Abstract
Background:
Influenza virus, associated with high level of morbidity and mortality, has been recently considered a public health concern while the choices for the control and treatment of the disease are limited. The present study was conducted to evaluate activity of pomegranate peel extract and its fractions against Influenza A virus in vitro .
Methods:
In this research, ethyl alcohol extract of pomegranate peel was prepared and subjected to fractionation with different polarities. The potential in vitro anti-influenza A virus activity of the extract and fractions was assessed using Cytopathic Effect (CPE) reduction assay, Hemagglutinin Assay (HA), and 50% Tissue Culture Infectious Doses (TCID50) method in Madin-Darby Canine Kidney (MDCK) cells.
Results:
The crude pomegranate peel extract and its n-butanol and ethyl acetate fractions had the highest inhibitory effect against influenza A virus with IC50 value of 6.45, 6.07 and 5.6 μg/ml in MDCK cells, respectively. Our results also showed that, the production of virus was significantly reduced upon treatment with crude extract, n-butanol and ethyl acetate fractions in a dose-dependent manner (p<0.05).
Conclusion:
Based on our results, the ethyl alcohol extract and its polar fractions of pomegranate peel can inhibit influenza A virus replication in vitro. Therefore, further characterization of its active ingredients and the mechanism of action should be carried out.
Keywords: Antiviral Agents, Pomegranate, Punica granatum L
Introduction
Pomegranate (Punica granatum L., belonging to the Punicaceae family) is one of the oldest edible fruits and is widely cultivated in many tropical and subtropical countries 1, including Iran, Egypt, Russia, Spain, France, China, Japan, Argentina, USA, and India. Pomegranate has been used extensively in the folk medicine of Iranians and many other countries. Many studies have indicated the antioxidant, antiatherogenic, anticancer, anti-inflammatory, antimicrobial and anti-infective effects of pomegranate peel and fruit extracts 2–6.
Although pomegranate peel is sometimes considered an agro-waste, it is indeed as a source of different flavonoids with antibacterial, antiviral, antioxidant, anti-inflammatory and antineoplastic bioactivities 7,8.
One of the most common human respiratory tract pathogens, associated with high morbidity and mortality, namely, influenza virus, has been recently considered a public health concern. Although vaccination is a suitable approach to prevent influenza, this method should be updated to be effective on new subtypes due to constant changes in virus surface 9. Currently, there are two groups of anti-influenza agents available for the management of influenza infection. One class includes amantadine and rimantadine which are matrix protein (M2) ion-channel inhibitors and interfere with viral un-coating within the host cells. They are effective only against influenza virus A with the risk of widespread drug resistance. The other group includes oseltamivir and zanamivir which are Neuraminidase (NA) inhibitors and are widely used in the treatment of both seasonal and pandemic influenza virus infections 10. However, oseltamivir resistant H1N1 strains were found to be circulated since 2007-08 11,12. As the options for the control and treatment of the disease are limited, use of herbal extracts such as pomegranate seems to be an alternative. This research was conducted to evaluate the anti-influenza A virus activity of crude hydro alcoholic extract and the four corresponding fractions of Punica granatum L. peel in vitro.
Materials and Methods
Plant collection, extraction and fractionation
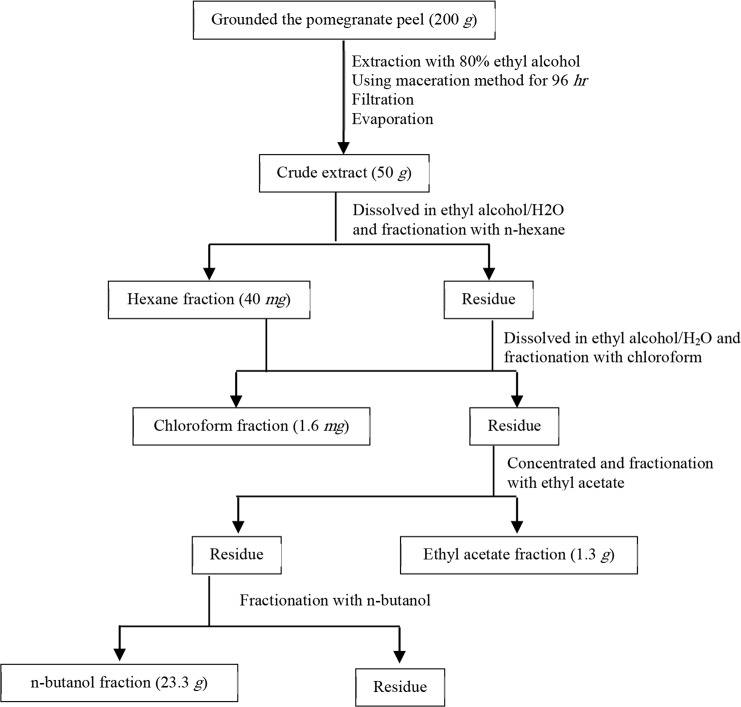
The pomegranate (Punica granatum L.) was from the Malas variant obtained from Shahreza, a central region of Iran (October 2015). Then, in the Herbarium of Medical Plants Research Center of the Shahrekord University of Medical Sciences (Iran), genus and species of the plant were identified and confirmed. The peel powder was dissolved in 80% ethanol and kept at room temperature for 96 hr. Then, the mixture was filtered and concentrated under nearly vacuum pressure at 40°C in the rotary evaporator. Four fractions of the crude extract, with different polarities were prepared by in-solution isolation and using the difference in various secondary metabolites’ polarities (Figure 1). The crude extract was dissolved in ethyl alcohol/H2O and fractionated by consecutive liquid/liquid partitioning with n-hexane (Merck, Germany), and then with chloroform (Merck, Germany), ethyl acetate (Merck, Germany) and n-butanol (Merck, Germany) with increasing order of polarity 13.
Figure 1.
Flow chart for the extraction and fractionation of pomegranate peel
Determination of total phenolic content
The total phenolic content of pomegranate peel extract and its fractions was determined using Folin-Ciocalteu method 14. Gallic acid was used as a standard reference for plotting calibration curve. Briefly, the dried extract/fractions or gallic acid were dissolved in 80% methanol for preparing 1 mg/ml of solutions. 0.2 ml of the diluted extract/fractions or standard solution of gallic acid (250, 125, 62.5, 31.2, and 15.6 μg/ml) were added to 1 ml of 10% (v/v) Folin-Ciocalteu reagent and kept at room temperature for 3–8 min. Next, 0.8 ml of 7.5% (w/v) sodium carbonate solution was added to the mixture. After the reaction solution was kept in total darkness for 30 min, its optical absorbance was measured at 765 nm using a UV-Vis spectrophotometer (UNICO 2100: USA). Total phenolic content was calculated using a gallic acid calibration curve. The results were expressed as mg gallic acid equivalents per g of dry plant extract/fractions (mg GAE/g).
Determination of total flavonoid content
The total flavonoid content of pomegranate peel extract and its fractions was measured according to previously described methods 15. Rutin was used as a standard reference for plotting calibration curve. Briefly, 0.2 ml of the diluted extract/fractions (1 mg/ml) or standard solution of rutin (125, 62.5, 31.2, 15.6, and 7.8 μg/ml) were separately mixed with 0.2 ml of 2% (w/v) aluminum chloride and 1.2 ml of 5% (w/v) potassium acetate. After the reaction solution was incubated at Room Temperature (RT) for 40 min, its optical absorbance was read at 415 nm using a UV-Vis spectrophotometer (UNICO 2100: USA). The results were expressed as mg of rutin equivalents per g of dry plant matter (mg RUT/g) in comparison with the standard curve, which was developed under the same conditions.
Cell culture and influenza virus propagation
Madin-Darby Canine Kidney (MDCK) cell line and influenza virus A/Puerto Rico/8/34 (H1N1; PR8) were obtained from Influenza Unit, Pasteur Institute of Iran. MDCK cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, USA), supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, USA) and 1% penicillin streptomycin (Gibco, USA) at 37°C in a 5% CO2 atmosphere and humidified incubator.
Cytotoxicity assay
The effect of pomegranate peel extract and its fractions on the viability of MDCK cells was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra zoliumbromide (MTT; Sigma, USA) assay, by a previously described method 16 with some modifications. Briefly, when the cell monolayer was confluent, the cells were incubated with 200 μl/well of various concentrations of the extract/fractions (200, 100, 50, 25, 12.5, 6.25 and 3.1 μg/ml) in 96-well plates for 48 hr. Afterwards, cell monolayers were incubated with 50 μl of 1 mg/ml MTT in Phosphate-Buffered Saline (PBS) at 37oC for 4 hr, and then treated with 100 μl of acidic isopropanol (0.05 N HCl in absolute isopropanol). After the plates were shaken for 15 min, the absorbance was read using a reference filter at 640 nm using microplate reader (StataFax2100, USA).
Cytopathic effect (CPE) reduction assay
When the cell monolayer was confluent in 96-well plates, the cell culture medium was aspirated and washed with PBS and infected with 100TCID50 of Influenza A (H1N1) virus for 1 hr, and then the virus was removed and the cells were treated with serial two fold dilutions of nontoxic concentration of the extract/fractions (200 μl/well) in serum-free DMEM containing 2 μg/ml of TPCK-trypsin and 0.3% BSA. 48 hr post infection, cell viability was also determined using previously described MTT assay 16. Various concentrations of oseltamivir (10, 5, 2.5, 1.25, 0.62 and 0.31 μmol) (Sigma, USA) were used as positive controls. The procedure was carried out in triplicate. The 50% cytotoxic concentration (CC50) and 50% inhibitory concentration (IC50) were calculated using GraphPad Prism 6 (Graph-Pad Software, La Jolla, CA). Selectivity index (SI) was calculated as ratio of CC50 to IC50.
Hemagglutination assay
MDCK cells in 24-well plates were infected with PR8 virus at 100 TCID50, incubated with virus for 1 hr at 37°C and cultured in DMEM and TPCK trypsin (0.5 μg/ml; Sigma, USA) either with or without extract/fractions treatment. The cell culture supernatants were harvested 24 and 48 hr post infection. Fifty μl of the two fold serial dilutions of the cell culture supernatants were mixed with the same volume of 0.5% chicken Red Blood Cells (RBCs) in U-bottomed 96-well plate for 45 min at room temperature. The HA was performed by measuring the dilution factor of the samples required for complete HA-mediated chicken RBC agglutination 17.
TCID50 virus titration
Confluent MDCK cells monolayer in 24-well plates were infected with PR8 virus (100 TCID50) in the presence of the extract/fractions or control compounds for 24 hr at 37oC. Standard 50% Tissue Culture Infectious Doses (TCID50) were used for virus titration in culture supernatants 18. Briefly, when 90%-confluent MDCK cells were prepared in 96 well plates, the cell culture medium was aspirated and washed with PBS twice, and then 100 μl of a series of 10-fold dilutions was added into the wells and left to incubate for 2 days. After the incubation, virus replication was detected by HA 18–20. TCID50 calculated based on the Reed and Muench method was expressed as log10 21.
Statistical analysis
The data were analyzed using Kruskal-Wallis test to compare differences between the groups. The IC50 and CC50 values were calculated by regression analysis using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Results
Total phenolic and flavonoid content
The results on total phenolic and total flavonoid content in the extract and fractions are presented in table 1. The results showed that pomegranate peel extract was the richest source of phenolic (233 mg GAE/g) and flavonoid (60.6 mg RUT/g) content. The highest phenolic (692 mg GAE/g) and flavonoid (93.7 mg RUT/g) content was obtained for ethyl acetate fraction (Table 1).
Table 1.
Total phenolic and flavonoid values of pomegranate peel crude extract and its fractions
| Sample | Total phenolics (mg GAE/g)* | Flavonoid content (mg RUT/g)** |
|---|---|---|
| Crude extract | 233±2.4 | 60.6.1±1.4 |
| n-Hexan fraction | 22.1±1.5 | 11.5±2.75 |
| Choloroform fraction | 221.9±3.5 | 83.2±1.9 |
| Ethyl acetate fraction | 476±4.2 | 60.6±2.7 |
| n-Butanol fraction | 199±1.8 | 77.1±2.5 |
| p-value# | < 0.05 | < 0.05 |
mg gallic acid equivalent/g of extract/fractions,
mg rutin equivalent/g of extract/fractions;
according to Kruskal-Wallis; all results are presented as mean (± standard error of the three measurements).
Cytotoxicity and anti-influenza A virus activity
Based on the CPE reduction assay results and probit analysis, the CC50 of crude extract and n-butanol and ethyl acetate fractions was 55.66, 55.61, and 29.7 μg/ml, respectively (Table 2). The analysis showed that there was a direct, significant relationship between the concentration of the extract/fractions and cell death (p< 0.05, Figure 2). The antiviral activities of the extract and the four fractions against the influenza A/PR/8/34 virus were investigated 48 hr after treatment using an MTT-based CPE reduction assay. Results indicated that the crude extract and n-butanol and ethyl acetate fractions produced antiviral effect against influenza virus with the SIs of 8.63, 9.16 and 5.3, respectively (Table 2).
Table 2.
Cell cytotoxicity and anti-influenza virus activity of pomegranate peel extract and fractions
| Extract/fractions | CC50aμg/ml (CI95%) | IC50bμg/ml (CI95%) | SIc |
|---|---|---|---|
| Crude extract | 55.6 (48.4–64) | 6.4 (4.5–9.2) | 8.63 |
| n-hexane fraction | 238.2 (142.4–398.4) | >238.2 | - |
| Chloroform fraction | 34.1 (30.1–38.6) | >34.1 | - |
| Ethyl acetate fraction | 29.7(24.9–35.3) | 5.6 (3.9–7.9) | 5.3 |
| n-butanol fraction | 55.61 (47.1–65.6) | 6.1 (4.5–8.13) | 9.16 |
| Oseltamivir (μmol)* | 539.4 (378.9–768.5) | 0.87 (0.55–1.4) | 617.8 |
CC50: 50% cytotoxic concentration (MDCK cell);
IC50: 50% inhibitory concentration (PR8 influenza virus);
SI: Selectivity index, i.e., the ratio of CC50 to IC50; CI95%: 95% confidence interval;
oseltamivir used as positive control.
Figure 2.
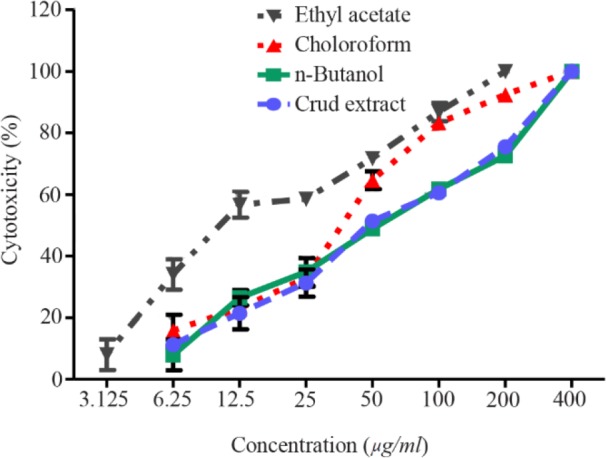
Cytotoxicity of pomegranate peel extract and its fractions on MDCK cells. Confluent MDCK cells were exposed to different concentrations of crude extract and its fractions for 48 hr. Cytotoxi-city was measured in MTT assay; experiments were carried out in triplicate.
Inhibition of influenza virus replication
Based on the CPE reduction assay results (Table 2), the crude extract and the n-butanol and ethyl acetate fractions underwent additional antiviral assays. The HA titers of the extract and the fractions on influenza virus were assessed by hemagglutination endpoint test. According to the results, the viral titer decreased dose dependently after treatment with the crude extract and the n-butanol and ethyl acetate fractions (Table 3).
Table 3.
Hemagglutination titers of PR8-infected MDCK cell supernatants in the presence of the pomegranate peel extract and its more effective fractions
| Extract/fractions | Concentration (μg/ml) | Log2 HA titer/50 μl supernatant | |||
|---|---|---|---|---|---|
| 24 hra | 48 hra | ||||
| Crude extract | |||||
| 50 | 0 | 0 | |||
| 25 | 0.67±1.15 | 4±3.46 | |||
| 12.5 | 1.67±1.15 | 6.33±0.58 | |||
| 6.25 | 2.67±1.15 | 6.67±1.15 | |||
| virus control | 5±1.4 | 7.33±.58 | |||
| n-butanol fraction | |||||
| 50 | 0 | 0 | |||
| 25 | 0 | 2.5±0.71 | |||
| 12.5 | 1.5±0.71 | 6 | |||
| 6.25 | 3.5±0.71 | 6.5±0.71 | |||
| virus control | 6 | 7.5±0.71 | |||
| Ethyl acetate fraction | |||||
| 25 | 0 | 1±1.73 | |||
| 12.5 | 0.33±0.58 | 4±1.43 | |||
| 6.25 | 1.67±1.53 | 6.33±0.58 | |||
| 3.12 | 2.67±0.58 | 6.67±1.15 | |||
| virus control | 4±1.73 | 7.33±0.58 | |||
| Oseltamivir (μmol)b | |||||
| 5 | 0 | 0 | |||
| 2.5 | 0 | 0.5±0.71 | |||
| 1.25 | 0 | 4 | |||
| virus control | 5±1.4 | 8 | |||
Hours post-infection;
Oseltamivir used as positive control.
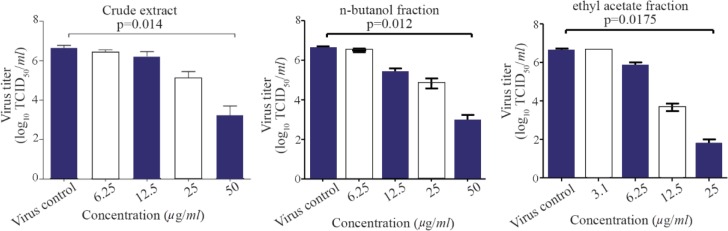
To investigate whether pomegranate peel extract and n-butanol fraction and ethyl acetate fraction could have inhibitory effect on infectious virus yield, the virus was titrated by TCID50 method. Consistent with the results of the HA, the production of virus was significantly reduced upon treatment with pomegranate peel extract and n-butanol and ethyl acetate fractions in a dose-dependent manner (p<0.05; Figure 3).
Figure 3.
Reduction of influenza viral titers in the culture supernatants by the pomegranate peel extract and its more effective fractions. PR8-infected MDCK cells were incubated with different concentrations of the extract/fractions for 24 hr and the supernatants were used for TCID50 titration. The data are the mean values of three independent experiments (mean±SEM). p-values were calculated against virus control (untreated sample) using Kruskal-Wallis test.
Discussion
Pomegranate is a highly active and important medicinal plant in folk medicine and its antibacterial, antiparasitic, apoptotic, antifungal, antiproliferative, and anti-viral activities have recently been studied 22–24.
Although few studies reported the inhibitory effects of pomegranate fruit against herpes virus, influenza virus, poxviruses, and human immunodeficiency virus 24,25, this is the first report on the antiviral activity of corresponding fractions of pomegranate peel. Our aim, therefore, was to study the anti-influenza activity of pomegranate peel extract and its fractions in the MDCK cell line.
In the present study, the crude extract inhibited influenza A PR8 virus replication in the MDCK cell line [IC50: approximately 6.45 (4.5–9.23)]. According to the results of antiviral assays to measure the titers of HA or infectious viral particles in the culture supernatants, it was observed that pomegranate peel could suppress the amplification of the infectious influenza viruses. Because the IC50 of an herbal extract for infectious diseases is conventionally less than 100 μg/ml 26 and SI over 4 27, pomegranate peel extract with IC50 of 6.45 and SI of 8.63 can be considered a potent agent to fight influenza virus.
Our results showed that the crude extract and n-butanol and the ethyl acetate fractions exerted more potent antiviral effects than other fractions. Other studies have also shown that the antiviral property of pomegranate extract may be due to hydrolysable tannins and polyphenols, especially punicalagin and gallagic acid, which have also been found in this extract 23. A study indicated that out of the four flavonoids of pomegranate, i.e. ellagic acid, caffeic acid, luteolin, and punicalagin, only punicalagin had inhibitory effect against influenza virus 25. Pomegranate peel’s polyphenol compounds such as punicalagin, ellagic acid, and hydroxy-benzoic acid that are extracted from both n-butanol and ethyl acetate fractions are probably associated with antiviral activity of pomegranate peel.
Conclusion
Based on our results, both n-butanol and ethyl acetate fractions of pomegranate peel, with high inhibitory effect against influenza virus replication, could be a new promising anti-influenza agent. More understanding of the mechanism of action and the natural components of these fractions seems necessary. The results of this study also showed the presence of high amounts of polyphenols in the fractions. As a result, the antiviral activity of the plant could be partly attributed to its polyphenol content.
Acknowledgement
We thank Dr. Fatemeh Fotouhi (Pasteur Institute of Iran, Tehran, Iran) for kindly providing the virus. This work was supported by Shahrekord University of Medical Science, Shahrekord, Iran (Grant No.:2489). Authors are thankful to the Director of Medical Plants Research Center and the Deputy of Research and Technology of Shahrekord University of Medical Sciences, Shahrekord, Iran.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Elyatem SM, Kader AA. Post-harvest physiology and storage behaviour of pomegranate fruits. Scientia Horticulturae 1984;24(3):287–298. [Google Scholar]
- 2.Asmaa MJ, Ali AJ, Farid JM, Azman S. Growth inhibitory effects of crude pomegranate peel extract on chronic myeloid leukemia, K562 cells. Int J Appl Basic Med Res 2015;5(2):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haber SL, Joy JK, Largent R. Antioxidant and antiatherogenic effects of pomegranate. Am J Health Syst Pharm 2011;68(14):1302–1305. [DOI] [PubMed] [Google Scholar]
- 4.Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol 2012;143(2):397–405. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahium MI. Efficiency of pomegranate peel extract as antimicrobial, antioxidant and protective agents. World J Agricultural Sci 2010;6(4):338–344. [Google Scholar]
- 6.Colombo E, Sangiovanni E, Dell'agli M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid Based Complement Alternat Med 2013;2013:247145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plumb GW, de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC, Williamson G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Rep 2002;7(1):41–46. [DOI] [PubMed] [Google Scholar]
- 8.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 2007;109(2):177–206. [DOI] [PubMed] [Google Scholar]
- 9.Hayden FG. Respiratory viral threats. Curr Opin Infect Dis 2006;19(2):169–178. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, Read RC, et al. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect 2011;62(1):14–25. [DOI] [PubMed] [Google Scholar]
- 11.van der Vries E, Schutten M, Fraaij P, Boucher C, Osterhaus A. Influenza virus resistance to antiviral therapy. Adv Pharmacol 2013;67:217–246. [DOI] [PubMed] [Google Scholar]
- 12.Dapat C, Kondo H, Dapat IC, Baranovich T, Suzuki Y, Shobugawa Y, et al. Neuraminidase inhibitor susceptibility profile of pandemic and seasonal influenza viruses during the 2009–2010 and 2010–2011 influenza seasons in Japan. Antiviral Res 2013;99(3):261–269. [DOI] [PubMed] [Google Scholar]
- 13.Moradi MT, Karimi A, Alidadi S, Ghasemi-Dehkordi P, Ghaffari-Goosheh MS. Cytotoxicity and in vitro anti-oxidant potential of Quercus Brantii acorn extract and the corresponding fractions. Int J Pharmacogn Phytochem Res 2016;8(4):558–562. [Google Scholar]
- 14.Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem 1927;73(2):627–650. [Google Scholar]
- 15.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal 2002;10(3):178–182. [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65(1–2):55–63. [DOI] [PubMed] [Google Scholar]
- 17.Jang YJ, Achary R, Lee HW, Lee HJ, Lee CK, Han SB, et al. Synthesis and anti-influenza virus activity of 4-oxo- or thioxo-4,5-dihydrofuro[3,4-c]pyridin-3(1H)-ones. Antiviral Res 2014;107:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Narayanan S, Chang KO. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res 2010;88(2):227–235. [DOI] [PubMed] [Google Scholar]
- 19.WHO Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Global Influenza Surveil-lance and Response System (GISRS) 2011. http://apps.who.int/iris/bitstream/handle/10665/44518/9789241548090_eng.pdf;jsessionid=06602DB20D114E1880E85A1CF842476B?sequence=1.
- 20.Matusevich OV, Egorov VV, Gluzdikov IA, Titov MI, Zarubaev VV, Shtro AA, et al. Synthesis and antiviral activity of PB1 component of the influenza A RNA polymerase peptide fragments. Antiviral Res 2015;113:4–10. [DOI] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938;27(3):493–497. [Google Scholar]
- 22.Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat 2002;71(3):203–217. [DOI] [PubMed] [Google Scholar]
- 23.Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med 2007;73(5):461–467. [DOI] [PubMed] [Google Scholar]
- 24.Howell AB, D'Souza DH. The pomegranate: effects on bacteria and viruses that influence human health. Evid Based Complement Alternat Med 2013;2013: 606212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidari M, Ali M, Ward Casscells S, Madjid M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009;16(12):1127–1136. [DOI] [PubMed] [Google Scholar]
- 26.Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 2006;106 (3):290–302. [DOI] [PubMed] [Google Scholar]
- 27.Debiaggi M, Tateo F, Pagani L, Luini M, Romero E. Effects of propolis flavonoids on virus infectivity and re-plication. Microbiologica 1990;13(3):207–213. [PubMed] [Google Scholar]