Abstract
OBJECTIVE
To compare diabetic kidney disease (DKD) rates over 5 years of follow-up in two cohorts of severely obese adolescents with type 2 diabetes (T2D) undergoing medical or surgical treatment for T2D.
RESEARCH DESIGN AND METHODS
A secondary analysis was performed of data collected from obese participants of similar age and racial distribution enrolled in the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and the Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) studies. Teen-LABS participants underwent metabolic bariatric surgery (MBS). TODAY participants were randomized to metformin alone or in combination with rosiglitazone or intensive lifestyle intervention, with insulin therapy given for glycemic progression. Glycemic control, BMI, estimated glomerular filtration rate (eGFR), urinary albumin excretion (UAE), and prevalence of hyperfiltration (eGFR ≥135 mL/min/1.73 m2) and elevated UAE (≥30 mg/g) were assessed annually.
RESULTS
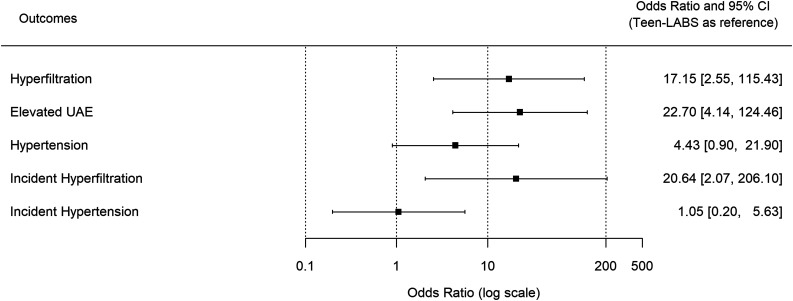
Participants with T2D from Teen-LABS (n = 30, mean ± SD age, 16.9 ± 1.3 years; 70% female; 60% white; BMI 54.4 ± 9.5 kg/m2) and TODAY (n = 63, age 15.3 ± 1.3 years; 56% female; 71% white; BMI 40.5 ± 4.9 kg/m2) were compared. During 5 years of follow-up, hyperfiltration decreased from 21% to 18% in Teen-LABS and increased from 7% to 48% in TODAY. Elevated UAE decreased from 27% to 5% in Teen-LABS and increased from 21% to 43% in TODAY. Adjusting for baseline age, sex, BMI, and HbA1c, TODAY participants had a greater odds of hyperfiltration (odds ratio 15.7 [95% CI 2.6, 94.3]) and elevated UAE (27.3 [4.9, 149.9]) at 5 years of follow-up.
CONCLUSIONS
Compared with MBS, medical treatment of obese youth with T2D was associated with a higher odds of DKD over 5 years.
Introduction
Diabetic kidney disease (DKD) is the leading cause of renal failure in the U.S. and develops at an alarming rate in adolescents with youth-onset type 2 diabetes (T2D) (1–4). Current medical treatments are only partially protective against DKD in the setting of T2D. Compared with adult-onset T2D, youth with T2D have a more aggressive phenotype, with greater insulin resistance, more rapid β-cell failure, and higher prevalence of DKD (2–5), supporting a need for dedicated studies in youth.
The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study prospectively evaluated outcomes of adolescents who clinically qualified for and underwent metabolic bariatric surgery (MBS) at one of five U.S. centers. A longitudinal analysis in Teen-LABS established that urinary albumin excretion (UAE) and estimated glomerular filtration rate (eGFR) decreased over 3 years following MBS in severely obese adolescents without T2D (6). The Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) study was a multicenter randomized controlled trial designed to investigate strategies to achieve durable glycemic control and demonstrated that almost 50% of youth with T2D progressed to requiring insulin after a median follow-up of 11 months (7). Longitudinal data from the TODAY study also demonstrated that the cumulative incidences of elevated UAE (≥30 mg/g) and hypertension were 18% and 37%, respectively, during nearly 6 years of follow-up (8). Yet, there are no data that have specifically examined kidney outcomes after MBS versus medical therapy in adolescents with T2D.
A recent analysis of severely obese youth with T2D in Teen-LABS and TODAY found that over 2 years of follow-up, hemoglobin A1c (HbA1c) increased significantly in TODAY, while it decreased in Teen-LABS (9). Furthermore, BMI increased by 3.7% in TODAY but decreased by 29% in Teen-LABS over 2 years (9). Studies are now needed to understand differences in the effect of MBS and medical therapy on other T2D-related comorbidities, including DKD. Accordingly, the objective of the current study was to define DKD rates over 5 years of follow-up in these two cohorts (Teen-LABS and TODAY) of severely obese adolescents with T2D undergoing medical versus MBS interventions. We hypothesized that youth with T2D undergoing MBS would experience improvement in DKD outcomes, whereas youth with T2D treated medically would experience worsening of DKD outcomes over 5 years.
Research Design and Methods
Study Design and Participants
Teen-LABS enrolled 242 adolescents (≤19 years of age) from 1 March 2007 to 31 December 2011. TODAY enrollment started on 1 May 2004 and ended on 31 December 2009, with a total of 699 randomized participants (ages 10–17 years). Postintervention follow-up, wherein participants were provided with standard medical therapy, lasted 3 years and began immediately after the TODAY clinical trial was completed. Study details for both Teen-LABS and TODAY have been published elsewhere (7,8,10). The TODAY and Teen-LABS protocols were approved by the institutional review boards of each participating institution. Participants provided written informed parental consent and child assent. The participants provided consent for identifiers to be maintained at the data coordinating centers for each study. Deidentified data were used for the purposes of the current analysis.
Pertinent to this analysis, there were 30 Teen-LABS participants with T2D at the time of MBS. Of these, 24 underwent Roux-en-Y gastric bypass and 6 underwent vertical sleeve gastrectomy procedures. Participants with T2D in TODAY (irrespective of treatment group assignment) were frequency matched to the 30 Teen-LABS participants with T2D using the following matching criteria: baseline age (13–18 years), race/ethnicity, sex, and baseline BMI (>35 kg/m2). Through this process, a total of 63 TODAY participants were identified. This secondary analysis included data collected from the 30 MBS-treated and 63 medically treated individuals with T2D at the baseline and 1-, 2-, 3-, 4-, and 5-year study visits.
T2D Definition
Standard conventions were followed for the assessment and prevalence of conditions over time. In brief, presence of T2D in Teen-LABS participants was defined as use of medications for diabetes, baseline HbA1c concentration of ≥6.5% (to convert to proportion of hemoglobin, multiply by 0.01), fasting glucose concentration of ≥126 mg/dL (to convert to g/L, multiply by 10), or 2-h glucose value >200 mg/dL (to convert to mmol/L, multiply by 0.0555) during an oral glucose tolerance test in the 6 months before enrollment. T2D in TODAY was defined by standard American Diabetes Association (ADA) glucose and HbA1c criteria (11) except that asymptomatic patients with a normal fasting glucose but elevated 2-h glucose concentration during an oral glucose tolerance test were also required to have an HbA1c ≥6% to limit enrollment of patients with prediabetes (12).
Laboratory Assessments
All laboratory assays for the Teen-LABS and TODAY cohorts were performed by the Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA) as previously described (9). HbA1c and insulin assays were performed by high-performance liquid chromatography and double-antibody radioimmunoassay, respectively, as previously described (7,8,10). Insulin sensitivity was calculated annually as 1 / fasting insulin (mL/μU), which correlates strongly with hyperinsulinemic-euglycemic clamp–derived in vivo insulin sensitivity in obese youth with or without T2D (13). Concentrations of creatinine in serum and urine were determined annually by using the Creatinine Plus ver.2 enzymatic reagent on a Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). The results of this procedure are traceable to the isotope dilution mass spectrometry reference method and allow for accurate eGFR. The reportable range of creatinine in serum/plasma samples is 0.03–60.0 mg/dL and in urine samples, 0.03–1,200.0 mg/dL. Concentration of cystatin C in serum was determined immunochemically at baseline and annually by using Siemens reagents (Siemens Healthcare Diagnostics, Newark, DE) on a Siemens BN II nephelometer autoanalyzer. This method is standardized against the International Federation of Clinical Chemistry and Laboratory Medicine ERM DA-471 Reference Material (RT Corp, Laramie, WY).
Elevated UAE and Hypertension Definition
Because of the expected normal to elevated glomerular filtration rates for age, we calculated eGFR by the full age spectrum (FAS)–combined serum creatinine (SCr) and cystatin C (ScysC) equation, which has been validated in both children and adults and lends itself well to studies examining the transition from pediatrics to early adulthood (14):
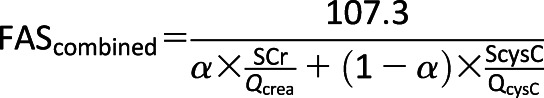 |
The FAS equation is based on normalized serum creatinine (SCr / Qcrea), where Qcrea is the median SCr from healthy populations to account for age and sex, and QcysC is 0.82 mg/L for ages <70 years. The coefficient α in the denominator is a weighting factor for the normalized renal biomarkers. We used α = 0.5, which means that the denominator is equal to the average of both normalized biomarkers. We defined hyperfiltration as eGFR ≥135 mL/min/1.73 m2 (15). Urine albumin-to-creatinine ratio (UACR) was measured at baseline and annually thereafter, unless a result was abnormal. Spot urine samples were obtained after a 10–14-h overnight fast. Elevated UAE (previously known as microalbuminuria) was defined as a UACR ≥30 μg/mg (8). Participants who developed elevated UAE in TODAY were promptly treated according to ADA recommendations, which included starting an ACE inhibitor.
Hypertension was defined per protocol as use of blood pressure (BP)–lowering medications or 1) systolic blood pressure (SBP) ≥95th percentile or diastolic blood pressure (DBP) ≥95th percentile (for age, sex, height) if <18 years of age or 2) SBP >140 mmHg or DBP >90 mmHg if ≥18 years of age. Antihypertensive therapy was initiated in TODAY according to ADA adult guidelines, with the addition of treatment of BP from the 90th to 95th percentile (dietary) and >95th percentile (dietary and pharmacologic) for those in whom 130/80 mmHg would have been too high a threshold on the basis of age, as previously described (16).
Assessment of Adverse Clinical Events
The procedures for assessment of adverse events in Teen-LABS (10) and TODAY (7) have been previously described.
Statistical Analysis
Continuous variables are presented as means and SDs, except those with highly skewed distributions, which are summarized by median and interquartile range (IQR). Categorical variables are presented by numbers and percentages. Baseline variable comparisons between the Teen-LABS and TODAY groups were accomplished by F test and χ2 test. For categorical measures with a limited number of observations, Fisher exact test was used. For continuous variables without normal distribution, Wilcoxon rank sum test was used. Generalized estimating equation (GEE) was used for categorical outcomes (elevated UAE, hyperfiltration, hypertension, incident hyperfiltration, and incident hypertension), with a group ∗ month interaction included to measure the differences between groups at each time point. Sex, age, BMI, HbA1c, insulin sensitivity, triglycerides, and antihypertensive medication use were included in the multivariable models. Linear mixed-effects (LME) models were used for continuous variables, including eGFR, UACR, SBP, and DBP. Log values were used instead of original UACR values to fit the normality assumption. Autoregressive(1) covariance structure was used for within-subject variations. The same set of covariates as used in the GEE was adjusted for LME. Mediation analysis based on GEE was conducted to obtain the direct group effect (Teen-LABS vs. TODAY) and the combined group effects (direct effect + indirect effect) through the change of HbA1c or BMI or estimated insulin sensitivity or triglycerides. The strongest mediator of the group effect was obtained by comparing the odds ratio (OR) of outcomes between direct and combined group effects (change in HbA1c, BMI, insulin sensitivity, and triglycerides). Complete data were used for the main study of this article. Sensitivity analyses were performed with SAS PROC MI. Fifty imputed data sets were generated. SAS PROC MIANALYZE was used to estimate the pooled results of 50 fittings. All statistical analyses were performed using SAS 9.4 software (SAS Institute).
Results
Baseline Comparison Between Youth With T2D in TODAY Versus Teen-LABS
Severely obese participants in Teen-LABS were older and had a higher mean BMI, SBP, DBP, and triglycerides at baseline than those in TODAY (Table 1). Additionally, there were more female participants in Teen-LABS versus TODAY, although this did not reach statistical significance (70% vs. 56%, P = 0.18). There were no statistically significant differences in HbA1c, insulin sensitivity, HDL cholesterol, eGFR, or UACR at baseline (Table 1).
Table 1.
Baseline participant characteristics stratified by study
| Teen-LABS (n = 30) | TODAY (n = 63) | P value | |
|---|---|---|---|
| Age (years) | 16.9 ± 1.3 | 15.4 ± 1.3 | <0.0001 |
| Female sex | 70.0 | 55.6 | 0.18 |
| Race/ethnicity | |||
| Black non-Hispanic | 30.0 | 28.6 | 0.89 |
| Hispanic | 3.3 | 0 | 0.32 |
| White non-Hispanic | 60.0 | 71.4 | 0.27 |
| Other | 6.7 | 0 | 0.10 |
| BMI (kg/m2) | 54.4 ± 9.5 | 40.5 ± 4.9 | <0.0001 |
| BP (mmHg) | |||
| Systolic | 129 ± 12 | 119 ± 11.4 | 0.0002 |
| Diastolic | 76 ± 12 | 70 ± 9 | 0.02 |
| Hypertension | 66.7 | 25.4 | 0.0001 |
| Antihypertensive medication use | 60.0 | 12.7 | <0.0001 |
| ACE inhibitor/ARB use | 30.0 | 12.7 | 0.04 |
| Lipid-lowering medication use | 10.0 | 0.0 | 0.03 |
| UACR (mg/g) | 11 (5–32) | 10 (5–22) | 0.66 |
| Elevated UAE (≥30 mg/g) | 26.7 | 21.3 | 0.57 |
| ScysC (mg/L) | 0.80 ± 0.18 | 0.79 ± 0.14 | 0.94 |
| SCr (mg/dL) | 0.66 ± 0.15 | 0.67 ± 0.14 | 0.88 |
| eGFR-FAS (mL/min/1.73 m2) | 118 ± 22 | 115 ± 15 | 0.53 |
| Hyperfiltration | 20.7 | 7.1 | 0.15 |
| HbA1c | 6.8 ± 1.9 | 6.2 ± 0.7 | 0.53 |
| Insulin sensitivity (1/IF) (mL/μU) | 0.04 ± 0.04 | 0.04 ± 0.04 | 0.14 |
| HDL cholesterol (mg/dL) | 40 ± 10 | 39 ± 9 | 0.50 |
| Triglycerides (mg/dL) | 153 ± 68 | 132 ± 100 | 0.03 |
| High triglycerides (≥200 mg/dL) | 20.7 | 15.9 | 0.57 |
| Very high triglycerides (≥500 mg/dL) | 0.0 | 1.6 | 0.99 |
Data are mean ± SD, median (IQR), or percent. 1/If, 1 / fasting insulin; ARB, angiotensin receptor blocker.
Medical Versus Surgical Intervention on Metabolic Control, DKD, and Hypertension
BMI, HbA1c, Insulin Sensitivity, and Triglycerides
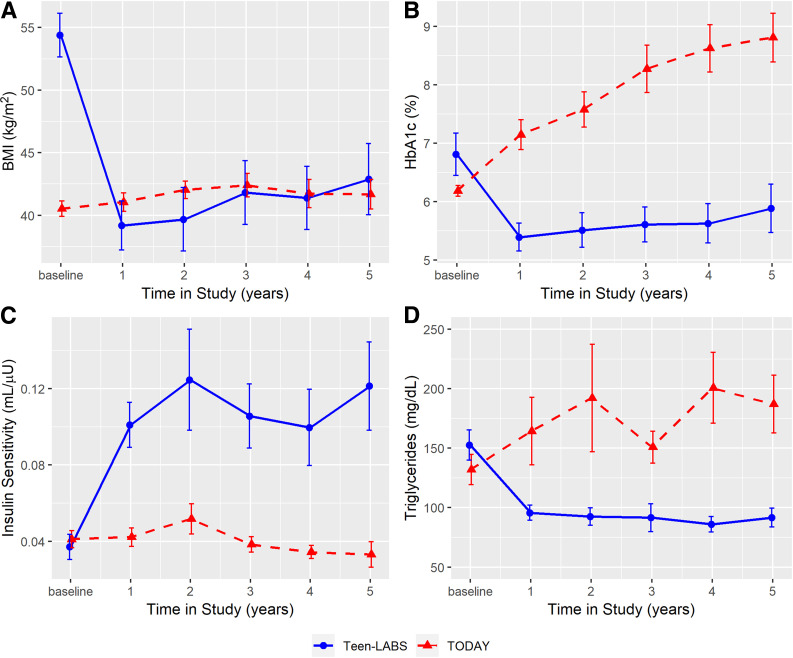
Following MBS, participants in Teen-LABS demonstrated significant improvements in BMI, HbA1c, and insulin sensitivity over the 5-year follow-up compared with their TODAY counterparts (Fig. 1 and Supplementary Table 2). In Teen-LABS, mean BMI decreased from 54.4 kg/m2 at baseline to 39.2 kg/m2 at year 1 and then modestly increased to 42.9 kg/m2 at year 5. In TODAY, mean BMI increased from 40.5 kg/m2 at baseline to 41.7 kg/m2 at year 5. Mean HbA1c decreased from 6.8% at baseline to 5.4% at year 1 and then modestly increased to 5.9% at year 5 in Teen-LABS. Conversely, in TODAY, mean HbA1c progressively increased from 6.2% at baseline to 8.8% at year 5. Mean insulin sensitivity improved from 0.04 mL/μU at baseline to 0.12 mL/μU at year 5 in Teen-LABS and worsened from 0.04 mL/μU at baseline to 0.03 mL/μU at year 5 in TODAY. Finally, mean triglycerides decreased from 153 mg/dL at baseline to 92 mg/dL at year 5 in Teen-LABS and increased from 132 mg/dL at baseline to 187 mg/dL at year 5 in TODAY.
Figure 1.
BMI, HbA1c, insulin sensitivity, and triglycerides over 5 years in TODAY and Teen-LABS. Line charts for BMI (P < 0.0001) (A), HbA1c (P < 0.0001) (B), insulin sensitivity (P = 0.0012) (C), and triglycerides (P < 0.0001) (D). Error bars indicate ±SEM. Means and error bars were jittered to avoid overlapping. P values are provided for the test of trajectory difference between groups over the study period (i.e., test for the existence of group ∗ time interaction terms).
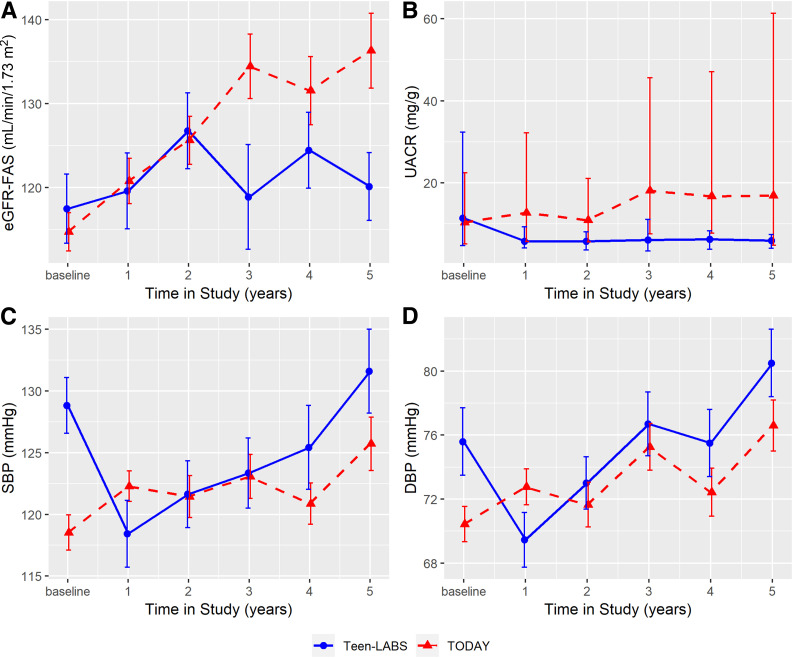
eGFR and Hyperfiltration
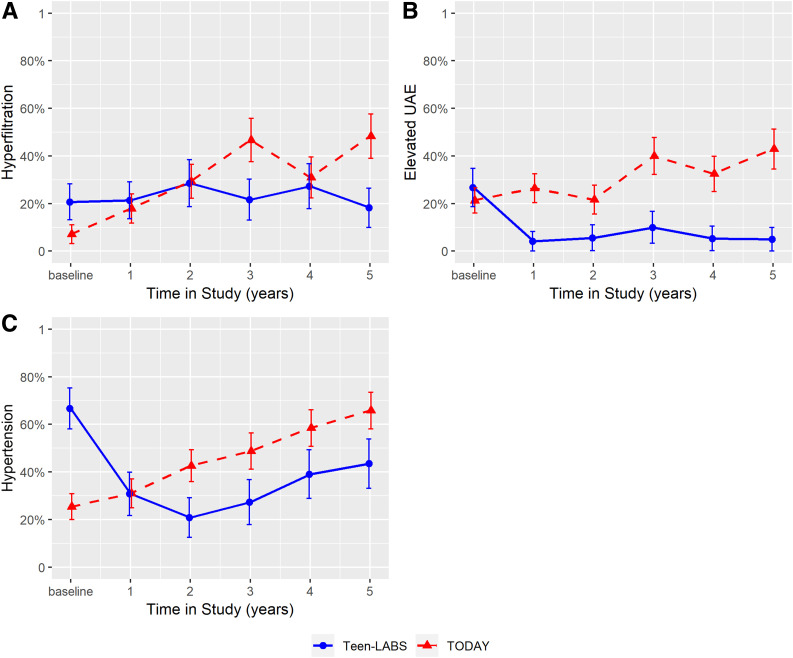
Renal function remained stable in participants in Teen-LABS, whereas eGFR increased over 5 years in youth in TODAY (Fig. 2). Additionally, the prevalence of hyperfiltration increased by 41% (7% to 48%) in TODAY compared with a decrease of 3% (21% to 18%) in Teen-LABS (P = 0.04). The cumulative incidence of hyperfiltration was 26% in Teen-LABS and 46% in TODAY. At 5-years of follow-up, TODAY participants had a 17-fold greater odds of hyperfiltration (OR 17.2 [95% CI 2.6, 114.5], P = 0.003) (Fig. 3) after adjusting for baseline age, sex, BMI, HbA1c, insulin sensitivity, and antihypertensive medication use. TODAY participants also had a 21-fold greater odds of incident hyperfiltration over 5 years compared with Teen-LABS (21.2 [2.2, 202.8], P = 0.008) (Fig. 4) in adjusted models. In mediation analyses, the change in HbA1c was the strongest mediator of developing hyperfiltration (Supplementary Table 1).
Figure 2.
eGFR, UACR, SBP, and DBP over 5 years in TODAY and Teen-LABS. Line charts for eGFR-FAS (P = 0.0243) (A), UACR (medians and IQRs) (B), SBP (P < 0.0001) (C), and DBP (P = 0.0245) (D). Error bars indicate ±SEM except for UACR. Means and error bars were jittered to avoid overlapping. P values are provided for the test of trajectory difference between groups over the study period (i.e., test for the existence of group ∗ time interaction terms). All models were adjusted for baseline age, sex, BMI, HbA1c, insulin sensitivity, and antihypertensive medication use.
Figure 3.
Hyperfiltration, elevated UAE, and hypertension over 5 years in TODAY and Teen-LABS. Line charts for hyperfiltration (P = 0.0364) (A), elevated UAE (P = 0.0003) (B), and hypertension (P = 0.0002) (C). Error bars indicate ±SE. Prevalence and error bars were jittered to avoid overlapping. P values are provided for the test of trajectory difference between groups over the study period (i.e., test for the existence of group ∗ time interaction terms). Hyperfiltration and elevated UAE were adjusted for baseline age, sex, BMI, HbA1c, insulin sensitivity, triglycerides, and antihypertensive use. Baseline antihypertensive use was not included in model for hypertension because of collinearity.
Figure 4.
Odds of DKD and hypertension at year 5. Forest plot for ORs of group effect. Teen-LABS is the reference group. The x-axis is in log scale. ORs on the left-hand side of 1 favor TODAY; ORs on the right-hand side of 1 favor Teen-LABS. Hyperfiltration, elevated UAE, and incident hyperfiltration models were adjusted for baseline age, sex, BMI, HbA1c, insulin sensitivity, triglycerides, and antihypertensive medication use. Baseline antihypertensive medication use was not included in hypertension and incident hypertension models because of collinearity.
UACR and Elevated UAE
UACR decreased in Teen-LABS participants compared with TODAY participants over the 5-year follow-up (Fig. 2). Similarly, the prevalence of elevated UAE increased by 22% (21% to 43%) in TODAY compared with a decrease of 22% (27–5%) in Teen-LABS (P = 0.003) (Fig. 3). The cumulative incidence of elevated UAE was 48% in TODAY participants, but none of the youth in Teen-LABS developed elevated UAE after MBS. At 5 years of follow-up, TODAY participants had a 27-fold greater odds of elevated UAE compared with Teen-LABS participants (OR 27.3 [95% CI 5.2, 146.2], P = 0.0001) (Fig. 4) in adjusted models. Accordingly, we did not calculate the differences in incident elevated UAE between the two cohorts. Mediation analyses suggested that change in HbA1c was the strongest mediator (Supplementary Table 1).
Hypertension
The prevalence of hypertension increased by 40% (25% to 66%) from baseline to 5-year follow-up in TODAY compared with a decrease of 23% (67% to 44%) in Teen-LABS (P = 0.0002) (Fig. 3). The cumulative incidence of hypertension was 51% in TODAY and 56% in Teen-LABS. At 5 years of follow-up, TODAY participants had a fourfold greater odds of hypertension, but this was not significant after multivariable adjustments (OR 4.0 [95% CI 0.7, 21.4], P = 0.11) (Fig. 4). For hypertension, BMI was the strongest mediator, although it did not reach statistical significance (Supplementary Table 1).
Missing Data and Sensitivity Analyses
Data on hyperfiltration were missing in 31% of participants in TODAY and 36% of participants in Teen-LABS at 5-year follow-up. For elevated UAE, 9% and 50% of data were missing for TODAY and Teen-LABS, respectively, at 5-year follow-up. Finally, hypertension was missing in 0% of participants in TODAY and 30% of participants in Teen-LABS at 5-year follow-up. To account for the missing data, we reran our models with multiple imputations under the missing-at-random assumption (Supplementary Fig. 1) as sensitivity analyses. The associations remained significant, but the magnitude was attenuated. We also performed sensitivity analyses with imputed values under missing-not-at-random adjustments, and the ORs for elevated albumin excretion and hyperfiltration at 5 years were within all CIs, which support the conclusions made (Supplementary Figs. 2 and 3).
We also performed sensitivity analyses excluding the six participants who underwent vertical sleeve gastrectomy. Limiting our analyses to participants who underwent Roux-en-Y gastric bypass did not meaningfully change the output of the multivariable models (data not shown).
Conclusions
Adolescents with severe obesity and T2D receiving medical treatment in TODAY experienced increased rates of hyperfiltration, elevated UAE, and hypertension over 5 years of follow-up. In contrast, adolescents with severe obesity and T2D undergoing MBS in Teen-LABS experienced regression of hyperfiltration, elevated UAE, and hypertension over the same time period, despite having worse markers of kidney health at baseline. Change in HbA1c was the strongest mediator of the differences observed in TODAY and Teen-LABS, yet it remains incompletely understood how MBS provides dramatic attenuation of DKD in youth with T2D beyond the impact of improved glycemic control and weight loss. Accordingly, a better understanding of the effects of bariatric surgery on renal health is critical to helping to define mechanisms of surgical benefits and to identify potential novel future nonsurgical approaches to DKD.
Youth-onset T2D represents a substantial percentage of new cases of diabetes in children and adolescents, ranging from 14% in non-Hispanic whites to 86% in American Indians (4,17,18). Youth-onset T2D is characterized by a suboptimal response to currently approved medical therapies and major challenges in adherence and management. Although major therapeutic advances have been made in diabetes care for adults with T2D, the only U.S. Food and Drug Administration–approved medications as of June 2019 for youth-onset T2D were metformin and insulin. Furthermore, in the recently completed Restoring Insulin Secretion (RISE) study, where youth and adult participants were randomly assigned to receive either 12 months of metformin or 3 months of insulin glargine followed by 9 months of metformin, early insulin glargine and metformin both failed to improve β-cell function in youth-onset T2D (5,19). Moreover, RISE demonstrated that insulin resistance and insulin secretion were markedly higher in youth with T2D compared with adults, calling for novel approaches to youth-onset T2D (5,19).
Given the suboptimal control achieved with lifestyle and medical therapy in youth-onset T2D to date, novel therapies are required. MBS is currently the only treatment available in obese youth with T2D that leads to considerable weight loss and at least a substantial improvement in glycemic control in the majority of patients as well as potential remission of diabetes (9). Additionally, a recent analysis suggested that adolescents were more likely than adults to experience remission of T2D in response to Roux-en-Y gastric bypass (20). However, the LABS and Teen-LABS studies almost exclusively used Roux-en-Y gastric bypass, whereas vertical sleeve gastrectomy is now the procedure of choice in youth because of its improved safety profile. Therefore, more data on vertical sleeve gastrectomy in youth and on longer-term outcomes of MBS are needed to determine its impact on youth-onset T2D and its complications. In adults with T2D, MBS is recommended for those with poorly controlled diabetes and a BMI of ≥30 kg/m2, as supported by several studies that have demonstrated durable diabetes remission (21,22). On the other hand, indications for MBS in adolescents currently include T2D with a BMI of ≥35 kg/m2 (23,24) but are extrapolated from adult data (25,26) because of the paucity of adolescent studies. However, youth with BMI values <35 kg/m2 may well experience equal or greater metabolic and renal benefits compared with those with BMI values ≥35 kg/m2, arguing for prospective, longitudinal, controlled studies to better define the role of MBS in youth-onset T2D.
In a prior analysis comparing metabolic outcomes over 2 years of follow-up in adolescents with severe obesity and T2D from the TODAY and Teen-LABS studies, we demonstrated worsening of glycemic control and increased BMI in participants receiving medical therapy compared with remission of diabetes in the majority of participants who had MBS (almost exclusively Roux-en-Y gastric bypass) (9). In the current analysis, we demonstrated a progressive worsening of glycemic control, insulin sensitivity, hypertension, and DKD in adolescents with T2D receiving medical therapy, and substantial improvements of these outcomes in those undergoing MBS over 5 years of follow-up. In our mediation analyses, reduction in HbA1c was the most influential mediator associated with attenuation of elevated UAE and hyperfiltration in adolescents undergoing MBS. To a lesser extent, change in triglycerides, BMI, and insulin sensitivity were associated with attenuation of elevated UAE and change in BMI and HbA1c with hypertension. The role of BMI as a mediator may relate to obesity-related nephropathy separate from DKD, a finding consistent with prior Teen-LABS data demonstrating a decline in UAE over 3 years in response to MBS in severely obese adolescents without T2D (6). Although mean BMI differed at baseline between the two study populations, BMI values at 5 years of follow-up were not statistically significantly different between Teen-LABS and TODAY participants, which probably contributed to the nonsignificant differences in prevalence of hypertension at 5 years of follow-up. The increase in SBP and DBP observed in Teen-LABS participants from 1 year after MBS to 5 years of follow-up may relate to discontinuation of antihypertensive medications.
Currently, it is incompletely understood how MBS provides dramatic attenuation of DKD. Proposed mechanisms of MBS include improved insulin sensitivity and insulin secretion through incretin mediators such as glucagon-like peptide 1 and peptide YY; enhanced secretion of fibroblast growth factor, which regulates bile acid synthesis with effects on glucose and lipid metabolism; impact on the microbiome; and improvements in endothelial and vascular function, among others (10,27–31). Albeit speculative, the net effect of these metabolic changes would likely be improved substrate utilization and ATP generation, which when coupled with reduced renal energy expenditure from attenuated hyperfiltration could collectively mitigate renal hypoxia risk.
Our study has important strengths and limitations. In terms of study design, it is a secondary analysis of two different subcohorts derived from two different studies with distinct objectives. Baseline matching for the two subcohorts were based on age, sex, race/ethnicity, and baseline BMI distributions in youth with T2D, with additional confounders accounted for in the multivariable models. Despite matching, there were important baseline differences between the two cohorts, including higher BMI in the Teen-LABS cohort at baseline compared with TODAY. Yet, both studies were prospective and had important similarities in methodology. For instance, both used the same central laboratory for analyses, which provided an ideal opportunity to compare eGFR and UACR outcomes between these cohorts of adolescents with T2D. The small sample size, particularly in the surgical group, limited our power to detect changes in some outcomes. However, this limitation was offset by the large effect sizes for important kidney outcomes. The wide CIs for the ORs in the fully adjusted models are also likely a function of the small sample size and should be acknowledged when interpreting the output from these models. Other limitations of our methods include use of eGFR and estimated insulin sensitivity rather than directly measured GFR and insulin sensitivity. However, repeated gold-standard assessments of GFR and insulin sensitivity would have been difficult within such large, longitudinal studies. Hyperfiltration may not imply progressive nephropathy on the basis of recent data from adults with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) (32), although the opposite has also been demonstrated in adults with T2D (33). Moreover, our data were limited to random UACR collections rather than to timed urine collections, and while UACR was paired in TODAY, it was determined from a single urine sample in Teen-LABS. The strengths of our study include 5 years of longitudinal data from the largest multicenter studies of youth-onset T2D to date, rigorously conducted with available data at several times points for UACR, SBP, DBP, eGFR, and other important covariates (e.g., HbA1c, BMI). Data on serum uric acid were not available in Teen-LABS, limiting our ability to examine change in serum uric acid as a mediator of group effect.
Although our data demonstrate a marked attenuation of DKD in response to MBS, the potential risks associated with MBS should be recognized. Complications of MBS include the possibility of the need for repeat surgery, the requirement for lifelong nutrient supplementation to prevent or treat dietary deficiencies, deleterious implications on bone health, potential impacts on offspring, and the increasingly recognized mental health burden. Clinical adverse events in severely obese adolescents with T2D in TODAY and Teen-LABS have previously been reported (9). These data demonstrated that 23% of participants in Teen-LABS experienced complications that required subsequent operation and/or readmission related or possibly related (e.g., cholecystectomy for gallstones) to their prior bariatric surgery (9). However, Roux-en-Y gastric bypass, the surgery done predominantly in Teen-LABS, appears to have more complications than the currently preferred vertical sleeve gastrectomy procedure. We do not yet know the extent of complications with vertical sleeve gastrectomy in adolescents with T2D. Furthermore, MBS incurs a substantial initial cost. However. if evaluated over a period of 5 years, current cost predictions argue that MBS in severely obese adolescents would be cost-effective (34). Therefore, the benefit of MBS in youth-onset T2D may outweigh the potential morbidity and initial costs for the carefully chosen patient in a specialized and experienced medical center.
In summary, we demonstrate for the first time to our knowledge that surgical treatment of severely obese youth with T2D is associated with substantially lower odds of DKD over 5 years of follow-up compared with standard medical therapy. Further long-term outcome studies for adolescents with T2D undergoing bariatric surgery are needed to confirm and refine these preliminary results in addition to studies comparing bariatric surgery to newer antidiabetic drugs, including sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 agonists. Furthermore, studies are needed to determine the nephroprotective effects of gastric bypass versus vertical sleeve gastrectomy in youth with T2D. Finally, future directions should also include translational studies dedicated to enhancing our understanding of the mechanisms of surgical benefit and identify potential novel nonsurgical approaches to DKD in youth-onset T2D.
Supplementary Material
Article Information
Acknowledgments. The authors gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective tribes or the Indian Health Service.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu. A complete list of the members of the TODAY study group can be found in the Supplementary Data online. Materials developed and used for Teen-LABS are available to the public at www.teen-labs.org.
Funding. The TODAY study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This work was completed with funding from NIDDK grants K23-DK-116720, U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and 01-DK-61254 and from National Center for Research Resources General Clinical Research Centers Program grants M01-RR-00036 (Washington University School of Medicine), M01-RR-00043-45 (Children’s Hospital Los Angeles), M01-RR-00069 (University of Colorado Denver), M01-RR-00084 (Children’s Hospital of Pittsburgh), M01-RR-01066 (Massachusetts General Hospital), M01-RR-00125 (Yale University), and M01-RR-14467 (University of Oklahoma Health Sciences Center) and Clinical and Translational Science Awards UL1-RR-024134 (Children’s Hospital of Philadelphia), UL1-RR-024139 (Yale University), UL1-RR-024153 (Children’s Hospital of Pittsburgh), UL1-RR-024989 (Case Western Reserve University), UL1-RR-024992 (Washington University in St. Louis), UL1-RR-025758 (Massachusetts General Hospital), and UL1-RR-025780 (University of Colorado Denver). The Teen-LABS consortium is funded by cooperative agreements with the NIDDK through grants UM1-DK-095710 (C.X., principal investigator, University of Cincinnati) and UM1-DK-072493 (T.I., principal investigator, University of Colorado Denver).
NIDDK had no role in the study design; collection, analysis, and interpretation of data; or writing of the report.
Duality of Interest. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi. P.B. has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk, and Horizon Pharma and serves on the advisory board of XORTX. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.B. wrote the manuscript and researched data. K.H., M.M.K., A.S.S., J.L., E.N., and M.M. contributed to the discussion and reviewed/edited the manuscript. T.J. contributed to the analysis and discussion and reviewed/edited the manuscript. P.X. and C.X. were responsible for data analyses, contributed to the discussion, and reviewed/edited the manuscript. T.I. and K.N. research data, contributed to the discussion, and reviewed/edited the manuscript. P.X. and C.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00081328 and NCT00474318, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0708/-/DC1.
T.I. and K.N. are co-senior authors.
References
- 1.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 2011;57(Suppl. 1):A8, e1-526. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TODAY Study Group Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial [published correction appears in Diabetes Care 2013;36:2448] Diabetes Care 2013;36:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 2016;39:823–829 [DOI] [PubMed] [Google Scholar]
- 5.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nehus EJ, Khoury JC, Inge TH, et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int 2017;91:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, Nehus E, El Ghormli L, et al.; TODAY Study Group . Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis 2018;71:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inge TH, Laffel LM, Jenkins TM, et al.; Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) Consortia . Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 2018;172:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S13–S28 [DOI] [PubMed] [Google Scholar]
- 12.Zeitler P, Epstein L, Grey M, et al.; TODAY Study Group . Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017;28:1023–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker-Smith CM, Flinn SK, Flynn JT, et al.; Subcommittee on Screening and Management of High BP in Children . Diagnosis, evaluation, and management of high blood pressure in children and adolescents. Pediatrics 2018;142:e20182096. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Bell RA, D’Agostino RB Jr., et al.; Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States [published correction appears in JAMA 2007;298:627] JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 18.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RISE Consortium Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen–LABS Consortium . Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med 2019;380:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis 2018;14:259–263 [DOI] [PubMed] [Google Scholar]
- 22.Mechanick JI, Youdim A, Jones DB, et al.; American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery . Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl. 1):S1–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalsky M, Reichard K, Inge T, Pratt J, Lenders C; American Society for Metabolic and Bariatric Surgery . ASMBS pediatric committee best practice guidelines. Surg Obes Relat Dis 2012;8:1–7 [DOI] [PubMed] [Google Scholar]
- 24.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics 2004;114:217–223 [DOI] [PubMed] [Google Scholar]
- 25.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inge TH, Prigeon RL, Elder DA, et al. Insulin sensitivity and β-cell function improve after gastric bypass in severely obese adolescents. J Pediatr 2015;167:1042–1048.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chondronikola M, Harris LL, Klein S. Bariatric surgery and type 2 diabetes: are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? J Intern Med 2016;280:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fjeldborg K, Pedersen SB, Møller HJ, Richelsen B. Reduction in serum fibroblast growth factor-21 after gastric bypass is related to changes in hepatic fat content. Surg Obes Relat Dis 2017;13:1515–1523 [DOI] [PubMed] [Google Scholar]
- 30.Gastaldelli A, Iaconelli A, Gaggini M, et al. Short-term effects of laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass. Diabetes Care 2016;39:1925–1931 [DOI] [PubMed] [Google Scholar]
- 31.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes 2016;40:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molitch ME, Gao X, Bebu I, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Early glomerular hyperfiltration and long-term kidney outcomes in type 1 diabetes: the DCCT/EDIC experience. Clin J Am Soc Nephrol 2019;14:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melsom T, Nair V, Schei J, et al. Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis 2019;73:777–785 [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff MJ, Chhatwal J, Nudel JD, Corey KE, Kaplan LM, Hur C. Cost-effectiveness of bariatric surgery in adolescents with obesity. JAMA Surg 2017;152:136–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.