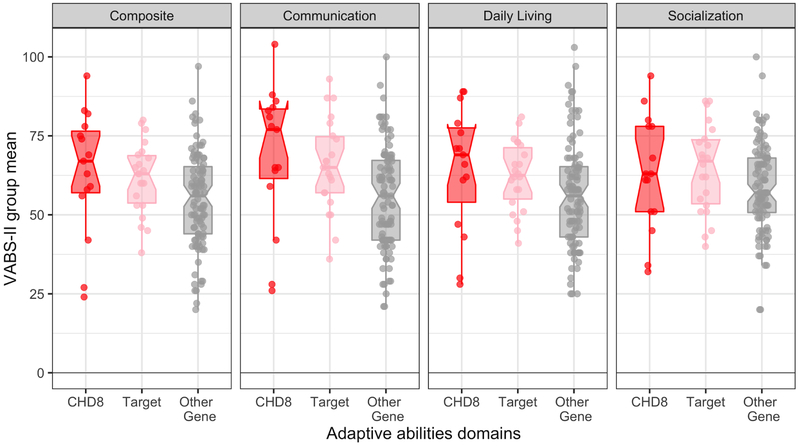
Group differences in overall adaptive functioning were significant at an omnibus level but pairwise comparisons did not survive correction for multiple comparisons (p’s > .13, Bonferroni), (see Figure 2). Consistent with functional language differences found on ADOS-2 module, a pattern of less severe deficits in the CHD8 and Target groups relative to the Other Gene group was observed specifically for the communication domain of the Vineland-II (p = .002), such that CHD8 and Target groups had substantially less severe communication challenges than the Other Gene group, p = .015 and p = .035, respectively. There were no group differences in socialization or daily living skills.
Figure 2. Vineland-II standard scores.
Adaptive abilities as measured by the Vineland-II. Composite and domain standard scores are illustrated for CHD8 (red, left), Target (pink, middle), and Other Gene (grey, right) groups. Error bars represent standard error of the mean. Horizontal bars reflect significant (solid) or trending (dashed) group differences with Bonferroni correction applied. The CHD8 group did not differ from the Target group on any of the domains. The Other Gene group had significantly greater adaptive deficits compared to the CHD8 group on the composite score as well as in the communication domain; the Other Gene group had a trend (p’s < .089) towards greater adaptive deficits compared to the Target group on the composite score as well as in the daily living skills domain.
Groups differed on parent ratings of externalizing behavior problems (p = .020) but not internalizing problems (p =.592) such that the CHD8 group had similar scores to the Target group (p > .999) and had fewer problems than the Other Gene group (p = .036). There was no difference in ratings of externalizing behavior between the Target and Other Gene groups (p = .294).
Medical Conditions
Aligned with prior work associating larger head sizes with the CHD8 phenotype (11), head circumference differed across groups, (Figure 3). Specifically, the CHD8 group had comparable head size to the Target group, (p = .405) but larger head size than the Other Gene group (p =.002). The Target and Other Gene group did not differ (p = .156). Similarly, the CHD8 group had similar body weight (i.e., z-score body weight) compared to the Target group (p = 1.000) but were heavier than the Other Gene group, (p = .017). The Target and Other Gene group did not differ (p = . 183). CHD8 carriers alone were taller (i.e., z-score body height) than both comparison groups, F(2, 82) = 11.95, p < .001.
Figure 3. Head circumference distribution.

Horizontal bars reflect significant group differences between CHD8 (red, left), Target (pink, middle), and Other Gene (grey, right) groups. Distribution of head circumference z-scores indicates larger head sizes for the CHD8 and Target groups relative to the Other Gene group. Horizontal dashed lines indicate clinical criterion for macrocephaly (z-score = 2) and microcephaly (z-score = −2). Solid horizontal lines indicate significant group differences with Bonferroni correction applied.
Seizure activity was more prevalent in the Other Gene group compared to the CHD8 group (p = .038). No difference between the CHD8 group and Targets (p = .70). There were no significant group differences in prevalence of sleep problems or gastrointestinal concerns.
Supplemental analyses found consistent results across medical conditions, even when considering the potential influence of a microcephaly-associated gene (i.e., DYRK1A, (29), SI Materials II) or a seizure-associated gene (i.e., SCN2A, (30), SI Materials III) and the potential influence of ethnicity on growth parameters (SI Materials IV).
Discussion
In this study, we characterized phenotypic profiles of groups of individuals with genetic mutations associated with ASD to evaluate a possible converging biological pathway associated with disruptive mutations to CHD8 and genes directly regulated by CHD8. Overall, phenotypic comparisons supported our hypothesis that the CHD8 and Target groups were more similar to one another than to the Other Gene group. Specifically, we found the CHD8 and Target groups were characterized by increased social motivation problems in those diagnosed with ASD, similar functional language, less severe adaptive deficits in the area of communication skills, less severe externalizing symptoms, large head circumference, higher weight, and a lower incidence of seizures compared to the Other Gene group. Although rates of sleep and GI problems have previously been reported as common in the CHD8 group, our analyses indicated these symptoms are equally prevalent across groups.
Increased head circumference among individuals with CHD8 mutations is the most replicated finding across human and animal studies (11; 31). Neuroimaging of Chd8 haploinsufficient mice (32; 33), measurement of chd8 zebrafish interorbital distance (11), postmortem examination of children with or without ASD (34), and tabulation of shared ontological properties of CHD8 and its targets are all strongly indicative of a model wherein brain overgrowth in early development explains head enlargement. CHD8 target genes have been independently associated with macrocephaly, including PTEN and ARID1B (35; 36). Thus, one hypothesis is the rates of macrocephaly in individuals with CHD8 mutations may be secondary to disrupted downstream modulation of these targets. Alternately, each ASD-associated disruptive gene event may be independently affecting head growth, which contributes to the overall heterogeneity in head size among individuals with ASD. Given higher rates of ASD in our CHD8 and Target groups, we cannot rule out that this shared pattern of larger head circumference may be due to an unknown third factor explaining higher rates of macrocephaly in ASD more broadly (37-47). Considering that in addition to increased head circumference in the CHD8 and Target groups, these two groups also evince larger body weights than the Other Gene group, overgrowth may be a key phenotypic commonality between individuals with a mutation to CHD8 and those with a mutation to a gene that is regulated by CHD8. Overall, with the CHD8 group also measuring significantly taller than the other two groups, results are consistent with prior research that describes individuals with CHD8 mutations as tall and lean (11).
Rates of ASD were highest in the CHD8 group, and this group also demonstrated more severe restricted and repetitive behaviors. Because the pattern of higher CSS for the restricted and repetitive behavior domain held even when participants without ASD were excluded from the analyses, it is possible the repetitive qualities are specific to the CHD8 presentation. Platt and colleagues (48) found disruption to Chd8 in mice results in impairments in the ventral striatum. In humans, the striatum, and specifically, the caudate nucleus, have been implicated in the ontogeny of restricted and repetitive behavior (48-50). Taken together, these results may help link converging biological explanations of ASD, such that the potential shared genetic pathways associated with CHD8 may have consequences for the striatal system that in turn yield a specific constellation of elevated restricted and repetitive behaviors.
In contrast, all genetic groups scored similarly in the social affect domain of the ADOS-2, as well as on the SRS-2 with the exception of the social motivation subscale where the CHD8 groups showed more problems than the other two groups. Because all groups evince similarly high amounts of social difficulty overall, it may be that these measures are tapping into an overall deficit rather than social problems consistent with ASD. Hus and colleagues (51) found that SRS scores may reflect overall impairment in groups that have significant deficits in language or cognition, or when there are significant behavior problems. In the present study, it is also possible that the elevated externalizing behavior problems for children in the Other Gene Group may inflate challenges as reported by parents on the SRS-2. Of note, when considering only those individuals with ASD, the CHD8 and Target groups had similar elevated scores on the social motivation subscale, suggesting the possibility of a shared etiological subtype of ASD associated with poorer social motivation and resulting from or consistent with a shared mechanism. Paired with other work evaluating how potential autism subgroups are linked to genetic etiologies for ASD-specific traits (52), these results may facilitate a deeper understanding of the genetic underpinnings of the social problems in ASD.
Overall functioning level did not differ among groups in terms of presence of ID or IQ scores, and adaptive skill differences were limited to the area of communication with significant differences in gross indicators of language abilities as measured by the Communication domain of the Vineland-II and differences in the module of the ADOS-2 that was administered. Within a larger sample, particularly within the CHD8 and Target groups, it is possible we may see more nuanced differences in terms of global functioning between groups. The bimodal distribution observed within Other Gene group may indicate a potential subgroup within our designated group, such that better specifying functioning ability may better elucidate classification between groups. It is also possible that language skills are more sensitive to our level of measurement.
Contrary to our hypothesis, we found similar rates of GI and sleep problems across groups. Elevated GI problems have previously been reported in individuals with disruptive mutations to CHD8 and recapitulated in an animal model (11); however, the current study reports similarly high rates of GI problems associated with other disruptive genetic events. Similarly, while sleep issues are known to be more prevalent and more severe in ASD (53; 54), the extent to which genetic etiology dictates sleep mechanisms is unclear. Chromatin modification is linked to the maintenance of circadian rhythms (55), which may explain high rates of sleep disruptions in children with CHD8 mutations (11). However, both Target and Other Gene groups exhibited similarly high prevalence of sleep issues to CHD8 carriers, potentially driven by a different kind of shared disruption (e.g., dysregulation of fundamental neurotransmitters (56)) though other alternatives beyond neurotransmission may also be implicated (e.g., medical and behavioral conditions (57)).
It may be worth considering moderating or mediating factors that may impact overall functioning for individuals with mutations to ASD risk genes. For one, CHD8 predominantly downregulates genes involved in cell adhesion, axonal guidance, and calcium signaling pathways (31) that are functions critical for cortical development. However, there may be differential phenotypic patterns based upon the regulating mechanism. Future work would benefit from careful evaluation of specific variants to identify alternative pathways and developmental processes, as well as explore other potential mechanisms (e.g., protein-protein interactions).
There are several limitations to the current study. The size of the CHD8 and Target groups relative to the Other Gene group are small, potentially making it difficult to elucidate differences statistically due to power limitations. Future work will be necessary to replicate and more deeply evaluate subtle aspects of the phenotype, considering that the implication of the significant findings here should be tempered given our sample size and statistical limitations (i.e., Bonferroni correction). In addition, although the group designations were generated based upon conservative expectations of molecular pathway given criteria per (13; 14), it is possible that these groups are improperly specified. The conservative approach may have resulted in genes being included in the Other Gene group that are, in fact, regulated by CHD8. In addition, this strategy does not account for other indirect regulatory relationships. Further, it is important to note possibilities of ascertainment bias, such that participants were recruited from previous studies on ASD, specifically. Lastly, there is a need for more nuanced measures of social functioning to enable clearer phenotypic characterization of social deficits associated with etiological subtypes of ASD. There are potential concerns regarding using the SRS-2 in severely affected populations (58), and it is possible that the social features measured in the current study (e.g., CSS of the ADOS-2, socialization domain of the Vineland-II) are not sufficient to adequately describe the specific deficit areas. Future work should evaluate other implicit measurements that may better capture social characteristics that are impacted in individuals with these gene events with and without ASD, especially in a globally impaired sample.
Conclusions
Whereas recent efforts have focused on phenotypic characterization of small groups of individuals with mutations to a specific gene or genomic region, in this study we hypothesized a neurodevelopmental subtype defined by gene-gene interactions and shared genetic ontology. We found support for a pathogenic effect of early, atypical neurogenesis shared by individuals with disruptions to CHD8 or CHD8 Targets. Functional genetic clustering is a promising step toward development of precision medicine approaches to ASD and associated neurodevelopmental disorders.
Supplementary Material
Key Resources Table
| Resource Type | Specific Reagent or Resource |
Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs athttps://scicrunch.org/resources | Include any additional information or notes if necessary. |
| Software; Algorithm | SPSS version 19 | IBM | RRID:SCR_002865 | |
| Deposited Data; Public Database | Rare Mutations and Autism Spectrum Disorder #2627 | National Database for Autism Research (NDAR) | RRID:SCR_004434 |
Acknowledgements
We thank the children and families for their participation in this study. This work was supported by the National Institutes of Mental Health [MH100047 Bernier; MH101221 Eichler] and by the National Institute of Child Health and Human Development to the University of Washington’s Center on Human Development and Disability [U54 HD083091]. E.E.E. is an investigator of the Howard Hughes Medical Institute. Data presented previously on two occasions:
Bernier, R.A. (October, 2018). The Human CHD8 phenotype. Oral presentation for symposium on “A genotype-first approach for defining phenotypic subtypes in ASD” at the 65th annual meeting for the American Academy of Child and Adolescent Psychiatry in Seattle, WA.
Beighley, J. S., (April, 2018). The CHD8 phenotype: Relationship to shared molecular subtypes. Oral presentation for symposium on “Uncovering Genetic Subtypes of Neurodevelopmental Disorders” at the 51st annual Gatlinburg Conference in San Diego, CA.
Footnotes
Disclosures
E.E.E. is on the Scientific Advisory Board of DNAnexus, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. (2011): Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 43: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Ercument Cicek A, et al. (2014): Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 515: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. (2014): Convergence of Genes and Cellular Pathways Dysregulated in Autism Spectrum Disorders. The American Journal of Human Genetics. 94: 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. (2010): Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 466: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. (2011): Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 70: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. (2015): Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 87: 1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. (2012): De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 485: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. (2012): Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 338: 1619–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramaswami G, Geschwind DH (2018): Genetics of autism spectrum disorder. Handb Clin Neurol. 147: 321–329. [DOI] [PubMed] [Google Scholar]
- 10.King BH, Navot N, Bernier R, Webb SJ (2014): Update on diagnostic classification in autism. Curr Opin Psychiatry. 27: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, et al. (2014): Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 158: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. (2014): The contribution of de novo coding mutations to autism spectrum disorder. Nature. 515: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, et al. (2015): The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nature Communications. 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugathan A, Biagioli M, Golzio C, Erdin S, Blumenthal I, Manavalan P, et al. (2014): CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proceedings of the National Academy of Sciences. 111: E4468–E4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard RA, Pomaville MB, O’Roak BJ (2015): Mutations and Modeling of the Chromatin Remodeler CHD8 Define an Emerging Autism Etiology. Front Neurosci. 9: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stessman HAF, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, et al. (2017): Targeted sequencing identifies 91 neurodevelopmental- disorder risk genes with autism and developmental-disability biases. Nat Genet. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord C, Rutter M, Goode S (1989): Autism Diagnostic Observation Schedule: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 24: 659–685. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- 20.Constantino JN, Gruber CP (2012): Social Responsiveness Scale.
- 21.Elliott CD (2007): Differential Ability Scales-ll. San Antonio, TX: Pearson. [Google Scholar]
- 22.Weschler D (2011): Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). [Google Scholar]
- 23.Sparrow SS, Balla DA, Cicchetti DV (2005): Vineland II: Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service. [Google Scholar]
- 24.Achenbach TM, Rescorla LA (2001): Manual for the ASEBA School-Age Forms & Profiles. University of Vermont. [Google Scholar]
- 25.Rescorla LA, Achenbach TM (2004): The Achenbach System of Empirically Based Assessment (ASEBA) for Ages 18 to 90 Years In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment Instruments for adults. Mahwah, NJ, pp 115–152. [Google Scholar]
- 26.Fischbach GD, Lord C (2010): The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 68: 192–195. [DOI] [PubMed] [Google Scholar]
- 27.Roche AF, Mukherjee D, Guo SM, Moore WM (1987): Head circumference reference data: birth to 18 years. PEDIATRICS. 79: 706–712. [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. (2002): 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 1–190. [PubMed] [Google Scholar]
- 29.Earl RK, Turner TN, Mefford HC, Hudac CM, Gerdts J, Eichler EE, Bernier RA (2017): Clinical phenotype of ASD-associated DYRK1A haploinsufficiency Molecular Autism, 5 ed. 8. doi: 10.1186/s13229-017-0173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders SJ, Campbell AJ, Cottrell JR, Møller RS, Wagner FF, Auldridge AL, et al. (2018): Progress in Understanding and Treating SCN2A-Mediated Disorders. Trends in Neurosciences. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suetterlin P, Hurley S, Mohan C, Riegman KLH, Pagani M, Caruso A, et al. (2018): Altered Neocortical Gene Expression, Brain Overgrowth and Functional Over-Connectivity in Chd8 Haploinsufficient Mice. Cereb Cortex. 28: 2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gompers AL, Su-Feher L, Ellegood J, Copping NA, Riyadh MA, Stradleigh TW, et al. (2017): Germline Chd8 haploinsufficiency alters brain development in mouse. Nat Neurosci. 20: 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gompers AL, Su-Feher L, Ellegood J, (null) TS, Zdilar I, Copping NA, et al. (2016): Heterozygous mutation to Chd8 causes macrocephaly and widespread alteration of neurodevelopmental transcriptional networks in mouse. bioRxiv. doi: 10.1101/076158. [DOI] [Google Scholar]
- 34.Yang G, Smibert CA, Kaplan DR, Miller FD (2014): An eIF4E1/4E-T complex determines the genesis of neurons from precursors by translationally repressing a proneurogenic transcription program. Neuron. 84: 723–739. [DOI] [PubMed] [Google Scholar]
- 35.Klein S, Hannauer PS, Agosto JAM (2013): Macrocephaly as a Clinical Indicator of Genetic Subtypes in Autism. Autism Res. 6: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vals MA, Oiglane-Shlik E, Nõukas M, Shor R, Peet A, Kals M, et al. (2014): Coffin-Siris Syndrome with obesity, macrocephaly, hepatomegaly and hyperinsulinism caused by a mutation in the ARID1B gene. Eur J Hum Genet. 22: 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995): Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 25: 63–77. [DOI] [PubMed] [Google Scholar]
- 38.Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. (1994): A case-control family history study of autism. J Child Psychol & Psychiat. 35: 877–900. [DOI] [PubMed] [Google Scholar]
- 39.Davidovitch M, Patterson B, Gartside P (1996): Head circumference measurements in children with autism J Child Neurol, 3rd ed. 11: 389–393. [DOI] [PubMed] [Google Scholar]
- 40.Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE (1997): Macrocephaly in Children and Adults With Autism. J Am Acad Child Adolesc Psychiatry. 36: 282–290. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ (1997): Autism and macrocephaly. The Lancet. 349: 1744–1745. [DOI] [PubMed] [Google Scholar]
- 42.Fombonne E, Rogé B, Claverie J, Courty S, Frémolle J (1999): Microcephaly and macrocephaly in autism. Journal of Autism and Developmental Disorders. 29: 113–119. [DOI] [PubMed] [Google Scholar]
- 43.Deutsch CK, Joseph RM (2003): Brief report: Cognitive correlates of enlarged head circumference in children with autism. Journal of Autism and Developmental Disorders. 33: 209–215. [DOI] [PubMed] [Google Scholar]
- 44.Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, et al. (2005): Accelerated head growth in early development of individuals with autism. Pediatric Neurology. 32: 102–108. [DOI] [PubMed] [Google Scholar]
- 45.Ververi A, Vargiami E, Papadopoulou V, Tryfonas D, Zafeiriou DI (2012): Clinical and laboratory data in a sample of Greek children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 42: 1470–1476. [DOI] [PubMed] [Google Scholar]
- 46.van Daalen E, Swinkels SHN, Dietz C, van Engeland H, Buitelaar JK (2007): Body Length and Head Growth in the First Year of Life in Autism. Pediatric Neurology. 37: 324–330. [DOI] [PubMed] [Google Scholar]
- 47.Miles JH, Hadden LL, Takahashi TN, Hillman RE (2000): Head circumference is an independent clinical finding associated with autism. Am J Med Genet. 95: 339–350. [PubMed] [Google Scholar]
- 48.Platt RJ, Zhou Y, Slaymaker IM, Shetty AS, Weisbach NR, Kim J-A, et al. (2017): Chd8 Mutation Leads to Autistic-like Behaviors and Impaired Striatal Circuits. Cell Rep. 19: 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langen M, Bos D, Noordermeer DS, Nederveen H, van Engeland H, Durston S (2014): Changes in the Development of Striatum Are Involved in Repetitive Behavior in Autism. Biological Psychiatry. 76: 405–411. [DOI] [PubMed] [Google Scholar]
- 50.Kohls G, Yerys BE, Schultz RT (2014): Striatal development in autism: repetitive behaviors and the reward circuitry. Biological Psychiatry. 76: 358–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hus V, Bishop S, Gotham K, Huerta M, Lord C (2013): Factors influencing scores on the social responsiveness scale. J Child Psychol & Psychiat. 54: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH (2015): Social Responsiveness, an Autism Endophenotype: Genomewide Significant Linkage to Two Regions on Chromosome 8. AJP. 172: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gregory AM, Sadeh A (2012): Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Medicine Reviews. 16: 129–136. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Cho SC, Cho IH, Kim BN, Kim JW, Shin MS, et al. (2012): Sleep problems and their correlates and comorbid psychopathology of children with autism spectrum disorders. Research in Autism Spectrum Disorders. 6: 1068–1072. [Google Scholar]
- 55.Aguilar-Arnal L, Sassone-Corsi P (2013): The circadian epigenome: how metabolism talks to chromatin remodeling. Curr Opin Cell Biol. 25: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crocker A, Sehgal A (2010): Genetic analysis of sleep. Genes Dev. 24: 1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maxwell-Horn A, Malow BA (2017): Sleep in Autism. Semin Neurol. 37: 413–418. [DOI] [PubMed] [Google Scholar]
- 58.Constantino JN, Przybeck T, Friesen D, Todd RD, Perv CADFWG (2000): Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental & Behavioral Pediatrics. 21: 2–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.