Abstract
Described is a general method for the installation of a minimal 6-methyltetrazin-3-yl group via the first example of a Ag-mediated Liebeskind-Srogl cross-coupling. The attachment of bioorthogonal tetrazines on complex molecules typically relies on linkers that can negatively impact the physiochemical properties of conjugates. Cross-coupling with arylboronic acids and a new reagent, 3-((p-biphenyl-4-ylmethyl)thio)-6-methyltetrazine (b-Tz), proceeds under mild, PdCl2(dppf)-catalyzed conditions to introduce minimal, linker-free tetrazine functionality. Safety considerations guided our design of b-Tz which can be prepared on decagram scale without handling hydrazine and without forming volatile, high-nitrogen tetrazine byproducts. Replacing conventional Cu(I) salts used in Liebeskind-Srogl cross-coupling with a Ag2O mediator resulted in higher yields across a broad library of aryl and heteroaryl boronic acids and provides improved access to a fluorogenic tetrazine-BODIPY conjugate. A covalent probe for MAGL incorporating 6-methyltetrazinyl functionality was synthesized in high yield and labeled endogenous MAGL in live cells. This new Ag-mediated cross-coupling method using b-Tz is anticipated to find additional applications for directly introducing the tetrazine subunit to complex substrates.
Graphical Abstract
The bioorthogonal reactions of tetrazines have emerged as important tools for chemical biology over the last decade.1–6 Cycloadditions involving a range of dienophiles including trans-cyclooctenes1,7–10, cyclopropenes11–12 and norbornenes13 have been developed as tools for a variety of applications including cellular labeling14–17, in vivo imaging18–20, unnatural amino acid mutagenesis3,21–22, targeted drug delivery23–25, proteomics26, as well as in the fabrication and patterning of biomaterials27. Tetrazines themselves have also found applications in explosives technology28, in metal-organic frameworks29 and in natural product synthesis.30
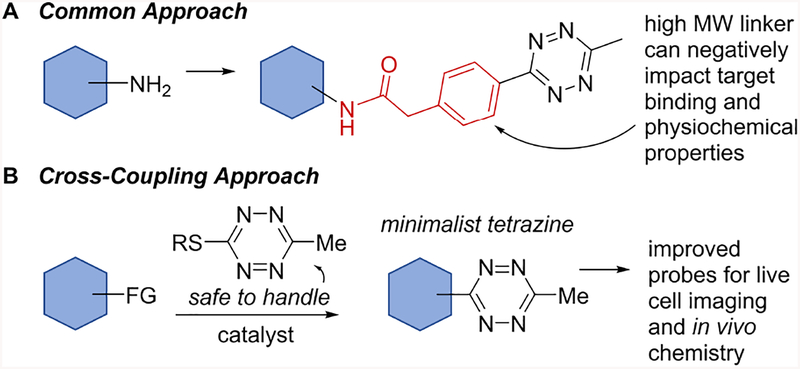
Conjugates of tetrazines are frequently prepared by amide bond forming reactions as represented in Figure 1A. A major limitation of this approach is that large and hydrophobic linkers can negatively impact the physiochemical properties of an attached ligand.6, 17 Complementary new methods for the introduction of minimal tetrazines to small molecules may further advance their potential as bioorthogonal probes and chemical reporters. The replacement of bulky derivatives with smaller tetrazines has resulted in fluorophores with improved fluorogenic and cellular wash-out properties31–33, better substrates for enzyme-catalyzed protein modification,17, 34 and probes for 18F-PET imaging35. However, there are currently few methods for the direct attachment of ‘minimal’ tetrazine fragments to target molecules.36 Additionally, many approaches to tetrazine synthesis produce high-nitrogen byproducts and involve harsh reaction conditions that can limit scalability and scope. Herein, we describe the decagram synthesis and thermal stability of 3-((p-biphenyl-4-ylmethyl)thio)-6-methyltetrazine, (b-Tz, 1a) and a method to directly introduce the 6-methyltetrazin-3-yl group to arylboronic acids through the first example of a Ag-mediated Liebeskind-Srogl reaction (Fig 1B).
Figure 1:
(A) The most common approach to tetrazine conjugation uses bulky linkers to attach molecules of interest. (B) Cross-coupling approach described here.
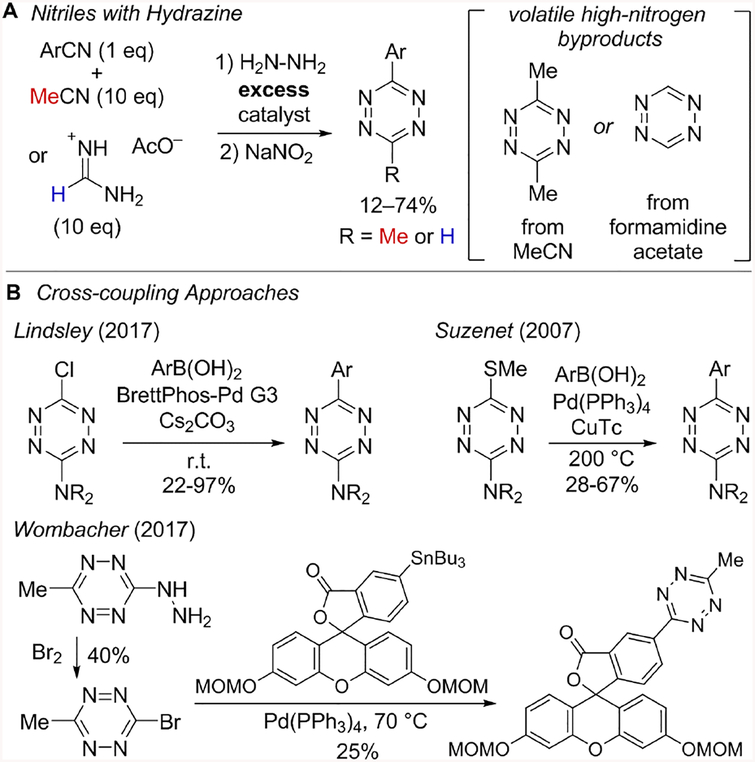
Classical tetrazine synthesis involves the condensation of Pinner salts or nitriles with excess hydrazine followed by oxidation.37–38 Catalytic nitrile condensation with neat anhydrous hydrazine, most notably with Zn(OTf)2 and Ni(OTf)2, has expanded access to unsymmetrical tetrazines.39 Further, thiol catalysis has been shown to promote tetrazine synthesis from nitriles using hydrazine-hydrate.40 The most practiced procedures utilize excess acetonitrile or formamidine acetate and produce volatile tetrazine byproducts with high-nitrogen content (Fig 2A). Recently, a sulfur-catalyzed reaction of nitriles with hydrazine hydrate and dichloromethane has been described for 3-aryltetrazine synthesis.41 A safety consideration for all of these procedures is the direct addition of an oxidant to a reaction mixture containing hydrazine. While these methods for preparing tetrazines have been transformative to the field of bioorthogonal chemistry, there is a continuing need for safer alternatives with complementary functional group compatibility.
Figure 2:
(A) Tetrazine synthesis based on condensation of nitriles or Pinner reagents with hydrazine (B) Cross-couplings of tetrazine electrophiles with arylboronic acids have been limited to N-substituted tetrazines, which are deactivated for bioorthogonal chemistry applications. Stille coupling has been used to couple 3-bromo-6-methyl-tetrazine to fluorophores.
Tetrazines have been used in a limited number of metal catalyzed CH activations,42–43 cross-couplings,32–33,44–51 and in Heck reactions with in situ generated 3-vinyl-6-methyltetrazine32. Recently, 3-amino-6-chlorotetrazines have been cross-coupled under Suzuki conditions (Fig 2B).45 Liebeskind-Srogl cross-couplings have also been reported with 3-amino-6-thiomethyl-tetrazines at 200 °C (Fig 2B).46 The 3-aminotetrazine products of these methods are valuable in medicinal chemistry, but their utility in bioorthogonal chemistry is attenuated by the deactivating amino substituent.21, 32 The tetrazines most useful to bioorthogonal chemistry are also sensitive to basic conditions, making them incompatible with many conditions commonly associated with cross-coupling chemistry. Currently, there is a single method of cross-coupling to introduce a 3-methylte-trazine fragment via Stille coupling with 3-bromo-6-methylte-trazine, which is prepared from 3-hydrazino-6-methylte-trazine (Fig 2B).33
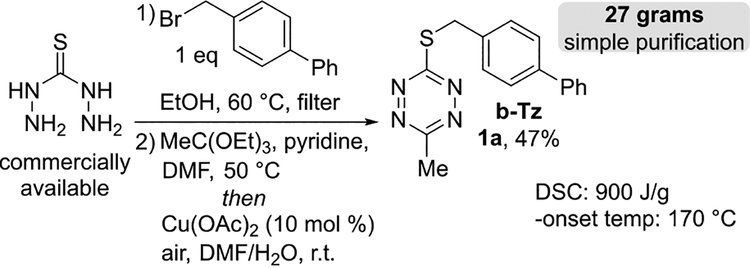
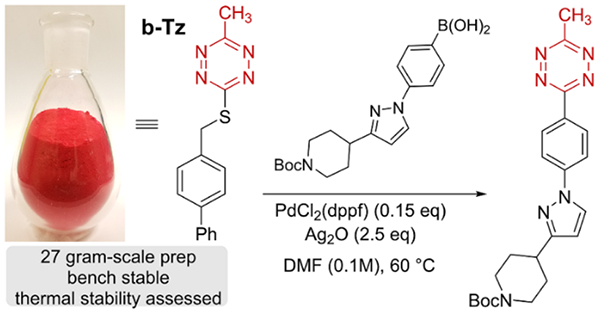
We considered that 3-thioalkyl-6-methyltetrazines might serve as useful reagents for the preparation of 3-aryl-6-methylte-trazines, which are attractive bioorthogonal reagents due to their balance of rapid kinetics toward dienophiles and high stability in the cellular environment.17, 21, 52 By modifying a method for the synthesis of 3-thiomethyl-6-methyltetrazine,53 we prepared compounds 1a-g with the rationale that a sacrificial S-benzylic substituent could serve to tune cross-coupling efficiency and improve the safety profile of the tetrazine. As shown in Figure 3, the 4-phenylbenzyl derivative b-Tz (1a) was prepared on large scale via alkylation of commercially available thiocarbohydrazide54 with 4-bromomethylbiphenyl followed by one-pot condensation with triethylorthoacetate and a novel Cu(OAc)2-catalyzed air-oxidation of the dihydrotetrazine intermediate. b-Tz was isolated on 27 gram scale with a 47% overall yield after simple silica plug filtration and is a bench-stable crystalline solid (m.p. 141°C). The differential scanning calorimetry (DSC) profile of b-Tz has an onset temperature of 170 °C and a transition enthalpy of 900 J/g and is not flagged as potentially shock sensitive or explosive by a modified Yoshida correlation (Fig S-11).55
Figure 3:
Decagram synthesis and thermal stability of b-Tz (1a).
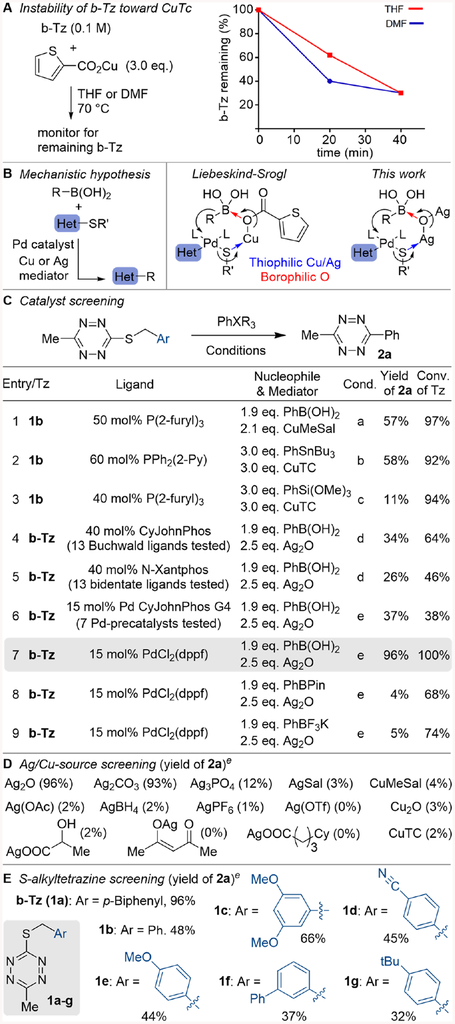
After extensive screening (Fig S-3 thru S-8), we found copper(I)-mediated Liebeskind-Srogl conditions56,57 could promote cross-coupling of benzylic thioether tetrazines with PhB(OH)2, PhSnBu3, and PhSi(OMe)3 (Fig 4C entries 1–3). Under Cu-mediated conditions tetrazine 1b was the best substrate; however, the generality under these conditions was modest. The rapid consumption of tetrazine starting materials during the reaction led us to test if Cu(I) was causing decomposition of the reagent. Indeed, heating b-Tz with Cu(I)-thiophene carboxylate (CuTC) at 70 °C resulted in rapid decomposition and produced 4-phenylbenzaldehyde as the only identifiable side product (Fig 4A).
Figure 4:
(A) Rapid decomposition of b-Tz in CuTC. (B) Proposed Liebeskind-Srogl transmetallation mechanism. (C) Optimized Pd-catalyzed cross-coupling of tetrazines b-Tz and 1b with various nucleophiles (yields determined by GC w/dodecane as a standard). Conditions: (a) Pd2dba3 (12.5 mol%), Cs2CO3 (3.0 eq.), dioxane, 70 °C, 90 min. (b) [Pd(allyl)Cl]2 (10 mol%), THF, 70 °C, 2 h. (c) Pd(OAc)2 (10 mol%), TBAF (1.0 eq.), dioxane, 70 °C, 2.5 h. (d) Pd2dba3 (15 mol%), DMF, 60 °C, 20 h. (e) DMF, 60 °C, 20 h. (D) Screening of silver(I) and copper(I) additives for condition e. (E) Screening of tetrazines 1a-g under condition e.
Copper has been proposed to promote the Liebeskind-Srogl reaction by facilitating transmetallation as shown in Figure 4B.57–58 We hypothesized that silver(I) salts might be similarly capable as promotors, whereby transmetallation would be promoted in a dual role by the thiophilic capture of benzylic thiolate by silver and the borophilic capture by oxygen. Ag(I) additives have been shown to promote Rh-catalyzed coupling of arylboronic acids with arylmethylsulfides bearing ortho-directing groups59–60, and the Cu-catalyzed coupling of arylboronic acids with aromatic thioesters61. To our knowledge, a Ag-mediated variation of the Liebeskind-Srogl reaction has not been reported. After extensive optimization (Fig 4C entries 4–7, S-1 and S-2), PdCl2(dppf) (15 mol%) was found to be especially effective for cross-coupling of 3-thioalkyl-6-methylte-trazines with arylboronic acids in polar, aprotic solvents (DMF, DMSO) at 60 °C. A screening of silver(I) additives revealed Ag2O as the most general promotor, although Ag2CO3 was also effective (Fig 4D). Substitution of Ag2O by Cu2O gave only trace product formation. Arylboronic acids are particularly effective nucleophiles, whereas PhBF3K and PhBPin were both less effective under identical reaction conditions (Fig 4C entries 8–9). Further, a series of 3-arylmethyl-6-methyltetrazines 1a-g were evaluated as coupling partners (Fig 4E). Of these, the 4-phenylbenzyl derivative b-Tz (1a) was identified as the substrate with both the best cross-coupling yield as well as most favorable thermal stabiliy. We also note that the cost of Ag2O (currently <$3/g) is similar to the common promotor CuTC, and is minor in the context of bioorthogonal chemistry reagents which are typically required only in small amounts.
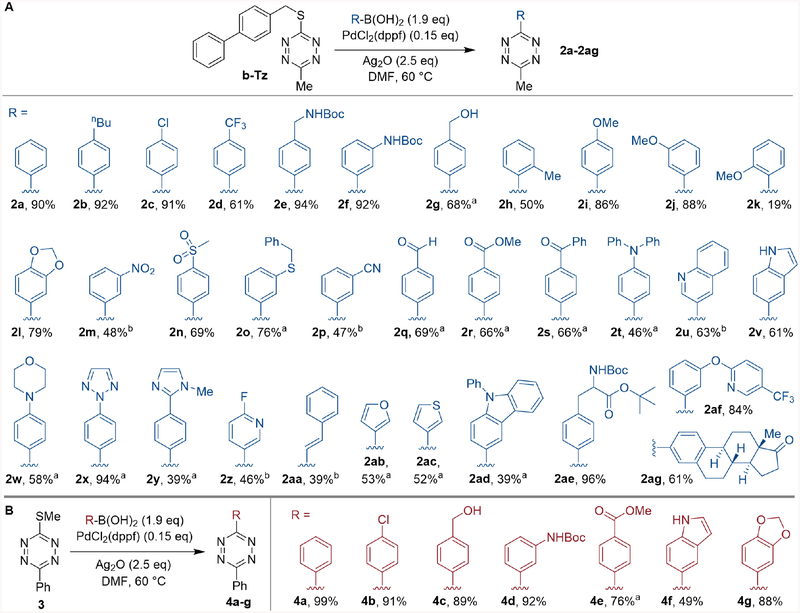
The scope of the Ag-mediated, Pd-catalyzed coupling of b-Tz with arylboronic acids is summarized in Figure 5A. Successful reactions were observed for arylboronic acids containing chloro-, fluoro-, secondary and tertiary amino-, alcohol, ether, nitro, sulfonyl, thioether, nitrile, aldehyde, ester, ketone, carbamate, and styryl groups. Heterocyclic functionality tolerated on the boronic acid component included quinoline, indole, pyridine, triazole, N-methylimidazole, furan and thiophene groups. The protected amino acid 2ae coupled with b-Tz in 96% yield. Estrone-tetrazine 2ag was also synthesized in 61%. In general, couplings were carried out using 1.9 equiv. of boronic acid, but 3.0 equiv. were utilized in reactions where homocoupling of the boronic acid was pronounced. ortho-Substituted heteroatoms had a deleterious impact with a relatively low yield observed for ortho-methoxy tetrazine 2k and only trace product with N-Boc-2-aminophenylboronic acid and 2-hydroxyphenylboronic acid. While protected thiol and amine functionality was well tolerated (Fig 5), additives with free thiol or primary alkyl amine groups were not (Fig S-19). Also unsuccessful were 2-pyridyl- and 4-pyridylboronic acids which are regarded as problematic across other cross-coupling reactions.62
Figure 5:
Reaction scope of b-Tz (5A) and 3 (5B). Typical conditions: thioalkyl tetrazine b-Tz or 3 (1.0 equiv.), RB(OH)2 (1.9 equiv.), PdCl2(dppf) (15 mol%), Ag2O (2.5 equiv.), DMF (0.1M), 60°C, 19–21h, average isolated yields of duplicate synthesis (± 5%). a 3.0 equiv. of RB(OH)2. b 3.0 equiv. of RB(OH)2 did not significantly improve yield (< 5%), 1.9 equiv. of RB(OH)2 was used.
This cross-coupling method is not limited to S-benzylic thioethers or methyl-substituted tetrazines. 3-(Methylthio)-6-phenyl-tetrazine (3) was prepared from triethyl orthobenzoate and evaluated as a reagent in the synthesis of diaryltetrazines (Fig 5B). Successful reactions were observed for arylboronic acids bearing chloro-, alcohol, carbamate, ester, indole and ether groups with yields comparable to b-Tz. Included is an improved synthesis of 3-(4-hydroxymethylphenyl)-6-phenyltetrazine (4c), which is used to create cell-contact guiding micro-fibrous materials for tissue-culture applications.27
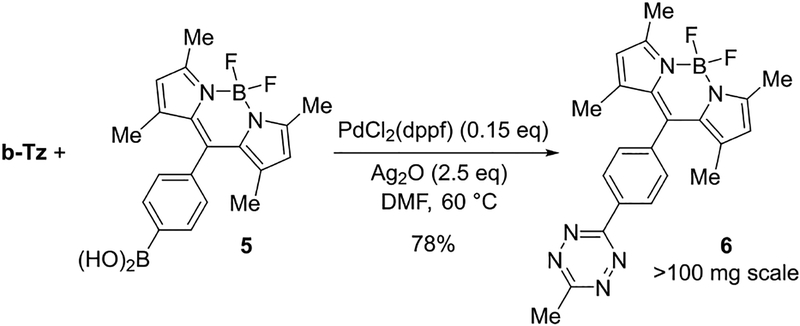
We sought to demonstrate the application of b-Tz for the construction of fluorophore-tetrazine conjugates—compounds that have utility in live cell imaging.15 BODIPY-dye 6 with a directly attached tetrazine has been developed as ‘superbright’ bioorthogonal probe for fluorogenic labeling in live cells.31 The condensation of nitriles with hydrazine produces 6 in 8% yield.31 As shown in Figure 6, compound 6 can be accessed in 78% yield through the Ag-mediated cross-coupling of boronic acid 5 with b-Tz.
Figure 6:
Synthesis of 3-BODIPY-6-methyltetrazine 6.
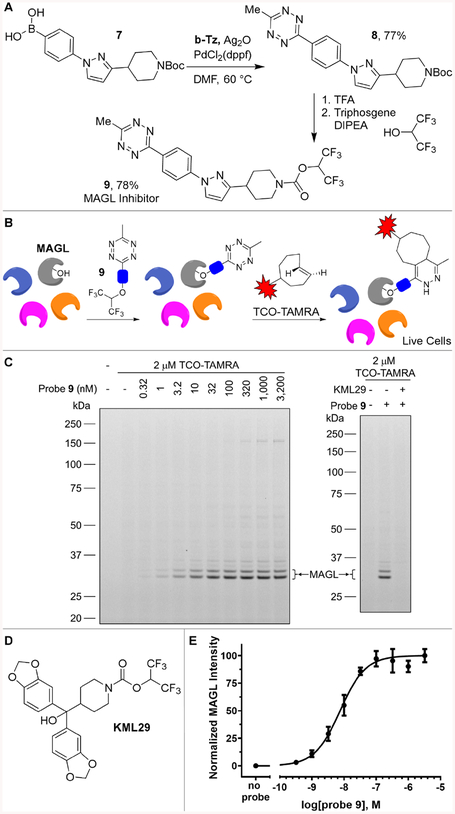
To demonstrate the utility of b-Tz in synthesizing chemical probes for studying endogenous levels of a protein in a native biological system, we constructed a tetrazine probe for monoacylglycerol lipase (MAGL). MAGL is a serine hydrolase in the endocannabinoid signaling pathway, and has attracted increasing interest as a target for neurological and metabolic disorders.63 We designed a MAGL probe (9) by appending a 6-methyltetrazine moiety to a pyrazolylpiperidine scaffold with an electrophilic hexafluoroisopropyl (HFIP) carbamate warhead for covalently labeling the active site serine (Fig 7A).64 Synthesis was accomplished by cross-coupling of b-Tz with boronic acid 7 resulting in a 77% yield of 8. The reactive HFIP carbamate was installed by Boc deprotection followed by in situ addition to a triphosgene and hexafluoroisopropanol mixture, giving the MAGL reactive probe 9 in 78% yield. The reaction rate of 9 toward trans-cyclooctene is similar to that of 3-methyl-6-[4-aminomethyl]tetrazine (krel 1.1, Fig S-20).65 Probe 9 inhibited MAGL activity with 31 nM IC50 in an in vitro assay.66
Figure 7:
(A) Synthesis of MAGL reactive probe 9. (B) Live cells were treated with probe 9 for 1 h, followed by 2 μM TCO-TAMRA for 30 min, cell lysis, and analysis by in-gel fluorescence (C) In-gel fluorescence signals for a dose response of probe 9. Probe 9 (32 nM, 1 h) was competed by pre-treatment with MAGL inhibitor KML29 (300 nM, 1 h). (D) KML29 also incorporates a HFIP carbamate warhead. (E) Dose response fitting of the fluorescence signals of MAGL normalized by the total protein amount indicated by Coomassie staining. Data are reported as mean ± SEM (n = 2). See Fig S-21 for Coomassie staining.
To test the labeling of endogenous MAGL in live cells, human brain vascular pericytes were treated with probe 9 for 1 h, followed by labeling with 2 μM of TCO-TAMRA for 30 min in live cells (Fig 7B). After cell lysis, MAGL labeling was assessed with a gel-based activity-based protein profiling (ABPP) analysis (Fig 7C–E).67 Strong fluorescence signals were observed for MAGL with minimal non-specific labeling from TCO-TAMRA. The labeling by probe 9 was dose responsive with a cellular IC50 of 8 nM, and was competed by a MAGL inhibitor, KML29.64 The HFIP warhead also labeled an additional protein at ~35 kDa, which is consistent with its reactivity with α/β-hydrolase domain 6 (ABHD6), and other off-targets at higher concentrations.64, 67
In summary, a method has been described for installing a minimal 6-methyltetrazinyl-3-yl group through the first Ag-mediated Liebeskind-Srogl cross-coupling. A combination of PdCl2(dppf) catalyst and Ag2O mediator was found to be uniquely effective for coupling 3-thioalkyl-6-methyltetrazines with arylboronic acids. Safety-testing guided our design of the reactive substrate b-Tz (1a), which can be synthesized from commercially available materials on decagram scale in 47% overall yield. Cross-coupling of b-Tz with boronic acids proceeds under mild conditions with broad functional group tolerance. Alternatively, 3-(Methylthio)-6-phenyl-tetrazine (3) undergoes cross-coupling with arylboronic acids to give 3,6-diaryltetrazines. Application to the synthesis of chemical biology tools was demonstrated. A BODIPY-tetrazine conjugate was synthesized in 78% yield—substantially higher than what is possible using traditional hydrazine-based synthesis. Finally, a tetrazine-functionalized probe for MAGL was synthesized in high yield and was shown to covalently label endogenous MAGL with good selectivity in live cells. We anticipate that this method for introducing minimal tetrazines to chemical probes will serve as an important tool for studying protein targets at endogenous levels in their native environment.
Supplementary Material
ACKNOWLEDGMENT
We would like to thank Jeffery Sperry for helpful discussions, Remzi Duzguner for DSC analysis, and Lucy Stevens for the in vitro potency data on probe 9. This work was supported by NIH GM132460, NSF 1809612 and Pfizer. Instrumentation was supported by NIH P20GM104316, P30GM110758, S10RR026962, S10OD016267 and NSF CHE-0840401, CHE-1229234, and CHE-1048367.
Footnotes
Supporting Information
Optimization tables, DSC data, experimental procedures, kinetics, inhibition assays, and 1H and 13C NMR data are displayed. The Supporting Information is available free of charge on the ACS Publications website at DOI: xxxxxxxxxxxxx
The authors declare no competing financial interests.
REFERENCES
- (1).Selvaraj R; Fox JM, trans-Cyclooctene--a stable, voracious dienophile for bioorthogonal labeling. Curr. Opin. Chem. Biol 2013, 17, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Patterson DM; Nazarova LA; Prescher JA, Finding the right (bioorthogonal) chemistry. ACS Chem. Biol 2014, 9, 592–605. [DOI] [PubMed] [Google Scholar]
- (3).Lang K; Chin JW, Bioorthogonal reactions for labeling proteins. ACS Chem. Biol 2014, 9, 16–20. [DOI] [PubMed] [Google Scholar]
- (4).Oliveira BL; Guo Z; Bernardes GJL, Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev 2017, 46, 4895–4950. [DOI] [PubMed] [Google Scholar]
- (5).Wu H; Devaraj NK, Advances in Tetrazine Bioorthogonal Chemistry Driven by the Synthesis of Novel Tetrazines and Dienophiles. Acc. Chem. Res 2018, 51, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Cañeque T; Müller S; Rodriguez R, Visualizing biologically active small molecules in cells using click chemistry. Nat. Rev. Chem 2018, 2, 202–215. [Google Scholar]
- (7).Blackman ML; Royzen M; Fox JM, Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Taylor MT; Blackman ML; Dmitrenko O; Fox JM, Design and synthesis of highly reactive dienophiles for the tetrazine-trans-cyclooctene ligation. J. Am. Chem. Soc 2011, 133, 9646–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Darko A; Wallace S; Dmitrenko O; Machovina MM; Mehl RA; Chin JW; Fox JM, Conformationally Strained trans-Cyclooctene with Improved Stability and Excellent Reactivity in Tetrazine Ligation. Chem. Sci 2014, 5, 3770–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lambert WD; Scinto SL; Dmitrenko O; Boyd SJ; Magboo R; Mehl RA; Chin JW; Fox JM; Wallace S, Computationally guided discovery of a reactive, hydrophilic trans-5-oxocene dienophile for bioorthogonal labeling. Org. Biomol. Chem 2017, 15, 6640–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Patterson DM; Nazarova LA; Xie B; Kamber DN; Prescher JA, Functionalized cyclopropenes as bioorthogonal chemical reporters. J. Am. Chem. Soc 2012, 134, 18638–18643. [DOI] [PubMed] [Google Scholar]
- (12).Yang J; Seckute J; Cole CM; Devaraj NK, Live-cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew. Chem. Int. Ed 2012, 51, 7476–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Devaraj NK; Weissleder R; Hilderbrand SA, Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconjug. Chem 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Devaraj NK; Upadhyay R; Haun JB; Hilderbrand SA; Weissleder R, Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew. Chem. Int. Ed 2009, 48, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Devaraj NK; Hilderbrand S; Upadhyay R; Mazitschek R; Weissleder R, Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew. Chem. Int. Ed 2010, 49, 2869–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Murrey HE; Judkins JC; am Ende CW; Ballard TE; Fang Y; Riccardi K; Di L; Guilmette ER; Schwartz JW; Fox JM; Johnson DS, Systematic Evaluation of Bioorthogonal Reactions in Live Cells with Clickable HaloTag Ligands: Implications for Intracellular Imaging. J. Am. Chem. Soc 2015, 137, 11461–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Baalmann M; Ziegler MJ; Werther P; Wilhelm J; Wombacher R, Enzymatic and Site-Specific Ligation of Minimal-Size Tetrazines and Triazines to Proteins for Bioconjugation and Live-Cell Imaging. Bioconjug. Chem 2019, 30, 1405–1414. [DOI] [PubMed] [Google Scholar]
- (18).Rossin R; Verkerk PR; van den Bosch SM; Vulders RC; Verel I; Lub J; Robillard MS, In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed 2010, 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- (19).Zeglis BM; Sevak KK; Reiner T; Mohindra P; Carlin SD; Zanzonico P; Weissleder R; Lewis JS, A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med 2013, 54, 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang M; Vannam R; Lambert WD; Xie Y; Wang H; Giglio B; Ma X; Wu Z; Fox J; Li Z, Hydrophilic (18)F-labeled trans-5-oxocene (oxoTCO) for efficient construction of PET agents with improved tumor-to-background ratios in neurotensin receptor (NTR) imaging. Chem. Commun 2019, 55, 2485–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Blizzard RJ; Backus DR; Brown W; Bazewicz CG; Li Y; Mehl RA, Ideal Bioorthogonal Reactions Using A Site-Specifically Encoded Tetrazine Amino Acid. J. Am. Chem. Soc 2015, 137, 10044–10047. [DOI] [PubMed] [Google Scholar]
- (22).Hoffmann JE; Plass T; Nikic I; Aramburu IV; Koehler C; Gillandt H; Lemke EA; Schultz C, Highly Stable trans-Cyclooctene Amino Acids for Live-Cell Labeling. Chemistry 2015, 21, 12266–12270. [DOI] [PubMed] [Google Scholar]
- (23).Li J; Chen PR, Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol 2016, 12, 129–137. [DOI] [PubMed] [Google Scholar]
- (24).Versteegen RM; Rossin R; ten Hoeve W; Janssen HM; Robillard MS, Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed 2013, 52, 14112–14116. [DOI] [PubMed] [Google Scholar]
- (25).Mejia Oneto JM; Khan I; Seebald L; Royzen M, In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma. ACS Cent. Sci 2016, 2, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kang K; Park J; Kim E, Tetrazine ligation for chemical proteomics. Proteome. Sci 2016, 15, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Liu S; Moore AC; Zerdoum AB; Zhang H; Scinto SL; Zhang H; Gong L; Burris DL; Rajasekaran AK; Fox JM; Jia X, Cellular interactions with hydrogel microfibers synthesized via interfacial tetrazine ligation. Biomaterials 2018, 180, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).DeLuca LT; Shimada T; Sinditskii VP; Calabro M, Chemical Rocket Propulsion: A Comprehensive Survey of Energetic Materials Springer International Publishing: 2017. [Google Scholar]
- (29).Xue M; Ma S; Jin Z; Schaffino RM; Zhu GS; Lobkovsky EB; Qiu SL; Chen B, Robust metal-organic framework enforced by triple-framework interpenetration exhibiting high H2 storage density. Inorg. Chem 2008, 47, 6825–6828. [DOI] [PubMed] [Google Scholar]
- (30).Hamasaki A; Zimpleman JM; Hwang I; Boger DL, Total synthesis of ningalin D. J. Am. Chem. Soc 2005, 127, 10767–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Carlson JC; Meimetis LG; Hilderbrand SA; Weissleder R, BODIPY-tetrazine derivatives as superbright bioorthogonal turn-on probes. Angew. Chem. Int. Ed 2013, 52, 6917–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wu H; Yang J; Seckute J; Devaraj NK, In situ synthesis of alkenyl tetrazines for highly fluorogenic bioorthogonal live-cell imaging probes. Angew. Chem. Int. Ed 2014, 53, 5805–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wieczorek A; Werther P; Euchner J; Wombacher R, Green- to far-red-emitting fluorogenic tetrazine probes - synthetic access and no-wash protein imaging inside living cells. Chem. Sci 2017, 8, 1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Best M; Degen A; Baalmann M; Schmidt TT; Wombacher R, Two-step protein labeling by using lipoic acid ligase with norbornene substrates and subsequent inverse-electron demand Diels-Alder reaction. Chembiochem 2015, 16, 1158–1162. [DOI] [PubMed] [Google Scholar]
- (35).Denk C; Svatunek D; Filip T; Wanek T; Lumpi D; Frohlich J; Kuntner C; Mikula H, Development of a (18) F-labeled tetrazine with favorable pharmacokinetics for bioorthogonal PET imaging. Angew. Chem. Int. Ed 2014, 53, 9655–9659. [DOI] [PubMed] [Google Scholar]
- (36).For the introduction of bioorthogonal triazines via Suzuki coupling, see:; Kamber DN; Liang Y; Blizzard RJ; Liu F; Mehl RA; Houk KN; Prescher JA, 1,2,4-Triazines Are Versatile Bioorthogonal Reagents. J. Am. Chem. Soc 2015, 137, 8388–91. [DOI] [PubMed] [Google Scholar]
- (37).Pinner A, Ueber die Einwirkung von Hydrazin auf Imidoather. Ber. Dtsch. Chem. Ges 1893, 26, 2126. [Google Scholar]
- (38).Tolshchina SG; Rusinov GL; Charushin VN, 1,2,4,5-Tetrazines and Azolo[1,2,4,5]tetrazines: Synthesis and Reactions with Nucleophiles. Chem. Heterocycl. Compd 2013, 49, 66–91. [Google Scholar]
- (39).Yang J; Karver MR; Li W; Sahu S; Devaraj NK, Metal-catalyzed one-pot synthesis of tetrazines directly from aliphatic nitriles and hydrazine. Angew. Chem. Int. Ed 2012, 51, 5222–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mao W; Shi W; Li J; Su D; Wang X; Zhang L; Pan L; Wu X; Wu H, Organocatalytic and Scalable Syntheses of Unsymmetrical Tetrazines by Thiol-Containing Promotors. Angew. Chem. Int. Ed 2019, 58, 1106–1109. [DOI] [PubMed] [Google Scholar]
- (41).Qu Y; Sauvage FX; Clavier G; Miomandre F; Audebert P, Metal-Free Synthetic Approach to 3-Monosubstituted Unsymmetrical Tetrazines Useful for Bioorthogonal Reactions. Angew. Chem. Int. Ed 2018, 57, 12057–12061. [DOI] [PubMed] [Google Scholar]
- (42).Testa C; Gigot E; Genc S; Decreau R; Roger J; Hierso JC, Ortho-Functionalized Aryltetrazines by Direct Palladium-Catalyzed C-H Halogenation: Application to Fast Electrophilic Fluorination Reactions. Angew. Chem. Int. Ed 2016, 55, 5555–5559. [DOI] [PubMed] [Google Scholar]
- (43).Mboyi CD; Testa C; Reeb S; Genc S; Cattey H; Fleurat-Lessard P; Roger J; Hierso J-C, Building Diversity in ortho-Substituted s-Aryltetrazines By Tuning N-Directed Palladium C-H Halogenation: Unsymmetrical Polyhalogenated and Biphenyl s-Aryltetrazines. ACS Catalysis 2017, 7, 8493–8501. [Google Scholar]
- (44).Novak Z; Kotschy A, First Cross-Coupling Reactions on Tetrazines. Org. Lett 2003, 5, 3495–3497. [DOI] [PubMed] [Google Scholar]
- (45).Bender AM; Chopko TC; Bridges TM; Lindsley CW, Preparation of Unsymmetrical 1,2,4,5-Tetrazines via a Mild Suzuki Cross-Coupling Reaction. Org. Lett 2017, 19, 5693–5696. [DOI] [PubMed] [Google Scholar]
- (46).Suzenet F; Leconte N; Keromnes-Wuillaume A; Guillaumet G, Efficient Palladium-Catalyzed Synthesis of Unsymmetrical (Het)aryl-tetrazines. Synlett 2007, 2007, 204–210. [Google Scholar]
- (47).Pellegatti L; Vedrenne E; Leger J-M; Jarry C; Routier S, New heteroaromatic aminations on 5-aryl-1,2,4-triazines and 1,2,4,5-tetrazines by palladium catalysis. Tetrahedron 2010, 66, 4383–4389. [Google Scholar]
- (48).Quinton C; Alain-Rizzo V; Dumas-Verdes C; Clavier G; Vignau L; Audebert P, Triphenylamine/tetrazine based π-conjugated systems as molecular donors for organic solar cells. New J. Chem 2015, 39, 9700–9713. [Google Scholar]
- (49).Wieczorek A; Buckup T; Wombacher R, Rigid tetrazine fluorophore conjugates with fluorogenic properties in the inverse electron demand Diels-Alder reaction. Org. Biomol. Chem 2014, 12, 4177–4185. [DOI] [PubMed] [Google Scholar]
- (50).Soloducho J; Doskocz J; Cabaj J; Roszak S, Practical synthesis of bis-substituted tetrazines with two pendant 2-pyrrolyl or 2-thienyl groups, precursors of new conjugated polymers. Tetrahedron 2003, 59, 4761–4766. [Google Scholar]
- (51).Li Z; Ding J; Song N; Lu J; Tao Y, Development of a New s-Tetrazine-Based Copolymer for Efficient Solar Cells. J. Am. Chem. Soc 2010, 132, 13160–13161. [DOI] [PubMed] [Google Scholar]
- (52).Karver MR; Weissleder R; Hilderbrand SA, Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconjug. Chem 2011, 22, 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Fields SC; Parker MH; Erickson WR, A Simple Route to Unsymmetrically Substituted 1,2,4,5-Tetrazines. J. Org. Chem 1994, 59, 8284–8287. [Google Scholar]
- (54).DSC data for commercially available Thiocarbohydrazide: onset temp. of 172 °C, transition enthalpy of 1634 J/g. As a precaution, all experiments involving thiocarbohydrazide were carried out below 72 °C (100 °C below the DSC transition onset).
- (55).Sperry JB; Minteer CJ; Tao J; Johnson R; Duzguner R; Hawksworth M; Oke S; Richardson PF; Barnhart R; Bill DR; Giusto RA; Weaver JD, Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing. Org. Process Res. Dev 2018, 22, 1262–1275. [Google Scholar]
- (56).Cheng H-G; Chen H; Liu Y; Zhou Q, The Liebeskind-Srogl Cross-Coupling Reaction and its Synthetic Applications. Asian J. Org. Chem 2018, 7, 490–508. [Google Scholar]
- (57).Liebeskind LS; Srogl J, Heteroaromatic Thioether-Boronic Acid Cross-Coupling under Neutral Reaction Conditions. Org. Lett 2002, 4, 979–981. [DOI] [PubMed] [Google Scholar]
- (58).Musaev DG; Liebeskind LS, On the Mechanism of Pd(0)-Catalyzed, Cu(I) Carboxylate-Mediated Thioorganic-Boronic Acid Desulfitative Coupling. A Non-innocent Role for Carboxylate Ligand. Organometallics 2009, 28, 4639–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Hooper JF; Young RD; Pernik I; Weller AS; Willis MC, Carbon-carbon bond construction using boronic acids and aryl methyl sulfides: orthogonal reactivity in Suzuki-type couplings. Chem. Sci 2013, 4, 1568–1572. [Google Scholar]
- (60).Pan F; Wang H; Shen P-X; Zhao J; Shi Z-J, Cross coupling of thioethers with aryl boroxines to construct biaryls via Rh catalyzed C-S activation. Chem. Sci 2013, 4, 1573–1577. [Google Scholar]
- (61).Ghosh P; Ganguly B; Perl E; Das S, A synthesis of biaryl ketones via the C-S bond cleavage of thiol ester by a Cu/Ag salt. Tetrahedron Lett 2017, 58, 2751–2756. [Google Scholar]
- (62).Markovic T; Rocke BN; Blakemore DC; Mascitti V; Willis MC, Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides. Chem. Sci 2017, 8, 4437–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Blankman JL; Cravatt BF, Chemical probes of endocannabinoid metabolism. Pharmacol. Rev 2013, 65, 849–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chang JW; Niphakis MJ; Lum KM; Cognetta AB 3rd; Wang C; Matthews ML; Niessen S; Buczynski MW; Parsons LH; Cravatt BF, Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem. Biol 2012, 19, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Electron withdrawing aromatic substitutents have an accelerating effect on Diels-Alder rate constants with tetrazines, see ref 9 and ref 52.
- (66).Butler CR; Beck EM; Harris A; Huang Z; McAllister LA; Am Ende CW; Fennell K; Foley TL; Fonseca K; Hawrylik SJ; Johnson DS; Knafels JD; Mente S; Noell GS; Pandit J; Phillips TB; Piro JR; Rogers BN; Samad TA; Wang J; Wan S; Brodney MA, Azetidine and Piperidine Carbamates as Efficient, Covalent Inhibitors of Monoacylglycerol Lipase. J. Med. Chem 2017, 60, 9860–9873. [DOI] [PubMed] [Google Scholar]
- (67).Chang JW; Cognetta AB 3rd; Niphakis MJ; Cravatt BF, Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition. ACS Chem. Biol 2013, 8, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.