Abstract
Purpose
To evaluate fine motor ability in children treated for unilateral congenital or infantile cataract.
Methods
Twenty-three children 3–13 yearsof agewho were treated for unilateral congenital or infantile cataract and 38 age-similar control children were enrolled. Children completed five fine motor skills tasks (unimanual dexterity, bimanual dexterity, drawing trail, aiming, catching) from the Movement Assessment Battery for Children-2. Raw scores were converted into standardized scores, with higher scores indicating better performance.
Results
Compared with controls, children treated for unilateral cataract scored lower on drawing trail (P = 0.009), aiming (P = 0.009), and catching (P < 0.001) but not on unimanual (P = 0.77) or bimanual dexterity (P = 0.31). Poorer affected eye visual acuity was moderately related to poorer performance for unimanual dexterity (r = −0.47; P = 0.025), bimanual dexterity (r = −0.50; P = 0.014), and catching (r = −0.41; P = 0.051). Those with a poor visual outcome (>0.6 logMAR) had worse performance than those with a good visual outcome (≤0.6 logMAR) for all tasks (all P values, 0.008–0.09) except aiming. Cataract type (congenital, 9; infantile, 14) and sensory fusion by Worth 4-Dot testing at 33 cm (pass, 10; fail, 13) had no effect on fine motor performance (all P values, 0.12–0.98).
Conclusions
In our study cohort, fine motor deficits were found in children treated for congenital or infantile unilateral cataract.
Normal binocular vision during childhood provides important sensory input for optimal development of fine motor ability. Discordant input from eye conditions such as strabismus, ansiometropia, or unilateral cataract often results in deficits in visual acuity (ie, amblyopia) and stereoacuity that persist throughout childhood.1 Discordant binocular experience also affects motor development; adults and children with anisometropic and strabismic amblyopia have been shown to have impaired fine motor skills—placing pegs into holes, threading beads, pouring water into a container, and ball catching—even with both eyes open.2–6 These impairments have been linked to the poor visual acuity and stereoacuity deficits typical of amblyopia.4,5,7
Visual outcomes following extraction of a dense congenital or infantile unilateral cataract are typically more severe than strabismic or anisometropic amblyopia.8 Deprivation amblyopia from unilateral cataract may also affect the development of fine motor ability. The only study of motor skills in children with deprivation amblyopia (Infant Aphakia Treatment Study [IATS]) found delayed motor outcomes in 4-year-olds using the Movement Assessment Battery for Children, 2nd edition (MABC-2).9 Children scored lower than established norms for manual dexterity, aiming/catching, and balance, with worse performance for manual dexterity. However, visual acuity outcomes in this study were overwhelmingly poor (60% with >0.6 logMAR or worse [>20/80]), some had bilateral visual impairment at the time of testing, and there was no control group. In this study, we evaluated fine motor skills in children 3–13 years of age treated for unilateral congenital or infantile cataract using MABC-2 tasks. We predicted that children treated for unilateral congenital or infantile cataract would also have poorer performance for fine motor tasks compared with controls when completing tasks with both eyes open.
Subjects and Methods
The research protocol for this study observed the tenets of the Declaration of Helsinki and was approved by the University of Texas Southwestern Medical Center Institutional Review Board and conformed to the requirements of the US Health Insurance Portability and Accountability Act of 1996. Informed consent was obtained from a parent or legal guardian and written assent was obtained from children ≥10 years of age prior to testing.
A total of 23 children (12 female; mean patient age ± standard deviation = 6.9 ± 3.1 years; range, 3.1–13.5 years) were enrolled following extraction of a dense unilateral congenital or infantile cataract. Congenital cataracts were dense nuclear cataracts associated with mild microphthalmia diagnosed <6 months of age. Infantile cataracts were anterior subcapsular, nuclear sclerosis, or posterior lenticonus/posterior subcapsular cataracts diagnosed between 6 months and 28 months of age. Amblyopia was defined as best-corrected visual acuity in the affected eye of 0.2 logMAR (Snellen equivalent, 20/32) or worse, best-corrected visual acuity in the nonaffected eye of 0.1 logMAR (Snellen, 20/25) or better, and an interocular difference of ≥0.2 logMAR (Snellen, ≥2 lines). Diagnosis, cataract extraction date, and current eye alignment were obtained from the patient records of referring ophthalmologists. A group of 38 age-similar controls (20 female; mean control age, 8.2 ± 2.9 years; range, 3.9–13.0 years) with age-normal visual acuity and stereoacuity and no history of vision disorders were also enrolled. No child was born <32 weeks’ postmenstrual age or had coexisting ocular or systemic disease, congenital infections/malformations, or developmental delay. English was the primary language for all children.
Vision Assessment
Crowded monocular best-corrected visual acuity was assessed using the e-ETDRS protocol for children ≥7 years of age10 or the Amblyopia Treatment Study HOTV protocol for children <7 years of age.11 Stereoacuity was measured using the Randot Preschool Stereoacuity and Stereo Butterfly tests (Stereo Optical Co, Chicago, IL). Sensory fusion was measured at near (33 cm) using the Worth 4-Dot test.12
Each child was tested with both eyes open using the MABC-213 (administered by KK or SM), a standardized test used to identify children with delay or impairment in motor development that is administered in three age bands (3–6, 7–10, 11–16 years). The MABC-2 consists of manual dexterity, aiming and catching, and balance subscales (see Table 1). For this study, testing was limited to fine motor skills, that is, the three manual dexterity tasks and two aiming and catching tasks. The raw score for each task was converted into a standardized score using the look-up tables provided with the MABC-2. Higher standardized scores indicate better performance. To compare a child’s motor proficiency with published age-matched normative data,13 standardized scores were summed per subscale and converted to percentiles (standard score of 10 = 50th percentile). According to MABC-2 instructions, children scoring ≤5th percentile have a significant motor impairment, children scoring between 6th and 15th percentile are considered at-risk for impairment, and children scoring ≥16th percentile are typically developed. Test time is approximately 20–30 mins.
Table 1.
Description of each task per subscale for three age bands
| Subscale and tasks | Age band | Outcome measure | ||
|---|---|---|---|---|
| 3 to 6 years | 7 to 10 years | 11–13 years | ||
| Manual dexterity | ||||
| Unimanual | Post coins, preferred hand | Place pegs, preferred hand | Turn pegs, preferred hand | Latency |
| Bimanual | Thread beads | Thread lace | Build triangle | Latency |
| Drawing trail | Stay inside lines, preferred hand | Stay inside lines, preferred hand | Stay inside lines, preferred hand | No. errors |
| Aiming and catching | ||||
| Aiming | Throw beanbag on mat | Throw beanbag on mat | Throw ball at wall target | No. successful hits |
| Catching | Catch beanbag, two hands | Catch ball, two hands | Catch ball, one hand | No. successful catches |
Statistical Analyses
Stereoacuity was converted to log arcseconds (range, 1.3–3.3 log arcsec). Nil stereoacuity (ie, not measurable) was arbitrarily assigned a value of 4 log arcsec. Sensory fusion (Worth 4-Dot) was categorized as fail if there was no fusion at near (ie, suppression) and pass if there was fusion at near. Independent t tests were used to evaluate group differences in standardized scores for each subscale (Bonferroni-corrected α = 0.025) and for each task (unimanual dexterity, bimanual dexterity, drawing trail, aiming, and catching; Bonferroni-corrected α = 0.010). Independent t tests were conducted to determine the effect of cataract type (congenital vs infantile), affected eye visual acuity (good visual outcome of ≤0.6 logMAR [≤20/80)] vs poor visual outcome of >0.6 logMAR [>20/80]),14 and sensory fusion (pass vs fail) on task performance (Bonferroni-corrected α = 0.010). Pearson’s r correlations were calculated for the patient group only to examine relationships of motor performance with affected eye visual acuity. Because the number of patients with measurable stereoacuity was small, no comparisons were conducted to determine relationship between stereoacuity and fine motor performance.
Results
Of the 23 subjects, 9 were treated for congenital cataract (mean age at cataract extraction, 1.6 ± 1.6 months) and 14 for infantile cataract (mean age at cataract extraction, 14.5 ± 5.8 months). Children treated for unilateral cataract had mean visual acuity of 0.7 ± 0.5 in the affected eye; 15 had good visual outcomes ≤0.6 logMAR14 (3 had no amblyopia), and 8 had poor visual outcomes >0.6 logMAR. Two children had subnormal stereoacuity (2.3, 2.9 log arcsec), and 21 had no measurable stereoacuity. Thirteen children failed and 10 children passed the Worth 4-Dot test. Group descriptive statistics are reported in Table 2 (individual patient characteristics can be found in eSupplement 1, available at jaapos.org).
Table 2.
Group characteristics
| Characteristic | Unilateral cataract (n = 23) | Control (n = 38) |
|---|---|---|
| Sex, female, no. (%) | 12 (52) | 20 (53) |
| Age, years, mean ± SD (range) | 6.9 ± 3.1 (3.1–13.5) | 8.2 ± 2.9 (3.9–13.0) |
| VA, logMAR, mean ± SD [Snellen] (range) | ||
| Affected eyea | 0.7 ± 0.5 [20/100 ± 5 lines] (0.1 to 1.9) | 0.0 ± 0.1 [20/20 ± 1 line] (−0.1 to 0.1) |
| Fellow eyeb | 0.0 ± 0.1 [20/20 ± 1 line] (−0.1 to 0.3) | 0.0 ± 0.1 [20/20 ± 1 line] (−0.1 to 0.1) |
| Stereoacuity, no. (%) | ||
| Normal (≤1.78 log arcsecs or age-normal)c | 0 (0) | 38 (100) |
| Subnormal (2–3.3 log arcsecs) | 2 (9) | 0 (0) |
| Nil (not measurable) | 21 (91) | 0 (0) |
| Sensory fusion (Worth 4-Dot), no. (%) | ||
| Fail (suppression) | 13 (57) | 0 (0) |
| Pass (fusion) | 10 (43) | 38 (100) |
| Cataract type, no. (%) | ||
| Congenital | 9 (39) | N/Ad |
| Infantile | 14 (61) | N/A |
| Age at extraction, mos, mean ± SD (range) | ||
| Congenital | 1.6 ± 1.6 (0.3–5.6)e | N/A |
| Infantile | 14.5 ± 5.8 (6.4–27.9) | N/A |
LogMAR, logarithm of the minimum angle of resolution; SD, standard deviation; VA, visual acuity.
Right eye for controls.
Left eye for controls.
Three controls ≤5 years of age had stereoacuity of 2 log arcsec (normal for their age).
Not applicable
In 8/9 children, congenital cataract was extracted by 1.8 months. One child had a dense cataract first noted by primary care physician at 4 months, with a nuclear cataract and mild microphthalmia diagnosed by a pediatric ophthalmologist at 5 months and extraction at 5.6 months.
Children treated for unilateral cataract did not differ in age from controls (T59 = 1.67; P = 0.10). Of children treated for unilateral cataract, no sex differences were found in standard scores for the manual dexterity (T21 = 1.04; P = 0.31) or aiming and catching (T21 = 1.09; P = 0.29) subscales. For control children, no sex differences were found for the manual dexterity (T36 = 1.90; P = 0.07) or aiming and catching subscales after Bonferroni correction (T36 = 2.04; P = 0.05); however, these trended toward significance, with females scoring higher for manual dexterity and males scoring higher for aiming and catching. Sex differences have been previously reported for normal control children.15
Manual Dexterity
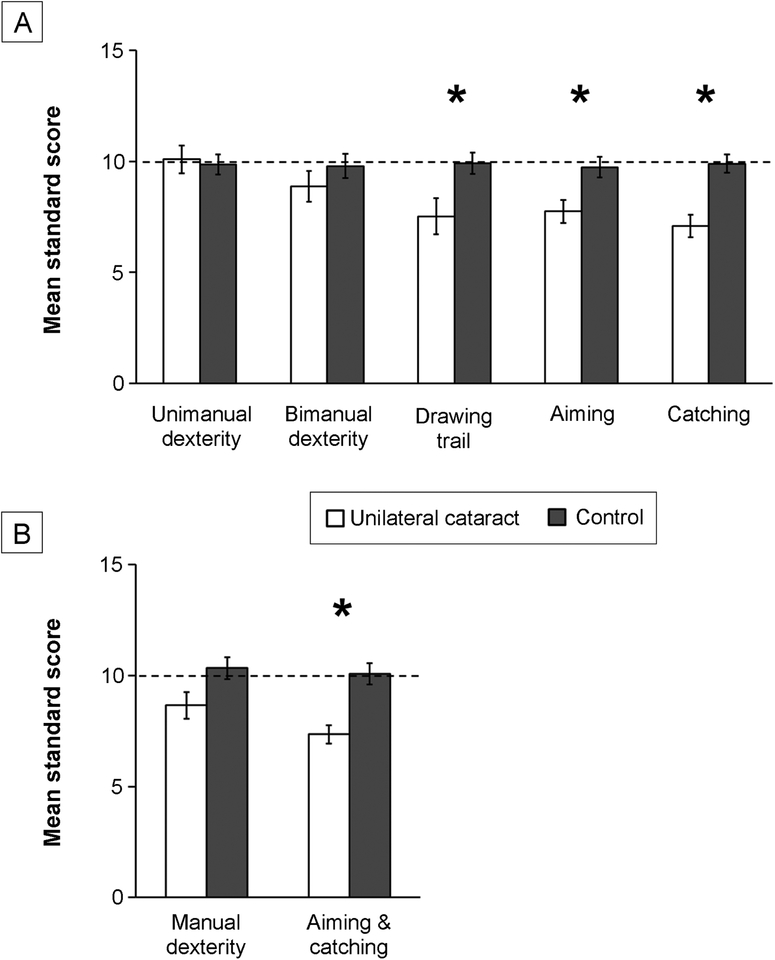
Children treated for unilateral cataract scored lower than controls on the drawing trail (cataract = 7.52 ± 3.92 vs control = 9.92 ± 2.96; T59 = 2.71; P = 0.009). However, no group differences were found for unimanual dexterity (10.09 ± 2.95 [cataract] vs 9.87 ± 2.78 [control]; T59 = 0.29; P = 0.77) or for bimanual dexterity (8.87 ± 3.32 [cataract] vs 9.79 ± 3.43 [control]; T59 = 1.03; P = 0.31; Figure 1A). Although children treated for unilateral cataract trended toward scoring lower total on the manual dexterity subscale than did controls (8.65 ± 2.89 [cataract] vs 10.34 ± 3.03 [control]; T59 = 2.15; P = 0.036; Figure 1B), this trend was not statistically significant after Bonferroni correction. Further, 26% scored ≤16th percentile compared with 16% of controls; however, the difference in these proportions was not significant (mean difference = 0.10, 95% CI for the difference = −0.10 to 0.32; Z = 0.98; P = 0.33).
FIG 1.
Fine motor performance. Mean standard scores per task for unimanual dexterity, bimanual dexterity, drawing trail, aiming, and catching tasks (A) and per subscale for manual dexterity and aiming and catching (B) for children treated for unilateral cataract (white bars) compared with control children (dark gray bars). The dotted line represents the 50th percentile. Errors bars represent standard error of the mean (SEM). *Group differences are significant after Bonferonni-correction.
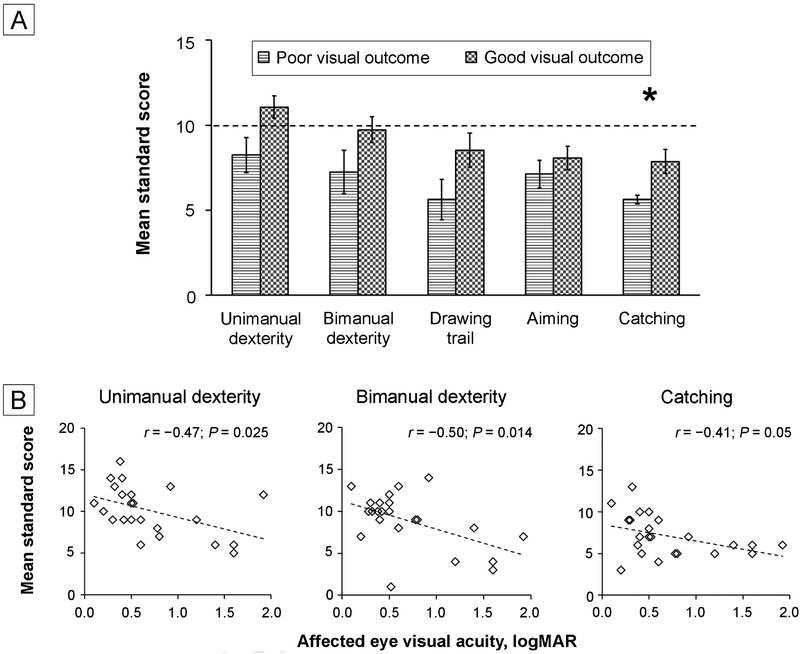
Children treated for unilateral cataract with a poor visual outcome trended toward scoring lower compared to those with a good visual outcome on unimanual dexterity (8.25 ± 2.92 [poor] vs 11.07 ± 2.55 [good]; T21 = 2.40; P = 0.026), bimanual dexterity (7.25 ± 3.62 [poor] vs 9.73 ± 2.91 [good]; T21 = 1.79; P = 0.088), and the drawing trail (5.62 ± 3.38 [poor] vs 8.53 ± 3.91 [good]; T21 = 1.78; P = 0.09; Figure 2A); these trends were not statistically significant after Bonferroni correction. No group differences for cataract type (all P values, 0.24–0.88) or sensory fusion on the Worth 4-Dot test (all P values, 0.12–0.98) were found. Poorer affected eye visual acuity was moderately correlated with worse performance for unimanual dexterity (r = −0.47; P = 0.025; 95% CI, −0.74 to −0.07) and bimanual dexterity (r = −0.50; P = 0.014; 95% CI, −0.76 to −0.11; Figure 2B). No significant correlation was found with performance on the drawing trail and visual acuity (r = −0.20; P = 0.36; 95% CI, −0.57 to 0.10).
FIG 2.
Factors affecting fine motor performance. A, Mean standard scores for children treated for unilateral cataract who have a poor visual outcome of >0.6 logMAR (horizontal line bars) compared with those who have a good visual outcome of ≤0.6 logMAR (checkerboard bars) for unimanual dexterity, bimanual dexterity drawing trail, aiming, and catching tasks. The dotted line represents the 50th percentile. Error bars represent SEM. *Group differences are significant after Bonferonni-correction. B, Scatterplots showing correlations of affected-eye visual acuity (logMAR) with mean standard scores for unimanual dexterity, bimanual dexterity, and catching tasks. Poorer affected eye visual acuity was related to poorer performance on these tasks. Dotted line indicates the trend. Overlapping points have been slightly jittered horizontally for clarity.
Aiming and Catching
Children treated for unilateral cataract scored lower than controls on aiming (7.74 ± 2.53 [cataract] vs 9.74 ± 2.94 [control]; T59 = 2.71; P = 0.009) and catching (7.09 ± 2.45 [cataract] vs 9.89 ± 2.58 [control]; T59 = 4.21; P = 0.00009). See Figure 1A. Children treated for unilateral cataract scored lower on the aiming and catching subscale than controls (mean, 7.35 ± 1.99 [cataract] vs 10.08 ± 2.92 [control]; T59 = 3.96; P = 0.0002; Figure 1B), with 39% scoring ≤16th percentile compared with 13% of controls. The difference in these proportions was significant (mean difference, 0.26; 95% CI for difference, 0.04–0.47; Z = 2.34; P = 0.02).
Children treated for unilateral cataract with a poor visual outcome scored lower compared to those with a good visual outcome on catching (mean, 5.63 ± 0.74 [poor] vs 7.87 ± 2.70 [good]; T17.7 = 3.01; P = 0.008), but not aiming (7.13 ± 2.30 [poor] vs 8.07 ± 2.66 [good]; T21 = 0.85; P = 0.41; Figure 2A). Poorer affected eye visual acuity was moderately correlated with worse performance for catching (r = −0.41; P = 0.051; 95% CI, −0.70 to 0.00; Figure 2B). No significant correlation was found with performance for aiming and visual acuity (r = −0.17; P = 0.44; 95% CI, −0.54 to 0.26). No group differences for cataract type (all P values > 0.20) or sensory fusion on the Worth 4-Dot test (all P values > 0.12) were found.
Discussion
Our findings suggest disrupted maturation of many aspects of visuomotor ability as a result of binocularly discordant input during a critical period of development. Our data are consistent with fine motor deficits in strabismic and anisometropic amblyopia and expand on the data showing fine motor deficits in 4-year olds in the IATS study.2–6,9
Although drawing trail performance was worse in children treated for unilateral cataract compared with controls, scores for unimanual and bimanual dexterity were similar to controls. This finding may stem from the difference in task nature and scoring method (accuracy for the former; timed for the latter). This difference is not surprising, given previous studies. For example, overall manual dexterity was affected in 4-year-olds in the IATS study, with scores for unimanual and bimanual dexterity being higher than the drawing trail,9 consistent with our findings. However, all children in the IATS study were treated for a congenital cataract, with no child having cataract extraction >6 months. In our study, only 39% of patients were treated for congenital cataract and had cataract extraction before 6 months of age; yet, there was no effect of cataract type, suggesting no difference between those who had a cataract from birth and those who developed one before 2 years of age.
A subgroup of 9 amblyopic children treated for childhood unilateral cataract in Webber and colleagues3 had equal performance for fine motor tasks compared with controls. However, differences existed between this study and ours. For example, Webber used tasks from the Bruininks-Osteretsky Test of Motor Proficiency, with some similar tasks but many that differ from MABC-2 (eg, cutting circles, drawing or copying shapes, dots in circles). Scoring for timed tasks relied on how many units were completed in a set time, rather than the duration of time spent completing the task. Our cohort may have differed since Webber’s sample size was much smaller, there was no information on alignment status or age of onset, and mean affected eye visual acuity was poorer than in our study (1.1 ± 0.2 logMAR vs 0.7 ± 0.5 logMAR). In another study, Caputo and colleagues6 found that manual dexterity deficits present at baseline in strabismic children (baseline deviation, 14Δ−40Δ) were resolved following eye alignment surgery. Most of the children in our study were orthotropic or had a small tropia, which may have contributed to better scores for some manual dexterity tasks.
In our study, children treated for unilateral cataract had poor catching and aiming performance. Limited research has been conducted on the effects of amblyopia on ball skills, with most research focusing rather on impaired stereo vision (eg, Mazyn and colleagues16). Caputo and colleagues6 found that catching, but not aiming, was poorer in strabismic children compared with controls, and that this impairment was not resolved following eye alignment surgery. In contrast, the IATS study concluded that 4-year-olds with deprivation amblyopia did not have an aiming and catching impairment. However, there was no control group, and mean scores were lower than published data for the normative 50th percentile (7.5 for catching, 8.7 for aiming).
Fine motor impairments have been linked to deficits in visual acuity and stereoacuity.4,5,7 We found that poorer visual acuity was related to poorer performance on most tasks completed with both eyes open, even though the fellow eye had normal visual acuity. Further, those with a poor visual outcome (>0.6 logMAR) scored consistently lower than those with a good visual outcome (≤0.6 logMAR), especially for manual dexterity. IATS reported that better performance for aiming/catching was related to better stereoacuity. However, only 11% of children had measurable stereoacuity, and the real-depth Frisby stereoacuity test used was different than the Randot stereoacuity test used in our study.9 We were unable to correlate performance with stereoacuity because most of our patients (21/23) had no measurable stereoacuity. Since amblyopic eye visual acuity is strongly related to the degree of interocular suppression,17 fine motor deficits may also be a consequence of this suppression. However, we did not find an effect of suppression (ie, no sensory fusion measured with the Worth 4-Dot at 33 cm) on fine motor performance. Lastly, those treated for congenital or infantile cataracts often have ocular motor dysfunction, including strabismus and nystagmus,18 which may contribute to fine motor impairments. Adults with strabismic and anisometropic amblyopia have increased secondary reach-related saccades, a compensatory strategy to increase reach accuracy and grasp precision.19,20
Hand kinematics are also important in evaluating underlying mechanisms of fine motor deficits. Adults with anisometropic or strabismic amblyopia have reduced hand peak acceleration and prolonged acceleration duration during reaching and make more grasping errors.21–23 Children with strabismic or anisometropic amblyopia are slower at planning and executing reaching movements and have less precise grasp than controls, although compensatory strategies may improve performance with age.7,24 For children with deprivation amblyopia, the same may be true; altered hand kinematics may limit their performance on fine motor tasks. Future research should investigate the influence of eye and hand kinematics on fine motor skills in deprivation amblyopia.
Fine motor impairments in children treated for unilateral cataract may affect self-perception of academic, athletic, and social competence. Lower self-perception has been found in children with various forms of amblyopia.25,26 Lower academic and physical competence scores were related to slower reading speed and worse aiming and catching skills in children 8–13 years, respectively.26 Lower peer acceptance scores were related to worse aiming and catching skills in children 3–7 years old.25 Lower peer acceptance and physical competence scores were also related to worse manual dexterity and aiming/catching scores, respectively, in children 3–7 years old with deprivation amblyopia (Birch EB, et al, IOVS 2019;60:ARVO E-Abstract 1028).
Our study had several limitations. We were unable to assess the role that stereoacuity played in our findings since most of the patients had no measurable stereoacuity. Further, we did not assess gross motor skills (eg, balance or walking), which may also be impacted by deprivation amblyopia. Lastly, we did not control for experience with motor skills. Many of the children in both groups were enrolled in physical recreational activities.
Supplementary Material
Acknowledgments
This research was supported by the Thrasher Research Fund (13441) and the National Eye Institute (EY022313 and K99EY028224).
Footnotes
Presented as a poster at the 44th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Washington, DC, March 18–22, 2018.
References
- 1.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res 2013;33:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus 2011;19:119–28. [DOI] [PubMed] [Google Scholar]
- 3.Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008;49:594–603. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci 2010;87:942–7. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor AR, Birch EE, Anderson S, Draper H; FSOS Research Group. The functional significance of stereopsis. Invest Ophthalmol Vis Sci 2010;51:2019–23. [DOI] [PubMed] [Google Scholar]
- 6.Caputo R, Tinelli F, Bancale A, et al. Motor coordination in children with congenital strabismus: effects of late surgery. Eur J Paediatr Neurol 2007;11:285–91. [DOI] [PubMed] [Google Scholar]
- 7.Grant S, Suttle C, Melmoth DR, Conway ML, Sloper JJ. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest Ophthalmol Vis Sci 2014;55:5687–57015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raab EL. Amblyopia In: Raab EL, ed. Pediatric Ophthalmology and Strabismus. 2012–2013 ed. San Fransico, CA: American Academy of Ophthalmology; 2012:61–7. [Google Scholar]
- 9.Celano M, Hartmann EE, DuBois LG, Drews-Botsch C; Infant Aphakia Treatment Study Group. Motor skills of children with unilateral visual impairment in the Infant Aphakia Treatment Study. Dev Med Child Neurol 2015;58:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to < 13 years old. Am J Ophthalmol 2003;136:655–61. [DOI] [PubMed] [Google Scholar]
- 11.Holmes JM, Beck RW, Repka MX, et al. ; Pediatric Eye Disease Investigator Group. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol 2001;119:1345–53. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum A, Santiago A, eds. Clinical Strabismus Management. Philadelphia: WB Saunders; 1999. [Google Scholar]
- 13.Henderson SE, Sugden DA, & Barnett AL. Movement Assessment Battery for Children-2. 2nd ed. London: Pearson Assessment; 2007. [Google Scholar]
- 14.Birch EE, Swanson WH, Stager DR, Woody M, Everett M. Outcome after very early treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci 1993;34:3687–99. [PubMed] [Google Scholar]
- 15.Flatters I, Hill LJ, Williams JH, Barber SE, Mon-Williams M. Manual control age and sex differences in 4 to 11 year old children. PLoS One 2014;9:e88692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazyn LI, Lenoir M, Montagne G, Savelsbergh GJP. The contribution of stereo vision to one-handed catching. Exp Brain Res 2004;157:383–90. [DOI] [PubMed] [Google Scholar]
- 17.Birch EE, Morale SE, Jost RM, et al. Assessing suppression in amblyopic children with a dichoptic eye chart. Invest Ophthalmol Vis Sci 2016;57:5649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch EE, Wang J, Felius J, Stager DR Jr, Hertle RW. Fixation control and eye alignment in children treated for dense congenital or developmental cataracts. J AAPOS 2012;16:156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z, Wong AMF. Effects of anisometropic amblyopia on visuomotor behavior, III: Temporal eye-hand coordination during reaching. Invest Ophthalmol Vis Sci 2011;52:5853–61. [DOI] [PubMed] [Google Scholar]
- 20.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior: III. Temporal eye-hand coordination during reaching. Invest Ophthalmol Vis Sci 2014;55:7831–8. [DOI] [PubMed] [Google Scholar]
- 21.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of strabismic amblyopia on visuomotor behaviour: part II. Visually-guided reaching. Invest Ophthalmol Vis Sci 2014;55:3857–65. [DOI] [PubMed] [Google Scholar]
- 22.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, part 2: visually guided reaching. Invest Ophthalmol Vis Sci 2011;52:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant S, Conway ML. Reach-to-precision grasp deficits in amblyopia: Effects of object contrast and low visibility. Vision Res 2015;114:100–10. [DOI] [PubMed] [Google Scholar]
- 24.Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci 2011;52:1851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception in children aged 3 to 7 years with amblyopia and its association with deficits in vision and fine motor skills. JAMA Ophthalmol 2019;137:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birch EE, Castañeda YS, Cheng-Patel CS, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol 2019;137:16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.