Abstract
Purpose of review
Stem cell-derived islets are likely to be useful as a future treatment for diabetes. However, the field has been limited in the ability to generate β-like cells with both phenotypic maturation and functional glucose-stimulated insulin secretion that is similar to primary human islets. The field must also establish a reliable method of delivering the cells to patients while promoting rapid in-vivo engraftment and function. Overcoming these barriers to β cell differentiation and transplantation will be key to bring this therapy to the clinic.
Recent findings
The ability to generate stem cell-derived β-like cells capable of dynamic glucose-responsive insulin secretion, as well as β-like cells expressing key maturation genes has recently been demonstrated by several groups. Other groups have explored the potential of vascularized subcutaneous transplant sites, as well as endothelial cell co-transplant to support β cell survival and function following transplantation.
Summary
The generation of stem cell-derived islets with dynamic glucose-responsive insulin secretion has brought the field closer to clinical translation, but there is still need for improving insulin content and secretory capacity, as well as understanding the factors affecting variable consistency and heterogeneity of the islet-like clusters. Other questions remain regarding how to address safety, immunogenicity and transplantation site moving forward.
Keywords: beta cells, diabetes, insulin secretion, pancreatic islets, stem cell-derived islets, stem cells
INTRODUCTION
Stem cell-derived islets (SC-islets) are a potential alternative to pancreas and islet transplantation as a therapy for diabetes. Despite significant advances in the generation of β-like cells from human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs) over the past 10 years, only recent protocols have derived SC-islets with dynamic glucose responsive function and rapid reversal of diabetes in mice after transplantation. In this review, we highlight these recent accomplishments and discuss what the next steps are for the field, with a focus on improved phenotype and function, as well as future considerations of safety, immunogenicity and translatable strategies for transplantation.
GENERATION OF INSULIN-PRODUCING CELLS
For decades, researchers have investigated the directed differentiation of hESCs and hiPSCs to SC-islets and have published protocols demonstrating progress toward this goal. These protocols utilize step-wise exposure to growth factors to mimic or closely resemble the temporal and spatial chemical gradients that exist throughout development. A schematic representation of a selection of these protocols can be found in Fig. 1 with Tables 1 and 2 comparing the in-vitro and in-vivo outcomes [1▪▪,2▪▪,3▪,4,5▪▪].
FIGURE 1.
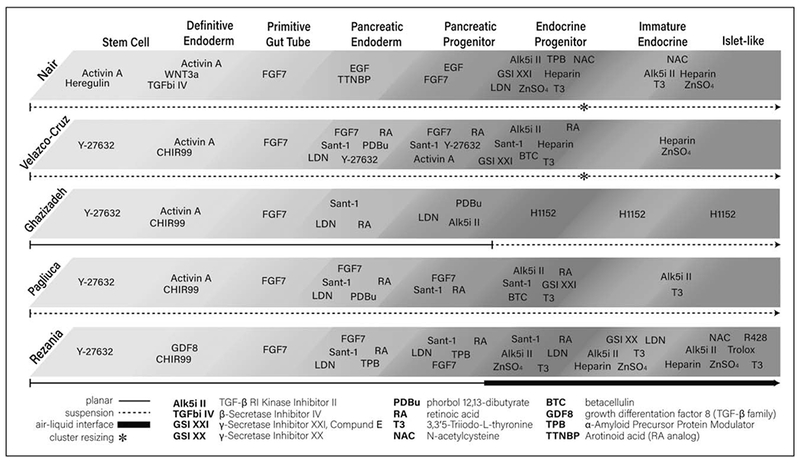
An outline of stem cell-derived islet differentiation protocols from five prominent publications. Data from [1▪▪,2▪▪,3▪,4,5▪▪].
Table 1.
Summary of the in vitro properties of stem cell-derived beta-cells from five prominent stem cell-derived beta cell publications
| Article | Main cell line(s) used | Protocol length (days) | Platform | % Ins+ cells | Differentiation efficiency | Static GSIS SI (High/low) | Dynamic GSIS SI (phase 1) | INS1 mRNA | MAFA mRNA | UCN3 mRNA |
|---|---|---|---|---|---|---|---|---|---|---|
| Nair et al. (2019) [2▪▪] | Mel1 INSGFP/W | 26–27 | Susp | 92% Ins+ | 54% C-Pep+ at d20 FACS | NR | ~ 4 | ~0.1× islets | ~0.1× islets | NR |
| Velazco-Cruz et al. (2019) [5▪▪] | HUES8 | 28–41 | Susp | 73% C-Pep+ | NR | = 3 | = 7.6 | ~0.4× islets | <0.1× islets | <0.1× islets |
| Ghazizadeh et al. (2017) [1▪▪] | HUES8; HES3; INSGFP/W | 28 | 2D - Susp | 34% C-Pep+ | NR | ~ 3 | NR | ~1.0× islets (nsd) | ~0.2× islets | ~0.8× islets (nsd) |
| Pagliuca et al. (2014) [3▪] | HUES8 | 27—34 | Susp | 53% C-Pep+ | NR | = 2.2 | NR | NR | NR | NR |
| Rezania et al. (2014) [4] | H1 | 28—41 | ALI (TW) | 55% Ins+ | 1 β-like cell/2 hESCs | ~ 1.5 | <2 | ~1.0× islets (nsd) | ~1.2× islets (nsd) | <0.1× islets |
Table 2.
Summary of the in vivo properties of stem cell-derived beta-cells posttransplant from 5 prominent stem cell-derived beta cell publications
| Article | Number of cells transplanted | Strain/diabetes model | Txp site | Earliest detected human C-Pep | Latest time point in vivo | Number of days until diabetes reversal | Average in-vivo SI (preglucose to postglucose) |
|---|---|---|---|---|---|---|---|
| Nair et al. (2019) [2▪▪] | 0.7 × 106 - 6.0 × 106 cells | NSG/STZ | KSC | 3 days | 8 months | Unclear timeline | ~5 (8 months posttxp) (5/5 mice stimulated) |
| Velazco-Cruz et al. (2019) [5▪▪] | 5 × 106 cells | SCID-beige/STZ | KSC | 10 days | 6 months | Unclear timeline | ~2 (10 weeks posttxp) (5/5 mice stimulated) |
| Ghazizadeh et al. (2017) [1▪▪] | 2 × 106 cells | SCID-beige/STZ | KSC | 5 weeks | 4.5 months | Unclear if reversal was achieved | <2 (5 weeks posttxp) (5/8 mice stimulated) |
| Pagliuca et al. (2014) [3▪] | 5 × 106 cells | NRG-Akita | KSC | 2 weeks | ~4.5 months | ~18 days | ~2 (18 weeks posttxp) (5/6 mice stimulated) |
| Rezania et al. (2014) [4] | 1.25 × 106 cells | NSG/STZ | KSC | 2 weeks | 2.5 months | ~45 days | <2 (16 weeks posttxp) (n = 10 mice) |
In 2006, D’Amour et al. published the directed differentiation toward a β-cell fate, which resulted in a population of cells, which were enriched for Chromogranin A (CHGA)-positive cells but only 7% were insulin-positive. Not unexpectedly, these cells were poorly glucose responsive [6]. Successive protocols have since improved the yield and functionality of SC-islets. In 2014, Pagliuca et al. established a scalable protocol, working toward large-scale production, which will ultimately be necessary for clinical use, resulting in efficient β-cell production with functional static glucose stimulated insulin secretion (GSIS). These SC-islets had similar gene expression profiles to primary human islets for several key β-cell genes [3▪]. In the same year, Rezania et al. [4] published their protocol describing differentiation toward an enriched insulin+ cell population. Both groups achieved approximately 50% insulin+ cells, which when transplanted into diabetic mice resulted in a gradual reduction of blood glucose to normal, nondiabetic levels over the course of several weeks [3▪,4]. However, both Rezania et al. and Pagliuca et al. reported phenotypes of the cells that still suggested immaturity, such as elevated NGN3 and low UCN3 transcript levels [4], blunted dynamic insulin secretory profiles [4], elevated proinsulin:C-Pep ratio [4], and overall significantly lower insulin secreted per cell compared to islets [3▪,4]. Moreover, total insulin content per islet equivalent was not reported [3▪,4]. Thus, protocols were able to achieve improved directed beta cell differentiation in vitro, but cells still appeared phenotypically and functionally immature compared with normal islets.
Whereas many other early protocols led to the development of primarily polyhormonal cells, such as INS+/GCG+ cells, which are poorly functional, several important studies identified a key link between enriching for NKX6.1+ progenitors during differentiation and ultimately achieving improved generation of β-like cells. Rezania et al. [7] were the first to identify this link, demonstrating improved in-vivo maturation and function following NKX6.1 enrichment prior to transplantation of stem cell-derived pancreatic endoderm cells. Nostro et al. identified key signaling pathways that contribute to NKX6.1 enrichment and determine the hormonal fate of progenitor cells, demonstrating that ineffective induction of NKX6.1 prior to endocrine hormone production led to cells that could never become monohormonal β-like cells. They also tested the differentiation of monohormonal cells from multiple hPSC lines [8]. Russ et al. [9] also studied the induction of NKX6.1 prior to the endocrine progenitor stage and found that increased early NKX6.1 expression lead to a reduced polyhormonal population in the final islet-like clusters, and more glucose-responsive β-like cells. These studies significantly advanced our understanding of the complexities of β-cell differentiation and the importance of NKX6.1 expression in PDX1+ progenitors for generating monohormonal β-like cells.
ACQUISITION OF MATURE PHENOTYPES AND FUNCTION
Three recent publications have significantly pushed the boundaries of stem cell-derived β-cell maturation. In 2017, Ghazizadeh et al. [1▪▪] published the first evidence of in-vitro expression of the maturation-associated gene, UCN3. This was accompanied with an improvement in static GSIS function, however dynamic GSIS was not studied. Velazco-Cruz et al. and Nair et al. were the first studies to show functional dynamic GSIS, approaching levels comparable with human islet function. Interestingly, these changes were not accompanied by significant improvement in expression of the genes MAFA and UCN3, thought to be associated with β-cell maturation [2▪▪,5▪▪].
In their 2017 study, Ghazizadeh et al. screened a library of 4000 chemicals and identified 5 that stimulated at least a five-fold increase in INS+ cell production from PDX1+ progenitors. The chemical with the highest efficiency was the molecule H1152, a ROCK II inhibitor. The addition of H1152 into their differentiation protocol, increased the efficiency from only 12.2% INS+ cells to 29.8% INS+ cells. Although the proportion of INS+ cells is relatively low, they found that the expression of MAFA and UCN3, which otherwise had been found to be poorly expressed, were upregulated following culture with H1152. For the first time, UCN3 mRNA expression was detectable at levels similar to primary islets. MAFA expression increased with H1152 from a nearly undetectable level to approximately five-fold lower than primary islets. Using their unique differentiation protocol with H1152 in the final stages (see Fig. 1), they were able to generate glucose-responsive cells with a three-fold stimulation index (SI) in static GSIS, and total insulin content comparable with human islets [1▪▪]. They validated their study with several stem cell lines and also added H1152 to the differentiation protocol published by Rezania et al., which resulted in SC-islets with improved static GSIS performance and UCN3 expression. The study did not assess whether H1152 improved the dynamic GSIS of the cells, which was shown to be deficient in Rezania’s initial publication. Furthermore, UCN3 is expressed in mature human α and β cells [10]; immunofluorescent staining did not conclusively or quantitatively demonstrate that the protein was present in the Ins+ cell population [1▪▪].
Key features of normal islet function in vitro are rapid first phase insulin release response to a glucose stimulus followed by a more sustained second phase release, rapid and complete turn off of insulin release in response to low glucose, and responses to other chemical secretagogues. Achieving these types of dynamic functional insulin secretory responses of SC-islets is a critical goal. Velazco-Cruz et al. [5▪▪] and Nair et al. [2▪▪] recently made significant advances toward this goal, describing strategies for improving beta-like cell function. Nair et al. implemented a unique strategy utilizing insulin-driven GFP labeling and Ins+ cell enrichment by FACS, followed by reaggregation of clusters from single cells and continuation of in-vitro culture, thereby attempting to recapitulate the in-vivo endocrine clustering process. After an additional 6–8 days of culture, this purification step resulted in highly enriched clusters (eBCs) with 99% endocrine cells (99% CHGA+), which on average had better gene expression profiles than the unsorted aggregates. The eBC clusters performed successfully in dynamic GSIS, with a stimulation of ~4 in a first phase response, but with a very weak second phase. Compared with islets, with a first phase stimulation of ~12, the cells are clearly less potent, but the total C-peptide secreted in response to depolarization (KCl) was similar, ~1.2 pg⋅min⋅ng−1 DNA for eBCs compared with ~1.8 for islets, suggesting that the potential of the eBCs is close to that of islets. Total insulin content of the eBCs was not reported, although the INS1 mRNA expression levels of the eBCs were reported and were nearly 10-fold lower than primary islets. This study is arguably the most complete metabolic study of SC-islets, with an analysis of calcium signaling as well as mitochondrial health and function. eBCs displayed similar levels of mitochondrial energization and increased oxygen consumption in response to high glucose. However, these parameters were only assessed comparing immature cells (day 20) to eBCs (days 26–27), so it is difficult to know whether these mitochondrial properties are unique to eBCs, or are also present in other SC-islets. Finally, this protocol was developed and tested solely with one cell line, dependent on the genetically engineered Ins-GFP cassette. It is difficult to know how well it would work with other nongenetically modified stem cell lines, but based on the unsorted ‘nonenriched clusters’ in the Nair et al. [2▪▪] study, the success of the protocol is completely dependent on GFP-labeled cell sorting.
Velazco-Cruz et al. [5▪▪] were able to show improved dynamic insulin secretion without cell selection and demonstrated that their protocol could be applied to multiple cell lines. Most significantly, the study convincingly demonstrates that the TGFβR1 inhibitor, Alk5 inhibitor II, has a detrimental effect in the final stage of differentiation. This is significant because Alk5i II has been included in the final stage of many of the leading differentiation protocols [2▪▪,3▪,4,5▪▪,11]. Through the use of a serum-free media, and by removing T3 and Alk5i II in the final stage of differentiation, the protocol derived from Pagliuca et al. was significantly improved based on gene expression profiles, and insulin secretion patterns. In addition to removing these two components in the final stage of differentiation, they also included a resizing step of the cell aggregates in the final stage and continued the last stage of differentiation for 9–35 days. In the end, their extended culture protocol generated cells with approximately a 10-fold increase in INS1 gene expression and two-fold higher insulin content, and more importantly displayed improved static and dynamic insulin secretion in response to high glucose compared with Pagliuca et al. The SC-islets had a static stimulation index of 3.0, whereas cadaveric islets stimulation index averaged 3.2. The stimulation index of the first phase of dynamic GSIS was 7.6 for SC-islets, which was lower than an average stimulation of 15 for primary islets. The second phase response was also lower for SC-islets. This study demonstrates an important step toward achieving dynamic β-cell function, as compared with realistic and high-functioning islet controls. Further, in-vivo data demonstrated that by 10 days after transplantation into diabetic mice, a glucose-tolerance test was similar to nontransplanted, nondiabetic controls, suggesting early function, which is believed to be critical for successful clinical translation of this therapy [5▪▪].
SC-islets generated by both Nair et al. [2▪▪] and Velazco-Cruz et al. [5▪▪] had negligible levels of MAFA and UCN3 mRNA expression [3▪,4]. Although both groups cited a 2016 study (Arda et al. [12]) revealing that human MAFA levels peak during puberty as an explanation for the low SC-islet MAFA levels, this study does not suggest that human juvenile β cells have zero MAFA expression, but rather contain about half that of adult β cells. This suggests that better MAFA expression would be expected in β cells even at the juvenile stage of development. Furthermore, the Arda et al. [12] study identified several other genes associated with juvenile to adult β cell maturation, such as SIX2, SIX3, and ONECUT2, which have not been assessed in these recent SC-islet articles.
Together, these three reports have confirmed the ability to achieve better gene expression and dynamic function than previously demonstrated in the SC-islet field. The advances presented by each of these studies have still not achieved a cell population, which recapitulates the human islet in every aspect, but each contribute an important piece of the puzzle for future investigation. It is now clear that dynamic in-vitro function can be achieved, even with expression of MAFA, UCN3 and INS1 at levels significantly lower than human islets. Though the reasons for, and implications of, these lower gene expression levels are not clear, it is evident that better insulin secretory function is associated with a more rapid restoration of normoglycemia in diabetic mouse models. A more in depth understanding of the functional and phenotypic differences in cadaver islet and islet-like clusters at the single cell and cluster level will likely pave the way towards generating even more robust function. Increasing the insulin content, MAFA and UCN3 expression may lead to further improvements in SC-islet function.
NEXT STEPS IN STEM CELL-DERIVED ISLET DIFFERENTIATION
In consideration of islet biology, there are further steps that could be taken toward creating more mature and/or more enriched islet-like cell clusters. Other important cell types exist within the islet, including α and δ cells, endothelial cells and neurons, which are known to affect islet function [13,14]. Furthermore, there is a wealth of evidence that both islet composition and architecture is important in islet development, health and function [13,15,16]. The presence of gap junctions between islet endocrine cells helps synchronize and regulate secretory function, and functionally heterogeneous subpopulations exist even with the β-cell population alone [16–18]. Many of the current protocols do not produce pure populations of INS+ cells, but also produce INS+ cells that co-express GCG+ or SST+, which are generally considered immature polyhormonal cells. That the total levels of GCG and SST mRNA levels are lower than in islet correlates with the relatively few GCG+ and SST+ monohormonal cells present [2▪▪,3▪,4,5▪▪]. Veres et al. reported single cell transcriptomics of SC-islets and demonstrated significant cell type heterogeneity within the clusters [19▪▪]. It would be important, either through a protocol that generates all three cell types, or the aggregation of β cells with stem cell-derived α and δ cells to study how this may affect gene expression profiles and islet function. Finally, adding endothelial cells or neural cell types into the mix may also provide important growth factor signals and cell-cell interactions, or may facilitate better engraftment after transplantation. Regulating extracellular matrix-mediated signals may also be critical and important at multiple levels, including differentiation of progenitors to β cells, extending islet-like cluster survival in culture and improving engraftment after transplantation [20,21,22▪,23].
In addition to obvious next steps in improving insulin production and glucose-sensing function, there are many other important considerations as the field moves forward toward a cell therapy. The aim of this article is not to delve into these deeply. One concern that has been addressed by some, but not all, SC-islet investigators is the need to develop a ‘universal’ differentiation protocol that is effective with a wide variety of cell types. Using either patient-derived iPSCs or a universal donor cell line [24▪▪,25], it will be important to develop a differentiation protocol that is not specifically optimized for use with one cell line, but is also highly efficient for multiple cell lines. Although noting a reduction in efficiency and potency, Ghazizadeh et al. [1▪▪] and Velazco-Cruz et al. [5▪▪] both tested their protocols with a variety of cell lines, including iPSCs. Most protocols published to date have not used GMP-compliant cell lines, and optimization of protocols for use with good manufacturing practice (GMC)-compliant cell lines may be necessary. It may be worthwhile for more investigators to begin doing comparative studies of several protocols to generate broad data about the performance of these protocols with various cell types, reagents and laboratory settings.
Furthermore, there has been a movement in regenerative medicine toward xeno-free cell culture systems, with concerns that xenoantigens at any stage in the differentiation may complicate translation to human transplantation. None of the leading published protocols to date have achieved xeno-free SC-islets, although suitable reagents are available and would simply require methodical testing to replace the mouse feeder cells, mouse-derived Matrigel, or bovine serum and albumin, which are currently in use. As demonstrated by Velazco-Cruz et al. [5▪▪], the move toward a serum-free final stage may be useful in this regard. Finally, few authors report differentiation efficiency (number of Ins+ cells/starting number of ESCs) of their protocols, which is important when considering scalability for the differentiation of a large bank of transplantable cells. Rezania et al. [4] reported a 50% efficiency in this regard, but without data from other protocols, it is difficult to know how they compare.
STEM CELL-DERIVED ISLET TRANSPLANT SITE
The SC-islet studies discussed thus far have had degrees of success with transplantation into diabetic mice or rats. All of these studies were able to detect human C-peptide in the blood stream, and almost all were able to reverse diabetes. However, the number of cells transplanted, timeline and method of diabetes induction, and time points measured are variable among research groups (Table 2), so it is difficult to compare the efficacy of the various SC-islet studies. Furthermore, all groups are using a kidney subcapsule (KSC) transplantation strategy, which is not currently used in clinical settings. As the SC-islet field begins clinical trials, it will be important to consider the limitations that clinical islet transplantation has faced [26,27] and develop a strategy that will be most effective for the survival and function of the cells in vivo. Intraportal islet transplantation has been the only clinically reliable site for achieving insulin independence following islet transplantation, so it offers proof-of-principle. Yet, it is clear this site is associated with significant loss of viability [26,27]. To ensure the optimal impact of SC-islets in clinical trials and onward, alternative transplantation options are being investigated. Due to an initial need to monitor stem-cell derived cells for safety purposes, a retrievable site may be desired. Subcutaneous, intramuscular or omental transplantation strategies have been proposed, but intraperitoneal sites are being tested as well. Despite easy accessibility, the subcutaneous site is notoriously slow to vascularize, leading to reduced survival. To address this issue, a number of experimental approaches are being tested to provide rapid or immediate vascular supply in the subcutaneous space. These include strategies to prevascularize the subcutaneous space [28,29,30▪,31], co-transplant endothelial cells along with β cells to induce vascularization at the time of transplant [32–34,35▪], or provide oxygen via oxygen-generating biomaterials [36,37]. A variety of promising encapsulation (macro and micro) techniques have been tested in small and large animals with promising results [38–41]. Although encapsulation materials protect the β cells from the host immune system, they by and large prevent direct vascularization. Many of these strategies have already shown success with transplanting SC-islets [30▪,38,40,41], however, further optimization will likely be required for clinical application.
CONCLUSION
In conclusion, recent advances in both SC-islet in-vitro differentiation and novel strategies for transplanting the cells into animals are major steps toward the ultimate goal of designing a reliable strategy for clinical stem-cell derived beta cell therapy. Figure 1 and Tables 1 and 2 summarize the various approaches and outcomes among some of the leading differentiation protocols. Each protocol is able to achieve a population of cells with the described β-cell phenotypes and functionality, yet none of the protocols demonstrates superior performance in all categories. As we advance toward better islet-like phenotype and function, the next steps include developing universal xeno-free protocols for broad applicability, optimizing clinically applicable transplantation platforms, evaluating innate and adaptive immune responses and designing strategies to promote immune acceptance. Though achieving these additional goals will be important to making this therapy a clinical reality, tangible recent progress in attaining better functional cells offers real promise.
KEY POINTS.
Recent evidence indicates that stem cell-derived islets are capable of dynamic insulin secretion in response to glucose.
Stem cell-derived beta cells have still not been shown to have gene expression profiles and function that are fully comparable to native beta cells.
More work is needed to address transplantation strategy, safety, and immunogenicity of these cells.
Stem cell-derived islets have a promising future as a cell therapy for diabetes.
Footnotes
Conflicts of interest
J.S.O. declares that he is scientific co-founder, is chair of the Scientific Advisory Board, is Chief Scientific Officer and has equity in Regenerative Medical Solutions, Inc. The remaining authors of this manuscript have no conflicts of interest to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.▪▪.Ghazizadeh Z, Kao DI, Amin S, et al. ROCKII inhibition promotes the maturation of human pancreatic beta-like cells. Nat Commun 2017; 8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a method for improving Ins-positive cell yield and the first to achieve expression of the maturation gene Ucn3 in stem cell-derived islets.
- 2.▪▪.Nair GG, Liu JS, Russ HA, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat Cell Biol 2019; 21:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a method for deriving beta cells with dynamic glucose-responsive insulin secretion that utilizes a GFP-based cell sorting mechanism to enrich the final population of cells to be the highest percentage of Ins+ cells reported thus far.
- 3.▪.Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article was among the first to publish the derivation of functional glucose-responsive beta cells. Along with Rezania et al. in the same year, it set the standard for β-cell differentiation moving forward.
- 4.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32:1121–1133. [DOI] [PubMed] [Google Scholar]
- 5.▪▪.Velazco-Cruz L, Song J, Maxwell KG, et al. Acquisition of dynamic function in human stem cell-derived beta cells. Stem Cell Reports 2019; 12:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article refines methods for deriving beta cells with improved, near-normal dynamic glucose-responsive insulin secretion, without requiring the use of a genetically modified stem cell line or reporter. This protocol was validated with several cell lines and suggests that the removal of several key media components in the final stages and resizing of clusters is sufficient for improving secretory function.
- 6.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24:1392–1401. [DOI] [PubMed] [Google Scholar]
- 7.Rezania A, Bruin JE, Xu J, et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 2013; 31:2432–2442. [DOI] [PubMed] [Google Scholar]
- 8.Nostro MC, Sarangi F, Yang C, et al. Efficient generation of NKX6–1þ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports 2015; 4:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015; 34:1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meulen T, Xie R, Kelly OG, et al. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS One 2012; 7:e52181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millman JR, Xie C, Van Dervort A, et al. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat Commun 2016; 7:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arda HE, Li L, Tsai J, et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human beta cell function.Cell Metab 2016; 23:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan MF, Hull RL. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia 2017; 60:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman-Francis K, Koffler J, Weinberg N, et al. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS One 2012; 7:e40741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huising MO, van der Meulen T, Huang JL, et al. The difference delta-cells make in glucose control. Physiology (Bethesda) 2018; 33:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roscioni SS, Migliorini A, Gegg M, Lickert H. Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nat Rev Endocrinol 2016; 12:695–709. [DOI] [PubMed] [Google Scholar]
- 17.Benninger RK, Head WS, Zhang M, et al. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 2011; 589:5453–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benninger RKP, Hodson DJ. New understanding of beta-cell heterogeneity and in situ islet function. Diabetes 2018; 67:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.▪▪.Veres A, Faust AL, Bushnell HL, et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature 2019; 569:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article analyzes the stem cell-derived islet transcriptome at a single cell level, and identifies populations of a variety of endocrine, enterochromaffin, endocrine progenitor and nonendocrine cell types that co-exist in the differentiated clusters.
- 20.Cleaver O Specifying the pancreatic islet through biomechanical forces. N Engl J Med 2019; 380:1281–1283. [DOI] [PubMed] [Google Scholar]
- 21.Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 2018; 61:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.▪.Mamidi A, Prawiro C, Seymour PA, et al. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 2018; 564:114–118. [DOI] [PubMed] [Google Scholar]; This article describes a mechanosignalling-based mechanism, which can induce Pdx1, Ngn3 and other crucial genes in the pathways toward beta-cell differentiation.
- 23.Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis 2018; 14:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.▪▪.Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 2019; 37:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a novel genetically modified cell line with superior immune-evading capabilities, based on the removal of MHC Class 1 and 2 molecules from the cell surface, and the overexpression of CD47.
- 25.Sackett SD, Rodriguez A, Odorico JS. The nexus of stem cell-derived beta-cells and genome engineering. Rev Diabet Stud 2017; 14:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anazawa T, Okajima H, Masui T, Uemoto S. Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg 2019; 3:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets 2018; 10:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komatsu H, Rawson J, Barriga A, et al. Posttransplant oxygen inhalation improves the outcome of subcutaneous islet transplantation: a promising clinical alternative to the conventional intrahepatic site. Am J Transplant 2018; 18:832–842. [DOI] [PubMed] [Google Scholar]
- 29.Luan NM, Iwata H. Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant 2014; 14:1533–1542. [DOI] [PubMed] [Google Scholar]
- 30.▪.Pepper AR, Bruni A, Pawlick R, et al. Post-transplant characterization of long-term functional hESC-derived pancreatic endoderm grafts. Diabetes 2019; 68:953–962. [DOI] [PubMed] [Google Scholar]; This article describes the successful transplantation of hESC-derived progenitors into a translatable subcutaneous device-less site.
- 31.Pepper AR, Gala-Lopez B, Pawlick R, et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 2015; 33:518–523. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi Y, Sekine K, Kin T, et al. Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep 2018; 23:1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlahos AE, Cober N, Sefton MV. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc Natl Acad Sci U S A 2017; 114:9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver JD, Headen DM, Aquart J, et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv 2017; 3:e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.▪.Citro A, Moser PT, Dugnani E, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 2019; 199:40–51. [DOI] [PubMed] [Google Scholar]; This article describes a novel islet culture and transplantation strategy utilizing a decellularized lung scaffold revascularized with human endothelial cells and implanted with islet cells that is capable of short-term islet function in in-vitro culture and posttransplantation.
- 36.Coronel MM, Geusz R, Stabler CL. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 2017; 129:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coronel MM, Liang JP, Li Y, Stabler CL. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 2019; 210:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alagpulinsa DA, Cao JJL, Driscoll RK, et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant 2019; 19:1930–1940. [DOI] [PubMed] [Google Scholar]
- 39.Stock PG, German MS. A path to insulin independence: ‘the end of the beginning’. Cell Stem Cell 2016; 18:431–433. [DOI] [PubMed] [Google Scholar]
- 40.Vegas AJ, Veiseh O, Doloff JC, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol 2016; 34:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]


