Abstract
OBJECTIVE
We examined trends in diabetic ketoacidosis (DKA) at diagnosis of type 1 diabetes at a large pediatric diabetes center between 2010 and 2017, overlapping with the Affordable Care Act’s overhaul of U.S. health care.
RESEARCH DESIGN AND METHODS
Colorado residents <18 years old who were diagnosed with type 1 diabetes from 2010 to 2017 and subsequently followed at the Barbara Davis Center for Diabetes were included. Logistic regression models were used to test associations among age, sex, race/ethnicity, insurance, language, year of diagnosis, and rural/nonrural residence and DKA at diagnosis. Linear regression models were used to test the association of each predictor with HbA1c at diagnosis.
RESULTS
There were 2,429 subjects who met the inclusion criteria. From 2010 to 2017, the rate of DKA increased from 41 to 58%. It increased from 35.3 to 59.6% among patients with private insurance (odds ratio 1.10 [95% CI 1.05–1.15]; P < 0.0001) but remained unchanged (52.2–58.8%) among children with public insurance (1.03 [0.97–1.09]; P = 0.36). In the multivariable model, public insurance (1.33 [1.08–1.64]; P = 0.007), rural address (1.42 [1.08–1.86]; P = 0.013), and HbA1c (1.32 [1.26–1.38]; P < 0.0001) were positively associated with DKA, whereas age, race/ethnicity, sex, and primary language were not.
CONCLUSIONS
The increase in the rate of DKA in patients with newly diagnosed type 1 diabetes was driven by patients with private insurance. This paradoxically occurred during a time of increasing health insurance coverage. More study is needed to understand the factors driving these changes.
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening complication of type 1 diabetes due to a critical deficiency of insulin. Children with DKA at diagnosis of type 1 diabetes have poorer glycemic trends for at least the following 15 years, increasing the risk for long-term complications (1). Additionally, moderate to severe DKA in young children at diagnosis is associated with lower cognitive scores and altered brain growth (2).
While many children with new-onset type 1 diabetes receive insulin therapy in time to avoid DKA, many do not. The rate of DKA at type 1 diabetes diagnosis widely varies by country (3,4). In Colorado, it increased from 35% in 2007 to 46% in 2012, higher than many other industrialized nations (5).
In 2010, the U.S. Congress passed the Affordable Care Act (ACA) to improve access to affordable health care insurance coverage (6). From 2014 to 2017, this law required all U.S. legal residents to have health insurance. Paradoxically, the rise in DKA at type 1 diabetes diagnosis in youth between 2007 and 2012 was primarily driven by those with private health insurance despite a simultaneous increase from 17 to 38% of youth being covered by public health insurance (5). It is unknown if the rate has changed since the implementation of the ACA. Therefore, we evaluated trends in DKA at diagnosis of type 1 diabetes from a large pediatric diabetes center in Colorado between 2010 and 2017.
Research Design and Methods
The clinical database at the Barbara Davis Center for Diabetes was queried to identify subjects who were diagnosed with type 1 diabetes between 2010 and 2017, <18 years of age, and Colorado residents at the time of diagnosis. A total of 2,485 eligible children were identified. Of those, 56 (2.3%) had insufficient medical records to rule in or out the presence of DKA at diagnosis and were excluded from the analyses; their age at diagnosis, sex, and ethnicity did not differ from those of the remaining 2,429 children included in the analyses. The study population comprised >90% of all Colorado children diagnosed with type 1 diabetes in 2010–2016, according to the population-based SEARCH for Diabetes in Youth (SEARCH) registry (7).
All laboratory results and clinical documentation in the record were manually reviewed, including scanned media. DKA was defined by venous pH <7.3 or HCO3− <15 mmol/L (8). This study was approved by the Colorado Multiple Institutional Review Board.
Laboratory data were not available for some children seen in emergency departments or hospitalized outside of our health system. For these subjects, we relied on clinical documentation to establish whether the patient experienced DKA. This included reviewing all available physician and nursing notes from the emergency room, transport team, inpatient units at Children’s Hospital Colorado, and the Barbara Davis Center for Diabetes to ascertain the clinical judgement of the medical team at the time of diagnosis.
Insurance at the time of outpatient diabetes education was categorized as public, private, or unknown, with unknown assigned to subjects whose insurance field was blank at the time of the first outpatient diabetes encounter. Tricare, the program insuring children of U.S. Department of Defense employees, was categorized as private insurance. Address was categorized as rural or nonrural according to the Centers for Medicare & Medicaid rural zip code list (9).
Statistical Analyses
Logistic regression models were used to test the univariable and multivariable associations of age, sex, race/ethnicity, insurance, primary language, year of diagnosis, rural residence, and initial HbA1c with DKA at diagnosis in all patients and in patients with private and public insurance separately.
Results
There were 2,429 subjects who met inclusion criteria. Forty-nine percent of all patients experienced DKA upon the diagnosis of type 1 diabetes. Laboratory data were available to confirm or exclude DKA for 91.1% of patients in the DKA group and 61.4% of patients in the non-DKA group, respectively. Demographic characteristics are shown in Table 1. Overall, 32.1% of patients were publicly insured, 69.1% were non-Hispanic white, 12.7% had a rural address, and English was the primary language for 94.3%. From 2010 to 2017, the number of subjects with public insurance increased from 29 to 36%. There were 88 individuals who were uninsured or whose insurance could not be determined.
Table 1.
Demographic characteristics
| DKA (n = 1,187) | No DKA (n = 1,242) | Unknown (n = 56) | |
|---|---|---|---|
| Age, years, mean (SD) | 9.4 (4.3) | 9.7 (4.2) | 8.9 (4.3) |
| HbA1c, %, mean (SD) | 12.3 (1.8) | 10.9 (2.5) | — |
| HbA1c, mmol/mol, mean (SD) | 111 (20) | 96 (27) | — |
| Female | 539 (45.4) | 581 (46.8) | 26 (46.4) |
| Race/ethnicity | |||
| Non-Hispanic white | 785 (66.1) | 898 (72.3) | 34 (60.7) |
| Hispanic | 201 (16.9) | 166 (13.4) | 8 (14.3) |
| Non-Hispanic black | 60 (5.1) | 38 (3.1) | 2 (3.6) |
| Other | 141 (11.9) | 140 (11.3) | 12 (21.4) |
| Insurance | |||
| Private | 676 (57.0) | 823 (66.3) | 10 (17.9) |
| Public | 442 (37.2) | 341 (27.5) | 14 (25.0) |
| Unknown or none | 69 (5.8) | 78 (6.3) | 32 (57.1) |
| Primary language | |||
| English | 1,115 (93.9) | 1,176 (94.7) | 52 (92.9) |
| Non-English | 72 (6.1) | 66 (5.3) | 4 (7.1) |
| Zip code | |||
| Nonrural | 941 (79.3) | 1,056 (85.0) | 22 (39.3) |
| Rural | 173 (14.6) | 139 (11.2) | 4 (7.1) |
| Unknown | 73 (6.1) | 47 (3.8) | 30 (53.6) |
Data are n (%) unless otherwise indicated. Only one subject with unknown status at presentation had an initial HbA1c value available.
The prevalence of DKA increased from 41.0 to 59.3% (odds ratio 1.07 [95% CI 1.04–1.11]; P = 0.0001). The increase among those with private insurance was from 35.3 to 59.6% (1.10 [1.05–1.15]; P < 0.0001) and from 52.2 to 58.8% in those with public insurance (1.03 [0.97–1.090]; P = 0.36). Figure 1 shows the rate of DKA by insurance in each year. There was a bimodal distribution of DKA rate by age, with the highest rate <1 year of age, falling by mid-childhood, and rising in the adolescent years (Fig. 2).
Figure 1.
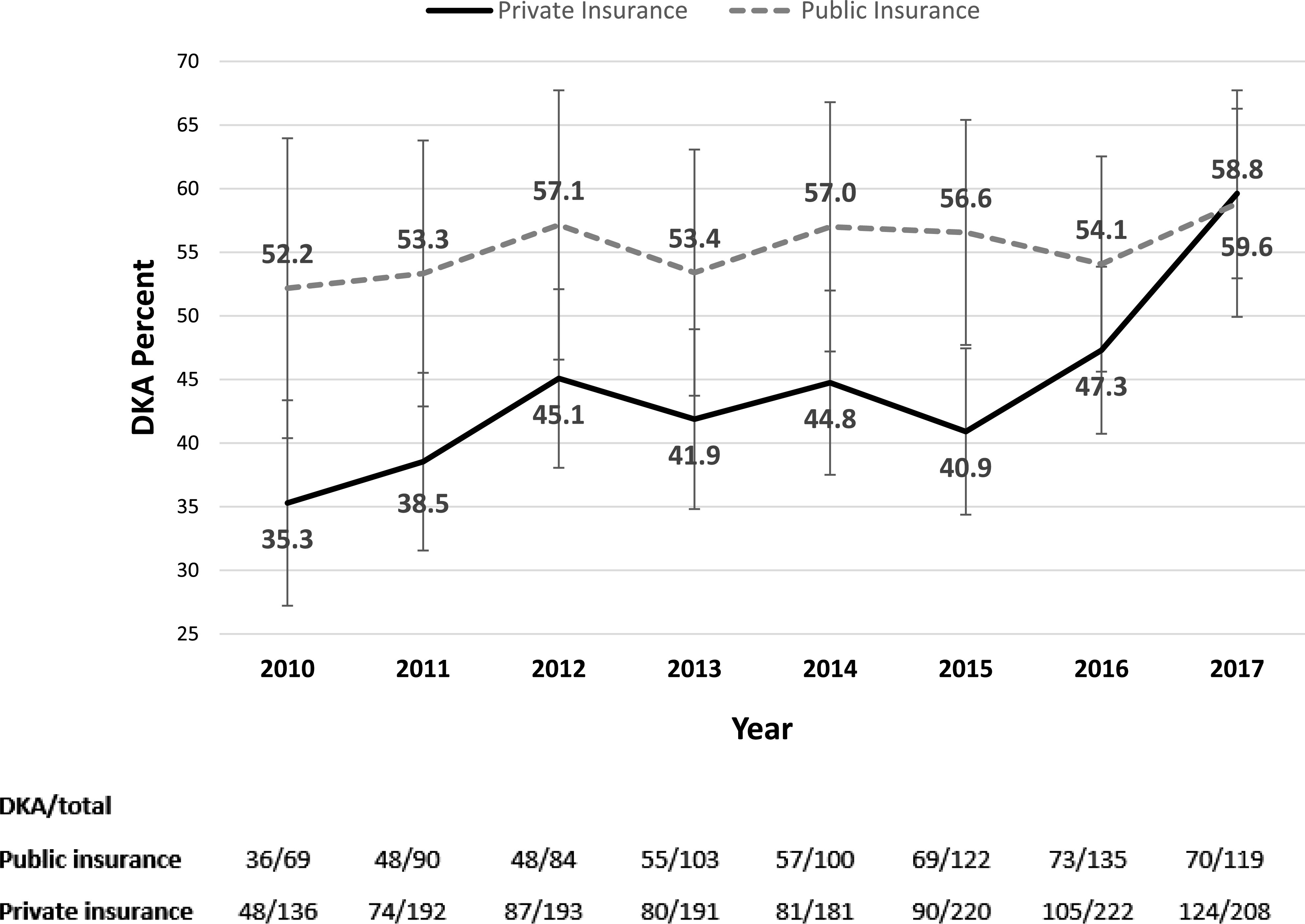
Percent of children with DKA by year and insurance. Bars represent 5th and 95th percentiles.
Figure 2.
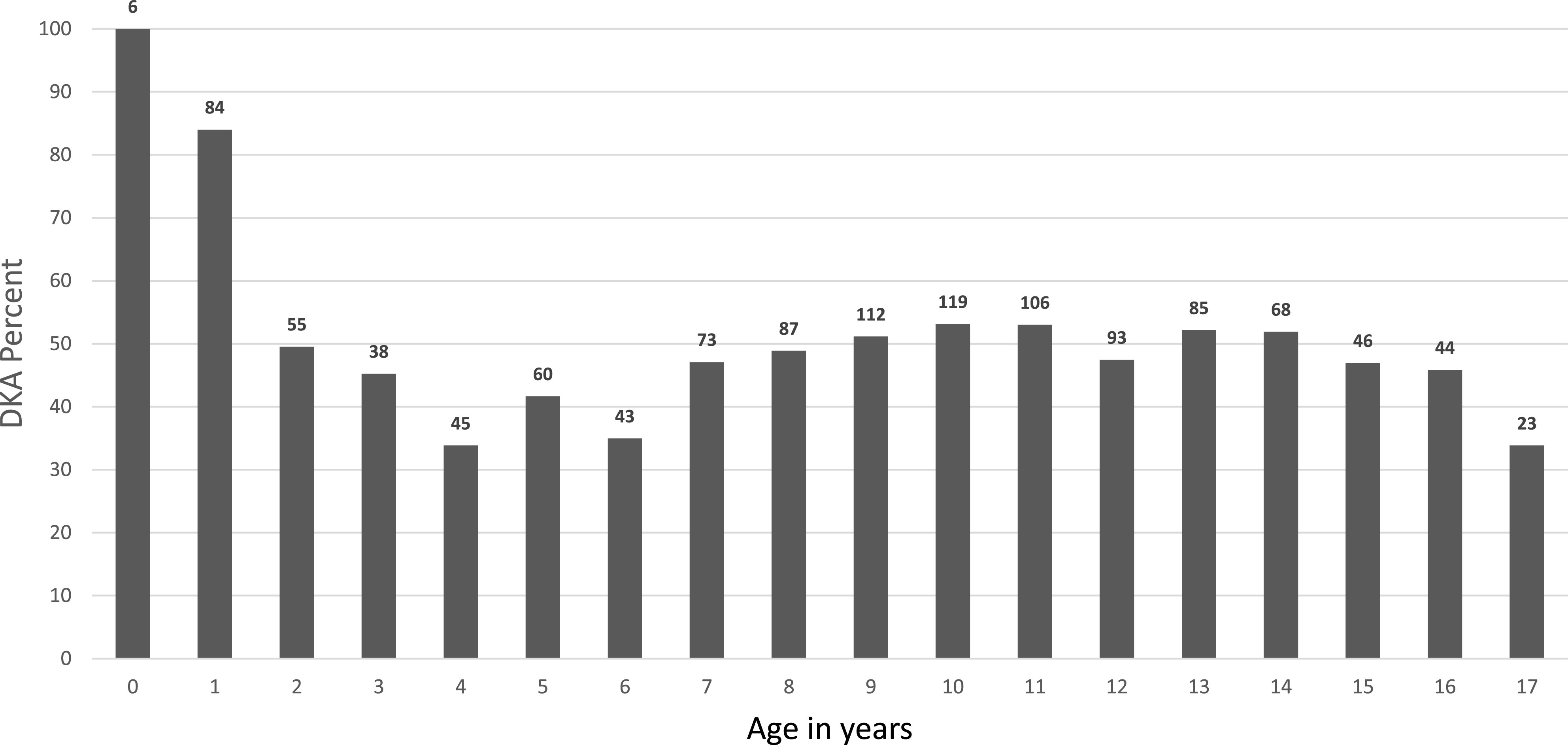
Percent of children with DKA by age. Numbers above bars represent number of cases per age-group.
In univariable logistic regression analyses among all patients, age as a continuous (P = 0.15) and categorical variable (<6 vs. ≥13, <6 vs. 6 to <13; P = 0.80), sex (P = 0.50), and primary language (P = 0.42) were not significantly associated with DKA. Race/ethnicity (P = 0.002), public insurance (1.59 [1.34–1.88]; P = 0.0001), year of diagnosis (1.07 [1.04–1.11]; P = 0.0001), rural address (1.40 [1.10–1.78]; P = 0.006), and initial HbA1c (1.34 [1.29–1.40]; P < 0.0001) were associated with increased rate of DKA.
In the multivariable analyses for the association with DKA, race/ethnicity was not associated with increased risk for DKA (P = 0.49), but public insurance (1.33 [1.08–1.64]; P = 0.007), year of diagnosis (1.07 [1.03–1.11]; P = 0.002), rural address (1.42 [1.08–1.86]; P = 0.013), and HbA1c at diagnosis (1.32 [1.27–1.38]; P < 0.0001) were (Table 2).
Table 2.
Multivariable analyses for DKA at diagnosis of type 1 diabetes
| OR (95% CI) | P value | |
|---|---|---|
| Race/ethnicity | ||
| H vs. NHW | 1.21 (0.93–1.58) | 0.49 |
| NHB vs. NHW | 1.23 (0.76–1.97) | |
| Other/unknown vs. NHW | 1.10 (0.81–1.49) | |
| Public vs. private insurance | 1.33 (1.08–1.64) | 0.007 |
| Year of diagnosis | 1.07 (1.03–1.11) | 0.002 |
| Rural address | 1.42 (1.08–1.86) | 0.013 |
| HbA1c at diagnosis | 1.32 (1.26–1.38) | <0.0001 |
H, Hispanic; NHB, non-Hispanic black; NHW, non-Hispanic white; OR, odds ratio.
Conclusions
From 2010 to 2017, the rate of DKA at presentation of type 1 diabetes in youth continued to increase, reaching ∼60% in 2017. In the U.S., private insurance is provided as an employment benefit or purchased on the open market, while people with low incomes may qualify for public insurance programs that have no out-of-pocket costs. Privately insured patients represent a wealthier and higher educated group with fewer ethnic minorities than those with public insurance. Therefore, it was surprising that patients with private insurance had a rapid rise in DKA incidence, which, in 2017, was as high as their publicly insured counterparts. As the rate of public health insurance increased during this period, we may have expected the overall DKA incidence to decrease due to improved access, but this was not the case.
Implementation of the ACA has been followed by increased enrollment in high-deductible insurance plans, which could disincentivize privately insured families from seeking timely care (10). In a recent study at our center, high insurance deductibles were a commonly reported barrier to seeking medical care for their ill child. Other frequent barriers were difficulty getting an appointment with a primary care provider, difficulty taking time off from work, and not having the time to take the child to a medical appointment (11).
Educational campaigns to increase awareness of type 1 diabetes symptoms have had variable success at reducing the incidence of DKA, but they appear to require sustained effort to maintain effect (12–14). Diabetes autoimmunity screening has repeatedly shown rates of DKA at diabetes diagnosis between 5 and 10%, but widespread, general population screening remains restricted to the research setting (15–20). In our study, children <2 years of age experienced DKA at high rates, yet they would be less likely than older children to benefit from educational campaigns through public schools or autoimmunity screening unless they were conducted very early in life. More study is warranted to understand how to address barriers and develop broad, scalable, and sustainable interventions.
The increased risk of DKA and higher HbA1c in disadvantaged groups are consistent with known inequities in health care delivery and outcomes in the U.S. (21). Relatively small numbers of subjects with rural zip codes and non-English primary language may have limited our ability to detect associations between these predictors and HbA1c.
In the T1D Exchange clinic registry, a network of >70 U.S. diabetes centers, <25% of children meet recommended glycemic targets (22,23). Given the relationship between DKA at diabetes diagnosis and future HbA1c trajectory, current DKA trends may portend an increasingly difficult challenge of improving the proportion of patients reaching glycemic goals.
Although this was a single-center study, strengths include the large sample size and the fact that our center cares for >90% of youth with type 1 diabetes in Colorado (D. Dabelea, personal communication). All available data were manually reviewed. Although discrete fields exist, some laboratory data for patients initially seen outside of our health system were not available or were only found on scanned records or sections not accessible by automated search. Billing codes were an imperfect source, with laboratory data at the time of presentation contradicting the recorded diagnosis in 1.3% of cases.
Only 3.6% of all subjects were categorized as unknown insurance, but some of these may have been insured given limited data availability. Also, because Colorado’s public insurance programs reimburse retrospectively for 30 days from the start of coverage, some subjects in the public insurance group may have been uninsured upon presentation to the hospital. Therefore, we cannot know from these data the number of children whose care may have been delayed due to lack of insurance coverage. Insurance plan data, such as how much each patient was billed and how much was paid by the patient and the insurer, were not available. Given regional demographics and variability in Medicaid expansion across states, our data may not be nationally representative.
In conclusion, the increasing rate of DKA at diabetes diagnosis suggests the need for continuous national tracking to understand what factors are responsible and facilitate evidence-based interventions. Interventions may include increased public awareness, reducing barriers to medical care, or diabetes autoimmunity screening for selected populations.
Article Information
Acknowledgments. The authors thank Bing Wang from the University of Colorado for database support.
Funding. This study received funding from the National Institute of Diabetes and Digestive and Kidney Diseases Medical Student Research Program.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.T.A. researched the data and wrote the manuscript. A.C., K.M., and S.T. researched data and reviewed and edited the manuscript. L.P. performed the statistical analyses. A.R. researched the data and contributed to the discussion. G.T.A. and A.R. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in poster form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 2017;40:1249–1255 [DOI] [PubMed] [Google Scholar]
- 2.Aye T, Mazaika PK, Mauras N, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care 2019;42:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ 2011;343:d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012;55:2878–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA 2015;313:1570–1572 [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum S The Patient Protection and Affordable Care Act: implications for public health policy and practice. Public Health Rep 2011;126:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;377:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfsdorf JI, Allgrove J, Craig ME, et al.; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes 2014;15(Suppl. 20):154–179 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services DME_Rural Zip and Formats [Internet], 2015. Available from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSFeeSched/DMEPOS-Fee-Schedule-Items/DME-Rural-Zip-and-Formats.html. Accessed 30 August 2018.
- 10.Cohen RA, Zammitti EP, Martinez ME; National Center for Health Statistics . Health insurance coverage: early release of estimates from the National Health Interview Survey, 2016 [Internet], 2017. Available from https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201705.pdf. Accessed 30 August 2018
- 11.Baldelli L, Flitter B, Pyle L, et al. A survey of youth with new onset type 1 diabetes: opportunities to reduce diabetic ketoacidosis. Pediatr Diabetes 2017;18:547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derraik JGB, Cutfield WS, Maessen SE, et al. A brief campaign to prevent diabetic ketoacidosis in children newly diagnosed with type 1 diabetes mellitus: the NO-DKA Study. Pediatr Diabetes 2018;19:1257–1262 [DOI] [PubMed] [Google Scholar]
- 13.Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care 1999;22:7–9 [DOI] [PubMed] [Google Scholar]
- 14.Choleau C, Maitre J, Elie C, et al.; le Groupe d’étude de l’aide aux jeunes diabétiques (AJD Study Group) . Ketoacidosis at time of diagnosis of type 1 diabetes in children and adolescents: effect of a national prevention campaign. Arch Pediatr 2015;22:343–351 [in French] [DOI] [PubMed] [Google Scholar]
- 15.Barker JM, Goehrig SH, Barriga K, et al.; DAISY study . Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404 [DOI] [PubMed] [Google Scholar]
- 16.Triolo TM, Chase HP, Barker JM; DPT-1 Study Group . Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care 2009;32:769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elding Larsson H, Vehik K, Gesualdo P, et al.; TEDDY Study Group . Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes 2014;15:118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren M, Sahlin Å, Svensson C, et al.; DiPiS study group . Reduced morbidity at diagnosis and improved glycemic control in children previously enrolled in DiPiS follow-up. Pediatr Diabetes 2014;15:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012;13:308–313 [DOI] [PubMed] [Google Scholar]
- 20.Elding Larsson H, Vehik K, Bell R, et al.; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry JG, Bloom S, Foley S, Palfrey JS. Health inequity in children and youth with chronic health conditions. Pediatrics 2010;126(Suppl. 3):S111–S119 [DOI] [PubMed] [Google Scholar]
- 22.Wood JR, Miller KM, Maahs DM, et al.; T1D Exchange Clinic Network . Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013;36:2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]


