Abstract
Like cervical cancer, anal cancer is caused by human papillomavirus (HPV). HPV is the most common sexually transmitted agent and is found in the anal canal of almost all HIV-positive men who have sex with men (MSM). Rates of HPV anal cancer are disproportionately higher in this population. Although the nanovalent HPV vaccine is efficacious in protecting against oncogenic HPV types, a substantial proportion of MSM remains unvaccinated and anal HPV infection continues to be an important public health burden. Therefore, it is important to identify strategies to prevent HPV infection. We report on two promising and interlinked strategies: (1) the development of a cell-based Renilla luminescence reporter assay using HPV-16 pseudovirions that encapsidate SV40-driven Renilla luminescence reporter expression plasmid and (2) use of this assay for high-throughput screening (HTS) of FDA- and internationally approved drugs to identify those that could be repurposed to prevent HPV infection. We conducted a screen of 1906 drugs. The assay was valid with a Z′ of 0.67 ± 0.04, percent coefficient of variance of 10.0, and signal-to-background noise window of 424.0 ± 8.0. Five drugs were chosen for further analyses based on selection parameters of ≥77.0% infection of HPV-16 pseudovirion-driven Renilla expression with <20.0% cytotoxicity. Of these, the antifungal pentamidine and a gamma-amino butyric acid receptor agonist securinine exhibited ≥90.0% infection with <10.0% cytotoxicity. This luminescent cell-based reporter expression plasmid assay for HTS is a valid method to identify FDA- and internationally approved drugs with the potential to be repurposed into prevention modalities for HPV infection.
Keywords: papillomavirus, high-throughput screening, pseudovirus, repurposed, HPV-16
Introduction
Human papillomavirus (HPV) is a small double-stranded DNA nonenveloped virus and the etiologic agent of anal cancer.1 HPV has more than 100 different genotypes, of which at least 12 are classified as high risk because of their ability to cause cancer.2
Anal carcinogenesis begins with anal HPV infection with persistence over time, followed by the development of precancerous anal lesions, and progression of these precancerous lesions to cancer.3 HPV-16 is found in 88.0% of all high-grade anal lesions.4 Approximately 90.0% of anal cancers occur in HIV-positive men who have sex with men (MSM).5 The incidence of anal cancer in HIV-positive MSM is 131/100,000 person-years, a rate that is 60–100 times higher than that of the general population.2,6 Primary prevention of HPV infection through vaccination is the best long-term approach to reducing the incidence of HPV-associated cancers. Given the limited uptake of HPV vaccines that prevent infection, and the high proportion of adult MSM who already have anal HPV infection, MSM will continue to be at risk for HPV infection and anal cancer for decades to come.7,8 To date, there is no effective cure for an active HPV infection.
High-throughput screening (HTS) is a commonly used method to identify new compounds or new purposes for drugs from existing drug libraries to prevent or treat various diseases, including those associated with HPV.9,10 Several HPV HTS assays have been developed focusing on inhibiting different parts of the HPV life cycle for both types, HPV 16 and HPV 18.2,11-13 These include assays of novel compounds targeting full-length genome replication13 and E1/E2 replication proteins.12 To date, no targeted therapies for HPV have been proven effective to prevent or treat HPV infection, thus creating an opportunity to screen drug libraries for FDA-approved drugs with the potential to be repurposed into HPV prevention.14 Repurposing of drugs refers to discovering new uses for approved drugs to provide the quickest possible transition from bench to bedside. This innovative approach has the potential to take advantage of existing industry and academic drug libraries that represent untapped resources. Repurposing drugs with known human safety profiles could accelerate the transition of drug and disease mechanisms coupled with clinical translation to human applications and decreased cost and time of bringing a new drug to market.10
Therefore, our overarching conceptualization and goal was to develop an HTS assay to identify FDA- and internationally approved drugs able to prevent HPV infection. Ideally, the drugs would have the potential to be developed into self-protective lubrication or gel to be administered prior to intercourse. Since the mechanism of HPV infection varies (e.g., cell surface receptor attachment or pinocytosis), our initial target validation focused on a dichotomous outcome of drugs that prevented and did not prevent infection. Given that HPV-16 is the most common cause of cervical and anal cancer, we used HPV-16 pseudovirions (HPV16-PsV). HPV16-PsV is composed of the HPV-16 major capsid protein L1 and minor capsid protein L2. Co-expression of the L1 and L2 genes in mammalian cultures, alongside a Renilla reporter plasmid, leads to capsid assembly and packaging of heterologous nonviral DNA into infectious particles that resemble authentic HPV virions.1 We based initial selectivity of drugs able to inhibit infection on the strength of the Renilla signal. Our assays led to the identification of two drugs displaying promising inhibition of HPV infection with minimal cell toxicity: pentamidine and securinine.
Material and Methods
Compound Library
The screen was optimized and carried out at the University of California, San Francisco Small Molecule Discovery Center (SMDC). The screen was conducted using a library of FDA- and internationally approved drugs (n = 1906). The SMDC’s library was assembled from the commercially available Pharmakon 1600 collection (Micro Source Discovery Systems, Gaylordsville, CT) and supplemented with the addition of approved small-molecule drugs sourced directly from vendors (Enzo Life Sciences, Farmingdale, NY; Tocris Bioscience, Bristol, UK) and smaller historical collections (Iconix FDA drug set).
Cell Line
Human embryonal kidney cells (293FT) transformed with the SV40 large T antigen were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10.0% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 1.0% penicillin/streptomycin (P/S) 100×, and 1.0% G418 50 mg/mL (Invitrogen). The cells were cultured at 37 °C in a humidified atmosphere of 5.0% CO2.
Production of Pseudovirions
HPV16-PsV was engineered to encapsidate a reporter plasmid that expresses the Renilla reporter gene upon cell entry.15 HPV16-PsVs were produced in 293FT cells as previously described by Buck et al.1 An expression plasmid encoding the Renilla luciferase reporter gene under the control of pRL-SV40 (Promega, Madison, WI) was co-transfected into 293FT cells, along with a second plasmid, pl6LlL2 (Addgene, Watertown, MA), which drives expression of the HPV-16 major (L1) and minor (L2) capsid proteins leading to capsid formation. Cells were incubated for 48 h at 37 °C, harvested, washed with DPBS-Mg (Dulbecco’s phosphate-buffered saline [DPBS] supplemented with 9.5 mM MgCl2), and lysed with an equal volume of lysis buffer (DPBS supplemented with 0.5% w/v Brij58 and 0.2% v/v benzonase). For PsV maturation, cell lysates were incubated for 24 h at 37 °C and adjusted to 850 mM NaCl. The resultant crude HPV16-PsV stock was then purified by Opti-prep as previously described by Buck and Thompson.15 The crude HPV16-PsV extraction lysates were centrifuged at 10,000g for 10 min. Clarified supernatants were loaded onto a 12 mL step gradient of Opti-prep (39%, 33%, 27% w/v) and spun at 30,000/rpm for 6 h at 16 °C with an SW55Ti rotor (Beckman Coulter Inc., Fullerton, CA). After centrifugation, 1 mL of Opti-prep fractions was collected from the top layer and the fraction demonstrating the highest infectivity was selected for further experiments.
Opti-prep fractions were quantified in relative light units (RLU) by measuring Renilla luciferase expression. A total of 1 × 104 293FT cells were seeded onto a 96-well plate overnight at 37 °C. Five microliters of each fraction was transferred to the 96-well plate with 293FT cells and incubated for 72 h at 37 °C. Luminescence was measured 72 h postincubation using the Dual-Glo Luciferase Assay System (Promega). All samples were analyzed using a Glomax Multi Detection System (Promega). Fractions with the highest magnitude of Renilla luciferase expression following infection of 293FT cells were pooled together and screened for the presence of encapsidated plasmid. We assumed one copy of DNA per pseudovirion particle. The viral copy number was quantified from the pooled HPV16-PsV stock and run against known pRL-SV40 plasmid standards on a 1% precast agarose gel (Bio-Rad Laboratories, Hercules, CA) by electrophoresis. Briefly, a master mix with 100 μL of PBS and 5 μL each of proteinase K, 0.5 M EDTA, and 10% sodium dodecyl sulfate (SDS) was made, and 5 μL of the master mix was distributed to 10 microcentrifuge tubes. A 15 μL sample of each gradient fraction was added to separate tubes and vortexed. The tubes were then incubated for 10 min at 56 °C. Five microliters of DNA loading dye was distributed to each tube. Twenty microliters of each sample was loaded into the gel. We included a lane with 10 ng of DNA marker ladder. The gel was run at 120 V for ~60 min. Fractions with peak plasmid content were pooled, divided into 100 μL aliquots, and frozen at −80 °C.15
HTS HPV-16 Cell-Based Renilla Luminescent Reporter Plasmid Assay
Briefly, 1 × 104 293FT cells were seeded onto 384-well plates. The optimal HPV16-PsV viral copy number for the assay was selected by performing twofold serial dilutions of the pooled HPV16-PsV stock. A total of 420 viral particles were chosen based on a magnitude expression of 10 times that of the control (cells alone), a fold difference that is considered ideal for a cell-based luminescent assay.9,11 Each twofold serial dilution of HPV16-PsV was applied in triplicate wells and allowed to incubate for 72 h, which was used as a standard incubation time for all future assays. Luminescence was measured at 72 h post-HPV16PsV incubation using the Dual-Glo Luciferase Assay System (Promega). All samples were analyzed using a Glomax Multi Detection System (Promega).
Infection and Cell Viability Assay
Three hundred eighty-four-well assay plates (Greiner BioOne CellStar cat. 781080, Monroe, NC) were filled with 50 ×L of 1 × 104 293FT cells in G418 media (DMEM + 10.0% FBS + 1.0% P/S + 1.0% 50 mg/mL of G418) using a WellMate microplate dispenser (Thermo Scientific, Waltham, MA) and incubated overnight at 37 °C in 5.0% CO2 (culture condition). An aliquot of 50.0 nL (10.0 mM stock containing 0.3% DMSO) of each library compound was then transferred to 384-well plates with a fixed-volume pin tool (V&P Scientific, San Diego, CA) loaded to the BioMek-FXP liquid handling automation workstation (Beckman Coulter, Brea, CA). Assay plates were incubated for 2 h at 37 °C to allow compounds and 293FT cells to interact and potentially prevent HPV16-PsV infection (e.g., compound may block viral receptor site on the cell surface). After 2 h, 10.0 μL of HPV16-PsV (~400 HPV16-PsV/cell) was added. The positive control (no infection) wells received 10.0 μL of growth medium alone. The final assay volume was 60.0 μL, with an assay concentration of test compounds of 8.3 μM. After an additional 72 h of incubation in culture conditions, reagents from the Dual-Glo Reporter Assay System (Promega) were added to the assay plates in accordance with the manufacturer’s protocol. Renilla luciferase luminescence was measured with an Envision multimode microplate reader. Drugs with a cytotoxic effect on the cells may produce a nonspecific Renilla signal and thus be identified as a “hit.” Therefore, we conducted a parallel cell viability assay, as determined by the ATP content of living cells, using the Cell Titer Glo Assay Kit (Promega). ATP content was measured with an Envision multimode microplate reader.
The luminescent signals of test compounds were normalized as percent inhibition relative to both positive (no infection) and negative (infection) controls. For both infection and cell viability assays, the dose-response of active compounds was first tested in singlicate, eight concentrations in threefold serial dilution from 0.007 to 15.0 μM. Select active compounds were subsequently repurchased from chemical vendors and retested in triplicate dose-responses, from 0.00152 to 30.0 μM. The screening was performed in the SMDC at the University of California, San Francisco.
Hit Threshold Criteria
The hit threshold criteria were determined by a four-parameter nonlinear regression analysis including hill slope, log IC50, bottom plateau, and top plateau16,17 using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA).18 The four-parameter analysis provided a mathematical model to describe the assay’s biological process. The overall key quality indicators of the HPV HTS cell-based assay are summarized in Table 1.
Table 1.
Assay Key Quality Indicators.
| Parameter | Key Quality Indicator |
|---|---|
| Z′ | 0.67 ± 0.04 |
| %CV | 10.0 |
| s/b | 424.0 ± 8.0 |
Key quality indicators for HTS assay optimization are determined by three parameters: Z′, percent coefficient of variance (% CV), and signal-to-background noise window (s/b). A Z′ of 0.5 to 1.0 is statistically significant. A % CV of < 15% is statistically significant. A s/b ratio higher than 1:1 indicated more signal than noise, suggesting a high quality of the measurement.
Results
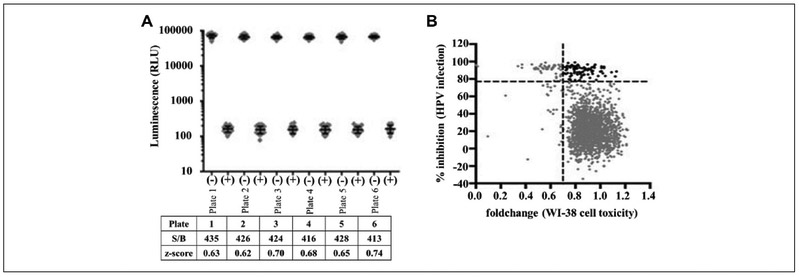
We developed a cell-based Renilla luminescence reporter plasmid assay HTS. The assay focused on identifying FDA-approved drugs able to inhibit HPV infection ( Fig. 1). The assay showed excellent discrimination between active and inactive FDA drugs based on Renilla luminescent expression of the HPV16-PSV reporter plasmid. Overall, assay performance was measured by Z′ as defined by Zhang et al.19 The screen was robust with a wide assay window and excellent discrimination. The Z′ of 0.67 ± 0.05 is statistically significant and has a large signal-to-background noise window (s/b) of 424.0 ± 8.0 (Fig. 2). The HTS screen performance ( Fig. 2A) shows the s/b and assay performance parameter Z′ positive (no infection) and negative (infection) controls in each assay plate (n = 32 for each control per plate, with mean and standard deviation bars).
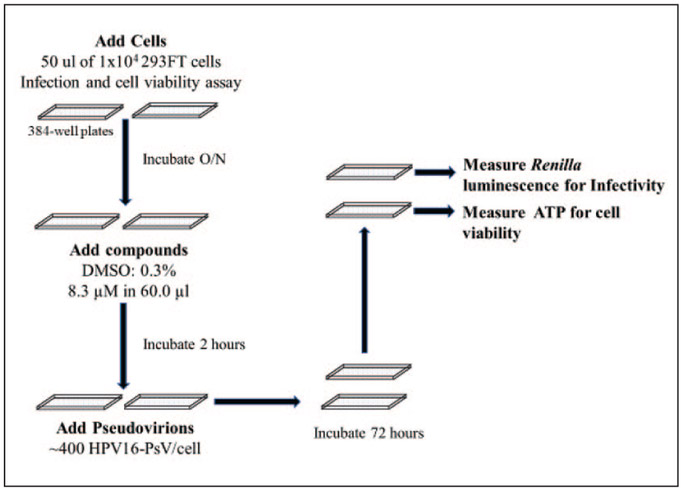
Figure 1.
High-throughput screen for identifying inhibitors of HPV infection. Shown is a schematic illustrating the basic steps in the HTS. See Materials and Methods for additional details. Briefly, HPV16-PsVs containing the Renilla reporter genes are analyzed for their ability to infect as judged by the levels of luminescence. Cell viability was assessed in parallel 384-well plates. In the specific HTS carried out in this study, the compounds were added at a 8.3 μM/0.3% DMSO final concentration 2 h prior to infection.
Figure 2.
Assay validation. (A) Screen performance. Luminescence signals (gray diamonds) of positive and negative controls in six plates assayed during the screen (n = 32 for each control per plate, with mean and standard deviation bars). Panel A shows the signal-to-background window (S/B) and assay performance parameter Z′ of controls in each assay plate. The screen was robust with a wide assay window (S/B > 400) and excellent assay performance (control Z′ > 0.5). (B) Hit selection for inhibitors of HPV infection. Screening of 1906 clinical drugs (gray diamonds). The y axis represents percent inhibition of HPV infections and the x axis represents the fold change of cell toxicity. The black diamonds represent hits. WI-38 cells, normal human lung cells, were used as a positive control in a corresponding counterscreen to filter out compound toxicity.
The primary screen of 1906 FDA drugs identified “hits” based on IC50 and at least a 1.7-fold percent inhibition of HPV (≥70.0% infectivity) at the 3 μM HTS standard concentration. Figure 2B displays the percent inhibition for all 1906 FDA-approved drugs. The positive control WI-38, normal human lung cells, was used as a corresponding counterscreen to filter out compound toxicity. The inclusion of WI-38 cells, as a normal counter screen, is justified by the selective indices in long-term proliferation and cellular viability assays.2,20,21
A total of 152 compounds met the hit threshold criteria and were selected for a secondary screen to confirm dose-response activity and investigate cytotoxicity. The selection parameters for the secondary screen were increased to FDA drugs demonstrating ≥77.0% HPV inhibition of HPV-16PsV with <20.0% cellular cytotoxicity. Ninety-eight drugs met these conditions (black diamonds. Fig. 2B). The 98 active drugs were ranked according to highest percentage inhibition and the highest level of cell viability.
Next, we manually examined them for interesting drug classes (e.g., antihypertensive, antidepressant, antiemetic) with no known HPV inhibitory properties, the strength of inhibitory activity, and minimal cellular cytotoxicity. A selection of 25 drugs was made (Table 2). We retested the 25 drugs with secondary stock in a smaller-scale format in which we monitored infectivity and cell viability in 293FT cells using HPV16-PsVs.
Table 2.
Top 25 Active FDA-Approved Drugs from Primary Screen.
| No. | SMDC ID | Name | HPV Infection IC50 (μM) | 293FT Cell Viability IC50 (μM) |
|---|---|---|---|---|
| 1 | 131248 | Floxuridine | <0.007 | >15 |
| 2 | 735569 | Ancitabine | <0.007 | 5 |
| 3 | 131375 | Bleomycin | <0.007 | 2 |
| 4 | 255720 | Etoposide | 0.01 | 1 |
| 5 | 735623 | Amsacrine | 0.01 | 1 |
| 6 | 915004 | Clofarabine | 0.02 | 2 |
| 7 | 285708 | Cladribine | 0.05 | 4 |
| 8 | 285803 | Irinotecan | 0.06 | 3 |
| 9 | 735553 | Thiostrepton | 0.1 | 3 |
| 10 | 131546 | Trifluridine | 0.1 | >15 |
| 11 | 131324 | Hycanthone | 0.1 | 6 |
| 12 | 756738 | Clofarabine | 0.4 | 2 |
| 13 | 285957 | Rubitecan | 0.8 | >15 |
| 14 | 285779 | Oxaliplatin | 1 | 8 |
| 15 | 130676 | Fluorouracil | 1 | 13 |
| 16 | 131779 | Trichlormethine | 1 | 9 |
| 17 | 255957 | Securinine | 2 | 4 |
| 18 | 757032 | Tolonium chloride | 2 | 4 |
| 19 | 130032 | Ciclopirox | 2 | 8 |
| 20 | 130690 | Mechlorethamine | 2 | >15 |
| 21 | 130651 | Pentamidine | 2 | 9 |
| 22 | 757019 | Bronopol | 3 | 7 |
| 23 | 285650 | Fluorodeoxyuridine | 4 | 13 |
| 24 | 131711 | Doxifluridine | 5 | 16 |
| 25 | 41379 | Ondansetron | 5 | >15 |
| 26 | 130726 | Nortriptyline | 6 | >15 |
The table displays the diverse drug classifications identified. A subset of the top 25 drugs from the primary screen of 1906 was compiled to select a smaller set of drugs for secondary stock dose–response activity. Securinine (no. 17) displayed IC50 = 2 μM HPV infection compared with IC50 = 4 μM cell viability and pentamidine (no. 21) displayed IC50 = 2 μM HPV infection compared with IC50 = 9 μM cell viability in 293FT cells.
The final selection of five FDA-approved drugs was selected for secondary stock dose–response activity: tricyclic antidepressant (nortriptyline), antiemetic (ondansetron), antibiotic (thiostrepton), antiprotozoal (pentamidine), and a plant-derived alkaloid a-γ-amino butyric acid (GABA) antagonist drug (securinine). Pentamidine and securinine are the focus of the current study.
Control Validation
Several compounds are known to prevent HPV infection, including carrageenan,11 heparin,22 and human SERA.23 Carrageenan, heparin, and human sera from women vaccinated with the quadrivalent HPV vaccine were added as positive controls to determine if our HPV-16 cell-based assay could confirm known inhibitors of HPV-16. In our assay, the inhibition of carrageenan, heparin, and human sera was consistent with that in previous studies and the literature.11,23,24 Carrageenan exhibited 100-fold selective inhibition of HPV 16-PsV infection (IC50 = 0.05 μM) compared with cell viability (IC50 ≥ 2 μg/mL), and heparin exhibited 95-fold selective inhibition of HPV 16-PsV infection (IC50 = 24 μg/mL) compared with cell viability (IC50 = 83.0 μg/mL). Human sera showed 98.0% inhibition at a 1:2560 μL dilution. In our assay, the three control drugs inhibited HPV-16PsV infection and induced <10.0% cellular cytotoxicity.
FDA Drugs Pentamidine and Securinine
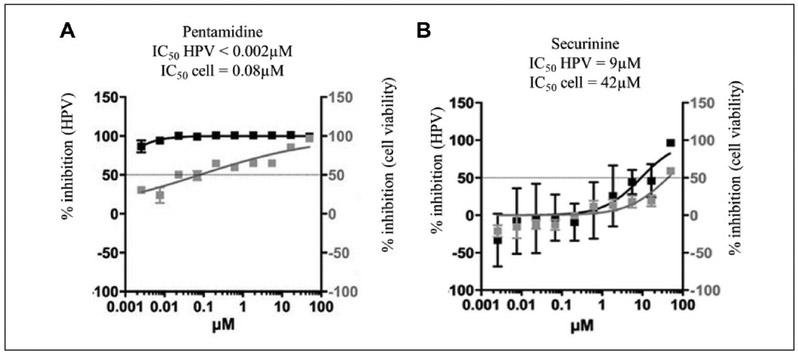
Pentamidine exhibited a > 100-fold selective inhibition of HPV16-PsV infection (IC50 ≤ 0.002 μM) compared with cell viability (IC50 = 0.08 μM) (Fig. 3A). Securinine exhibited a >90-fold selective inhibition of HPV16-PsV infection (IC50 = 9.0 μM) compared with cell viability (IC50 > 42.0 μM) (Fig. 3B). Additionally, pentamidine and securinine represented drug classifications not previously associated with antiviral properties.
Figure 3.
Two lead FDA drugs. (A) Pentamidine exhibited more than 100-fold selective inhibition of HPV16-PsV infection (IC50 ≤ 0.002 μM) compared with cell viability (IC50 = 0.08 μM). (B) Securinine exhibited 90-fold selective inhibition of HPV 16-PsV infection (IC50 = 9 μM) compared with cell viability (IC50 = 42.0 μM).
Pentamidine and securinine were tested in a secondary stock threefold dose–response for their ability to prevent HPV-16PsV infection and preserve cell viability (Fig. 4). We chose a high concentration of 30 μM to test dose–response activity on cell viability in order to elucidate optimal formulation concentration. At 30 μM, pentamidine reduced HPV-16PsV infection by a twofold change, whereas securinine reduced HPV-16PSV infection by a 1.5-fold change compared with the control of cells only (Fig. 4A). Both drugs displayed >95.0% cellular viability even at the highest concentration of 30 μM compared with the control of cells only (Fig. 4B). Taken together, these results suggest that pentamidine and securinine prevent HPV-16PsV infection in a range of micromolar concentrations while preserving cell viability.
Figure 4.
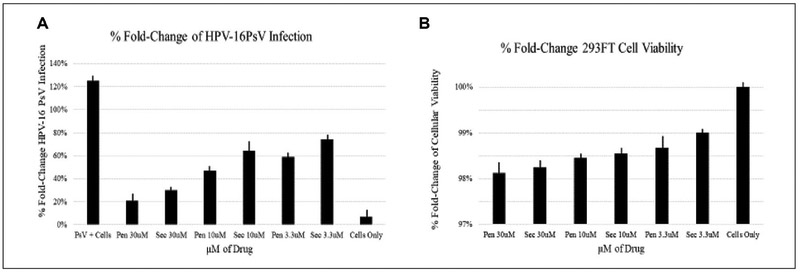
Percent fold change of infection and cell viability. (A) Pentamidine and securinine were tested in a secondary stock threefold dose–response for their ability to prevent HPV infection compared with the positive control (PsV + cells) and negative control (cells only). (B) Pentamidine and securinine were tested in a secondary stock threefold dose–response for cell viability compared with the positive control (cell only).
Discussion
In this study, we report on two promising and interlinked strategies: (1) the development of an innovative cell-based Renilla luminescence reporter assay using HPV16-PsVs that encapsidate an SV40-driven Renilla expression reporter expression plasmid, and (2) the potential to repurpose FDA- and internationally approved drugs identified by HTS for the prevention of HPV infection.
A major limitation to developing an HTS assay with HPV centered around the ability to produce enough of the viral reagent to conduct the screens. The contribution of the efficient intracellular assembly of papillomaviral vectors protocol by Buck et al. made it possible to produce pseudovirions with a high degree of viral packaging and to convert transfected reporter plasmids into papillomaviral vector stock capable of delivering the encapsidated plasmid to a wide variety of cell types.1,15 We optimized human embryonal kidney cells (293FT) transformed with the SV40. We chose SV40 given its robust promoter strength of Renilla luciferase expression in 293FT cells. 293FT cells were selected because they are of human origin, highly infectable by HPV16-PsV, rapidly grown, and can endure the mechanical challenges of the HTS format.
The assay was validated using an FDA- and internationally approved drug library of 1906 drugs. Our data demonstrate that the assay is both a valid and reliable tool to repurpose FDA-approved drugs for their potential to inhibit HPV infection of epithelial cells. Several prominent classes of drugs were identified, including antibiotic, antihypertensive, antidepressant, antiprotozoal, and GABA antagonist drugs. The secondary stock of drugs was used for dose–response activity confirmation.
Two lead FDA drugs, pentamidine and securinine, displayed superior biological reproducibility in triplicate, >90.0% inhibition of HPV16-PsV Renilla expression, and <10.0% cellular cytotoxicity. Pentamidine is an antiprotozoal agent and is known to have activity against the fungus Pneumocystis carinii. The medication is also useful in leishmaniosis and in prophylaxis against sleeping sickness caused by Trypanosoma brucei gambiense. Securinine is the major alkaloid natural product from the root of the plant Securinega suffruticosa. It was originally discovered over 50 years ago and reported to have potent biological activity and is considered one of the 50 fundamental Chinese herbs and is used in Chinese herbal medicine. To date, neither pentamidine nor securinine has been evaluated as a preventative agent for HPV-related infection. Pentamidine and securinine are examples of the reposed drugs pentamidine and securinine. The repurposing of FDA drugs for HPV prevention has the potential to substantially decrease the costs and delivery time associated with bringing a new drug to market. This offers a promising opportunity for the prevention of HPV-related infections and cancer in sexually active individuals.
With evidence of lack of cell cytotoxicity even at 30 μM, we next investigated the effect of the pentamidine and securinine on the SV40 promoter. The SV40 promoter enhances and drives expression of the luciferase gene Renilla. We performed transfection of the SV40 promoter in 293FT cells. We compared SV40 with 30.0 μM pentamidine and SV40 with 30.0 μM securinine to the positive control of SV40 with no drug. HPV16-PsV + cells were included as a second positive control. Our results showed that 30.0 μM pentamidine and securinine did not significantly affect SV40 promoter activity, indicating inhibition of Renilla signal was not due to cell toxicity or inhibition of the SV40 promoter. However, we have not yet proven that the drugs work by directly inhibiting binding of PsV to the cell receptors (data not shown). Further studies are required to investigate if the drugs prevent infection by inactivating virions on the cell surface before viral attachment and entry, preventing attachment of virions to epithelial cell surfaces, or inhibiting virion entry by endocytosis.
The assay also identified 5-fluorouracil (5-FU). 5-FU is currently used topically in the clinical setting to treat anal high-grade intraepithelial squamous lesions of the vulva, vagina, and anus.25 5-FU exhibited 100-fold selective inhibition of HPV-16PsV infection (IC50 = 0.4 μM) compared with cell viability (IC50 ≥ 50 μM). The identification of 5-FU represents proof of concept (data not shown).
MSM living with HIV remain at an increased risk of developing HPV-related precancerous anal lesions and anal cancer. Drugs shown to be safe and active may be tested in a lubricant that could be applied prior to intercourse to reduce oncogenic HPV infection. Repurposed drugs represent cost-effective strategies of bringing drugs to market, and thus our assay may be widely generalizable to both industry and academic HTS drug libraries.
Acknowledgments
The authors would like to thank Dr. Carol Dawson-Rose for her input on vulnerable high-risk populations.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health: NINR (5T32NR007081-15 and 5T32NR007091-22), Ruth L. Kirschstein Research Service Awards for Individual Predoctoral Fellow in Nursing Research (F31) (5F31NR013863), and American Cancer Society (Doctoral Degree Scholarship in Cancer Nursing). This project was partially supported by a National Institutes of Health: National Cancer Institute grant (R01 CA232887).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Buck CB; Pastrana DV; Lowy DR; et al. Efficient Intracellular Assembly of Papillomaviral Vectors. J. Virol. 2004, 78, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CJ; Sparano J; Palefsky JM Human Immunodeficiency Virus/AIDS, Human Papillomavirus, and Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervaz P; Hirschel B; Morel P Molecular Biology of Squamous Cell Carcinoma of the Anus. Br. J. Surg. 2006, 93, 531–538. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi M-P; Ngan SY; Michael M; et al. Molecular Biology of Anal Squamous Cell Carcinoma: Implications for Future Research and Clinical Intervention. Lancet Oncol. 2015, 16, e611–e621. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health, National Cancer Institute. HPV and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer?redirect=true (accessed June 30, 2019).
- 6.Thompson AB; Gillespie SE; Mosunjac MB; et al. Prevalence of Anal Squamous Intraepithelial Lesions in HIV-1-Infected Young Men Who Have Sex with Men and Transwomen. J. Low. Genit. Tract Dis. 2018, 22, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver SE; Hoots BE; Paz-Bailey G; et al. Increasing Human Papillomavirus Vaccine Coverage among Men Who Have Sex with Men—National HIV Behavioral Surveillance, United States, 2014. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, S370–S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrosky E; Bocchini JA; Hariri S; et al. Centers for Disease Control and Prevention (CDC). Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H-S; Pyeon D; Pearce SM; et al. Novel Antivirals Inhibit Early Steps in HPV Infection. Antiviral Res. 2012, 93, 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macarron R; Banks MN; Bojanic D; et al. Impact of High-Throughput Screening in Biomedical Research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [DOI] [PubMed] [Google Scholar]
- 11.Buck CB; Thompson CD; Roberts, et al. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fradet-Turcotte A; Morin G; Lehoux M; et al. Development of Quantitative and High-Throughput Assays of Polyomavirus and Papillomavirus DNA Replication. Virology 2010, 399, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toots M; Ustav M; Mannik A; et al. Identification of Several High-Risk HPV Inhibitors and Drug Targets with a Novel High-Throughput Screening Assay. PLOS Pathog. 2017, 13, e1006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris V; Rao X; Pickering C; et al. Comprehensive Genomic Profiling of Metastatic Squamous Cell Carcinoma of the Anal Canal. Mol. Cancer Res. 2017, 15, 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck CB; Thompson CD Production of Papillomavirus-Based Gene Transfer Vectors. Curr. Protoc. Cell Biol. 2007, 37, 26.1.1–26.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Gubler H; Schopfer U; Jacoby E Theoretical and Experimental Relationships between Percent Inhibition and IC50 Data Observed in High-Throughput Screening. J. Biomol. Screen. 2013, 18, 1–13. [DOI] [PubMed] [Google Scholar]
- 17.Walters WP; Namchuk M Designing Screens: How to Make Your Hits a Hit. Nat. Rev. Drug Discov. 2003, 2, 259–266. [DOI] [PubMed] [Google Scholar]
- 18.GraphPad Software. Principals of Fitting a Curve. https://cdn.graphpad.com/docs/prism/6/Prism-6-Curve-Fitting-Guide.pdf (accessed June 30, 2019).
- 19.Zhang JH; Chung TD; Oldenburg KR A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [DOI] [PubMed] [Google Scholar]
- 20.Vassilev LT Small-Molecule Antagonists of P53-MDM2 Binding: Research Tools and Potential Therapeutics. Cell Cycle 2004, 3, 417–419. [PubMed] [Google Scholar]
- 21.Frankfurt OS; Krishan A Apoptosis Enzyme-Linked Immunosorbent Assay Distinguishes Anticancer Drugs from Toxic Chemicals and Predicts Drug Synergism. Chem. Biol. Interact. 2003, 145, 89–99. [DOI] [PubMed] [Google Scholar]
- 22.Giroglou T; Florin L; Schafer F; et al. Human Papillomavirus Infection Requires Cell Surface Heparan Sulfate. J. Virol. 2001, 75, 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastrana DV; Gambhira R; Buck CB; et al. Cross-Neutralization of Cutaneous and Mucosal Papillomavirus Types with Anti-Sera to the Amino Terminus of L2. Virology 2005, 337, 365–372. [DOI] [PubMed] [Google Scholar]
- 24.Huang K-S; Holmgren J; Reik L; et al. High-Throughput Methods for Measuring Heparanase Activity and Screening Potential Antimetastatic and Anti-Inflammatory Agents. Anal. Biochem. 2004, 333, 389–398. [DOI] [PubMed] [Google Scholar]
- 25.Long K; Menon R; Bastawrous A; et al. Screening, Surveillance, and Treatment of Anal Intraepithelial Neoplasia. Clin. Colon Rectal Surg. 2016, 29, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]