Abstract
Objective
The analysis of epidemiology, risk factors and outcome of viral infections in children and adolescents after hematopoietic cell transplantation (HCT).
Methods
In this multicenter nationwide study a total of 971 HCT procedures (741 allo-HCT; 230 auto-HCT) over a period of 6 years were analyzed.
Results
During this period 801 episodes of viral infections were diagnosed in 442 patients. The incidence of viral infections was 57.9% in allo-HCT and 4.8% in auto-HCT patients. The most frequent infections after allo-HCT were caused by cytomegalovirus (CMV), polyoma BK virus (BKV) and Epstein–Barr virus (EBV). The majority of infections occurred within the first 4 months after allo-HCT and over 80% required pharmacotherapy or symptomatic therapy. The median time of treatment of specific viral infection ranged from 7 (for EBV) to 24 (for CMV) days. The highest mortality was observed in case of CMV infection. The risk factors for viral infections were allo-HCT, acute leukemia, acute and chronic graft versus host disease (a/cGVHD), and matched unrelated donor (MUD)/mismatched unrelated donor (MMUD)-HCT. The risk factor for death from viral infection were CMV-IgG seropositivity in acute lymphoblastic leukemia recipient, and MUD/MMUD-HCT. The incidence of EBV infection requiring pre-emptive treatment with rituximab in allo-HCT children was 19.3%. In 30.8% cases of EBV infection, these episodes were preceded by other viral infection and treated with antivirals, which did not prevent development of EBV-DNA-emia with need of rituximab treatment in 81.5% cases. In 47.7% of these cases, GVHD was a factor enabling development of significant EBV-DNA-emia during antiviral therapy of other infection.
Conclusion
We have shown that antiviral drugs do not prevent EBV reactivation in allo-HCT pediatric patients.
Keywords: children, EBV, HCT, infectious complications, risk factors analysis, viral infections
Introduction
Infections are the major cause of mobility and mortality in children who are undergoing hematopoietic cell transplantation (HCT) or chemotherapy due to malignancy.1–3 According to the Center for International Blood and Marrow Transplant Research (CIBMTR), infectious complications were the cause of death in 7% of autologous HCT (auto-HCT), 11% of matched sibling donor HCT (MSD-HCT) and 13% of matched unrelated donor HCT (MUD-HCT) recipients.2 Infectious complications in children after HCT were noted in 82% of children after allogeneic HCT (allo-HCT)4 and in 21% of children with solid tumor or lymphoma in pediatric auto-HCT setting.5 The incidence of viral infection after allo-HCT was observed in 19.3% of patients during the first 30 days after transplantation, 18.5% between 31 and 100 days, and in 20% after day +101 up to 2 years after HCT.4 In the auto-HCT setting viral infections were observed only in 11% of patients with median onset of 10 days post-transplant.5
Viruses that cause infection after HCT can be classified as latent or “episodic” in nature, with the latter acquired typically after exposure rather than as a result of a reactivation event.6 In the early post-transplant period herpes simplex virus (HSV) reactivation was the most frequent viral infection both in allo-HCT and auto-HCT patients.4,5 Overall, 8% of allo-HCT patient died primarily due to infections and 24% of these infections were viral.4 In auto-HCT patients deaths due to infections were episodic.5
Some major improvements in outcomes were associated with the application of drugs and molecular tests to detect and prevent early bacterial infections, HSV, cytomegalovirus (CMV), and pre-engraftment candidal infections.6 Screening for reactivation with pre-emptive treatment or application of prophylaxis in seropositive recipients plays a role in preventing diseases caused by latent herpesviruses such as CMV, human herpesvirus 6 (HHV-6), and varicella-zoster virus (VZV).6 Implementation of diagnostic and therapeutic strategies for management of Epstein–Barr virus (EBV) infection, on the basis of monitoring of EBV-DNA-emia and pre-emptive or targeted therapy with rituximab, has reduced the incidence of mortality from post-transplant lymphoproliferative disease (EBV-PTLD) from 84% before the year 2000 to 30% in 2013.7 Ganciclovir (GCV) can reduce EBV replication, but neither ganciclovir/foscarnet (FCV) nor cidofovir (CDV) therapy/prophylaxis have any impact on development of EBV-PTLD, so antiviral agents are not recommended.8
In this multicenter nationwide study we present analysis of the epidemiology, risk factors and outcome of viral infections in children and adolescents after HCT over a period of 72 consecutive months. Special attention was given to antiviral drugs usage and incidence of EBV infection after HCT.
Materials and Methods
Design of the Study
The study was designed as multicenter nationwide cohort analysis performed on behalf of the Polish Society of Pediatric Oncology and Hematology. Viral infections diagnosed during a six-year period (between January 1, 2012 and December 31, 2017), were reported by all Polish pediatric HCT centers (Bydgoszcz, Krakow, Lublin, Poznan, Wroclaw) and data were analyzed centrally by two independent researchers. Data from the first 2-year period were collected retrospectively, then prospectively afterwards.
Pathogen Diagnosis
Viral infections were classified as episodic (diagnosed on the basis of clinical manifestation and supplemented with appropriate tests) or latent (requiring monitoring at the molecular level).6,8,9 The following latent viruses were included in the analysis: CMV, EBV, HHV6, HSV, VZV, polyoma BK virus (BKV), while episodic viruses included influenza A and B (FLUAV and FLUBV, respectively) and other community-acquired respiratory viruses (CARV), such as parainfluenza (HPIV), human metapneumovirus (hMPV), respiratory syncytial virus (RSV), rhinovirus (RhV), as well as parvovirus B19 (PVB19), hepatotropic viruses, adenovirus (ADV), rotavirus (RV), and norovirus (NoV). Detection of RV and NoV in the stool was performed using a serological method. For all other viruses polymerase chain reaction (PCR)-based analysis was used for the diagnosis of viral infections of material derived from blood, urine, cerebrospinal fluid, respiratory swabs or bronchoalveolar lavage. According to recommendations of the European Conference on Infections in Leukaemia (ECIL), a pre-emptive quantitative approach was introduced for infections with CMV and EBV in allo-HCT patients.8,9
Recurrent viral infection was defined as a new episode of viral infection in a patient with previous evidence of the same viral infection in whom the virus has not been detected for at least 4 weeks during active surveillance. Recurrent infection could have resulted from reactivation of latent virus or exogenous reinfection.10 Multiple viral infections were defined as infections with two or more viruses in one patient in any time in the study period. Significant plasma CMV-DNA-emia and EBV-DNA-emia were defined with the threshold value ≥1000 copies/mL.
Anti-Infective Management
Standard uniform prophylaxis has been applied for all patients undergoing HCT.8,9,11 Commonly accepted strategies were performed for prophylactic, empirical and targeted anti-infectious therapy with various agents.8,9,11,12 In the case of significant CMV-DNA-emia and EBV-DNA-emia and/or CMV- or EBV-disease, specific treatment was implemented.
Statistical Analysis
An event was defined as the diagnosis of a first specific infectious disorder. Categorical variables were compared by the chi-square test, while non-categorical variables were compared by the Mann–Whitney U test. Hazard ratio (HR) and confidence intervals (CI) were calculated for the difference in occurrence of infections in patients. Cumulative incidences of viral infections were calculated using competing risk analysis,13 starting from the day of transplantation to the day of the first specific infection. Death was considered as the competing event. The Kaplan–Meier method was used to determine infection-related mortality and overall survival.14 Logistic regression method was used for the multivariate analysis. All reported p-values are two-sided; p<0.05 was considered statistically significant.
Results
Demographics
Over the analysis period of 72 consecutive months, a total of 971 HCT (741 allo-HCT; 230 auto-HCT) were performed. Indications for HCTs were: acute lymphoblastic leukemia (ALL, n=233), acute myeloblastic leukemia (AML, n=151), neuroblastoma (n=138), primary immunodeficiencies (n=113), bone marrow failure syndromes (n=101), Non-Hodgkin Lymphoma and Hodgkin Lymphoma (NHL/HD, n=65), Ewing sarcoma (n=44), myelodysplastic syndrome (n=38) and other indications (n=88).
Incidence of Infection
A total of 801 episodes of viral infections were diagnosed in 442 patients (431 allo-HCT and 11 auto-HCT). Overall, 788 infections (726 primary infections and 62 reinfections) (Table 1) were diagnosed in allo-HCT, and 13 (11 primary infections and 2 recurrent infections) in auto-HCT patients (Supplementary Table 1).
Table 1.
Specification of Viral Infection and Therapy in Allo-HCT Setting
| Number of Infectious Episodes | Number of Primary Infections | Number of Relapsed Infections | Symptomatic Therapy | Number of Therapies | Pharmacotherapy | Survival (%) | |
|---|---|---|---|---|---|---|---|
| CMV* | 256 | 214 | 42 | 0 | 250 | Ganciclovir (n=218) | 93.9 |
| Foscarnet (n=84) | |||||||
| Cidofovir (n=31) | |||||||
| Valganciclovir (n=25) | |||||||
| IVIG (n=11) | |||||||
| Acyclovir (n=1) | |||||||
| CMV-CTL (n=1) | |||||||
| BKV* | 181 | 176 | 5 | 5 | 96 | Cidofovir (n=71) | 90.7 |
| Ciprofloxacin (n=18) | |||||||
| Ganciclovir (n=11) | |||||||
| Foscarnet (n=10) | |||||||
| IVIG (n=5) | |||||||
| EBV* | 175 | 168 | 7 | 2 | 143 | Rituximab (n=143) | 93.4 |
| Cidofovir (n=2) | |||||||
| ADV* | 85 | 79 | 6 | 10 | 51 | Cidofovir (n=48) | 90.3 |
| Ganciclovir (n=5) | |||||||
| Foscarnet (n=1) | |||||||
| RV | 37 | 37 | 0 | 19 | 0 | – | 100.0 |
| FLUAV | 14 | 14 | 0 | 1 | 13 | Oseltamivir (n=13) | 92.8 |
| HHV-6* | 10 | 9 | 1 | 1 | 10 | Foscarnet (n=5) | 100.0 |
| Ganciclovir (n=5) | |||||||
| Cidofovir (n=1) | |||||||
| VZV* | 9 | 9 | 0 | 0 | 9 | Aciclovir (n=9) | 100.0 |
| VZIG (n=1) | |||||||
| RSV* | 5 | 5 | 0 | 1 | 5 | IVIG (n=3) | 100.0 |
| Ribavirin (n=2) | |||||||
| Ganciclovir (n=2) | |||||||
| RhV | 4 | 3 | 1 | 4 | 0 | - | 100.0 |
| NoV | 4 | 4 | 0 | 4 | 0 | - | 100.0 |
| HPIV* | 3 | 3 | 0 | 0 | 3 | Ribavirin (n=2) | 66.7 |
| Ganciclovir (n=2) | |||||||
| IVIG (n=1) | |||||||
| HSV | 2 | 2 | 0 | 0 | 2 | Aciclovir (n=2) | 100.0 |
| hMPV | 1 | 1 | 0 | 0 | 1 | Ganciclovir (n=1) | 100.0 |
| PVB19 | 1 | 1 | 0 | 1 | 0 | – | 100.0 |
| HBV | 1 | 1 | 0 | 0 | 0 | – | 100.0 |
Note: *Some of the patients were treated with more than one drug.
Abbreviations: ADV, adenovirus; BKV, BK virus; CMV, cytomegalovirus; CMV-CTL, cytomegalovirus specific T lymphocytes; EBV, Epstein–Barr virus; FLUAV, influenza A virus; HBV, hepatitis B virus; HHV-6, human herpesvirus 6; hMPV, human metapneumovirus; HPIV, parainfluenza; IVIG, intravenous human immunoglobulins; NoV, norovirus; PVB19, parvovirus B19; RhV, rhinovirus; RSV, respiratory syncytial virus; RV, rotavirus; VZIG, varicella-zoster human immune globulin; VZV, varicella-zoster virus.
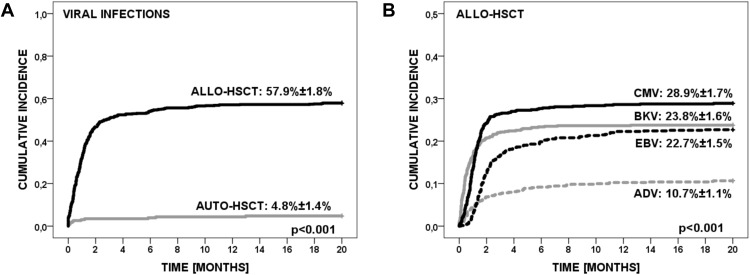
The cumulative incidence of viral infections was 57.9% (95% CI=56.1–59.7) in allo-HCT patients, and 4.8% in auto-HCT patients (95% CI=3.4–6.2) (p<0.001) (Figure 1A). Due to the small number of infections in the auto-HCT setting, these data were excluded from further analysis and are presented in Supplementary Table 1.
Figure 1.
(A). The cumulative incidence of viral infections in allo-HCT and auto-HCT patients. (B). The cumulative incidence of four most frequent viral infections in allo-HCT setting.
In the allo-HCT setting, viral infection with a single pathogen occurred in 54.0% of patients, while there were multiple viral infections in 46.0% of patients (29.0% with two viruses, 13.7% with three viruses, 2.6% with four viruses and 0.7% with five viruses).
The cumulative incidence of viral infections was 28.9% for CMV, 23.8% for BKV, 22.7% for EBV, 10.7% for ADV, 5.0% for RV, 1.9% for FLUAV, 1.2% for VZV, 1.2% for HHV-6, 0.7% for RSV, 0.5% NoV, 0.4% for HPIV, 0.4% for RhV, 0.3% for HSV, 0.1% for hMPV, 0.1% for PVB19 and 0.1% for HBV. The incidence of CMV, BKV and EBV infections were significantly higher than ADV (p<0.001) and other viral infections. The cumulative incidence of four most frequent viral infections is shown in Figure 1B.
Most of the viral infections occurred within the first 4 months from allo-HCT. There were no differences in the median patient age and median time from allo-HCT to specific viral infection, with respective values ranging from 0.4 to 3.8 months. Only in cases of VZV, HSV and NoV were late infections observed, with a median time 6.5, 7.4 and 7.9 months, respectively (Supplementary Table 2).
Treatment
Of 788 specific viral infections (726 primary infections and 62 recurrent infections) in 631 (80.1%) episodes therapies were performed: 583 (92.4%) pharmacotherapies and 48 (7.6%) symptomatic therapies. In all episodes of HHV-6, VZV, HPIV, HSV, hMPV infections, and in 97.6% of CMV, 92.6% FLUAV, 81.7% EBV, 60.0% ADV and 53.0% BKV infectious episodes specific antiviral pharmacotherapy was used. No specific pharmacotherapy was used for RV, RhV, NoV, PVB19 and HBV. The most frequent symptomatic therapies were used in RV (19/48), ADV (10/48), BKV (5/48) infections (Table 1). The median time of treatment of specific viral infection range from 7 days for EBV, RV, FLUAV, RhV, NoV to 22.5 days for BKV and 24 days for CMV infections (Supplementary Table 2).
Survival After Infections
There were no deaths related to RV, HHV-6, VZV, RSV, RhV, NoV, HSV, hMPV, and PVB19 infection. For episodic viral infections one death was related to FLUAV (survival 92.9%) and one to HPIV (survival 66.7%). For the four most frequent viral infections (CMV, BKV, EBV and ADV) survival rate ranged between 90.3% for ADV infection and 95.4 for EBV infection and did not differ between specific viral infection (p=0.3) (Figure 2A). With respect to primary diagnosis, survival from viral infection was 97.4% (95% CI=95.9–98.9) in AML and 92.4% (95% CI=90.6–94.2%) in ALL patients and did not statistically differ between these two (p=0.073) (Figure 2B) and other primary diseases.
Figure 2.
(A). Survival from four most frequent viral infections in allo-HCT setting. (B). Survival from viral infections in AML and ALL patients.
Risk Factor Analysis
Risk Factors for Viral Infections
In multivariate analysis, the risk of infections was higher after allo-HCT (HR=18.0; p<0.001) than auto-HCT. In allo-HCT patients, the risk was higher in acute leukemia patients vs other (HR=1.9; p<0.001), MUD vs MSD (HR=2.6; p<0.001), MMUD (mismatched unrelated donor) vs MSD (HR=4.2; p<0.001), acute and chronic graft versus host disease (a/cGVHD) before infection (HR=1.9; p<0.001 and HR=3.4; p<0.001, respectively) (Table 2).
Table 2.
Multivariate Analysis of Risk Factors for Viral Infection
| Risk Factor | HR (95%CI) | p |
|---|---|---|
| Allo- vs auto-HCT | 18.0 (13–22) | <0.001 |
| allo-HCT | ||
| Sex male vs female | 0.9 (0.7–1.1) | 0.698 |
| Acute leukemia vs other | 1.9 (1.4–2.4) | <0.001 |
| NHL/HD vs other | 1.2 (0.6–1.8) | 0.751 |
| Haplo vs other | 0.8 (0.2–1.4) | 0.825 |
| MUD vs MSD | 2.6 (1.6–3.6) | <0.001 |
| MMUD vs MSD | 4.2 (2.5–5.8) | <0.001 |
| BM vs PB | 0.9 (0.6–1.2) | 0.682 |
| MAC vs RIC | 1.3 (0.8–1.9) | 0.348 |
| TBI vs chemotherapy | 1.2 (0.6–1.8) | 0.676 |
| ANC recovery: >D21 vs ≤D21 | 1.3 (0.7–1.9) | 0.513 |
| aGVHD before infection: yes vs no | 1.9 (1.2–2.6) | <0.001 |
| cGVHD before infection: yes vs no | 3.4 (1.9–5.0) | <0.001 |
Note: All statistically significant p-values are given in bold font.
Abbreviations: a/cGVHD, acute/chronic graft versus host disease; ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; BM, bone marrow; D, days; HCT, hematopoietic cell transplantation; HD, Hodgkin lymphoma; MAC, myeloablative conditioning; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; MSD, matched sibling donor; NHL, Non-Hodgkin lymphoma; RIC, reduced-intensity of conditioning; PB, peripheral blood; TBI, total body irradiation.
Risk Factor for Death from Viral Infection After HCT
Among auto-HCT patients, no child died of viral infection. For allo-HCT patients, a CMV-IgG positive recipient with ALL as primary disease was a risk factor for death (HR=1.5; p=0.045) in multivariate analysis. Other risk factors for death were MUD vs MSD (HR=2.3; p=0.049) and MMUD vs MSD (HR=3.8; p<0.001) (Table 3).
Table 3.
Multivariate Logistic Regression Analysis for Risk Factors for Death from Viral Infection After HCT
| Risk Factor | HR (95%CI) | p |
|---|---|---|
| CMV-IgG+ in recipient and ALL | 1.5 (1.1–2.0) | 0.045 |
| Sex male vs female | 1.1 (0.8–1.5) | 0.829 |
| Acute leukemia vs other | 1.5 (0.8–2.4) | 0.413 |
| NHL/HD vs other | 0.9 (0.4–1.6) | 0.918 |
| Haplo vs other | 1.2 (0.6–1.9) | 0.353 |
| MUD vs MSD | 2.3 (1.0–3.8) | 0.049 |
| MMUD vs MSD | 3.8 (1.5–7.1) | <0.001 |
| BM vs PB | 0.9 (0.4–1.8) | 0.740 |
| MAC vs RIC | 1.2 (0.6–1.9) | 0.762 |
| TBI vs chemotherapy | 1.3 (0.5–2.2) | 0.541 |
| aGVHD before infection: yes vs no | 1.0 (0.5–1.6) | 0.849 |
| cGVHD before infection: yes vs no | 1.6 (0.7–2.5) | 0.092 |
| ANC recovery: >D21 vs ≤D21 | 1.0 (0.5–1.5) | 0.955 |
| First infection: <D30 vs ≥D30 | 1.2 (0.6–2.1) | 0.834 |
| Treatment duration of infection: >D21 vs ≤D21 | 1.7 (0.8–2.6) | 0.171 |
Note: All statistically significant p-values are given in bold font.
Abbreviations: a/cGVHD, acute/chronic graft versus host disease; ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; BM, bone marrow; CMV, cytomegalovirus; D, days; HCT, hematopoietic cell transplantation; HD, Hodgkin lymphoma; HR, hazard ratio; MAC, myeloablative conditioning; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor; NHL, Non-Hodgkin lymphoma; PB, peripheral blood; RIC, reduced-intensity of conditioning; TBI, total body irradiation.
Viral Infections Preceding EBV Infection
In order to assess the impact of antivirals on the reactivation of EBV-DNA-emia, we analyzed the use of all antiviral drugs within 60 days before the onset of significant EBV-DNA-emia. Of 175 EBV infectious episodes, in 66 (37.7%) cases, the infection was preceded by another viral infection, including CMV (n=21), BKV (n=20), ADV (n=4), RV (n=3), FLUAV (n=1), HPIV (n=1), RSV (n=1), VZV (n=1), or multiple infections (BKV+CMV, n=8; BKV+RV, n=3; ADV+CMV, n=1; ADV+HHV6, n=1; ADV+RV, n=1) within 7–56 (median 24) days before the diagnosis of EBV-DNA-emia. In 54/66 (81.8%) cases, a significant EBV-DNA-emia has occurred and treatment with rituximab was required. In 44/54 (81.5%) of these cases viral infections preceding EBV infection were treated with antiviral drugs, including: CDV (n=18), GCV (n=17), aciclovir (ACV) (n=1), oseltamivir (n=1) or drug combinations (FCV+GCV, n=5; FCV+CDV, n=2; GCV+CDV, n=2). Antivirals were used for treatment of: CMV (n=16), BKV (n=11), ADV (n=4), FLUAV (n=1), VZV (n=1) or multiple infections (BKV+CMV, n=7; BKV+RV, n=1; ADV+CMV, n=1; ADV+HHV-6, n=1; ADV+RV, n=1). Additionally, in 21/44 (47.7%) cases, development of significant EBV-DNA-emia occurred during GVHD (18 acute, 3 chronic). With respect to antiviral drugs, significant EBV-DNA-emia has developed in 24/242 (9.9%) GCV therapies, 22/153 (14.4%) CDV therapies, 9/100 (9.0%) FCV therapies, 1/12 (8.3%) ACV therapies and 1/13 (7.7%) of oseltamivir therapies.
Discussion
The primary causes of morbidity and non-relapse mortality following HCT are acute and chronic GVHD, organ dysfunction and infection.15 Changes in the transplantation procedure and the implementation of effective supportive care strategies have decreased the incidence of infectious complications early after conditioning therapy for allo-HCT, but have also prolonged the risks beyond day +100.6 These late infections might be caused by all types of microorganisms; however, the risks are predictable and surmountable with the use of tailored prevention strategies.6 There are only a few articles focused on viral complications in children after HCT.4,5,16,17 Herein we reported the results of multicenter nationwide study of the epidemiology, risk factors and outcome of viral infections in children and adolescents after HCT over a period of six consecutive years. All patients were treated with the same therapeutic protocols, using comparable principles of supportive therapy.
We shown high incidence of viral infections in allo-HCT patients whereas in auto-HCT patients the incidence was episodic, comparably with other studies.4,5 This disproportion is a result of allogeneic source of stem cells, T-cell depletion or CD34 selection, moderate-to-severe GVHD and use of immunosuppressive drugs (especially steroids) in allo-HCT patients.11
We observed that CMV had the highest incidence from all viral infections. CMV is one of the most difficult infections that occur after allo-HCT.18 The CMV serostatus of the donor and recipient before transplantation significantly influence the incidence of CMV recurrence, whereas the immunosuppressive status of the recipient is the most important factor for CMV infection.10 Monitoring by a sensitive technique such as PCR tests of whole blood allows intervention before development of CMV disease. Pre-emptive therapy can be used as a standalone strategy or combined with antiviral prophylaxis. Recently, letermovir, given as prophylaxis, was shown to reduce the risk of clinically significant CMV infection.18 We have shown that ganciclovir and foscarnet were the most frequently used drugs in our cohort for the therapy of CMV reactivation; accordingly to currently recommended first-line pre-emptive treatment.18 Nevertheless, management of patients with resistant or refractory CMV infection or CMV disease is a challenge. Combination therapy (ganciclovir and foscarnet), cidofovir, leflunomide or artesunate can be considered in patients resistant or refractory to other second-line and third-line antiviral drugs, and immunosuppression should always be reduced, if possible.18
We observed that cumulative incidence of BKV infection was 23.8% with survival rate of 90.7%. Cesaro et al. reported that BKV-related hemorrhagic cystitis occurred in 8–25% of pediatric and 7–54% of adult recipients undergoing allo-HCT.19 Specific anti-BKV prophylaxis is not available and fluoroquinolones are not recommended given the lack of significant effects on BKV replication and hemorrhagic cystitis severity, and the selection of antibiotic resistance.19 In our study cidofovir was the most frequently used drug in the treatment of BKV infection. Cidofovir at a dose of 3–5 mg/kg every 1–2 weeks with probenecid, or 0.5–1.5 mg/kg 1–3 times/week without probenecid, were used by other authors for the treatment of BKV hemorrhagic cystitis, although without strong recommendation on its use in these doses.19
The clinical manifestations of ADV infections in immunocompetent hosts include upper respiratory disease, gastroenteritis or (kerato-)conjunctivitis and are self-limited in most cases, although severe manifestations including encephalitis, myocarditis, and pneumonia have been sporadically observed. In immunocompromised patients, ADV can cause systemic disease and lethal organ damage.20 Prophylactic antiviral therapy with available antiviral drugs is currently not recommended and intravenous cidofovir is currently regarded as a standard of care in cases of ADV disease.20 Children are more frequently affected with ADV than adults (6–28% vs 0–6%, respectively);20 we observed 10.7% incidence of ADV infection with 90.3% survival rate.
A relatively low percentage of infections with CARV origin was observed in our analysis. CARV respiratory tract infections have been recognized as a significant cause of morbidity and mortality in patients with leukemia and those undergoing HCT.12,21 In the late 1990s the frequency of documented respiratory virus infections was 3.5% among allo-HCT and 0.4% among auto-HCT.22 However, in that time period, viral antigen detection by immunofluorescence or enzyme immunoassays were used in diagnostics.22 During the last decade, rapid and highly sensitive molecular tests have been developed and made available, with the most recent multiplex PCR platform that can detect multiple viral pathogens.21 Choi et al. reported in allo-HCT children the incidence 28.1% of RhV infection, 25.8% RSV, 18% HPIV, 1.1% hMPV; yet more than half of the infections were acquired during hospitalization.17 In the case of CARV infections, ribavirin and intravenous immunoglobulin are recommended in hMPV, HPIV and RSV infections, while there is insufficient evidence for the specific recommendation against infections caused by coronavirus and RhV.12
We observed a relatively low (1.2%) rate of influenza A infection, with no cases of influenza B infection, which may be due to environmental prophylaxis (annual vaccination of patients, household contacts, and hospital and prophylactic use of neuraminidase inhibitors during influenza season in some cases). The current guidelines of the 4th European Conference of Infections in Leukemia (ECIL-4) recommend diagnosis based on PCR of material collected from the respiratory tract, especially broncho-alveolar lavage.23 Neuraminidase inhibitors (oral oseltamivir or inhalation of zanamivir) are currently the most effective therapeutic agents for influenza.23
RV and NoV are important pathogens of viral gastroenteritis in children. We observed that cumulative incidence of RV was 5% while NoV was 0.5%, treated only with symptomatic therapy and no deaths were observed. In other studies the incidence of RV infection varied from 2.3% in adult allo-HCT and auto-HCT recipients up to 19.6% in pediatric allo-HCT recipients.24,25 In pediatric HCT, the incidence of NoV-associated gastroenteritis was reported in 12.9% with no NoV-related mortality.26 PCR as well virus antigen detection in stool are commonly used in diagnosis of RV and NoV infection. No specific prophylaxis against RV- and NoV-related gastroenterocolitis is available; however, oral immunoglobulins and nitazoxanide were used in some studies.24,27
In immunocompetent individuals primary EBV infection or reactivation induces usually asymptomatic infection or infectious mononucleosis.28 In immunocompromised patients most EBV reactivations are subclinical and require no therapy.8 However, it may be manifested as encephalitis/myelitis, pneumonia, hepatitis and EBV-associated tumors as PTLD.8,29 After transplantation 14–65% of recipients developed EBV reactivation,28 while the incidence of EBV-PTLD varies from 0.45% to 29%, according to the source of hematopoietic cells, the associated cell manipulation, and the details of immunosuppressive regimens used.30 We observed high incidence of EBV infection (22.7%) in allo-HCT patients, while there were no cases in auto-HCT setting. In 80.3% (143/175) of EBV reactivations rituximab was used and 93.3% of patients survived this infection. We also observed that in almost 1/3 of EBV reactivations, these episodes were preceded by other viral infection treated with antivirals, which did not prevent the development of significant EBV-DNA-emia with the need of rituximab treatment. This is clinically proven evidence that antiviral drugs do not prevent EBV reactivation in allo-HCT pediatric patients. Antiviral agents, such as ganciclovir, are not active against EBV, presumably because of low levels of viral thymidine kinase expression during lytic phase, and a lack of expression during latency.8 Intravenous immunoglobulins also have no impact in PTLD. Neither ganciclovir/foscarnet nor cidofovir therapy/prophylaxis have any impact on the development of EBV-PTLD, so antiviral agents are not recommended.8 Prospective monitoring of EBV-DNA-emia is recommended in patients after allo-HCT, and these patients should be closely monitored for symptoms and/or signs attributable to EBV infection and PTLD.8 The following pre-emptive therapies are recommended after high-risk allo-HCT: rituximab (375 mg/m2/weekly), reduction of immunosuppressive therapy (if possible), donor EBV-specific cytotoxic T-cells (EBV-CTL) infusion (if available).8 Compared with rituximab, adoptive cellular immunotherapy has higher response rate (50–88%) and fewer relapse.28
We found that allo-HCT, acute leukemia, a/cGVHD, MUD/MMUD-HCT were the risk factors for viral infections. CMV donor/recipient serostatus, era of transplantation, MUD-HCT, and cGVHD were found in other studies as risk factors for viral infection.4 We have also found that CMV-IgG seropositivity in ALL recipients, and MUD/MMUD-HCT were the risk factors for deaths from viral infection in children after HCT. This is a unique observation, as there is no study available analyzing this issue.
Conclusions
The incidence of viral infections was high in allo-HCT patients while in auto-HCT patients viral infections were episodic. The most frequent viral infection after allo-HCT were CMV, BKV and EBV. Most viral infections occurred within the first 4 months after allo-HCT and over 80% required pharmacotherapy or symptomatic therapy. The median time of treatment of specific viral infection ranged from 7 (for EBV) to 24 days (for CMV). The highest infection-related mortality was observed in case of CMV infection, whereas primary diagnosis did not influence survival from viral infection. The risk factors for viral infections were allo-HCT, acute leukemia, a/cGVHD, MUD/MMUD vs MSD. The risk factor for death from viral infection were CMV-IgG seropositivity in acute lymphoblastic leukemia recipients, MUD/MMUD vs MSD. The incidence of EBV infection requiring pre-emptive treatment with rituximab in allo-HSCT children was 19.3%. In 30.8% of cases of EBV infection, these episodes were preceded by other viral infection treated with antivirals, which did not prevent development of significant EBV-DNA-emia with the need of rituximab treatment in 81.5% of cases. This is clinically proven evidence that antiviral drugs do not prevent EBV reactivation in allo-HCT pediatric patients. Additionally, in 47.7% of these cases GVHD was a risk factor, possibly facilitating development of significant EBV-DNA-emia during antiviral therapy of other infections.
Acknowledgments
This study was created on behalf of the Polish Society of Pediatric Oncology and Hematology. The authors thanks to Mariusz Wysocki, Krzysztof Kalwak, Jacek Wachowiak and Jerzy Kowalczyk for their support in the implementation of the project.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun. All patient data were analyzed in anonymity. As neither individual data were published nor any intervention was performed on patients, patient consent was waived by the ethics committee.
Abbreviations
ACV, aciclovir; ADV, adenovirus; a/cGVHD, acute/chronic graft versus host disease; ALL, acute lymphoblastic leukemia; allo-, allogeneic; AML, acute myeloblastic leukemia; ANC, absolute neutrophil count; ARAC, cytarabine; auto-, autologous; BCNU, carmustine; BKV, polyoma BK virus; BM, bone marrow; CARV, community-acquired respiratory virus; CI, confidence intervals; CIBMTR, Center for International Blood and Marrow Transplant Research; CMV, cytomegalovirus; D, days; EBV, Epstein–Barr virus; ECIL, European Conference on Infections in Leukaemia; FCV, foscarnet; FLUAV, influenza A virus; FLUBV, influenza B virus; GCV, ganciclovir; HBV, hepatitis B virus; HCT, hematopoietic cell transplantation; HD, Hodgkin lymphoma; HHV-6, human herpesvirus 6; hMPV, human metapneumovirus; HPIV, parainfluenza; HR, hazard ratio; HSV, herpes simplex virus; IQR, quartiles; MAC, myeloablative conditioning; MEL, melphalan; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor; NHL, Non-Hodgkin Lymphoma; NoV, norovirus; PB, peripheral blood; PTLD, post-transplant lymphoproliferative disease; PVB19, parvovirus B19; RhV, rhinovirus; RIC, reduced-intensity of conditioning; RSV, respiratory syncytial virus; TBI, total body irradiation; VP, etoposide; VZV, varicella-zoster virus.
Author Contributions
KC and JS were responsible for concept of study and design. KC wrote the manuscript. All authors contributed toward data collection, data analysis, critical revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no potential conflict of interest relevant to this article.
References
- 1.Styczynski J, Czyzewski K, Wysocki M, et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol Infect. 2016;22(2):179 e171–179 e110. doi: 10.1016/j.cmi.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 2.D’Souza A, Fretham C. Current uses and outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR summary slides. 2018. Available from: https://www.cibmtr.org. Accessed November11, 2019.
- 3.Czyzewski K, Galazka P, Zalas-Wiecek P, et al. Infectious complications in children with malignant bone tumors: a multicenter nationwide study. Infect Drug Resist. 2019;12:1471–1480. doi: 10.2147/IDR.S199657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan A, Wang C, Srivastava DK, et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(1):94–101. doi: 10.1016/j.bbmt.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan A, McLaughlin L, Wang C, et al. Early infections after autologous hematopoietic stem cell transplantation in children and adolescents: the St. Jude experience. Transpl Infect Dis. 2014;16(1):90–97. doi: 10.1111/tid.2014.16.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marr KA. Delayed opportunistic infections in hematopoietic stem cell transplantation patients: a surmountable challenge. Hematology Am Soc Hematol Educ Program. 2012;2012:265–270. doi: 10.1182/asheducation.V2012.1.265.3800160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styczynski J, Tridello G, Gil L, et al. Impact of donor epstein-barr virus serostatus on the incidence of graft-versus-host disease in patients with acute leukemia after hematopoietic stem-cell transplantation: a study from the acute leukemia and infectious diseases working parties of the European society for blood and marrow transplantation. J Clin Oncol. 2016;34(19):2212–2220. doi: 10.1200/JCO.2015.64.2405 [DOI] [PubMed] [Google Scholar]
- 8.Styczynski J, Reusser P, Einsele H, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43(10):757–770. doi: 10.1038/bmt.2008.386 [DOI] [PubMed] [Google Scholar]
- 9.Ljungman P, de la Camara R, Cordonnier C, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42(4):227–240. doi: 10.1038/bmt.2008.162 [DOI] [PubMed] [Google Scholar]
- 10.Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1–16. doi: 10.1007/s40121-017-0180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styczynski J, Gil L. Prevention of infectious complications in pediatric HSCT. Bone Marrow Transplant. 2008;42(Suppl 2):S77–S81. doi: 10.1038/bmt.2008.289 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258–266. doi: 10.1093/cid/cis844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(ISSN)1097-0258 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 15.Miller HK, Braun TM, Stillwell T, et al. Infectious risk after allogeneic hematopoietic cell transplantation complicated by acute graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(3):522–528. doi: 10.1016/j.bbmt.2016.12.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48(6):803–808. doi: 10.1038/bmt.2012.221 [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Choi EH, Kang HJ, et al. Respiratory viral infections after hematopoietic stem cell transplantation in children. J Korean Med Sci. 2013;28(1):36–41. doi: 10.3346/jkms.2013.28.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e260–e272. doi: 10.1016/S1473-3099(19)30107-0 [DOI] [PubMed] [Google Scholar]
- 19.Cesaro S, Dalianis T, Hanssen Rinaldo C, et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018;73(1):12–21. doi: 10.1093/jac/dkx324 [DOI] [PubMed] [Google Scholar]
- 20.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis. 2012;14(6):555–563. doi: 10.1111/tid.2012.14.issue-6 [DOI] [PubMed] [Google Scholar]
- 21.Mikulska M, Del Bono V, Gandolfo N, et al. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014;93(4):669–676. doi: 10.1007/s00277-013-1912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28(5):479–484. doi: 10.1038/sj.bmt.1703139 [DOI] [PubMed] [Google Scholar]
- 23.Engelhard D, Mohty B, de la Camara R, Cordonnier C, Ljungman P. European guidelines for prevention and management of influenza in hematopoietic stem cell transplantation and leukemia patients: summary of ECIL-4 (2011), on behalf of ECIL, a joint venture of EBMT, EORTC, ICHS, and ELN. Transpl Infect Dis. 2013;15(3):219–232. doi: 10.1111/tid.2013.15.issue-3 [DOI] [PubMed] [Google Scholar]
- 24.Flerlage T, Hayden R, Cross SJ, et al. Rotavirus infection in pediatric allogeneic hematopoietic cell transplant recipients: clinical course and experience using nitazoxanide and enterally administered immunoglobulins. Pediatr Infect Dis J. 2018;37(2):176–181. doi: 10.1097/INF.0000000000001740 [DOI] [PubMed] [Google Scholar]
- 25.van Kraaij MG, Dekker AW, Verdonck LF, et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant. 2000;26(3):299–303. doi: 10.1038/sj.bmt.1702484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2012;18(12):1883–1889. doi: 10.1016/j.bbmt.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Williams D. Treatment of rotavirus-associated diarrhea using enteral immunoglobulins for pediatric stem cell transplant patients. J Oncol Pharm Pract. 2015;21(3):238–240. doi: 10.1177/1078155214522313 [DOI] [PubMed] [Google Scholar]
- 28.Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2013;6:94. doi: 10.1186/1756-8722-6-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czyzewski K, Styczynski J, Krenska A, et al. Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk Lymphoma. 2013;54(3):503–506. doi: 10.3109/10428194.2012.718342 [DOI] [PubMed] [Google Scholar]
- 30.Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis. 2009;11(5):383–392. doi: 10.1111/tid.2009.11.issue-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- D’Souza A, Fretham C. Current uses and outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR summary slides. 2018. Available from: https://www.cibmtr.org. Accessed November11, 2019.