Abstract
The study of individuals with autosomal dominant Alzheimer’s disease affords one of the best opportunities to characterize the biological and cognitive changes of Alzheimer’s disease that occur over the course of the preclinical and symptomatic stages. Unifying the knowledge gained from the past three decades of research in the world’s largest single-mutation autosomal dominant Alzheimer’s disease kindred — a family in Antioquia, Colombia with the E280A mutation in the Presenilin1 gene — will provide new directions for Alzheimer’s research and a framework for generalizing the findings from this cohort to the more common sporadic form of Alzheimer’s disease. As this specific mutation is virtually 100% penetrant for the development of the disease by midlife, we use a previously defined median age of onset for mild cognitive impairment for this cohort to examine the trajectory of the biological and cognitive markers of the disease as a function of the carriers’ estimated years to clinical onset. Studies from this cohort suggest that structural and functional brain abnormalities — such as cortical thinning and hyperactivation in memory networks — as well as differences in biofluid and in vivo measurements of Alzheimer’s-related pathological proteins distinguish Presenilin1 E280A mutation carriers from non-carriers as early as childhood, or approximately three decades before the median age of onset of clinical symptoms. We conclude our review with discussion on future directions for Alzheimer’s disease research, with specific emphasis on ways to design studies that compare the generalizability of research in autosomal dominant Alzheimer’s disease to the larger sporadic Alzheimer’s disease population.
Keywords: autosomal dominant Alzheimer’s disease, dementia, biomarkers, cognitive markers
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is associated with the accumulation of amyloid-beta (Aβ1–42) plaques and intra-neuronal tau tangles which are hypothesized to damage brain structures, impair brain metabolism, and result in the clinical manifestation of the disease [1,2]. Most individuals develop the sporadic form AD, which for the purpose of this review we operationally define as the form of AD with a late onset of clinical symptoms (i.e., >65 years old) and an etiology that is believed to not solely be determined by genetic influences [3]. A small percentage of AD cases, however, are caused by autosomal dominant mutations that typically result in an earlier onset of clinical symptoms (i.e. <65 years of age) [4], with age of symptom onset varying by mutation type [5–7]. Autosomal dominant mutations in genes such as the Presenilin1 (PSEN1) are nearly 100% penetrant and confer the development of AD with virtual certainty by midlife [8,9], providing a unique opportunity to study the biological and cognitive markers of AD across the preclinical and symptomatic stages of the disease. Beyond aiming to find treatments that benefit mutation-carriers, ADAD investigators work to elucidate the trans-diagnostic disease mechanisms between ADAD and the more common sporadic form of the disease. As ADAD kindreds provide a quasi-experimental framework to investigate AD mechanisms and treatments, to the degree which ADAD and sporadic AD are similar it is possible through ADAD research to generate testable research hypotheses for new ways to identify and treat individuals at risk for the sporadic form of AD.
The largest identified single-mutation ADAD kindred in the world is a family of individuals with the E280A mutation in PSEN1 from Antioquia, Colombia [8–10]. Over 1,800 Colombian PSEN1 E280A carriers and 4,000 non-carrier family members who trace their lineage back to a single-common ancestor with the mutation participate in research at the University of Antioquia and collaborating sites in the United States [11]. As research in this cohort progresses – including a five-year phase 2 clinical trial of the anti-amyloid agent Crenezumab involving 300 Colombian kindred members, 200 of whom are mutation carriers [12] – a review that unifies our understanding of the PSEN1 E280A literature is needed.
Here, we synthesize all previous studies from the Colombian kindred, using a previously determined median estimated years until onset (EYO) of mild cognitive impairment (MCI) for this cohort (44 years) as a reference point to understand the progression of the cognitive and biological makers of the disease [10]; importantly, negative EYO indicates years before the median age of clinical onset of MCI, while positive EYO represents years after the median age of onset of MCI. We also provide directions for future research in this and other ADAD cohorts, including suggestions for generalizing ADAD findings to sporadic AD.
Methods
Thirty-four original reports (including published abstracts) on the cognitive and biological markers of PSEN1 E280A were identified, dating to 1997, which was the year of the first published research investigation in the cohort [13]. Studies from this cohort were defined using cohort characteristic criteria described in Lopera et al. (1997) and Acosta-Banea et al. (2011) [10,13]. All studies were found using Google Scholar, PubMed, and PsycInfo searches between November, 2017 and May, 2018. Search terms included: Presenilin1 E280A, Colombian kindred, autosomal dominant Alzheimer’s disease, familial Alzheimer’s disease. Articles found included works in both English and Spanish. All published reports on the biological and cognitive markers of this cohort were included in this review. We break down our review by the type of measurement used in each study: clinical/cognitive, psychophysiological, functional neuroimaging, structural neuroimaging, and fluid and brain-based markers of AD pathology.
As all the studies included in this review draw upon participants in the same cohort, identifying the exact number of unique participants is challenging. We can infer that some participants, such as those in the studies of child mutation carriers, were not included in other studies (i.e., studies of carriers with MCI or dementia due to AD, but otherwise the number of unique participants across all studies is difficult to identify. The median sample sizes of carriers and non-carriers across the cognitive studies were 40 and 30, respectively, including two large (n > 400 carriers) database-wide retrospective clinical and cognitive studies [10,14]. In comparison, for the psychophysiological studies the median sample size of carriers and non-carriers were 15 individuals in each group. Across the functional imaging reports, there were median sample sizes of 21 carriers and 20 non-carriers. A median of 29 carriers and 23 non-carriers were included in the structural imaging studies. A median of 25 carriers and 21 non-carriers were used in the studies of the plasma, cerebral spinal fluid, and in vivo studies of this cohort. Table 1 provides a summary of the characteristics (number of published reports, EYO range, median sample size) and outstanding research questions for each cognitive and biological marker domain. For a synopsis statement on each published report from specific studies with the Colombian kindred, please see Supplementary Table A.
Table 1:
Summary of Characteristics of Published Cognitive and Biological Marker Reports of PSEN1 E280A ADAD
| Modality of study | Number of published reports* | EYO range of mutation carriers | Median sample size of mutation carriers (and non-carriers) | Questions that need further investigation |
|---|---|---|---|---|
| Cognitive tests | 13 | −26 EYO to dementia due to ADAD | 40(30)** | Are there cognitive markers that occur earlier than verbal memory impairment that extant measures are not sensitive enough to detect? What cognitive differences distinguish child mutation carriers from non-carrier children? |
| Psychophysiological (EEG) | 7 | −18 EYO to dementia due to ADAD | 15(15) | Can EEG serve as proxy for preclinical aggregation of cortical amyloid-beta? What does EEG activation look like in child carriers of the mutation? |
| Brain function (fMRI; SPECT; FDG-PET) | 6 | −35 EYO to dementia due to ADAD | 21(20) | Are there SPECT and FDG-PET differences seen in younger carriers (e.g., −25 EYO) than those in two published reports? |
| Brain structure (MRI; DTI) | 6 | −35 EYO to dementia due to ADAD | 29(23) | At what age does the paradoxically greater cortical thickness in child carriers convert to cortical thinning? Do specific pathological processes drive this change? |
| Biomarkers (Plasma, CSF, amyloid-beta and tau PET) | 7 | −35 EYO to dementia due to ADAD | 25(21) | How do changes in blood and CSF markers of AD pathology relate to preclinical differences in cortical activation and functional connectivity? |
Note:
Some reports share overlapping modalities (e.g., brain function and biomarkers).
The median sample size of the studies of cognitive testing is driven by two large (n > 400) retrospective studies.
Abbreviations: EYO = estimated years until onset of mild cognitive impairment; EEG = electroencephalogram; fMRI = functional magnetic resonance imaging; SPECT = single-photon emission computed tomography; FDG-PET = fludeoxyglucose positron emission tomography; MRI = magnetic resonance imaging; DTI = diffusion tensor imaging; CSF = cerebral spinal fluid; PET = position emission tomography
Results
Cognitive Function
The age of onset of dementia and rate of disease progression in this kindred was found to be variable [15], but a large, retrospective study of 449 mutation carriers identified a median age of onset of MCI of 44 years old, with a symptomatic disease course averaging 15 years thereafter [10]. Verbal memory deficits appeared in carriers between −9 to −14 EYO [10,16–20]. In addition, subjective memory complaint (SMC) scores based on study-partner report began to differ from non-carriers approximately −6 EYO and followed a linear function, correlating with hippocampal volume in these carriers in the preclinical stage of the disease [21]. Self-reported SMC was elevated in preclinical carriers relative to non-carriers, though a linear relationship with age was not found [21].
Poor performance on tests of attention, concentration, and semantic recognition was also observed in carriers approximately −5 EYO [16,17,22]. As mutation carriers progressed to dementia, memory and language problems worsened, and changes in personality, behavior and physical symptoms occurred [13]. Carriers of the PSEN1 E280A mutation with mild dementia (mean EYO from MCI = 5 years) performed worse than non-carriers on two thirds of tests from a battery of 43 neuropsychological variables [23].
The visual short-term memory test by Parra and colleagues [24] also demonstrated potential as a possible marker of early cognitive decline in preclinical carriers [24–26]. Performance on the color-shape binding condition of this test reliably discriminated preclinical carriers from non-carriers, even when other traditional neuropsychological tests could not, though a precise age at which this test begins to reveal deficits in carriers was not identified [24].
Psychophysiological Measures
Electroencephalogram (EEG) studies in the Colombian kindred, when considered together, provide evidence of a shift from hyperactivity to hypoactivity among frontal [27], perceptual [28,29], and memory-related [27,30–32] brain networks in carriers in the preclinical stage of the disease. Increased theta synchronization [33] and alterations in beta-band frequencies [34] in frontal regions of preclinical carriers occurred approximately −10 EYO [27,35], suggesting that abnormal cortical activity may occur in younger mutation carriers, despite similar cognitive performance to non-carriers. The right visuo-perceptual area, which showed greater activity in younger carriers, was conversely found to have decreased activity in carriers close to clinical onset [29]. In the context of other markers of AD, the change from EEG-measured cortical hyperactivity to hypoactivity in PSEN1 E280A carriers appeared to coincide with elevated levels of in vivo cortical Aβ1–42 in mutation carriers [36,37].
Structural Imaging
Structural magnetic resonance imaging (MRI) studies across ADAD mutations consistently suggest a pattern of decreased cortical thickness in the posterior parietal lobe of PSEN1 E280A mutation carriers before significant atrophy in other memory-related brain structures (e.g., the hippocampus and perihippocampus). The initial MRI study of kindred members showed that perihippocampal fissures and the distance between the unci of the temporal lobe distinguished symptomatic mutation carriers from cognitively unimpaired carriers and non-carrier family members, and that temporal lobe atrophy and ventricular enlargement (likely driven by the grade of atrophy) also correlated with disease severity [38]. Subsequent studies revealed decreased cortical thickness in the parietal lobe of young carriers (−18 to −26 EYO) [39] and broader temporal and parietal reductions in thickness in carriers a decade before the median age of onset of MCI [40,41]. Complementing these studies of grey matter volume, one diffusion tensor imaging study measuring white matter integrity found significantly degenerated white matter only in carriers with dementia due to AD (mean EYO = 3 years) relative to non-carriers and preclinical carriers [26]. Decreased white matter integrity was observed most saliently in the mid-frontal lobe and the genu of the corpus callosum [26].
Child PSEN1 E280A carriers (9–17 years old, −27 to −35 EYO) paradoxically showed greater grey matter volume compared to age-matched non-carriers in parietal and temporal areas [42], consistent with what has been observed in infant carriers of the E4 variant of the Apolipoprotein (ApoE4) who have greater grey matter in frontal areas relative to non-ApoeE4 carrier infants [43]. Evidence from the ApoE4 literature is mixed about whether greater frontal lobe matter in ApoE4-carrying infant relates to a potential executive-function-related compensatory neurodevelopmental process in these individuals at-risk for AD [44,45]. A study of child PSEN1 E280A carriers is currently underway to attempt to similarly determine if Colombian kindred child mutation carriers who have greater grey matter in the parietal and temporal lobes show any cognitive deficits or strengths relative to non-carrier children.
Functional Imaging
The use of functional MRI (fMRI), hexamethylpropyleneamine oxime spectroscopy (SPECT), and fludeoxyglucose PET (FDG-PET) propelled the discovery of AD-related disruptions in the metabolism and activity of memory-related brain structures (e.g., the hippocampus) and in connectivity within networks associated with memory performance (e.g., the default mode network, [DMN]) in PSEN1 E280A carriers. Prior studies have shown that, relative to non-carriers, increased blood oxygenation level-dependent (BOLD) fMRI activation during the encoding phase of a face-name association task was evident in the right cingulate gyrus and the right anterior hippocampus in mutation carriers a mean of −11 EYO [46]. Younger carriers (−18 to −26 EYO) also showed greater BOLD activation during encoding in the right hippocampus and parahippocampal gyrus on this task, as well as less deactivation in the precuneus and posterior cingulate cortex relative to matched non-carriers [39]. Child carriers (−27 to −35 EYO) similarly had less encoding-related deactivation of parietal regions and increased connectivity in the DMN between the posterior cingulate cortex and medial temporal lobe relative to non-carriers during this task [42]. A scene-encoding BOLD fMRI task yielded similar encoding-related hippocampal and parahippocampal gyri hyperactivation patterns as the face-name task, discriminating mutation carriers in the preclinical stage with a median of −10 EYO from non-carriers [47].
In a SPECT study, preclinical mutation carriers in the decade before the estimated onset of MCI exhibited decreased cerebral perfusion in the posterior parietal lobe, the anterior frontal lobe, the posterior and anterior cingulate cortex, and throughout the hippocampal complex [48]. FDG-PET was used in a different study with adult carriers and revealed lower glucose metabolism in temporal and parietal regions (e.g., the posterior cingulate and precuneus) among carriers with approximately −18 EYO relative to non-carriers [49]. Carriers with dementia showed a similar pattern of decreased glucose metabolism as preclinical carriers [49], but had further decreased cerebral perfusion in the superior frontal cortex and posterior parietal lobe [48]. Findings of brain hypometabolism among PSEN1 E280A carriers are generally consistent with the literature from individuals at risk for sporadic AD [50].
Plasma, CSF, and other in vivo markers of AD pathology
Plasma, CSF, and in vivo PET studies provide evidence of AD pathogenesis in mutation carriers decades before the onset of clinical symptoms. Increased CSF Aβ1–42 distinguished young adult mutation carriers (−18- to −26 EYO) from non-carriers [39] while child mutation carriers (−27 to −35 EYO) showed elevated plasma Aβ1–42 and elevated ratios of plasma Aβ1–42 to Aβ1–40 when compared to non-carrier children [42]. Florbetapir PET imaging revealed Aβ1–42 aggregation in carriers with an average of −16 EYO, which increased steeply for almost 10 years until plateauing at −7 EYO [36]. Aβ1–42 aggregation was most evident in the anterior cingulate and precuneus [36]. Consistent with this initial study, a recent report using the Pittsburgh-compound-B (PiB) tracer showed cortical Aβ1–42 aggregation beginning in carriers with an average of −15 EYO [37]. Flortaucipir PET imaging of tau in the brain of carriers has shown progressive accumulation of tau in the entorhinal cortex and inferior temporal lobe an average of −6 EYO, and an estimated 10 years after the first evidence of cortical Aβ1–42 [37]. Tau aggregation was strongly related to performance on tests of verbal memory and global cognition, and could be seen in the junctions of the parietal, temporal, and occipital lobes, as well as in the posterior cingulate cortex and precuneus among carriers with MCI [37].
A single study extracted the sequential change points (and their 95% confidence intervals) for the CSF and brain biomarkers in carriers, revealing that the CSF Aβ1–42 was the earliest biomarker to show abnormality at −19 EYO, followed by PET markers of in vivo AD pathology, and finally hippocampal volume at −5 EYO [51]. Further work with biomarkers has shown that baseline cortical Aβ1–42 and CSF phosphorylated-tau/Aβ1–42 ratios predicted cognitive decline over 2–3 years among carriers with EYOs ranging from −24 to 0, whereas CSF Aβ1–42 and tau measurement levels (total tau and phosphorylated-tau [p-tau]) did not [52].
Discussion
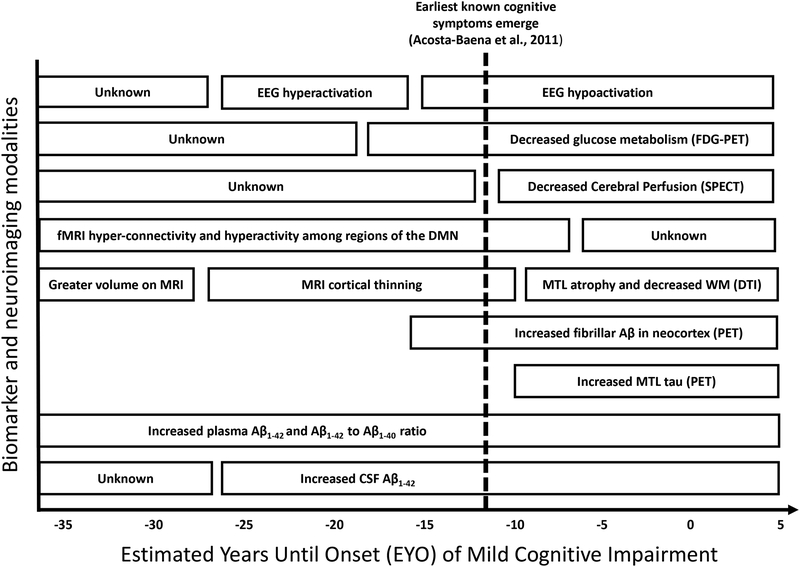
The near 100% penetrance of the PSEN1 E280A ADAD mutation has provided investigators with a unique framework to examine the course of the cognitive and biological markers of AD across the preclinical and symptomatic stages of the disease, something that cannot be readily done in sporadic AD populations without multi-decade longitudinal studies. Research in the Colombian kindred has included participants with a wide age range, including children, young cognitively unimpaired adults, and individuals with MCI and dementia due to AD. Figure 1 draws from the cumulative studies across this wide age range and provides a cross-sectional synthesis of the findings from Colombian ADAD kindred based on the EYO of MCI for carriers of the PSEN1 E280A mutation. Ordering these findings based on EYO suggests that plasma and CSF Aβ1–42 are clinically abnormal early in the lives of PSEN1 E280A carriers, corresponding with early-life increases in connectivity (as seen on fMRI) within the DMN and hyperactivity (as measured by EEG) throughout the brain. Cortical Aβ1–42 deposition becomes evident a decade-and-a-half later (~ −16 EYO), followed by decreased cerebral perfusion and glucose metabolism in brain regions impacted by cortical Aβ deposition, suggesting a potential connection between cortical Aβ1–42 and decreased parietal, frontal, and temporal metabolic activity in preclinical ADAD. Significantly decreased cortical thickness in parietal regions was also evident as carriers approached the median age of onset of MCI, while child mutation carriers showed paradoxically greater grey matter than non-carriers in regions impacted by AD pathology.
Figure 1. Hypothetical Model of Progression of Biological Markers of PSEN1 E280A Autosomal Dominant Alzheimer’s Disease Relative to Earliest Known Signal of Cognitive Decline.
A synthesis of the Colombian kindred biological marker literature is presented as a function of the estimated years until the median age of onset of mild cognitive impairment in this cohort. The hypothetical trajectory of the biological markers of ADAD in this cohort are displayed relative to the earliest known signs of cognitive decline at a median age of −12 EYO (dashed line; Acosta-Baenta et al., 2011). Figure 1a examines biological markers of brain function, while Figure 1b charts biological markers of brain structure and AD-related pathology.
Note: fMRI = EEG = electroencephalogram; FDG-PET = fludeoxyglucose positron emission tomograph; SPECT = single-photon emission computerized tomography; FMRI = functional magnetic resonance imaging; DMN = default mode network; MRI = structural magnetic resonance imaging; WM = white matter; DTI = diffusion tensor imaging; Aβ = amyloid-beta; PET = positron emission tomography; MTL = medial temporal lobe; CSF = cerebral spinal fluid.
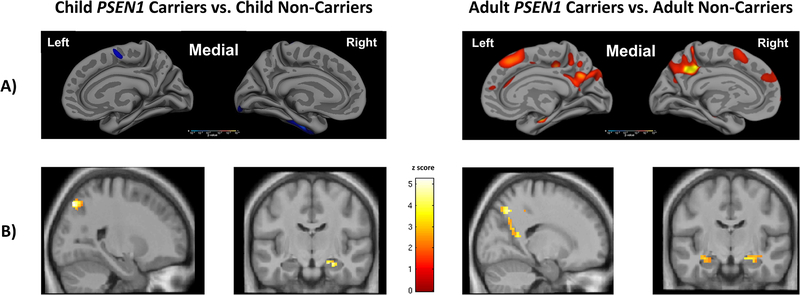
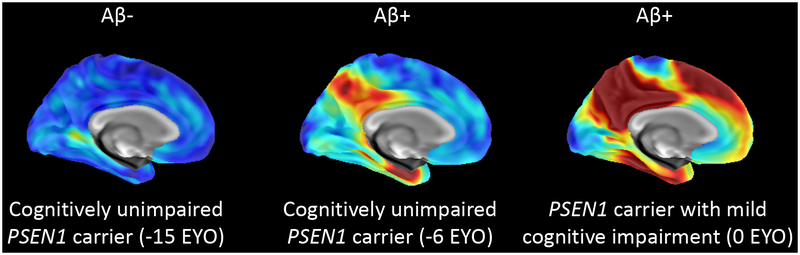
The differences seen between younger and older carriers of the PSEN1 E280A mutation suggest that the course of this ADAD mutation has a temporal order in which biofluid markers and electrophysiological measures may become abnormal early in the disease process. Structural imaging markers (e.g., grey matter volume, white matter integrity) provide evidence of degeneration much later in the preclinical stage of the disease close to the onset of clinical symptoms, although child mutation carriers show paradoxically greater cortical thickness in temporal and parietal regions. Figure 2 illustrates the course of structural MRI and fMRI signatures of PSEN1 E280A from childhood into adulthood, while Figure 3 shows the spatial pattern of cortical Aβ1–42 and temporal lobe tau deposition from the late preclinical stage of the disease to MCI. Synthesizing findings across PSEN1 E280A ADAD is essential to future research that will compare findings from this mutation to sporadic AD. Until longitudinal data are available for this cohort, using cross-sectional findings and EYO to model the hypothetical progression of disease is the best way to understand the earliest biological and cognitive events in ADAD at which pharmacological and non-pharmacological interventions may be most efficacious.
Figure 2.
A) Child mutation carriers (−36 to −27 EYO), relative to non-carrier children, show paradoxically greater cortical thickness in the parietal and temporal lobe, as indicated by the color blue; cognitively unimpaired adult mutation carriers, however, exhibit decreased cortical thickness relative to non-carriers in the posterior parietal lobe, as indicated by the colors red and yellow. B) Relative to non-carrier children, child PSEN1 mutation carriers show decreased deactivation of the posterior parietal lobe and increased hippocampal activation during memory encoding (Quiroz et al., 2015); cognitively unimpaired adult PSEN1 mutation carriers also exhibit decreased deactivation of the posterior parietal lobe and increased hippocampal activation during memory encoding (Reiman et al., 2012).
Figure 3. Comparison of the spatial patterns of tau deposition in PSEN1 E280A mutation carriers.
Amyloid-positive PSEN1 mutation carriers display greater tau levels in the entorhinal cortex and regions of the inferior temporal lobe; deposition is believed to begin around −6 EYO (Quiroz et al., 2018).
Through the cumulative, synthesized findings of our review, we propose a hypothetical “tipping point” of hyper to hypo-activity in memory and visuo-perceptual regions in carriers in their mid-thirties (~10 -EYO), which aligns with the mid-point between cortical Aβ deposition (median of −16 EYO) and regional tau deposition (median of −6 EYO) [36,37]. Significant debate persists, however, about whether changes in cortical activation and functional activity are not compensatory, but reflective of poor brain maintenance. A prevailing counter-argument to the posterior-to-anterior compensatory shift in preclinical AD is that increased prefrontal activity in healthy older adults corresponds with decreased efficiency in neural networks [53]. While this question is still unresolved in the fields of sporadic AD and ageing, we note that the cognitively unimpaired PSEN1 E280A mutation carriers who participated in the studies of brain function were between 20–30 years younger than the earliest age at which one can be diagnosed with sporadic AD (65 years old) and were likely unaffected by ageing processes discrete from AD pathology. Studying cognitively unimpaired adult ADAD mutation carriers from this kindred mitigates many of the confounds of advancing age that are common in sporadic AD research.
Several questions remain about how cortical Aβ1–42 relates to changes in cortical activation and functional connectivity in preclinical AD. For example, little is known about the deposition of Aβ1–42 or tau in the occipital lobe, so understanding changes in occipital lobe functioning in the context of AD pathologic change is an important area of future work in in the Colombian kindred. Grey and white matter degeneration and the aggregation of cortical Aβ1–42 and temporal lobe tau are also likely interrelated in ADAD; future work should further explore the relation between white matter integrity and AD pathologic change. A longitudinal study of members from the Colombian kindred that examines plasma, CSF, imaging, and cognitive function is currently underway with the hope of more definitively addressing these questions and elucidating the temporal relations between the cognitive and biological markers of preclinical ADAD. Similarly, studying EEG and in vivo brain pathology in the same sample could determine whether EEG hyper- or hypoactivity can reliably serve as a proxy for the aggregation of AD-related cortical Aβ1–42. The implications of such a study could facilitate the potential clinical and research use of EEG as a precursor assessment to more expensive (and less widely available) positron emission tomography (PET) imaging. Future research should also seek to integrate new behavioral measures and biomarker and imaging methods that will continue to expand our knowledge about the progression of AD, such as functional near-infrared spectroscopy (fNIRS) and PET imaging of other AD-related proteins. Understanding how health behaviors, like aerobic fitness and sleep hygiene, impact the course of AD is also a goal of ongoing research efforts in this cohort; two pilot studies currently underway to that explore how these health behaviors impact the biological and cognitive course of AD in PSEN1 E280A carriers.
In addition to identifying the earliest imaging marker abnormalities of ADAD, cognitive and other clinical markers can play an important role as well. Studies from this cohort have suggested that short-term memory deficits in verbal [14] and visual memory [24–26] strongly discriminate preclinical PSEN1 E280A carriers from non-carriers, and are likely to be cost-effective screeners for preclinical AD. Continued detailed investigation of cognitive and behavioral markers sensitive to changes early in preclinical AD is warranted, with specific emphasis on exploring preclinical subclinical changes (i.e., close to the threshold but below cutoffs for statistical significance) in cognitive domains, such as executive function, that may underpin episodic memory problems in the early symptomatic stage of the disease.
Our review also highlights the utility of examining PSEN1 E280A ADAD fluid and imaging progression in children younger than 8 years old, even younger than those investigated by Quiroz and colleagues (2015) [42]. Extending ADAD research with larger samples and longitudinal follow up in younger children could provide the strongest evidence for neurodevelopmental changes in PSEN1 E280A ADAD. If such changes were identified, this would underscore the importance of developing early-life interventions for these individuals. Extrapolating from the EYO of children carriers, ADAD-related abnormal memory network activity, as well as elevations of plasma Aβ1–42 and elevated ratios of plasma Aβ1–42 to Aβ1–42 may inform potential longitudinal or retrospective studies of sporadic AD that explore these abnormalities approximately 30 years before the onset of clinical symptoms.
Like other narrative reviews, our approach is limited in that we pool research studies from the same kindred that vary in methodological design and participant age to extrapolate a hypothesized sequential change model across the disease. In addition, the normally inferable strength of replication by multiple studies is weakened in the set reviewed here, due to the high likelihood that some participants were measured in several studies. Nonetheless, given the robust characterization of this cohort and, in particular, the virtual guarantee that carriers of this mutation will develop clinical symptoms by mid-life, the data reviewed here can inform ongoing research on ADAD and sporadic AD.
Findings to date from this PSEN1 E280A kindred generally support the prevailing models of AD pathogenesis and largely align with research from other ADAD groups, such as the Dominantly Inherited Alzheimer’s Network (DIAN), which suggest that CSF markers of amyloid-beta change earliest in the disease process, followed by decline in brain metabolism, and lastly by atrophy in memory-related brain structures (e.g., the hippocampus) which is closer to the age of onset of cognitive decline [54]. Reports from the Colombian kindred also generally align with studies of sporadic AD [55,56]. The most precise understanding of disease-course will, however, come from longitudinal studies. We are collecting such data now, and these data as well as those from DIAN other ADAD research groups will facilitate comparisons with longitudinal studies of sporadic AD (e.g., the Harvard Aging Brain Study, the Alzheimer’s Disease Neuroimaging Initiative, and the Australian Imaging, Biomarker & Lifestyle study). Studies that compare data from ADAD kindreds and sporadic AD groups should ensure that, when feasible, parallel protocols for imaging, biomarker collection, and cognitive testing are followed. While the clinical and cognitive course of ADAD appears to follow a similar course to that in sporadic AD, initial studies comparing the two etiologically distinct forms of AD should prioritize testing whether specific biological and cognitive markers in the preclinical stage of ADAD occur at similar points before clinical onset and predict risk for clinical progression in sporadic AD populations. Studying ADAD kindreds, like the Colombian PSEN1 E280A cohort, is an approach that affords an understanding of the cognitive and biological course of AD separate from age-related processes and co-morbidities (e.g., stroke and white matter hyperintensities). As carriers of the PSEN1 E280A mutation are virtually guaranteed to develop MCI and dementia due to AD by their mid-to-late 40s, the effects age-related processes on the cognitive and biological changes seen in ADAD are believed to be low to non-existent.
Over the next decade, collaborations between ADAD and sporadic AD research groups may substantially advance our understanding of the generalizability of ADAD findings to the more common sporadic form of the disease, while also highlighting those changes that are specific to early onset forms of the disease. As we approach the new frontier of AD research that aims to find an effective prevention or treatment for AD by 2025 [57], longitudinal research and comparisons between PSEN1 E280A ADAD and sporadic AD cohorts will be essential to advancing our understanding of when the AD pathophysiological process begins, how to most accurately identify individuals at risk for AD in late life early on, where in the disease process intervention will be most efficacious, and what the most effective and stage-specific interventions might be.
Conclusions
Significant abnormalities in plasma, CSF, and brain-based AD pathology, as well as differences in brain structure and function (despite preserved cognition) are evident in carriers of the PSEN1 E280A mutation as early as three-and-a-half decades before the median age of onset of AD-related cognitive decline. Findings from the Colombian kindred have laid the groundwork for better understanding the prognostic value of fluid and in vivo imaging markers of ADAD. However, continued integration and comprehensive evaluation of the biological and cognitive, and other clinical markers of ADAD will inform the search for the earliest sensitive and specific markers of the disease and direct the development of interventions for the preclinical stage of AD. Generalizing findings from the Colombian kindred to sporadic AD will be the next wave of research in this kindred, carrying significant implications for clinical trials, research, and treatment of the disease.
Supplementary Material
Acknowledgements:
We thank the members of the Colombian families with ADAD for their invaluable dedication to research and inspiration.
Funding and financial disclosures:
This review was supported by grants from the NIH Office of the Director (DP5OD019833 to YTQ); the NIH National Institute on Aging (R01AG054671 to YTQ; 1RF1AG041705 to EMR, FL, and PNT; 1R01AG055444 to EMR, FL, and PNT; AG058805-01 to JRG; Massachusetts General Hospital ECOR (1200-228010 and 1200-228767 to YTQ) and Rappaport Fellowship (JRG); the Alzheimer’s Association Clinical Fellowship (AACF-16-440965 to JRG); the Alzheimer’s Association (EMR, KJ); project 111565741185 from the Administrative Department of Science, Technology, and Innovation (Colciencias Colombia) (FL); the European Union¹s Horizon 2020 Research and Innovation Programme (Marie Sklodowsk; a-Curie Grant agreement [IF-2015-GF, 706714] to HILJ); the Belgian Foundation for Scientific Research (FNRS grant# SPD28094292 to BJH); the Belgian Foundation for Alzheimer Research (SAO-FRA grant# P16.008 to BJH); the Banner Alzheimer’s Foundation (EMR); GHR Foundation (EMR); F-Prime Biosciences Research Initiative (EMR); NOMIS Foundation (EMR), the FIL Foundation (EMR), Fidelity Biosciences (KJ), Harvard Neurodiscovery Center (KJ), the Marr Foundation (KJ and RAS) and the Phi Kappa Phi Graduate Student Fellowship (JTF). PNT reports receiving consulting fees from Abbott Laboratories, AbbVie, AC Immune, Acadia, Auspex, Boehringer Ingelheim, Chase Pharmaceuticals, Corium, Eisai, GliaCure, INSYS Therapeutics, Pfizer, T3D; receiving consulting fees and research support from AstraZeneca, Avanir, Biogen, Cognoptix, Eli Lilly, H. Lundbeck A/S, Merck and Company, Roche, and Takeda; receiving research support only from Amgen, Avid, Functional Neuromodulation, GE Healthcare, Genentech, Novartis, Roche, Targacept, the National Institute on Aging, and the Arizona Department of Health Services; owning stock options in Adamas; and being listed as a contributor to a patent owned by the University of Rochester. Banner Alzheimer’s Institute also has contracts with Genentech/Roche, Novartis/Amgen and Avid/Lilly. KJ has provided consulting services for Lilly, Novartis, Janssen, Roche, Piramal, GE Healthcare, Siemens, ISIS Pharma, AZTherapy, and Biogen; has received support from a joint National Institutes of Health- and Lilly-sponsored clinical trial (Anti-Amyloid Treatment in Asymptomatic Alzheimer’s [A4] Study); and has received research support from the National Institute on Aging (grants U19AG10483 and U01AG024904-S1), Fidelity Biosciences, the Michael J. Fox Foundation, and the Alzheimer’s Association. RAS receives research support from grants U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, and U19 AG010483 from the National Institutes of Health. She is also a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. EMR reports that he is a compensated Scientific Advisor with: Alkahest, Alzheon, Axovant, Denali, Green Valley, United Neuroscience and Zinfandel Pharma. Authors JTF, ACG, JRG, DJN, EGV, EPD, HILJ, BJH, AA, AB, YB, KK, KC, FL, and YTQ report no financial disclosures.
References
- [1].Stelzmann RA, Norman Schnitzlein H, Reed Murtagh F (1995) An English translation of Alzheimer’s 1907 paper,“Über eine eigenartige Erkankung der Hirnrinde”. Clin. Anat 8, 429–431. [DOI] [PubMed] [Google Scholar]
- [2].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [3].Zetterberg H, Mattsson N (2014) Understanding the cause of sporadic Alzheimer’s disease. Expert Rev. Neurother 14, 621–630. [DOI] [PubMed] [Google Scholar]
- [4].Awada A (2015) Early and late-onset Alzheimer’s disease: What are the differences? J. Neurosci. Rural Pract 6, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JBS, Lopera F, Martins R, Masters CL, Mayeux RP, McDade E, Moreno S, Reiman EM, Ringman JM, Salloway S, Schofield PR, Sperling R, Tariot PN, Xiong C, Morris JC, Bateman RJ (2014) Symptom onset in autosomal dominant Alzheimer disease. Neurology 83, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tang Y-P, Gershon ES (2003) Genetic studies in Alzheimer’s disease. Dialogues Clin. Neurosci 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun L, Zhou R, Yang G, Shi Y (2017) Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl. Acad. Sci 114, E476–E485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cornejo W, Lopera F, Uribe C, Salinas M (1987) Descripción de una familia con demencia presenil tipo Alzheimer. Acta Médica Colomb. 12,. [Google Scholar]
- [9].Lopera F, Arcos M, Madrigal L, Kosik K, Cornejo W, Ossa J (1994) Demencia tipo Alzheimer con agregación familiar enAntioquia, Colombia. Acta Neurol Colomb 10,. [Google Scholar]
- [10].Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, Saldarriaga A, Lopera F (2011) Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 10, 213–220. [DOI] [PubMed] [Google Scholar]
- [11].Reiman EM, Langbaum J, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN (2011) Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J. Alzheimers Dis. 26, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tariot PN, Lopera F, Langbaum JB, Thomas RG, Hendrix S, Schneider LS, Rios-Romenets S, Giraldo M, Acosta N, Tobon C, Ramos C, Espinosa A, Cho W, Ward M, Clayton D, Friesenhahn M, Mackey H, Honigberg L, Sanabria Bohorquez S, Chen K, Walsh T, Langlois C, Reiman EM (2018) The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. Transl. Res. Clin. Interv 4, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lopera F, Ardilla A, Martínez A, Madrigal L, Arango-Viana JC, Lemere CA, Arango-Lasprilla JC, Hincapié L, Arcos-Burgos M, Ossa JE (1997) Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. Jama 277, 793–799. [PubMed] [Google Scholar]
- [14].Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, Bangdiwala SI, Reiman EM, Tariot PN, Langbaum JB (2016) Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol. 73, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sepulveda-Falla D, Glatzel M, Lopera F (2012) Phenotypic profile of early-onset familial Alzheimer’s disease caused by presenilin-1 E280A mutation. J. Alzheimers Dis. 32, 1–12. [DOI] [PubMed] [Google Scholar]
- [16].Ardila A, Lopera F, Rosselli M, Moreno S, Madrigal L, Arango-Lasprilla JC, Arcos M, Murcia C, Arango-Viana JC, Ossa J (2000) Neuropsychological profile of a large kindred with familial Alzheimer’s disease caused by the E280A single presenilin-1 mutation. Arch. Clin. Neuropsychol 15, 515–528. [PubMed] [Google Scholar]
- [17].Arango-Lasprilla JC, Cuetos F, Valencia C, Uribe C, Lopera F (2007) Cognitive changes in the preclinical phase of familial Alzheimer’s disease. J. Clin. Exp. Neuropsychol 29, 892–900. [DOI] [PubMed] [Google Scholar]
- [18].Tirado V, Motta M, Aguirre-Acevedo DC, Pineda D, Lopera F (2008) Analysis of intrusive errors in a memory test as possible pre-clinical marker of familial Alzheimer disease, in E280A presenilin-1 mutation carrier. Rev. Neurol 47, 290–294. [PubMed] [Google Scholar]
- [19].Rosselli M, Ardila A, Moreno S, Standish V, Arango-Lasprilla JC, Tirado V, Ossa J, Goate AM, Kosik KS, Lopera F (2000) Cognitive decline in patients with familial Alzheimer’s disease associated with E280a presenilin-1 mutation: a longitudinal study. J. Clin. Exp. Neuropsychol 22, 483–495. [DOI] [PubMed] [Google Scholar]
- [20].Cuetos F, Arango-Lasprilla J, Uribe C, Valencia C, Lopera F (2007) Linguistic changes in verbal expression: A preclinical marker of Alzheimer’s disease. J. Int. Neuropsychol. Soc. JINS 13, 433–9. [DOI] [PubMed] [Google Scholar]
- [21].Norton DJ, Amariglio R, Protas H, Chen K, Aguirre-Acevedo DC, Pulsifer B, Castrillon G, Tirado V, Munoz C, Tariot P (2017) Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. Neurology 89, 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tirado V, Munoz C, Aguirre C, Pineda D, Lopera F (2004) Performance of carriers and non-carriers of the E280A mutation for familial Alzheimer’s disease in a naming test. Rev. Neurol 39, 322–326. [PubMed] [Google Scholar]
- [23].Lasprilla JCA, Iglesias J, Lopera F (2003) Neuropsychological stydy of familial Alzheimer’s disease caused by mutation E280A in the presenilin 1 gene. Am. J. Alzheimers Dis. Dementiasr 18, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S (2010) Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 133, 2702–2713. [DOI] [PubMed] [Google Scholar]
- [25].Parra MA, Della Sala S, Abrahams S, Logie RH, Méndez LG, Lopera F (2011) Specific deficit of colour–colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia 49, 1943–1952. [DOI] [PubMed] [Google Scholar]
- [26].Parra MA, Saarimäki H, Bastin ME, Londoño AC, Pettit L, Lopera F, Della Sala S, Abrahams S (2015) Memory binding and white matter integrity in familial Alzheimer’s disease. Brain 138, 1355–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodriguez R, Lopera F, Alvarez A, Fernandez Y, Galan L, Quiroz Y, Bobes MA (2014) Spectral analysis of EEG in familial Alzheimer’s disease with E280A presenilin-1 mutation gene. Int. J. Alzheimer’s Dis. 2014,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Quiroz YT, Ally BA, Celone K, McKeever J, Ruiz-Rizzo AL, Lopera F, Stern CE, Budson AE (2011) Event-related potential markers of brain changes in preclinical familial Alzheimer disease. Neurology 77, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suárez-Revelo JX, Ochoa-Gómez JF, Duque-Grajales JE, Tobón-Quintero CA (2016) Biomarkers identification in Alzheimer’s disease using effective connectivity analysis from electroencephalography recordings. Ing. E Investig 36, 50–57. [Google Scholar]
- [30].Ochoa JF, Alonso JF, Duque JE, Tobón CA, Mañanas MA, Lopera F, Hernández AM (2017) Successful Object Encoding Induces Increased Directed Connectivity in Presymptomatic Early-Onset Alzheimer’s Disease. J. Alzheimers Dis 55, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Penny W, Iglesias-Fuster J, Quiroz YT, Lopera FJ, Bobes MA (2018) Dynamic Causal Modeling of Preclinical Autosomal-Dominant Alzheimer’s Disease. J. Alzheimers Dis 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bobes MA, García YF, Lopera F, Quiroz YT, Galán L, Vega M, Trujillo N, Valdes-Sosa M, Valdes-Sosa P (2010) ERP generator anomalies in presymptomatic carriers of the Alzheimer’s disease E280A PS-1 mutation. Hum. Brain Mapp 31, 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev 29, 169–195. [DOI] [PubMed] [Google Scholar]
- [34].Wróbel A (2000) Beta activity: a carrier for visual attention. Acta Neurobiol. Exp. (Warsz.) 60, 247–260. [DOI] [PubMed] [Google Scholar]
- [35].Duque-Grajales JE, Tobon C, Aponte-Restrepo CP, Ochoa-Gómez JF, MUÑOZ-ZAPATA C, Valdivieso H, Quiroz-Zapata YT, Lopera F (2014) Quantitative EEG analysis disease during resting and memory task in carriers and non-carriers of PS-1 E280A mutation of familial Alzheimer’s. CES Med. 28, 165–176. [Google Scholar]
- [36].Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Ayutyanont N, Roontiva A, Thiyyagura P (2012) Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol. 11, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Quiroz YT, Sperling RA, Norton DJ, Baena A, Arboleda-Velasquez JF, Cosio D, Schultz A, Lapoint M, Guzman-Velez E, Miller JB (2018) Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lopera F, Tobon N, Arcos-Burgos M, Vargas S, Gutiérrez J, Rosselli M, Adrilla A (1999) Caracterización imagenológica de la enfermedad de Alzheimerasociada a la mutación E280A-PS1. Estudio caso-control:hallazgos en la resonancia magnética. Rev. Neurol 29, 6–12. [PubMed] [Google Scholar]
- [39].Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A (2012) Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 11, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Quiroz Y, Reiman E, Brickhouse M, Chen K, Fleisher A, Munoz C, Langbaum J, Alvarez S, Tariot P, Lopera F (2012) Trajectory of the Alzheimer’s-signature MRI biomarker in familial Alzheimer’s disease, for the Alzheimer’s Prevention Initiative. Alzheimers Dement. J. Alzheimers Assoc 8, P36. [Google Scholar]
- [41].Quiroz YT, Stern CE, Reiman EM, Brickhouse M, Ruiz A, Sperling RA, Lopera F, Dickerson BC (2013) Cortical atrophy in presymptomatic Alzheimer’s disease presenilin 1 mutation carriers. J Neurol Neurosurg Psychiatry 84, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Quiroz YT, Schultz AP, Chen K, Protas HD, Brickhouse M, Fleisher AS, Langbaum JB, Thiyyagura P, Fagan AM, Shah AR (2015) Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross-sectional study. JAMA Neurol. 72, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dean D, Jerskey B, Chen K, Protas H, Thiyyagura P, Roontiva A, O’muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Siniard A (2014) Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M (2003) Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr. Res 54, 819–825. [DOI] [PubMed] [Google Scholar]
- [45].Ihle A, Bunce D, Kliegel M (2012) APOE ε4 and cognitive function in early life: a meta-analysis. Neuropsychology 26, 267–277. [DOI] [PubMed] [Google Scholar]
- [46].Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillón G, Lopera F, Stern CE (2010) Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol 68, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Quiroz YT, Willment KC, Castrillon G, Muniz M, Lopera F, Budson A, Stern CE (2015) Successful scene encoding in presymptomatic early-onset Alzheimer’s disease. J. Alzheimers Dis. JAD 47, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Johnson KA, Lopera F, Jones K, Becker A, Sperling R, Hilson J, Londono J, Siegert I, Arcos M, Moreno S (2001) Presenilin-1–associated abnormalities in regional cerebral perfusion. Neurology 56, 1545–1551. [DOI] [PubMed] [Google Scholar]
- [49].Fleisher A, Chen K, Quiroz Y, Jakimovich L, Gutierrez M, Langbaum J, Roontiva A, Thiyyagura P, Luo J, Liu X (2013) Pre-symptomatic functional brain changes in PS1 E280A mutation carriers compared with other biomarkers: Pilot data from the Alzheimer’s Prevention Initiative Biomarker project. Alzheimers Dement. J. Alzheimers Assoc 9, P729. [Google Scholar]
- [50].Mosconi L, Pupi A, De Leon MJ (2008) Brain Glucose Hypometabolism and Oxidative Stress in Preclinical Alzheimer’s Disease. Ann. N. Y. Acad. Sci 1147, 180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Roontiva A, Thiyyagura P, Lee W (2015) Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. 72, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Quiroz YT, Protas H, Chen K, Roontiva A, Thiyyagura P, Fagan AM, Shah A, Gutierrez M, Londono M, Giraldo M (2015) Relationships between baseline biomarkers and subsequent cognitive decline in cognitively unimpaired PSEN1 E280A mutation carriers from the colombian kindred with autosomal dominant Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc 11, P888. [Google Scholar]
- [53].Morcom AM, Henson RN. Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation (2018) Journal of Neuroscience. 15;38(33): 7303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, Fagan AM, Holtzman DM, Cairns NJ, Goate AM, Marcus DS, Morris JC, Paumier K, Xiong C, Allegri R, Berman SB, Klunk W, Noble J, Ringman J, Ghetti B, Farlow M, Sperling RA, Chhatwal J, Salloway S, Graff-Radford NR, Schofield PR, Masters C, Rossor MN, Fox NC, Levin J, Jucker M, Bateman RJ, for the Dominantly Inherited Alzheimer Network (2018) Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 91, e1295–e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vradenburg G (2015) A pivotal moment in Alzheimer’s disease and dementia: how global unity of purpose and action can beat the disease by 2025. Expert Rev. Neurother 15, 73–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.