Abstract
Purpose:
To quantify the distribution profile of glycosaminoglycans (GAGs) in articular cartilage with the magnetic resonance imaging (MRI) gadolinium (Gd) contrast method (the dGEMRIC procedure in clinical MRI) and correlate with histochemical results.
Materials and Methods:
Fresh canine cartilage from seven humeral heads was harvested. Sixteen cartilage specimens were imaged at 13 μm pixel resolution using the μMRI T1-Gd method to generate 2D GAG maps in cartilage. Nineteen cartilage specimens from adjacent locations on the same joints were papain-digested to quantify the bulk GAG content in tissue. In addition, six cartilage specimens were microtomed into 40-μm serial sections that were parallel with the articular surface. These sections were biochemically analyzed individually to determine the depth-dependent profiles of GAG concentration.
Results:
The GAG concentrations between the μMRI measurement and the bulk biochemical method have statistically significant agreement. The depth-dependent GAG profiles from the histochemical method (40 μm depth resolution) have similar line shapes as that determined by μMRI at 13 μm resolution.
Conclusion:
The GAG concentration as measured by μMRI T1-Gd contrast method provides an accurate account of the macromolecular content in articular cartilage.
Keywords: cartilage, microscopic imaging, glycosaminoglycan, MRI, histochemistry, dGEMRIC
ARTICULAR CARTILAGE is a thin layer of connective tissue covering the ends of bones in joints. Even though the tissue is thin (typically 0.5–2 mm), articular cartilage has a unique depth-dependent structure, both biochemically (eg, concentration variations) and morphologically (eg, subtissue structural zones) (1–4). This depth-dependent organization of cartilage essentially defines its properties as measured by any technique in laboratories and hospitals. The ability to resolve the tissue’s depth-dependent structure will therefore add to the comprehension of the various mechanisms that govern the natural functions of the tissue and signal the onset of tissue degradation. In recent years many studies have documented the depth-dependent properties of articular cartilage by biochemical (2,5–8), physical and optical (9–17), and mechanical means (5,18–23).
Proteoglycans are one of the three major molecular components in articular cartilage (the other two being water and collagen) and have a bottle-brush-like structure with a central protein core and the sidechains of glycosaminoglycan (GAG). The heavily sulfated GAG molecules carry a high concentration of negative charges and are closely packed in the tissue, thereby generating an osmotic pressure that contributes to the stiffness of articular cartilage as a load-bearing material. The reduction of GAG in the tissue will result in a biochemically and biomechanically weakened cartilage, eventually leading to clinical diseases such as osteoarthritis (OA) and other joint diseases. Since early degradations of the tissue usually occur localized at certain depths (24–26), it is critically important to measure not only the bulk GAG concentrations but also the GAG profiles across the tissue depth in cartilage.
There are several approaches to obtain a depth-dependent profile of GAG in articular cartilage, mainly 1) biochemical digestion, 2) light microscopy of stained sections, and 3) magnetic resonance imaging (MRI). The biochemical approach can provide an accurate account of the total GAG content in cartilage as a bulk quantity. This destructive digestion method can also be made into a ‘low-resolution imaging’ mode if one slices a tissue block into multiple sections that are parallel with the articular surface and analyze the bulk GAG content in each thin section (1,3,5,6,20,27,28). The light microscopy approach uses thin tissue sections that are purposely stained. If the staining protocol is specific to GAG, it is possible to obtain the molecular concentration from the intensity of the stained image (6,8). There is also a new method in the light microscopy approach that uses fluorescence immunolabeling to identify the chondrocytes under compression (18,23). The MRI approach, which is totally nondestructive, is known in clinical MRI literature as the dGEMRIC procedure (delayed gadolinium enhanced magnetic resonance imag ing of cartilage). Based on the assumption that charged mobile ions will distribute in cartilage in an inverse relation to the concentration of the negatively charged GAG molecules, this MRI technique constructs the T1 images before and after the patient is injected with a charged MRI contrast agent, Gd(DTPA)2− (10,11,13–16). Since Gd3+ is a paramagnetic ion that can shorten the T1 relaxation significantly and a low quantity of Gd(DTPA)2− is usually harmless to humans, the dGEMRIC protocol has become an important clinical procedure in the detection and management of joint diseases.
This study concerns the quantitative determination of the GAG concentration profile as measured by the in vitro version of the dGEMRIC protocol, in which an intact block of tissue is soaked directly in the Gd(DTPA)2− solution between the before/after imaging experiments. The microscopic resolution GAG profiles from μMRI were correlated with the GAG contents and profiles from the histochemical analysis. We hypothesized that the MRI T1-Gd approach can provide an accurate assessment of the GAG concentration profiles in cartilage nondestructively.
MATERIALS AND METHODS
Tissue Specimens
Fresh humeral heads were harvested within 3 hours of the animal death from canines that were sacrificed for unrelated cardiovascular experiments. The dogs were ≈ 1–2 years old and musculoskeletally healthy. Rectangular blocks of tissue with full thickness of cartilage still attached to the underlying bone were cut from the central load-bearing region of humeral heads. Each specimen, which had dimensions of about 2 × 2 × 10 mm, was bathed in saline and sealed in a precision glass tube. The results in this report, which came from 41 specimens harvested from the same area of the humeral surfaces from 7 dogs, were obtained with the same parameters and conditions. Among the specimens, which were adjacent to each other, 16 were used for μMRI experiments and the rest for biochemical experiments.
Microscopic MRI (μMRI) Determination of GAG
Several series of NMR spectroscopy experiments were performed to determine the relaxivity of Gd(DTPA)2− in solutions of skim-milk powder at the concentrations of 0%–40%. As discussed in several reports in literature (13,33), these concentrations cover the approximate range of the macromolecular contents in most biological tissues (the solid content in cartilage is about 20%–35% (34,35)). R-values of 4.2 (mM sec)−1 was found in our experiment for saline at 7T and 25°C, which agrees with the relaxivity values found in literature (14,29,30,33).
The μMRI T1-GAG imaging followed the well-documented dGEMRIC procedure in the literature (10,11,29–31). Briefly summarized, each cartilage block was T1-imaged before (T1before) and after (T1after) a 10-hour immersion in 1 mM solution of a commercially available Gd(DTPA)2− contrast agent (Magnevist, Berlex, NJ) at the room temperature ([H11015]25°C). The T1 im ages were converted to the Gd(DTPA)2− concentration image of cartilage and subsequently to the GAG concentration image of cartilage (30).
The μMRI experiments were performed using a Bruker AVANCE II MRI console interfaced to a 7T/89 mm superconducting magnet, commercial microimaging accessory, and a home-built 4-mm solenoid coil. The tissue block was placed in the magnet at the magic angle, which minimizes the influence of the dipolar interactions (32). The echo time (TE) of the imaging sequence was 8.6 msec and the repetition time (TR) of the imaging experiment was 1.5 and 0.5 seconds for the before- and after-soaking experiments, respectively. The 1-mm-thick imaging slice was transversely located in the middle of the 10-mm-long specimen. The 2D in-plane pixel size was 13 μm. The measurement of 2D T1 images used the inversion-recovery pulse sequence with five inversion points (for the T1before, they were 0, 0.4, 1.1, 2.2, 4.0 sec; for the T1after, they were 0, 0.1, 0.3, 0.5, 1 sec), which allowed the calculation of T1 relaxation in the tissue through a single exponential equation on a pixel-by-pixel basis. (To save time, the repetition time TR in μMRI was less than 5T1. In this case, we used a modified T1 fitting function, Y = A(1 – B exp (-t/T1), where B is less than 2 when TR [H11021] 5T1.)
Biochemical Determination of GAG
The specimens for biochemistry experiments, which were harvested from adjacent locations on the same joints used in μMRI specimens, were analyzed in two ways, parallel section analysis and bulk analysis. For parallel sections, a fresh specimen was frozen in water and sliced into a series of 40-μm parallel sections using a cryotome (Reichert HistoStat Cryotome). These sequenced thin sections were individually dried in the oven at 70°C for 48 hours, weighed using a Cahn-2000 Micro Balance, and papain-digested in a 1:10 ratio at 67°C in an oven for 18 hours. For bulk analysis the whole-thickness fresh tissue was separated from the underlining bone and papain-digested immediately using the same approach. The digested cartilage extract was clarified by using 0.22 μm cellulose acetate filter to remove any suspended particles (eg, residuals of bone) and a Millipore filter used for histochemical analysis. The total volume of the clarified extract was measured.
Aliquot volumes of 5–100 μL from the test samples were taken for the glycosaminoglycan measurement spectrophotometrically by a modified DMMB (1,9-dimethylmethylene blue) protocol as follows. A Blyscan dye reagent (BioColor, Northern Ireland) containing an inorganic buffer with suitable surfactants and stabilizers was formulated for specific binding to GAGs. Absorbance values were measured at 656 nm using a Shimadzu UV-1700 spectrophotometer. Standard curves for the GAG analysis were generated by assaying known concentrations of Chondroitin 4-Sulfate, a GAG standard obtained from bovine trachea (BioColor). Separate standard calibration curves were generated for every individual measurement. The GAG content in the aliquot taken for analysis was then used to obtain the total GAG concentration present in the specimen and is expressed as μg/mg or mg/ml wet/dry weights of the tissue.
RESULTS
μMRI Determination of GAG
Cartilage imaging experiments using multiple concentrations of Gd(DTPA)2− solution were performed. Despite the difference in Gd(DTPA)2− concentrations (hence the difference in the T1after values), the calculated GAG concentration in cartilage is independent of the Gd(DTPA)2− concentrations. The μMRI results in this report came from 16 specimens that were soaked in 1 mM Gd(DTPA)2− solution. Figure 1 shows a set of quantitative images from one representative cartilage-bone specimen. Although there is no T1 anisotropy in normal (healthy) cartilage (32,36), the existence of the magic angle effect in cartilage due to the inevitable T2 weighting in imaging influences the signal intensity. The T1 imaging experiments were therefore carried out at the magic angle (≈55°), and the T1 images in Fig. 1a,b were rotated in the computer to the 0° orientation to simplify the data extraction. Since the paramagnetic Gd3+ ions shorten the T1 relaxation, the difference between the T1before and T1after can be used to calculate the Gd(DTPA)2− concentration in the tissue (Fig. 1c), which can subsequently be converted to the GAG concentration (Fig. 1d) via the ideal electrochemical equilibrium theory (30).
Figure 1.
T1 images of cartilage before (a) and after (b) the specimens were immersed in 1 mM Gd(DTPA)2− solution. c: The image of gadolinium concentration in cartilage. d: The image of the GAG concentration in cartilage.
Since there was no obvious topographical variation at the direction that was parallel with the articular surface within any single specimen (37), a rectangular region of interest (10 pixel width) was selected from the middle region of each tissue image. At each pixel depth (13 μm) an averaged number was obtained from the 1D array of 10 pixels. The 1D profiles of the tissue properties as a function of the tissue depth therefore facilitate the quantitative evaluation. Figure 2 shows the averaged profiles from three independent specimens. Three features were observed. First, the error bars for the T1before and T1after measurement are small, which indicates the consistency of our quantitative T1 measurement and the small GAG variation among the specimens that were from different animals. (Reproducibility measurement on the same specimen was also carried out for a number of specimens; the reproducibility in our experiment was excellent.) Second, the concentration of the Gd(DTPA)2− in the tissue decreases monotonically as the function of tissue depth. Finally, the concentration of the GAG in cartilage is approximately a linear function, increasing from the superficial zone to the radial zone.
Figure 2.
a: T1 profiles before and after the Gd(DTPA)2− immersion and (b) the concentration profiles of gadolinium and GAG in articular cartilage.
Biochemical Determination of Bulk GAG
Nineteen fresh specimens from seven animals were digested to determine the bulk GAG concentration in cartilage. Table 1 compares the GAG concentration as determined by the bulk biochemical method and the μMRI method. Despite the inevitable influence of specimen-site/subject variations, a good agreement was found between the results from these two methods, which indicates that the GAG concentration as measured by MRI method is an accurate account of the GAG content in cartilage tissue.
Table 1.
Bulk GAG Concentrations in Cartilage as Determined by the Histochemical and μMRI Methods
| GAG Concentration (mg/ml, w.w.) | Sample No. | Statistics | ||
|---|---|---|---|---|
| Summation of μMR imaging profiles | 69.1 ± 6.4 | 16 | * | |
| Biochemistry of bulk specimens | 64.9 ± 6.8 | 19 | ||
| Summation of biochemistry of 40-μm parallel sections | 36.2 ± 9.6 | 6 |
w.w. = wet weight.
The correlation between these two groups of data was analyzed using the nonparametric Wilcoxon-Mann-Whitney test in commercial software, KaleidaGraph 4.0 (Synergy Software, Reading, PA). The P-value of the test was 0.076, which indicates that there is no statistically significant difference between the medians of these two groups.
Biochemical Determination of GAG in Parallel Sections
In addition to the bulk GAG measurement, six specimens (each was adjacent to a specimen used in the bulk measurement) were microtomed into 40-μm-thick serial sections that were parallel to the articular surface, where the depth-dependent bulk values could mimic the quantitative GAG profiles from MRI. Table 1 contains the summation of the bulk GAG concentrations from all parallel sections, which is about 46% lower than the GAG concentrations from the bulk specimens from their adjacent locations. Table 2 contains the detailed biochemical data from one specimen as well as the averaged GAG concentration from all six specimens. (In the calculation of GAG concentration the water concentration in cartilage was assumed to vary from 75% near the surface to 65% near the bone, and the density of cartilage was assumed to be 1.12 g/ml (38)). Figure 3 illustrates the depth-dependent profiles of tissue’s dry weight and GAG concentration from one specimen. It is interesting to note that while the dry weight of the tissue sections is not a linear function of the tissue depth, the GAG concentration in the tissue varies approximately linearly as a function of the tissue depth (the correlation coefficient of a linear fitting is 0.964).
Table 2.
Histochemical Results From 40-μm Parallel Sections
| Depth (μm) | Details of One Specimen | GAG Concentration of Six Specimens | ||||
|---|---|---|---|---|---|---|
| Tissue Weight (μg, d.w.) | Total GAG (μg) | GAG Concentration (mg/ml) | Mean | Standard Deviation | ||
| (d.w.) | (w.w.) | (mg/ml, w.w.) | ||||
| 20 | 10 | 0.201 | 22.45 | 5.61 | 6.79 | 5.21 |
| 60 | 23 | 0.875 | 42.59 | 10.94 | 7.89 | 4.58 |
| 100 | 35 | 1.551 | 49.63 | 13.08 | 16.03 | 7.95 |
| 140 | 41 | 2.389 | 65.27 | 17.65 | 18.27 | 7.71 |
| 180 | 47 | 2.085 | 49.67 | 13.77 | 21.72 | 8.60 |
| 220 | 39 | 2.471 | 70.95 | 20.15 | 23.97 | 10.15 |
| 260 | 56 | 4.811 | 96.22 | 27.98 | 27.64 | 13.98 |
| 300 | 50 | 3.608 | 80.83 | 24.05 | 33.49 | 18.66 |
| 340 | 55 | 4.361 | 88.81 | 27.04 | 36.36 | 19.46 |
| 380 | 50 | 5.473 | 122.59 | 38.15 | 41.89 | 14.50 |
| 420 | 55 | 5.770 | 117.50 | 37.37 | 45.71 | 13.72 |
| 460 | 55 | 6.664 | 135.70 | 44.08 | 53.82 | 11.01 |
| 500 | 57 | 7.574 | 148.83 | 49.35 | 55.56 | 7.27 |
| 540 | 66 | 6.762 | 114.74 | 38.83 | 48.10 | 8.73 |
| 580 | 60 | 7.558 | 141.08 | 48.70 | 46.15 | 15.15 |
| Total | 699 | 62.15 | ||||
| Mean | 89.79 | 27.78 | ||||
d.w. = dry weight; w.w. = wet weight.
Figure 3.
The profiles of biochemical details of the 40-μm parallel sections microtomed from a single cartilage section. Although the dry weight of the tissue is not a linear function of the tissue depth, the GAG concentration varies approximately linearly.
Correlation Between the μMRI Method and Histochemical Method
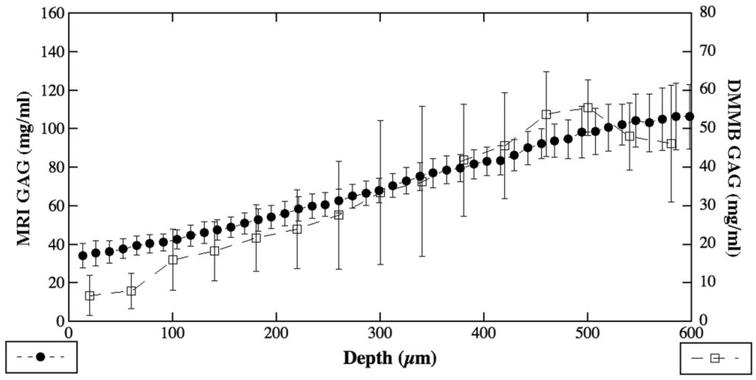
Figure 4 compares the averaged GAG profiles as determined by the μMRI method and the ‘histochemical imaging’ method using the parallel sections. As one can see, the shapes of the GAG profiles from these two methods were largely consistent with each other. However, the average measured values from the biochemical ‘imaging’ were about 46% of the corresponding values from the MRI method. Since the data from MRI is in statistical agreement with the biochemical data using the bulk specimens from the same joints, it is clear that a portion of the tissue GAG has been lost during the process of analyzing the thin parallel sections (see Discussion).
Figure 4.
The correlation of the quantitative GAG profiles from MRI and biochemistry. The difference of the total quantity could be from the additional process in preparation of the thin sections. See text for discussion.
DISCUSSION
This study demonstrates that the GAG concentration as determined by MRI T1-Gd contrast method using the cartilage-bone blocks is an accurate determination of the true GAG content in cartilage tissue (Table 1). From the available literature and the best of our knowledge, this is the first quantitative microimaging study that directly correlates the distribution profiles of GAG content in cartilage from these two distinctly different but important biomedical techniques.
Despite the fact that the biochemical data from the bulk specimens correlates well with the μMRI data, the summation of the spatially resolved GAG profiles from the biochemical method of the parallel sections, however, generates a measured ‘bulk’ value that is lower than the true bulk experiments. Since the specimens in the bulk and parallel specimens were from the adjacent locations of the same joints, it is clear that there is a GAG loss during the process of analyzing the parallel sections, which could have several possible sources.
First, several reports in the literature have documented the histochemical analysis of parallel sections in cartilage study. The thickness of the sections in these reports varies from 70–300 μm (1,3,5,6,20,27,28). Our section thickness of 40 μm is probably the thinnest section thickness ever reported in the literature. A thinner section results in a better ‘resolution’ in histochemical ‘imaging’; however, a thinner section, where each section needs to be analyzed individually, introduces a larger experimental error (a random error). Second, the bulk specimens were enzyme-digested immediately after a minimal time delay and with no additional process, while obtaining and analyzing the parallel sections requires the specimens to be frozen for embedding, with a number of additional processes. In particular, some trace amount of GAG could be measured in the solution where the specimen was immersed. The amount of the GAG lost in the immersion solution depends on several experimental factors, including the temperature and duration of the immersion period, and the type of the solution. This will introduce another experimental error in the GAG determination by histochemistry, a systematic error. Third, the method of measuring tissue’s wet weight was different between the bulk specimens and parallel sections. For bulk specimens the fresh tissue blocks were directly weighed using a microbalance. For parallel sections, getting a reliable measurement of the wet-weight of any thin section was nearly impossible with our equipment—the 40-μm thin section gets dehydrated quickly and constantly while it is being weighed. For this reason, the thin sections were dried in the oven to obtain its dry weight, which was later converted into wet weight based on the assumption of ≈70% water concentration in articular cartilage, which could introduce another systematic error. Finally, when one microtomes the parallel sections, it is often difficult to know where is the start of the tissue block’s surface, which can result in a lower dry weight for the first several sections (Table 2). In addition, any surface curvature of a tissue block further contributes to the uncertainty of the dry tissue amount for the first several sections. For these reasons, the percentage errors in the GAG concentration were highest for the first several sections and were lowest for the deepest sections. This large error near the surface region might explain the difference in the GAG quantity between the two GAG profiles in Figure 4.
Although the physical principle of this in vitro T1-Gd contrast method is identical to that of the in vivo dGEMRIC protocol, the mechanisms that transport the gadolinium ions into the tissue are quite different. In the in vitro case (this report), the small tissue blocks are soaked directly in ample volume of Gd(DTPA)2− solution where self-diffusion is probably the only mechanism responsible for the ion transportation into the tissue. In the in vivo case (dGEMRIC), the gadolinium ions are injected into either a vein or the joint cavity where a much more complicated set of mechanisms are involved in the ion transportation. There are several complications in the transportation of the gadolinium ions in the in vivo dGEMRIC procedure, such as the diffusion limitation of gadolinium ions into cartilage and the deviation of a true R-value due to the changes in the solid concentration in a lesioned tissue. These complications would result in a less accurate correlation between the gold-standard measurement (biochemistry) and the clinical imaging. The fact that the summation of the spatially resolved GAG profiles by μMRI experiments is in statistical agreement with the bulk GAG determination by the classical biochemical method offers an assurance that the physical mechanisms in the dGEMRIC method can serve as a truthful tool to quantitatively monitor the tissue’s GAG content, which is considered to be responsible for the mechanical strength of articular cartilage as a load-bearing material.
The high sensitivity to GAG content using the in vitro T1-Gd contrast method has two implications. First, since the μMRI T1-Gd method has high spatial resolution and can map the GAG concentration in cartilage with high sensitivity nondestructively, this work validates the potential of the high-resolution imaging procedure, which can be directly applied to monitoring of cartilage development, degeneration, and repair in vitro in laboratories. Second, the in vitro validation in this report lends strong indirect support to the clinical potential of the in vivo dGEMRIC procedure since the same physical mechanism is used in both environments. Although a less direct correlation could be assumed between the gold-standard measurement (biochemistry) and the clinical imaging due to the practical complication discussed above, the unique value of clinical detection of early osteoarthritic lesion relies on the visualization and comparison of cartilage in a whole joint nondestructively. Comparing a local tissue with its surrounding tissue is an equally invaluable approach that offers complementary information to the detailed knowledge of the actual GAG content in cartilage, which does vary among individual animals and humans.
In conclusion, this study demonstrated that the GAG concentration as measured by μMRI is an accurate account of the macromolecular content in articular cartilage. To the best of our knowledge, this is the first study in cartilage research that correlates directly the GAG distribution profiles between the gold standard in histochemistry and the noninvasive MRI at microscopic resolutions. By analyzing parallel sections of cartilage, this work demonstrates that the GAG concentration profile in articular cartilage varies as a function of the tissue depth approximately linearly in cartilage.
ACKNOWLEDGMENTS
Y. Xia thanks Drs. C. Les and H. Sabbah (Henry Ford Hospital, Detroit) for providing the canine joints. The authors thank Dr. Nagarajan Ramakrishnan and Mr. Farid Badar (Oakland University) for critical discussions during the course of this work, and Ms. Janelle Spann (Michigan Resonance Imaging, Rochester Hills, MI) for providing the contrast agent. We thank Mr. Farid Badar for assistance in specimen harvesting.
Contract grant sponsor: National Institutes of Health (NIH); Contract grant number: R01 AR 45172 (Y.X.).
REFERENCES
- 1.Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis 1977;36:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. Ann Rheum Dis 1977;36: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroudas A, Bayliss MT, Venn M. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis 1980;39: 514–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Mankin HJ. Articular cartilage. Part I: Tissue design and chondrocyte-matrix interactions. J Bone Joint Surg (Am) 1997;79:600–611. [Google Scholar]
- 5.Roberts S, Weightman B, Urban J, Chappell D. Mechanical and biochemical properties of human articular cartilage from the femoral head after subcapital fracture. J Bone Joint Surg Br 1986;68: 418–422. [DOI] [PubMed] [Google Scholar]
- 6.Kiviranta I, Jurvelin J, Tammi M, Saamanen AM, Helminen HJ. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry 1985;82:249–255. [DOI] [PubMed] [Google Scholar]
- 7.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res 1989;19:149–176. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann MD, Maurer AM, Berger E, Perumbuli P, Hunziker EB. Ruthenium hexaammine trichloride chemography for aggrecan mapping in cartilage is a sensitive indicator of matrix degradation. J Histochem Cytochem 2000;48:81–88. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Farquhar T, Burton-Wurster N, Lust G. Origin of cartilage laminae in MRI. J Magn Reson Imaging 1997;7:887–894. [DOI] [PubMed] [Google Scholar]
- 10.Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 1997;205:551–558. [DOI] [PubMed] [Google Scholar]
- 11.Trattnig S, Mlynarik V, Breitenseher M, et al. MRI visualization of proteoglycan depletion in articular cartilage via intravenous administration of Gd-DTPA. Magn Reson Imaging 1999;17:577–583. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage 2001;9: 393–406. [DOI] [PubMed] [Google Scholar]
- 13.Nieminen MT, Rieppo J, Silvennoinen J, et al. Spatial assessment of articular cartilage proteoglycans with Gd-DTPA-enhanced T1 imaging. Magn Reson Med 2002;48:640–648. [DOI] [PubMed] [Google Scholar]
- 14.Nieminen MT, Toyras J, Laasanen MS, Silvennoinen J, Helminen HJ, Jurvelin JS. Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech 2004;37:321–328. [DOI] [PubMed] [Google Scholar]
- 15.Samosky JT, Burstein D, Eric Grimson W, Howe R, Martin S, Gray ML. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res 2005;23:93–101. [DOI] [PubMed] [Google Scholar]
- 16.Wedig M, Bae W, Temple M, Sah R, Gray M. [GAG] profiles in “normal” human articular cartilage In: Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society (Washington, DC: ); 2005:358. [Google Scholar]
- 17.Xia Y, Ramakrishnan N, Bidthanapally A. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI). Osteoarthritis Cartilage 2007;15:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage 2001;9:561–569. [DOI] [PubMed] [Google Scholar]
- 19.Laasanen MS, Toyras J, Korhonen RK, et al. Biomechanical properties of knee articular cartilage. Biorheology 2003;40:133–140. [PubMed] [Google Scholar]
- 20.Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech 2007;40:182–190. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Zheng YP, Niu HJ, Mak AF. Extraction of mechanical properties of articular cartilage from osmotic swelling behavior monitored using high frequency ultrasound. J Biomech Eng 2007; 129:413–422. [DOI] [PubMed] [Google Scholar]
- 22.Wilson W, Huyghe JM, van Donkelaar CC. Depth-dependent compressive equilibrium properties of articular cartilage explained by its composition. Biomech Model Mechanobiol 2007;6:43–53. [DOI] [PubMed] [Google Scholar]
- 23.Choi JB, Youn I, Cao L, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech 2007;40:2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang WS, Li B, Jin LH, Ngo K, Schachar NS, Hughes GN. Collagen fibril structure of normal, aging, and osteoarthritic cartilage. J Pathol 1992;167:425–433. [DOI] [PubMed] [Google Scholar]
- 25.Buckwalter JA, Mankin HJ. Articular cartilage. Part II: Degeneration and osteoarthritis, repair, regeneration, and transplantation. J Bone Joint Surg (Am) 1997;79:612–632. [Google Scholar]
- 26.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum 2003;48:1261–1270. [DOI] [PubMed] [Google Scholar]
- 27.Venn MF. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis 1978;37:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayliss M, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartialge. Biochem J 1983; 209:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donahue KM, Burstein D, Manning WJ, Gray ML. Studies of Gd-DTPA relaxivity and proton exchange rates in tissue. Magn Reson Med 1994;32:66–76. [DOI] [PubMed] [Google Scholar]
- 30.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med 1996;36:665–673. [DOI] [PubMed] [Google Scholar]
- 31.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med 1999;41:857–865. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y Magic angle effect in MRI of articular cartilage — a review. Investig Radiol 2000;35:602–621. [DOI] [PubMed] [Google Scholar]
- 33.Stanisz GJ, Henkelman RM. Gd-DTPA relaxivity depends on on macromolecular content. Magn Reson Med 2000;44:665–667. [DOI] [PubMed] [Google Scholar]
- 34.Brocklehurst R, Bayliss MT, Maroudas A, et al. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am 1984;66:95–106. [PubMed] [Google Scholar]
- 35.Lüsse S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 2000;18:423–430. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y Relaxation anisotropy in cartilage by NMR microscopy ([H9262]MRI) at 14 [H9262]m resolution. Magn Reson Med 1998;39:941–949. [DOI] [PubMed] [Google Scholar]
- 37.Xia Y Heterogeneity of cartilage laminae in MR imaging. J Magn Reson Imaging 2000;11:686–693. [DOI] [PubMed] [Google Scholar]
- 38.Loret B, Simoes FM. Articular cartilage with intra- and extrafibrillar waters: a chemo-mechanical model. Mech Mater 2004;36: 515–541. [Google Scholar]