Abstract
Many microbes exhibit nutrient preferences, exemplified by the “hierarchical” consumption of certain carbon substrates. Here we systematically investigate under which physiological conditions hierarchical substrate utilization occurs and its mechanisms of implementation. We show utilization hierarchy of Escherichia coli to be ordered by the carbon-uptake flux rather than the identity of the substrates. A detailed study of glycerol uptake finds that it is fully suppressed if the uptake flux of another glycolytic substrate exceeds a threshold, set to the influx obtained when grown on glycerol alone. Below this threshold, limited glycerol uptake is “supplemented” such that the total carbon uptake is maintained at the threshold. This behavior results from total-flux feedback mediated by cAMP-Crp signaling, but also requires inhibition by regulator fructose-1,6-bisphosphate, which senses the upper glycolytic flux and ensures that glycerol uptake defers to other glycolytic substrates but not to gluconeogenic ones. A quantitative model reproduces all observed utilization patterns including those of key mutants. The proposed mechanism relies on differential regulation of uptake enzymes and requires a specific operon organization. This organization is found conserved across related species for several uptake systems, suggesting the deployment of similar mechanisms for hierarchical substrate utilization by a spectrum of microbes.
INTRODUCTION
Bacteria grown on multiple carbon substrates often consume them sequentially, using some only after others are depleted1,2. This “hierarchical” order of utilization has been studied most intensely in E. coli1,3–5, particularly through the iconic diauxic growth on lactose and glucose6,7. However, several studies have demonstrated that mixtures of carbon substrates that are utilized hierarchically in batch cultures are utilized simultaneously in carbon-limited chemostat cultures8–10. Likewise, if multiple substrates are present at low concentrations, they are often utilized simultaneously even in batch culture8,11–13. Intriguingly, hierarchical utilization appears to be limited to combinations of glycolytic substrates: gluconeogenic substrates tend to be co-utilized with other substrates14. Together, these observations raise questions regarding the strategies and mechanisms bacteria use to control their carbon utilization.
In this work, we address these questions for several exemplary substrate pairs in E. coli. After showing similar physiological behavior on a variety of substrate pairs, we focus on glycerol uptake in the presence of glycolytic substrates glucose or lactose. As shown in Fig. 1a,b and Extended Data 1, the utilization of these substrate pairs is hierarchical: glycerol is not consumed until glucose or lactose is depleted. Through quantitative characterization, we establish that glycerol uptake responds to the total carbon-uptake flux, including glycerol uptake itself. Glycerol uptake is turned off if this total carbon-uptake flux exceeds a threshold set slightly above that on glycerol alone. If the total flux is below that threshold, glycerol is co-utilized with the other substrates, but only such that the total flux is maintained at approximately the same level as that on glycerol alone. This flux-based regulation dictates that glycerol is simultaneously utilized with other substrates when their flux is sufficiently low, explaining why simultaneous utilization is rather general for mixtures of substrates present at low concentrations, including continuous cultures.
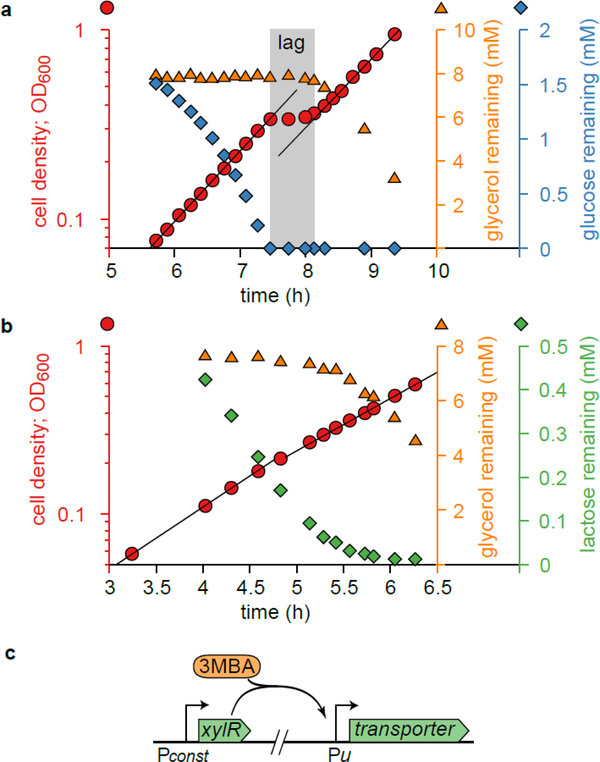
Figure 1. Diauxie and the titratable LacY or PtsG strains.
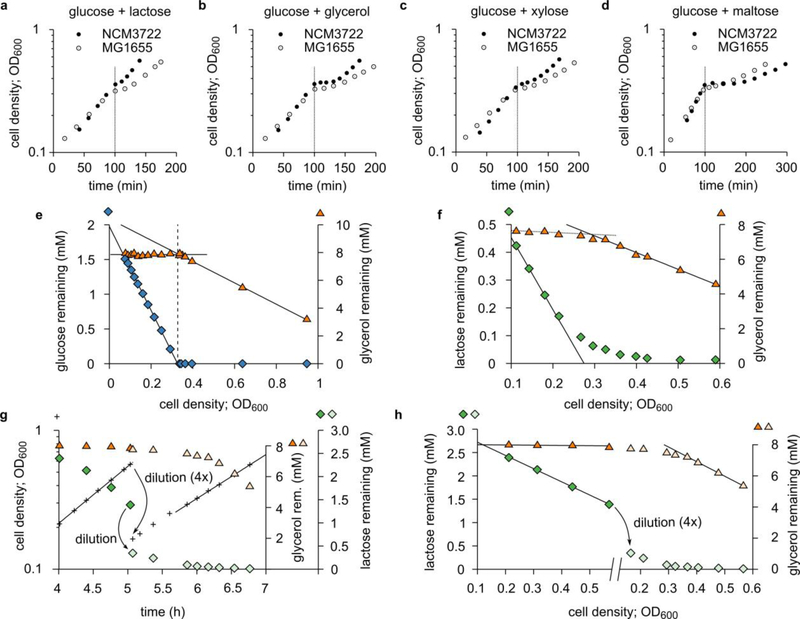
a. Diauxic growth of E. coli (NCM3722) grown on glycerol and glucose. The growth curve (OD600 vs. time, red circles) is shown together with measured concentrations of glycerol (orange triangles) and glucose (blue diamonds) remaining in the medium. Two growth phases—black lines are exponential fits—are separated by a lag time of about 40 min (gray shading). During the first growth phase, glucose is consumed, but glycerol is not. The lag phase starts when glucose is depleted; it ends when glycerol consumption begins.
b. As Panel a, but this time using lactose (green diamonds) instead of glucose. Despite the two distinct growth phases, no clear lag phase is observed. The slight consumption of glycerol (orange triangles) in the first phase is due to the low starting concentration of lactose (relative to the Michaelis constant of the lactose transporter), which is necessary for displaying diauxic growth; no glycerol is utilized at high enough lactose concentrations (see Extended Data 1g,h). Panel a,b clearly demonstrate E. coli’s preference for glucose and lactose over glycerol; yet, the growth curve of Fig. 1b does not exhibit the signature diauxic lag phase. This illustrates that the relation between hierarchical utilization and diauxic growth is not one-to-one and depends on the specifics of the system.
c. Illustration of the genetic constructs used to titrate the expression of substrate uptake (transporter) enzymes by varying the amount of inducer 3MBA in the medium. See Supplementary Figure 1a–c and Supplementary Table 1 for details.
We also address how this flux-based regulation of glycerol uptake is implemented molecularly, using a computational model based on known regulatory interactions15,16. We demonstrate that the response of glycerol uptake to other substrates is implemented by feedback loops mediated by cAMP–Crp, reflecting the total carbon-uptake flux, and fructose-1,6-bisphosphate (FBP), reflecting the upper-glycolytic flux. The latter elucidates how the cell distinguishes glycolytic from gluconeogenic substrates and accordingly switches between hierarchical and simultaneous utilization.
We also show that implementation of the total-flux feedback is facilitated by the chromosomal organization of the glycerol degradation pathway in two separate operons. Strikingly, this organization is found for many other degradation pathways and conserved in related species, suggesting that total-flux feedback is employed for hierarchical substrate utilization by a spectrum of microbes.
RESULTS
Patterns of hierarchical and simultaneous utilization
Titratable uptake of the preferred substrate
Since its discovery, diauxic growth has been the primary way to study hierarchy in carbon-substrate utilization1. However, to elucidate the mechanisms involved, it is necessary to study the regime where the preferred substrate is running out and the cell begins to metabolize the second substrate. The transient nature of this “diauxic shift” makes a quantitative characterization difficult to perform. Here, we exploit strains that allow the cellular response to the depletion of the preferred substrate to be studied in batch cultures under balanced exponential growth. Because maintaining fixed low substrate concentrations during exponential growth is difficult, we use E. coli strains in which the expression of a substrate uptake system—and hence that substrate’s uptake flux—can be controlled by varying the concentration of an inducer (3-methylbenzyl alcohol, or 3MBA) in the growth medium (Fig. 1c)16. Thus, we mimicked the reduced uptake flux of the preferred substrate when it runs out while keeping its actual concentration at saturating levels. We employed three such strains (NQ917, NQ1243, NQ399; see Supplementary Figure 1), in which the expression of LacY, PtsG, and GlpF/GlpK, the uptake systems for lactose, glucose, and glycerol, can be finely titrated.
A tight growth-rate crossover
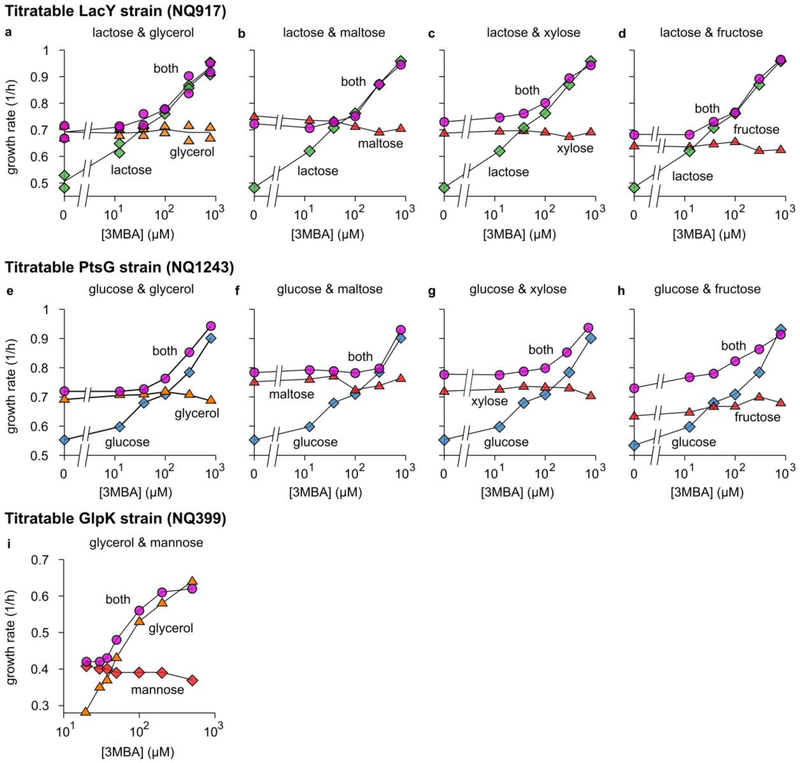
We first studied the growth of the titratable LacY strain (NQ917), using minimal medium with glycerol, lactose, or both, at various concentrations of 3MBA (Fig. 2a). At low 3MBA concentrations (low LacY expression), the growth rate on the two substrates (pink circles) was similar to that on glycerol only (orange triangles), while at high 3MBA concentrations (high LacY expression) it was similar to that on lactose only (green diamonds). A tight crossover between these regimes occurred at [3MBA] ≈ 100 μM and a growth rate of 0.7—0.8/h, near the intersection of the pink circles and the orange triangles (indicated with a red arrow). Remarkably, the growth rate never dipped below that on glycerol only (≈ 0.7/h). A very similar behavior was found using the titratable PtsG strain (NQ1243) with glucose instead of lactose (Fig. 2b) and, importantly, for many other substrate combinations (see Extended Data 2).
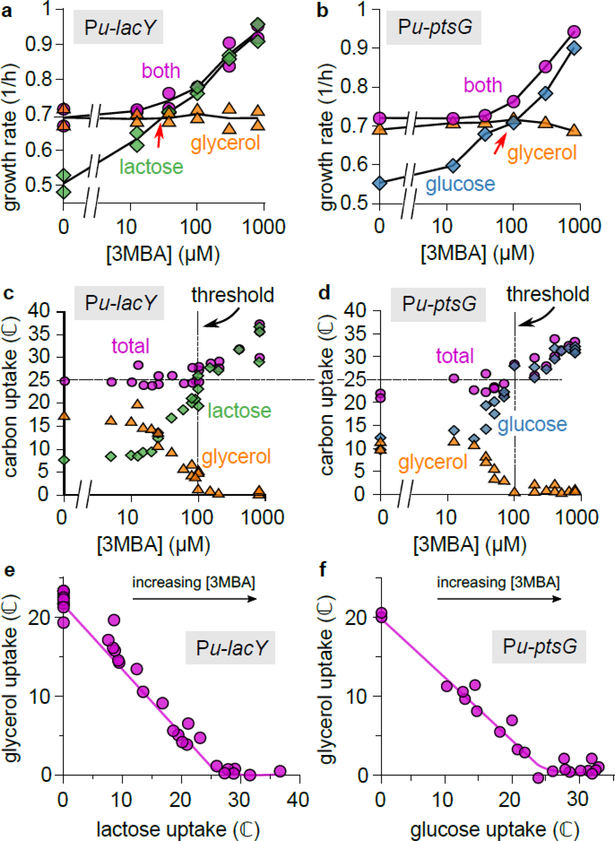
Figure 2. Uptake fluxes reveal hierarchical and simultaneous utilization regimes.
a. Growth rates for the titratable LacY strain (NQ917), grown on lactose, glycerol, or both, at various 3MBA concentrations. To demonstrate the reproducibility of the data, results from two independent experiments are shown for each condition.
b. As Panel a, but for the titratable PtsG strain (NQ1243), with glucose instead of lactose. Other substrate combinations show similar patterns; see Extended Data 2.
c. Lactose and glycerol uptake (in ℂ = mM of carbon atoms per OD600 per hour) by the titratable LacY strain (NQ917) at various 3MBA concentrations. If lactose uptake is below a threshold of ≈ 25 ℂ (horizontal dashed line), glycerol is consumed, too. In this regime (left of the dashed vertical line), the total carbon uptake stays approximately constant despite a threefold change in lactose uptake. Also see Supplementary Figure 2.
d. As Panel c, but using the titratable PtsG strain (NQ1243) and glucose instead of lactose. A strikingly similar threshold of ≈ 25 ℂ is found for the onset of glycerol utilization.
e. Plotting glycerol uptake versus lactose uptake (the “flux relation”) of the titratable LacY strain reveals a threshold-linear relation. (The solid line is a guide to the eye.)
f. As Panel e, but for the titratable PtsG strain with glucose instead of lactose. The flux relation is remarkably similar to that of Panel e.
A threshold separates hierarchical and simultaneous utilization
To elucidate the growth-rate crossovers, we measured the uptake of glycerol and lactose (in the presence of both) at various 3MBA levels (Fig. 2c). For lactose uptake above a threshold (dashed horizontal line at jth ≈ 25 ℂ, where ℂ =1 mM of carbon atoms per OD600 per hour), no glycerol was taken up. But when it fell below the threshold, the cells supplemented it with glycerol. The threshold occurred at [3MBA] ≈ 100 μM (vertical dashed line), corresponding to the occurrence of the growth-rate crossovers shown in Fig. 2a,b. Using single-cell measurements, we ruled out that this supplementation effect arises from population heterogeneity (Supplementary Figure 2).
Strikingly, throughout the supplementation regime glycerol uptake adapted such that the total carbon-uptake flux (Fig. 2c) remained approximately constant despite a three-fold change in lactose uptake, at a level corresponding to that of growth on glycerol alone, (23 ± 1)ℂ (error represents 95% CI, n = 11 independent measurements). The same pattern was found for the titratable PtsG strain (NQ1243), with a remarkably similar threshold value jth ≈ 25 ℂ (Fig. 2d). Because glycerol and glucose are co-utilized in the supplementation regime, the diauxic lag vanishes there (see Extended Data 3).
A threshold-linear flux relation as a hallmark of hierarchical utilization
If glycerol uptake is plotted against lactose uptake (Fig. 2e) or glucose uptake (Fig. 2f), a nearly threshold-linear relation is seen. This “flux relation” reveals the transition from a supplementation regime (a straight line with a slope of approximately −1) to a hierarchical regime (no glycerol uptake) if the uptake of the preferred substrate exceeds the threshold jth ≈ 25 ℂ. This flux relation is more revealing than the diauxic lag, which is exhibited only under carefully chosen conditions (Fig. 1a,b, Extended Data 1, Supplementary Figure 3).
Regulation of glycerol uptake
To elucidate the features of hierarchical utilization described in Fig. 2, we turn to the regulation of glycerol uptake. The catabolism of glycerol has been studied in detail15 (Fig. 3a and Supplementary Figure 4). The assimilation of glycerol into glycolysis requires two reactions, catalyzed by kinase GlpK and dehydrogenase GlpD, which are both transcriptionally repressed by specific repressor GlpR17,18 and activated by cAMP–Crp. Repression by GlpR is relieved by inducer sn-glycerol-3-phosphate (G3P), which is the product of GlpK and the substrate of GlpD. GlpK is allosterically inhibited by fructose-1,6-bisphosphate (FBP), an intermediate of glycolysis19. (The PTS enzyme EIIAGlc is also known to inhibit GlpK allosterically, but this interaction has little or no effect on glycerol uptake during glucose-glycerol diauxie20; data presented below support this.)
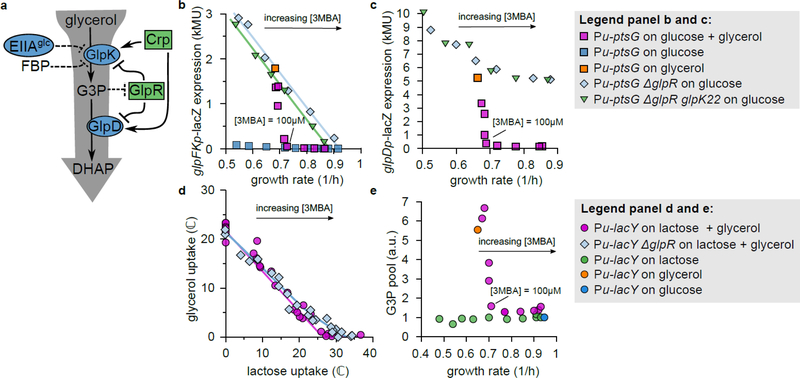
Figure 3. Glycerol uptake involves derepression of glycerol catabolic genes.
a. Known regulation of glycerol uptake under aerobic conditions. Blue ovals represent enzymes, green rectangles transcriptional regulators. Dashed lines indicate allosteric inhibition18,19,49. See Supplementary Figure 4 for more details.
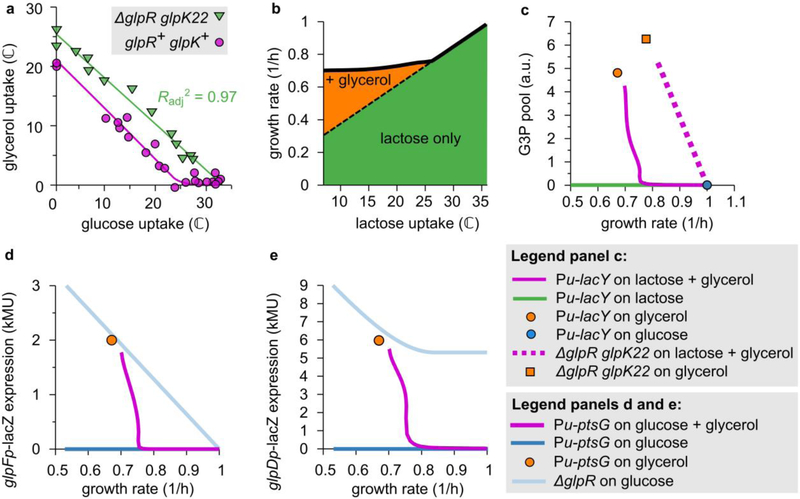
b. LacZ reporter expression from the glpFK promoter versus growth rate in titratable PtsG strains (HE305 and mutants thereof) with varying 3MBA levels. In the presence of glucose + glycerol (purple squares) expression fell abruptly if the growth rate exceeded that on glycerol alone (orange square). In contrast, in the ΔglpR or ΔglpR glpK22 background (pale blue diamonds and green triangles) the expression followed a straight line (a “C-line”16). (Regression lines are shown. ΔglpR strain:, n = 6 conditions, t = 17.6, df = 4, two-sided p = 6.1 × 10−5. ΔglpR glpK22 strain:, n = 6 conditions, t = 46.0, df = 4, two − sided p = 1.3 × 10−6.) Little expression was observed on glucose alone (blue squares).
c. As Panel b, except that expression from the glpD promoter was measured (strain HE397 and mutants thereof). Again, in the presence of glucose + glycerol (purple squares) expression turned off abruptly if the growth rate exceeded that on glycerol alone (orange square). However, reporter expression in the ΔglpR or ΔglpR glpK22 background (pale blue diamonds and green triangles) responded more moderately and displayed a high background level.
d. Comparison between the flux relations of the ΔglpR strain (NQ958) and the glpR+ strain (NQ917) in the titratable LacY background. The threshold-linear shape is less prominent in ΔglpR (blue diamonds) than in glpR+ (purple circles). (Solid lines are guides to the eye.)
e. Intracellular G3P pool versus growth rate in the titratable LacY strain (NQ917) grown on lactose + glycerol with various 3MBA concentrations (purple circles), lactose alone with various 3MBA concentrations (green circles), glycerol alone without 3MBA (orange circle), and glucose alone without 3MBA (blue circle). The measurements for each mass spectrometry run were normalized by the result for growth on lactose alone with [3MBA] = 800 mM. In the presence of lactose + glycerol, the G3P pool abruptly drops if the growth rate exceeds that on glycerol alone.
Onset of glycerol uptake is due to GlpR derepression upon abrupt increase of its inducer G3P
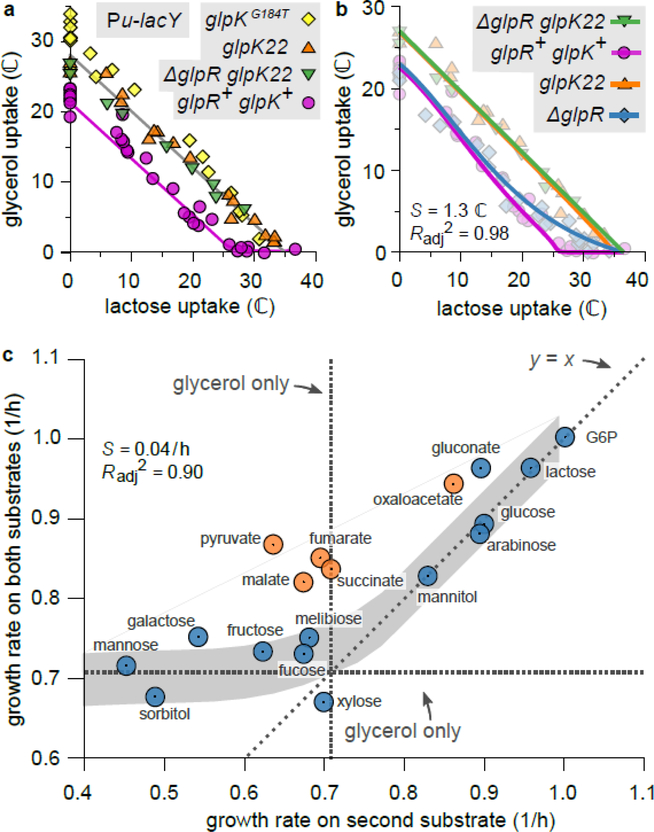
To establish whether the abrupt onset in glycerol uptake at the threshold (Fig. 2c,d) is tied to the expression of the GlpK and GlpD enzymes, we measured LacZ reporter expression from the glpFK (strain HE305) and glpD (strain HE397) promoters in titratable PtsG strains. During growth on glucose + glycerol with various 3MBA concentrations, the LacZ expression from both promoters sharply increased when the 3MBA concentration was reduced below the threshold of 100 μM (Fig. 3b,c, purple squares). The abrupt onset of expression resulted from the relief of repression by GlpR, because in ΔglpR mutant strains (HE308 and HE398) this behavior is abolished (Fig. 3b,c, light-blue diamonds). Consistent with this, the flux relation of the ΔglpR strain (NQ958) shows a more gradual transition to glycerol consumption (Fig. 3d, light-blue diamonds).
The relief of repression by GlpR is due to an abrupt rise of the inducer G3P, as determined by mass spectrometry (see Materials & Methods): in the titratable LacY strain (NQ917) grown on lactose + glycerol, the G3P pool increased abruptly when the 3MBA level was reduced below the threshold of 100 μM (Fig. 3e, pink circles).
The tight coupling between onset of glycerol uptake and the relief of GlpR-dependent repression suggests that the lactose/glucose flux inhibits glycerol uptake by affecting the G3P pool. We therefore asked whether the remaining regulators, FBP and cAMP–Crp, could transmit information on the lactose/glucose flux to the G3P pool.
FBP senses upper-glycolytic flux and directly inhibits glycerol uptake
A recent study reported that the intracellular FBP pool increases linearly with the glycolytic flux and hence called FBP a glycolytic flux sensor21. To verify this, we measured the intracellular FBP pool in the titratable LacY strain (NQ917) grown on lactose alone and on lactose + glycerol, with various 3MBA concentrations. Across conditions, the FBP pool increased consistently with the lactose uptake flux (Fig. 4a) but not with the total glycolytic flux (including the glycerol flux) (Fig. 4b). This indicates that the FBP pool is sensitive to the flux through upper glycolysis (i.e., to substrates entering glycolysis upstream of FBP) rather than to the total glycolytic flux (including substrates entering downstream). Since FBP is known to inhibit GlpK activity19 (Fig. 3a and Supplementary Figure 4) and hence the synthesis of G3P, it can transmit information about the upper-glycolytic flux to the G3P pool.
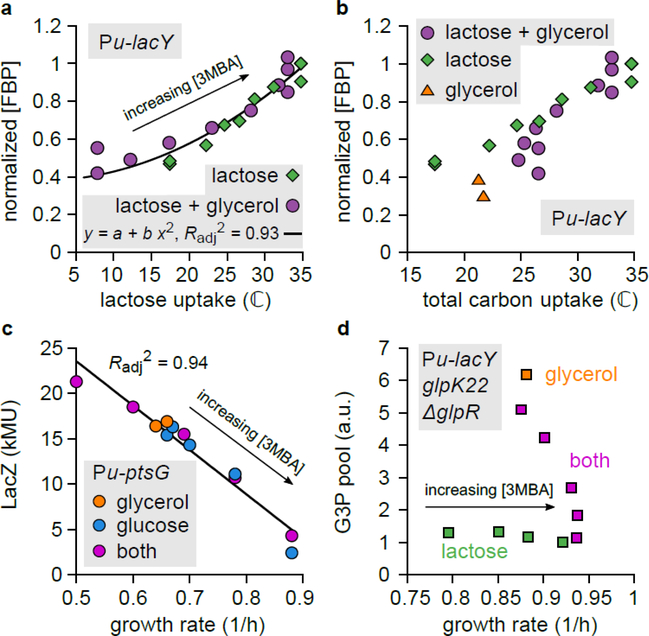
Figure 4. Glycerol uptake is regulated by two flux sensors: FBP and cAMP–Crp.
a. Intracellular FBP pool in the titratable LacY strain (NQ917) grown on lactose + glycerol or lactose alone, plotted against the lactose uptake flux. The results of each run of mass spectrometry were normalized by the result on lactose alone with [3MBA] = 800 mM. The black line is a quadratic fit ( y = a + b x2; , n = 22 experimental conditions, t = 16.7, df = 20, two-sided p = 3 × 10−13). The FBP pool can be considered a function of the lactose uptake flux alone.
b. As Panel a, but now with total carbon flux on the horizontal axis. The FBP pool cannot be considered a function of the total carbon-uptake flux because the three datasets do not collapse.
c. LacZ expression levels from the native lac promoter in the titratable PtsG strain (NQ1243) grown on glucose alone, glycerol alone, or both, with various 3MBA concentrations, plotted against the growth rate. Because IPTG (1 mM) was added to remove repression by LacI, expression is a proxy for cAMP–Crp activity. The black line is a linear regression (, n = 12 experimental conditions, t = 13, df = 10, two-sided p = 1.3 × 10−7 ). The data collapse on a single trend line (a “C-line”16), demonstrating that activation by cAMP–Crp can be considered a function of the growth rate or the total carbon-uptake flux.
d. G3P pool in the titratable LacY strain with ΔglpR glpK22 mutations (NQ1187). When grown on lactose + glycerol with varying amounts of 3MBA, the G3P pool responds sharply to changes in the growth rate. Because the ΔglpR glpK22 mutations remove GlpR repression and allosteric inhibition by FBP, this demonstrates that cAMP-Crp signaling affects the G3P level.
cAMP–Crp senses the total carbon-uptake flux and sharply affects the G3P pool
Recent studies reported that, under variation of carbon sources and uptake rates, transcriptional activation by cAMP–Crp is a decreasing function of the growth rate; in particular, the expression levels of many catabolic genes regulated by cAMP-Crp decrease linearly with the growth rate14,16,22. We verified that this linear relation (called the “C-line”16) also applies under the growth conditions of this study (Fig. 4c). Because the growth rate correlates strongly with the total carbon-uptake rate (Supplementary Figure 5a,b), this also implies that cAMP–Crp can be considered a sensor of the total carbon-uptake flux.
cAMP–Crp activates the transcription of the two operons containing the glycerol degradation pathway (Supplementary Figure 4). To test whether cAMP–Crp signaling also affects the intracellular G3P pool, we studied a titratable LacY strain carrying ΔglpR glpK22 double mutations. In this strain, the glpK22 mutation renders GlpK insensitive to allosteric inhibition by EIIAGlc and FBP 19,23, and the glpR deletion removes specific repression by GlpR, so that GlpK and GlpD activity is controlled by cAMP–Crp only (Fig. 3a and Supplementary Figure 4). When this strain was grown on lactose + glycerol with various 3MBA concentrations, the intracellular G3P pool responded sharply to changes in the growth rate (Fig. 4d, purple squares), demonstrating that cAMP–Crp signaling affected the G3P pool. Such an increase was not observed for growth on lactose alone (Fig. 4d, green squares), ruling out effects due to conversion of other internal metabolites to G3P. The response differed markedly from that of the glpR+ glpK+ strain, where the G3P pool fell to the basal level as soon as the growth rate exceeded the threshold of 0.7/h (Fig. 3e). This confirms again that GlpR and/or allosteric inhibition by FBP are necessary for strict inhibition at the threshold.
Because the synthesis and turnover of G3P are dictated by GlpK and GlpD, the G3P level is affected by the ratio of GlpK to GlpD. Since their genes glpK and glpD reside in different operons, the effect of cAMP–Crp signaling on the G3P pool could be due to a difference in its effect on the expression of these operons (Extended Data 4). Indeed, in the ΔglpR and ΔglpR glpK22 strains, in which glpD and glpK expression are controlled by cAMP–Crp only, reporter expression from the glpK promoter follows a C-line whereas reporter expression from the glpD promoter does not (compare light-blue diamonds in Fig. 3b and c). This demonstrates that differential regulation of the two operons by cAMP–Crp affects the ratio of GlpK and GlpD expression and thus transmits information on the total carbon uptake to the G3P pool, hence affecting glycerol uptake.
Analysis and quantitative molecular model
The total-flux feedback strategy
Above, we observed that glycerol uptake (i) responds nearly identically to lactose and glucose uptake, (ii) is completely inhibited if lactose or glucose flux exceed a threshold jth, and (iii) is supplemented if lactose or glucose flux is below jth, such that total carbon-uptake flux is approximately constant and the growth rate is near the growth rate on glycerol alone.
In theory, observations (i)—(iii) can be implemented by a simple scheme that we call the total-flux feedback strategy (Extended Data 5). It is characterized by a single negative feedback loop: glycerol uptake is inhibited by a signal that represents the total carbon-uptake flux including glycerol uptake itself. Observations (i)—(iii) are attained generically provided the response of glycerol uptake to the total-flux sensor is sensitive enough and has its response threshold jth tuned slightly above the uptake flux on glycerol alone, jG,0. In the actual system, cAMP–Crp could implement the total-flux feedback strategy. The required sensitive response of glycerol uptake to cAMP–Crp can be achieved by differential regulation of glpK and glpD by GlpR (see Supplementary Discussion D).
Distinguishing glycolytic and gluconeogenic carbon fluxes
While the total-flux feedback strategy alone can produce the hierarchical and supplementation regimes, it cannot explain the observed simultaneous utilization of gluconeogenic substrates with other substrates14 because a total-flux sensor does not distinguish glycolytic and gluconeogenic substrates. Specifically, cAMP–Crp signaling is known to respond equally to glycolytic and gluconeogenic substrates (Supplementary Figure 5e)14.
The situation changes, however, if FBP is added to the scheme. During growth on gluconeogenic substrates the FBP pool is much lower than during growth on glycolytic substrates24. Glycolytic and gluconeogenic substrates could therefore be distinguished if the inhibitory effect of FBP on GlpK activity is necessary for the inhibition of glycerol uptake (on top of cAMP–Crp signaling). Consistent with this, a strain carrying the glpKG184T mutation (NQ959), which renders GlpK insensitive to inhibition by FBP25, lost the hierarchical utilization of glycerol with lactose, instead showing a strikingly linear flux relation (Fig. 5a). We note that the glpK22 mutation, which renders GlpK insensitive to inhibition by both EIIAGlc and FBP23, resulted in a similar linear flux relation (Fig. 5a), confirming that EIIAGlc plays a minor role.
Figure 5. Analysis, modeling and predictions.
a. Comparison between the flux relations of strains harboring the glpKG184T mutation (NQ959), the glpK22 mutation (NQ1186), or the ΔglpR glpK22 double mutation (NQ1187), and the wild-type glpK (NQ917, glpR+ glpK+) in the titratable LacY background. In the mutant strains the threshold-linear shape is lost, and the flux relation is nearly linear. (Linear regression for glpKG184T:, n = 18 conditions, t = 22.6, df = 16, p = 1 × 10−13. For glpK22:, n = 18, t = 29.7, df = 16, conditions, p = 2 × 10−15. For ΔglpR glpK22:, n = 8 conditions, t = 27.8, df = 6, p = 1 × 10−7.) Lines are guides to the eye.
b. Model fits of the flux relations for various strains (solid lines) together with the corresponding measurements (background symbols). A single set of parameter values (given in Supplementary Table 3) fits the data of all strains (, standard error of the regression S = 1.3 ℂ, n = 84 experimental conditions). The same model and parameters also reproduce G3P measurements and expression data (see Extended Data 6).
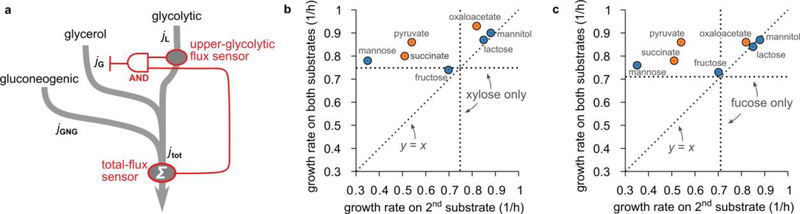
c. Growth rate of WT cells (NCM3722) in the presence of glycerol plus a “second” substrate, versus growth rate on the second substrate only, for many second substrates. Plotted is the mean of n = 2 to 4 independent replicates (see Source Data). Horizontal and vertical dashed lines indicate the growth rate on glycerol only. If the second carbon substrate is processed by upper glycolysis (blue circles; errors are of the order of the symbol size), the model predicts hierarchical utilization (gray band; width is estimated SE of the prediction, assuming an independent error in the predicted uptake of each substrate of S = 1.3 ℂ, as in Panel b; fit quality: , S = 0.04 /h, n = 13 independent measurements). Therefore, the growth rate on both substrates is approximately the larger of the two single-substrate growth rates. In contrast, if the second substrate is gluconeogenic (orange circles), the growth rate on both substrates is larger than on either substrate alone (two-sided paired t-test, n = 5 cases, t = 9.26, df = 4, p = 8 × 10−4), reflecting simultaneous utilization. A similar trend is seen if xylose or fucose are used instead of glycerol (Extended Data 7b,c).
Quantitative molecular model of the regulation of glycerol consumption
To verify that specific repression by GlpR, allosteric inhibition of GlpK by upper-glycolytic flux sensor FBP, and total-flux feedback through differential regulation by cAMP–Crp can together account for all experimental observations in Fig. 1 to 4, we constructed a mathematical model; see Supplementary Discussion. For a single set of physiologically reasonable parameters (Supplementary Table 3; see Supplementary Figure 6 for a parameter sensitivity analysis), the model reproduces the threshold-linear flux relation of Fig. 2e, as well as the flux relations of the ΔglpR, glpK22, and ΔglpR glpK22 strains (see Fig. 5b). Moreover, the model predicts the flux relation of the ΔglpR glpK22 strain to be linear (see Supplementary Discussion B), as observed for the titratable LacY strain in Fig. 5a and the titratable PtsG strain in Extended Data 6a. With the same parameters, the model also reproduces (Extended Data 6) the salient features of the growth-rate crossover seen in Fig. 2a,b, the G3P pool as a function of the growth rate presented in Fig. 3e and 4d, and the response of the glpK and glpD expression levels as a function of the growth rate shown in Fig. 3b,c.
Simultaneous utilization of glycolytic and gluconeogenic substrates
The model can also predict the growth rate on glycerol + a second substrate (See Supplementary Discussion F, and Extended Data 7a). If the second substrate is glycolytic, the utilization is dictated by the threshold-linear flux relation (Fig. 2e,f), which implies two regimes: (i) If the second substrate provides an uptake flux (and hence growth rate) larger than that on glycerol alone, glycerol consumption is fully inhibited, so that the predicted growth rate is simply the growth rate on the second substrate alone. (ii) If the substrate provides an uptake flux (and hence growth rate) below that on glycerol alone, glycerol consumption is supplemented such that the growth rate is approximately as obtained on glycerol alone. In contrast, if the second substrate is gluconeogenic, allosteric inhibition of GlpK by FBP is minimal, the substrates are co-utilized, and the resulting growth rate should be higher than on each of the individual substrates alone. Note that these predictions do not rely on any details of the model and are completely immune to uncertainty in the model parameters.
To test this, we grew wild-type cells on glycerol plus one of a variety of other substrates. Fig. 5c plots the measured growth rate on two substrates against that on the second substrate alone (colored circles). For second substrates that are processed (at least partly) by the upper-glycolytic pathway (blue circles) the results are consistent with the model prediction (gray band). In contrast, adding a gluconeogenic substrate (orange circles) consistently yields a higher growth than on either substrate alone. We conclude that the joint regulation by FBP and cAMP–Crp limits hierarchical utilization of glycerol to combinations of upper-glycolytic carbon substrates.
Remarkably, a very similar utilization pattern was observed for cells grown on xylose or fucose plus a variety of second substrates (Extended Data 7b,c), suggesting that the regulation strategy of glycerol uptake is not an exception (see Discussion).
DISCUSSION
Flux-based regulation underlies transitions between hierarchical and simultaneous utilization of glycolytic substrates
In this study, we investigated the regulatory strategies underlying the utilization of multiple carbon substrates. Titratable uptake systems allowed us to control the uptake rate of a preferred substrate (lactose or glucose) in the presence of a less preferred one (glycerol) under balanced exponential growth. Glycerol uptake was completely suppressed if the lactose or glucose uptake flux exceeded that on glycerol alone. Otherwise, glycerol uptake was curbed such that the total carbon-uptake flux was maintained near the flux obtained on glycerol alone (Fig. 2c,d). This pattern is reflected by the threshold-linear shape of the flux relation (Fig. 2e,f) and readily accounts for known differences between batch and continuous cultures: Hierarchical utilization dominates in batch cultures where substrate concentrations and hence uptake rates are high, whereas simultaneous utilization dominates in continuous cultures, where substrate concentrations and hence uptake rates are low.
Flux-based regulation can be efficient because it allows many carbon substrates (glucose, lactose, …) to inhibit the uptake of another substrate (glycerol) through a single regulatory system. As such, it could be used to efficiently establish a hierarchy among a collection of substrates (see Supplementary Figure 7). We saw that many pairs of carbon substrates showed a growth-rate crossover similar to those involving glycerol (Extended Data 2), and that the growth rates on xylose or fucose plus a second substrate exhibit the same pattern as observed for glycerol (Extended Data 7b,c). This suggests that a fluxbased mechanism may be employed in the uptake of substrates other than glycerol, although it is unknown whether FBP or another upper-glycolytic flux sensor (e.g., EIIAGlc)26 is involved in those cases. It also justifies the ad hoc rule used in the previous work27 modeling the kinetics of growth transitions, where a threshold in the total carbon uptake was assumed to control the uptake of less preferred carbon source.
We previously published a very simple model that predicts the growth rate of E. coli grown on one glycolytic and one gluconeogenic substrate14. Supplementary Figure 8 illustrates the relationship between that model and the one presented here. The mechanistic models developed here are based on knowledge of biological processes. As such, in addition to successfully reproducing complex biological phenomena, they also shed light on the mechanistic origins of these responses.
Total-flux feedback is enabled by differential regulation of two operons
We established that cAMP–Crp causes sharp changes in the pool of inducer G3P (Fig. 4d) through differential regulation of glpK and glpD. This differential regulation is easily implemented because these enzymes are encoded on different operons. Strikingly, the uptake systems of many other carbon substrates show the same operon organization: separate operons for the enzymes upstream and downstream of the specific inducer, each regulated by cAMP–Crp and a specific repressor. Supplementary Figure 9 illustrates several examples. It is thus possible that the strategy of total-flux feedback through differential regulation is also implemented in these uptake systems.
Also, if the placement of glpK and glpD in different operons is physiologically important, one would expect this to be preserved in E. coli’s pangenome and in related species. An analysis of EcoCyc database (version 23.0) confirmed this28. This database returned 300 genomes that contain an ortholog of glpD and belong to the order of Enterobacteriales. (Of these, 228 are strains of E. coli, 38 are other Enterobacteriacea, and 34 are Enterobacteriales outside of the Enterobacteriacea.) In none of these, glpK is in the same operon as glpD. A similar analysis for the xylose and fucose uptake systems (Extended Data 7b,c) again yielded no exceptions within the Enteriobacteriales: the enzymes xylA and xylB (downstream of inducer D-xylose) are never combined with xylE, xylF, xylG or xylH (upstream), and fucA (downstream of inducer L-fucose-1-phosphate) is never combined with fucP, fucI, fucK or fucU (upstream).
In contrast, the famous lac system is encoded on a single operon. It is noteworthy, however, that the lac system has several exceptional features. First, both our strain (NCM3722) and the strain originally studied by Monod (ML308) grow faster on lactose than on glucose16,29, but glucose is nevertheless preferred. Thus, cells growing on lactose and glucose cannot possibly follow the growth-rate crossover shown in Fig. 2a,b. Second, the inducer of the lac system, allolactose, is synthesized and degraded by one and the same enzyme LacZ, which rules out differential regulation. These features suggest that the lac system is an exception to the rule described here.
Physiological rationalization of hierarchical and simultaneous utilization
Recent studies suggest that simultaneous utilization of glycolytic and gluconeogenic substrates is advantageous, allowing cells to save resources that would otherwise be used to express enzymes necessary to connect the upper and the lower parts of carbon catabolism30–32. Our study shows that E. coli uses the upper-glycolytic sensor FBP as a cue to discriminate glycolytic or gluconeogenic substrates and to choose hierarchical or simultaneous utilization accordingly.
Future experiments of the type presented here for these and other substrates will tell how widely the strategy employing flux sensors is used. Characterization of such strategies for different microbes in a community will reveal a detailed map of who consumes what in which order. It may also open up rational, synthetic biology approaches to manipulating the order of substrate hierarchy, e.g., the efficient breakdown of cellulose for biofuel production33–35.
METHODS
Reagents and E. coli strains
Isopropyl-β-D-1-thiogalactopyranoside (IPTG) was purchased from Bio Basic Inc. (Ontario, Canada). Tetrabuthylammoniumhydrogensulfate (TBAS) was purchased from Waters Corp (Milford, MA). Adenosyne 5’-triphosphate, o-nitrophenyl-β-galactoside, 4-amino-antipyrine, N-ethyl-N-(3-sulfopropyl) m-anisidine, chloroacetaldehyde, glycerol 3-phosphate oxidase from Aerococcus viridans, horseradish peroxidase, glycerol kinase from Cellulomonas sp., and β-galactosidase from E. coli were purchased from Sigma-Aldrich (St Louis, MO). Restriction enzymes were purchased from New England Biolabs (Ipswich, MA).
All E. coli strains and oligonucleotides are described in Supplementary Tables 1 and 2, respectively. Unless stated otherwise, all strains were derived from the prototrophic E. coli K-12 strain NCM372236. For details on reagents and strain construction, see “Strain construction” below.
Growth conditions
Unless stated otherwise, the nitrogen- and carbon-free minimal-medium base was N−C− as described in Ref. 37. As nitrogen source, 20 mM of ammonium chloride was used. All growth experiments were conducted at 37°C under vigorous shaking at 250 rpm in a water bath shaker. In growth-rate measurements and diauxie measurements, each carbon substrate was supplied at the carbon-atom concentration of 120 mM, unless stated otherwise. For measurements of uptake fluxes, the concentrations of carbon substrates were adjusted at each 3MBA concentration such that they were high enough to achieve balanced exponential growth, but low enough to obtain sufficient resolution for uptake measurements (see Source Data). OD600 was measured with a spectrophotometer Genesys 20 (Thermoscientific). With this spectrophotometer, one OD600 × ml is equivalent to 0.44 mg dry weight38.
Strain construction
Transfer of glpK mutations to NCM3722 background
The glpK point mutations glpK22 and glpKG184T were moved from one strain to another as follows. A kanamycin-sensitive parental strain was first transduced with P1vir phage prepared from JW3887–1 (ΔpfkA775::kan). The kanamycin-resistant pfkA transductant cannot grow on minimal agar plates supplemented with 20 mM mannitol as the sole carbon substrate 39. The pfkA transductant was further transduced with P1vir phage prepared from the glpK mutant strain and selected on the minimal agar plate supplemented with 20 mM mannitol as the sole carbon substrate. Since pfkA and glpK are genetically linked, the glpK mutant frequently replaces the wild-type glpK in Mtl+ (pfkA+) transductants. The Mtl+ KanS transductant carrying the glpK allele of interest was selected by DNA sequencing of the region surrounding the mutation.
PLtetO-1-xylR
The PLtetO-1-xylR allele was made as follows. The xylR gene 40 was cloned as a KpnI-BamHI fragment on pKD13-rrnBt:PLtet-O1 41. The resulting plasmid was used as a template to insert a tandem array of the kan gene, the rrnB terminator, and PLtetO-1:xylR between ycaC and ycaD (zca locus) and between intS and yfdG (zfd locus) by primer sets ycaD-P1-S1 & ycaD-P4-A1 and intC-P1-S1 & intC-P4-A1, respectively. P1vir phages were prepared from these strains and used to transduce NCM3722. Using these phages, NQ914 and NQ915 were created by respectively one and two cycles of transduction and the flip-out of the kan gene.
Pu-ptsG
The ΔptsG468::Φ(kan:Pu) allele in which the ptsG promoter is replaced by the Pu promoter was made as follows. The region containing the kan gene and Pu promoter was PCR amplified by primers SDY158 and SDY159 from NQ38116 and integrated at the ptsG locus, resulting in the replacement of 342 base before the open reading frame with a tandem array of kan gene and Pu promoter (zah locus) by using the λ Red system 42, which results in the replacement of ptsG promoter with Pu promoter.
glpFp-gfp
NQ1344 was made as follows. The plasmid pKD13-rrnBt:PLtet-O1:gfp was made by inserting the gfpmut3b structural gene immediately downstream of the PLtetO-1 promoter in pKD13-rrnBt:PLtet-O141. The PLtetO-1 in the plasmid pKD13-rrnBt:PLtet-O1:gfp was replaced by an XhoI-KpnI fragment containing the glpF promoter that was amplified from NCM3722 by primers PglpF-XhoI-S2 and PglpF-KpnI-A4. The resulting plasmid pKD13-rrnBt:glpFp:gfp was used to produce a donor DNA fragment. The host strain was made by flipping out the kan gene from the strain carrying a tandem array of kan gene, rrnB terminator, glnK promoter, the 5’ untranslated region (5’ UTR) from the PLtetO-1 promoter and the structural gene of gfpmut3b between intS and yfdG (zfd locus) 43 and transforming the flipped-out strain with pKD46 for λ Red recombination 43. This strain was transformed with the donor DNA fragment that was amplified by primers intC-P1-S1 and gfp-Ptet-PglpF-A1 using pKD13-rrnBt:glpFp:gfp as a template. The resulting strain carries a tandem array between intS and yfdG consisting of the kan gene, the rrnB terminator, the glpF promoter spanning −246 to −1, the 5’ UTR from PLtetO-1 promoter, and the structural gene of gfpmut3b. P1vir phage was prepared from this strain and used to transduce the strain with the kan gene flipped out from NQ916.
glpFp-lacZ and glpDp-lacZ
The glpFp-lacZ reporter strain HE305 was made by transducing NQ1332, a strain made by flipping out of kan gene from NQ1243, with P1vir phage containing a tandem array of the kan gene, the rrnB terminator, and the glpF promoter spanning −273 to −1 bp relative to glpF translational start site16.
The glpDp-lacZ reporter strain HE397 was made as follows. The PLtetO-1 in the plasmid pKD13-rrnBt:PLtet-O1 was replaced with an XhoI-KpnI fragment containing the glpD promoter that was amplified from NCM3722 by primers PH008 and PH009. The resulting plasmid pKD13-rrnBt:glpDp was used to produce a DNA as follows. First, a DNA fragment was amplified by PH019 and PH020 using pKD13-rrnBt:glpDp as a template. This fragment was further amplified by primers PH025 and PH026 to produce the DNA donor. The host strain NQ309 was transformed with the donor DNA fragment to replace a part of lacI gene and the entire lac promoter (from +134 bp after lacI translational start codon to lacZ translational start codon) with a tandem array of the kan gene, the rrnB terminator, and the glpD promoter spanning −292 to −1 bp relative to glpD translational start site. P1vir phage was prepared from this strain and used to transduce NQ1332 to obtain HE397.
Measurements of glucose, lactose, and glycerol uptake
A fraction of an exponentially growing culture was collected and kept on ice for < 0.5 h. The incubation on ice caused a decrease in the concentration of each carbon substrate by only 2–3%. After the sample was centrifuged at 16,110 × g for 1 min, the supernatant was taken, frozen on dry ice and kept at −80°C. Typically, four samples were taken at OD600 between 0.15 and 0.60.
Glucose was assayed enzymatically using a commercially available kit (Glucose Assay Kit, GAHK20; Sigma-Aldrich). For the lactose assay, samples were first digested by β-galactosidase in Z-buffer at 37°C for 20 min and the released glucose was measured using the glucose assay described above. As a control, the sample was treated in the same way without β-galactosidase. Little glucose was detected in the control. Glycerol was measured essentially as described in Ref. 44. The assay was performed by adding 7.5 μl sample to 225 μl reaction mixture containing 50 mM MOPS [pH7.0], 0.75 mM ATP, 3.75 mM MgSO4, 0.188 mM 4-aminoantipyrine, 2.11 mM N-ethyl-N-(3-sulfopropyl) m-anisidine, sodium salt, 2.5 U/ml glycerol phosphate oxidase, and 2.5 U/ml peroxidase with or without 1.25 U/ml glycerol kinase. After incubation at room temperature for 30–60 min, A540 was measured and converted to the glycerol concentration based on standards.
The carbon-uptake rate was calculated as the slope of carbon concentration versus OD600, multiplied by the specific growth rate.
G3P pool measurements by enzymatic assay
G3P pools reported in Fig. 4d were measured as follows. The culture of NQ1187 was grown to OD600 = 0.5, and the cells were harvested by filtration of 2.5 ml culture through the membrane filter (25 mm-disc with 0.45 μm pore size, HAWP02500; Millipore) pre-wetted with warmed culture medium, and washed by 2.5 ml warmed culture medium. The filter was quickly immersed in 4 ml of extraction solution (40% (v/v) methanol, 40% (v/v) acetonitrile, and 20% (v/v) water) precooled at −20°C, and incubated at −20°C for 2 h. The extract was dried in a vacuum concentrator and stored at −80 °C. Immediately prior to the assay, the samples were dissolved in 170 μl phosphate-buffered saline (D1408; Sigma-Aldrich). G3P was assayed enzymatically using a commercially available kit (Amplite Fluorimetric Glycerol 3-Phosphate Assay Kit, 13827; AAT Bioquest).
Measurements of intracellular metabolites by mass spectrometry
G3P and FBP pools reported in Fig. 3e and Fig. 4a,b were measured as follows. Cultivations were performed in M9 minimal media in 96-deep-well plates as described in24. During mid-exponential phase (OD600 = 0.5), cells were harvested by fast filtration using 1 mL culture as described previously24, and the filter was quickly immersed in 4 ml of extraction solution (40% (v/v) methanol, 40% (v/v) acetonitrile, and 20% (v/v) water) precooled to −20°C and incubated at −20°C for 2 h. Samples were dried completely at 120 lbar (Christ RVC 2–33 CD centrifuge and Christ Alpha 2–4 CD freeze dryer), and stored at −80°C until measurements. Before measurements, samples were resuspended in 100 μL water, centrifuged for 5 min (5,000 × g, 4°C) to remove residual particles, diluted 1:10 in water, and transferred to V-bottomed 96 well sample plates (Thermo Fisher Scientific). Samples were measured by flow-injection time-of-flight mass spectrometry with an Agilent 6550 QToF instrument operated in negative ionization mode at 4 GHz high-resolution in a range of 50–1,000 m/z as described before45. Sample processing and ion annotation was performed based on accurate mass within 0.001 Da using the KEGG eco database46 as reference and accounting for single deprotonated forms of the respective metabolite (M-H+) as described before46.
Total and lactose uptake rates used to plot (in Fig. 4a, b) against FBP pools were estimated from the relation between uptake rates [ℂ] and 3MBA concentrations [μM] in Fig. 2c. Lactose uptake rates above 12.5 μM 3MBA on lactose + glycerol and lactose alone were estimated as −0.495 × ln([3MBA])3 + 6.326 × ln([3MBA])2 − 18.745 × ln([3MBA]) + 23.499 and 0.252 × ln([3MBA])3 − 3.446 × ln([3MBA])2 + 18.452 × ln([3MBA]) − 9.881, respectively. Glycerol uptake rates on lactose + glycerol below and above 100 μM 3MBA were estimated as 34.091 − 6.679 × ln([3MBA]) and 0, respectively. When estimating uptake rates at 0 μM 3MBA, we used 10 μM (instead of 0 μM) in the natural logarithm.
Single-cell analysis of GFP expression from the glpF promoter
Cultures of strain NQ1344, carrying a chromosomal glpFp-gfp reporter gene together with the titratable LacY system, were grown on N−C− medium supplemented with 20 mM NH4Cl, 3 mM lactose (Supplementary Figure 2a, c–l) or 10 mM lactose (Supplementary Figure 2b,n), and 4 mM glycerol (Supplementary Figure 2c–e) or 8 mM glycerol (Supplementary Figure 2f–k, n). At OD600 = 0.5, a fraction of the culture was taken and kept on ice until images were taken. GFP fluorescence and phase contrast (PC) images were acquired by a Clara charge-coupled device camera (Andor, Belfast, UK) for Supplementary Figure 2c–l or QImaging Retiga 2000R MONO (Teledyne Qimaging, Surrey, Canada) for Supplementary Figure 2n connected to an Eclipse Ti inverted microscopic system (Nikon Inc., Melville, NY) under the control of NIS Elements software (Nikon Inc., Melville, NY).
Images were analyzed using the open-source platform software Fiji47 as follows. Individual cells were identified as region of interest (ROI) on a PC image with an appropriate threshold set. To minimize the number of misidentified cells, the gate for the cell size was set with the ratio of maximal to minimal cell size set to 4, and remaining misidentified cells were manually removed. The ROIs thus obtained on a PC image were overlaid to the corresponding GFP fluorescence image and mean intensity in each ROI was measured. For series A (see Supplementary Figure 2) background fluorescence was subtracted from the signal and used to normalize the intensities. For series B, no background was subtracted; instead, the fluorescence level of cells grown on lactose only with 500 μM of 3MBA was measured to determine the fluorescence level of cells with minimal glpFp expression.
Glycerol kinase assay
Cell extracts were prepared essentially as described before 17. A 25 mL culture was grown exponentially until OD600 = 0.5. Twenty mL of the culture was transferred to a tube precooled on ice water and chloramphenicol was added to give a final concentration of 40 μg/mL. The cells were harvested by centrifugation at 0°C, washed with 40 mL of 1% NaCl, resuspended in 200 μL extraction buffer containing 0.1 M MOPS-NaCl at pH7.0, 1 mM 2-mercaptoethanol, 1 mM ethylenediaminetetraacetic acid, and 2 mM glycerol, and stored at −80°C.
Before the assay, the cell suspension was thawed, disrupted by sonication on ice water for 4 times 5 s at the amplitude of 4 at low mode in a MSE sonicator, centrifuged at 13,600 × g for 30 min at 4°C, and the supernatant was frozen at −80°C. The GlpK activity was stable for one day at −80°C.
The cell extract was thawed immediately before the assay and diluted in the extraction buffer. The reaction was started by adding 40 μL of cell extract to 200 μL of assay buffer, both of which were preincubated at 37°C for 5 min, to give final concentrations of 0.1 M MOPS-NaCl at pH7.0, 0.167 mM 2-mercaptoethanol, 0.167 mM ethylenediaminetetraacetic acid, 2 mM glycerol, 2.5 mM ATP, 13.5 mM MgSO4, 0.188 mM 4-aminoantipyrine, 2.11 mM N-ethyl-N-(3-sulfopropyl) m-anisidine, 5 U/mL glycerol phosphate oxidase, and 5 U/mL peroxidase. A540 was recorded every 6 s. To confirm that the activities were proportional to the concentrations of the cell extracts added to the reaction mixture, the assays were repeated with four different dilutions of the cell extract for each. The GlpK activity was reported as A540 per min per mg of total protein. Total amounts of protein in a cell extract were determined by Biuret method 48.
cAMP assay
The cAMP concentrations in media were assayed as described before 16.
A fraction of an exponentially growing culture was collected and filtered through a 0.22-μm-pore-size nylon membrane filter, and the filtrate was frozen on dry ice and kept at −80°C. Four samples were taken at OD600 between 0.15 and 0.60.
cAMP in the filtrate was ethenylated by incubation of 80 μL filtrate for 30 min at 70°C in the presence of 1.2 M chloroacetaldehyde, 25 mM Na2HPO4 [pH 4.0], and 5 mM EDTA in a final volume of 200 μL. The reaction mixture was transferred to ice, neutralized by adding one-third volume of 0.5 M NH4HCO3, filtered through a 0.22-μm-pore-size nylon membrane filter, and analyzed by high performance liquid chromatography (HPLC). The HPLC system used was Shimadzu Prominence HPLC system composed of LC-20AB binary pump, SIL-10AF autosampler, and RF-10AxL fluorescence detector as main modules. Eluent flow rate was 1.5 mL/min. The eluent used was a TBAS buffer (5.7 mM TBAS, 30.5 mM KH2PO4 adjusted to pH 5.8 with phosphoric acid) and an acetonitrile buffer (acetonitrile:TBAS buffer, 2:1). Seventy μL sample aliquot was injected on XTerra MS C18 column (3.0 × 50 mM, I.D. 3 mM, 5 μm particle size; Waters Corp.) equipped with its guard column and maintained at 40°C during separation. The elution was isocratic with 90% TBAS buffer (10% acetonitrile buffer) for 3 min after injection, decreased to 50% TBAS buffer in 3 sec, remaining isocratic for 1.9 min, and re-equilibrated with 90% TBAS buffer for 2 min. The fluorescence signal was monitored at an excitation wavelength of 280 nm and an emission wavelength of 410 nm. Ethenylated cAMP typically eluted between 1.5 and 1.6 min.
The cAMP excretion rate was calculated as the slope of the plot of cAMP concentration in a filtrate versus OD600, multiplied by the specific growth rate.
Statistics and Reproducibility
Fig. 1: The data shown in Panel a and b are from a single series of experiments.
Fig. 2: In all panels, each datapoint derives from a single experiment. In Panels a and b, a complete data series (composed of 18 datapoints in total: the growth rates on lactose/glucose, glycerol, and both, each at six 3MBA concentrations) was typically obtained through two independent batches of experiments. In Panel a, this two-batch series was repeated and hence two data series are shown for each carbon substrate. The full dataset in Panels c and e was obtained through ten independent batches of growth-rate measurement and sampling which include replicates of the same or similar culture conditions, seven independent batches of the lactose uptake measurements, and seven independent batches of the glycerol uptake measurements. The full dataset in Panels d and f was obtained through four independent batches of growth-rate measurement and sampling which include replicates of the same or similar culture conditions, five independent batches of lactose uptake measurements, and four independent batches of glycerol uptake measurements. See Source Data for details.
Fig. 3: In all panels, each datapoint derives from a single experiment. The full dataset in Panel b was obtained through six independent batches of growth-rate measurements and sampling, and six independent batches of LacZ assays. The full dataset in Panel c was obtained through four independent batches of growth-rate measurements and sampling, and four independent batches of LacZ assays. The full dataset in Panel d was obtained through eight independent batches of growth-rate measurement and sampling which include replicates of the same or similar culture conditions, seven independent batches of lactose uptake measurements, and seven independent batches of glycerol uptake measurements. The full dataset in Panel e was obtained through three independent series of growth-rate measurement and sampling which include replicates of the same culture conditions and two independent sets of mass spectrometry experiments. See Source Data for detail.
Fig. 4: In all panels, each datapoint represents a single experiment. The full dataset in Panel a and b was obtained through three independent batches of growth-rate measurements and sampling which include replicates of the same culture conditions and two independent sets of mass spectrometry experiments. In Panel c and d, the full datasets were obtained through two independent batches of sampling and a single batch of measurement. See Source Data for detail.
Fig. 5: In Panel a, each datapoint represents a single experiment and the full datasets for glpKG184T, glpK22, and ΔglpR glpK22 were obtained through six, six, and three independent batches of growth-rate measurement and sampling, respectively, which include replicates of the same or similar culture conditions, five, four, and one independent batches of lactose uptake measurements, respectively, and five, five, and one independent batches of glycerol uptake measurements, respectively. In Panel c, the x- and y-values of each datapoint represents the average of the growth rates obtained from at least two independent experiments. See Source Data for details.
Extended Data 1: In Panels a and d, the growth experiments were repeated twice and similar growth curves were obtained as shown in Source Data. In the other panels, each data set shown derives from a single experiment.
Extended Data 2: In all panels, each datapoint derives from a single experiment. A full data series, composed of the growth rates on three carbon-substrate conditions (1st substrate, 2nd second substrate, and both) at six (Panel a-e) or seven (Panel i) 3MBA concentrations (18 or 21 datapoints in total) was typically obtained through two independent batches of experiments. In Panel a, this two-batch series was repeated and hence the two datasets are shown for each carbon substrate.
Extended Data 3: Each growth curve derives from a single experiment.
Extended Data 6: In Panel a, each datapoint derives from a single experiment and the full dataset was obtained through four independent batches of growth-rate measurements and sampling which include replicates of the same or similar culture conditions, four independent batches of the lactose uptake measurements, and four independent batches of the glycerol uptake measurements. See Source Data for details.
Extended Data 7: In Panel b and c, the x- and y-values of each datapoint represents the average of the growth rates obtained from two independent experiments. See Source Data for details.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
DATA AVAILABILITY
The datasets corresponding to all figures (including Extended Data Figures and Supplementary Figures) are available online as Source Data.
CODE AVAILABILITY
Numerical analyses of the mathematical model were carried out using Wolfram Mathematica 11.3, gnuplot, and R (version 3.5.1). A Mathematica notebook that reproduces the central modeling results is shared at: https://doi.org/10.5281/zenodo.3462129. Other code will be shared upon reasonable request.
Extended Data
Extended Data Figure 1. Examples of diauxic (“double growth”) curves.
a—d. Diauxic growth curves1 on glucose and one additional carbon substrate, for E. coli strains NCM3722 (closed circles) and MG1655 (open circles). A diauxic curve consists of two exponential growth phases separated by a lag phase during which the culture hardly grows. (For comparison, all data series are shifted horizontally such that the lag begins at ~100 min.)
The duration of the lag phase varies between strains and substrate pairs, from a few minutes (NCM3722 in Panel a) to over an hour (NCM3722 in Panel d).
e. Hierarchical growth on glucose plus glycerol. The same data as Fig. 1a are now plotted against OD600. The glucose concentration (blue diamonds) initially decreases linearly (black solid lines are linear fits): during balanced exponential growth, producing a unit of cell mass consumes a fixed amount of substrate. After glucose runs out, glycerol (orange triangles) is consumed, again linearly.
f. Similar to Panel e, but with lactose (green diamonds) instead of glucose, using the same data as Fig. 1b. Here, the transition to glycerol utilization is more gradual than Panel e. Lactose uptake slows down before lactose is used up, likely due to the large Michaelis constant of the lactose permease50: KM = 0.1 to 1 mM. The reduction in lactose uptake relieves the inhibition of glycerol uptake before lactose is fully depleted, resulting in a smooth transition. In contrast, the KM of the glucose transporter PtsG51,52 is 3—10 μM; therefore, cells do not sense that glucose is running out until the glucose concentration is very low (Panel e and Fig. 1a).
g. As Fig. 1b, except the higher initial lactose concentration (3 mM instead of 0.7 mM) to fully inhibit glycerol uptake. To observe the transition to glycerol utilization before the culture reaches high OD600, the culture was diluted, at OD600 = 0.5, four-fold in fresh medium containing glycerol (green diamonds before dilution, pale-green diamonds after) but no lactose (orange triangles before dilution, pale-orange triangles after).
h. Same data as Panel g, except that lactose and glycerol concentrations are plotted against OD600, revealing straight lines similar to Panel e and f.
Extendend Data Figure 2. Growth-rate crossover patterns for various combinations of substrates.
a—d. The growth rate of the titratable LacY strain (Supplementary Figure 1a) grown on lactose and a second substrate (glycerol, maltose, xylose or fructose) as a function of 3MBA concentration. Each plot shows the measured growth rate on lactose only (green diamonds), the growth rate on the second substrate only (orange or red triangles), and the growth rate in the presence of both (pink circles).
e—h. As Panels a—d, but for the titratable PtsG strain (Supplementary Figure 1b) and glucose (green diamonds) instead of lactose.
i. Similar results for the titratable GlpK strain (Supplementary Figure 1c) growing on glycerol (orange triangles), mannose (red diamonds), or both (pink circles).
In all cases (Panels a—i), if the 3MBA concentration is reduced sufficiently, the growth rate on the preferred carbon substrate eventually becomes smaller than the growth rate on the non-preferred one. Yet, the growth rate in the presence of both substrates never drops below the growth rate on the non-preferred one, indicating that the uptake of the non-preferred substrate is induced. In almost all cases, the crossover regime is rather narrow. The glucose-fructose hierarchy is an apparent exception.
Extended Data Figure 3. The diauxic lag disappears in the supplementation regime.
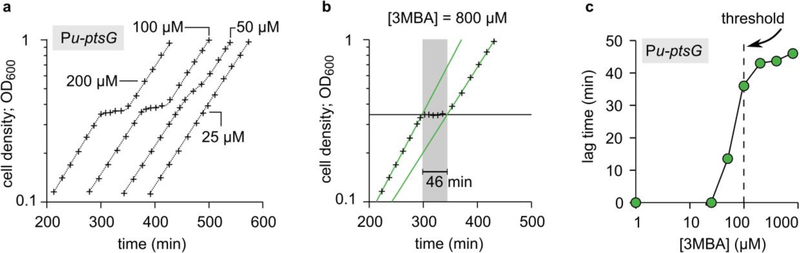
a. Diauxic growth curves (+) of the titratable PtsG strain (NQ1243) in medium with 1.7 mM glucose and saturating glycerol. Each curve is for a different 3MBA concentration (indicated in the figure) and horizontally shifted for convenience. The data shown is from a single series of experiments.
b. Lag times were determined for each diauxic growth curve; the method is illustrated here using the condition [3MBA] = 800 μM as an example. We fitted exponential curves through the two growth phases (green lines) and a horizontal line through the lag phase (horizontal black line). The lag time (indicated in gray) is heuristically defined as the horizontal distance between the intersections of the green lines with the horizontal one. In this case, a lag time of 46 minutes is found.
c. Lag times of the growth curves versus [3MBA]. The diauxic lag time vanishes precipitously when [3MBA] is tuned below 100 μM, where glycerol and glucose are co-utilized.
Extended Data Figure 4. How differential regulation by cAMP-Crp can affect G3P concentration.
As demonstrated in Fig. 3b,c using the ΔglpR and ΔglpR glpK22 background, cAMP-Crp signaling affects the expression of the glpK and glpD genes differently: with increasing growth rate (decreasing cAMP-Crp transcriptional activation), glpK expression vanishes whereas glpD expression maintains a significant basal level. The figure illustrates how this differential regulation explains the marked growth-rate dependence of the G3P concentration observed in the ΔglpR glpK22 background (Fig. 4d).
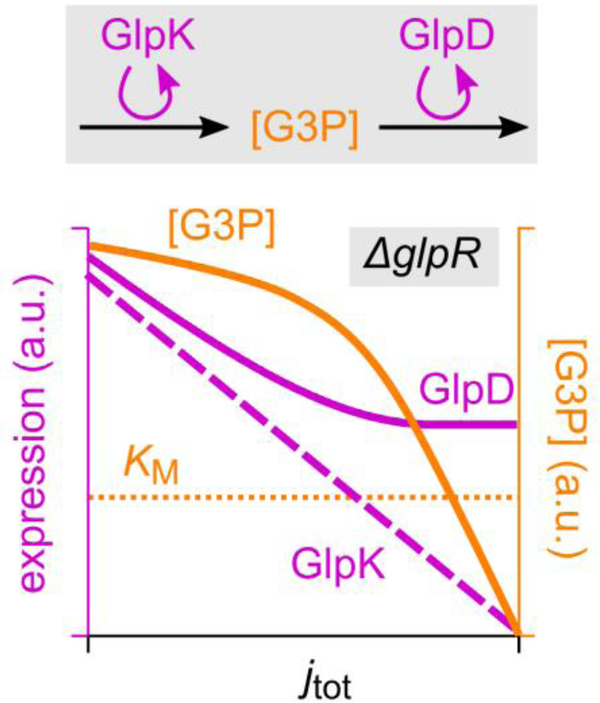
G3P is the product of GlpK and the substrate of GlpD. The synthesis of G3P should therefore be proportional with the abundance of GlpK, while its turnover increases with both GlpD abundance and substrate concentration [G3P]. Flux balance then implies that [G3P] increases with the ratio of GlpK (purple dashed line; sketch based on Fig. 3b) to GlpD abundance (purple solid line; sketch based on Fig. 3c). This ratio reduces with increasing growth rate, so that the G3P concentration (solid orange line; sketch) reduces as well.
In strains without the ΔglpR mutation the same mechanism should act, but with an additional layer of amplification: Because both glpK and glpD expression are repressed by GlpR, an increase in its inducer G3P due to differential regulation has little effect until it is of the order of the Michaelis constant KM (horizontal dotted line) associated with the induction of GlpR, upon which glpK and glpD expression are induced and glycerol uptake is turned on.
Extended Data Figure 5. The total-flux feedback model.
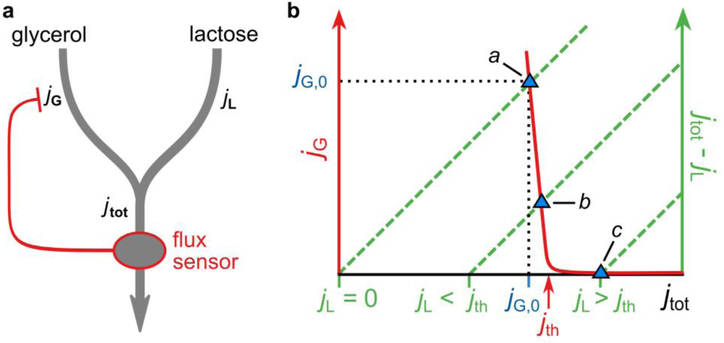
Several key observations can be explained by a highly simplified regulatory scheme in which glycerol uptake is inhibited by a signal that reflects the total carbon-uptake flux—a total-flux sensor. In this scheme (Panel a), glycerol and lactose uptake jG and jL both contribute to the total carbon-uptake flux jtot. This total flux is sensed by a total-flux sensor, which represses glycerol uptake, but only if jtot exceeds a threshold that is set to coincide with the carbon flux obtained on glycerol alone. Thus, glycerol uptake is suppressed by any substrate that supplies a larger carbon-uptake flux than glycerol can provide, but not by substrates that produce a smaller flux.
Panel b demonstrates graphically how total-flux feedback determines the uptake of glycerol. Because jG = jtot − jL, the steady-state value of jG obtained for a given jL can be found by plotting both jG(jtot) (red solid curve) and jtot − jL (green dashed lines, for various values of jL ) as a function of jtot and determining their intersection (blue triangles). We assume that jG responds sensitively to jtot, with a threshold jth (red arrow) set slightly above the flux on glycerol alone, jG,0. Thus, it is seen that glycerol uptake is inhibited if jL > jth (the hierarchical utilization regime, intersection c). Yet, if jL < jth, glycerol uptake is adjusted such that jtot ≈ jth ≈ jG,0 (the supplementation regime; intersection a and b).
cAMP–Crp signaling can function as a total-flux sensor because transcriptional activation by cAMP–Crp is a decreasing function of jtot (Fig. 4c)5. It transmits information on jtot to the glycerol uptake through differential regulation of glpK and glpD expression (Fig. 3b,c).
Extended Data Figure 6. Model predictions.
With a single parameter set, our mathematical model reproduces the main features of various measurements in addition to the flux relations of the various mutants (Fig. 5b).
a. The flux relation of the titratable PtsG strain with the ΔglpR glpK22 mutations (NQ1264) is linear (Pearson correlation:, n = 13 experimental conditions, t = 21, df = 11, p = 3 × 10−10), as predicted by the model. The flux relation for the glpR+ glpK+ strain (NQ1243) is also plotted for comparison.
b. The growth-rate crossover of the titratable LacY strain. The model predicts that, on lactose only, the growth rate decreases linearly (dashed line) on lactose only as lactose uptake (green area) is reduced.. On lactose and glycerol, the growth rate initially follows the same trend (solid line), but below the threshold lactose flux of ≈ 25 ℂ glycerol uptake (orange area) is gradually induced such that the growth rate remains approximately constant. This behavior underlies the observations in Fig. 2a and c. (The model makes identical predictions for the titratable PtsG strain.)
c. The G3P pools. The model predicts that in the titratable LacY strain grown on lactose + glycerol (purple solid line), as the lactose uptake is reduced, the G3P pool remains low until the growth rate approaches that on glycerol only, ≈ 0.7/h; it then sharply increases and converges to the level obtained during growth on glycerol only (orange circle). This closely resembles the measured behavior in Fig.3e (purple and orange circles). The sensitive response is disrupted in the ΔglpR glpK22 double mutant (purple dotted line and orange square), in agreement with Fig. 4d (purple and orange squares).
d-e. Expression from glpF and glpD promoters. If the titratable PstG strain is grown on glucose + glycerol and glucose uptake is reduced, expression levels from both glpF and glpD promoters are negligible until the growth rate approaches the growth rate on glycerol only, ~0.7/h (purple solid lines in both figures). The sudden onset of expression is completely lost in the ΔglpR mutation strain (light blue curves in both figures). This agrees with the measured expression levels in Fig. 3b and c.
Extended Data Figure 7. Growth on glycerol, xylose or fucose with various second substrates.
a. This diagram illustrates the difference in the effect of glycolytic and gluconeogenic substrates on glycerol uptake. The uptake and catabolism of glycerol, gluconeogenic substrates and glycolytic substrates are drawn as three pathways (gray arrows) that merge at different places. In the regulation (red lines), two flux-sensors are involved: one (FBP) senses the upper-glycolytic flux jL, the other (cAMP-Crp) the total carbon flux jtot. Crucially, both flux sensors are required to fully suppress glycerol uptake; this is symbolized in the diagram using the symbol of a logical AND gate. Glycolytic substrates contribute both to the upper-glycolytic flux and the total carbon flux. A sufficiently large glycolytic flux therefore activates both the upper-glycolytic flux sensor AND the total-flux sensor, which together suppress glycerol uptake. In contrast, gluconeogenic substrates do not contribute to the upper-glycolytic flux and will not fully inhibit glycerol uptake even if they provide a large carbon flux. (Through the total-flux sensor cAMP-Crp, gluconeogenic substrates will affect glycerol uptake mildly, but both substrates remain co-utilized.) This difference between glycolytic and gluconeogenic substrates underlies the pattern in Fig. 5c.
b. A pattern similar to Fig. 5c is obtained if glycerol is replaced by xylose. Shown here is the growth rate of WT cells (NCM3722) in M9 medium24 on xylose plus a second substrate plotted against growth rate with the second substrate only, for a variety of “second” substrates. The growth rate on xylose only is indicated by horizontal and vertical dotted lines. The growth rate on both substrates shows a similar dependence on the “second” substrate species as seen in Fig. 5c of the main text: If the second carbon substrate is processed at least partially by upper glycolysis (blue circles) the growth rate is approximately the larger of the two single-substrate growth rates, possibly with an exception for mannose. If on the other hand the second substrate is a gluconeogenic substrate (orange circles) the growth rate on both substrates is usually larger than either of the two single-substrate growth rates.
c. Same as Panel b, but for fucose as the “first” substrate.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Uwe Sauer for his generous support and encouragement, to Thomas Egli, Luca Gerosa, Joshua Silverman, and members of the Hwa lab for helpful discussions, to Kenyon Applebee for providing the glpKG184T strain, and to Lin Chao and Camilla Ulla Rang for helping to acquire single-cell GFP images. This research is supported by the NIH to TH (R01GM095903). RH was supported by NWO (VENI 680-47-419). TH additionally acknowledges the hospitality of the Institute for Theoretical Studies at ETH where some of this work was carried out.
Footnotes
COMPETING INTEREST STATEMENT
The authors declare that the authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
REFERENCES
- 1.Monod J Recherches sur la croissance des cultures bactériennes, 210 p. (Hermann & cie, Paris, 1942). [Google Scholar]
- 2.Monod J The phenomenon of enzymatic adaptation - And its bearings on problems of genetics and cellular differentiation. Growth 11, 223–289 (1947). [Google Scholar]
- 3.Müller-Hill B The lac Operon : a short history of a genetic paradigm, ix, 207 p. (Walter de Gruyter, Berlin; New York, 1996). [Google Scholar]
- 4.Deutscher J, Francke C & Postma PW How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70, 939–1031 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narang A & Pilyugin SS Bacterial gene regulation in diauxic and non-diauxic growth. J Theor Biol 244, 326–48 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Loomis WF & Magasanik B Glucose-lactose diauxie in Escherichia coli. J Bacteriol 93, 1397–401 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inada T, Kimata K & Aiba H Mechanism responsible for glucose-lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells 1, 293–301 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Lendenmann U, Snozzi M & Egli T Kinetics of the simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture. Appl Environ Microbiol 62, 1493–9 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baidya TKN, Webb FC & Lilly MD The utilization of mixed sugars in continuous fermentation. I. Biotechnol. Bioeng. 9:195–204. Biotechnol. Bioeng.9, 195–204 (1967). [Google Scholar]
- 10.Harte MJ & Webb FC Utilisation of mixed sugars in continuous fermentation. II. Biotechnol. Bioeng. 9, 205–221 (1967). [Google Scholar]
- 11.Harder W & Dijkhuizen L Strategies of mixed substrate utilization in microorganisms. Philos Trans R Soc Lond B Biol Sci 297, 459–80 (1982). [DOI] [PubMed] [Google Scholar]
- 12.Wanner U & Egli T Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol Rev 6, 19–43 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Egli T, Lendenmann U & Snozzi M Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek 63, 289–98 (1993). [DOI] [PubMed] [Google Scholar]
- 14.Hermsen R, Okano H, You C, Werner N & Hwa T A growth-rate composition formula for the growth of E.coli on co-utilized carbon substrates. Mol Syst Biol 11, 801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin EC Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30, 535–78 (1976). [DOI] [PubMed] [Google Scholar]
- 16.You C et al. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch JP, Hayashi S & Lin EC The control of dissimilation of glycerol and L-alpha-glycerophosphate in Escherichia coli. J Biol Chem 239, 3106–8 (1964). [PubMed] [Google Scholar]
- 18.Weissenborn DL, Wittekindt N & Larson TJ Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J Biol Chem 267, 6122–31 (1992). [PubMed] [Google Scholar]
- 19.Zwaig N & Lin EC Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science 153, 755–7 (1966). [DOI] [PubMed] [Google Scholar]
- 20.Holtman CK, Pawlyk AC, Meadow ND & Pettigrew DW Reverse genetics of Escherichia coli glycerol kinase allosteric regulation and glucose control of glycerol utilization in vivo. J Bacteriol 183, 3336–44 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochanowski K et al. Functioning of a metabolic flux sensor in Escherichia coli. Proc Natl Acad Sci U S A 110, 1130–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui S et al. Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol Syst Biol 11, 784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettigrew DW, Liu WZ, Holmes C, Meadow ND & Roseman S A single amino acid change in Escherichia coli glycerol kinase abolishes glucose control of glycerol utilization in vivo. J Bacteriol 178, 2846–52 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochanowski K et al. Few regulatory metabolites coordinate expression of central metabolic genes in Escherichia coli. Mol Syst Biol 13, 903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Applebee MK, Joyce AR, Conrad TM, Pettigrew DW & Palsson B Functional and metabolic effects of adaptive glycerol kinase (GLPK) mutants in Escherichia coli. J Biol Chem 286, 23150–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettenbrock K et al. Correlation between growth rates, EIIACrr phosphorylation, and intracellular cyclic AMP levels in Escherichia coli K-12. J Bacteriol 189, 6891–900 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson DW et al. A global resource allocation strategy governs growth transition kinetics of Escherichia coli. Nature 551, 119–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keseler IM et al. The EcoCyc database: reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res 45, D543–D550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandelstam J The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J 82, 489–93 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller S, Regensburger G & Steuer R Enzyme allocation problems in kinetic metabolic networks: optimal solutions are elementary flux modes. J Theor Biol 347, 182–90 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Wortel MT, Peters H, Hulshof J, Teusink B & Bruggeman FJ Metabolic states with maximal specific rate carry flux through an elementary flux mode. FEBS J 281, 1547–55 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Xia K, Yang X & Tang C Growth strategy of microbes on mixed carbon sources. Nat Commun 10, 1279 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R, Singh S & Singh OV Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol 35, 377–91 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Block DE & Mills DA Simultaneous consumption of pentose and hexose sugars: an optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl Microbiol Biotechnol 88, 1077–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinuselvi P, Kim MK, Lee SK & Ghim CM Rewiring carbon catabolite repression for microbial cell factory. BMB Rep 45, 59–70 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Soupene E et al. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol 185, 5611–26 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csonka LN, Ikeda TP, Fletcher SA & Kustu S The accumulation of glutamate is necessary for optimal growth of Salmonella typhimurium in media of high osmolality but not induction of the proU operon. J Bacteriol 176, 6324–33 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basan M et al. Inflating bacterial cells by increased protein synthesis. Mol Syst Biol 11, 836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrissey AT & Fraenkel DG Suppressor of phosphofructokinase mutations of Escherichia coli. J Bacteriol 112, 183–7 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lorenzo V, Herrero M, Metzke M & Timmis KN An upstream XylR- and IHF-induced nucleoprotein complex regulates the sigma 54-dependent Pu promoter of TOL plasmid. EMBO J 10, 1159–67 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klumpp S, Zhang Z & Hwa T Growth rate-dependent global effects on gene expression in bacteria. Cell 139, 1366–75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datsenko KA & Wanner BL One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–5 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M et al. Need-based activation of ammonium uptake in Escherichia coli. Mol Syst Biol 8, 616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGowan MW, Artiss JD, Strandbergh DR & Zak B A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29, 538–42 (1983). [PubMed] [Google Scholar]
- 45.Sévin DC & Sauer U Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nat Chem Biol 10, 266–72 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Ogata H et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 27, 29–34 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gornall AG, Bardawill CJ & David MM Determination of serum proteins by means of the biuret reaction. J Biol Chem 177, 751–66 (1949). [PubMed] [Google Scholar]
- 49.Saier MH & Roseman S Sugar transport. Inducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem 251, 6606–15 (1976). [PubMed] [Google Scholar]
- 50.Sandermann H Jr. beta-D-Galactoside transport in Escherichia coli: substrate recognition. Eur J Biochem 80, 507–15 (1977). [DOI] [PubMed] [Google Scholar]
- 51.Stock JB, Waygood EB, Meadow ND, Postma PW & Roseman S Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem 257, 14543–52 (1982). [PubMed] [Google Scholar]
- 52.Misset O, Blaauw M, Postma PW & Robillard GT Bacterial phosphoenolpyruvate-dependent phosphotransferase system. Mechanism of the transmembrane sugar translocation and phosphorylation. Biochemistry 22, 6163–70 (1983). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets corresponding to all figures (including Extended Data Figures and Supplementary Figures) are available online as Source Data.