Abstract
Aims
This observational study characterized cardiovascular disease (CVD) mortality risk for multiple cancer sites, with respect to the following: (i) continuous calendar year, (ii) age at diagnosis, and (iii) follow-up time after diagnosis.
Methods and results
The Surveillance, Epidemiology, and End Results program was used to compare the US general population to 3 234 256 US cancer survivors (1973–2012). Standardized mortality ratios (SMRs) were calculated using coded cause of death from CVDs (heart disease, hypertension, cerebrovascular disease, atherosclerosis, and aortic aneurysm/dissection). Analyses were adjusted by age, race, and sex. Among 28 cancer types, 1 228 328 patients (38.0%) died from cancer and 365 689 patients (11.3%) died from CVDs. Among CVDs, 76.3% of deaths were due to heart disease. In eight cancer sites, CVD mortality risk surpassed index-cancer mortality risk in at least one calendar year. Cardiovascular disease mortality risk was highest in survivors diagnosed at <35 years of age. Further, CVD mortality risk is highest (SMR 3.93, 95% confidence interval 3.89–3.97) within the first year after cancer diagnosis, and CVD mortality risk remains elevated throughout follow-up compared to the general population.
Conclusion
The majority of deaths from CVD occur in patients diagnosed with breast, prostate, or bladder cancer. We observed that from the point of cancer diagnosis forward into survivorship cancer patients (all sites) are at elevated risk of dying from CVDs compared to the general US population. In endometrial cancer, the first year after diagnosis poses a very high risk of dying from CVDs, supporting early involvement of cardiologists in such patients.
Keywords: Neoplasm, SEER, Heart disease, Epidemiology, Cardio-oncology
Introduction
Heart disease and cancer are the leading causes of mortality, both in the USA and worldwide.1 During 2015, in the USA, 633 842 deaths were due to heart disease, while 595 930 deaths were due to cancer.2 Worldwide, in 2015, 17.7 million deaths were due to cardiovascular diseases (CVDs),3 while 8.8 million were due to cancer.4
Cancer survivors have an increased risk for CVDs,5–7 either from shared lifestyles or from toxicities of cancer treatment.8,9 With recent progress in screening, diagnosis, and treatment of many cancers, the population of cancer survivors is steadily increasing.10 It is expected that by 2040, the number of Americans with a history of cancer will increase to more than 26 million.11 Therefore, cardiology care of cancer survivors becomes increasingly important. For most cancer survivors, the most effective strategy for primary prevention, and or management, of CVD is likely achieved through modification of traditional risk factors.12,13
Long-term cancer survivorship care is an advancing field of research and the delineation of responsibility between primary care physicians (PCPs) and specialists (oncologists, cardiologists, etc.) is evolving.14 Primary care physicians are to a large extent tasked with primary prevention and cardiologists oversee management of CVDs. Due to the complexities of specific cancer treatment effects, there may be uncertainty across a multidisciplinary care team as to the role of managing CV health for cancer survivors. Underestimation of the elevated risk that cancer survivors face may result in (i) missed opportunities for early intervention or (ii) treatment approaches that may not be aggressive enough. To better characterize the landscape of CVD mortality in cancer survivors, we conducted a comprehensive overview analysis of the risk of dying from CVDs in cancer survivors. Our goals were to analyse mortality due to CVDs in cancer patients (28 cancer sites) as a function of (i) calendar year, (ii) age at diagnosis, and (iii) follow-up time after cancer diagnosis. This study fills an important gap in the literature for both PCPs and specialists as we highlight both historical trends and observations regarding basic clinical presentations (age at cancer diagnosis and follow-up time after cancer diagnosis) which together may influence patient-level decisions on cardiovascular care.
Methods
Patients with invasive cancer, diagnosed between 1973 and 2015, were analysed from the Surveillance, Epidemiology, and End Results (SEER) program. The SEER program is a network of population-based incident tumour registries, covering 28% of the US population, including incidence, survival, and treatment.15,16 Cause of death was categorized by the International Classification of Diseases (ICD)-9 code. Cause of death from clinician or coroner coded CVDs (heart disease, hypertension, cerebrovascular disease, atherosclerosis, aortic aneurysm/dissection, and other diseases of arteries, arterioles, or capillaries) was used. The methods and limitations for mortality rate calculation are described in the Supplementary material online, Text and Figure S1. Comorbidities, performance status, surgical pathology, margin status, doses, or agents are not coded in the SEER registry. SEER*Stat 8.2.2 was used for analysis.15 Patients whose cancer were diagnosed only through autopsy or death certificate were excluded. Institutional Review Board approval was not necessary for publicly available information.
The analysis consists of three objectives. In Objective 1, we characterized CVD mortality risk in each continuous calendar year by cancer type. Death, based on death certificate, was coded as being due to ‘index-cancer’ or ‘death from CVD’ (defined above). Using standard methods, a competing risk model was also conducted for cancers at higher than average risk for death from CVD. The Fine and Gray model was applied to estimate the cumulative incidence function, which was implemented using SAS 9.4 software.17,18 For Objectives 2 and 3, we describe the risk of death due to CVD as a function of age at cancer diagnosis, and follow-up time after cancer diagnosis, respectively. Standardized mortality ratios (SMRs) were used to estimate CVD mortality risk in cancer survivors using SEER data between 1973 and 2012 as well as SEER data in the modern treatment era from 2000 to 2015. Standardized mortality ratios provide the relative risk of death from CVD for cancer survivors as compared to all US residents, adjusted by age, race, and sex over the same time.19 The reference cohort was US mortality as reported in the National Vital Statistics System and maintained by the National Center for Health statistics. Ninety-five percent confidence intervals (CIs) of the SMRs were calculated using SEER*Stat 8.2.1 and Microsoft Excel 15.0.4 (Microsoft, Redmond, WA, USA).19–21 At least 1000 person-years at risk were necessary for each CVD cause of death with respect to cancer type in order to be included in the analysis.
Results
Patient characteristics
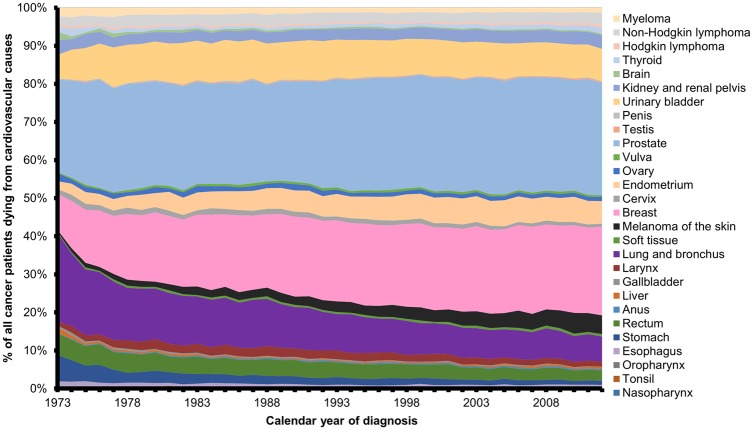
We identified 3 234 256 cancer patients among 28 cancer sites that were registered in the SEER program between years 1973 and 2012. In this representative sample, 49.3% of cancer patients died from either their cancer or from CVD during this time period (Table 1). The plurality (76.3%) of all cardiovascular-related deaths in cancer patients was from heart disease. We observed that increasing age at diagnosis was associated with both increased percent of cancer patients dying from index-cancer and increased percent of cancer patients dying from CVD. We also observed a trend for more recent years of cancer diagnosis to be associated with both decreased percent of cancer patients dying from index-cancer and decreased percent of cancer patients dying from CVD. These observations indicate that (i) with increasing age there is increased risk of dying, either from cancer or CVD; and (ii) cancer patients with a recent diagnosis have neither died of their cancer nor CVD yet. Consistent with these observations, there was a decrease in death from index-cancer and an increase in CVD death as months since diagnosis increased. Urinary bladder cancer patients have the highest risk of dying from a CVD with 19.4% of bladder cancer patients having died from CVD. The following cancer sites also displayed a higher than average (11.3%) risk for death from CVD in cancer patients: larynx (17.3%), prostate (16.6%), corpus uteri (15.6%), colorectal (13.7%), and breast (11.7%). To further contextualize our observations of decreased death from index-cancer and increased CVD death as time since diagnosis increases, we conducted a competing risk analysis for the above six cancer sites which have an elevated risk for death from CVD (Supplementary material online, Figure S2). Prostate and breast cancer patients have the largest absolute number of patients who have died of CVDs between 1973 and 2012, and therefore make up the largest percent (49.2%) of all cancer patients among the 28 cancer sites who have died from cardiovascular causes (Figure 1).
Table 1.
Cancer patients and death from cardiovascular disease between 1973 and 2012
| No. of cancer patients | Index-cancer deaths |
CVD deaths |
Heart disease deaths |
||||
|---|---|---|---|---|---|---|---|
| No. | % of cancer patients | No. | % of cancer patients | No. | % of all CVD deaths | ||
| All patients | 3 234 256 | 1 228 328 | 38.0 | 365 689 | 11.3 | 279 060 | 76.3 |
| Sex | |||||||
| Male | 1 662 864 | 651 517 | 39.2 | 200 610 | 12.1 | 157 349 | 78.4 |
| Female | 1 571 392 | 576 811 | 36.7 | 165 079 | 10.5 | 121 711 | 73.7 |
| Age at diagnosis | |||||||
| 0–19 years | 43 263 | 10 156 | 23.5 | 278 | 0.6 | 232 | 83.5 |
| 20–39 years | 210 699 | 54 047 | 25.7 | 2845 | 1.4 | 2330 | 81.9 |
| 40–59 years | 932 545 | 326 003 | 35.0 | 41 356 | 4.4 | 32 622 | 78.9 |
| 60–79 years | 1 636 489 | 656 668 | 40.1 | 224 173 | 13.7 | 171 137 | 76.3 |
| 80+ years | 411 260 | 181 454 | 44.1 | 97 037 | 23.6 | 72 739 | 75.0 |
| Year of diagnosis | |||||||
| 1973–82 | 564 129 | 294 723 | 52.2 | 109 144 | 19.3 | 82 941 | 76.0 |
| 983–92 | 766 593 | 356 095 | 46.5 | 127 992 | 16.7 | 97 955 | 76.5 |
| 1993–2002 | 911 959 | 338 260 | 37.1 | 97 014 | 10.6 | 73 780 | 76.1 |
| 2003–2012 | 991 575 | 239 250 | 24.1 | 31 539 | 3.2 | 24 384 | 77.3 |
| Race | |||||||
| White | 2 731 980 | 1 027 442 | 37.6 | 319 537 | 11.7 | 243 887 | 76.3 |
| Black | 297 188 | 127 077 | 42.8 | 30 924 | 10.4 | 24 233 | 78.4 |
| Other | 205 088 | 73 809 | 36.0 | 15 228 | 7.4 | 10 940 | 71.8 |
| Marital status | |||||||
| Single | 399 184 | 141 885 | 35.5 | 28 848 | 7.2 | 22 463 | 77.9 |
| Married | 1 894 691 | 699 628 | 36.9 | 200 897 | 10.6 | 153 711 | 76.5 |
| Separated | 42 498 | 20 794 | 48.9 | 6740 | 15.9 | 5244 | 77.8 |
| Divorced | 237 000 | 98 579 | 41.6 | 18 723 | 7.9 | 14 557 | 77.7 |
| Widowed | 484 770 | 224 723 | 46.4 | 91 850 | 18.9 | 68 835 | 74.9 |
| Stage at presentation | |||||||
| Localized | 1 059 957 | 169 107 | 16.0 | 148 767 | 14.0 | 111 465 | 74.9 |
| Regional | 632 242 | 282 521 | 44.7 | 65 304 | 10.3 | 49 420 | 75.7 |
| Distant | 528 923 | 389 794 | 73.7 | 25 220 | 4.8 | 20 052 | 79.5 |
| Months since diagnosis | |||||||
| 2–11 months | 3 234 256 | 519 279 | 16.1 | 45 592 | 1.4 | 35 925 | 78.8 |
| 12–59 months | 2 533 198 | 498 282 | 19.7 | 105 581 | 4.2 | 81 355 | 77.1 |
| 60–179 months | 1 493 858 | 176 561 | 11.8 | 148 842 | 10.0 | 112 673 | 75.7 |
| 180–239 months | 490 191 | 20 554 | 4.2 | 34 398 | 7.0 | 25 802 | 75.0 |
| 240+ months | 257 961 | 13 652 | 5.3 | 31 276 | 12.1 | 23 305 | 74.5 |
| Top 10 cancer sites with the largest percent of CVD deaths | |||||||
| Urinary bladder | 143 846 | 34 079 | 23.7 | 27 907 | 19.4 | 21 618 | 77.5 |
| Larynx | 32 564 | 12 019 | 36.9 | 5648 | 17.3 | 4393 | 77.8 |
| Prostate | 509 128 | 89 722 | 17.6 | 84 534 | 16.6 | 65 355 | 77.3 |
| Corpus uteri | 113 614 | 19 809 | 17.4 | 17 692 | 15.6 | 12 947 | 73.2 |
| Rectum | 76 448 | 31 266 | 40.9 | 10 440 | 13.7 | 8057 | 77.2 |
| Breast | 516 225 | 119 981 | 23.2 | 60 409 | 11.7 | 44 475 | 73.6 |
| Kidney and renal pelvis | 77 882 | 27 165 | 34.9 | 8032 | 10.3 | 6198 | 77.2 |
| Non-Hodgkin lymphoma | 125 567 | 50 504 | 40.2 | 10 989 | 8.8 | 8634 | 78.6 |
| Melanoma of the skin | 121 290 | 17 904 | 14.8 | 9315 | 7.7 | 6968 | 74.8 |
| Lung and bronchus | 377 956 | 289 842 | 76.7 | 22 463 | 5.9 | 18 076 | 80.5 |
In the SEER program, between 1973 and 2012, 3 234 256 cancer patients representing 28 different cancer sites were identified. Morality rates from index-cancer, cardiovascular diseases, and particularly heart disease are presented as a function of demographic variables.
Figure 1.
Yearly cardiovascular death in cancer patients by cancer site. All deaths from cardiovascular causes in cancer survivors between 1973 and 2012 are categorized by cancer site. Cancer site and its representative colour are listed to the right of the graph and in descending order of appearance on the graph.
Objective 1: Cardiovascular deaths per calendar year
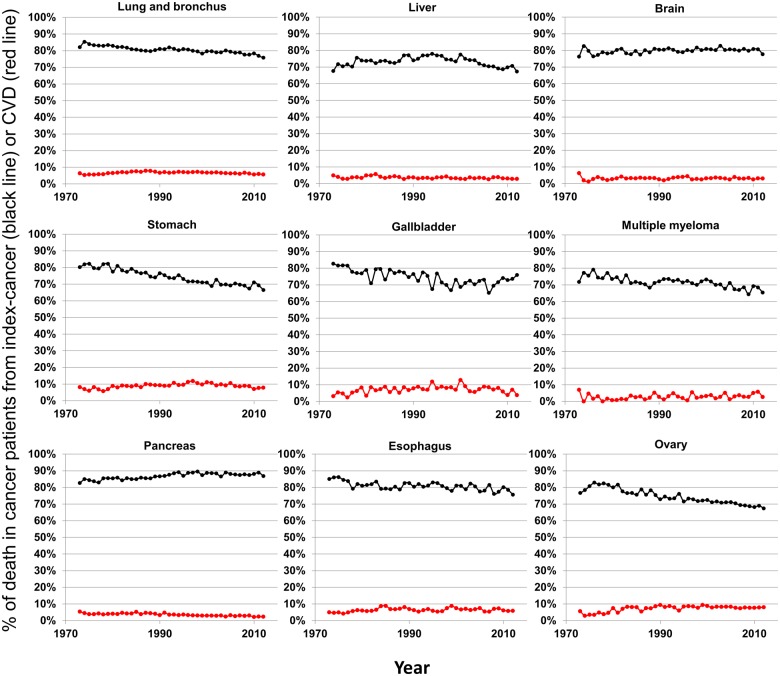
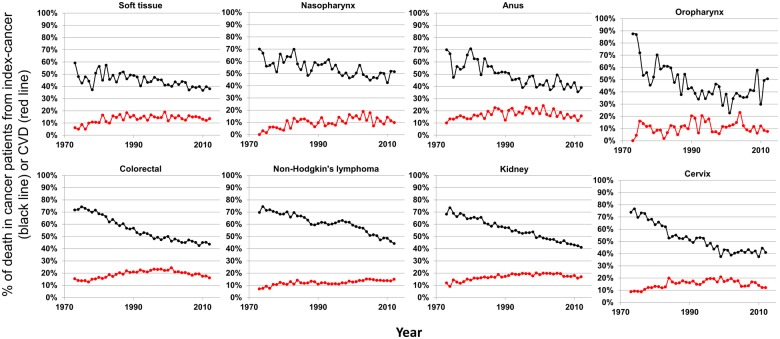
We examined historical trends in cancer patients by assessing deaths due to index-cancers (black lines) and deaths due to CVDs (red lines) in all 28 cancer sites (Figures 2, 3, and 4). Survivors who are least likely to die from CVDs (<10%) are those with cancers of the lung, liver, brain, stomach, gallbladder, multiple myeloma, pancreas, oesophagus, and ovary (Figure 2). These cancers are all associated with high mortality from index-cancer, and the prognosis has been relatively stable in the past decades. As prognosis for cancers improve (specifically: soft tissue, nasopharynx, anus, oropharynx, colorectal, non-Hodgkin’s lymphoma, kidney, and cervix), there appears to be a concomitant increase in deaths due to CVD, yet death from index-cancer still remains >10% higher than death from CVD (Figure 3).
Figure 2.
Plots of patient death vs. attained calendar year (from 1973 to 2012), for cancer sites with <20% decline in death due to index-cancer. Death was characterized as due to ‘index-cancer’, (black lines; i.e. the cancer originally diagnosed in the patient) and ‘cardiovascular disease’ (red lines). Attained calendar year refers to the year in which the death occurred.
Figure 3.
Plots of patient death vs. attained calendar year (from 1973 to 2012), for cancer sites with decreasing deaths due to index-cancer, yet, death from index-cancer still remains >10% higher than death from cardiovascular disease. Death was characterized as due to ‘index-cancer’, (black lines; i.e. the cancer originally diagnosed in the patient) and ‘cardiovascular disease’ (red lines). Attained calendar year refers to the year in which the death occurred.
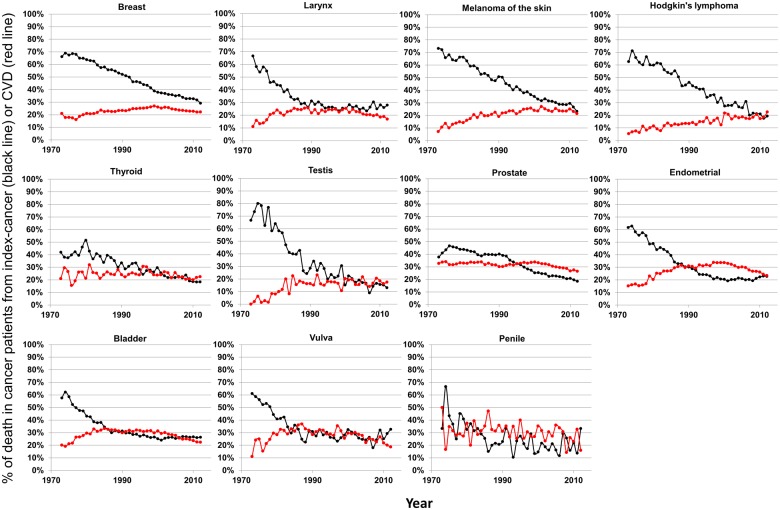
Figure 4.
Plots of patient death vs. attained calendar year (from 1973 to 2012), for cancer sites where death from index-cancer is either <10% higher than death from cardiovascular disease, or death from cardiovascular disease has surpassed death from index-cancer. Death was characterized as due to ‘index-cancer’, (black lines; i.e. the cancer originally diagnosed in the patient) and ‘cardiovascular disease’ (red lines). Attained calendar year refers to the year in which the death occurred.
Figure 4 presents cancer sites where death from index-cancer is either <10% higher than death from CVD, or death from CVD has surpassed death from index-cancer. Variation by calendar year between CVD or index-cancer as the leading cause of death occurs for several cancers. In 2012, death from index-cancer was the leading cause of death among most cancer patients (24 of 28 cancer sites). The four cancer sites with CVD as the leading cause of death for cancer patients in 2012 were prostate, thyroid, Hodgkin’s lymphoma, and testis (Figure 4).
Objective 2: Cardiovascular deaths by age at cancer diagnosis
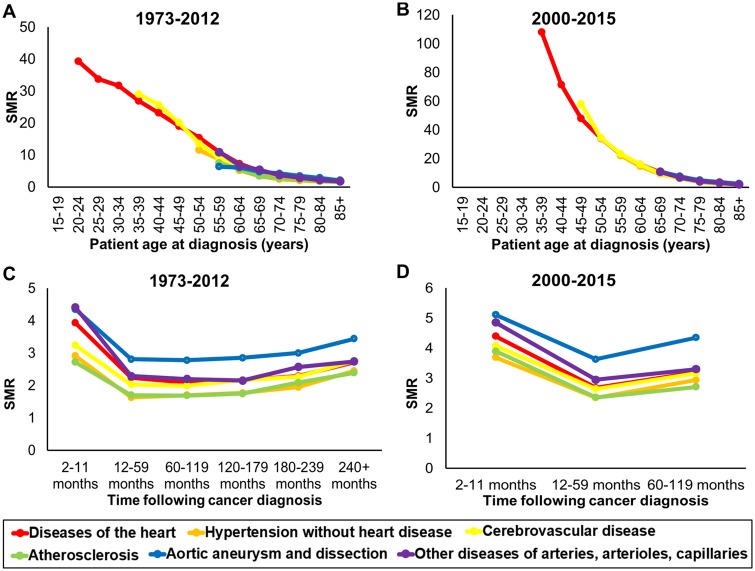
Cancer patients diagnosed at age 85 or younger (all sites) have an increased risk of death from heart disease compared to men and women in the general US population (Figure 5A, B). The younger a cancer survivor is diagnosed (all sites), the higher their risk of death from heart disease. However, the prevalence of death from heart disease in cancer survivors is very low with 340 cases of death in patients 15–35 years of age between 1973 and 2012. For survivors with cancers (all sites) diagnosed before 55 years old, the risk of CV mortality is more than 10-fold greater than the general population (Figure 5A). Risk of death from CV causes in cancer survivors (all sites) gradually decreases as age at cancer diagnosis increases (55–64 years of age: SMR 7.5; 65–74 years of age: SMR 3.8; 75–84 years of age: SMR 2.4), and this trend is maintained in the modern treatment area (Figure 5B). This is due to the risk of CVD death increasing in the general population as age increases. Standardized mortality ratios and CIs for risk of CVD death by age at cancer diagnosis are presented in Supplementary material online, Table S1.
Figure 5.
Cardiovascular disease mortality ratio by age at diagnosis and follow-up time in cancer patients. Standardized mortality ratios for the six types of cardiovascular diseases were characterized after diagnosis (all cancer sites), binned by patient age at diagnosis (A and B) and binned by period of follow-up time (C and D). Risk for cardiovascular disease mortality was assessed both historically (A and C) and specifically restricted to the modern treatment era (B and D). A standardized mortality ratio above 1 represents a higher relative risk of death for a type of cardiovascular cause, when compared to the general population (>1000 person years of risk for graphical inclusion).
Objective 3: Cardiovascular deaths during follow-up
The first year following a cancer diagnosis (all sites) represents the period with the highest risk of CV mortality (Figure 5C, D). Additionally, cancer survivors (all sites) displayed SMRs that remained higher than the general population from diagnosis forward (Figure 5C, D). Standardized mortality ratios and CIs for risk of CVD death by follow-up time after cancer diagnosis are presented in Supplementary material online, Table S2.
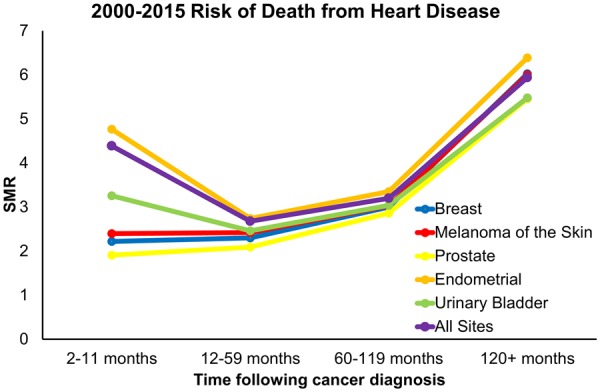
Furthermore, we assessed cancer sites in which patients had both (i) ≤30% risk of death from the index-cancer and ≥20% risk of mortality from CVD (same cancer sites as Figure 4) and (ii) at least 1000 person-years of data at each time point in the modern treatment era (2000–15). In Take home figure, we observed that relative to the average SMR for all 28 cancer sites, endometrial cancer has the greatest risk of mortality from heart disease at all time points following diagnosis. We also observed that compared to the first year following a cancer diagnosis, breast, melanoma, and prostate cancer patients have a continually elevated risk of mortality from heart disease.
Take home figure.

Standardized morality ratios for cancer sites with both ≤30% risk of death from the index-cancer and ≥20% risk of mortality from heart disease were calculated and binned by follow-up time. Cancers sites with at least 1000 person years of risk for death from heart disease between 2000 and 2015 were displayed.
Discussion
Previous studies reported CVDs in malignancies of breast,22,23 head and neck,24 prostate,24 testicular,25 renal cell carcinoma,26 endometrium,27 and Hodgkin lymphoma.28 A previous report by Abdel-Rahman assessed heart disease-specific mortality for 10 cancer sites in cancer survivors whom have lived 5+ years following diagnosis.29 However, the current study is the largest and most comprehensive characterization of cardiovascular mortality among 28 individual cancer sites using a national cancer registry with 40 years of data. These data underscore the importance of multidisciplinary care for cancer patients. Cardio-oncology is a developing standard of care for cancer patients at the point of diagnosis, but it is also important to highlight the role of PCPs and cardiologists throughout survivorship as we have observed that cancer patients remain at elevated risk for CV mortality compared to the general US population.
In 2012, we observed that 61% of all cancer patients whom died from CVDs were diagnosed with either breast, prostate, or bladder cancer. For patients with cancers of the penis, vulva, bladder, endometrium, prostate, testes, thyroid, or Hodgkin’s lymphoma, CV mortality has surpassed death from index-cancer during at least 1 year between 1973 and 2012. This study also describes the SMR of CVDs as a function of age at diagnosis and follow-up after diagnosis. We observed that cancer patients are perpetually at elevated risk of dying from CVDs compared to the general US population and the risk is negatively associated with age at diagnosis. Additionally, the first year following a cancer diagnosis represents the period with the highest SMR of CVDs.
We observed that in cancers with ≥20% risk of CVD mortality, these cancer were also all characterized by <30% risk of dying from index-cancer. Thus, this relationship seems to be the result of better prognosis in those cancers. For cancers with a more favourable prognosis, but still elevated risk of CV deaths, patients may benefit from clinical intervention by cardiologists at the point of diagnosis. Indeed, more research is needed to investigate the optimal approach in managing these patients and the collaboration between cardiologists and oncologists. Further, this study also highlights the need for ongoing and proactive surveillance by PCPs during cancer survivorship.
Evaluation of SMRs in this study provides important preliminary population-level data to support development of clinical screening tools which may aid clinicians in identifying cancer patients at risk for elevated CVD mortality. Consistent with previous reports, younger age of diagnosis is associated with higher SMR, both historically and in the modern treatment era.6 Similarly, the first year of cancer diagnosis represents the greatest risk for CVD mortality. This finding may be explained by the aggressive treatment shortly after disease discovery and long-time at risk until death of the general population.30,31 It could also be from the fact that patients who die early are those with the most severe co-existing CVDs, particularly in those with adult cancers.32 Patients with co-existing CVDs at cancer diagnosis are also at high risk of cardiotoxicity from cancer treatment.33,34 Unfortunately, pre-existing diseases prior to cancer diagnosis are not entered into the SEER program. Nonetheless, this finding supports early involvement of cardiologists. Of particular importance would be future studies addressing early cardiology evaluation and how aggressive cardiology care should be in cancer patients. Such studies of interventional cardio-oncology following cancer diagnosis might focus on cancers of the larynx and endometrium as these patients have a good cancer prognosis but relatively high risk of CVD mortality, particularly in the first year following diagnosis.
This study has several limitations. First, death due to CVDs may be miscoded and bias may arise towards an overestimate of heart disease in death certificate data.35 The long-range of accruing patients in the SEER database may contribute to the higher risk of death because of the smaller denominator. Patients diagnosed in recent years have short follow-up and therefore lower chance of dying from any cause (as observed in Table 1). We sought to mitigate this limitation by specifically examining a more modern treatment timeframe of 2000–15. All patients in the SEER registry were included to reduce the selection bias. For studies based on large population-based cancer registries, care is needed in interpretation as patients who are at increased risk of CVDs at cancer diagnosis, will tend not to be given potentially cardiotoxic chemotherapy if it can possibly be avoided. It is unknown what type of cancer treatment patients in the SEER database received though, and there is significant heterogeneity in cancer care and resulting impacts on cardiovascular biology. Moreover, our study did not reveal the influence of immune checkpoint inhibitors on the trend of CVD mortality as recent studies showed these medications have potential cardiac toxicity.36 Further, our analysis does not provide insight on the role of socioeconomic status (SES) in risk of CVD mortality following cancer diagnosis. Populations with low SES are more likely to have cancer and SES disparity may continue to contribute to CVD mortality risk in cancer survivors.37,38 In line with these limitations, it is acknowledged that the SEER database does not provide information on comorbidities or modifiable risk factors. However, the broad presentation of mortality patterns holds considerable importance for physician approach to CV health in cancer patients.
The foundational evidence provided by this investigation is a seminal characterization of 28 cancer sites for CVD mortality risk and provides insight on the scope of CVD mortality risk in cancer patients that has not yet been fully reported. Future directions include assessing patient risk stratification (sex, race, ethnicity, disease stage, and geographical location). Such an analysis is possible without risking unstable estimates and will improve identification of patients at highest risk for CVD mortality.
Conclusion
The majority of deaths (absolute numbers) from CVD occur in patients diagnosed with breast, prostate, or bladder cancer. For patients with cancers of the penis, vulva, bladder, endometrium, prostate, testes, thyroid, or Hodgkin’s lymphoma, there is year-to-year variation in leading cause of death (CV mortality or index-cancer mortality). We observed that from the point of cancer diagnosis forward into survivorship cancer patients (all sites) are at elevated risk of dying from CVDs compared to the general US population. In endometrial cancer patients, the first year of diagnosis poses a very high risk of dying from CVDs, which remains elevated compared to other cancer sites, supporting early involvement of cardiologists in such patients. Our observations highlight the need for earlier and more aggressive cardiovascular care in cancer patients which may require enhanced coordinated care between oncologists, cardiologists, and PCPs.
Supplementary Material
See page 3898 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz781)
Funding
National Center for Advancing Translational Sciences (NCATS) at the National Institute of Health (5 UL1 TR002014, 5 KL2 TR002015 to K.S.), National Institute of Health (LRP 1 L30 CA231572-01 to N.Z.); and the American Cancer Society (ACS) (MSRG-18-136-01-CPPB to S.B.). The National Center for Advancing Translational Sciences and the American Cancer Society had no role in the design of this study nor the execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest: Dr D.M.T. reports clinical research support from Novocure, outside the submitted work.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: Final Data for 2015. Natl Vital Stat Rep 2017;66:1–75. https://stacks.cdc.gov/view/cdc/50011. [PubMed] [Google Scholar]
- 3.WHO. Cardiovascular diseases. http://www.who.int/mediacentre/factsheets/fs317/en/ (18 October 2019).
- 4.WHO. Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/ (18 October 2019).
- 5. Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C.. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol 2016;34:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM.. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, Wilsgaard T, Bremnes RM, Fossa SD.. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol 2010;28:4649–4657. [DOI] [PubMed] [Google Scholar]
- 8. Lenihan DJ, Cardinale DM.. Late cardiac effects of cancer treatment. J Clin Oncol 2012;30:3657–3664. [DOI] [PubMed] [Google Scholar]
- 9. Rothe D, Paterson I, Cox-Kennett N, Gyenes G, Pituskin E.. Prevention of cardiovascular disease among cancer survivors: the role of pre-existing risk factors and cancer treatments. Current Epidemiol Rep 2017;4:239–247. [Google Scholar]
- 10. Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, Allemani C, Ciccolallo L, Santaquilani M, Berrino F, Group EW.. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol 2003;14 Suppl 5:v128–v149. [DOI] [PubMed] [Google Scholar]
- 11. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swiger K. Integration of CVD prevention into cancer survivorship In: Latest in Cardiology. Washington DC: American College of Cardiology; 2016. [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group E.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 14. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Baron-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol C, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GY, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S; Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), Document Reviewers. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 15.Surveillance Research Program, National Cancer Institute SEER*Stat Software Version 8.2.1. www.seer.cancer.gov/seerstat (18 October 2019).
- 16. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF.. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(8 Suppl):IV–3–IV18.. [DOI] [PubMed] [Google Scholar]
- 17. Kalbfleisch JD, Prentice RL.. The Statistical Analysis of Failure Time Data. 2nd ed Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 18. Klein JP, Moeschberger ML.. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed New York: Springer; 2003. [Google Scholar]
- 19. Koepsell TD, Weiss NS.. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 20. Breslow NE, Day NE.. Statistical Methods in Cancer Research: Volume II—The Design and Analysis of Cohort Studies. New York, NY: Oxford University Press; 1987. p1–406. [PubMed] [Google Scholar]
- 21. Ury HK, Wiggins AD.. Another shortcut method for calculating the confidence interval of a Poisson variable (or of a standardized mortality ratio). Am J Epidemiol 1985;122:197–198. [DOI] [PubMed] [Google Scholar]
- 22. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P.. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 23. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH; Pharmacovigilance Study Team . Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dubec JJ, Munk PL, Tsang V, Lee MJ, Janzen DL, Buckley J, Seal M, Taylor D.. Carotid artery stenosis in patients who have undergone radiation therapy for head and neck malignancy. Br J Radiol 1998;71:872–875. [DOI] [PubMed] [Google Scholar]
- 25. Sagstuen H, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, Wilsgaard T, Bremnes RM.. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol 2005;23:4980–4990. [DOI] [PubMed] [Google Scholar]
- 26. Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H.. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204–5212. [DOI] [PubMed] [Google Scholar]
- 27. Felix AS, Bower JK, Pfeiffer RM, Raman SV, Cohn DE, Sherman ME.. High cardiovascular disease mortality following endometrial cancer diagnosis: results from the Surveillance, Epidemiology, End Results Database. Int J Cancer 2017;140:555–564. [DOI] [PubMed] [Google Scholar]
- 28. Hull MC, Morris CG, Pepine CJ, Mendenhall NP.. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831–2837. [DOI] [PubMed] [Google Scholar]
- 29. Abdel-Rahman O. Risk of cardiac death among cancer survivors in the United States: a SEER database analysis. Expert Rev Anticancer Ther 2017;17:873–878. [DOI] [PubMed] [Google Scholar]
- 30. Girardi F, Franceschi E, Brandes AA.. Cardiovascular safety of VEGF-targeting therapies: current evidence and handling strategies. Oncologist 2010;15:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang K, Eblan MJ, Zagar TM, Lee CB, Deal AM, Jensen BC, Mavroidis P, Rosenman JG, Stinchcombe TE, Marks LB.. Cardiac toxicity after radiation for stage III non-small cell lung cancer: pooled analysis of several prospective dose-escalation trials delivering 70 to 90 Gy. J Radiat Oncol 2016;96:S130. [Google Scholar]
- 32. Koene RJ, Prizment AE, Blaes A, Konety SH.. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamnvik OP, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, Kaymakcalan MD, Williams JS.. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015;121:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morris PG, Hudis CA.. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol 2010;28:3407–3410. [DOI] [PubMed] [Google Scholar]
- 35. Lloyd-Jones DM, Martin DO, Larson MG, Levy D.. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med 1998;129:1020–1026. [DOI] [PubMed] [Google Scholar]
- 36. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, Pauschinger M, Gajewski TF, Lipson EJ, Luke JJ.. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A.. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol 2009;6:712–722. [DOI] [PubMed] [Google Scholar]
- 38. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P, Goda A, Demiraj AF, Weidinger F, Metzler B, Ibrahimov F, Pasquet AA, Claeys M, Thorton Y, Kusljugic Z, Smajic E, Velchev V, Ivanov N, Antoniades L, Agathangelou P, Táborský M, Gerdes C, Viigima M, Juhani PM, Juilliere Y, Cattan S, Aladashvili A, Hamm C, Kuck K-H, Papoutsis K, Bestehorn K, Foussas S, Giannoulidou G, Varounis C, Kallikazaros I, Kiss RG, Czétényi T, Becker D, Gudnason T, Kearney P, McDonald K, Rozenman Y, Ziv B, Bolognese L, Luciolli P, Boriani G, Berkinbayev S, Rakisheva A, Mirrakhimov E, Erglis A, Jegere S, Marinskis G, Beissel J, Marchal N, Kedev S, Xuereb RG, Tilney T, Felice T, Popovici M, Bax J, Mulder B, Simoons M, Elsendoorn M, Steigen TK, Atar D, Kalarus Z, Tendera M, Cardoso JS, Ribeiro J, Mateus C, Tatu-Chitoiu G, Seferovic P, Beleslin B, Simkova I, Durcikova P, Belicova V, Fras Z, Radelj S, Gonzalez Juanatey JR, Legendre S, Braunschweig F, Kaufmann UP, Rudiger-Sturchler M, Tokgozoglu L, Unver A, Kovalenko V, Nesukay E, Naum A, de Courtelary PT, Martin S, Sebastiao D, Ghislain D, Bardinet I, Logstrup S.. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.