Abstract
Within the human pancreas, exocrine and endocrine cells control secretion of digestive enzymes and production of hormones to maintain metabolic homeostasis, respectively. While the vast majority of type 1 diabetes research efforts have focused on endocrine function and autoimmunity, recent studies identified a series of unique features (e.g., reduced weight and volume, increased density of leukocytes) within the exocrine pancreas in this disease, but the mechanisms underlying these aberrancies are unknown. Therefore, we histologically assessed amylase, insulin, glucagon, lipase, and/or trypsinogen in 78 organ donor pancreata from birth through adulthood in control subjects and those at various stages of type 1 diabetes. While amylase-positive (AMY+) acinar cells were detectable in pancreata from all study groups, tissues from individuals >2 years of age contained clusters of acinar cells devoid of amylase (AMY−). A majority of these AMY− cell clusters localized proximal to islets (i.e., peri-islet). Additionally, most AMY− clusters were positive for the exocrine enzymes lipase and trypsinogen. Interestingly, type 1 diabetes pancreata displayed significant reductions in the frequency of these AMY− cell clusters. These results support a contribution of the islet-acinar axis in pancreatic development and underscore a potential role for the exocrine pancreas in the pathogenesis of type 1 diabetes.
Introduction
The human pancreas is comprised of two main compartments in terms of its secretory functions. The first, an exocrine component, is composed of acinar cells that produce digestive enzymes alongside ductal cells that generate bicarbonate. The second, endocrine in nature, consists of the islets of Langerhans, cells that manufacture hormones to regulate metabolism and maintain glycemic homeostasis (1,2). Most studies of the pancreas in diabetes, in any form, have historically focused on the endocrine compartment, with the aim of understanding islet cell dysfunction or loss in the setting of this disease.
Previously, we and others have noted that pancreas weight and relative pancreas volume are significantly reduced in individuals with and at risk for type 1 diabetes compared with control subjects without diabetes (3–7). These observations suggest that pancreas mass is lost prior to disease onset, possibly as the result of undefined genetic, maternal, or environmental factors. In addition, patients with type 1 diabetes have been noted to exhibit exocrine insufficiency, including a reduction in exocrine enzyme production, as assessed in serum and stool (8–10), albeit to levels not routinely subject to clinical significance. It remains unclear whether these alterations result from disrupted islet-acinar interactions secondary to the loss of functional β-cell mass or contribute directly to type 1 diabetes development. For interrogation of the potential relationship linking islet and acinar cell mass and function, a foundational understanding of cell phenotype and morphological organization within the exocrine pancreas is necessary.
Research Design and Methods
Human Subjects
Pancreata (n = 78) were recovered from organ donors who had type 1 diabetes and from autoantibody-positive (Aab+) and control organ donors aged 35 weeks’ gestation (G35w) through 65 years with informed consent from next of kin and processed by the Network for Pancreatic Organ donors with Diabetes (nPOD) program as previously described (11). Quantitative analysis was divided into two parts, with 37 control donors aged 2–65 years examined for age-related changes in amylase expression (Supplementary Table 1) and 34 age-matched donors (12 control and 10 Aab+ donors and 12 with type 1 diabetes) evaluated to ascertain whether alterations in amylase expression are related to type 1 diabetes (Supplementary Table 2). Pancreatic samples from five patients who underwent partial pancreatectomy were obtained from the University of Florida Clinical and Translational Science Institute (CTSI) Biorepository (https://www.ctsi.ufl.edu/research/laboratory-services/ctsi-biorepository-2/). Samples from donors aged G35w to 1 year (N = 7) and from pancreatic biopsies (N = 5) were subjected to qualitative analysis only (Supplementary Table 1). All studies were approved by the University of Florida Institutional Review Board.
Immunohistochemistry and Immunofluorescence
Consecutive whole 4-μm formalin-fixed paraffin-embedded cross-sections were deparaffinized, rehydrated with serial passage through changes of xylene and graded ethanol, and stained for several antibody panels: 1) amylase, insulin, glucagon, and cytokeratin 19 (CK19); 2) amylase, insulin, and laminin 1/2; 3) amylase, insulin, and e-cadherin; and 4) amylase, lipase, trypsinogen, insulin, and glucagon. Both chromogen-based immunohistochemistry (IHC) and immunofluorescence (IF) methods used heat-induced epitope retrieval. Detection kits, antibodies, and antigen retrieval buffers were utilized according to the manufacturer’s instructions and are listed in Supplementary Table 3.
Quantification of Amylase-Negative Areas
Slides stained by quadruple IHC for amylase, insulin, glucagon, and CK19 were digitized at ×20 magnification using an Aperio CS2 slide scanner (Leica Biosystems Imaging, Inc.). Whole slide images were analyzed using the HALO next-generation quantitative image analysis platform (Indica Labs) to detect cell nuclei and positively stained cells. Separate layers of annotations were created and annotations automatically counted for three categories: 1) peri-islet AMY-negative (AMY−) cell clusters (AMY− cell clusters directly associated with islets), 2) tele-islet AMY− cell clusters (AMY− cell clusters not directly associated with islets), and 3) AMY+/islets+ (islets surrounded by AMY-positive acinar cells). For each layer, the number of annotations divided by total tissue area (mm2) was used to calculate the density of each category. The frequency (percentage) of peri-islet AMY− cell clusters out of total AMY− cell clusters was calculated as follows: (N peri-islet AMY− cell clusters)/(N peri-islet AMY− cell clusters + N tele-islet AMY− cell clusters). The frequency (percentage) of peri-islet AMY− cell clusters out of total islets was calculated as follows: (N peri-islet AMY− cell clusters) / (N peri-islet AMY− cell clusters + N AMY+/islets+). Morphometric analyses were carried out by three independent observers, and automatic counts were averaged across all three observers for each slide image.
RNA In Situ Hybridization
Triple-fluorescent RNA in situ hybridization was performed for amylase, insulin, and glucagon on formalin-fixed paraffin-embedded pancreatic sections for using RNAscope Multiplex Florescent Reagent Kit v2 (cat. no. 323110; Advanced Cell Diagnostics) per the manufacturer instructions. Probe accession numbers are listed in Supplementary Table 3.
Statistics
Data were analyzed with GraphPad Prism 8 (GraphPad Software), using one-way ANOVA followed by Tukey post hoc test for multiple comparisons with significance defined as P < 0.05.
Data and Resource Availability
The data sets presented herein are available from the corresponding author upon reasonable request. Tissue samples utilized in the current study are available from the nPOD biorepository (https://www.jdrfnpod.org/). Antibody information is available in the Antibody Registry (https://antibodyregistry.org/) (Research Resource Identification [RRID] listed in Supplementary Table 3).
Results
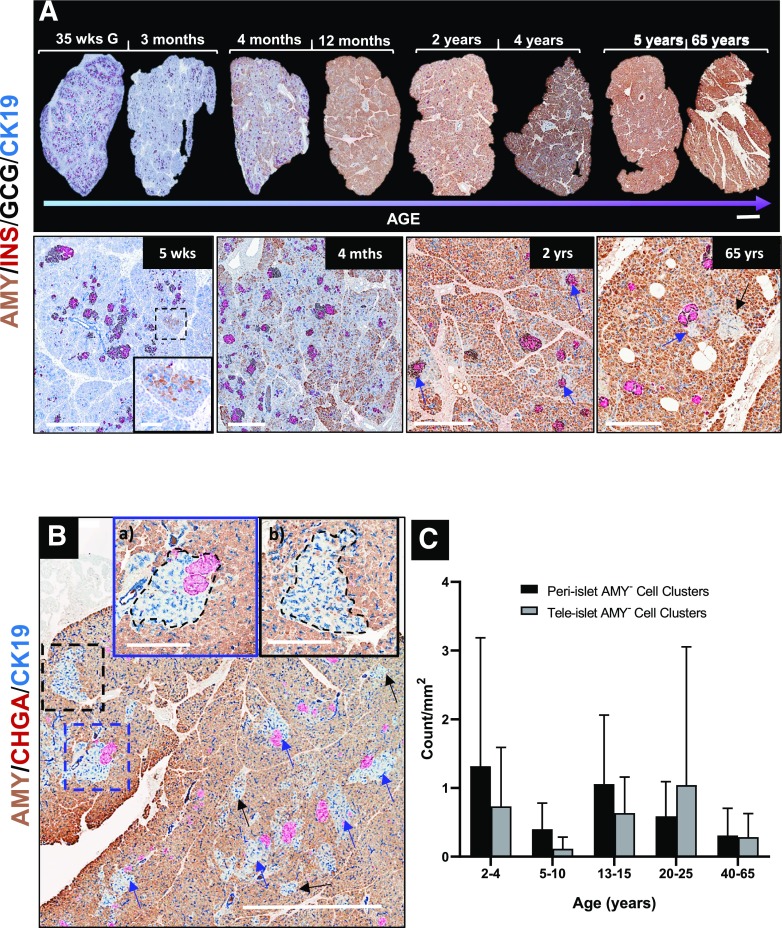
We investigated the amylase expression pattern in acinar cells of control organ donors without diabetes, ranging from G35w through 65 years of age, along with donors with and at risk for type 1 diabetes (Supplementary Tables 1 and 2). Using quadruple IHC staining for human pancreatic amylase, insulin, glucagon, and CK19, we confirmed previous studies (12) noting that small numbers of AMY+ cells are scattered throughout the pancreatic parenchyma in control human pancreas at birth until ∼3 months of age (Fig. 1A [5 weeks]). Throughout normal pancreas development during the first year of life, the number of AMY+ cells dramatically increased, with acinar cells located at a distance from islets (i.e., tele-islet) acquiring amylase positivity earlier than peri-islet acinar cells, giving the pancreas a patchy appearance (Fig. 1A [4 months]). The vast majority of acinar cells expressed amylase by age 2 years and continued to do so throughout the life span in control donors (Fig. 1A). Strikingly, an appreciable number of acinar cells that appeared devoid of amylase were consistently observed in aggregate clusters of varying size in pancreatic tissue samples collected from control organ donors (Fig. 1B) as well as living individuals at the time of partial pancreatectomy (Supplementary Fig. 1A and B). Two distinct patterns for AMY− cell cluster distribution emerged: most AMY− cell clusters were immediately associated with pancreatic islets (peri-islet [Fig. 1Ba]), while others had no direct islet contact (tele-islet [Fig. 1Bb]). Quantification analysis of pancreatic tissue from all control organ donors >2 years of age revealed that mean ± SD 63 ± 20% (range 11.9–100) of AMY− clusters were located proximal to the islets (Supplementary Fig. 2A). However, upon examination at different depths within the same tissue (serial sections), many AMY− clusters originally identified as tele-islet were observed to be peri-islet (Supplementary Fig. 2B), implying that the average percentage of peri-islet AMY− cell clusters could be higher than found in our analyses. We then examined whether the number of peri- and tele-islet AMY− cell clusters progressively changed with age (2–65 years) in control donors without diabetes and observed no significant associations with age (Fig. 1C). These findings appeared to be species specific, as AMY− cell clusters were not observed in mouse, porcine, or Rhesus monkey pancreas (Supplementary Fig. 3).
Figure 1.
Representative images of pancreatic tissue sections from donors with ages ranging from G35w (35 wks G) to 65 years, showing progressive developmental changes in amylase expression. A: Amylase production increases over the first year of development to include all exocrine tissue with the exception of peri- and tele-islet AMY− cell clusters that are apparent by age 2 years. B: Peri-islet AMY− cell clusters are found adjacent to the islet, while tele-islet clusters have no associated islet. C: Peri- and tele-islet AMY− cluster density varied across all ages analyzed: 2–65 years. Data points represent density per whole tissue section for individual donors. Data are presented as mean ± SD and analyzed by one-way ANOVA with Tukey post hoc test for multiple comparisons. Scale bars: 2 mm (A, upper panel, and B); 600 µm, with inset 100 μm (A, lower panel [5 weeks]); 600 μm (4 months–2 years); 300 μm (65 years); and 500 μm (Ba and Bb).
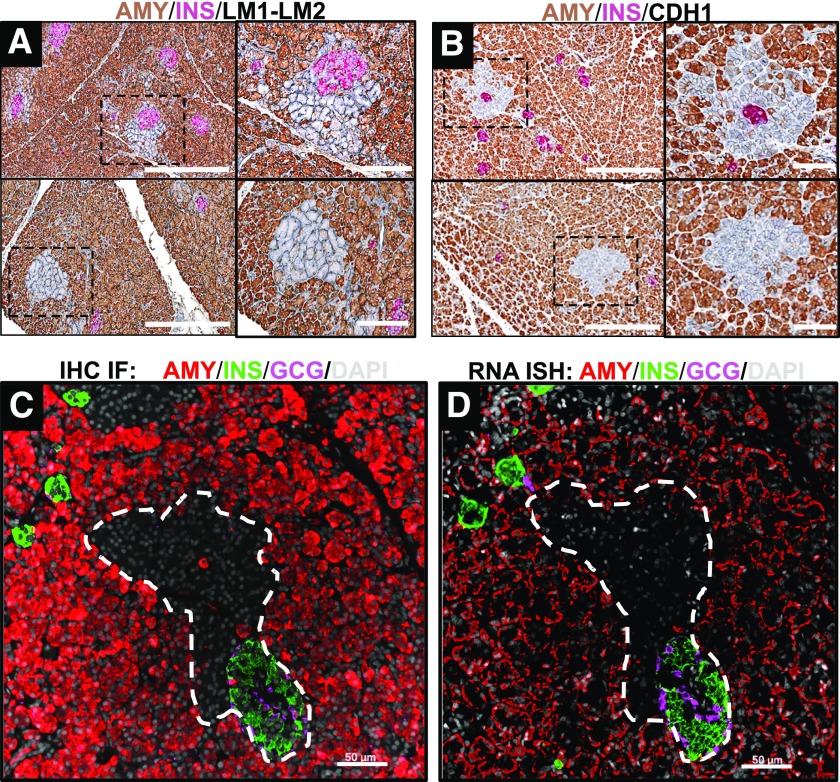
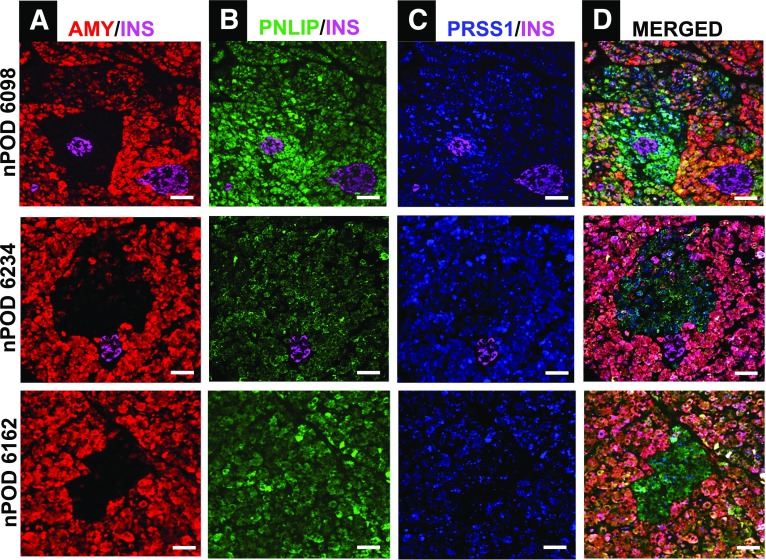
Cells in peri- and tele-islet AMY− clusters displayed similar laminin 1/2 (Fig. 2A) and e-cadherin (Fig. 2B) staining patterns that were comparable with those of AMY+ acinar cells, implying that AMY− cell basement membranes were preserved and cell-to-cell contacts maintained. The absence of detectable amylase protein in peri- and tele-islet cell clusters prompted us to examine amylase mRNA expression. While pancreatic mRNA for amylase was readily detectable in the majority of acinar cells, it was undetectable in the AMY− cell clusters (Fig. 2C and D). To determine whether cells in AMY− cell clusters produce other digestive enzymes, we stained pancreatic tissue for amylase, lipase, and trypsinogen, as well as the islet marker, insulin. Lipase and trypsinogen proteins were expressed in the vast majority of AMY− cells, confirming their acinar origin (Fig. 3).
Figure 2.
Representative images of pancreatic tissue sections stained for exocrine enzymes, extracellular matrix proteins, and endocrine hormones. A and B: Pancreas tissue sections were stained by IHC for amylase, insulin, and laminin 1/2 (LM1-LM2) (A) or e-cadherin (CDH1) (B). Both laminin 1/2 and e-cadherin were evident in AMY− acinar cell clusters, indicating intact cell membranes. There were no histological differences between peri-islet AMY− clusters (A and B, upper panels) and tele-islet AMY− clusters (A and B, lower panels). C: Additional sections were stained using IF for amylase, insulin, glucagon, and DAPI, showing peri-islet AMY− regions. D: In a consecutive slide, the same peri-islet region had negative amylase mRNA expression by in situ hybridization (ISH). These findings confirm the absence of amylase protein and mRNA in these clusters. Scale bars: 300 μm, with insets 100 μm (A and B), and 50 μm (C and D).
Figure 3.
IF staining of peri- and tele-islet AMY− acinar cell clusters in human pancreas. A: Representative images of peri- and tele-islet AMY− cell clusters are shown for nPOD donors 6098 (upper panel), 6234 (middle panel), and 6162 (lower panel). The expression of pancreatic digestive enzymes lipase (PNLIP) (B) and trypsinogen (PRSS1) (C) was evident in the AMY− areas (merged image, D). Scale bars: 50 μm.
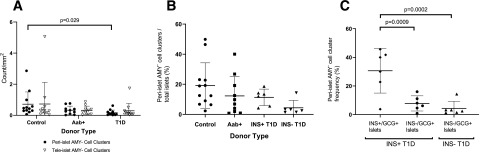
We then questioned whether AMY− cell cluster abundance and peri- versus tele-islet distribution might be altered in pancreata of those with type 1 diabetes. Therefore, we examined the pancreas of 12 donors with type 1 diabetes, 10 Aab+ donors without diabetes, and 12 age- and sex-matched control donors for amylase, insulin, glucagon, and CK19. The proportion of peri-islet AMY− cell clusters normalized against total AMY− cell clusters (mean ± SD 44.73 ± 26.26 vs. 65.49 ± 20.32, P = 0.009 [Supplementary Fig. 2A]) as well as density of peri-islet AMY− cell clusters per tissue area (0.16 ± 0.18 vs. 0.71 ± 0.79, P = 0.029 [Fig. 4A]) were significantly decreased in donors with type 1 diabetes versus control donors. We did not, however, observe significant differences in the density of tele-islet AMY− cell clusters among Aab+, type 1 diabetes, and control groups (Fig. 4A). Because donors with type 1 diabetes have fewer islets than donors without diabetes and Aab+ donors, we compared the frequency of peri-islet AMY− cell clusters normalized against total number of islets. The percentages of AMY− peri-islet clusters were decreased in patients with type 1 diabetes without residual insulin–containing islets (residual ICIs) versus donors without diabetes (Fig. 4B). To analyze this further, the percentages of peri-islet AMY− cell clusters associated with insulin+/glucagon+ islets, as well as peri-islet AMY− cell clusters associated with insulin−/glucagon+ islets, were calculated. As shown in Fig. 4C, the frequency of peri-islet AMY− cell clusters associated with insulin+ islets was four times higher (P = 0.0009) than that of peri-islet AMY− cell clusters associated with insulin− islets in donors with type 1 diabetes with residual ICIs and sevenfold higher (P = 0.0002) than in insulin− subjects with type 1 diabetes.
Figure 4.
Quantitative results from whole-tissue sections analyzed for peri- and tele-islet AMY− cell clusters, insulin+/glucagon− islets, and insulin−/glucagon+ islets for control and Aab+ donors and donors with type 1 diabetes. A: The densities of both peri- and tele-islet AMY− clusters are shown. Donors with type 1 diabetes had a significantly lower density of peri-islet AMY− clusters than control donors without diabetes (P = 0.029). B: With expression as a percentage of total islets per section, donors with type 1 diabetes without residual ICIs (INS− T1D) had a smaller proportion of peri-islet AMY− cell clusters than control donors (4.3 ± 4.9% vs. 19.1 ± 15.1%), which trended toward significance (P = 0.084). C: A higher proportion of peri-islet AMY− clusters were found in association with insulin+/glucagon+ (Ins+/Gcg+) islets in donors with type 1 diabetes with residual ICIs (INS+T1D) than in association with insulin− (Ins−/Gcg+) islets in both INS+ and INS− donors with type 1 diabetes. A–C: Data points represent whole tissue sections for individual donors. Data are presented as mean ± SD and analyzed by one-way ANOVA with Tukey post hoc test for multiple comparisons.
Discussion
The exocrine pancreas has historically been thought to be homogeneous in function, supported by the relatively uniform structural organization of acini throughout the organ alongside the capacity for a single cell type to produce all four digestive enzymes (i.e., trypsin, chymotrypsin, amylase, and lipase) (13). This notion was, however, challenged in the 1980s–1990s by studies establishing that acinar cells or whole acini may differ in terms of their enzyme production and secretory response to immediate digestive requirements (14,15), potentially depending on innervation or gut hormones (16,17). Moreover, acinar cells differ in their response to certain secretagogues (18,19), which is particularly relevant to the exocrine-endocrine cell interactions occurring within the islet-acinar axis (20,21). However, the majority of these findings originated from studies on animal models due to scarcity of available human pancreatic tissue. Hence, very little is known, on a morphological level, regarding expression patterns for the major digestive enzymes in human pancreas.
Herein, we describe pancreatic amylase expression patterns throughout the human life span from individuals with and individuals without type 1 diabetes, representing what we believe to be the largest cohort and most extensive histological analysis of exocrine human pancreas reported to date. These efforts agreed with a previous report that acinar cells expressing amylase are sparse and scattered in the first few months of life (12). However, we expanded on these findings in two key ways. First, AMY+ cells accumulate during early life development with the majority of acinar cells expressing amylase by age 2 years, which is then maintained throughout the life span. Second and most significantly, we identified peri- and tele-islet AMY− cell clusters (directly associated with islets and without islet contact, respectively) that lack amylase protein and mRNA expression. As an indication of cell viability and the uniqueness of AMY− cell clusters, the vast majority of cells in these clusters continue to express the enzymes lipase and trypsinogen. Additionally, these cells are negative for insulin and glucagon, altogether supporting their exocrine lineage. After conducting an extensive literature search, we were unable to identify any reports involving pancreata, from humans or other species, having similar morphological findings. Indeed, we did not observe AMY− cell clusters in mouse, porcine, or Rhesus monkey pancreas samples.
Given the proximity of many AMY− cell clusters to the islets of Langerhans and taking into account previous findings from animal models that insulin augments both basal and secretagogue-stimulated amylase secretion in vivo and in vitro (22,23), we investigated whether peri- and tele-islet AMY− cell clusters have an altered distribution or frequency in the pancreata of “pre-diabetic” (i.e., Aab+) subjects as well as subjects with type 1 diabetes. The proportion and density of peri-islet AMY− clusters appeared to decrease with type 1 diabetes progression. It has been well-documented that the type 1 diabetes pancreas has significantly lower islet density than pancreas of control and Aab+ donors (24). Thus, we compared peri-islet AMY− cell cluster frequency normalized against total islet number. While our studies were not sufficiently powered to detect significant differences in the Aab+ group (due to rare case availability), the percentage of peri-islet AMY− cell clusters was reduced in tissues from type 1 diabetes donors, particularly in those donors completely lacking residual ICIs, as compared with control donors. We observed ∼75% decrease in the number of peri-islet AMY− cell clusters associated with insulin− versus AMY− cell clusters associated with insulin+ islets, potentially implying a role for insulin in amylase expression. This is consistent with rodent studies demonstrating insulin-dependent amylase gene expression via signaling along an islet-exocrine axis (23). The existence of this axis is anatomically supported by an islet-acinar portal blood system (25) and innervation network (26). Alternatively, the presence of AMY− cell clusters could be a consequence of a lack in production of incretin hormones in the pancreas of organ donors sustained by total parenteral nutrition as opposed to oral feeding. Consistent with this notion are similar observations in the biopsy samples obtained from the patients in the fasting state at the time of pancreatic surgery (27).
Recent findings in patients with type 1 diabetes and Aab+ subjects indicate smaller relative pancreas weight and volume (3–7) and lower serum trypsinogen levels (10), perhaps even prior to diagnosis. Taken together, our current observations regarding amylase expression patterns strongly implicate the interaction between endocrine and exocrine cells and alterations in the exocrine compartment in human type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Rhonda Bacher (University of Florida, Gainesville, FL) and John Kaddis (Diabetes and Metabolism Research Institute, City of Hope/Beckman Research Institute, Duarte, CA) for assistance with statistical analysis.
Funding. Research reported in this publication was supported by nPOD (RRID-SCR_014541), a collaborative type 1 diabetes research project sponsored by the JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (grant 2018PG-T1D053). Organ procurement organizations partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/, by the University of Florida CTSI, which is supported in part by the National Institutes of Health (NIH), National Center for Advancing Translational Science, under award number UL1TR001427.
The content is solely the responsibility of the authors and does not necessary represent the official view of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. I.K. researched the data and wrote the manuscript. M.B., H.H., M.P., and S.S. researched the data and reviewed and edited the manuscript. A.P., H.S.N., M.C.-T., D.A.S., and M.J.H. contributed to discussion and reviewed and edited the manuscript. C.H.W. and M.A.A. conceived of the study and reviewed and edited the manuscript. M.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0554/-/DC1.
References
- 1.Hart P, Conwell D. Secretion of the human exocrine pancreas in health and disease. Pancreapedia. Pancreapedia: Exocrine Pancreas Knowledge Base, 2017 [Google Scholar]
- 2.Mann E, Bellin M. Secretion of insulin in response to diet and hormones. Pancreapedia: Exocrine Pancreas Knowledge Base, 2016. [Google Scholar]
- 3.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 4.Williams AJ, Thrower SL, Sequeiros IM, et al. . Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 5.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. . The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virostko J, Williams J, Hilmes M, et al. . Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care 2019;42:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell-Thompson ML, Filipp SL, Grajo JR, et al. . Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care 2019;42:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandre-Heymann L, Mallone R, Boitard C, Scharfmann R, Larger E. Structure and function of the exocrine pancreas in patients with type 1 diabetes. Rev Endocr Metab Disord 2019;20:129–149 [DOI] [PubMed] [Google Scholar]
- 9.Piciucchi M, Capurso G, Archibugi L, Delle Fave MM, Capasso M, Delle Fave G. Exocrine pancreatic insufficiency in diabetic patients: prevalence, mechanisms, and treatment. Int J Endocrinol 2015;2015:595649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Campbell-Thompson M, Wasserfall CH, et al. . Serum trypsinogen levels in type 1 diabetes. Diabetes Care 2017;40:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugliese A, Yang M, Kusmarteva I, et al. . The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes 2014;15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riopel M, Li J, Fellows GF, Goodyer CG, Wang R. Ultrastructural and immunohistochemical analysis of the 8-20 week human fetal pancreas. Islets 2014;6:e982949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelson JW, Miller PE. Heterogeneity of the exocrine pancreas. Am J Physiol 1989;256:G817–G825 [DOI] [PubMed] [Google Scholar]
- 14.Beaudoin AR, Vachereau A, St-Jean P. Evidence that amylase is released from two distinct pools of secretory proteins in the pancreas. Biochim Biophys Acta 1983;757:302–305 [DOI] [PubMed] [Google Scholar]
- 15.Adelson JW, Clarizio R, Coutu JA. Pancreatic digestive enzyme secretion in the rabbit: rapid cyclic variations in enzyme composition. Proc Natl Acad Sci U S A 1995;92:2553–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreto SG, Woods CM, Carati CJ, et al. . Galanin inhibits caerulein-stimulated pancreatic amylase secretion via cholinergic nerves and insulin. Am J Physiol Gastrointest Liver Physiol 2009;297:G333–G339 [DOI] [PubMed] [Google Scholar]
- 17.Coutu J, Adelson J. Heterogeneous distribution of digestive enzymes in rat pancreas is highly influenced by diet (Abstract). Gastroenterology 1998;114:A872 [Google Scholar]
- 18.Gingras D, Bendayan M. Differences in secretory granule content in pancreatic acinar cells from peri-insular and tele-insular regions. Pancreas 1992;7:477–485 [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Kim K, Lee H, Song C, Ryu H, Hyun J. Potentiation of cholecystokinin and secretin-induced pancreatic exocrine secretion by endogenous insulin in humans. Pancreas 1999;18:410–414 [DOI] [PubMed] [Google Scholar]
- 20.Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 1985;34:980–986 [DOI] [PubMed] [Google Scholar]
- 21.Barreto SG, Carati CJ, Toouli J, Saccone GT. The islet-acinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol 2010;299:G10–G22 [DOI] [PubMed] [Google Scholar]
- 22.Kim SK, Cuzzort LM, Allen ED. Effects of age on diabetes- and insulin-induced changes in pancreatic levels of alpha-amylase and its mRNA. Mech Ageing Dev 1991;58:151–161 [DOI] [PubMed] [Google Scholar]
- 23.Korc M, Owerbach D, Quinto C, Rutter WJ. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science 1981;213:351–353 [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami T, Fujita T, Taguchi T, Nonaka Y, Orita K. The blood vascular bed of the human pancreas, with special reference to the insulo-acinar portal system. Scanning electron microscopy of corrosion casts. Arch Histol Cytol 1992;55:381–395 [DOI] [PubMed] [Google Scholar]
- 26.Babic T, Travagli RA. Neural control of the pancreas. Pancreapedia: Exocrine Pancreas Knowledge Base, 2016 [Google Scholar]
- 27.Weimann A, Braga M, Carli F, et al. . ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017;36:623–650 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.