Abstract
Purpose
Despite the increasing number of research studies of cardiopulmonary coupling (CPC) analysis, an electrocardiogram-based technique, the use of CPC in underserved population remains underexplored. This study aimed to first evaluate the reliability of CPC analysis for the detection of obstructive sleep apnea (OSA) by comparing with polysomnography (PSG) derived sleep outcomes.
Methods
205 PSG data (149 males, age 46.8 ± 12.8 years) were used for the evaluation of CPC regarding the detection of OSA. Automated CPC analyses were based on ECG signals only. Respiratory events index (REI) derived from CPC and apnea-hypopnea index (AHI) derived from PSG were compared for agreement tests.
Results
CPC-REI positively correlated with PSG-AHI (r = 0.851, p < 0.001). After adjusting for age and gender, CPC-REI and PSG-AHI were still significantly correlated (r = 0.840, p < 0.001). The overall results of sensitivity and specificity of CPC-REI were good.
Conclusion
Compared to the gold standard PSG, CPC approach yielded acceptable results among OSA patients. ECG recording can be used for the screening or diagnosis of OSA in the general population.
Keywords: autonomic nervous system, obstructive sleep apnea, cardiopulmonary coupling, electrocardiogram, polysomnography, portable monitoring
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a major form of sleep-disordered breathing (SDB) with an estimated prevalence ranging from 9% to 38% among the general population [1]. OSA causes or contributes to hypoxemia, hypercapnia, nocturia, sleep fragmentation, morning headaches, and excessive daytime sleepiness. It also increases the risks of cardiovascular disease, neurocognitive dysfunction, mood or psychiatric disorders, metabolic syndrome or diabetes, gastroesophageal reflux disease, impaired work performance, degraded quality of life, all-cause mortality or even sudden death. Screening to identify unrecognized OSA followed by appropriate treatment may improve sleep quality and normalize the respiration and oxygen saturation levels to prevent adverse health outcomes.
Although attended in-lab polysomnography (PSG) is the current gold standard diagnostic test for SDB, the high cost, requirement of multiple sensors during an overnight stay in the laboratory, and hours of manual scoring, make it difficult to expand services. On the other hand, there is uncertainty about the accuracy or clinical utility of all potential screening tools. There is increasing demand for simple, readily obtained and cost-effective screening approaches for OSA diagnosis, particularly in China, where OSA and its comorbid are significantly underdiagnosed [2], and the large population overwhelms its limited medical resources.
In recent years, several new techniques have been proposed for OSA screening or diagnosis [3,4]. Although most of them are portable, they require several sensors for accuracy, or scarify accuracy for easy use. Home screening tools based on a few or single signals include devices using respiratory flow and/or respiratory effort, peripheral arterial tonometry (PAT) or Watch-PAT [5–7], pulse transit time (PTT) [8], photoplethysmography (PPG) [9] and actigraphy [10]. Another approach is to detect SDB by altered heart rate dynamics. Since the autonomic nervous system (ANS) dynamics vary according to sleep depth and states [11,12], ECG-based approaches can be used for sleep studies. However, traditional heart rate variability (HRV) has many limitations [13–19], and does not distinguish the power spectra due to signal non-stationarity or cyclic variation patterns resulting from repeated sleep apneas [20]. In addition, some HRV measures (e.g. rMSSD, pNN50, HF, etc.) can be exaggerated due to scanning error (uneven beat detection, missed or misclassified beats) or from irregular HR patterns (erratic rhythm) that are not reflective of better parasympathetic nervous systems functioning. Although commonly reported and potentially meaningful, HRV measures need to be interpreted with caution [13]. Many years ago, cyclic variation of heart rate (CVHR) [21,22] was proposed as a marker of SDB because apnea/hypopnea episodes result in repeated autonomic arousals associated with cyclic changes in heart rate. More recently, a novel approach, known as Cardiopulmonary Coupling (CPC), incorporates the respiration coupling concept into HRV analysis [20], thus is able to enhance the potential diagnostic utility by “filtering” out power spectra due to non-respiratory induced heart rate changes [13].
As the CPC technique was first developed [20] and evaluated by cyclic alternating pattern identified from electroencephalography (EEG), it is often utilized as sleep stability assessment, and has been introduced in a series of publications describing potential clinical applications. The agreement in the detection of stable/unstable sleep of CPC sleep states was previously proven by comparing with EEG-based measures [20]. In addition, CPC may also be used for sleep apnea detection and classification, since SDB is associated with predictable characteristics from the ANS dynamics. By now, there are little published studies showing the sensitivity and specificity of CPC in the use of OSA detection. In this study, we aimed to evaluate the reliability of CPC analysis, and assess the agreement of the respiratory events index derived from CPC (CPC-REI) and apnea-hypopnea index derived from conventional PSG (PSG-AHI) in the general population to fill the gaps.
2. METHODS
The datasets included in this study were collected previously from clinical studies with separate protocols approved by different Institutional Review Boards (IRB) accordingly, and all experiments were performed in accordance with relevant guidelines and regulations. All the data we used in this secondary analysis study were de-identified. Therefore, additional IRB approval can be waived.
2.1. Database for PSG vs CPC comparison
The accuracy of the cardiopulmonary coupling measure was evaluated on data from two sleep centers, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, and Nanjing First Hospital. All included data were from out-patients referred for evaluation of suspected OSA. One hundred and twenty polysomnograms from each hospital were randomly acquired backward from September 2014, by using random number sequence (A001-A120 and B001-B120). The data analyzed in this study were selected using the following criteria: (1) subjects with age over 18 years, (2) standard overnight diagnostic PSG study with sleep time no less than four hours, and (3) continuous ECG signal can be extracted from the PSG recording for no less than 4 hours. Data were excluded for (1) patients with use of ventricular pacing, atrial fibrillation, severe arrhythmia, or severe comorbidities, such as symptomatic coronary heart disease, congestive heart failure, uncontrolled pulmonary disease, wearing pacemaker, or pregnancy and (2) those who currently under interventions including oxygen therapy or positive airway pressure.
2.2. Polysomnography Protocols and Scoring
All subjects from the two sleep centers underwent attended overnight PSG in the sleep laboratories. Sleep studies was performed using the Compumedics E-Series and Compumedics Siesta (Compumedics Ltd, Abbotsford, Australia), SW-SM2000C (Curative Medical Technology Inc, China), or Respironics Alice 5 Diagnostic Sleep System (Philips Respironics, United States). PSG montages were placed according to American Academy of Sleep Medicine (AASM) recommendations [23], at least including six EEG channels, two electromyogram channels, a vibration snore sensor, nasal pressure airflow, oronasal thermocouple, submental electromyography, one ECG channel, dual thoracoabdominal respiratory inductance plethysmography belts, finger pulse oximetry, bilateral anterior tibialis electromyography, and body position.
All PSG studies were independently and manually scored by three registered polysomnographic technologists (RPSGT) following the AASM recommendations (AASM Manual for the Scoring of Sleep and Associated Events, version 2.3) [23]. Every PSG recording was scored by two RPSGTs. To reduce inter-scorer difference, once disagreement occurred between the two RPSGTs, the third RPSGT will involve, and the scoring agreed by two RPSGTs was used as the final result. All the RPSGTs were blind to any results from CPC analyses. After manual PSG scoring and CPC automated analyses were completed, the results were collected by an independent statistician for agreement analysis.
An apneic event was defined when all of the following criteria are met [23,9]: (a) there is a drop in the peak signal excursion by ≥ 90% of pre-event baseline using an oronasal thermal sensor; (b) the duration of the ≥ 90% drop in sensor signal lasts at least the minimum duration as specified by obstructive, mixed, or central apnea duration criteria; and (c) the event meets respiratory effort criteria for obstructive, central or mixed apnea. Hypopnea is scored if all of the following criteria are met: (a) the peak signal excursions drop by ≥ 30% of pre-event baseline using nasal pressure or an alternative hypopnea sensor; (b) the duration of the ≥ 30% drop in signal excursion is ≥ 10 seconds; and (c) there is a ≥ 3% oxygen desaturation from pre-event baseline or the event is associated with an arousal.
PSG derived apnea-hypopnea index (PSG-AHI) was defined as the total number of apnea and hypopnea events per hour of sleep, and OSA diagnosis is based on the International Classification of Sleep Disorders, Third Edition (ICSD-3) [24]. Mild, moderate and severe OSA were defined using PSG-AHI cut-off points of 5, 15 and 30 respectively [25].
2.3. Cardiopulmonary Coupling Analysis
The Cardiopulmonary Coupling analysis technique is based on two features of a continuous ECG signal: heart rate variability and the respiratory modulation of QRS waveform on a beat-to-beat basis. These signals have two basic patterns: a high-frequency component due to physiological sinus arrhythmia that reflects intra-breath fluctuations and a low-frequency component that reflected cyclic variation across multiple breaths. The cross-power and coherence between these two signals can be calculated as a way of quantification of cardiac and respiratory interactions. By analyzing coupling between heart rate variability (HRV) and ECG-derived respiration (EDR), the CPC algorithm can generate an ECG-derived sleep spectrogram. Physiological sleep states derived from CPC analysis include stable sleep (indicated by high frequency coupling, or HFC, 0.1–0.4 Hz), unstable or fragmented sleep (indicated by low frequency coupling, or LFC, 0.01–0.1 Hz), and REM/wakeful states (indicated by very low frequency coupling, or VLFC, 0.001–0.01 Hz). Elevated-low frequency coupling (e-LFC), a subset of LFC, reflects predominant obstructive upper airway and sustained strong chemoreflex effects on sleep respiration [26]. When sleep apnea occurs, heart rate typically shows cyclic increases and decreases associated with apneic phase and resumption of breathing. Therefore, respiratory events during sleep can be detected and quantified. CPC derived respiratory event index (CPC-REI) is defined as the total number of respiratory events per hour of sleep. It is an automated measure detecting the changes in heart rate that occur during apneas, called Cyclic Variation of Heart Rate (CVHR), which consists of bradycardia during apnea followed by abrupt tachycardia near the end of the apnea [27]. CPC-REI was defined as the total number of respiratory events per hour of sleep.
2.4. Statistical Analyses
Statistical analyses were performed using SPSS version 19.0 (IBM SPSS Statistics, NY, United States), and receiver operating characteristic (ROC) analysis was done using R (R 3.4.0 for Windows). Descriptive statistics were reported as mean ± standard deviation (SD) for normal distributed data, or median [interquartile range (IQR)] for skewed data, and number (percentage) for categorical data. Correlations between continuous variables were tested by Pearson correlation. Partial correlation and linear regression models were used to adjust covariates. Comparisons of categorical variables were made using the chi-squared or Fisher’s exact test. Comparisons of continuous variables were assessed by t-test or non-parametric test (Mann-Whitney U). Agreement analysis included calculations of sensitivity, specificity, positive and negative likelihood ratios (LR+, LR−), negative predictive value, positive predictive value, accuracy and Kappa test by SPSS using the PSG-AHI as the referenced standard. Analyses were performed based on the diagnosis by comparing the final OSA severity categorization (normal, mild, moderate, severe) of CPC-REI and PSG-AHI. Bland Altman plot and ROC curves were generated to show the agreement between CPC-REI and PSG-AHI. All statistical tests were 2-tailed and a p value <0.05 was considered statistically significant.
3. RESULTS
3.1. Subject Characteristics
A total of 205 overnight attended polysomnograms were included in the study. Demographic characters of the included subjects from the test database were shown in Table 1. As for OSA severity indicated by PSG-AHI, there were 49 (23.9%) normal subjects, 29 (14.1%) mild, 33 (16.1%) moderate, and 94 (45.9%) severe OSA among all.
Table 1.
Characteristics of the study cohort.
| All subjects | non-OSA | Mild OSA | Moderate OSA | Severe OSA | |
|---|---|---|---|---|---|
| Demographics | |||||
| N (%) | 205 (100%) | 49 (23.9%) | 29 (14.1%) | 33 (16.1%) | 94 (45.9%) |
| Sex, F/M | 56/149 | 22/27 | 7/22 | 9/24 | 18/76 |
| Age, yr | 46.8 ± 12.8 | 41.1 ± 13.0 | 44.9 ± 13.7 | 51.5 ± 14.2 | 48.7 ± 10.9 |
| BMI, kg/m2 | 27.53 ± 4.28 | 25.20 ± 3.38 | 26.57 ± 5.30 | 27.46 ± 4.40 | 29.22 ± 4.28 |
| Sleep measures | |||||
| TIB, min | 498.6 ± 52.6 | 489.5 ± 42.4 | 481.6.0 ± 52.0 | 504.6 ± 46.9 | 506.8 ± 58.2 |
| PSG-TST, min | 357.3 ± 91.7 | 387.5 ± 56.0 | 368.0 ± 87.5 | 366.6 ± 98.4 | 333.8 ± 101.2 |
| Sleep latency (min) | 20.4 ± 41.4 | 26.2 ± 35.3 | 42.0 ± 91.3 | 16.3 ± 18.5 | 13.7 ± 24.7 |
| Sleep efficiency (%) | 70.2 ± 16.7 | 70.2 ± 15.6 | 65.8 ± 24.5 | 70.5 ± 16 | 71.2 ± 15.1 |
| N1 (%) | 32.4 ±18.2 | 27.0 ± 12.5 | 31.9 ± 20.9 | 34.6 ± 21.7 | 34.2 ± 18.3 |
| N2 (%) | 44.2 ± 13.8 | 50.4 ± 12.0 | 41.5 ± 13.9 | 42.0 ± 14.8 | 42.9 ± 13.7 |
| N3 (%) | 15.4 ± 14.0 | 15.5 ± 10.8 | 17.8 ± 16 | 12.2 ± 13.5 | 15.7 ± 14.8 |
| REM (%) | 7.8 ± 8.9 | 7.3 ± 9.8 | 9.2 ± 9.9 | 11.1 ± 10.4 | 6.6 ± 7.6 |
| Apnea/hypopnea related measures | |||||
| PSG-AHI, /h | 30.5 (0.1–96.0) | 2.4 ± 1.5 | 10.8 ± 2.5 | 22.0 ± 3.8 | 54.3 ± 17.1 |
| CPC-REI, /h | 27.0 (0–107.5) | 3.6 ± 5.8 | 8.1 ± 9.6 | 19.1 ± 16.1 | 47.7 ± 24.5 |
| 3% ODI, /h | 31.1 ± 28.3 | 5.2 ± 13.9 | 7.7 ± 4.7 | 21.8 ± 13.3 | 50.2 ± 24.4 |
| SaO2 nadir, % | 77.4 ± 16.5 | 90.0 ± 5.9 | 86.1 ± 6.0 | 82.2 ± 6.6 | 68.5 ± 18.3 |
| RERA, /h | 7.6 ± 13.2 | 1.2 ± 1.4 | 2.9 ± 4.5 | 5.7 ± 5.4 | 12.3 ± 17.1 |
Data are presented as mean ± standard deviation of the mean if normally distributed, or presented as median (interquartile range) if skewed; AHI, apnea-hypopnea index; BMI, body mass index; CPC, cardiopulmonary coupling; OSA, obstructive sleep apnea; ODI, oxygen desaturation index; PSG, polysomnogram; RERA, respiratory effort related arousal; SaO2, oxygen saturation; TIB, time in bed; TST, total sleep time; REI, respiratory event index.
3.2. Correlations between PSG-based and CPC-based Measures
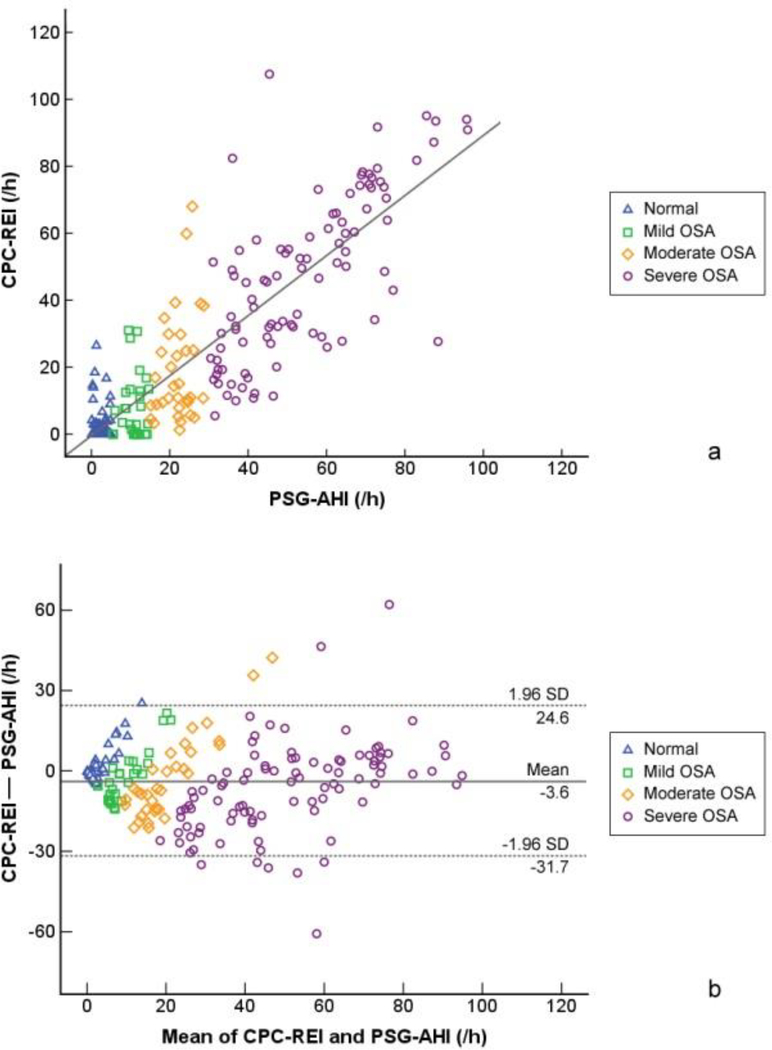
As shown in Figure 1a, significant correlations were found between CPC-REI and PSG-AHI (r = 0.851, p < 0.001). After adjusting for age, gender and TST, CPC-REI and PSG-AHI were still significantly correlated (r = 0.838, p < 0.001). Although the Bland-Altman plots (Figure 1b) showed that most estimates were within two standard deviations of the mean, CPC-REI seemed to be overestimating normal and mild individuals.
Figure 1.
Comparison of CPC-REI and PSG-AHI. (a) Correlation of CPC-REI and PSG-AHI. The correlation coefficient (r) of CPC-REI and PSG-AHI is 0.851 (p < 0.001). After controlling for age and gender, the correlation coefficient (r) is 0.840 (p < 0.001). (b) Bland Altman plot for agreement of CPC-REI and PSG-AHI.
3.3. Diagnostic Accuracy of the CPC Measures
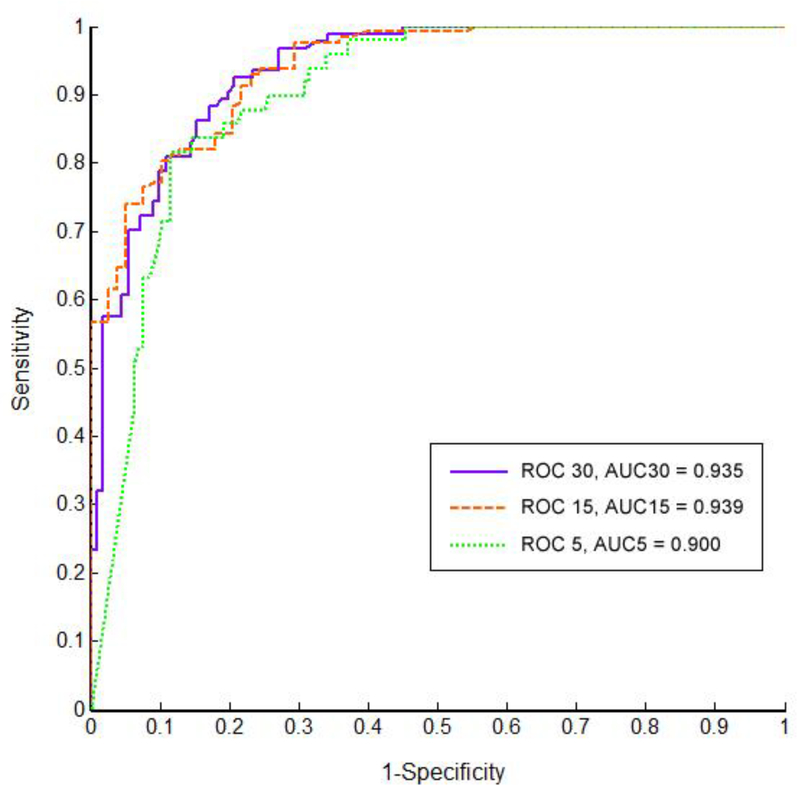
Compared with PSG-AHI, the performance of automated CPC analysis regarding the diagnostic accuracy of CPC-REI was evaluated by sensitivity, specificity, positive and negative predictive values, agreement, positive and negative likelihood ratios, and Kappa value (as shown in Table 2). By ROC analysis (Figure 2), the three curves were shown with the AHI cut-off points set at 5, 15 and 30 events/hour, and area under the curve (AUC) is 0.900, 0.939 and 0.935 respectively. According to ROC analysis, cut-off points of CPC-REI to distinguish none, mild, moderate and severe sleep apnea were found to be 4.5, 14.2 and 19.2 events/hour, respectively. Accordingly, the sensitivities are 88.5%, 81.1%, 86.2%, and specificities are 81.6%, 88.5%, 84.7%, respectively. Since CPC-REI and PSG-AHI were significantly correlated, and most of the current alternative approaches still take the same cut-off points as PSG-AHI (5, 15, and 30 events/hour), we also analyzed the diagnostic accuracy of CPC-REI using the same cut-off points (Table 2). For mild+ (AHI⩾5), moderate+ (AHI⩾15) and severe (AHI⩾30) sleep apnea, CPC presented a sensitivity of 93.8%, 92.7% and 89.5%, while a specificity of 67.8%, 72.2% and 79.8%, respectively.
Table 2.
Diagnostic accuracy of CPC-REI compared to PSG-AHI.
| Groups based PSG-AHI | AUC | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | LR+ | LR− | Kappa |
|---|---|---|---|---|---|---|---|---|---|---|
| AHI<5 vs AHI≥5 | 0.900 | 0.858–0.943 | 93.8 | 67.8 | 87.8 | 81.6 | 86.3 | 2.91 | 0.09 | 0.649 |
| AHI<15 vs AHI≥15 | 0.939 | 0.909–0.969 | 92.7 | 72.2 | 78.9 | 89.7 | 83.0 | 3.33 | 0.10 | 0.663 |
| AHI<30 vs AHI≥30 | 0.935 | 0.904–0.966 | 89.5 | 79.8 | 72.3 | 92.8 | 83.4 | 4.44 | 0.13 | 0.735 |
AHI, apnea-hypopnea index; AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; LR, likelihood ratio; +, positive; −, negative.
Figure 2.
The receiver operating characteristic curves for CPC-REI versus PSG-AHI.
3.4. Correlations of OSA severity and PSG- or CPC-derived parameters
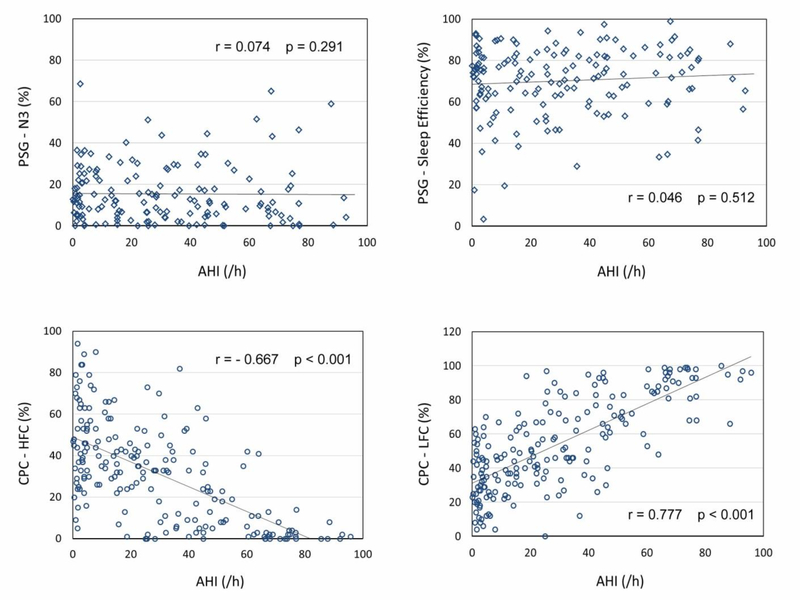
The parameters derived from PSG and CPC were included in correlation analyses (Table 3). Weak correlations were found between PSG-AHI and N1, N2, REM sleep, while no correlations were found between PSG-AHI and N3 sleep or sleep efficiency (Figure 3). In contrast, significantly strong correlations were found between PSG-AHI and all CPC derived parameters (e.g., CPC-REI, HFC, LFC, VLFC). PSG-AHI is negatively correlated with HFC (r = −0.641, p < 0.001, Figure 3) and VLFC (r = −0.412, p < 0.001), and positively correlated with LFC (r = 0.759, p < 0.001, Figure 3) with adjustment for age, gender, total sleep time (TST).
Table 3.
Correlations of PSG- or CPC-derived parameters.
| PSG-AHI | N1 | N2 | N3 | REM | ||
|---|---|---|---|---|---|---|
| PSG-AHI | unadjusted | 1 | 0.222* (0.001) | −0.257* (<0.001) | 0.074 (0.291) | −0.204* (0.003) |
| adjusted | 1 | 0.196* (0.005) | −0.235* (0.001) | 0.104 (0.139) | −0.198* (0.005) | |
| CPC-REI | unadjusted | 0.851* (<0.001) | 0.142* (0.042) | −0.195* (0.005) | 0.129 (0.065) | −0.222* (0.001) |
| adjusted | 0.838* (<0.001) | 0.103 (0.143) | −0.167* (0.017) | 0.167* (0.018) | −0.223* (0.001) | |
| HFC | unadjusted | −0.667* (<0.001) | −0.192* (0.006) | 0.265* (<0.001) | −0.062 (0.381) | 0.109 (0.118) |
| adjusted | −0.642* (<0.001) | −0.135 (0.056) | 0.236* (0.001) | −0.106 (0.133) | 0.080 (0.260) | |
| LFC | unadjusted | 0.777* (<0.001) | 0.214* (0.002) | −0.253* (<0.001) | 0.075 (0.287) | −0.197* (0.005) |
| adjusted | 0.759* (<0.001) | 0.147* (0.037) | −0.218* (0.002) | 0.1328 (0.060) | −0.175* (0.013) | |
| VLFC | unadjusted | −0.454* (<0.001) | −0.120 (0.088) | 0.043 (0.544) | −0.042 (0.550) | 0.260* (<0.001) |
| adjusted | −0.410* (<0.001) | −0.065 (0.355) | −0.002 (0.975) | −0.079 (0.264) | 0.258* (<0.001) | |
Abbreviations: PSG-AHI, apnea-hypopnea index derived from polysomnogram; CPC-REI, respiratory event index derived from cardiopulmonary coupling analysis; HFC, high frequency coupling; LFC, low frequency coupling; VLFC, very low frequency coupling.
Data reported in this table are results from correlation analyses, presented as r (p), where r is the Pearson correlation coefficient. Adjusted partial correlations were analyzed by controlling for age, gender and total sleep time.
indicates statistical significance when p < 0.05.
Figure 3.
Scatter plots to show correlations between AHI and sleep parameters derived from PSG and CPC.
4. DISCUSSION
This study included 205 subjects with PSG-based sleep studies. The performance of CPC-REI was compared with PSG-AHI. In moderate and severe OSA patients, CPC-based diagnostic accuracy was 83.0%, suggesting that the screening results from CPC were consistent with the currently recommended criteria for portable monitoring device to “rule-in” OSA (AHI ≥ 15 events/hour) in clinical settings [3]. In a recently published study with smaller sample size, Hilmisson et al [28] performed a similar evaluation on the accuracy of CPC and they reported that CPC identified patients with moderate to severe sleep apnea with sensitivity of 100%, specificity of 81%, agreement of 93%, and kappa of 0.85 compared with manual scoring of AHI.
Our results in the Chinese population are in line with existing evidence from Caucasians to support the reliability of CPC as a screening tool for sleep apnea. For sleep apnea detection, a recent study combined CPC and CVHR in the ambulatory screening for sleep apnea and found a high degree of agreement between the CPC+CVHR algorithms against both the manually rescored AHI (sensitivity 89%, specificity 79%, agreement 85%) and the computerized scored AHI (sensitivity 93%, specificity 79%, agreement 87%) to identify patients with moderate and severe sleep apnea (AHI > 15) [21]. For sleep quality in sleep apneic patients, Harrington et al [29] compared PSG- versus CPC-based sleep measures, and reported that CPC can be used to study sleep quality in patients with OSA, and distinguish successful and unsuccessful continuous positive airway pressure (CPAP) response. All these evidence yields comparable or more reliable results to other screening tools.
Beyond the screening of SDB, since the early publication on CPC technique [20], single-channel ECG has been used in many sleep-related studies, including sleep stability and quality in a broad range of conditions (e.g. insomnia [30–32], depression [33–36], fibromyalgia [37], heritability of abnormalities [38], etc), subtypes of SDB [39], assessment for treatment options [40,41], pre- and post-treatment evaluation [42–45].
EEG-based recording allows multiple approaches to analyze the dynamic changes of brain activities during sleep [46–51]. Our results indicate that ECG also provides possibilities to study sleep differently; an ECG-based monitor can be used to screen OSA in the general population., Main advantages of using ECG monitor as an OSA screening tool include (1) It reduces the number of electrodes attached to patients, and it is significantly easier for the patients, as there are only 3 electrodes that need to be attached to a patient for a one-lead ECG; (2) The algorithm is completely automated, no time-consuming manual scoring is needed, which also avoids inter-scorer discrepancies; and (3) The CPC technique can also provide valuable measures (e.g., HFC, LFC) that are related to sleep structure and quality.
PSG scoring rules require that the change of breathing has to meet the criteria (e.g. length and amplitude) to be marked as a respiratory event. By CPC techniques, when power on the e-LFC bands was detected, a respiratory event was automatically marked. Some events with breathing disturbance that barely meet the “at least 10-second” rule may be sensitively detected by CPC, leading to a possible overestimation by CPC-REI. In addition, we also noticed the lack of correlation between CPC-HFC and N3 sleep, which may be because effective sleep is restorative and spans both conventional SWS and periods of stage N2 [52]. Respiratory effort- related arousal (RERA) may induce LFC/e-LFC can cause discrepancy in such analyses. Further investigations on the correlation between RERA and CPC outcomes are encouraged.
Evidence from systematic reviews show that sensitivities decreased and specificities increased for detecting moderate or greater OSA (AHI≥15) or severe OSA (AHI≥30). The ranges of sensitivity and specificity reported across studies for type IV monitors were wide [53,9]. To meet the demand of home sleep testings, other alternative approaches are currently available with recording of different physiological signals. Actigraphy may assist to distinguish wake and sleep during the night, thus the combination of actigraphy and respiratory recording may allow a more accurate AHI or REI. The use of oximetry enables proper risk stratification, and can be added to OSA screening in the general population [54]. Wristband-based and ECG-based sleep analyses may provide comparable results in the screening of SDB [55]. An appropriate combination of the methods for the targeted population can improve the diagnostic accuracy and provide complementary values in clinical interventions [9].
A preferred approach for sleep evaluation is that the measures can objectively illustrate how abnormalities impact sleep quality or sleep stability. For example, sleep apnea is known to worsen deep sleep and sleep continuity. However, our results showed that PSG-AHI and slow wave sleep (i.e., N3 stage) is not correlated. This result is in agreement with the criticism that conventional sleep stages often cannot reflect disease severity [56–59]. In contrast, significant correlations have been found between PSG-AHI and HFC, LFC, VLFC derived from CPC, suggesting that a higher AHI negatively impact stable sleep or deep sleep, positively increase unstable sleep, which is in line with the existing findings from the clinical practice. Implying that CPC measures, which focus on physiologic aspects of sleep instead of EEG morphology, can bring valuable insight to a quantitative description of sleep quality.
SDB was linked to a higher prevalence of metabolic syndrome [60] and abnormal blood pressure patterns [2] in the general population. Therefore, large-scale screening of high risk population to identify subjects with SDB for appropriate management is warranted [60,2]. Previous studies have found ECG-based approach to be cost-efficient and may provide clinical insight into abnormal sleep, because it can be used in various populations, illustrate sleep states (stable or unstable sleep), and provide reliable estimation of SDB [61]. Our findings suggest that CPC offers opportunity for OSA screening or diagnosis to be simple, cost-effective, and less resource-intensive, and can also be potentially for monitoring the efficacy of intervention.
While we found significant association between CPC-REI and PSG-AHI, we were unable to evaluatethe compliance of ECG monitoring at home settings. However, previous studies using ECG at home may support that CPC techniques can be used for home monitoring. The predicting value in OSA screening is worth further studies. Second, in our analyses, only CPC-REI measures with PSG outcomes were compared. Future comparison studies are encouraged to include different portable approaches for the use of home testing. A combination of CPC with other signal-based measures may further improve the accuracy of home diagnosis, and such studies are encouraged given the increasing needs for SDB screening. For example, there are many situations when knowing the oxygenation profile matters, including for severity, which the ECG technique is blind to, including deep REM desaturation and hypoventilation. When ECG recording is combined with actigraphy, the accuracy of sleep period estimates may be improved. Third, the exclusion of subjects with comorbidities (e.g. symptomatic coronary heart disease, congestive heart failure, uncontrolled pulmonary disease) may limit the generalizability of this approach to the general population. CPC techniques are not applicable for patients with cardiac arrhythmia, especially atrial fibrillation and continuous bigeminy, and for patients using some medications like beta blockers. Future studies are encouraged to investigate whether this technique is also applicable across patients with these types of common comorbidities or conditions. In addition, severely fragmented sleep from other causes may cause an apnea-like signature on the CPC spectrum. Proper context (e.g. snoring, sleepiness, etc.) should be considered for clinical differential diagnosis.
5. CONCLUSION
The performance of CPC techniques with automated estimation of REI was shown with reliable diagnostic values that are consistent with standard PSG measures. CPC-REI has significant correlation and good agreement with PSG-AHI, and the sensitivity, specificity, positive and negative predictive values indicated good accuracy on the detection of respiratory events from ECG recordings. The use of ECG signals allow the possibilities for simple, less resource-intensive and cost-effective methods for OSA screening or treatment follow-ups.
Acknowledgements
This work was supported by grants from the National Institutes of Health of the United States (T32AT000051), and from National Natural Science Foundation of China (81273820). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NSFC.
Abbreviations
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AUC
area under the curve
- CPC
cardiopulmonary coupling
- e-LFC
elevated-low frequency coupling
- ECG
electrocardiogram
- EEG
electroencephalography
- FDA
Food and Drug Administration
- HFC
high-frequency coupling
- HRV
heart rate variability
- IRB
Institutional Review Board
- LFC
low frequency coupling
- LR
likelihood ratio
- NPV
negative predictive value
- OCST
out of center sleep testing
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PSG
polysomnography
- REI
respiratory events index
- ROC
receiver operating characteristic
- RPSGT
registered polysomnographic technologists
- SD
standard deviation
- SDB
sleep-disordered breathing
- TIB
time in bed
- TST
total sleep time
- VLFC
very low frequency coupling
Footnotes
Conflict of Interest:
Chung-Kang Peng is a co-patent holder for the ECG-based analytic technique for phenotyping sleep and sleep apnea, known as Cardiopulmonary Coupling (CPC) analysis. He also receives royalties from a license issued by Beth Israel Deaconess Medical Center to MyCardio, LLC. The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Yan Ma declares that she has no conflict of interest. Shuchen Sun declares that he has no conflict of interest. Ming Zhang declares that he has no conflict of interest. Dan Guo declares that she has no conflict of interest. Arron Runzhou Liu declares that he has no conflict of interest. Yulin Wei declares that she has no conflict of interest.
Compliance with Ethical Standards
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval and informed consent:
The datasets included in this study were collected previously from clinical studies with separate protocols approved by different Institutional Review Boards (IRB) accordingly, and all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the studies. All the data we used in this secondary analysis study were de-identified. Therefore, additional IRB approval was waived.
Comments:
With the caveats of limitations, cardiopulmonary coupling patterns may be a useful approach to screen for apnea in high risk populations. However, the addition of oximetry to the assessment would be complementary - thus those with mildly hypoxic disease will be detected, while those with severe hypoxia can be risk stratified.
Robert Thomas
MA, USA
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC (2016) Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep medicine reviews. doi: 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Sun S, Peng CK, Fang Y, Thomas RJ (2017) Ambulatory Blood Pressure Monitoring in Chinese Patients with Obstructive Sleep Apnea. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 13 (3):433–439. doi: 10.5664/jcsm.6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R (2007) Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 3 (7):737–747 [PMC free article] [PubMed] [Google Scholar]
- 4.Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA, Ojile JM (2011) Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 7 (5):531–548. doi: 10.5664/jcsm.1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuceege M, Firat H, Demir A, Ardic S (2013) Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 9 (4):339–344. doi: 10.5664/jcsm.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkuyu E, Duzlu M, Karamert R, Tutar H, Yilmaz M, Ciftci B, Guven SF (2015) The efficacy of Watch PAT in obstructive sleep apnea syndrome diagnosis. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology – Head and Neck Surgery 272 (1):111–116. doi: 10.1007/s00405-014-3097-0 [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Lee B, Lee JY, Kim HJ (2018) Validating the Watch-PAT for Diagnosing Obstructive Sleep Apnea in Adolescents. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 14 (10):1741–1747. doi: 10.5664/jcsm.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehring J, Gesche H, Drewniok G, Kuchler G, Patzak A (2018) Nocturnal blood pressure fluctuations measured by using pulse transit time in patients with severe obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung 22 (2):337–343. doi: 10.1007/s11325-017-1555-9 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Gao H, Ma Y (2017) Evaluation of pulse oximeter derived photoplethysmographic signals for obstructive sleep apnea diagnosis. Medicine 96 (18):e6755. doi: 10.1097/md.0000000000006755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr., Coleman J, Lee-Chiong T, Pancer J, Swick TJ (2007) Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30 (4):519–529 [DOI] [PubMed] [Google Scholar]
- 11.Dumont M, Jurysta F, Lanquart JP, Migeotte PF, van de Borne P, Linkowski P (2004) Interdependency between heart rate variability and sleep EEG: linear/non-linear? Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 115 (9):2031–2040. doi: 10.1016/j.clinph.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, Garcia C, Glos M, Fietze I, Schobel C (2016) Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography. Frontiers in physiology 7:460-460. doi: 10.3389/fphys.2016.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein PK, Pu Y (2012) Heart rate variability, sleep and sleep disorders. Sleep medicine reviews 16 (1):47–66. doi: 10.1016/j.smrv.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 14.Hill LK, Siebenbrock A (2009) Are all measures created equal? Heart rate variability and respiration - biomed 2009. Biomedical sciences instrumentation 45:71–76 [PubMed] [Google Scholar]
- 15.Billman GE (2011) Heart rate variability - a historical perspective. Frontiers in physiology 2:86. doi: 10.3389/fphys.2011.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaffer F, McCraty R, Zerr CL (2014) A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Frontiers in psychology 5:1040. doi: 10.3389/fpsyg.2014.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, McCraty R (2016) Heart rate variability in mind-body interventions. Complementary therapies in medicine 29:A1–A2. doi: 10.1016/j.ctim.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Tseng PH, Ahn A, Wu MS, Ho YL, Chen MF, Peng CK (2017) Cardiac Autonomic Alteration and Metabolic Syndrome: An Ambulatory ECG-based Study in A General Population. Scientific reports 7:44363. doi: 10.1038/srep44363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Wu CW, Peng CK, Ahn A, Bertisch SM, Lipsitz LA, Yeh GY, Manor B, Novak V, Hausdorff JM, Gow B, Wayne PM (2019) Complexity-Based Measures of Heart Rate Dynamics in Older Adults Following Long- and Short-Term Tai Chi Training: Cross-sectional and Randomized Trial Studies. Scientific reports 9 (1):7500. doi: 10.1038/s41598-019-43602-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas RJ, Mietus JE, Peng CK, Goldberger AL (2005) An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 28 (9):1151–1161 [DOI] [PubMed] [Google Scholar]
- 21.Magnusdottir S, Hilmisson H (2018) Ambulatory screening tool for sleep apnea: analyzing a single-lead electrocardiogram signal (ECG). Sleep & breathing = Schlaf & Atmung 22 (2):421–429. doi: 10.1007/s11325-017-1566-6 [DOI] [PubMed] [Google Scholar]
- 22.Hayano J, Watanabe E, Saito Y, Sasaki F, Fujimoto K, Nomiyama T, Kawai K, Kodama I, Sakakibara H (2011) Screening for obstructive sleep apnea by cyclic variation of heart rate. Circulation Arrhythmia and electrophysiology 4 (1):64–72. doi: 10.1161/circep.110.958009 [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 2016; American Academy of Sleep Medicine, Illinois:Version 2.3. Darien. [Google Scholar]
- 24.American Academy of Sleep Medicine. International classification of sleep disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; (2014). [Google Scholar]
- 25.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force (1999). Sleep 22 (5):667–689 [PubMed] [Google Scholar]
- 26.Gupta MA, Simpson FC (2015) Obstructive sleep apnea and psychiatric disorders: a systematic review. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 11 (2):165–175. doi: 10.5664/jcsm.4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A (1984) Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet (London, England) 1 (8369):126–131 [DOI] [PubMed] [Google Scholar]
- 28.Hilmisson H, Lange N, Duntley SP (2018) Sleep apnea detection: accuracy of using automated ECG analysis compared to manually scored polysomnography (apnea hypopnea index). Sleep & breathing = Schlaf & Atmung. doi: 10.1007/s11325-018-1672-0 [DOI] [PubMed] [Google Scholar]
- 29.Harrington J, Schramm PJ, Davies CR, Lee-Chiong TL Jr. (2013) An electrocardiogram-based analysis evaluating sleep quality in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung 17 (3):1071–1078. doi: 10.1007/s11325-013-0804-9 [DOI] [PubMed] [Google Scholar]
- 30.Thomas RJ, Wood C, Bianchi MT (2017) Cardiopulmonary coupling spectrogram as an ambulatory clinical biomarker of sleep stability and quality in health, sleep apnea and insomnia. Sleep. doi: 10.1093/sleep/zsx196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilmisson H, Sveinsdottir E, Lange N, Magnusdottir S (2019) Insomnia symptoms in primary care: A prospective study focusing on prevalence of undiagnosed co-morbid sleep disordered breathing. European journal of internal medicine. doi: 10.1016/j.ejim.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 32.Schramm PJ, Thomas R, Feige B, Spiegelhalder K, Riemann D (2013) Quantitative measurement of sleep quality using cardiopulmonary coupling analysis: a retrospective comparison of individuals with and without primary insomnia. Sleep & breathing = Schlaf & Atmung 17 (2):713–721. doi: 10.1007/s11325-012-0747-6 [DOI] [PubMed] [Google Scholar]
- 33.Yang AC, Yang CH, Hong CJ, Tsai SJ, Kuo CH, Peng CK, Mietus JE, Goldberger AL, Thomas RJ (2011) Sleep state instabilities in major depressive disorder: Detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiology 48 (2):285–291. doi: 10.1111/j.1469-8986.2010.01060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schramm PJ, Poland RE, Rao U (2014) Bupropion response on sleep quality in patients with depression: implications for increased cardiovascular disease risk. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24 (2):207–214. doi: 10.1016/j.euroneuro.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Yeung A, Yang AC, Peng CK, Clain A, Alpert J, Fava M, Yeung AS (2018) The Effects of Tai Chi on Sleep Quality in Chinese American Patients With Major Depressive Disorder: A Pilot Study. Behavioral sleep medicine 16 (4):398–411. doi: 10.1080/15402002.2016.1228643 [DOI] [PubMed] [Google Scholar]
- 36.Schramm PJ, Zobel I, Monch K, Schramm E, Michalak J (2016) Sleep quality changes in chronically depressed patients treated with Mindfulness-based Cognitive Therapy or the Cognitive Behavioral Analysis System of Psychotherapy: a pilot study. Sleep medicine 17:57–63. doi: 10.1016/j.sleep.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 37.Thomas RJ, Mietus JE, Peng CK, Goldberger AL, Crofford LJ, Chervin RD (2010) Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep medicine 11 (5):497–498. doi: 10.1016/j.sleep.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim LH, Jacono FJ, Patel SR, Thomas RJ, Larkin EK, Mietus JE, Peng CK, Goldberger AL, Redline S (2010) Heritability of abnormalities in cardiopulmonary coupling in sleep apnea: use of an electrocardiogram-based technique. Sleep 33 (5):643–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, Gottlieb DJ (2007) Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep 30 (12):1756–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schramm PJ, Thomas RJ (2012) Assessment of therapeutic options for mild obstructive sleep apnea using cardiopulmonary coupling measures. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 8 (3):315–320. doi: 10.5664/jcsm.1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WH, Hong SN, Kim HJ, Rhee CS, Lee CH, Yoon IY, Kim JW (2016) A Comparison of Different Success Definitions in Non-Continuous Positive Airway Pressure Treatment for Obstructive Sleep Apnea Using Cardiopulmonary Coupling. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 12 (1):35–41. doi: 10.5664/jcsm.5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi JH, Thomas RJ, Suh SY, Park IH, Kim TH, Lee SH, Lee HM, Yun CH, Lee SH (2015) Sleep quality change after upper airway surgery in obstructive sleep apnea: Electrocardiogram-based cardiopulmonary coupling analysis. The Laryngoscope 125 (7):1737–1742. doi: 10.1002/lary.25101 [DOI] [PubMed] [Google Scholar]
- 43.Lee WH, Ahn JC, We J, Rhee CS, Lee CH, Yun PY, Yoon IY, Kim JW (2014) Cardiopulmonary coupling analysis: changes before and after treatment with a mandibular advancement device. Sleep & breathing = Schlaf & Atmung 18 (4):891–896. doi: 10.1007/s11325-014-0961-5 [DOI] [PubMed] [Google Scholar]
- 44.Ramar K, Desrues B, Ramar P, Morgenthaler TI (2013) Analysis of cardiopulmonary coupling to assess adaptive servo-ventilation success in complex sleep apnea management. Sleep & breathing = Schlaf & Atmung 17 (2):861–866. doi: 10.1007/s11325-012-0780-5 [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Choi JH, Park IH, Lee SH, Kim TH, Lee HM, Park HK, Thomas RJ, Shin C, Yun CH (2012) Measuring sleep quality after adenotonsillectomy in pediatric sleep apnea. The Laryngoscope 122 (9):2115–2121. doi: 10.1002/lary.23356 [DOI] [PubMed] [Google Scholar]
- 46.Ma Y, Hou F, Yang AC, Ahn AC, Fan L, Peng C-K (2019) Symbolic dynamics of electroencephalography is associated with the sleep depth and overall sleep quality in healthy adults. Physica A: Statistical Mechanics and its Applications 513:22–31. doi: 10.1016/j.physa.2018.08.043 [DOI] [Google Scholar]
- 47.Xiong H, Shang P, Hou F, Ma Y (2019) Visibility graph analysis of temporal irreversibility in sleep electroencephalograms. Nonlinear Dynamics 96 (1):1–11. doi: 10.1007/s11071-019-04768-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou F, Yu Z, Peng CK, Yang A, Wu C, Ma Y (2018) Complexity of Wake Electroencephalography Correlates With Slow Wave Activity After Sleep Onset. Frontiers in neuroscience 12:809. doi: 10.3389/fnins.2018.00809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao X, Shang P, Wang J, Ma Y (2018) Characterizing time series by extended complexity-entropy curves based on Tsallis, Renyi, and power spectral entropy. Chaos (Woodbury, NY) 28 (11):113106. doi: 10.1063/1.5038758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y, Shi W, Peng CK, Yang AC (2018) Nonlinear dynamical analysis of sleep electroencephalography using fractal and entropy approach Sleep medicine reviews 37:85–93. doi: 10.1016/j.smrv.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 51.Shi W, Shang P, Ma Y, Sun S, Yeh C-H (2017) A comparison study on stages of sleep: Quantifying multiscale complexity using higher moments on coarse-graining. Communications in Nonlinear Science and Numerical Simulation 44:292–303. doi: 10.1016/j.cnsns.2016.08.019 [DOI] [Google Scholar]
- 52.Thomas RJ, Mietus JE, Peng CK, Guo D, Gozal D, Montgomery-Downs H, Gottlieb DJ, Wang CY, Goldberger AL (2014) Relationship between delta power and the electrocardiogram-derived cardiopulmonary spectrogram: possible implications for assessing the effectiveness of sleep. Sleep medicine 15 (1):125–131. doi: 10.1016/j.sleep.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, Lux L, Harris RP (2017) Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 317 (4):415–433. doi: 10.1001/jama.2016.19635 [DOI] [PubMed] [Google Scholar]
- 54.Ma Y, Yeh J, Sun S, Qiao J, Peng C (2013) Detecting pediatric sleep apnea: consistency on cardiopulmonary coupling and oximetry measurement. Sleep medicine 14:e189 [Google Scholar]
- 55.Ma Y, Wei Y, Peng C-K (2016) Comparison of wristband-based and ECG-based sleep analyses. Journal of Sleep Disorders & Therapy. doi: 10.4172/2167-0277.C1.005 [DOI] [Google Scholar]
- 56.Young A, Home M, Churchward T, Freezer N, Holmes P, Ho M (2002) Comparison of sleep disturbance in mild versus severe Parkinson’s disease. Sleep 25 (5):573–577 [PubMed] [Google Scholar]
- 57.Diederich NJ, Rufra O, Pieri V, Hipp G, Vaillant M (2013) Lack of polysomnographic Non-REM sleep changes in early Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 28 (10):1443–1446. doi: 10.1002/mds.25520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez-Ramirez D, De Jesus S, Walz R, Cervantes-Arriaga A, Peng-Chen Z, Okun MS, Alatriste-Booth V, Rodriguez-Violante M (2015) A Polysomnographic Study of Parkinson’s Disease Sleep Architecture. Parkinson’s disease 2015:570375. doi: 10.1155/2015/570375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parrino L, Smerieri A, Spaggiari MC, Terzano MG (2000) Cyclic alternating pattern (CAP) and epilepsy during sleep: how a physiological rhythm modulates a pathological event. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 111 Suppl 2:S39–46 [DOI] [PubMed] [Google Scholar]
- 60.Tseng PH, Lee PL, Hsu WC, Ma Y, Lee YC, Chiu HM, Ho YL, Chen MF, Wu MS, Peng CK (2017) A Higher Proportion of Metabolic Syndrome in Chinese Subjects with Sleep-Disordered Breathing: A Case-Control Study Based on Electrocardiogram-Derived Sleep Analysis. PloS one 12 (1):e0169394. doi: 10.1371/journal.pone.0169394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma Y, Yeung A, Yang AC, Peng CK, Clain A, Alpert J, Fava M, Yeung AS (2016) The Effects of Tai Chi on Sleep Quality in Chinese American Patients With Major Depressive Disorder: A Pilot Study. Behavioral sleep medicine:1–17. doi: 10.1080/15402002.2016.1228643 [DOI] [PubMed] [Google Scholar]