Abstract
OBJECTIVE
A subset of people with long-standing type 1 diabetes (T1D) appears to be protected from microvascular and macrovascular complications. Previous studies have focused on improved abilities to respond to glucose and its downstream effects as protective mechanisms. It is unclear whether lipoproteins play a role in the vascular health of these people. We therefore determined whether HDL particle concentration, size, function, and/or protein composition associate with protection from vascular complications.
RESEARCH DESIGN AND METHODS
We studied two independent cross-sectional cohorts with T1D: the T1D Exchange Living Biobank (n = 47) and the Joslin Medalist Study (n = 100). Some of the subjects had vascular complications, whereas others never exhibited vascular complications, despite an average duration of diabetes in the cohorts of 45 years. We assessed HDL particle size and concentration by calibrated ion mobility analysis, the HDL proteome by targeted mass spectrometry, and HDL function ex vivo by quantifying cholesterol efflux capacity and inhibition of monocyte adhesion to endothelial cells.
RESULTS
In both cohorts, people without vascular complications exhibited significantly higher concentrations of medium-sized HDL particles (M-HDL) independently of total and HDL cholesterol levels. While no consistent differences in HDL functions were observed ex vivo, people without vascular complications had higher levels of HDL-associated paraoxonase 1 (PON1), an enzyme that inhibits atherosclerosis in animal models.
CONCLUSIONS
Elevated concentrations of M-HDL particles and elevated levels of HDL-associated PON1 may contribute to long-term protection from the vascular complications of diabetes by pathways that are independent of total cholesterol and HDL cholesterol.
Introduction
Microvascular and macrovascular dysfunction in patients with type 1 diabetes (T1D) greatly increase the risk of nephropathy, retinopathy, and cardiovascular disease (1–3). However, some people with long-standing T1D are protected from vascular complications or have a much slower rate of complications development. The 50-Year Medalist Program of the Joslin Diabetes Center, studying patients with T1D for ≥50 years, has provided insights into mechanisms associated with long-term protection from vascular complications (4–6). These studies found no association between current or longitudinal glycemic control (HbA1c) and vascular complications. In contrast, elevated plasma levels of certain advanced glycation end products associated with increased risk (5). The Medalist studies also revealed that people who are protected from one type of complication are often protected from other types, suggesting that common mechanisms might be at play (7). By using induced pluripotent stem cells, the investigations showed that improved DNA repair mechanisms in response to genotoxic stressors, such as elevated glucose, associated with protection from complications (6). Increased glucose flux in renal glomeruli might also protect against nephropathy by preventing the accumulation of toxic glucose metabolites (8).
The roles of lipoproteins in long-term protection from complications of T1D are not well understood, in part because lipid levels are often normal in people with T1D (9). One study of the Medalists suggested that elevated HDL cholesterol (HDL-C) levels associate with protection from cardiovascular complications in women with long-standing T1D (10). However, there is strong evidence that HDL’s cholesterol content does not directly reflect its protective effects and that other metrics better predict the role of HDL in vascular protection (11–13). Furthermore, the concentration of the different HDL subpopulations varies markedly among humans, and HDL particle concentration is likely a better measure of HDL’s cardioprotective effects than its cholesterol content (14–17). In addition, the protein composition of HDL is altered with inflammatory disease, suggesting that protein composition may be a marker for, and perhaps a mediator of, vascular dysfunction (18). Little is known about any of these metrics in people with T1D protected from vascular complications. Our results, which are based on two different cohorts of people with long-standing T1D, demonstrate that increased levels of medium-sized HDL (M-HDL) particles, a specific HDL subpopulation with a mean diameter of 9.1–9.3 nm, and HDL enrichment of paraoxonase 1 (PON1), an atheroprotective enzyme, associate with protection from vascular complications.
Research Design and Methods
Human Subjects and Sample Collection
For the first cross-sectional study (discovery cohort), subjects were identified by T1D Exchange Living Biobank research coordinators to meet the following criteria: >20 years duration of T1D and never diagnosed with vascular complications (complications absent [C−]) and age-matched subjects with the same diabetes duration who had been diagnosed with at least two of the following four complications: nephropathy, retinopathy, neuropathy, cardiovascular disease (CVD) (myocardial infarction, stroke, or treatment for increased risk of cardiovascular disease by lipid-lowering or antihypertensive medications) (complications present [C+]). The discovery cohort was designed to identify large differences in susceptibility to multiple diabetes complications because common mechanisms might contribute to complications (7). Complications were self-reported through standardized questionnaires and were verified by study coordinators. Subjects with lupus, rheumatoid arthritis, and vasculitis were excluded. This process identified 47 subjects with an average T1D duration of 40 years (31 with complications and 16 who had remained complications free). After an overnight fast, blood samples (60 mL) were collected in prechilled EDTA tubes and centrifuged at 2,500g for 15 min at 4°C, and the plasma was frozen in 1.5-mL aliquots and shipped on dry ice. All subjects signed an informed consent. The study was approved by the Benaroya Research Institute institutional review board (IRB).
The second cohort (replication cohort) was selected from the Joslin Diabetes Center Medalist Study, which has been previously described (5,10). The Medalist Study consists of individuals from all U.S. states who were characterized at the Joslin Diabetes Center by completion of medical history questionnaires and clinical examinations to evaluate complications with collection of biospecimens. The cohort for the current study was randomly selected to include 50 women with >50 years of T1D with CVD (C+ group) and 50 women who were CVD free (C− group). CVD status was determined on the basis of standardized, validated, self-reported questions regarding history of coronary artery disease, angina, heart attack, prior cardiac or leg angioplasty, or bypass graft surgery. Several studies have demonstrated the validity of self-reported CVD (19,20). The groups were matched for diabetes duration and age (5-year frequency match), HbA1c, and range of insulin dose per kilogram. Subjects with nephropathy (estimated glomerular filtration rate <45 mL/min/1.73 m2) were excluded. Once-only thawed EDTA plasma samples and serum from a subset of the same subjects were used. Informed consent was obtained before participation in the study. All procedures were approved by the Joslin Diabetes Center Committee on Human Subjects IRB. Deidentified samples were used for all analyses.
HDL Particle Concentration and Size Measurement
HDL particle concentration was quantified by calibrated ion mobility analysis on a differential mobility analyzer (DMA) (TSI Inc., Shoreview, MN) as previously described (14). Five HDL subspecies (extra-small [XS-HDL] ∼7.6 nm, small [S-HDL] ∼8.3 nm, M-HDL ∼9.3 nm, large [L-HDL] ∼11.1 nm, extra-large [XL-HDL] ∼12.4 nm) were fitted to the DMA profiles by unsupervised, iterative curve fitting (14). Areas under the curve fitted for each subspecies were directly converted to HDL particle concentration using a glucose oxidase calibration curve. For total HDL particle concentration, intraday and interday coefficients of variation were <10%. The coefficients of variation were <10% for all HDL subpopulations except XS-HDL and S-HDL (14.8% and 18.5%, respectively).
HDL Protein Digestion
HDL (d = 1.063–1.21 g/mL) was isolated by sequential density ultracentrifugation from EDTA plasma samples (21). Ten micrograms of HDL protein were solubilized with 0.5% sodium deoxycholate (Sigma-Aldrich, St. Louis, MO) in 200 mmol/L NH4HCO3 spiked with 0.5 μg [15N]apolipoprotein (APO) A1 as internal standard (22), reduced with dithiothreitol, alkylated with iodoacetamide, and digested with two additions of trypsin (1:20 w/w HDL protein, sequencing grade; Promega, Fitchburg, WI) for 4 h and overnight. After precipitation of sodium deoxycholate with formic acid (8% final concentration), samples were frozen and stored at −20°C until analysis (<1 week). For the liquid chromatography–mass spectrometry (LC/MS) analysis, an equivalent of 200 ng HDL protein was injected (23).
Targeted Proteomics Analysis
The abundance of 35 HDL-associated proteins was quantified by MS using targeted proteomics as previously described (22,24). In short, tryptic digests of HDL were analyzed in a Thermo TSQ Vantage Triple Stage Quadrupole LC/MS with electrospray ionization. The instrument was operated in selected reaction monitoring mode with a 10-ms dwell time. Peptides were monitored, with collision energies optimized to maximize the signal for each peptide. Peak areas were integrated using Skyline (25), and responses of two measured peptides for each protein were averaged.
HDL Functional Assays
Cholesterol efflux capacity (CEC) of serum HDL was quantified using cAMP-stimulated J774 macrophages and baby hamster kidney cells with mifepristone-inducible overexpression of the cholesterol exporters ABCA1 or ABCG1, as previously described (21,26). To evaluate the effects of HDL on monocyte adhesion to endothelial cells, human monocytes were isolated from freshly collected blood from healthy volunteers by negative selection (RosetteSep Human Monocyte Enrichment Kit; Stemcell Technologies, Vancouver, British Columbia, Canada) and stained with calcein AM (Thermo Fisher Scientific, Waltham, MA). HDL (10 μg/mL), together with monocytes, was added to human umbilical vein endothelial cells after stimulation for 6 h in the presence or absence of 20 ng/mL human recombinant tumor necrosis factor-α (R&D Systems, Minneapolis, MN). Monocyte adhesion was determined by quantifying calcein AM fluorescence after removing unattached cells. The experiment was approved by the University of Washington IRB committee (protocol no. 31798).
Fractionation of Isolated HDL and Determination of Distribution of PON1 Mass and Activity
Isolated HDL was fractionated by size-exclusion fast-protein LC (FPLC-SEC) on tandem Superdex 200 and Superdex 75 columns in PBS. Fractions (250 μL) were collected, and PON1 immunoreactivity was measured by dot blot using a rabbit anti-PON1 antibody (P0123, 1:1,000 dilution; Sigma) and quantified by densitometry after normalization to a control sample included on all blots. Specificity of the PON1 antibody and integrity of PON1 were confirmed by Western blot. HDL-associated PON1 arylesterase activity was measured in HDL isolated and fractionated from serum using a published method (27).
PON1 Polymorphisms
Medalists were genotyped on Illumina HumanCoreExome BeadChip arrays followed by IMPUTE version 2 imputation, resulting in a genome-wide panel of 7 million polymorphisms. PON1 variants were extracted from this panel and tested for association with CVD in the overall Medalist cohort (n = 891) and in the subset (n = 100) used for this HDL study. These variants included the Gln192 coding sequence variant, the Leu55 coding sequence variant, and the −108C-T noncoding variant (rs662, rs854560, and rs705379).
Statistical Analysis
Differences between groups were assessed using two-tailed unpaired Student t test or Wilcoxon rank sum nonparametric test after testing for normality of distribution using Shapiro-Wilk tests. Categorical variables were compared using Fisher exact test. Proteomics variables were log-transformed and standardized for data analysis. Where noted, analyses were corrected for multiple comparisons. Multivariate models were built using logistic regression, with vascular complications status as a dependent variable. All statistical analyses were performed in R version 3.3 software with packages compareGroups and stargazer (28). By applying logistic regression models using PLINK 1.07 (Purcell, Boston, MA) (29), the additive effects of the PON1 risk variants were tested for association with CVD in the Medalists.
Results
C− Subjects With Long-standing T1D Have Metabolic Profiles Similar to Those of C+ Subjects
To begin investigating factors associated with long-term protection from vascular complications, we recruited volunteers from the T1D Exchange Living Biobank. We chose this biobank because larger volumes of plasma than what is usually available from clinical studies could be obtained, which allowed us to optimize methods and use the plasma for several different assays. This discovery cohort included 47 subjects with an average T1D duration of 40 years, with 16 in the C− group and 31 in the C+ group, who reported having been treated for or diagnosed with at least two of four diabetes complications. The C− group consisted of 14 females and 2 males with an average age of 54 years and a diabetes duration of 38 years. The C+ group consisted of 14 females and 17 males of age, diabetes duration, BMI, and HbA1c comparable to the subjects in the C− group (Table 1). Twenty-six of these 31 subjects had had a myocardial infarction or stroke or had been treated for increased CVD risk, 26 had been treated for retinopathy and 16 for nephropathy, and 27 had been diagnosed with diabetic neuropathy. Three of the subjects in the C+ group had undergone a kidney transplant, and one was on dialysis. The subjects in the C− and C+ groups exhibited plasma lipid levels within the normal range. HDL-C and total cholesterol levels were higher in the C− group. Because of the difference in sex distribution between the groups, we also stratified the analyses by sex. For females, there were no significant differences between the C− and C+ groups in lipids or other clinical characteristics (Table 1).
Table 1.
Demographic and clinical characteristics of the discovery and replication cohorts
| T1D Exchange Living Biobank cohort (discovery cohort) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All subjects | Females only | Joslin Medalist cohort (replication cohort) | |||||||
| C− (n = 16) | C+ (n = 31) | P value | C− (n = 14) | C+ (n = 14) | P value | C− (n = 50) | C+ (n = 50) | P value | |
| Female sex | 14 (87.5) | 14 (45.2) | 0.013 | 14 (100) | 14 (100) | 50 (100) | 50 (100) | ||
| Age (years) | 54.5 (46.0; 62.2) | 58.0 (51.5; 65.0) | 0.276 | 54.5 (47.2; 61.2) | 56.5 (51.2; 60.0) | 0.783 | 62.0 (60.0; 66.0) | 63.0 (60.0; 66.0) | 0.997 |
| T1D duration (years) | 38.0 (32.0; 44.8) | 45.0 (32.5; 50.0) | 0.317 | 38.0 (32.8; 43.2) | 46.0 (39.2; 49.8) | 0.062 | 51.0 (50.0; 53.8) | 52.0 (51.0; 54.8) | 0.120 |
| BMI (kg/m2) | 25.9 (23.8; 28.8) | 25.0 (23.3; 28.6) | 0.621 | 25.9 (23.5; 28.4) | 24.4 (23.2; 27.0) | 0.49 | 23.8 (21.9; 25.6) | 25.5 (22.5; 28.4) | 0.112 |
| HbA1c (%) | 6.75 (6.65; 7.51) | 7.40 (6.75; 8.20) | 0.090 | 6.70 (6.55; 7.33) | 7.20 (6.75; 8.07) | 0.101 | 7.10 (6.62; 7.30) | 7.30 (6.62; 8.00) | 0.143 |
| HbA1c (mmol/mol) | 50 (49; 59) | 50 (50; 66) | 0.090 | 50 (48; 57) | 55 (50; 65) | 0.101 | 54 (49; 56) | 56 (49; 56) | 0.143 |
| Total cholesterol (mg/dL) | 188 (162; 207) | 158 (132; 178) | 0.004 | 188 (167; 206) | 176 (143; 187) | 0.129 | 178 (161; 194) | 160 (144; 186) | 0.016 |
| Non-HDL-C (mg/dL) | 100 (84.5; 134) | 90.0 (76.5; 114) | 0.127 | 100 (84.5; 127) | 95.0 (87.0; 109) | 0.358 | 103 (86.5; 122) | 93.0 (76.8; 112) | 0.152 |
| HDL-C (mg/dL) | 75.5 (62.0; 89.8) | 58.0 (48.0; 74.5) | 0.031 | 81.0 (65.5; 93.2) | 74.5 (59.2; 89.2) | 0.408 | 71.5 (62.2; 84.0) | 65.0 (52.2; 83.2) | 0.147 |
| TGs (mg/dL) | 67.5 (50.0; 95.8) | 71.0 (58.0; 90.0) | 0.779 | 67.5 (54.2; 93.8) | 61.5 (49.5; 66.8) | 0.312 | 58.0 (49.2; 76.0) | 62.5 (53.2; 85.5) | 0.154 |
| VLDL-C (mg/dL) | 8.50 (6.50; 12.5) | 8.00 (7.00; 12.0) | 0.743 | 8.50 (7.00; 11.5) | 8.00 (5.50; 8.00) | 0.417 | ND | ND | — |
| LDL-C (mg/dL) | 84.0 (80.5; 110) | 81.0 (69.0; 98.5) | 0.151 | 90.0 (82.0; 118) | 86.5 (76.8; 101) | 0.462 | 76.0 (67.0; 90.0) | 76.0 (66.2; 96.0) | 0.831 |
| Medications | |||||||||
| β-Blockers | * | * | 4 (8.00) | 23 (46.9) | <0.001 | ||||
| Lipid lowering | 5 (31) | 23 (74) | 0.011 | 33 (66.0) | 42 (85.7) | 0.040 | |||
| HTN | 1 (6) | 27 (87) | <0.001 | 26 (53.1) | 32 (65.3) | 0.304 | |||
Data are median (interquartile range) or n (%). Data on sex, age, diabetes duration, HbA1c, BMI, and diabetes complications were obtained from the T1D Exchange Living Biobank or the Joslin Diabetes Center. Plasma levels of total cholesterol, VLDL-C, LDL-C, HDL-C, and TGs were measured by Northwest Lipid Research Laboratories (Seattle, WA) using standard biochemical methods in the discovery cohort. Plasma lipids in the replication study were measured at the Joslin Diabetes Center. Boldface type indicates significance at P < .05. HTN, hypertension; LDL-C, LDL cholesterol; ND, not determined; TG, triglyceride; VLDL-C, VLDL cholesterol.
β-Blockers and other anti-HTNs were not reported separately in this cohort.
An independent replication cohort of subjects with long-standing T1D with and without CVD was selected from the Joslin Medalist Program (n = 50/group). Because our data from the discovery cohort strongly implied that differences in HDL metrics were prevalent in women (as shown below) and because HDL-C had been previously associated with protection from CVD in women (10), we selected women for the replication study and focused on cardiovascular complications. The average age of subjects was 62 years, and the average diabetes duration was 52 years. There were no differences in the metabolic profiles between the C− and C+ groups except for higher total cholesterol in the C− group. Overall, both cohorts consisted of subjects with well-controlled, long-standing T1D (HbA1c ∼7%) among whom there were no consistent differences in clinical characteristics between those who remained free of complications and those who developed vascular complications (Table 1).
High Levels of M-HDL Particles Associate With Protection From Vascular Complications Independent of Plasma Total Cholesterol or HDL-C
In the discovery cohort, the C− group exhibited a higher total HDL particle concentration as a result of a selective increase in M-HDL (average diameter 9.3 nm) and XL-HDL (12.4 nm) particles (Table 2 and Supplementary Fig. 1A and B). The C− group also exhibited a reduced concentration of XS-HDL (7.6 nm). No differences were observed in concentrations of L-HDL (11.1 nm) or S-HDL (8.3 nm). In the sex-stratified analyses (females, n = 14/group), the total HDL particle concentration was again higher in the C− group, as was the M-HDL concentration (Table 2).
Table 2.
Elevated M-HDL particles are associated with protection from complications
| T1D Exchange Living Biobank cohort (discovery cohort) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All subjects | Females only | Joslin Medalist cohort (replication cohort) | |||||||
| C− (n = 16) | C+ (n = 31) | P value | C− (n = 14) | C+ (n = 14) | P value | C− (n = 50) | C+ (n = 50) | P value | |
| Total HDL (μmol/L) | 17.5 (14.4; 19.7) | 13.6 (12.0; 15.5) | 0.001 | 17.8 (15.2; 19.7) | 15.4 (13.6; 17.2) | 0.031 | 24.2 (21.3; 27.4) | 22.4 (19.1; 25.6) | 0.065 |
| XS-HDL (μmol/L) | 0.91 (0.84; 0.96) | 1.05 (0.94; 1.17) | 0.026 | 0.91 (0.83; 0.96) | 0.97 (0.86; 1.03) | 0.215 | 0.89 (0.70; 1.08) | 0.91 (0.71; 1.13) | 0.694 |
| S-HDL (μmol/L) | 2.70 (2.19; 4.13) | 2.68 (2.01; 3.45) | 0.559 | 2.68 (2.12; 3.84) | 2.06 (1.70; 2.90) | 0.232 | 2.50 (2.18; 2.84) | 2.73 (1.87; 3.66) | 0.22 |
| M-HDL (μmol/L) | 8.07 (7.02; 9.35) | 5.94 (5.14; 6.76) | <0.001 | 8.20 (7.65; 9.82) | 6.76 (5.88; 7.87) | 0.024 | 9.62 (8.66; 11.0) | 8.63 (7.31; 10.1) | 0.002 |
| L-HDL (μmol/L) | 3.73 (2.80; 5.13) | 2.70 (1.63; 4.06) | 0.101 | 3.82 (3.18; 5.30) | 4.06 (2.26; 5.48) | 0.713 | 9.82 (7.28; 12.3) | 9.09 (5.66; 11.6) | 0.156 |
| XL-HDL (μmol/L) | 0.75 (0.61; 0.91) | 0.56 (0.44; 0.67) | 0.015 | 0.79 (0.68; 0.94) | 0.66 (0.58; 0.86) | 0.27 | 0.69 (0.51; 1.05) | 0.68 (0.51; 1.04) | 0.669 |
| XS-HDL.sz (nm) | 7.61 (7.58; 7.65) | 7.59 (7.57; 7.67) | 0.719 | 7.61 (7.59; 7.65) | 7.60 (7.58; 7.70) | 0.963 | 7.60 (7.56; 7.64) | 7.57 (7.48; 7.63) | 0.036 |
| S-HDL.sz (nm) | 8.34 (8.32; 8.40) | 8.32 (8.27; 8.39) | 0.127 | 8.35 (8.33; 8.40) | 8.31 (8.27; 8.41) | 0.215 | 8.26 (8.22; 8.30) | 8.25 (8.20; 8.31) | 0.53 |
| M-HDL.sz (nm) | 9.31 (9.26; 9.32) | 9.24 (9.17; 9.28) | 0.026 | 9.31 (9.29; 9.32) | 9.25 (9.24; 9.29) | 0.089 | 9.08 (9.04; 9.13) | 9.09 (8.99; 9.15) | 0.736 |
| L-HDL.sz (nm) | 11.1 (11.0; 11.1) | 11.1 (11.0; 11.1) | 0.425 | 11.1 (11.0; 11.1) | 11.1 (11.0; 11.1) | 0.535 | 11.0 (10.9; 11.0) | 11.0 (10.9; 11.0) | 0.473 |
| XL-HDL.sz (nm) | 12.4 (12.2; 12.6) | 12.4 (12.3; 12.5) | 0.875 | 12.4 (12.2; 12.6) | 12.4 (12.3; 12.6) | 0.335 | 12.7 (12.7; 12.8) | 12.7 (12.6; 12.8) | 0.279 |
| ABCA1 (%) | 5.38 (4.69; 5.87) | 6.06 (4.64; 6.57) | 0.357 | 5.26 (4.59; 5.76) | 5.00 (4.11; 6.54) | 0.89 | 6.49 (5.56; 7.64) | 7.15 (5.67; 10.5) | 0.102 |
| ABCG1 (%) | 4.36 (3.94; 4.68) | 3.78 (3.24; 4.13) | 0.006 | 4.36 (3.94; 4.66) | 3.69 (3.31; 4.49) | 0.108 | 4.02 (3.59; 4.59) | 3.80 (3.42; 4.53) | 0.162 |
| J774 (%) | 13.9 (12.6; 14.4) | 11.6 (10.5; 14.6) | 0.061 | 13.9 (12.6; 14.1) | 12.8 (10.4; 14.8) | 0.383 | 14.9 (13.4; 16.6) | 15.8 (12.8; 18.7) | 0.368 |
Data are median (interquartile range). Boldface type indicates significance at P < 0.05. ABCA1, ABCA1-specific serum HDL CEC; ABCG1, ABCG1-specific serum HDL CEC; J774, J774 macrophage serum HDL CEC; sz, size.
In the Medalist replication study, a higher concentration of M-HDL particles in C− subjects confirmed the finding from the discovery cohort (Table 2). No significant differences were found in other HDL particles.
We further investigated possible confounding of the association of M-HDL concentration with protection from vascular complications by parameters that showed a strong trend for differences (P < 0.1) in the univariate analysis, using multivariate models. The association between higher concentrations of M-HDL particles and cardiovascular protection remained highly significant after adjustment for total cholesterol, HDL-C, lipid-lowering therapy, and use of β-blockers (Supplementary Table 1). Taken together, the data strongly suggest that women with long-standing T1D who are protected from vascular complications exhibit higher levels of HDL particles, specifically M-HDL, despite similar levels of HDL-C. This association is independent of total cholesterol, lipid-lowering therapies, or use of β-blockers.
Higher Levels of M-HDL Do Not Alter HDL CEC or Its Ability to Prevent Monocyte Adhesion to Endothelial Cells
Next, we assessed parameters believed to contribute to the cardioprotective functions of HDL, namely CEC (30) and the ability of HDL to reduce monocyte adhesion to endothelial cells (31). In the discovery cohort, serum HDL CEC mediated by ABCG1 was higher in the C− group compared with the C+ group (Table 2). In contrast, ABCA1-specific CEC and CEC in J774 macrophages were not different between the groups. The increase in ABCG1-specific CEC by serum HDL from C− subjects was most likely explained by the increased concentration of M-HDL in this group because there were no differences between the groups when the assay was repeated with isolated HDL normalized for particle concentration (Supplementary Fig. 1C). In the female subgroup analysis, no significant differences in CEC were found with either serum HDL or isolated HDL (Table 2 and Supplementary Fig. 1D). In the Medalist replication cohort, none of the serum HDL CEC assays were different between the C− and C+ groups (Table 2).
Isolated HDL, normalized for HDL protein concentration, suppressed monocyte adhesion to endothelial cells by ∼50% compared with cells not treated with HDL. However, there were no differences in the ability of HDL to prevent monocyte adhesion to endothelial cells between the C− and C+ groups under basal conditions or after activation of endothelial cells with tumor necrosis factor-α (Supplementary Fig. 1E). These results indicate that HDL’s CEC and ability to protect from monocyte adhesion to endothelial cells are unlikely to explain the protection from vascular complications in people with long-standing T1D.
C− Subjects With Long-standing T1D Exhibit Increased Levels of HDL-Associated PON1
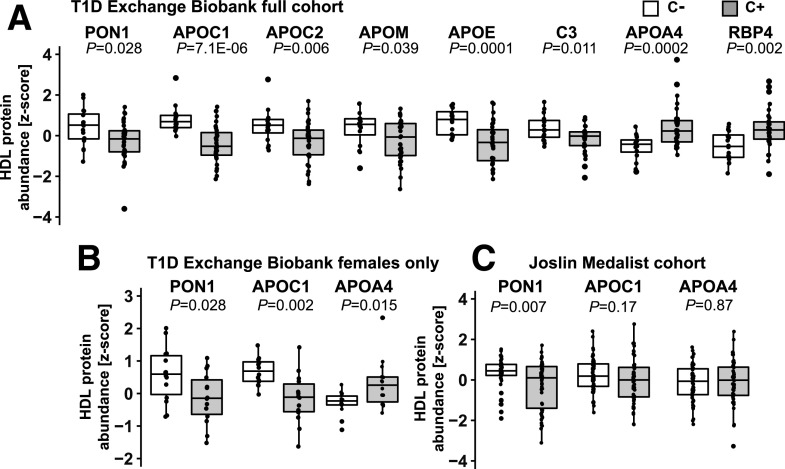
We next used targeted quantitative proteomics to quantify 35 proteins consistently found in HDL (22). These studies revealed higher levels of APOC1, APOE, complement C3, APOC2, APOM, and PON1 associated with HDL in the C− group compared with the C+ group in the discovery cohort in univariate analysis (Fig. 1A). In contrast, APOA4 and retinol-binding protein 4 (RBP4) were lower in the C− group. In the female subgroup analysis, only APOC1 and PON1 remained significantly higher and APOA4 significantly lower in the C− group (Fig. 1B). On the basis of these data, we prespecified the hypothesis that in the replication cohort, subjects in the C− group would exhibit higher HDL-associated APOC1 and PON1, while the HDL-associated APOA4 would be lower, compared with the C+ group.
Figure 1.
Differences in the HDL proteome in the two cohorts of people with long-standing T1D with and without vascular complications. A: Quantitative targeted proteomics of 35 major HDL proteins detected 8 proteins significantly different between the C− and C+ groups in the T1D Exchange Living Biobank discovery cohort, with 6 proteins with higher abundance and 2 proteins with lower abundance in the C− group (n = 16 in C− and n = 31 in C+ groups). B: When only females were analyzed (n = 14/group), two of these proteins remained significantly elevated in the C− group and one protein remained significantly reduced in the C− group. C: Quantification of PON1, APOC1, and APOA4 confirmed higher levels of PON1 in women protected from CVD complications of long-standing T1D in the Joslin Medalist replication study (n = 50/group). The protein abundance is presented as z scores, and data are median and quartiles. Significance was determined using nonparametric Wilcoxon rank sum test. P < 0.05 was considered statistically significant.
Targeted proteomic analysis of HDL-associated PON1 demonstrated higher levels in subjects protected from cardiovascular complications (C− group) in the replication cohort (Fig. 1C), confirming the results from the discovery cohort (Fig. 1A). However, APOC1 and APOA4 did not reveal significant differences (Fig. 1C). In a secondary analysis, we detected, after correction for multiple comparisons, differential abundance of several HDL-associated proteins that were not different in the smaller discovery cohort. These included higher levels of APOA2, APOA5, lecithin-cholesterol acyltransferase (LCAT), and PON3 in the C− group (Supplementary Fig. 2).
Multivariate logistic regression demonstrated that the association of higher HDL-associated PON1 in protected subjects is independent of HDL-C, total cholesterol, and lipid-lowering and β-blocker therapies (Supplementary Table 2).
The association of higher HDL-PON1 in the C− group was not due to differences in PON1 polymorphisms because there was no significant association between rs662, rs854560, or rs705379 and CVD status in unadjusted model analysis of the 100 Medalist subjects included in the current study (P = 0.99, P = 0.55, and P = 0.06, respectively) or in the whole Medalist cohort (891 subjects; P = 0.11, P = 0.59, and P = 0.27, respectively). Collectively, our results demonstrate that subjects with T1D who have remained complications free for decades exhibit elevated levels of HDL-associated PON1 in the two independent cohorts and that this association is independent of total cholesterol and HDL-C levels.
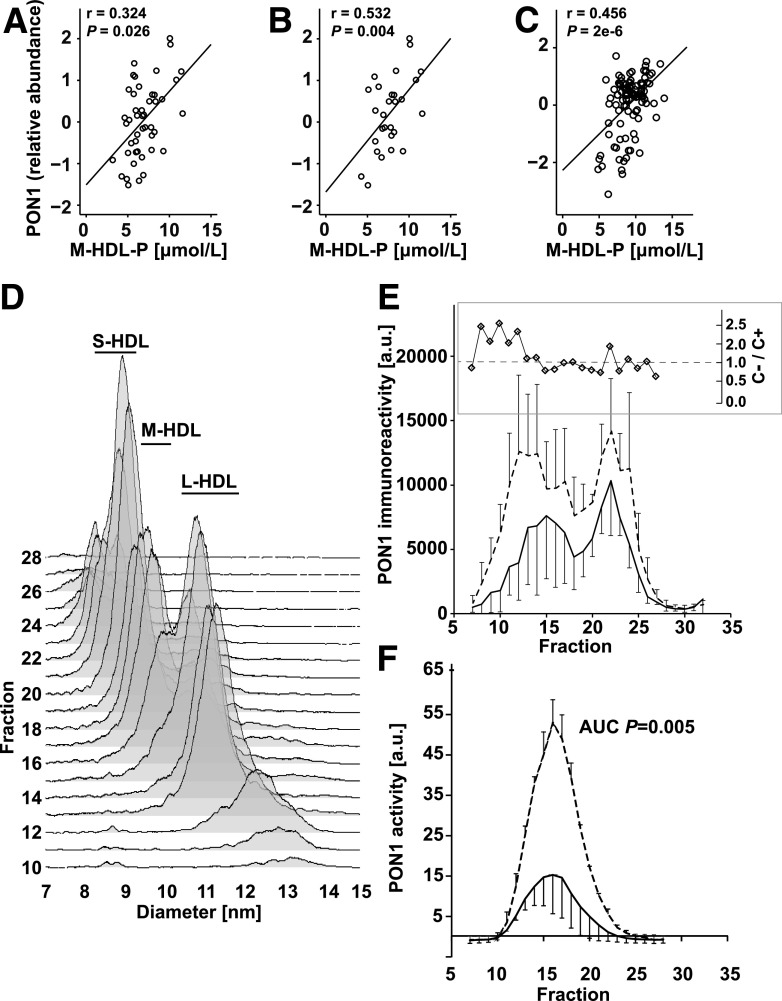
C− Subjects With Long-standing T1D Exhibit Increased PON1 Mass and Activity in M-HDL and L-HDL
There was a significant positive correlation between HDL-associated PON1 and M-HDL particle concentration in both cohorts as well as in the female subgroup analysis in the discovery cohort (Fig. 2A–C), suggesting that the increased concentration of M-HDL and increased HDL-associated PON1 may be related. To further investigate the relationship between PON1 and HDL subpopulations in the discovery cohort, the distribution of PON1 immunoreactivity in the different sizes of HDL in plasma was analyzed by high-resolution FPLC-SEC and dot blot analysis (Fig. 2D). Immunoreactive PON1 was higher in the C− group than in the C+ group across the whole range of HDL sizes. A large proportion of PON1 was carried by M-HDL; this size of HDL particles constituted as much as one-half of the HDL subspecies in the discovery cohort (Table 2). However, the relative enrichment of immunoreactive PON1, as a fraction of total HDL-associated PON1 in each group, was in larger HDL particles (Fig. 2E, inset).
Figure 2.
PON1 mass and activity associate with larger HDL particles in people with long-standing T1D protected from vascular complications. A–C: Correlation of PON1 and M-HDL particle (M-HDL-P) concentration in all subjects from the T1D Exchange Living Biobank discovery cohort (A), in women in the T1D Exchange Living Biobank discovery cohort (B), and in the Joslin Medalist replication cohort (C). Statistical analyses were performed by Spearman rank order test. D: Ultracentrifuge-isolated HDL was fractionated by FPLC-SEC on tandem Superdex 200 and Superdex 75 columns to maximize resolution of the HDL particles. Each fraction was then analyzed by DMA to determine the HDL particle profile. A representative HDL profile is shown. E: Three subjects in each group from the T1D Exchange Living Biobank cohort were pooled for each sample to yield n = 3 per group of pooled samples. An equal amount of the ultracentrifuge-isolated HDL was fractionated by FPLC-SEC as above. Distribution of PON1 in the different HDL subpopulations was quantified in the fractions using dot immunoblots after first ensuring specificity of the antibody and integrity of PON1 by Western blot. The inset shows relative PON1 enrichment in individual FPLC fractions in the C− vs. C+ groups. The amount of PON1 in each HDL fraction was first expressed as a fraction of total HDL-associated PON1 within each group, and the ratio C−/C+ was calculated to derive the relative enrichment in each HDL fraction. F: PON1 arylesterase activity measured in the FPLC-SEC fractions of HDL isolated from serum samples of subjects in the Joslin Medalist cohort. HDL-associated PON1 activity was normalized using a serum serial dilution standard curve to control for variability in the assay between different assay plates. Three pooled samples from the C+ and C− groups, each consisting of four subjects/pool, were analyzed. All analyses were performed in the linear range of the assay. Data are mean ± SD in panels E and F. a.u., arbitrary unit; AUC, area under the curve.
Serum samples from the replication study were used to investigate the distribution of PON1 enzymatic activity across HDL subpopulations separated by FPLC-SEC. PON1 activity was associated with larger HDL particles (approximately corresponding to M-HDL and L-HDL) and was strikingly higher in subjects in the C− group (Fig. 2F). Thus, protection from vascular complications associates with increased PON1 mass and activity in larger HDL subpopulations as opposed to smaller HDL particles.
Conclusions
We demonstrate that people with long-standing T1D without evidence of microvascular or macrovascular disease have higher concentrations of M-HDL particles, a specific HDL population, than people with evidence of vascular disease. We also found higher levels of HDL-associated PON1 mass and activity in people protected from vascular disease. Importantly, we observed these differences in two independent cohorts of people with long-standing T1D. The observed differences were independent of plasma cholesterol and HDL-C levels as well as of lipid-lowering and antihypertensive therapies.
The association of specific HDL subpopulations with vascular complications is not well understood. We previously showed that patients with coronary endothelial dysfunction had reduced levels of M-HDL and L-HDL particles (21). The independence of M-HDL concentration from HDL-C levels may relate to the fact that HDL particles in this population contribute less to total HDL-C than do L-HDL and XL-HDL.
Possible mechanisms explaining the higher concentration of M-HDL particles in people with T1D protected from vascular complications include increased activity of LCAT, which esterifies free cholesterol, converting S-HDL to M-HDL, or reduced activity of cholesterol ester transfer protein (CETP), which converts M-HDL to L-HDL by adding cholesterol esters. The higher level of LCAT in HDL of protected subjects in the replication study may partly explain the higher concentration of M-HDL, although LCAT was not higher in the smaller discovery cohort. A more likely mechanism is suggested by the higher HDL-associated PON1 in protected subjects in both the discovery and the replication cohorts. PON1 is an HDL-associated enzyme implicated in some of HDL’s proposed protective effects (32), and its overexpression protects against atherosclerosis in mouse models (33). We have recently shown that a low concentration of PON1 in HDL is associated with both albuminuria and coronary artery calcification in patients with T1D in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study (34). PON1 hydrolyzes multiple substrates. However, endogenous PON1 substrates are unknown, although in vitro studies point to oxidized lipids (32). Lower HDL-associated PON1 activity has been linked to impaired endothelial nitric oxide synthase activity and endothelial dysfunction in subjects with CVD (35), and it is possible that higher levels of PON1 could reduce oxidative stress. Our study suggests that PON1 does not act by increasing HDL’s CEC, as found in mouse studies (36), or by directly boosting HDL’s ability to prevent monocytes from adhering to endothelial cells.
Previous studies suggested that PON1 resides predominantly in small HDL3a particles isolated by isopycnic gradient ultracentrifugation (37) or in L-HDL and M-HDL particles when isolated by SEC (38). M-HDL most closely corresponds to HDL2a as defined by gradient gel electrophoresis (39), a fraction that appears in the middle range of HDL on SEC. Our studies reveal that people protected from vascular complications carry relatively more PON1 in the larger HDL subpopulation, compared with people who have complications, and that PON1 associated with larger HDL particles (approximately corresponding to M-HDL and L-HDL) separated by FPLC-SEC is more active than PON1 associated with smaller HDL particles, as supported by in vitro studies (40). It is therefore possible that M-HDL and L-HDL carrying increased levels of PON1 have increased protective effects. Our results further suggest that the elevated PON1 in protected people is not explained by differences in PON1 polymorphisms. Future studies are needed to reveal the molecular mechanisms whereby PON1 might protect against diabetes complications and how this relates to PON1 distribution and activity in different HDL subpopulations.
A major strength of our study is the use of two independent cohorts of subjects with long-standing T1D. Importantly, we also used validated methods to quantify HDL’s protein cargo and the concentration and size of the HDL subspecies. A limitation is the study’s cross-sectional design, which enabled us to investigate associations but prevented us from predicting future vascular complications.
In summary, we demonstrate that higher levels of M-HDL particles associate with protection from vascular complications in people with long-standing T1D and that the association is independent of total cholesterol and HDL-C levels. Furthermore, HDL-associated PON1—an enzyme strongly antiatherogenic in animal studies—is increased in people with T1D protected from complications.
Supplementary Material
Article Information
Acknowledgments. The authors thank Åsa Davis (Fred Hutchinson Cancer Research Center, Seattle, WA) for assisting with the study design and selection of subjects from the T1D Exchange Living Biobank study. The authors also thank the individuals who generously provided blood samples for these studies, the T1D Exchange Living Biobank study coordinators, and the Benaroya Research Institute research coordinators. The authors thank the Medalists who are part of the Joslin Medalist Study.
Funding. This study was supported in part by the T1D Exchange through Benaroya Research Institute and the Leona M. and Harry B. Helmsley Charitable Trust and in part by National Heart, Lung, and Blood Institute grants R01-HL-144558 to T.V. and P01-HL-092969 and P01-HL-128203 to J.W.H. and K.E.B.; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, grant R21-AI-135447 to J.W.H and K.E.B; National Institute of Diabetes and Digestive and Kidney Diseases grant DP3-DK-108209 to J.W.H. and K.E.B.; and the Diabetes Research Center at the University of Washington (P30-DK-017047).
Duality of Interest. T.V. has received funding from MedImmune LLC for unrelated projects. J.E.K. and K.E.B. have received funding from Novo Nordisk A/S for unrelated projects. C.J.G. has received funding from Novo Nordisk and Janssen for unrelated projects. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.V. performed experiments, analyzed data, and wrote the manuscript. J.E.K. performed experiments and edited the manuscript. J.W. and A.D.I. performed experiments. J.G., E.W., V.B., I.-H.W., H.S., H.A.K., and G.L.K. coordinated the Joslin Medalist studies, analyzed data, and edited the manuscript. C.J.G. coordinated the T1D Exchange Living Biobank studies and edited the manuscript. J.W.H. was involved in study design and edited the manuscript. K.E.B. designed the study and edited the manuscript. K.E.B. is guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0772/-/DC1.
References
- 1.de Boer IH; DCCT/EDIC Research Group . Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group . Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan HA, Costacou T, Sun JK, et al. . Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year Medalist Study. Diabetes Care 2007;30:1995–1997 [DOI] [PubMed] [Google Scholar]
- 5.Sun JK, Keenan HA, Cavallerano JD, et al. . Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-Year Medalist Study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt S, Gupta MK, Khamaisi M, et al. . Preserved DNA damage checkpoint pathway protects against complications in long-standing type 1 diabetes. Cell Metab 2015;22:239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordin D, Harjutsalo V, Tinsley L, et al. . Differential association of microvascular attributions with cardiovascular disease in patients with long duration of type 1 diabetes. Diabetes Care 2018;41:815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi W, Keenan HA, Li Q, et al. . Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 2017;23:753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg HN Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care 1991;14:839–855 [DOI] [PubMed] [Google Scholar]
- 10.He ZH, D’Eon SA, Tinsley LJ, et al. . Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care 2015;38:e73–e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Kothari V, Bornfeldt KE. High-density lipoprotein function in cardiovascular disease and diabetes mellitus. Arterioscler Thromb Vasc Biol 2018;38:e10–e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera AV, Cuchel M, de la Llera-Moya M, et al. . Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjunatha S, Distelmaier K, Dasari S, Carter RE, Kudva YC, Nair KS. Functional and proteomic alterations of plasma high density lipoproteins in type 1 diabetes mellitus. Metabolism 2016;65:1421–1431 [DOI] [PubMed] [Google Scholar]
- 14.Hutchins PM, Ronsein GE, Monette JS, et al. . Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem 2014;60:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ditah C, Otvos J, Nassar H, Shaham D, Sinnreich R, Kark JD. Small and medium sized HDL particles are protectively associated with coronary calcification in a cross-sectional population-based sample. Atherosclerosis 2016;251:124–131 [DOI] [PubMed] [Google Scholar]
- 16.Khera AV, Demler OV, Adelman SJ, et al. . Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER trial (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 2017;135:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–870 [DOI] [PubMed] [Google Scholar]
- 18.Vaisar T, Pennathur S, Green PS, et al. . Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007;117:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 20.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol 1998;147:969–977 [DOI] [PubMed] [Google Scholar]
- 21.Monette JS, Hutchins PM, Ronsein GE, et al. . Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ Res 2016;119:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoofnagle AN, Becker JO, Oda MN, Cavigiolio G, Mayer P, Vaisar T. Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin Chem 2012;58:777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson CM, Vaisar T, Hoofnagle AN. Isolating and quantifying plasma HDL proteins by sequential density gradient ultracentrifugation and targeted proteomics. Methods Mol Biol 2016;1410:105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronsein GE, Pamir N, von Haller PD, et al. . Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J Proteomics 2015;113:388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLean B, Tomazela DM, Shulman N, et al. . Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010;26:966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res 2006;47:2433–2443 [DOI] [PubMed] [Google Scholar]
- 27.Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet 2008;1:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subirana IS, Sanz H, Vila J. Building bivariate tables: the compareGroups Package for R. J Stat Softw 2014;57:1–1625400517 [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 2010;30:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenson RS, Brewer HB Jr., Ansell BJ, et al. . Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 2016;13:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aviram M, Vaya J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr Opin Lipidol 2013;24:339–344 [DOI] [PubMed] [Google Scholar]
- 33.Tward A, Xia YR, Wang XP, et al. . Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002;106:484–490 [DOI] [PubMed] [Google Scholar]
- 34.Shao B, Zelnick LR, Wimberger J, et al. . Albuminuria, the high-density lipoprotein proteome, and coronary artery calcification in type 1 diabetes mellitus. Arterioscler Thromb Vasc Biol 2019;39:1483–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besler C, Heinrich K, Rohrer L, et al. . Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011;121:2693–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenblat M, Vaya J, Shih D, Aviram M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis 2005;179:69–77 [DOI] [PubMed] [Google Scholar]
- 37.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol 2009;29:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res 2010;9:5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanche PJ, Gong EL, Forte TM, Nichols AV. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta 1981;665:408–419 [DOI] [PubMed] [Google Scholar]
- 40.Josse D, Ebel C, Stroebel D, et al. . Oligomeric states of the detergent-solubilized human serum paraoxonase (PON1). J Biol Chem 2002;277:33386–33397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.