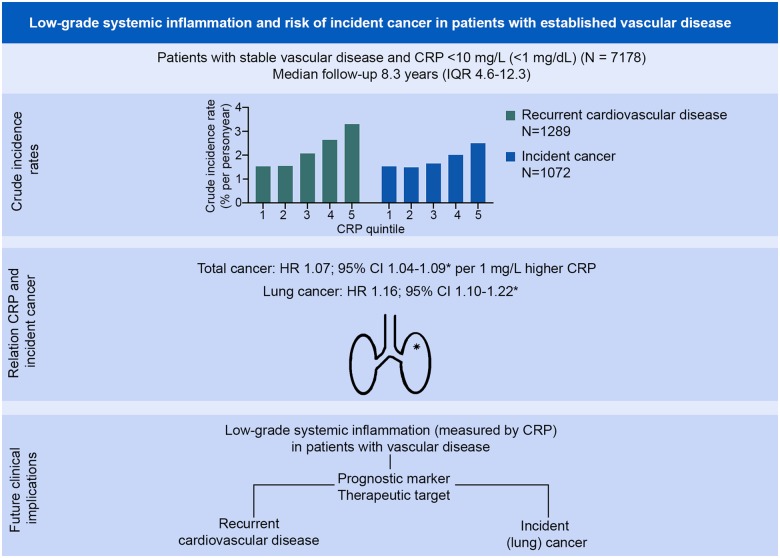
Abstract
Aims
Low-grade inflammation, measured by elevated plasma concentrations of high-sensitive C-reactive protein (CRP), is a risk factor for cardiovascular disease (CVD). There is evidence that low-grade inflammation is also related to a higher risk of cancer. The present prospective cohort study evaluates the relation between low-grade systemic inflammation and risk of cancer in patients with stable CVD.
Methods and results
In total, 7178 patients with stable CVD and plasma CRP levels ≤10 mg/L were included. Data were linked to the Dutch national cancer registry. Cox regression models were fitted to study the relation between CRP and incident CVD and cancer. After a median follow-up time of 8.3 years (interquartile range 4.6–12.3) 1072 incident cancer diagnoses were observed. C-reactive protein concentration was related to total cancer [hazard ratio (HR) 1.35; 95% confidence interval (CI) 1.10–1.65] comparing last quintile to first quintile of CRP. Especially lung cancer, independent of histopathological subtype, was related to CRP (HR 3.39; 95% CI 2.02–5.69 comparing last to first quintile of CRP). Incidence of epithelial neoplasms and especially squamous cell neoplasms were related to CRP concentration, irrespective of anatomical location. Sensitivity analyses after excluding patients with a cancer diagnosis within 1, 2, and 5 years of follow-up showed similar results. No effect modification was observed by smoking status or time since smoking cessation (P-values for interaction > 0.05).
Conclusion
Chronic systemic low-grade inflammation, measured by CRP levels ≤10 mg/L, is a risk factor for incident cancer, markedly lung cancer, in patients with stable CVD. The relation between inflammation and incident cancer is seen in former and current smokers and is uncertain in never smokers.
Keywords: Chronic systemic low-grade inflammation, High-sensitive C-reactive protein, Risk factor, Incident cancer, Patients with vascular disease
Introduction
Chronic systemic low-grade inflammation plays an important role in the aetiology of atherosclerotic disease by initiating and accelerating arterial plaque formation and transformation to vulnerable plaques.1 Besides the role in atherosclerotic disease, there is evidence that low-grade inflammation is related to a higher risk of incident cancer; previous prospective cohort studies found an increased risk of incident cancer related to higher C-reactive protein (CRP) levels in population-based cohorts or cohorts of apparently healthy people.2–10 Especially a higher risk of lung cancer was observed, with hazard ratios (HRs) of 2.2; 95% confidence interval (CI) 1.0–4.64 and 2.8; 95% CI 1.6–4.95 for patients with plasma CRP concentrations >3 vs. <1 mg/L. In the CANTOS trial, which randomized patients in the stable phase after myocardial infarction to placebo or canakinumab, lowering CRP with an interleukin (IL) 1β antibody lowered the incidence of cardiovascular disease (CVD)11 as well as lung cancer, lung cancer death, and total cancer mortality.12
C-reactive protein is part of the IL-1β, IL-6 inflammatory cascade, and can serve as a marker of systemic low-grade inflammation.13 It is unlikely that CRP itself is causally related to cancer development, as genetically elevated CRP is not related to risk of cancer in a Mendelian randomization study.14 Postulated mechanisms for the role of low-grade inflammation in the development of cancer are focused on the promotion phase, and include stimulation of tumour cell survival and proliferation, and promotion of metastatic spread.15,16 Chronic systemic low-grade inflammation, commonly defined as CRP levels ≤10 mg/L,17 is caused by various factors including smoking, abdominal obesity, atrial fibrillation, or heart failure.18 Shared risk factors for both CVD and cancer include smoking and (abdominal) obesity.19 In turn, these risk factors increase levels of systemic low-grade inflammation, further suggesting that low-grade inflammation could be a common pathway leading to CVD and cancer. Moreover, patients with stable CVD have a higher risk of cancer than the general population.20 These patients could benefit from therapy directed at lowering inflammation to reduce recurrent CVD risk and cancer risk.11,12 In the present study, the relation is evaluated between systemic low-grade inflammation and the risk of recurrent CVD and incident cancer in patients with stable CVD.
Methods
Study population
Patients originated from the Utrecht Cardiovascular Cohort - Second Manifestations of ARTerial disease (UCC-SMART) cohort, an ongoing prospective cohort study since 1996, including 18–79-year-old patients referred to the University Medical Centre Utrecht (UMCU), the Netherlands. Central aim of the UCC-SMART cohort is to gain insight into arterial disease occurrence and risk factors for (recurrent) cardiovascular events. For the current study, patients with established CVD at baseline between September 1996 and March 2017 were included (N = 8139). Inclusion in the UCC-SMART cohort occurs at least 2 months after the qualifying vascular event. The institutional review board of the UMCU approved the study and all patients gave written informed consent. Patients who did not give permission for data requests to other medical authorities were excluded (N = 269). Study design and rationale have been described previously.21 In short, information on medical history and lifestyle was acquired and physical examination measurements were obtained according to a standardized protocol. Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III definition.22 High-sensitive CRP level was determined by immunonephelometry (Nephelometer Analyzer BN II, Dade‐Behring). From 2013, high-sensitive CRP was determined in heparin plasma on an AU5811 routine chemistry analyzer (Beckman Coulter, Brea, CA, USA). Kidney function was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.23
Follow-up
During follow-up participants received questionnaires biannually, gathering information on occurrence of recurrent CVD, bleeding events, incident diabetes, and end-stage renal disease. Additional information was gained by collecting hospital or general practitioner’s data. Three physicians from the endpoint committee independently adjudicated all clinical events and conflicting classifications were discussed. The number of patients lost to follow-up was 412 (5.7%).
Data on cancer incidence and details of cancer types and histopathology were obtained by linking the UCC-SMART database to the Dutch National Cancer Registry (INKL), a national registry receiving notifications of all new cancer diagnoses in the Netherlands through the Nationwide Network and Registry of Histopathology and Cytopathology (PALGA), and hospital discharge diagnoses. For the current study, benign tumours, in situ neoplasms, non-melanoma skin cancer, and neoplasms of unknown or uncertain behaviour (e.g. polycythaemia vera) were excluded. Cancer diagnoses were classified according to anatomical location of origin and according to histopathology (Supplementary material online, Tables S1 and S2).
Data preparation
Missing data for high-sensitive C-reactive protein level [n = 97 (1.2%)], smoking [n = 28 (0.3%)], pack-years [n = 32 (0.4%)], body mass index [BMI; n = 18 (0.2%)], low-density lipoprotein cholesterol [LDL-c; n = 138 (1.7%)], and systolic blood pressure [SBP; n = 18 (0.2%)], were imputed. Single imputation was performed using bootstrapping and predictive mean matching based on multivariable regression including independent variables and outcome data (aregImpute-function in R, Hmisc-package). As CRP levels >10 mg/L are commonly associated with an acute inflammatory response,17 these patients (N = 690) were excluded. Two patients had a recurrence of the same cancer diagnosed before entering the cohort and were therefore excluded.
Data analyses
Patients were stratified by quintiles of CRP level and baseline characteristics were displayed accordingly. Kaplan–Meier survival curves were plotted per CRP quintile for recurrent CVD, CVD and/or cancer combined, total cancer, and lung cancer. Recurrent CVD was defined as the occurrence of myocardial infarction, stroke, or vascular death (Supplementary material online, Table S3).
Cox proportional hazard models were fitted to estimate HRs with 95% CIs describing the relation between CRP and recurrent CVD and incident cancer. With regard to the aetiologic nature of the study, there was no need to take competing risks into account. Adjusted HRs from Cox regression analyses were added to the Kaplan–Meier plots. C-reactive protein was added to the model as a continuous and categorical variable. Subjects who were exempt from the outcome, were lost to follow-up or died of another cause were censored. Total cancer incidence was analysed, as well as cancer types separately, if a sufficient number of cases (>60) was present. Cancer types classified according to anatomical location of origin were taken as primary endpoint. Secondary outcome was cancer type classified according to histopathology. For the analyses of specific cancer types, the first diagnosis of that particular cancer was taken as the outcome, possibly being the second or third diagnosis of cancer during follow-up for a certain patient. Hazard ratios were adjusted for age and sex in Model 1. Additionally, smoking status, pack-years of smoking, BMI, LDL-c, diabetes mellitus, SBP, and kidney function were considered potential confounders in the relation between CRP and CVD or cancer and were added to Model 2. Estimates did not change in exploratory models with addition of year of inclusion in the cohort, metabolic syndrome, or lipid-lowering or anti-platelet medication. To test potential effect modification by sex,24 multiplicative interaction terms with CRP level were added to the models, showing no significant interactions (P > 0.05).
Linearity assumption was tested visually by adding continuous CRP level as a restricted cubic spline function to the model. No violations were observed. The proportional hazards assumption, examined graphically by plotting scaled Schoenfeld residuals against time, was not violated.
Influence of BMI and smoking on the relation between CRP and cancer was evaluated. Multiplicative interaction terms with BMI and smoking status were added to the models to assess effect modification, and additional stratified analyses were performed for smoking status. Adjustment for BMI and smoking specifically was performed to evaluate mediation effects. For the relation between CRP and lung cancer, a multiplicative interaction term with time since smoking cessation was assessed, as well as additional adjustment for time since smoking cessation. To examine influence of CRP additional to smoking effects on (lung) cancer risk, analyses were performed with a categorical determinant combining smoking status with CRP quintile, using never smokers in the lowest CRP quintile as a reference group for total cancer. For lung cancer, due to the low event number in never smokers, former smokers in the lowest CRP quintile were taken as reference group.
To evaluate effect modification by interim non-fatal cardiovascular events, multiplicative interaction terms were added to models of total and lung cancer. Reverse causality was evaluated by repeating analyses after excluding patients diagnosed with cancer within 1, 2, and 5 year(s) after inclusion. Also, analyses were stratified for location of vascular disease (coronary artery disease, cerebrovascular disease, or peripheral vascular disease) at baseline. Additional sensitivity analyses were performed after exclusion of patients with any type of cancer (except non-melanoma skin cancer) before inclusion in the cohort, and after excluding patients with CRP levels >5 mg/L. Stability of CRP levels during follow-up was assessed in a subset of SMART patients who revisited for second measurements (N = 1794).
Results
In total, 7178 patients with stable vascular disease and CRP levels ≤10 mg/L were included. Baseline characteristics stratified for CRP quintiles are shown in Table 1. Patients in the highest CRP stratum were more likely to be current smokers, generally had a higher number of pack-years, and fewer patients used lipid-lowering and antiplatelet medication. Other unfavourable trends with regard to cardiovascular risk profile in the highest CRP stratum included a slightly higher SBP, LDL-c, and higher prevalence of diabetes.
Table 1.
Baseline characteristics stratified by quintiles of C-reactive protein level
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|
| Median CRP (mg/L) (range) | 0.50 (0.10–0.70) | 1.00 (0.71–1.39) | 1.80 (1.39–2.30) | 3.07 (2.31–4.10) | 5.90 (4.10–10.00) |
| n = 7178 | n = 1455 | n = 1417 | n = 1455 | n = 1426 | n = 1425 |
| Male, n (%) | 1129 (78) | 1075 (76) | 1094 (75) | 1044 (73) | 1008 (71) |
| Age (years)a | 58 ± 10 | 59 ± 10 | 61 ± 10 | 61 ± 10 | 61 ± 10 |
| Medical history | |||||
| Cancer (except non-melanoma skin cancer), n (%) | 45 (3) | 55 (4) | 53 (4) | 79 (6) | 68 (5) |
| Cerebrovascular disease, n (%) | 468 (32) | 404 (29) | 421 (29) | 431 (30) | 446 (31) |
| Coronary artery disease, n (%) | 930 (64) | 945 (67) | 929 (64) | 868 (61) | 756 (53) |
| Peripheral vascular disease, n (%) | 145 (10) | 186 (13) | 227 (16) | 298 (21) | 373 (26) |
| Diabetes mellitus, n (%) | 202 (14) | 223 (16) | 263 (18) | 248 (17) | 287 (20) |
| Current smoking, n (%) | 298 (20) | 333 (24) | 417 (29) | 519 (36) | 608 (43) |
| Former smoking, n (%) | 703 (48) | 724 (51) | 720 (49) | 662 (46) | 607 (43) |
| Number of pack-yearsa | 8 (0–23) | 12 (0–27) | 14 (3–31) | 20 (6–35) | 22 (9–37) |
| Metabolic syndrome, n (%) | 520 (36) | 640 (45) | 796 (55) | 844 (60) | 910 (64) |
| Physical examination | |||||
| Body mass index (kg/m2)a | 26 ± 3 | 26 ± 3 | 27 ± 4 | 27 ± 4 | 28 ± 4 |
| Waist circumference (cm)a | 91 ± 11 | 94 ± 11 | 97 ± 11 | 98 ± 12 | 98 ± 12 |
| Systolic blood pressure (mmHg)a | 136 ± 19 | 138 ± 20 | 140 ± 20 | 141 ± 20 | 142 ± 21 |
| Diastolic blood pressure (mmHg)a | 80 ± 11 | 80 ± 11 | 81 ± 11 | 81 ± 11 | 82 ± 11 |
| Laboratory measurements | |||||
| Triglycerides (mmol/L)a | 1.2 (0.9–1.6) | 1.3 (1.0–1.9) | 1.4 (1.0–2.1) | 1.5 (1.1–2.1) | 1.6 (1.2–2.3) |
| HDL-cholesterol (mmol/L)a | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (0.9–1.3) |
| LDL-cholesterol (mmol/L)a | 2.4 (1.9–3.1) | 2.5 (2.0–3.3) | 2.7 (2.1–3.5) | 2.8 (2.2–3.6) | 3.0 (2.3–3.9) |
| eGFR (CKD-EPI, mL/min/1.73 m²)a | 80 ± 16 | 78 ± 17 | 77 ± 17 | 76 ± 18 | 75 ± 20 |
| Medication | |||||
| Lipid-lowering medication, n (%) | 1123 (77) | 1070 (76) | 1021 (70) | 937 (66) | 843 (59) |
| Blood pressure-lowering medication, n (%) | 1052 (72) | 1081 (76) | 1117 (77) | 1063 (75) | 1047 (73) |
| Anti-platelet therapy, n (%) | 1190 (82) | 1158 (82) | 1138 (78) | 1078 (76) | 1004 (70) |
| Anti-coagulants, n (%) | 109 (7) | 133 (9) | 177 (12) | 156 (11) | 184 (13) |
CRP, C-reactive protein.
Data are expressed as means ± SD for normal distributed data and median (interquartile range) for unevenly distributed data.
Relation between C-reactive protein and risk of recurrent cardiovascular events
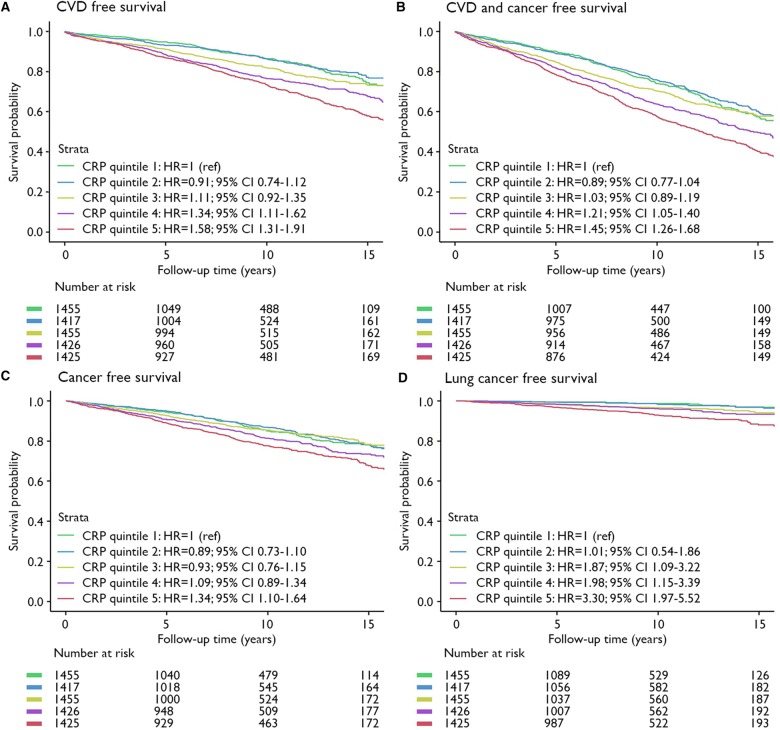
During a median follow-up of 8.3 years [interquartile range (IQR) 4.6–12.3] and a total of 58 568 person-years of follow-up, 1289 patients experienced a recurrent cardiovascular event. Crude incidence rates were 1.53%, 1.55%, 2.07%, 2.64%, and 3.30% across CRP quintiles. Patients in the highest CRP quintile had a higher cardiovascular risk compared to patients in the lowest quintile of CRP (HR 1.58; 95% CI 1.31–1.91) (Figure 1A). The risk of cancer and/or CVD was 45% higher in the highest CRP quintile compared to the lowest (HR 1.45; 95% CI 1.26–1.68) (Figure 1B). CRP was significantly related to risk of myocardial infarction, vascular death, and all-cause mortality, but not to risk of stroke in categorical and continuous analyses (Supplementary material online, Figure S1).
Figure 1.
Survival curves in CRP quintiles for (A) recurrent cardiovascular disease; (B) CVD and cancer free survival; (C) cancer free survival; and (D) lung cancer free survival. Hazard ratios are adjusted for age, sex, body mass index, smoking status, pack-years of smoking, low-density lipoprotein cholesterol, diabetes mellitus, systolic blood pressure, and kidney function. Quintile 1: C-reactive protein 0.50 (range 0.10–0.70); Quintile 2: C-reactive protein 1.00 (range 0.70–1.39); Quintile 3: C-reactive protein 1.80 (range 1.39–2.30); Quintile 4: C-reactive protein 3.07 (range 2.31–4.10); Quintile 5: C-reactive protein 5.90 (range 4.10–10.00).
Relation between C-reactive protein and risk of incident cancer according to anatomical location of origin
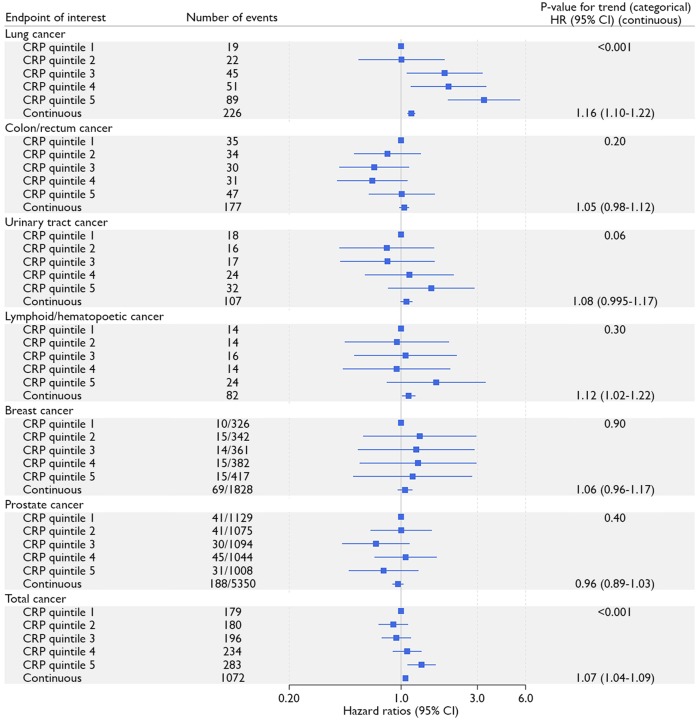
During follow-up 1072 incident malignancies were observed. Most frequently occurring diagnoses were cancer of the lung (n = 226), prostate (n = 188), and colon/rectum (n = 177). Crude incidence rates per person-year were 1.53%, 1.49%, 1.65%, 2.01%, and 2.50% across CRP quintiles. Patients with a higher CRP level had a higher risk of cancer, comparing patients in the highest CRP quintile to patients in the lowest quintile (HR 1.41; 95% CI 1.22–1.63) (Figures 1C and2), and per 1 mg/L higher CRP (HR 1.07; 95% CI 1.04–1.09) (Figure 2). Risk of incident lung cancer was higher in the last CRP quintile compared to the first (HR 3.39; 95% CI 2.03–5.69) (Figures 1D and2), and the risk increased 16% for each 1 mg/L higher CRP (HR 1.16; 95% CI 1.10–1.22) (Figure 2). Urinary tract cancer was possibly related to CRP concentration (HR 1.08; 95% CI 0.995–1.17 for every 1 mg/L higher CRP and HR 1.51; 95% CI 0.81–2.81 comparing last quintile with first CRP quintile) (Figure 2). Similarly, lymphoid/hematopoietic cancer was possibly related to CRP level, particularly in continuous analysis (HR 1.12; 95% CI 1.02–1.22 for every 1 mg/L higher CRP level, and HR 1.65; 95% CI 0.81–3.35 comparing fifth quintile with first CRP quintile; Figure 2). No relation was observed between CRP level and risk of breast or prostate cancer (in subgroups of women and men respectively), or incident colorectal cancer.
Figure 2.
Relation between CRP and incident cancer according to anatomical location of origin. Hazard ratios are adjusted for age, sex, smoking status, number of pack-years, body mass index, low-density lipoprotein cholesterol, diabetes mellitus, systolic blood pressure, and kidney function. Analyses for breast cancer and prostate cancer were performed in subgroups of women and men respectively. The number of events per number of women or men in C-reactive protein quintiles are given. Continuous analyses represent hazard ratios per 1 mg/L higher C-reactive protein concentration.
Relation between C-reactive protein and risk of incident cancer according to histopathology
The relation between plasma CRP and risk of lung cancer was similar for histopathological subtypes; small cell lung cancer and non-small-cell lung cancer, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (Supplementary material online, Table S4). In secondary outcome analyses with cancer types according to histopathology irrespective of anatomical location of origin, CRP was significantly related to risk of epithelial neoplasms not further specified (hereinafter referred to as epithelial neoplasms) (HR 1.17; 95% CI 1.08–1.27), and squamous cell neoplasms (HR 1.11; 95% CI 1.02–1.20) (Supplementary material online, Figure S2).
Smoking and body mass index, and the relation between C-reactive protein and risk of incident (lung) cancer
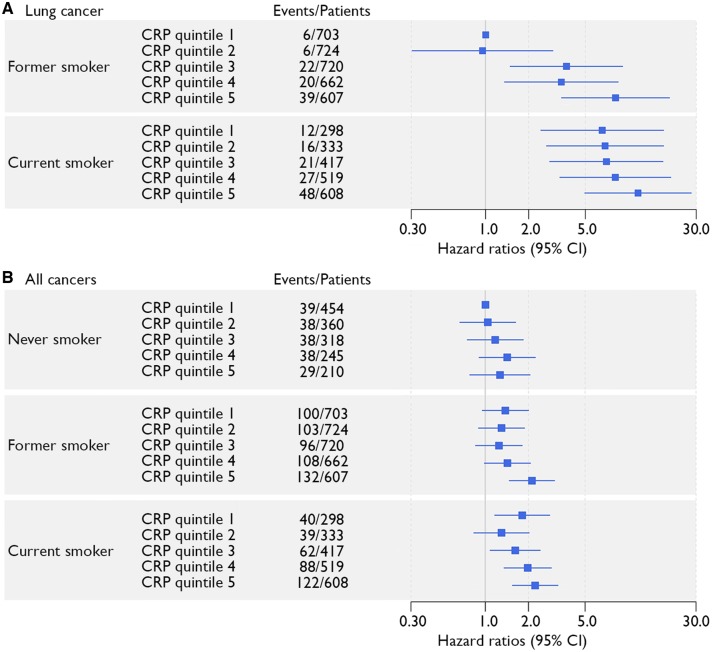
No significant interaction terms with BMI were observed (P > 0.05). Adjustment for BMI or smoking did not mitigate the relation between CRP and cancer (Supplementary material online, Table S5). For the relation between CRP and lung cancer, no effect modification was observed by time since smoking cessation in former smokers (P-value for interaction 0.44) and additional adjustment for time since smoking cessation showed similar results (HR 1.17; 95% CI 1.11–1.23 compared to HR 1.16; 95% CI 1.10–1.22 of the original adjusted model). Stratified analyses for smoking status showed similar HRs for lung and total cancer (P-values for interaction > 0.05) (Supplementary material online, Table S6). Current smokers in the highest quintile of CRP had the highest risk of lung cancer (HR 11.70; 95% CI 4.95–27.64 compared to former smokers in the lowest CRP quintile) (P-value for trend <0.0001) (Figure 3A) and total cancer (HR 2.23; 95% CI 1.55–3.22 compared to never smokers in the lowest quintile) (P-value for trend <0.0001) (Figure 3B).
Figure 3.
Relation between CRP quintiles within categories of smoking status and risk of (A) lung cancer and (B) all cancers. Hazard ratios are adjusted for age, sex, body mass index, low-density lipoprotein cholesterol, diabetes mellitus, systolic blood pressure, and kidney function.
Take home figure.
Low grade systemic inflammation and risk of incident cancer in patients with established vascular disease. CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; IQR, interquartile range. *Hazard ratios are adjusted for age, sex, body mass index, smoking status, pack-years of smoking, low-density lipoprotein cholesterol, diabetes mellitus, systolic blood pressure, and kidney function. Hazard ratios per 1 mg/L higher C-reactive protein are presented.
Sensitivity analyses
No significant interactions were observed with interim non-fatal CVD (P-values 0.72 and 0.33 for total cancer and lung cancer, respectively). Reverse causality was evaluated by repeating analyses after excluding patients who were diagnosed with cancer within 1 year (n = 102), 2 years (n = 193), and 5 years (N = 477) after entering the cohort, and showed similar results (Supplementary material online, Table S7). Analyses stratified for vascular disease location at baseline: coronary artery disease (n = 3931), cerebrovascular disease (n = 1904), or peripheral vascular disease (n = 1343) revealed similar results (Supplementary material online, Table S8). Similar results were observed after exclusion of patients with a history of cancer before inclusion (n = 300) or after exclusion of patients with CRP levels >5 mg/L (N = 985) (Supplementary material online, Figures S3 and S4). CRP levels were stable after a median of 9.9 years (IQR 5.4–10.8 years) with a mean difference of −0.18 mg/L (standard error of the mean 0.05).
Discussion
The present study shows that in patients with stable vascular disease plasma CRP concentration is related to risk of recurrent cardiovascular events, as well as risk of cancer, especially lung cancer. No effect modification by smoking status was observed. A potential relation was observed between CRP and lymphoid/hematopoietic and urinary tract cancer. The relation between plasma CRP and incident cancer was seen for epithelial neoplasms, especially squamous cell neoplasms, irrespective of anatomical location of origin.
Results of the present study support the role of chronic systemic low-grade inflammation as a stimulating factor in cancer development in a cohort of patients with established vascular disease. The observed relation between CRP and cancer risk cannot be explained by reverse causality, meaning that an elevated CRP would simply be a sign of occult cancer, as similar results were observed after exclusion of patients with a diagnosis of cancer within 1, 2, and 5 year(s) after inclusion. Results of the present study correspond to results of the CANTOS trial11,12 and previous prospective cohort studies performed in population-based cohorts or cohorts of apparently healthy people.2–6,9,10 To our knowledge, no previous studies investigated the relation between CRP and incident cancer in patients with established vascular disease specifically. Cancer incidence is higher in patients with established CVD compared to the general population, likely due to common risk factors,20 and the current study shows that systemic low-grade inflammation is a contributing factor in pathophysiology of CVD as well as cancer.
In accordance with previous observational studies,2–6,9,25 and in line with the CANTOS trial results,12 lung cancer risk was especially related to CRP levels. Chronic low-grade inflammation is previously considered to be one of the causal pathways by which smoking leads to lung cancer.15 Epithelial neoplasms and squamous cell neoplasms, irrespective of anatomical location of origin, were mostly respiratory tract cancers; lung carcinomas and carcinomas of the lip, oral cavity, pharynx, and glottis. The elevated systemic inflammatory levels as a risk factor for respiratory tract cancer might reflect a local inflammatory microenvironment caused by smoking26 that contributes to cancer development. It is possible that low-grade inflammation initiated by smoking is not reversed when quitting smoking, emphasizing the importance of smoking abstinence. In the present study, the relation between CRP and total cancer risk in never smokers was uncertain (HR 1.05; 95% CI 0.98–1.13). However, no significant interaction was observed for smoking status (P > 0.05) and the point estimate was the same as in current smokers (HR 1.05; 95% CI 1.01–1.10). The incidence of lung cancer (n = 9) in never smokers was too low for reliable analysis. A previous case–control study nested in population-based cohorts showed no relation between CRP and lung cancer in never smokers.25 However, that higher inflammation levels as a risk factor for cancer are a direct result of smoking is unlikely based on the results of this study. Adjustment for smoking status and pack-years did not mitigate the relation between CRP level and cancer risk, suggesting that other pathophysiological pathway, and possibly other inflammatory pathways, play a role in mechanisms leading from smoking to cancer. Furthermore, the combination of a CRP level in the highest quintile with current smoking, conferred the highest cancer risk, suggesting an additive effect of inflammation and smoking on cancer risk. Potential relations between CRP and lymphoid/haematopoietic and urinary tract cancer should be interpreted with caution, as the relations were not statistically significant in all analyses, but suggest that inflammation could be involved in the pathogenesis of these neoplasms.
The relation between CRP and cancer risk is of great importance for clinical practice. As treatment for CVD has improved substantially over the last decades, more patients survive acute manifestations of CVD and survive long enough to develop cancer. C-reactive protein is a marker for CVD risk and could potentially also serves as a prognostic marker to identify those at high risk of (lung) cancer. Since patients from the third CRP quintile and higher had an increased risk of lung cancer, CRP levels of ≥1.4 mg/L might be indicative of a higher risk of lung cancer. It could even be hypothesized that patients at high cardiovascular risk with high levels of inflammation are those that might benefit from anti-inflammatory treatment to reduce cardiovascular risk as well as risk of (lung) cancer. The CANTOS trial implicated that the IL-1β, IL-6, CRP inflammatory pathway is involved in cancer development.12 Results of trials studying other anti-inflammatory treatments could provide additional information on specific inflammatory pathways involved in cancer pathogenesis and the effectiveness of lowering inflammation on reduction of cancer risk, even though cancer was not the primary endpoint in these trials. However, the Cardiovascular Inflammation Reduction Trial (CIRT) was stopped due to ineffectiveness of methotrexate on CRP levels and CVD risk and no data are available yet on cancer incidence.27 The Low Dose Colchicine study (LoDoCo2, EudraCT Number: 2015-005568-40), trialling effect of colchicine on CVD risk is still ongoing and might provide additional information.
Strengths of the present study include the large patient population with established vascular disease and the prospective study design with long follow-up, large number of events, and histopathological cancer diagnoses. Potential limitations should be considered and include the single measurement of CRP level at baseline, as CRP levels might fluctuate during follow-up. However, patients were included in the cohort at least 2 months after the qualifying cardiovascular event, thus stable on medication that might influence CRP levels. Moreover, repeated CRP measurements over time are shown to be stable in a subset of UCC-SMART patients with repeated measurement as well as previous research.28 Data on other inflammatory markers, such as IL-6, was not available. Despite the large number of total cancer events, the number of certain specific cancer types was insufficient for reliable analyses. Additionally, subgroups of smaller size with limited number of events, for example, women, might be insufficient for reliable subgroup analyses. The number of lung cancer cases in never smokers was insufficient for reliable analysis, and the relation between CRP and lung cancer cannot be generalized to never smokers. Given the observational study design firm conclusions on causality should be made with caution as residual confounding cannot be ruled out.
Chronic systemic low-grade inflammation, measured by CRP levels ≤10 mg/L, is a risk factor for incident cancer, markedly lung cancer, in patients with stable CVD. The relation between inflammation and incident cancer is seen in former and current smokers and is uncertain in never smokers.
Supplementary Material
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We gratefully acknowledge the contribution of the research nurses; R. van Petersen (data-manager); B. van Dinther (study manager), and the members of the Utrecht Cardiovascular Cohort-Second Manifestations of ARTerial disease-study group (UCC-SMART-study group): F.W. Asselbergs and H.M. Nathoe, Department of Cardiology; G.J. de Borst, Department of Vascular Surgery; M.L. Bots and M.I. Geerlings, Department of Epidemiology; M.H. Emmelot, Department of Geriatrics; P.A. de Jong and T. Leiner, Department of Radiology; A.T. Lely, Department of Obstetrics/Gynaecology; N.P. van der Kaaij, Department of Cardiothoracic Surgery; L.J. Kappelle and Y. Ruigrok, Department of Neurology; M.C. Verhaar, Department of Nephrology, F.L.J. Visseren (chair) and J. Westerink, Department of Vascular Medicine, University Medical Center Utrecht and Utrecht University.
See page 3910 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz645)
Ethics approval and participants’ informed consent
The authors do hereby declare that the current study complies with the Declaration of Helsinki. The institutional review board of the University Medical Centre approved the study and all patients gave written informed consent.
Funding
The UCC-SMART study was financially supported by a grant of the University Medical Center Utrecht.
Conflict of interest: P.M.R. served as the Principle Investigator of CANTOS and CIRT, funded by Novartis and the NHLBI, respectively. P.M.R. is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and has served as a consultant to Novartis, Merck, Amgen, Corvidia, and Inflazome. F.W.A. is supported by UCL Hospitals NIHR Biomedical Research Centre. J.G.J.V.A. has served as a consultant for Eli-Lilly, MSD, BMS, Boehringer Ingelheim, Amphera, Astra-Zeneca, Takeda, and Roche and is stock owner of Amphera. All other authors have no conflict of interest to declare.
References
- 1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 2. Prizment AE, Folsom AR, Dreyfus J, Anderson KE, Visvanathan K, Joshu CE, Platz EA, Pankow JS.. Plasma C-reactive protein, genetic risk score, and risk of common cancers in the Atherosclerosis Risk in Communities study. Cancer Causes Control 2013;24:2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA.. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 2009;20:15–26. [DOI] [PubMed] [Google Scholar]
- 4. Allin KH, Bojesen SE, Nordestgaard BG.. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol 2009;27:2217–2224. [DOI] [PubMed] [Google Scholar]
- 5. Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA, Stricker BH.. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol 2006;24:5216–5222. [DOI] [PubMed] [Google Scholar]
- 6. Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB.. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 2005;14:2413–2418. [DOI] [PubMed] [Google Scholar]
- 7. Brasky TM, Kabat GC, Ho GYF, Thomson CA, Nicholson WK, Barrington WE, Bittoni MA, Wassertheil-Smoller S, Rohan TE.. C-reactive protein concentration and risk of selected obesity-related cancers in the Women's Health Initiative. Cancer Causes Control 2018;29:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izano M, Wei EK, Tai C, Swede H, Gregorich S, Harris TB, Klepin H, Satterfield S, Murphy R, Newman AB, Rubin SM, Braithwaite D, Health A.. Chronic inflammation and risk of colorectal and other obesity-related cancers: the health, aging and body composition study. Int J Cancer 2016;138:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allin KH, Bojesen SE, Nordestgaard BG.. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer 2016;139:1493–1500. [DOI] [PubMed] [Google Scholar]
- 10. Wang G, Li N, Chang S, Bassig BA, Guo L, Ren J, Su K, Li F, Chen S, Wu S, Zou Y, Dai M, Zheng T, He J.. A prospective follow-up study of the relationship between C-reactive protein and human cancer risk in the Chinese Kailuan Female Cohort. Cancer Epidemiol Biomarkers Prev 2015;24:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Ridker P, Lorenzatti A, Krum H, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Genest J, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Koenig W, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Kastelein J, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung K-B, Commerford P, Dellborg M, Donath M, Hwang J-J, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Everett B, Forgosh L, Glynn R, Harris B, Libby P, Ligueros M, Thuren T, Bohula E, Charmarthi B, Cheng S, Chou S, Danik J, McMahon G, Maron B, Ning M, Olenchock B, Pande R, Perlstein T, Pradhan A, Rost N, Singhal A, Taqueti V, Wei N, Burris H, Cioffi A, Dalseg AM, Ghosh N, Gralow J, Mayer T, Rugo H, Fowler V, Limaye AP, Cosgrove S, Levine D, Lopes R, Scott J, Thuren T, Ligueros M, Hilkert R, Tamesby G, Mickel C, Manning B, Woelcke J, Tan M, Manfreda S, Ponce T, Kam J, Saini R, Banker K, Salko T, Nandy P, Tawfik R, O'Neil G, Manne S, Jirvankar P, Lal S, Nema D, Jose J, Collins R, Bailey K, Blumenthal R, Colhoun H, Gersh B, Glynn RJ.. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allin KH, Nordestgaard BG, Zacho J, Tybjærg-Hansen A, Bojesen SE.. C-reactive protein and the risk of cancer: a Mendelian randomization study. J Natl Cancer Inst 2010;102:202–206. [DOI] [PubMed] [Google Scholar]
- 15. Coussens LM, Werb Z.. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balkwill F, Mantovani A.. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–545. [DOI] [PubMed] [Google Scholar]
- 17. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 18. Kushner I, Rzewnicki D, Samols D.. What does minor elevation of C-reactive protein signify? Am J Med 2006;119:166 e17–e28. [DOI] [PubMed] [Google Scholar]
- 19. Blaes A, Prizment A, Koene RJ, Konety S.. Cardio-oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin 2017;13:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Kruijsdijk RC, van der Graaf Y, Peeters PH, Visseren FL; Second Manifestations of ARTerial disease (SMART) study group. Cancer risk in patients with manifest vascular disease: effects of smoking, obesity, and metabolic syndrome. Cancer Epidemiol Biomarkers Prev 2013;22:1267–1277. [DOI] [PubMed] [Google Scholar]
- 21. Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y.. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol 1999;15:773–781. [DOI] [PubMed] [Google Scholar]
- 22. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F, American HA, National HL, Blood I.. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou B, Shu B, Yang J, Liu J, Xi T, Xing Y.. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control 2014;25:1397–1405. [DOI] [PubMed] [Google Scholar]
- 25. Muller DC, Larose TL, Hodge A, Guida F, Langhammer A, Grankvist K, Meyer K, Cai Q, Arslan AA, Zeleniuch-Jacquotte A, Albanes D, Giles GG, Sesso HD, Lee IM, Gaziano JM, Yuan JM, Hoffman Bolton J, Buring JE, Visvanathan K, Le Marchand L, Purdue MP, Caporaso NE, Midttun O, Ueland PM, Prentice RL, Weinstein SJ, Stevens VL, Zheng W, Blot WJ, Shu XO, Zhang X, Xiang YB, Koh WP, Hveem K, Thomson CA, Pettinger M, Engstrom G, Brunnstrom H, Milne RL, Stampfer MJ, Han J, Johansson M, Brennan P, Severi G, Johansson M.. Circulating high sensitivity C reactive protein concentrations and risk of lung cancer: nested case-control study within Lung Cancer Cohort Consortium. BMJ 2019;364:k4981.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z.. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget 2017;8:268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glynn RJ, MacFadyen JG, Ridker PM.. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem 2009;55:305–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.