Abstract
Background:
The study of Turner syndrome offers a unique window of opportunity for advancing scientific knowledge of how X chromosome gene imprinting, epigenetic factors, hormonal milieu and chronologic age affects brain development in females.
Methods:
Here, we describe brain growth trajectories in 55 girls with Turner syndrome (TS) and 53 typically developing (TD) girls (258 MR image datasets total) spanning 5 years. Using novel non-parametric and mixed effects analytic approaches we evaluate influences of X-chromosome genomic imprinting and hormone replacement therapy on brain development.
Results:
Parieto-occipital gray and white matter regions show slower growth during typical pubertal timing in TS relative to TD girls. In contrast, some basal ganglia, cerebellar and limited cortical areas showed enhanced volume growth with peaks around 10 years of age.
Conclusions:
The parieto-occipital finding suggests that girls with TS may be particularly vulnerable for altered brain development during adolescence. Basal ganglia regions may be relatively preserved in TS due to their maturational growth prior to, or early in typical pubertal years. Taken together, our findings indicate particular brain regions are more vulnerable to TS genetic and hormonal effects during puberty. These specific alterations in neurodevelopment may be more likely to affect long-term cognitive-behavioral outcomes in young girls with this common genetic condition.
Keywords: Turner Syndrome, Genomic Imprinting, Estrogen, X-chromosome, Neurodevelopment, Voxel-Based Morphometry
Introduction
Turner syndrome (TS) is caused by partial or complete absence of one of two X-chromosomes in a female serving, to some extent, as a human X chromosome “knockout” model. TS is common, affecting approximately 1 in every 2000–2500 live births, (1, 2) and is associated with high risk for impaired neurocognitive functions within the domains of visuo-spatial processing, executive function and social cognition (3). Despite consistent research indicating neurocognitive abnormalities are common in young girls with TS (3–6), only one preliminary study from our group has investigated disorder-specific variation in brain development over time. This study, limited to the parietal lobe only, assessed a small sample of pre-pubertal girls with TS (n=16) who had not begun hormone replacement therapy (7). Therefore, the study of brain development in larger samples of girls with TS over multiple time-points, in association with clinical and genetic information, holds promise for elucidating X-chromosome related influences on neurodevelopment in childhood and adolescence.
The neuroimaging literature has reported relatively consistent neuroanatomical features in cross-sectional studies of girls with TS, with evidence suggesting that the TS neurophenotype is influenced by age, genomic imprinting, and hormone replacement therapy (5, 8–12). Genomic imprinting in TS reflects a process whereby phenotypic features may be influenced by whether the retained X chromosome is of maternal or paternal origin. The study of genomic imprinting offers insight into potential differences in cognition, physical characteristics, and brain morphology in TS. Differences have been observed in visceral fat deposits, response to growth hormone, sensorimotor hearing loss, congenital heart and kidney malformations between girls with maternally retained (Xm) and paternally retained (Xp) X-chromosomes (13).
In healthy females, a large GWAS analysis from 57 studies identified parent-of-origin specific associations with pubertal timing (14). This finding suggests that pubertal timing in typically developing females is influenced by imprinted genes. The effects of parent-of-origin genetic factors on girls with hypogonadism is unknown, though such effects might lead to neurodevelopmental variation in individuals with TS. Therefore, one goal of this study was to examine regional brain growth in TS, with particular reference to brain areas that demonstrate puberty-associated temporal patterns of neurodevelopment or are sensitive to the effects of genomic imprinting.
The few studies that examined genomic imprinting effects on neuroanatomy indicate greater variation from typical development in girls with TS who have a maternally retained sex-chromosome (5, 11, 13–15). For example, genomic imprinting has been related to smaller brain volume in the temporal lobe and cerebellar gray matter (15, 16, 18), smaller volume of the caudate nucleus (18), and enlargement in the superior frontal regions in girls with a maternally (Xm) retained X chromosome relative to girls with a paternally (Xp) retained X chromosome and controls (11). No studies have yet evaluated the effects of imprinting on brain development over time in TS. In the present study, we assessed whether X-linked genomic imprinting led to differences in neurodevelopment within a cohort of girls with TS.
Hormone replacement therapy, in the form of estrogen treatment, represents another potential influence on brain and behavioral development in TS. Girls with TS, particularly those with X monosomy, have greatly reduced estrogen levels and premature ovarian failure (12, 19–22). Accordingly, current medical management guidelines for girls with TS emphasizes hormone replacement therapy with estrogen to induce and sustain puberty (22–24). Estrogen is known to significantly influence brain and behavioral development in typically developing girls (25, 26). Accordingly, Turner syndrome offers an opportunity to study the effects of exogenous and endogenous sex hormones on brain development.
One cross-sectional imaging study that examined brain related differences in separate groups of girls with TS prior to estrogen treatment and during treatment suggested neurodevelopmental trajectories could be related to estrogen deficiency (12). Due to the potential effects of estrogen treatment on brain maturation, we assessed here whether brain development differed in girls with TS depending on whether they were receiving estrogen therapy at the time of MRI acquisition.
To the best of our knowledge, the results presented here represent the first large-scale longitudinal study of brain volume growth over time in TS. Also, we employed an innovative, non-parametric analytic approach, the Sandwich Estimator (SwE) toolbox to more accurately characterize neurodevelopment trajectories in TS relative to a typically developing (TD), age and sex-matched control group (27). Based on prior research, we expected to observe slower gray and white volume growth in temporal, parietal, and cerebellar gray and white matter volume growth in girls with TS compared with TD girls. We also hypothesized differential neurodevelopmental trajectories related to parent-of-origin and estrogen therapy in TS.
Methods
Participant demographics
Fifty-five young females with TS associated with 45, X genotype (i.e., X monosomy) (Age: 11.5±2.6) and 53 young typically developing (TD) (Age: 11.0±2.1) females group matched for age met criteria for inclusion in the study and underwent structural MR scanning over 2–4 visits. In total, 149 scans from the participants with TS and 109 scans from the TD group were collected (Table 1). All participants underwent initial screening for potential MRI contraindications and medical history to exclude subjects with neurological injury and unrelated psychiatric illness. Average IQ scores from the Wechsler Intelligence Scales for Children, Fourth edition, are also presented in Table 1.
Table 1.
Demographics, parent-of-origin, estrogen status, brain volumes and IQ
| Turner | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| N | 55 | 44 | 38 | 12 | 53 | 31 | 19 | 6 |
| Age (years) | 10.6 ± 2.3 | 11.4 ± 2.6 | 12.2 ± 2.4 | 13.2 ± 2.6 | 10.2 ± 2 | 11.3 ± 2 | 12.1 ± 1.9 | 12.8 ± 1.6 |
| Parent of Origin | ||||||||
| (m/p/uk) | 35/14/6 | 30/9/5 | 30/6/2 | 10/1/1 | - | - | - | - |
| Estrogen Status | ||||||||
| (yes/no) | 28/27 | 25/19 | 19/19 | 6/6 | - | - | - | - |
| GMV (mL) | 802 ± 60 | 784 ± 59 | 804 ± 54 | 771 ± 46 | 803 ± 65 | 783 ± 51 | 780 ± 56 | 724 ± 64 |
| WMV (mL) | 438 ± 47 | 440 ± 43 | 438 ± 40 | 433 ± 28 | 443 ± 51 | 444 ± 42 | 442 ± 36 | 435 ± 39 |
| TIV (mL) | 1581 ± 123 | 1576 ± 121 | 1579 ± 101 | 1545 ± 80 | 1554 ± 132 | 1548 ± 98 | 1549 ± 93 | 1487 ± 72 |
| FSIQ | 93.8 ± 14.3 | 96.3 ± 14.1 | 95.9 ± 13.2 | 94.5 ± 12.5 | 113.8 ± 12.6 | 118.3 ± 9.7 | 117.4 ± 10.6 | 119.7 ± 11.6 |
| VCI | 106.4 ± 15.7 | 105.0 ± 15.9 | 105.9 ± 11.7 | 103.6 ± 17.3 | 115.8 ± 15.3 | 118.2 ± 12.7 | 118.3 ± 14.2 | 111.0 ± 13.0 |
| PRI | 92.5 ± 15.1 | 98.8 ± 15.3 | 99.7 ± 14.1 | 96.1 ± 15.9 | 112.6 ± 12.1 | 118.1 ± 11.2 | 117.2 ± 11.6 | 120.7 ± 10.8 |
| WMI | 91.7 ± 11.9 | 92.1 ± 15.3 | 91.4 ± 14.5 | 91.6 ± 10.1 | 107.7 ± 13.2 | 109.1 ± 10.8 | 105.6 ± 11.2 | 104.8 ± 7.8 |
| PSI | 84.7 ± 16.1 | 86.6 ± 13.1 | 85.3 ± 15.3 | 88.1 ± 11.5 | 102.7 ± 14.7 | 106.0 ± 14.9 | 108.6 ± 16.1 | 121.3 ± 13.0 |
Parent of Origin: m=Maternal, p=Paternal, uk=Unknown; GMV = Gray Matter Volume; WMV = White Matter Volume; TIV = Total Intracranial Volume; FSIQ = Full Scale IQ; VCI = Verbal Comprehension Index; PRI = Perceptual Reasoning Index; WMI = Working Memory Index; PSI = Processing Speed Index
MRI Acquisition
All MR images were acquired at the Stanford University Lucas Center for Medical Imaging. Imaging data were acquired on a 3-Tesla GE MR750 scanner (GE Healthcare, Wauwatosa, WI) with an eight-channel head coil, including high-resolution T1-weighted structural images (sagittal slices, repetition time 8.2 msec; echo time 3.2 msec; flip angle 12°; field of view 240 × 192 mm; matrix 256X256; 176 slices; voxel size = 1.0 × 1.0 × 1.0 mm).
Quality Assessment
MRI data were visually quality checked to eliminate scans with significant head motion or artifacts before pre-processing. Images were visually inspected for severe head motion and geometric distortions. Additional qualitative assessment was undertaken on segmented images to ensure valid skull-stripping, tissue contrast, and segmentation accuracy.
Tissue Probability Mapping
Tissue probability maps were used to delineate voxels associated with either gray matter (GM), white matter (WM), or cerebrospinal fluid (CSF). The template-o-matic (TOM) toolbox provides reference data based on an NIH study of normal brain development, using typically developing children ages 5–18 years (28). The same set of GM/WM/CSF tissue probability maps were used across all subjects and within-subjects across all time-points. Table 1 provides average total gray and white matter, and intracranial volume for each group and time point.
Voxel-Based Morphometry
Voxel-based morphometry (VBM) was used to quantify volume in GM and WM voxels for each structural scan (http://www.fil.ion.ucl.ac.uk/spm/). Volumetric pre-processing was implemented with the Computational Anatomy Toolbox (CAT12) with SPM12 using Matlab R2015b. Briefly, the pre-processing included skull-stripping of non-brain tissue, within-subject longitudinal image registration, and two rounds of tissue segmentation. A Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) study population template (averaged for all time points) was produced from the initial GM and WM segmentations to improve inter-subject alignment and the resolution of child and adolescent brain anatomy. Longitudinal image registration and normalization was applied with the DARTEL template. Finally, warped and modulated segmented GM and WM images were normalized from native space to MNI standard space, and all images were spatially smoothed using an 8mm full-width-at-half-maximum (FWHM) Gaussian smoothing kernel. The Computational Anatomy Toolbox (CAT12) provided the voxel-wise estimation of the local volumes (% volumes) of either gray or white matter tissue. The resulting data provides the fraction of the amount of pure tissue at the voxel-level.
Genetic analysis
Genetic analysis was undertaken to determine whether the retained X chromosome in our participants with TS was of maternal (Xm) or paternal (Xp) origin. Parental origin was determined by amplification of four polymorphic markers located exclusively on the X-chromosome and one marker in the pseudoautosomal region between the participant and mother. Parent-of-origin was determined for all but two participants (36 Maternal/17 Paternal). One participant was accompanied by a grandparent at data collection, thereby providing inadequate genetic data for determining parent-of-origin. Genetic testing from a different participant resulted in identification of individual markers from the mother with only one allele from the father. Therefore, this participant was also excluded from imprinting analyses as we could not determine a definitive maternal or paternal origin of their X chromosome. Table 1 presents summary information regarding TS group parent-of-origin by time point.
Statistics
The Sandwich Estimator (SwE) Toolbox for longitudinal and repeated measures neuroimaging data was employed for this study (27). The SwE is advantageous as it uses a marginal approach to measure how volume changes are associated with age, and can model unbalanced study designs where the number of scans may be variable across subjects and time. This technique is designed to improve upon standard regression models that assume neurodevelopment is spatially coherent, whereas the SwE method allows for non-parametric inferences for longitudinal data (27, 29).
There are two types of analyses in the SwE toolbox. The first is a voxel-wise analysis and the second tests for cluster-level differences. Within the voxel-wise analysis, the SwE method first estimates the parameters of interest with an ordinary least squares model, then uses sandwich estimation to account for the within-subject correlation in longitudinal data.
In this longitudinal study, we modeled two age covariates: between-subject (i.e., cross-sectional age effect) and within-subject (i.e., longitudinal visit effect of age) components (27). Of note, the time interval between visits across all subjects was consistent with a one year +/−30 day window for nearly all visits. The Age covariate was split into a between-subject measure reflecting the subject mean (Agei) and a within-subject component reflecting the difference with the mean Age - Agei (27, 30). These separate age covariates, along with total intracranial volume (TIV), were incorporated into our statistical design to model and assess between-group differences in longitudinal volume growth rates.
Regional volume may undergo maturation at the same rate in two populations, however one group may have a lower overall volume difference (i.e., height). To assess whether developmental trajectories were influenced by smaller or larger volumes as well as volume growth rates, a post-hoc analysis explored cross-sectional differences at visit 2 using the SwE. Specifically, we computed between group differences in GM and WM volume in girls with TS and TD girls when they were approximately 11 ± 2 years of age.
Genomic imprinting and effect of estrogen
Following the TS vs TD longitudinal brain volume analyses, volumetric data were derived from our statistically significant regions-of-interest (ROIs) for post-hoc analyses. Linear mixed effects models were used to assess the role of genomic imprinting and estrogen status on brain maturation. Our model follows previously described multi-level modeling by Mills and Tamnes (31). Prior to the mixed effects analysis, an Akaike information criterion estimator was carried out to determine the best model fit based on a linear, cubic, or quadratic model. A mixed effects analysis with a linear model resulted in the best model fit. Specifically, a three-group analysis was utilized to detect differences in brain volume by an age interaction across the three subgroups of interest: Xm, Xp, TD controls. Similarly, a linear mixed effects model with age centered at 10 and 14 was used to examine volume differences between two TS subgroups designated according to estrogen status (E+ or E−) and TD girls [Table 3; Figures 5, 6].
Table 3.
Effect of estrogen on brain volume change at age 10 and age 14.
| Age 10 | Age 14 | |
|---|---|---|
| Region | TS E− v TS E + T-value (p-value) |
TS E− v TS E + T-value (p-value) |
| Less Gray Matter Over Time | ||
| Right Calcarine-Lingual | 0.019 (p<0.98) Age Interaction: 2.43 (p<0.17) |
2.81 (p<0.0071) Age Interaction: 2.43 (p<0.01) |
| Left Postcentral-Inferior Parietal | 0.9 (p<0.3) Age Interaction: 2.5 (p<0.01) |
3.9 (p<0.0002) Age Interaction: 2.5 (p<0.01) |
| Right Postcentral Inferior Parietal | 1.1 (p<0.27) Age Interaction: 2.5 (p<0.01) |
4.1 (p<0.0001) Age Interaction: 2.5 (p<0.01) |
| Right Middle Temporal | 1.28 (p<0.2) Age Interaction: 2.0 (p<0.04) |
3.7 (p<0.0005) Age Interaction: 2.0 (p<0.04) |
| Increased Gray Matter Over Time | ||
| Left Lingual-Cerebellar | 0.63 (p<0.52) Age Interaction: 1.8 (p<0.07) |
2.6 (p<0.0097) Age Interaction: 1.8 (p<0.07) |
| Right Parahippocampal | 1.79 (p<0.07) Age Interaction: 1.36 (p<0.17) |
3.6 (p<0.0006) Age Interaction: 1.36 (p<0.17) |
| Left Putamen | −1.5 (p<0.13) Age Interaction: 1.14 (p<0.25) |
−0.59 (p<0.55) Age Interaction: 1.14 (p<0.25) |
| Right Insula-Superior Temporal | −1.5 (p<0.13) Age Interaction: 2.4 (p<0.1) |
0.8 (p<0.42) Age Interaction: 2.4 (p<0.01) |
| Less White Matter Over Time | ||
| Left Precentral | 0.67 (p<0.54) Age Interaction: 2.52 (p<0.01) |
3.68 (p<0.0006) Age Interaction: 2.52 (p<0.01) |
| Right Supramarginal | 0.30 (p<0.76) Age Interaction: 2.7 (p<0.008) |
3.66 (p<0.0006) Age Interaction: 2.72 (p<0.008) |
| Left Calcarine-Lingual | −0.08 (p<0.93) Age Interaction: 2.2 (p<0.02) |
2.58 (p<0.01) Age Interaction: 2.24 (p<0.02) |
| Left Cuneus-Precuneus | 0.37 (p<0.71) Age Interaction: 1.69 (p<0.09) |
2.49 (p<0.01) Age Interaction: 1.69 (p<0.09) |
| Left Postcentral | 0.63 (p<0.52) Age Interaction: 2.67 (p<0.009) |
0.44 (p<0.65) Age Interaction: 1.65 (p<0.1) |
Results
Genomic and Hormonal Status
Of the collected genomic data in the group with TS, 36 had a maternal (Xm) and 17 had a paternal (Xp) inherited sex chromosome. For the TS group, the number of girls who were receiving estrogen replacement therapy at each time-point is reported in Table 1.
Gray Matter Findings
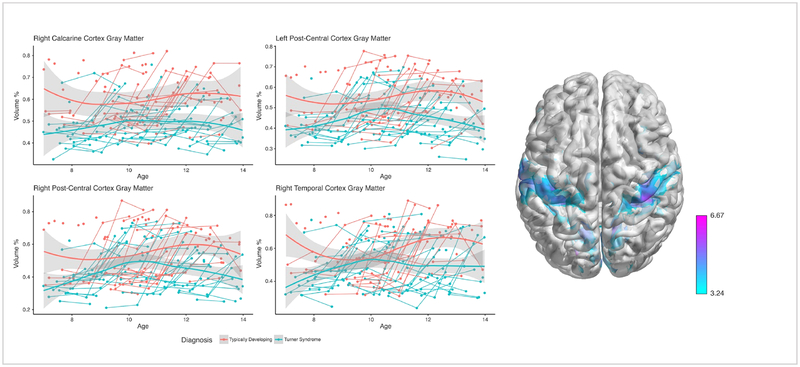
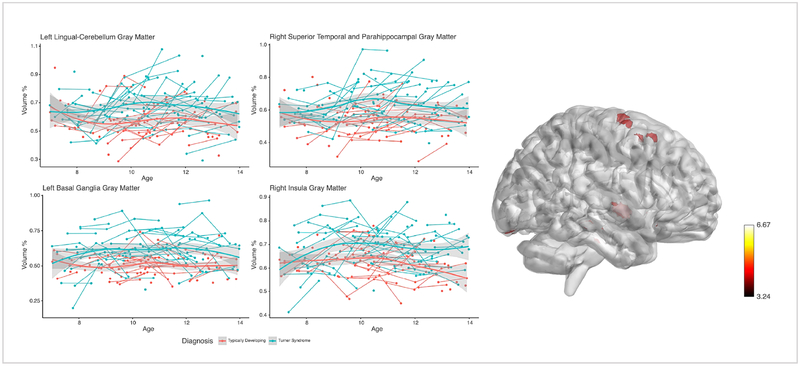
Voxel-Based Morphometry analysis demonstrated that the rate of GM volume growth was significantly slower in specific regions for the TS group compared with the TD group across the study period, based on a statistical threshold of p<0.01 FDR-corrected voxels. Voxel-level findings indicated four major regions showing a slower rate of volume growth: 1) right V1/calcarine cortex, 2) bilateral pre/postcentral gyrus, 3) right middle temporal gyrus, and 4) left middle cingulate cortex. Girls with TS also showed faster regional rates of GM volume growth compared with TD girls in 4 major clusters at p<0.01 FDR corrected height and clusters: 1) right parahippocampal cortex, 2) left lingual gyrus/cerebellum, 3) left putamen, 4) right insula/STG [Table 2; Figure 1, Figure 2]. Analyses were also evaluated with total intracranial volume as a covariate and results were unchanged.
Table 2.
VBM results from longitudinal age-related changes between TS and TD
| Cluster-size | Peak-level | MNI coordinates | Region | ||||
|---|---|---|---|---|---|---|---|
| kE | qFDR-corr | Z | mm | mm | mm | ||
| Gray Matter | |||||||
| TD > TS | 10026 | 0.000 | 6.67 | 21 | −66 | 15 | Right calcarine, lingual gyrus |
| 6177 | 0.000 | 5.97 | 42 | −36 | 62 | Right postcentral gyrus, inferior parietal | |
| 6886 | 0.000 | 5.61 | −66 | −20 | 26 | Left postcentral gyrus, inferior parietal | |
| 1022 | 0.000 | 5.41 | 46 | 0 | −28 | Right middle temporal gyrus | |
| TS > TD | 138 | 0.008 | 5.12 | 36 | −26 | −18 | Right parahippocampal gyrus |
| 189 | 0.008 | 4.97 | −8 | −87 | −22 | Left lingual gyrus, cerebellum | |
| 32 | 0.008 | 4.69 | −18 | 18 | −8 | Left putamen | |
| 16 | 0.008 | 4.56 | 45 | −3 | −3 | Right insula, superior temporal gyrus | |
| White Matter | |||||||
| TD > TS | 337 | 0.000 | 5.70 | −45 | −21 | 56 | Left post-central |
| 574 | 0.000 | 5.54 | −16 | −81 | 36 | Left cuneus | |
| 1290 | 0.000 | 5.48 | −58 | −10 | 24 | Left pre-central | |
| 149 | 0.000 | 5.09 | 57 | 3 | 14 | Right pre-central, rolandic operculum | |
| 955 | 0.000 | 4.95 | 60 | −27 | 33 | Right supramarginal | |
| 876 | 0.001 | 4.89 | −3 | −86 | −4 | Left calcarine, lingual | |
| 171 | 0.001 | 4.63 | 46 | −22 | 57 | Right post-central | |
| 50 | 0.001 | 4.51 | −32 | −16 | −26 | Left fusiform gyrus, parahippocampus | |
Fig. 1: Developmental trajectories for regions with less gray matter over time in girls with TS relative to TD girls.
Average and individual trajectories show local amount of gray matter in % volume (y-axis) by age in years (x-axis) in girls with X-monosomy (blue) and typically developing girls (red). Shaded area represents the 95% confidence intervals of the intercept. 3D brain image represents regions of slower GM growth, where T-values are represented by color bar.
Fig. 2: Developmental trajectories for regions with increased gray matter over time in girls with TS relative to TD girls.
Average and individual trajectories show local amount of gray matter in % volume (y-axis) by age in years (x-axis) in girls with X-monosomy (blue) and typically developing girls (red). Shaded area represents the 95% confidence intervals of the intercept. 3D brain image represents regions of increased GM growth, were T-values are represented by the color bar.
White Matter Findings
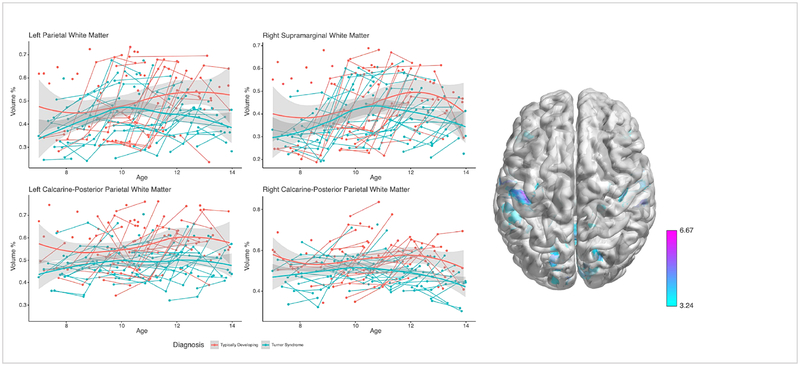
Whole brain VBM analysis of WM volume growth was significantly slower between the group with TS and the TD group (p<0.01 FDR-corrected). Participants with TS showed slower rates of volume growth in temporal and parieto-occipital regions including: 1) bilateral postcentral, 2) left cuneus, 3) bilateral precentral, 4) right supramarginal, 5) bilateral calcarine/lingual, 6) left fusiform/parahippocampal WM, 7) right middle temporal WM, and 8) right superior occipital WM expanding to the precuneus [Table 2, Figure 3]. Findings did not show an increased rate of WM growth in TS at the voxel-level after correcting for multiple testing. This was consistent when total intracranial volume was included as a covariate.
Fig. 3: Developmental trajectories for regions with less white matter over time in girls with TS relative to TD girls.
Average and individual trajectories show local amount of gray matter in % volume (y-axis) by age in years (x-axis) in girls with X-monosomy (blue) and typically developing girls (red). Shaded area represents the 95% confidence intervals of the intercept. 3D brain images represents regions of slower WM growth, where T-values are represented by color bar.
Genomic Imprinting
The mixed effects analysis indicated that imprinting status moderated region-specific neurodevelopment in girls with TS [Supplementary Figure 1]. From the ROI’s that showed slower GM growth in the overall TS group relative to TD controls, the Xp subgroup showed less GM growth in the right middle temporal gyrus relative to TD girls (t-value: −2.5, p<0.01). From the ROI’s that showed faster GM rate of growth in the overall TS group, the Xm group showed a more positive slope compared with TD girls in left lingual and cerebellar regions (t-value: 3.34, p<0.001) and right parahippocampal region (t-value: 3.31, p<0.001), and the Xp group showed a more positive slope compared with TD girls in the right parahippocampal region (t-value: 2.9, p<0.01) [Supplementary Figure 1]. Analysis of WM ROI’s that showed slower growth in the overall TS group indicated that the Xm group had a more negative slope relative to the TD group in left post central white matter (t-value, −2.95, p<0.001). Differences between Xm and Xp were not significantly different when slopes were compared directly.
Estrogen Status
The mixed effects analysis indicated that estrogen status moderated region-specific neurodevelopment in girls with TS. With respect to GM regions that showed slower growth in the overall group of girls with TS relative to TD controls, girls with TS who did not receive estrogen (TS E−) showed a more positive slope for volume growth over time relative to girls with TS receiving estrogen (TS E+) in right calcarinelingual, left postcentral-inferior parietal, right postcentral-inferior parietal, and right middle temporal gray matter [Table 3; Supplementary Figure 2]. Gray matter regions with faster growth rates in the overall TS group also showed a more positive slope for the TS E − group relative to the TS E+ group in the left lingual-cerebellar and right parahippocampal gray matter [Table 3; Supplementary Figure 3]. For WM ROI’s with slower rates of WM growth in the overall TS group, the TS E− subgroup showed a more positive slope of volume change relative to the TS E + subgroup in the left precentral, right supramarginal, left calcarine-lingual and left cuneus-precuneus WM areas [Table 3, Supplementary Figure 4].
Cross-sectional Analyses
A cross-sectional analysis at Visit 2 identified reduced volume in girls with TS (Age: 11.43±2.62) relative to TD girls (Age: 12.13±1.91) in GM and WM clusters including: 1) bilateral calcarine gyri, 2) right precuneus, 3) bilateral posterior cingulate gyrus, 4) left cuneus, 5) bilateral postcentral gyrus, and 6) left inferior temporal regions.
Discussion
Our longitudinal investigation of brain growth in TS provides novel and important information about regionally specific neurodevelopmental trajectories in this condition. We investigated regional brain volume growth from early childhood into adolescence in girls with TS relative to TD girls. GM growth showed both slower and faster rates of growth in TS across specific brain regions, whereas regional WM growth was slower in TS in comparison with TD girls. In addition, findings from the cross-sectional analysis at the second time-point are consistent with regions identified in the longitudinal analysis. Finally, we assessed the potential effects of genomic imprinting and estrogen on neurodevelopment. The results suggest that developmental trajectories of particular brain regions may be moderated by both genomic imprinting and estrogen status.
We found that girls with TS show smaller regional GM and WM volumes and that growth peaks in somatosensory and inferior frontal regions around age 10, while growth in these regions peaks by age 12 in TD girls. Previous studies of typical neurodevelopment also show inverted “U” shape growth in these regions around age 12 (32–34). This finding indicates that aberrant neurodevelopment is present early in childhood in TS and extends into adolescence. Our findings of slower brain volume growth in parieto-occipital regions are consistent with a longitudinal investigation across two time-points (7) and cross-sectional reports of reduced volume in these areas in girls (15, 35–38) and adult women (18, 39) with TS. These results provide additional evidence for a distinct developmental neuroanatomical profile, and indicate that these regions are sensitive to X-monosomy.
The TS group studied here demonstrated aberrant neurodevelopment in parietal regions implicated in somatosensory and visuo-spatial functions. Further research investigating associations between structure and function using multi-modal imaging would allow for more clinical inferences to be made. One study on the development of human somatosensory functions suggests that regions experiencing early neurodevelopmental insult may be predictive of later functional deficit (40). This hypothesis suggests sensory deficits may occur in later adolescence in girls with TS when cortical folding patterns become more complex (41). Our finding highlights the importance of long-term longitudinal investigations in TS as well as other cohorts who have genetic or medical risk factors for aberrant brain development.
Of interest, STG, lingual, insula, and parahippocampal regions showed faster rates of growth in girls with TS relative to TD girls with a plateau in volume around age 10 for both groups. This finding suggests specific brain regions that typically mature around age 10 may have faster volume growth in girls with TS. These regions are involved in verbal memory, speech production and object naming; supporting the behavioral finding of preserved or even enhanced verbal abilities in girls with TS (3, 16, 38, 42). Accordingly, the results presented here may represent a neuroanatomical phenotype associated with preserved cognitive abilities in girls with TS. Potentially, this represents a dynamic neurodevelopmental adaptation or compensation in these anatomical regions.
Genomic Imprinting
When we examined the influence of genomic imprinting, girls with a maternally retained X-chromosome (Xm) had significantly different trajectories relative to TD girls in the left lingual gyrus extending to the cerebellum and in the right parahippocampal area. These findings are consistent with previous cross-sectional investigations in the literature where girls with Xm showed the largest differences compared with typically developing girls (5, 15) and support the hypothesis that genomic imprinting may influence regional neurodevelopment in this condition. Additional longitudinal research in larger cohorts of individuals with TS could help to clarify associations among genomic imprinting effects, brain morphology and cognition. This topic was beyond the scope of our current study of genomic imprinting moderating neurodevelopment in TS.
Estrogen Status
Our results showed significant slope differences in specific brain regions based on the presence or absence of hormone replacement therapy in girls with TS. TS E− girls showed the largest slope differences relative to TS E+ in right calcarine-lingual, left postcentral-inferior parietal, right postcentral-inferior parietal, right middle temporal, left lingual-cerebellar, right parahippocampal GM, and left precentral, right supramarginal, left calcarine-lingual, and left cuneus-precuneus WM. Potentially, exogenous estrogen allows regional GM volume to undergo more normalized brain maturational processes around puberty. Based on animal studies, it has been posited that a second restructuring occurs during pubertal “hormonal events” (25, 43), where sex steroid hormones have been related to restructuring of the visual cortex in animals (44). The WM findings appear more complex. Girls with TS E+ showed a lower slope for volume change in left precentral, right supramarginal, left calcarine-lingual and left cuneus-precuneus WM relative to girls with TS E−. These results are potentially consistent with one longitudinal imaging study in typically developing adolescents that showed that girls with lower estradiol levels displayed increased WM growth relative to girls with higher estradiol levels (26). A possible explanation for these findings is that increased exogenous estrogen during adolescence may decrease myelination, a finding that has been reported in female rats during puberty (45). Our findings indicate that specific brain regions may be more sensitive to estrogen during development. This raises the potential for timing of hormone replacement therapy to not only induce puberty for feminization, but also normalize neurodevelopmental processes in regions that are more hormone dependent. Evaluation of specific estrogen dosage and therapy duration prior to MRI scanning in conjunction with our growth models could potentially contribute to more specific treatment recommendations for developing girls with TS. In summary, divergent trajectories observed within TS hormonal subgroups suggest that maturation of specific GM and WM areas are moderated by estrogen treatment.
Cross-sectional differences during peak development
When volume differences were compared cross-sectionally at the second visit we found less local GM volume in the right calcarine gyrus, bilateral post central gyri, right STG, left fusiform gyrus, and left cerebellum. These findings are consistent with previous cross-sectional studies in TS and support regional volume differences between these populations during ages of typical peak neurodevelopment. We demonstrate that volume differences and growth rates should both be considered when evaluating how brain development unfolds in longitudinal studies of young children through adolescence.
Non-parametric models
Finally, our study addresses the utility of non-parametric longitudinal models in longitudinal brain imaging research. Specifically, our analytic approach allowed us to probe regional trajectories of neurodevelopment and identify differential changes across brain regions over time. Standard statistical packages in popular neuroimaging software pipelines make parametric assumptions for longitudinal data (46). Such models can be useful in two time-point studies with matching samples; however, imaging data with heterogeneous visit and group variances are more complex. Accordingly, recent methodological advances have achieved fast and accurate modeling of longitudinal neuroimaging data through non-parametric inferences (27). Here, using non-parametric longitudinal analysis, we identified regions of aberrant growth rates during childhood and puberty in a large sample of girls with TS.
Study strengths and weaknesses
A particular strength of the study was the large-scale longitudinal design employed. This allowed us to show longitudinal volume change related to diagnosis, genomic imprinting, and hormone replacement therapy in girls with TS seven to 14 years of age. Furthermore, the statistical model allowed us to include individuals with scan drop-out over time with an alternative non-parametric procedure.
Only volume was employed as our neural measure of interest. Genomic imprinting and estrogen status also may affect morphological measures of cortical thickness and surface area12. Further research, including cortical thickness and surface area measures may distinguish different features of pathophysiological changes during development in TS. In addition, the population of girls who initiated estrogen treatment after study entry was limited. This was a naturalistic study and therefore hormone replacement therapy initiation was not controlled.
As expected (3), the two groups showed differences in average IQ with greater between-group divergence in non-verbal cognitive functions (Table1). However, all mean IQ scores for the TS group were in the average range of intelligence except for the Perceptual Reasoning Composite, which is comprised of subtests that measure nonverbal abstract reasoning skills, perceptual reasoning, and perceptual organization, known areas of cognitive weakness in TS (3).
Conclusion
The diagnosis of TS, along with genomic imprinting and estrogen treatment status significantly alter neurodevelopment from childhood into adolescence. Brain regions associated with verbal memory and encoding, which are relatively preserved neurocognitive functions in TS, displayed an increased rate of growth in girls with TS relative to TD girls. Girls with Xm show different growth trajectories than typically developing girls in lingual, cerebellar, and parahippocampal regions. Taken together, these findings indicate that regions with peak volume growth typically occurring during puberty may be particularly “at risk” for impaired development during adolescence in TS, while regions of earlier peak growth may be less hormone-dependent. Further research highlighting the role of sex-hormones and their interaction with X-chromosome expression is of interest to elucidate how typical neurodevelopment unfolds in females.
Supplementary Material
Acknowledgments:
The authors sincerely thank the participants and their families for their participation in this research study. The authors also thank the Turner Syndrome Society and the Turner Syndrome Foundation who assisted with participant recruitment. Dr. Reiss is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation. Dr. Tom Nichols and Dr. Bryan Guillaume contributed technical assistance in developing the Sandwich Estimator Anlaysis design used in this study.
Financial Disclosures: This project was supported by grants: Contract grant sponsor: NICHD; Contract grant number: HD049653; Contract grant sponsor: NIMH; Contract grant number: MH099630. The funding sources had no role in the study design, collection, analysis and interpretation of the data. S.O’D. was supported by a postdoctoral fellowship from a NIH T32 Training Grant. Dr. O’Donoghue, Dr. Green, Dr. Ross, Dr. Hallmayer, Ms. Lin, Dr. Jo, Dr. Huffman, Dr. Hong, and Dr. Reiss reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sybert VP, McCauley E (2004): Turner’s Syndrome. N Engl J Med. 351: 1227–1238. [DOI] [PubMed] [Google Scholar]
- 2.Stochholm K, Juul S, Juel K, Naeraa RW, Højbjerg Gravholt C (2006): Prevalence, Incidence, Diagnostic Delay, and Mortality in Turner Syndrome. J Clin Endocrinol Metab. 91: 3897–3902. [DOI] [PubMed] [Google Scholar]
- 3.Hong DS, Reiss AL (2014): Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol. 13: 306–318. [DOI] [PubMed] [Google Scholar]
- 4.Hong DS, Dunkin B, Reiss AL (2011): Psychosocial Functioning and Social Cognitive Processing in Girls with Turner Syndrome. J Dev Behav Pediatr. 32: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepage J-F, Hong DS, Hallmayer J, Reiss AL (2012): Genomic Imprinting Effects on Cognitive and Social Abilities in Prepubertal Girls with Turner Syndrome. J Clin Endocrinol Metab. 97: E460–E464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demily C, Poisson A, Peyroux E, Gatellier V, Nicolas A, Rigard C, et al. (2017): Autism spectrum disorder associated with 49,XYYYY: case report and review of the literature. BMC Med Genet. 18: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green T, Chromik LC, Mazaika PK, Fierro K, Raman MM, Lazzeroni LC, et al. (2014): Aberrant parietal cortex developmental trajectories in girls with turner syndrome and related visual-spatial cognitive development: A preliminary study. Am J Med Genet Part B Neuropsychiatr Genet. 165: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong DS, Hoeft F, Marzelli MJ, Lepage J-F, Roeltgen D, Ross J, Reiss AL (2014): Influence of the XChromosome on Neuroanatomy: Evidence from Turner and Klinefelter Syndromes. J Neurosci. 34: 3509–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong DS, Reiss AL (2012): Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev. 9 Suppl 2: 710–2. [PMC free article] [PubMed] [Google Scholar]
- 10.Mullaney R, Murphy D (2009): Turner syndrome: Neuroimaging findings: Structural and functional. Dev Disabil Res Rev. doi: 10.1002/ddrr.87. [DOI] [PubMed] [Google Scholar]
- 11.Lepage J-FJ-F, Hong DS, Mazaika PK, Raman M, Sheau K, Marzelli MJ, et al. (2013): Genomic Imprinting Effects of the X Chromosome on Brain Morphology. J Neurosci. 33: 8567–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepage J-F, Mazaika PK, Hong DS, Raman M, Reiss AL (2013): Cortical brain morphology in young, estrogen-naive, and adolescent, estrogen-treated girls with Turner syndrome. Cereb Cortex. 23: 2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespi B (2008): Turner syndrome and the evolution of human sexual dimorphism. Evol Appl. 1: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry JRB, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, et al. (2014): Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 514: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Patwardhan A, et al. (2002): Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiatry Res. 116: 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL (2003): Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry. 54: 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL (2004): Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 42: 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutter WJ, Daly EM, Robertson DMWW, Chitnis XA, Van Amelsvoort TAMJMJ, Simmons A, et al. (2006): Influence of X chromosome and hormones on human brain development: A magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol Psychiatry. 59: 273–283. [DOI] [PubMed] [Google Scholar]
- 19.Lim HH, Kil HR, Koo SH (2017): Incidence, puberty, and fertility in 45,X/47,XXX mosaicism: Report of a patient and a literature review. Am J Med Genet A. doi: 10.1002/ajmg.a.38276. [DOI] [PubMed] [Google Scholar]
- 20.Knickmeyer RC, Davenport M (2011): Turner syndrome and sexual differentiation of the brain: implications for understanding male-biased neurodevelopmental disorders. J Neurodev Disord. 3: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagata B, Barnea-Goraly N, Marzelli MJ, Park Y, Hong DS, Mimura M, Reiss AL (2012): White Matter Aberrations in Prepubertal Estrogen-Naive Girls with Monosomic Turner Syndrome. Cereb Cortex. 22: 2761–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backeljauw P, Klein K (2019): Sex hormone replacement therapy for individuals with Turner syndrome. Am J Med Genet Part C Semin Med Genet. doi: 10.1002/ajmg.c.31685. [DOI] [PubMed] [Google Scholar]
- 23.Hankus M, Soltysik K, Szeliga K, Antosz A, Drosdzol-Cop A, Wilk K, et al. (2017): Prediction of Spontaneous Puberty in Turner Syndrome Based on Mid-Childhood Gonadotropin Concentrations, Karyotype, and Ovary Visualization: A Longitudinal Study. Horm Res Paediatr. 89: 90–97. [DOI] [PubMed] [Google Scholar]
- 24.Trolle C, Hjerrild B, Cleemann L, Mortensen KH, Gravholt CH (2012): Sex hormone replacement in Turner syndrome. Endocrine. 41: 200–219. [DOI] [PubMed] [Google Scholar]
- 25.Blakemore S-J, Burnett S, Dahl RE (2010): The role of puberty in the developing adolescent brain. Hum Brain Mapp. 31: 926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER (2014): The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 35: 5633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE, Alzheimer’s Disease Neuroimaging Initiative (2014): Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. Neuroimage. 94: 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke M, Holland SK, Altaye M, Gaser C (2008): Template-O-Matic: A toolbox for creating customized pediatric templates. Neuroimage. 41: 903–913. [DOI] [PubMed] [Google Scholar]
- 29.Zhu H, Ibrahim JG, Tang N, Rowe DB, Hao X, Bansal R, Peterson BS (2007): A statistical analysis of brain morphology using wild bootstrapping. IEEE Trans Med Imaging. 26: 954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillaume B, Nichols TE, Adni (2015): Non-parametric Inference for Longitudinal and Repeated-Measures Neuroimaging Data with the Wild Bootstrap. Organ Hum Brain Mapp. Honolulu Retrieved June 5, 2017, from http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/presentations/ohbm2015/Guillaume-LongRepeatMeasWB-OHBM2015.pdf. [Google Scholar]
- 31.Mills KL, Tamnes CK (2014): Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci. 9: 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2: 861–863. [DOI] [PubMed] [Google Scholar]
- 33.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peper JS, Brouwer RM, Schnack HG, Van Baal GC, Van Leeuwen M, Van Den Berg SM, et al. (2009): Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 34. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Ross JL, Reiss AL, Freund L, Roeltgen D, Cutler GB (1993): Neurocognitive function and brain imaging in Turner syndrome--preliminary results. Horm Res. 39 Suppl 2: 65–9. [DOI] [PubMed] [Google Scholar]
- 36.Reiss AL, Mazzocco MMM, Greenlaw R, Freund LS, Ross JL (1995): Neurodevelopmental effects of X monosomy: A volumetric imaging study. Ann Neurol. 38: 731–738. [DOI] [PubMed] [Google Scholar]
- 37.Marzelli MJ, Hoeft F, Hong DS, Reiss AL (2011): Neuroanatomical spatial patterns in Turner syndrome. Neuroimage. 55: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q, Zhang Z, Xie S, Pan H, Zhang J, Gong G, Cui Z (2013): Cognitive impairment and gray/white matter volume abnormalities in pediatric patients with Turner syndrome presenting with various karyotypes. J Pediatr Endocrinol Metab. 26: 1111–21. [DOI] [PubMed] [Google Scholar]
- 39.Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, et al. (2010): Cortical anatomy in human X monosomy. Neuroimage. 49: 2915–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevalainen P, Lauronen L, Pihko E (2014): Development of Human Somatosensory Cortical Functions - What have We Learned from Magnetoencephalography: A Review. Front Hum Neurosci. 8: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, et al. (2014): Adolescent brain maturation and cortical folding: Evidence for reductions in gyrification. PLoS One. 9. doi: 10.1371/journal.pone.0084914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL (2006): Selective Alterations of White Matter Associated with Visuospatial and Sensorimotor Dysfunction in Turner Syndrome. J Neurosci. 26: 7007–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisk CL, Foster DL (2004): The neural basis of puberty and adolescence. Nat Neurosci. 7: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 44.Nuñez JL, Sodhi J, Juraska JM (2002): Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 52: 312–21. [DOI] [PubMed] [Google Scholar]
- 45.JURASKA JM, MARKHAM JA (2004): The Cellular Basis for Volume Changes in the Rat Cortex during Puberty: White and Gray Matter. Ann N Y Acad Sci. 1021: 431–435. [DOI] [PubMed] [Google Scholar]
- 46.Ashburner J, Friston KJ (2009): Computing average shaped tissue probability templates. Neuroimage. 45: 333–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.