Abstract
A unique O-mannose-linked glycan on the transmembrane protein dystroglycan binds a number of extracellular matrix proteins containing laminin G-like (LG) domains. The dystroglycan-matrix interaction is essential for muscle function: disrupted biosynthesis of the matrix-binding modification causes several forms of muscular dystrophy. The complete chemical structure of this modification has been deciphered in the past few years. We now know that LG domains bind to a glycosaminoglycan-like polysaccharide of [-3GlcAβ1,3Xylα1-] units, termed matriglycan, that is attached to a highly unusual heptasaccharide linker. X-ray crystallography has revealed the principles of Ca2+-dependent matriglycan binding by LG domains. In this review, the new structural insights are applied to the growing number of LG domain-containing proteins that bind dystroglycan. It is proposed that LG domains be recognised as “D-type” lectins to indicate their conserved function in dystroglycan binding.
Introduction
The proper functioning of skeletal muscle depends on mechanical linkage of the cytoskeleton of muscle cells to the extracellular matrix surrounding the cells. Disruption of this linkage underlies a group of inherited diseases characterised by progressive weakening and degeneration of skeletal muscle, termed muscular dystrophies [1]. The most common form is Duchenne muscular dystrophy, which results from mutations in the cytoplasmic protein dystrophin. A series of seminal studies by Kevin Campbell’s laboratory in the early 1990s identified the dystrophin-glycoprotein complex, which spans the muscle plasma membrane and provides the necessary linkage to the extracellular matrix [2]. The matrix-binding component was revealed to be dystroglycan (DG), which consists of a heavily glycosylated extracellular α subunit (α-DG) and a membrane-spanning β subunit; the two subunits are derived from a single gene product by post-translational cleavage. An anionic O-linked glycan on α-DG appeared to be mediating the interaction with the matrix protein laminin, but all of the plausible candidates (glycosaminoglycans, sialylated glycans) were eventually ruled out. Deciphering the chemical structure and biosynthesis of the laminin-binding carbohydrate modification of α-DG took nearly 25 years and was full of surprises. The problem was eventually solved by a combination of human genetics and mass spectrometry, with a good helping of traditional biochemistry. As these discoveries are described in detail in a number of recent reviews [3–6], only a brief outline will be given here. My review instead focuses on the recent advances in understanding laminin binding to α-DG.
Biosynthesis of α-DG’s laminin-binding modification
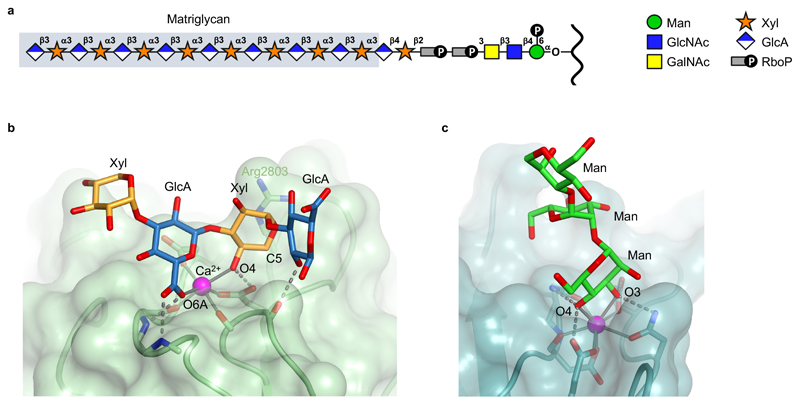
α-DG consists of two globular domains separated by a mucin-like region containing many serines and threonines. The N- and C-terminal halves of the mucin-like region are modified by O-Man and O-GalNAc cores, respectively, with a total of ~40 O-linked glycans identified in glycoproteomic studies [7–11]. Out of this number, only a few O-Man cores (Thr317, Thr319 and Thr379 in human α-DG) are extended to the laminin-binding modification (Figure 1A) [12,13]. O-mannosylation of α-DG is carried out by a complex of POMT1 and POMT2, which is embedded within the membrane of the endoplasmic reticulum [14]. Still within the endoplasmic reticulum, the O-Man cores destined to become the laminin-binding modification are extended by POMGNT2 and B3GALNT2 to form the so-called core M3 structure, GalNAcβ1-3GlcNAcβ1-4Man-O-Thr [13], which is then phosphorylated by POMK at position 6 of the mannose [15]. The remaining O-Man sites are elaborated to different structures, which are indirectly required for further synthesis of the laminin-binding modification [16,17•]. The Golgi-resident enzymes fukutin (FKTN) and fukutin-related protein (FKRP) now add a tandem ribitol-5-phosphate (RboP) moiety onto the phospho-core M3 [18••,19••,20••,21••,22•]. The presence of RboP in a vertebrate glycan was a major surprise, as this moiety previously was only known to exist in the teichoic acids of bacterial cell walls. TMEM5 next adds a xylose to the second RboP [20••,23••], and B4GAT1 adds a glucuronic acid to the xylose [24,25]. The resulting GlcAβ1-4Xyl terminal is a substrate for the bifunctional glycosyltransferase LARGE, which builds a polysaccharide of repeating [-3GlcAβ1,3Xylα1-] units [26]. It is this polysaccharide that confers laminin binding to α-DG. As we will see, other extracellular matrix proteins also bind to the LARGE-synthesised polysaccharide. Yoshida-Moriguchi and Campbell, therefore, coined the term matriglycan for the [-3GlcAβ1,3Xylα1-]n polysaccharide on α-DG [3].
Figure 1.
The matrix-binding modification of α-DG and its interaction with LG domain 4 of the laminin α2 chain. (a) Schematic structure of the O-linked glycan on α-DG [3–6]. The matriglycan polysaccharide is highlighted by grey shading. (b) Crystal structure of a matriglycan oligosaccharide bound to laminin α2 LG4-5 [43••]. A GlcAβ1,3Xyl disaccharide unit straddles the Ca2+ ion in LG4 (magenta sphere). Metal ion coordination bonds and hydrogen bonds are shown as solid and dashed lines, respectively. Selected atoms are labelled (see text). (c) Ca2+-dependent mannose binding to mannose-binding protein, a member of the C-type lectin subfamily [44,45].
Altogether, the biosynthesis of the matrix-binding modification on α-DG requires ten glycosyltransferases, one kinase, at least six enzymes involved in the synthesis of sugar donors, and most likely one or several transporters [3–6,27]. Mutation of any of the genes coding for this set of proteins results in the absence of functional α-DG modification and, hence, muscular dystrophy. Because α-DG also has important functions in the nervous system, these so-called dystroglycanopathies are frequently accompanied by brain and eye abnormalities [1].
There are several intriguing parallels between α-DG’s unique matrix-binding modification and the more widespread glycosaminoglycan (GAG) modifications of proteoglycans: in both cases, a linker is synthesised first, which is then extended by a bifunctional glycosyltransferase; phosphorylation of the linker is an obligatory step; and the anionic polysaccharides serve as a binding platform for a variety of extracellular matrix proteins [28–30]. For these reasons, it would make sense to consider matriglycan as a type of GAG, even though its disaccharide repeat does not contain an amino sugar. Interestingly, there may be overlap between the two types of modification: LARGE2 (the paralogue of LARGE) is able to attach matriglycan chains to proteoglycans, most likely by extending the GAG tetrasaccharide linkers [31•].
Structural basis of laminin binding to α-DG
Laminins are αβγ heterotrimers that polymerise on the extracellular face of the plasma membrane to form a cell-associated extracellular matrix, the basement membrane (BM) [32]. Mammalian genomes encode five laminin α chains, three β chains, and three γ chains, which assemble into 15 distinct laminin heterotrimers. The groundbreaking biochemical experiments on α-DG binding were all done using laminin-111 (α1β1γ1), which actually is not present in the BM of adult skeletal muscle; the predominant laminin isoforms in muscle contain the α2 chain [33]. Fortunately, the α-DG-binding properties of the laminin α1 and α2 chains have turned out to be quite similar.
The α-DG binding site(s) of laminins are located within the G (globular) region at the C-terminus of laminin α chains [34,35]. This region consists of five laminin G-like (LG) domains of ~200 residues each; in the laminin heterotrimer, LG1-3 forms a compact cloverleaf-shaped structure that is connected to LG4-5 through a flexible linker [36–38]. An important early observation was that laminin binding to α-DG strictly requires Ca2+ ions [39]. Intriguingly, the crystal structure of laminin α2 LG5 revealed a conserved Ca2+ binding site bound to one edge of the β-sandwich that makes up the LG fold [40]. Subsequent mutational studies established that the Ca2+ ion in LG4, and the region surrounding it, represents a major α-DG binding site in all laminin α chains [41,42]. An additional site is present in LG1-3 but has not been mapped to a single domain [35]. How LG domains recognise the carbohydrate modification of α-DG remained a mystery until recently.
The identification of LARGE as a bifunctional glucuronyl- and xylosyltransferase [26] made structural studies possible. When presented with only one type of sugar donor, LARGE will catalyse a single reaction, e.g. reacting GlcA-β-MU with UDP-Xyl will produce Xylα1-3GlcA-β-MU (MU denotes 4-methylumbelliferone). The reaction product can be purified and reacted with the other type of sugar donor, and so on. In this way, defined oligosaccharides can be obtained in sufficient quantity for X-ray crystallography and biophysical experiments. By soaking one such oligosaccharide into crystals of laminin α2 LG4-5, we were able to determine the structure of a laminin-carbohydrate complex at 1.4 Å resolution (Figure 1B) [43••].
In the structure of the complex, the carbohydrate ligand completes the octahedral coordination sphere of the Ca2+ ion in LG4. Specifically, a single GlcAβ1-3Xyl disaccharide unit chelates the Ca2+ ion, with coordination bonds formed by the carboxylate group of GlcA and the 4-OH group of Xyl. In addition, a total of four hydrogen bonds are formed between LG4 and the carbohydrate ligand, three with the Ca2+-chelating GlcA-Xyl disaccharide and one with the following GlcA. Water molecules are notably scarce at the binding site. This binding mode is similar to that employed by C-type lectins [44,45], but there is one important difference: the Ca2+ ion in C-type lectins is coordinated by a single sugar (Figure 1C), whereas the Ca2+ site in the LG4 domain is coordinated by a disaccharide. This difference likely accounts for the high specificity and affinity of the laminin-matriglycan interaction. Specificity for GlcA results from the two strong hydrogen bonds to the sugar’s carboxylate group; specificity for Xyl results from close contacts between the LG domain and the C5 atom of Xyl, which discriminate against hexoses; and specificity for the β1-3 glycosidic linkage is dictated by the Ca2+ coordination geometry. NMR titration of a GlcA-Xyl-GlcA-Xyl-GlcA-MU pentasaccharide with laminin α2 LG4-5 gave a dissociation constant of 230 nM [43••]. By comparison, solid-phase binding assays with full-length laminin and native dystroglycan typically give dissociation constants of 1-10 nM [46]. The tighter binding likely results from the presence of two independent matriglycan binding sites in laminin (LG1-3 and LG4-5) and the many GlcA-Xyl repeats in native matriglycan, i.e. from an avidity effect.
Laminin LG domains also bind other anionic carbohydrate ligands, namely heparan sulphate/heparin and sulphated glycolipids [32]. Heparin inhibits α-DG binding to laminin-111, but not to laminin-211 [47]. Importantly, however, heparin binding does not require Ca2+ [48]. Results from site-directed mutagenesis suggest that basic residues in laminin α1 LG4-5 and α2 LG4-5 are important for both heparin and matriglycan binding [41,42]. In our crystal structure of laminin α2 LG4-5 with a matriglycan oligosaccharide only Arg2803 interacts directly with the ligand [43••]. The role of the other basic residues may be to reduce the electrostatic repulsion between laminin and the polyanionic matriglycan chain.
A couple of anomalies remain in our current understanding of the laminin-matriglycan. First, it is not clear why certain Ca2+-binding LG domains do not seem to bind α-DG (e.g. α1 LG5 [35]). Second, α5 LG4 binds α-DG Ca2+-dependently [49], yet neither of the two aspartic acid residues that coordinate the Ca2+ ion in α1 LG4 and α2 LG4 are conserved in α5 LG4. Clearly, further structure-function studies are needed.
Other α-DG-binding proteins with LG domains
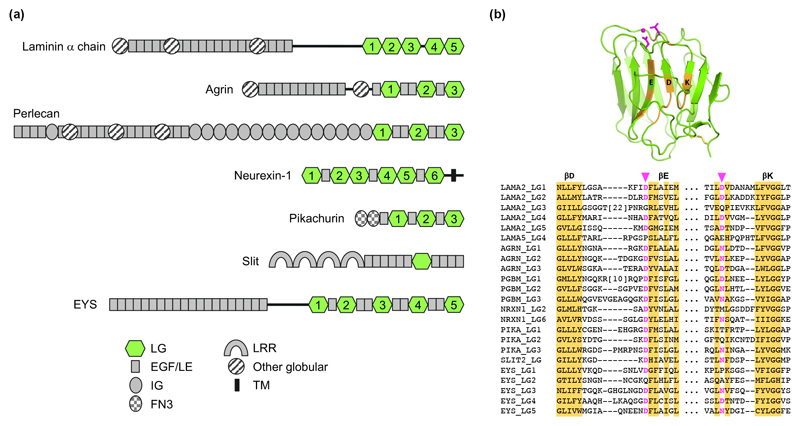
Apart from laminins, LG domains are present in a number of large multidomain proteins that have been shown to bind to α-DG (Figure 2A). These include: agrin and perlecan, the two major BM proteoglycans; neurexin, a synaptic transmembrane protein; pikachurin, a matrix protein located in the photoreceptor ribbon synapse; and Slit2, a matrix protein important for axon guidance. In all these cases, Ca2+ is essential for α-DG binding and suitable Ca2+ ligands are present in at least one of the LG domains (Figure 2B). These Ca2+ sites are predicted to bind matriglycan in the same way as in laminin α2 LG4 [43••].
Figure 2.
LG domain-containing proteins that bind α-DG. (a) Domain organisation in α-DG-binding proteins. The LG domains are highlighted in green. Note that α-DG binding by EYS has not been demonstrated; the protein is included as a likely candidate (see text). (b) Conservation of the Ca2+-binding site in many LG domains. The structure of laminin α2 LG4 is shown at the top (PDB 5IK5; magenta, Ca2+ ligands; orange, conserved residues). Below, a partial sequence alignment of the conserved regions is shown. Putative Ca2+-binding residues are highlighted in magenta. LAMA2, human laminin α2 chain; AGRN, human agrin; PGBM, human perlecan; NRXN1, human neurexin 1; PIKA, human pikachurin; SLIT2, human Slit2; EYS, human EYS.
Binding of agrin to α-DG was discovered independently by four laboratories in 1994 [50]. As for laminins, more than one LG domain of agrin is involved in α-DG binding, but additional complexity is introduced by alternative splicing of agrin’s LG domains. A splice insert in LG2 is required for heparin binding by agrin, and a splice insert in LG3 is required for the acetylcholine receptor-clustering activity of neuronal agrin. Both of these inserts reduce the affinity for α-DG, in agreement with the location of the splice sites close to the conserved Ca2+ sites in LG2 and LG3 [51,52]. Binding of perlecan α-DG also seems to involve more than one LG domain, but no detailed studies have been carried out [35].
Binding of neurexin to α-DG was reported in 2001 [53], but the physiological significance of the interaction is still unclear. The α-DG binding sites in LG2 and LG6 of neurexin-1 have been probed by site-directed mutagenesis and map to the immediate surroundings of the conserved Ca2+ sites [54]. Interestingly, the Ca2+ coordination in LG2 involves only one protein side chain instead of the usual two [55]. As for agrin, alternative splicing of neurexin LG domains has a negative impact on α-DG binding [54].
The interaction of pikachurin with α-DG is required for the proper synaptic connection between retinal photoreceptor and bipolar cells [56]. Dissection of pikachurin’s LG region revealed that no single LG domain is sufficient for α-DG binding and that the LG2-EGF-EGF-LG3 fragment fully retains the α-DG binding activity of pikachurin [57].
The interaction of Slit2 with α-DG was discovered in a genetic screen in mice [58], which revealed axon guidance defects resulting from mutations in B3gnt1 (now known to code for the glucuronyltransferase B4GAT1 [24,25]), ISPD (now known to code for the enzyme that synthesises the CDP-ribitol used by FKTN and FKRP [18••,19••,20••,59]), and dystroglycan itself. These defects resembled those of Slit/Robo mutants, and biochemical experiments indeed established a direct interaction of Slit2 with α-DG [58]. Slit2 is unique among α-DG binders in that it contains only a single LG domain.
Considering these examples, I would argue that the presence of multiple LG domains in a secreted protein is sufficient evidence to warrant an examination of α-DG binding. An intriguing candidate in this regard is EYS, a large extracellular matrix protein located in the connecting cilium and outer segment of retinal photoreceptor cells [60,61]. EYS contains five LG domains, the last three of which are predicted to have functional Ca2+ binding sites (Figure 2B). Mutations in EYS are a common cause of autosomal recessive retinitis pigmentosa [62–64]. Over 100 unique missense variants have been detected, two of which affect a putative Ca2+ ligand in LG4 (Asp2746). Functional studies in vertebrates are complicated by the lack of a functional EYS gene in mice, but EYS function can be studied in zebrafish, which have a functional O-mannosyl/matriglycan system [60,61,65].
Cooperativity in α-DG binding
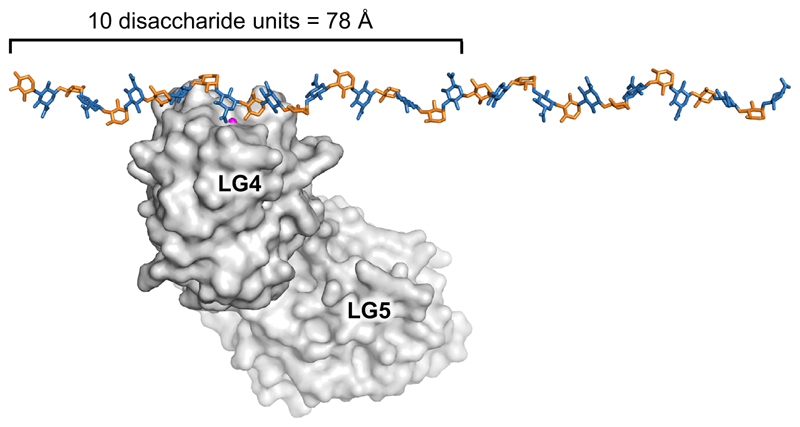
With the exception of Slit2 (and possibly the laminin α5 chain), proteins use multiple LG domains to bind α-DG. The length of the matriglycan chains in vivo is not known, but may exceed 100 disaccharide repeats [66]. By repeating the glycosidic angles observed in the oligosaccharide bound to laminin α2 LG4-5 [43••], the matriglycan chain can be modelled as a helix with a rotation of 107° and a translation of 7.8 Å per GlcA-Xyl disaccharide. This model does not take into account any flexibility, of course, but is nevertheless useful to appreciate the relative dimensions of protein and carbohydrate (Figure 3). A single LG domain will block access to no more than ten disaccharide units, allowing for multiple binding events on a matriglycan chain. The simultaneous binding of several extracellular matrix proteins to a single maytriglycan chain may be important for a compact BM structure [66].
Figure 3.
Relative dimensions of LG domains and the matriglycan polysaccharide. The matriglycan chain was modelled by repeating the tetrasaccharide observed in the crystal structure with laminin α2 LG4-5 [43••]. This procedure gives a helix with a rotation of 107° and a translation of 7.8 Å per GlcA-Xyl disaccharide.
Concluding remarks
The last few years have seen a remarkable confluence of genetic, biochemical and structural studies, which finally have demystified the matrix-binding modification of α-DG. Given the increasing number of LG domain-containing proteins that bind to this modification, it seems timely to award the LG domain its own class in the lectin family. The LG domain fulfils all of the necessary criteria: evolutionary relationship, conserved structure of the carbohydrate binding site, conserved function in different proteins, ligand specificity, and so on. I would like to propose the term “D-type lectins”: D for dystroglycan, of course, but also for Dave (Briggs), whose crystallographic skills were instrumental in unveiling their mode of carbohydrate binding.
What remains to be done? An obvious question is whether the twenty-odd gene products required to make the matrix-binding modification of α-DG are also used to modify other proteins. Is Nature really so wasteful as to use them only for α-DG? It is also important to stress that the recent ground-breaking studies all used recombinant α-DG fragments. The complete structure of the matrix-binding modification on tissue-derived α-DG still needs to be confirmed and may well throw up another few surprises.
Highlights.
The chemical structure of dystroglycan’s laminin-binding modification is now known
Laminin G-like domains bind to a polysaccharide of glucuronic acid-xylose units
Crystal structure analysis has revealed the atomic details of this interaction
Acknowledgments
Research in the author’s laboratory is supported by a Wellcome Trust Senior Investigator Award (101748/Z/13/Z).
Footnotes
Conflict of interest
The author declares no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- [1].Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- [2].Campbell KP. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- [3].Yoshida-Moriguchi T, Campbell KP. Matriglycan: A novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology. 2015;25:702–713. doi: 10.1093/glycob/cwv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manya H, Endo T. Glycosylation with ribitol-phosphate in mammals: New insights into the O-mannosyl glycan. Biochim Biophys Acta. 2017;1861:2462–2472. doi: 10.1016/j.bbagen.2017.06.024. [DOI] [PubMed] [Google Scholar]
- [5].Sheikh MO, Halmo SM, Wells L. Recent advancements in understanding mammalian O-mannosylation. Glycobiology. 2017;27:806–819. doi: 10.1093/glycob/cwx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kanagawa M, Toda T. Muscular dystrophy with ribitol-phosphate deficiency: A novel post-translational mechanism in dystroglycanopathy. J Neuromuscul Dis. 2017;4:259–267. doi: 10.3233/JND-170255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stalnaker SH, Aoki K, Lim JM, Porterfield M, Liu M, Satz JS, Buskirk S, Xiong Y, Zhang P, Campbell KP, Hu H, et al. Glycomic analyses of mouse models of congenital muscular dystrophy. J Biol Chem. 2011;286:21180–21190. doi: 10.1074/jbc.M110.203281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stalnaker SH, Hashmi S, Lim JM, Aoki K, Porterfield M, Gutierrez-Sanchez G, Wheeler J, Ervasti JM, Bergmann C, Tiemeyer M, Wells L. Site mapping and characterization of O-glycan structures on α-dystroglycan isolated from rabbit skeletal muscle. J Biol Chem. 2010;285:24882–24891. doi: 10.1074/jbc.M110.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harrison R, Hitchen PG, Panico M, Morris HR, Mekhaiel D, Pleass RJ, Dell A, Hewitt JE, Haslam SM. Glycoproteomic characterization of recombinant mouse α-dystroglycan. Glycobiology. 2012;22:662–675. doi: 10.1093/glycob/cws002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomez Toledo A, Raducu M, Cruces J, Nilsson J, Halim A, Larson G, Ruetschi U, Grahn A. O-mannose and O-N-acetyl galactosamine glycosylation of mammalian α-dystroglycan is conserved in a region-specific manner. Glycobiology. 2012;22:1413–1423. doi: 10.1093/glycob/cws109. [DOI] [PubMed] [Google Scholar]
- [11].Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci U S A. 2013;110:21018–21023. doi: 10.1073/pnas.1313446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hara Y, Kanagawa M, Kunz S, Yoshida-Moriguchi T, Satz JS, Kobayashi YM, Zhu Z, Burden SJ, Oldstone MB, Campbell KP. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc Natl Acad Sci U S A. 2011;108:17426–17431. doi: 10.1073/pnas.1114836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci U S A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshida-Moriguchi T, Willer T, Anderson ME, Venzke D, Whyte T, Muntoni F, Lee H, Nelson SF, Yu L, Campbell KP. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science. 2013;341:896–899. doi: 10.1126/science.1239951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kuwabara N, Manya H, Yamada T, Tateno H, Kanagawa M, Kobayashi K, Akasaka-Manya K, Hirose Y, Mizuno M, Ikeguchi M, Toda T, et al. Carbohydrate-binding domain of the POMGnT1 stem region modulates O-mannosylation sites of α-dystroglycan. Proc Natl Acad Sci U S A. 2016;113:9280–9285. doi: 10.1073/pnas.1525545113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Halmo SM, Singh D, Patel S, Wang S, Edlin M, Boons GJ, Moremen KW, Live D, Wells L. Protein O-linked mannose β-1,4-N-acetylglucosaminyl-transferase 2 (POMGNT2) is a gatekeeper enzyme for functional glycosylation of α-dystroglycan. J Biol Chem. 2017;292:2101–2109. doi: 10.1074/jbc.M116.764712. [• In vitro enzyme activity assays show that POMGNT1 is promiscuous for O-Man peptides, while POMGNT2 only reacts with those O-Man sites that are destined to become the matrix-binding modification of α-DG (Thr317, Thr379).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kanagawa M, Kobayashi K, Tajiri M, Manya H, Kuga A, Yamaguchi Y, Akasaka-Manya K, Furukawa JI, Mizuno M, Kawakami H, Shinohara Y, et al. Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep. 2016;14:2209–2223. doi: 10.1016/j.celrep.2016.02.017. [•• A biochemical tour de force that defines the enzyme activities of ISPD, FKTN and FKRP in the biosynthesis of α-DG's matrix-binding modification. Many of the findings are confirmed by independent studies published in the same year [19••,20••,21••].] [DOI] [PubMed] [Google Scholar]
- [19].Gerin I, Ury B, Breloy I, Bouchet-Seraphin C, Bolsee J, Halbout M, Graff J, Vertommen D, Muccioli GG, Seta N, Cuisset JM, et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto α-dystroglycan. Nat Commun. 2016;7 doi: 10.1038/ncomms11534. 11534. [•• ISPD is characterised as a CDP-ribitol pyrophosphorylase, in agreement with findings from other groups [18••,20••,59]. Purified FKTN and FKRP are shown to use CDP-ribitol to transfer RboP to α-DG, as independently shown by others [19••].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Praissman JL, Willer T, Sheikh MO, Toi A, Chitayat D, Lin YY, Lee H, Stalnaker SH, Wang S, Prabhakar PK, Nelson SF, et al. The functional O-mannose glycan on α-dystroglycan contains a phospho-ribitol primed for matriglycan addition. Elife. 2016;5:e14473. doi: 10.7554/eLife.14473. [•• Cleavage of phosphodiesters by aqueous HF treatment releases ribitol-Xyl-GlcA from α-DG, consistent with the chemical structure proposed by others [18••]. ISPD is characterised as a CDP-ribitol pyrophosphorylase, in agreement with results from other groups [18••,19••,59]. TMEM5 is shown to have xylose transferase activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yagi H, Kuo CW, Obayashi T, Ninagawa S, Khoo KH, Kato K. Direct mapping of additional modifications on phosphorylated O-glycans of α-dystroglycan by mass spectrometry analysis in conjunction with knocking out of causative genes for dystroglycanopathy. Mol Cell Proteomics. 2016;15:3424–3434. doi: 10.1074/mcp.M116.062729. [•• Mass spectrometry is used to demonstrate modification of phospho-core M3 with RboP and glycerol phosphate. Analysis of α-DG from mutant cell lines confirms the sequential incorporation of two RboP moieties by FKTN and FKRP [18••]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Imae R, Manya H, Tsumoto H, Osumi K, Tanaka T, Mizuno M, Kanagawa M, Kobayashi K, Toda T, Endo T. CDP-glycerol inhibits the synthesis of the functional O-mannosyl glycan of α-dystroglycan. J Biol Chem. 2018;293:12186–12198. doi: 10.1074/jbc.RA118.003197. [• Glycerol phosphate is shown to be a substrate for FKTN and FKRP, but its incorporation inhibits further elongation. This study is also significant because it provides, for the first time, kinetic data obtained with purified FKTN and FKRP.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Manya H, Yamaguchi Y, Kanagawa M, Kobayashi K, Tajiri M, Akasaka-Manya K, Kawakami H, Mizuno M, Wada Y, Toda T, Endo T. The muscular dystrophy gene TMEM5 encodes a ribitol β1,4-xylosyltransferase required for the functional glycosylation of dystroglycan. J Biol Chem. 2016;291:24618–24627. doi: 10.1074/jbc.M116.751917. [•• This study characterises the enzyme reaction that adds Xyl to the second RboP moiety. Because LARGE cannot extend from a β-linked Xyl, priming by B4GAT1 is required prior to extension [24,25].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Praissman JL, Live DH, Wang S, Ramiah A, Chinoy ZS, Boons GJ, Moremen KW, Wells L. B4GAT1 is the priming enzyme for the LARGE-dependent functional glycosylation of α-dystroglycan. Elife. 2014;3 doi: 10.7554/eLife.03943. 03943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Willer T, Inamori K, Venzke D, Harvey C, Morgensen G, Hara Y, Beltran Valero de Bernabe D, Yu L, Wright KM, Campbell KP. The glucuronyltransferase B4GAT1 is required for initiation of LARGE-mediated α-dystroglycan functional glycosylation. Elife. 2014;3:e03941. doi: 10.7554/eLife.03941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jae LT, Raaben M, Riemersma M, van Beusekom E, Blomen VA, Velds A, Kerkhoven RM, Carette JE, Topaloglu H, Meinecke P, Wessels MW, et al. Deciphering the glycosylome of dystroglycanopathies using haploid screens for Lassa virus entry. Science. 2013;340:479–483. doi: 10.1126/science.1233675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kreuger J, Kjellen L. Heparan sulfate biosynthesis: Regulation and variability. J Histochem Cytochem. 2012;60:898–907. doi: 10.1369/0022155412464972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [30].Wen J, Xiao J, Rahdar M, Choudhury BP, Cui J, Taylor GS, Esko JD, Dixon JE. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis. Proc Natl Acad Sci U S A. 2014;111:15723–15728. doi: 10.1073/pnas.1417993111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Inamori KI, Beedle AM, de Bernabe DB, Wright ME, Campbell KP. LARGE2-dependent glycosylation confers laminin-binding ability on proteoglycans. Glycobiology. 2016;26:1284–1296. doi: 10.1093/glycob/cww075. [• Over-expression of LARGE2, but not of LARGE, is shown to attach matriglycan chains to glypican-4 and other proteoglycans. LARGE2 most likely extends from the β1,3-linked GlcA in the tetrasaccharide linker of glycosaminoglycans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr. 2013;7:56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gullberg D, Tiger CF, Velling T. Laminins during muscle development and in muscular dystrophies. Cell Mol Life Sci. 1999;56:442–460. doi: 10.1007/PL00000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993;268:14972–14980. [PubMed] [Google Scholar]
- [35].Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pulido D, Hussain SA, Hohenester E. Crystal structure of the heterotrimeric integrin-binding region of laminin-111. Structure. 2017;25:530–535. doi: 10.1016/j.str.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tisi D, Talts JF, Timpl R, Hohenester E. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 2000;19:1432–1440. doi: 10.1093/emboj/19.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Takizawa M, Arimori T, Taniguchi Y, Kitago Y, Yamashita E, Takagi J, Sekiguchi K. Mechanistic basis for the recognition of laminin-511 by α6β1 integrin. Sci Adv. 2017;3:e1701497. doi: 10.1126/sciadv.1701497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol Cell. 1999;4:783–792. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- [41].Harrison D, Hussain SA, Combs AC, Ervasti JM, Yurchenco PD, Hohenester E. Crystal structure and cell surface anchorage sites of laminin α1LG4-5. J Biol Chem. 2007;282:11573–11581. doi: 10.1074/jbc.M610657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wizemann H, Garbe JH, Friedrich MV, Timpl R, Sasaki T, Hohenester E. Distinct requirements for heparin and α-dystroglycan binding revealed by structure-based mutagenesis of the laminin α2 LG4-LG5 domain pair. J Mol Biol. 2003;332:635–642. doi: 10.1016/s0022-2836(03)00848-9. [DOI] [PubMed] [Google Scholar]
- [43].Briggs DC, Yoshida-Moriguchi T, Zheng T, Venzke D, Anderson ME, Strazzulli A, Moracci M, Yu L, Hohenester E, Campbell KP. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat Chem Biol. 2016;12:810–814. doi: 10.1038/nchembio.2146. [•• Chemo-enzymatic synthesis of defined GlcA-Xyl oligosaccharides enables structural and biophysical studies. A high-resolution crystal structure shows that a GlcA-Xyl repeat wraps around the Ca2+ ion in the LG domain, explaining both the Ca2+-dependence and high affinity of the interaction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Drickamer K, Taylor ME. Recent insights into structures and functions of C-type lectins in the immune system. Curr Opin Struct Biol. 2015;34:26–34. doi: 10.1016/j.sbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- [46].Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- [47].Pall EA, Bolton KM, Ervasti JM. Differential heparin inhibition of skeletal muscle α-dystroglycan binding to laminins. J Biol Chem. 1996;271:3817–3821. doi: 10.1074/jbc.271.7.3817. [DOI] [PubMed] [Google Scholar]
- [48].Yurchenco PD, Cheng YS, Schittny JC. Heparin modulation of laminin polymerization. J Biol Chem. 1990;265:3981–3991. [PubMed] [Google Scholar]
- [49].Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, Li S, Wada Y, Combs AC, Ervasti JM, Sekiguchi K. Molecular dissection of the α-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279:10946–10954. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- [50].Fallon JR, Hall ZW. Building synapses: Agrin and dystroglycan stick together. Trends Neurosci. 1994;17:469–473. doi: 10.1016/0166-2236(94)90135-x. [DOI] [PubMed] [Google Scholar]
- [51].Stetefeld J, Alexandrescu AT, Maciejewski MW, Jenny M, Rathgeb-Szabo K, Schulthess T, Landwehr R, Frank S, Ruegg MA, Kammerer RA. Modulation of agrin function by alternative splicing and Ca2+ binding. Structure. 2004;12:503–515. doi: 10.1016/j.str.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [52].Scotton P, Bleckmann D, Stebler M, Sciandra F, Brancaccio A, Meier T, Stetefeld J, Ruegg MA. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J Biol Chem. 2006;281:36835–36845. doi: 10.1074/jbc.M607887200. [DOI] [PubMed] [Google Scholar]
- [53].Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reissner C, Stahn J, Breuer D, Klose M, Pohlentz G, Mormann M, Missler M. Dystroglycan binding to α-neurexin competes with neurexophilin-1 and neuroligin in the brain. J Biol Chem. 2014;289:27585–27603. doi: 10.1074/jbc.M114.595413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sheckler LR, Henry L, Sugita S, Sudhof TC, Rudenko G. Crystal structure of the second LNS/LG domain from neurexin 1α: Ca2+ binding and the effects of alternative splicing. J Biol Chem. 2006;281:22896–22905. doi: 10.1074/jbc.M603464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- [57].Kanagawa M, Omori Y, Sato S, Kobayashi K, Miyagoe-Suzuki Y, Takeda S, Endo T, Furukawa T, Toda T. Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization. J Biol Chem. 2010;285:31208–31216. doi: 10.1074/jbc.M110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron. 2012;76:931–944. doi: 10.1016/j.neuron.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Riemersma M, Froese DS, van Tol W, Engelke UF, Kopec J, van Scherpenzeel M, Ashikov A, Krojer T, von Delft F, Tessari M, Buczkowska A, et al. Human ISPD is a cytidyltransferase required for dystroglycan O-mannosylation. Chem Biol. 2015;22:1643–1652. doi: 10.1016/j.chembiol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- [60].Lu Z, Hu X, Liu F, Soares DC, Liu X, Yu S, Gao M, Han S, Qin Y, Li C, Jiang T, et al. Ablation of EYS in zebrafish causes mislocalisation of outer segment proteins, F-actin disruption and cone-rod dystrophy. Sci Rep. 2017;7 doi: 10.1038/srep46098. 46098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yu M, Liu Y, Li J, Natale BN, Cao S, Wang D, Amack JD, Hu H. Eyes shut homolog is required for maintaining the ciliary pocket and survival of photoreceptors in zebrafish. Biol Open. 2016;5:1662–1673. doi: 10.1242/bio.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Abd El-Aziz MM, Barragan I, O'Driscoll CA, Goodstadt L, Prigmore E, Borrego S, Mena M, Pieras JI, El-Ashry MF, Safieh LA, Shah A, et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–1287. doi: 10.1038/ng.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Collin RW, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, Zonneveld MN, Blokland EA, Strom TM, Hoyng CB, den Hollander AI, Cremers FP. Identification of a 2 mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 2008;83:594–603. doi: 10.1016/j.ajhg.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Messchaert M, Haer-Wigman L, Khan MI, Cremers FPM, Collin RWJ. EYS mutation update: In silico assessment of 271 reported and 26 novel variants in patients with retinitis pigmentosa. Hum Mutat. 2018;39:177–186. doi: 10.1002/humu.23371. [DOI] [PubMed] [Google Scholar]
- [65].Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna KV, Stevens CR, Partlow JN, Barry BJ, Rodriguez J, et al. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am J Hum Genet. 2012;91:541–547. doi: 10.1016/j.ajhg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Goddeeris MM, Wu B, Venzke D, Yoshida-Moriguchi T, Saito F, Matsumura K, Moore SA, Campbell KP. LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature. 2013;503:136–140. doi: 10.1038/nature12605. [DOI] [PMC free article] [PubMed] [Google Scholar]