Abstract
In humans, relapse to unhealthy eating habits following dieting is a significant impediment to obesity treatment. Food-associated cues are one of the main triggers of relapse to unhealthy eating during self-imposed abstinence. Here we report a behavioral method examining cue-induced relapse to food seeking following punishment-induced suppression of food taking. We trained male rats to lever press for food pellets that were delivered after a 10-s conditional stimulus (CS) (appetitive). Following training, 25% of reinforced lever presses resulted in the presentation of a compound stimulus consisting of a novel CS (aversive) and the appetitive CS followed by a pellet and footshock. After punishment-imposed abstinence, we tested the rats in an extinction test where lever pressing resulted in the presentation of either the appetitive or aversive CS. We then compared activity of lateral hypothalamus (LH) and associated extrahypothalamic regions following this test. We also assessed Fos expression in LH orexin and GABA neurons. We found that cue-induced relapse of food seeking on test was higher in rats tested with the appetitive CS compared to the aversive CS. Relapse induced by the appetitive CS was associated with increased Fos expression in LH, caudal basolateral amygdala (BLA), and medial amygdala (MeA). This relapse was also associated with increased Fos expression in LH orexin and VGAT-expressing neurons. These data show that relapse to food seeking can be induced by food-associated cues after punishment-imposed abstinence, and this relapse is associated with increased activity in LH, caudal BLA, and MeA.
Keywords: food, relapse, cue, punishment, lateral hypothalamus, amygdala
A key cause of the current obesity epidemic is the excessive consumption of unhealthy foods (Little, Horowitz, & Feinle-Bisset, 2007; Torres & Nowson, 2007). Relapse to unhealthy eating behaviors during dieting is one of the fundamental impediments to the successful treatment of this disorder (Brownell & Kramer, 1989; Calu, Chen, Kawa, Nair, & Shaham, 2014; Peterson & Mitchell, 1999; Skender et al., 1996). One of the main triggers of relapse during dieting is exposure to food-associated cues, such as TV advertisements (Kayman, Bruvold, & Stern, 1990; McGuire, Wing, Klem, Lang, & Hill, 1999). Such food-associated cues can provoke eating behaviors in the absence of hunger and metabolic need in both humans and rodent models (Birch, McPhee, Sullivan, & Johnson, 1989; Cornell, Rodin, & Weingarten, 1989; Holland, Petrovich, & Gallagher, 2002; Petrovich, Set-low, Holland, & Gallagher, 2002; Weingarten, 1983).
The reinstatement model, which has been used extensively to study relapse to drugs of abuse (Bossert, Marchant, Calu, & Shaham, 2013; Shaham, Shalev, Lu, De Wit, & Stewart, 2003; Venniro, Caprioli, & Shaham, 2016), has been adapted to study the neural mechanisms of relapse to palatable food seeking (Calu et al., 2014; Nair, Adams-Deutsch, Epstein, & Shaham, 2009). Like reinstatement of drugs of abuse, extinguished food seeking can be reinstated by food-associated cues, stress, or priming with food (Calu et al., 2014; Ghitza, Gray, Epstein, Rice, & Shaham, 2006; Nair et al., 2009). In this study, we sought to address a potential limitation of these models, the use of extinction to suppress reward seeking (Caprioli et al., 2015; Epstein, Preston, Stewart, & Shaham, 2006; Marchant, Khuc, Pickens, Bonci, & Shaham, 2013). Excessive food consumption, or overeating, is associated with an increased incidence of obesity, resulting in adverse health consequences (Klein et al., 2004). These adverse health consequences often motivate individuals to refrain from poor eating habits (Capaldi, 1996). In animal models, extinction does not adequately capture this aspect of excessive eating. To incorporate these negative consequences, we employed a punishment procedure to suppress food-reinforced operant responses (Azrin & Holz, 1966). In our previous studies using alcohol-trained rats, we used response-contingent punishment in a different context from alcohol self-administration to suppress alcohol taking (Marchant et al., 2013; Marchant et al., 2014; Marchant et al., 2016). In this study, we used two different response-contingent discrete cues to signal food reward or punishment, and reinstatement was induced by presentation of the food-associated cue after punishment-imposed suppression of food taking.
Brain regions important for feeding and food seeking prompted by food-associated cues include the lateral hypothalamus (LH) and basolateral amygdala (BLA; Petrovich & Gallagher, 2007). Using cue-potentiated feeding (Weingarten, 1983), Petrovich and colleagues showed that projections from BLA to LH are activated by food-associated cues, and lesions of these brain regions decrease cue-potentiated feeding (Petrovich, Holland, & Gallagher, 2005; Petrovich et al., 2002). The role of BLA and LH in reinstatement of food seeking is less well studied. Inactivation of BLA, with bupivacaine microinfusions, potentiates cue-induced reinstatement of extinguished food seeking (McLaughlin & Floresco, 2007). In terms of the LH, Nair, Golden, and Shaham (2008) examined the role of the hypothalamic neuropeptide orexin (hypocretin; de Lecea et al., 1998; Peyron et al., 1998) and demonstrated a reduction in food self-administration but not reinstatement of food seeking, with systemic injections of the orexin receptor 1 antagonist SB-334867. Given the role of BLA and LH in cue-potentiated feeding, we sought to examine their activity during cue-induced relapse to food seeking after punishment.
In this study, we examined the neural mechanisms of relapse to food seeking after suppression of food taking by adverse consequences (i.e., punishment). We first used a food self-administration procedure where a lever press resulted in the presentation of a 10-s conditioned stimulus (CS) followed by a food pellet reward. Then, in the next phase of training, we punished the operant response with mild footshock preceded by a compound stimulus comprising the initial food-associated appetitive CS and an alternative CS (aversive CS). After punishment-imposed suppression of food self-administration, we tested rats for cue-induced relapse in either conditioned reinforcement (appetitive CS) or conditioned punishment (aversive CS) tests. With this design, our aim was to measure neuronal activity that is associated with cues that signal the consequence (reward or punishment) of food seeking on test. We first used immunohistochemical detection of Fos-protein to measure brain activity in rats tested with either the appetitive CS or the aversive CS. We focused on several brain regions known to be responsive to appetitive and aversive cues, including LH, amygdala, nucleus accumbens (NAc), and periaqueductal gray (PAG; Nasser & McNally, 2013). Given the abundant literature implicating a role for orexin and recent evidence of a role of GABA in food-seeking behaviors (Jennings et al., 2015; Petrovich, Hobin, & Reppucci, 2012), we also characterized the phenotype of Fos-positive neurons in LH, focusing on orexin (hypocretin) and GABA.
Method
Subjects and Apparatus
Male (n = 22, ~300 g, ~8 weeks old) Long-Evans rats were obtained from Charles River. All rats were housed singly under reverse 12-hr light/dark cycle (lights off at 0800) with food and water available ad libitum. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Animal Care and Use Committee. Standard operant chambers (Med Associates, Georgia, VT) enclosed in a ventilated sound-attenuating cubicle illuminated by a house light were used for food self-administration. Each chamber was equipped with one retractable lever (designated as “active”) and one nonretractable lever (designated as “inactive”). Grid floors were connected to shockers. The stimuli used as appetitive and aversive cues were a white cue light (7.5 W white light) and a tone (2,900 Hz, 20 dB above background); both were located above the active lever.
Behavioral Procedure
All rats were given two 1-hr magazine-training sessions where one food pellet (Test Diet, 45 mg, cat. 1811155) was delivered noncontingently every 2.5 min. No cues were present during magazine training. Subsequently, rats were trained for twelve 1-hr self-administration sessions (6 days/week) under a fixed-ratio 1, fixed-interval 20 schedule of reinforcement where an active lever press resulted in the delivery of the 10-s appetitive CS (either light or tone counterbalanced), and the food pellet was delivered when the CS turned off. This was followed by a 10-s timeout period where lever presses were recorded but not reinforced. The initiation of each session was signaled by the illumination of the house light and the insertion of the active lever into the chamber. Inactive lever presses had no programmed consequences. Following FI-20 training, rats were given two 1-hr sessions of punishment, during which 25% of reinforced lever presses resulted in the presentation of the appetitive CS in compound with the aversive CS for 10 s followed by both pellet delivery and a 0.5-s footshock (0.5 mA). The footshock intensity was based on our previous studies (Marchant et al., 2013; Marchant et al., 2016). The remaining 75% of reinforced lever presses led to the appetitive CS and pellet delivery.
Cue-induced food-seeking test.
Rats were then tested for cue-induced food seeking (active lever presses under extinction conditions) in one 1-hr session. During the test, an active lever press resulted in the 10-s CS (appetitive or aversive) with no food or footshock delivered. See Figure 1. During the test session, we used freezing to assess fear expression in a subset of rats (n = 3) from each group. We manually scored freezing during the 10-s CS presentation from a video recording of the test session. Freezing was defined as the absence of movement other than that required for breathing for at least 2 s (Blanchard & Blanchard, 1969; Nasser & McNally, 2012); fear was scored as either freezing or not freezing during the 10-s CS presentation.
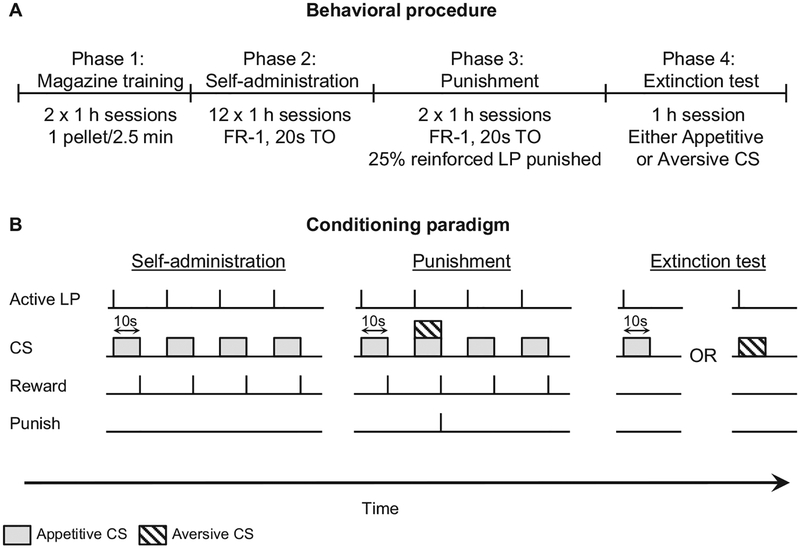
Figure 1.
Experimental design. (A, B) Outline of the experimental procedure. Phase 1 involved 2 × 1-hr magazine training sessions where one food pellet was delivered noncontingently every 2.5 min. In Phase 2, rats were trained to self-administer food pellets during 12 × 1-hr fixed-ratio 1 (FR-1) sessions where each lever press resulted in a 10-s conditioned stimulus (appetitive conditional stimulus [CS]) followed by a food reward and a 20-s timeout (TO) period. In Phase 3, rats had 2 × 1-hr punishment sessions where 25% of reinforced responses resulted in the presentation of the appetitive CS compounded with a novel aversive CS for 10 s followed by a 0.5-mA footshock, a food pellet, and a 20-s TO. The remaining 75% were identical to Phase 2. In Phase 4, rats were exposed to either the appetitive CS or the aversive CS during a 1-hr extinction test where neither food nor footshock was available.
Tissue Harvesting and Immunohistochemistry
One and a half hours following the initiation of the final test session, rats were deeply anesthetized with isofluorane and transcardially perfused with ~100 ml of 0.1M Diethylpyrocarbonate (DEPC)-treated phosphate buffer (PB) followed by ~400 ml of 4% paraformaldehyde in 0.1M PB (pH 7.4). A subset of rats was sacrificed directly from the home cage as a no-test control. Brains were removed and postfixed in 4% paraformaldehyde for 2 hr, then transferred to 18% sucrose in 0.1M DEPC-treated phosphate buffer (pH 7.4) for 48 hr at 4 °C. Brains were sagittally sectioned through the midline and stored at −80 °C until sectioning. Serial (40 μm) coronal sections of the right hemisphere were sectioned for immunohistochemistry using a Leica Microsystems cryostat and stored in 0.1M sodium phosphate (pH 7.4) containing 0.1% sodium azide at 4 °C. Serial (16 μm) coronal sections of the left hemisphere were sectioned into DEPC-treated PB, and sections were stored at −80 °C for in situ hybridization (see below).
Immunohistochemical staining of fos-protein and orexin.
A 1-in-4 series of the hypothalamus, amygdala, PAG, and NAc regions was processed for immunohistochemical detection of Fos-protein and orexin. Free-floating sections were rinsed for 30-min in phosphate-buffered saline (PBS) followed by two 30-min washes in 50% ethanol with the second wash containing 3% hydrogen peroxide and then incubated for 30 min in a blocking solution containing 5% normal horse serum (NHS) in PBS. Sections were then incubated for at least 48 hr at 4 °C in PBS-tx with 2% NHS, rabbit antic-Fos primary antibody (1:8,000, Phospho-c-Fos, 5348S; Cell Signaling). Following Fos primary incubation, sections were rinsed in PBS and incubated for 2 hr in biotinylated donkey antirabbit IgG (1:1,000, 711-065-152; Jackson ImmunoResearch) diluted in 2% NHS PBS-tx. The secondary antibody was rinsed off with PBS, and sections were incubated for 1 hr in ABC reagent (Vector Laboratories). Following this, sections were incubated in 0.1M sodium acetate with 0.025% diaminobenzodine (DAB) in 2% nickel sulfate containing 2 mg/ml D-glucose and 0.4 mg/ml ammonium chloride for 10 min before adding glucose oxidase (0.2 ~l per ml of solution) to visualize Fos. The reaction was stopped after ~10 min with sodium acetate washes. Sections were then rinsed in PBS followed by a 30-min wash in 0.3% hydrogen peroxide. Sections were then incubated for 1 hr in a blocking solution containing 5% NHS and 4 drops/ml of avidin blocking (Vector) in PBS-tx. Sections were incubated for ~48 hr at 4 °C in PBS-tx with 2% NHS, 4 drops/ml biotin (Vector), and rabbit antiorexin-A primary antibody (1:10,000, H-003–30; Phoenix). Following orexin-A primary incubation, sections were rinsed in PBS and processed in a similar manner to the Fos using biotinylated donkey antirabbit IgG (1:1,000, 711-065-152; Jackson ImmunoResearch) as the secondary for orexin. The DAB step of this reaction occurred without nickel sulfate in order to visualize orexin as a brown reaction product. Finally, sections were mounted onto gelatin-coated glass slides, air dried, and cover-slipped.
Image acquisition and neuronal quantification.
For orexin + Fos, we digitally captured bright-field images of immunoreactive (IR) cells in the different brain areas using an EXi Aqua camera (QImaging) attached to a Zeiss Axio Scope 2, Axio Imager M2. We captured and analyzed the images using iVision (Biovision). Each image analyzed comprised five images through the z plane that was digitally collapsed using iVision, giving a single plane view of in-focus cells. For each rat, Fos-positive cells were quantified in the BLA (rostral sections were Bregma −2.52 to −2.76, caudal sections were Bregma −2.92 to −3.12), central amygdala (CeA; Bregma −2.52 to −3.0), medial amygdala (MeA; Bregma −2.64 to −2.92), hypothalamus (Bregma −2.64 to −2.92), paraventricular thalamus (PVT; Bregma −2.64 to −2.92), NAc (Bregma 1.62 to 1.92), and PAG (Bregma −6.48 to −6.60). Orexin-positive cells were quantified in the perifornical/LH area (Bregma −2.64 to −2.92) and the dorsomedial hypothalamus (DMH; Bregma −2.64 to −2.92; Figure 5A). Counting was performed by manual identification of total orexin-IR, Fos-IR, and orexin + Fos-IR cells by an observer that was blinded to experimental conditions.
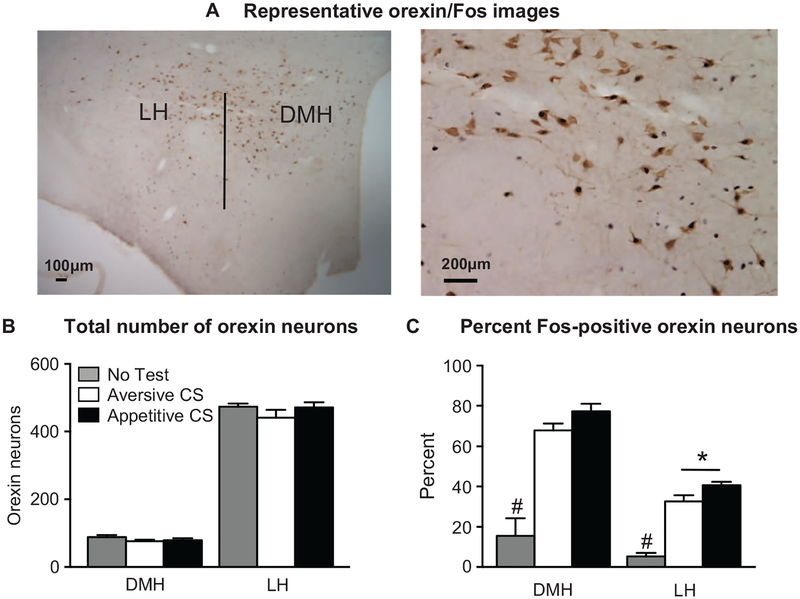
Figure 5.
Fos expression in orexin neurons during cue-induced food seeking after punishment. (A) Photomicrograph representing the hypothalamic subregions, including the lateral hypothalamus (LH) and dorsomedial hypothalamus (DMH) as well as a representative image of our Fos-orexin double-label immunohistochemistry. (B) There was no significant difference in the total number of orexin-positive cells across test cue groups in either the LH or DMH. In the LH, rats tested with the appetitive conditioned stimulus (CS) had a significantly greater percentage of Fos-positive orexin cells compared to those tested with the aversive CS. (C) In both LH and DMH, the no-test group had significantly lower percentages of Fos-positive orexin cells compared to rats tested with either the appetitive or aversive CS. * p < .001, #p < .001, no-test group significantly different from both the appetitive and aversive CS groups. Appetitive CS group, n = 9; aversive CS group, n = 8; no-test group, n = 4.
For in situ hybridization of LH sections for vesicular GABA transporter (VGAT) and Fos, we used a subset of animals (n = 6) to examine hypothalamic VGA messenger RNA (mRNA) expression. Serial 16-μm coronal LH sections of the left hemisphere were rinsed and treated with 0.2N HCL, rinsed, and then acetylated in 0.25% acetic anhydride in 0.1M triethanolamine. Subsequently, sections were rinsed and postfixed with 4% paraformaldehyde, rinsed, and then incubated in a hybridization buffer for 2 hr at 55 °C. Hybridization was then performed by in situ hybridization for radioactive detection of VGAT mRNA (vesicular GABA transporter) using previously detailed methods (Yamaguchi, Sheen, & Morales, 2007; Yamaguchi, Wang, Li, Ng, & Morales, 2011). For in situ hybridization, sections were hybridized for 16 hr at 55 °C with [35S]-and [33P]-labeled (107 c.p.m./ml) single-stranded antisense probes for VGAT (mouse vGAT probe: nucleotides 1–2,814; GenBank accession code: BC052020). Following hybridization, sections were treated with 4 μg/ml RNAse A at 37 °C for 1 hr, washed with 1× saline-sodium citrate and 50% formamide for 1 hr at 55 °C, and then with 0.1 × saline-sodium citrate at 68 °C for 1 hr. Subsequently, sections were incubated for 2 days in rabbit antic-fos (1:500, SC-52; Santa-Cruz Biotechnology), rinsed with PBS, incubated for 1 hr with biotinylated goat antirabbit secondary antibody, rinsed with PB, and then incubated for 1 hr at reaction time in avidin-biotinylated horseradish peroxidase (1:200, ABC kit; Vector Laboratories). Sections were then rinsed and the peroxidase reaction was developed with 0.05% 3,3’-DAB tetrahydrochloride and 0.003% H2O2. The tissue was then mounted on coated slides and photographed under bright-field illumination. Finally, slides were dipped in Ilford K.5 nuclear tract emulsion (Polysciences; 1:1 dilution in double-distilled water) and exposed in the dark at 4 °C for 3–4 weeks before development and photographs of silver-grain epiluminescence.
Study and consent procedures were approved in accordance with the National Institute on Drug Abuse Animal Care and Use Committee (protocol number 14-BNRB-175).
Data Analysis
Training and punishment data were analyzed using a repeated-measures analysis of variance (ANOVA) examining a main effect of session. For the behavioral test, the dependent variables were the total number of active lever presses, the number of normalized head entries during the cue; the number of inactive lever presses during testing was used as a covariate in the analyses to statistically control for the nonspecific (training independent) lever presses during the test. Normalized head entries were determined by calculating the number of head entries during the cue divided by the number of cues presented for each rat. In a subset of rats (n = 3/group), we assessed the percentage of time spent freezing throughout the cue-induced relapse test by calculating the total time spent freezing divided by the number of cues presented for each rat. We analyzed the immunohistochemical data with total cell counts of a given brain region as the dependent variable. We analyzed the number of Fos-positive cells, orexin-positive cells, and double-labeled cells using a mixed ANOVA with the within-subjects factor of brain region and the between-subjects factor of test cue (no test, aversive, appetitive). We subsequently analyzed main effects using one-way ANOVAs. We used Pearson’s correlations to examine the relationship between total Fos and behavioral responses to cue-induced food seeking after punishment.
Methods for analysis of in situ hybridization material have been described previously (Root et al., 2014). Radioactive in situ material was analyzed using epiluminescence to increase the contrast of silver grains, as described previously (Yamaguchi et al., 2011). Photographs of Fos-protein labeling (detected by brown DAB label) were overlaid with epiluminescent photos of silver grains and DAB labeling, as well as with dark-field images of silver grains (see Figure 6). For the radioactive in situ hybridization, a c-Fos-positive cell was considered to express VGAT when its c-Fos-positive nucleus contained concentric aggregates of silver grains that exceeded background levels. We performed all statistical analyses using IBM SPSS Version 21 and followed up on significant main or interaction effects (p < .05) with Fisher Protected Least Significant Difference (PLSD) post hoc tests. We present the data in the figures as mean ± SEM.
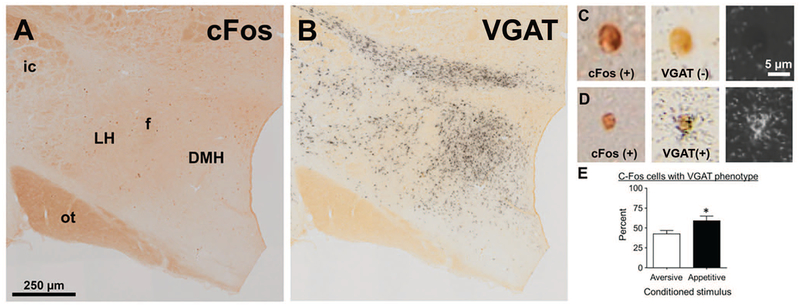
Figure 6.
Fos expression in vesicular GABA transporter (VGAT) messenger RNA (mRNA) neurons during cue-induced food seeking after punishment. (A, B) Photomicrograph illustrating immunohistochemical detection of Fos-protein (A) and in situ hybridization detection of VGAT mRNA (B) in lateral hypothalamus (LH). (C) A high-power photomicrograph of a representative VGAT-negative, Fos-positive neuron (D). A high-power photomicrograph of a representative VGAT-positive, Fos-positive neuron (E). We found increased Fos-protein expression in LH neurons expressing VGAT mRNA in rats tested with the appetitive conditioned stimulus (CS) compared to the aversive CS. Appetitive CS group, n = 3; aversive CS group, n = 3. DMH = dorsomedial hypothalamus; f = fornix; ot = optic tract; ic = internal capsule. * p < .05.
Results
Training and Punishment
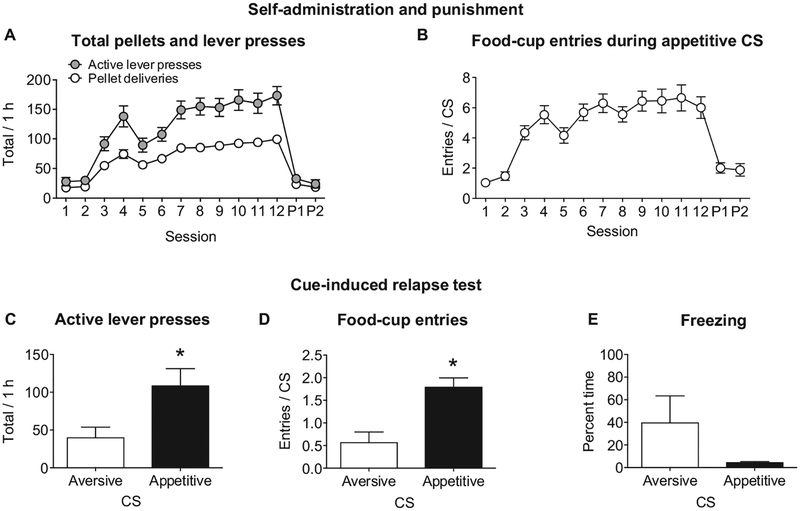
Rats reliably acquired food pellet self-administration in the sessions where lever press resulted in presentation of the appetitive CS for 10 s followed by food pellet reinforcement shown by a main effect of session (F11, 176 = 18.0, p < .0001; Figure 2A). We found that punishment of 25% of reinforced lever pressing resulted in a significant decrease in food self-administration (F1, 16 = 55.9, p < .0001; Figure 2A). During punishment, we observed evidence for generalization between the appetitive cue and the compound appetitive/aversive CS. Normalized food-cup entries during the appetitive CS significantly decreased compared to during self-administration training (F1, 16 = 28.4, p < .0001; Figure 1B).
Figure 2.
Cue-induced food seeking after punishment. (A) Following two 1-hr magazine training sessions, average number of active lever presses and food pellet deliveries during food self-administration training with an appetitive conditioned stimulus (CS) and punishment with an aversive CS. (B) The number of food-cup entries during the appetitive CS throughout self-administration training and punishment (C, D). Total number of active lever presses and food-cup entries during a 1-hr test of cue-induced food seeking after punishment (E). The percentage of time spent freezing during the appetitive or aversive CS throughout the 1-hr test. * p < .05; punishment Sessions 1 and 2 (P1, P2). Appetitive CS group, n = 10; aversive CS group, n = 8. Freezing behavior appetitive CS group, n = 3; aversive CS group, n = 3.
Cue-Induced Reinstatement of Palatable Food Seeking After Punishment
We observed higher cue-induced reinstatement of palatable food seeking after punishment in rats tested with the appetitive CS compared to the aversive CS. An analysis of covariance of active lever presses (inactive lever presses as covariate) showed a significant effect of test CS (F1, 15 = 5.8, p = .029), and there was higher lever pressing in the rats tested with the appetitive CS (Figure 2C). There was no effect of test CS on the number of inactive lever presses (F1, 16 = 0.1, p = .777; appetitive CS average = 2.6 ± 1.4; aversive CS average = 2.0 ± 1.6). An analysis of normalized food-cup entries showed an effect of test CS (F1, 16 = 15.6, p = .001) with rats having more food-cup entries during the appetitive CS compared to the aversive CS (Figure 2D). An analysis of freezing behavior in a subset of rats from each group during the cue showed no significant effect of test CS (F1, 4 = 2.1, p = .217), but we did observe a higher mean freezing behavior during the aversive CS compared to the appetitive CS (Figure 2E). Additionally, there was no significant effect of test CS on latency to the first lever press in the test session (F1, 16 = 3.2, p = .092).
Fos Expression in Response to Cue-Induced Food Seeking After Punishment
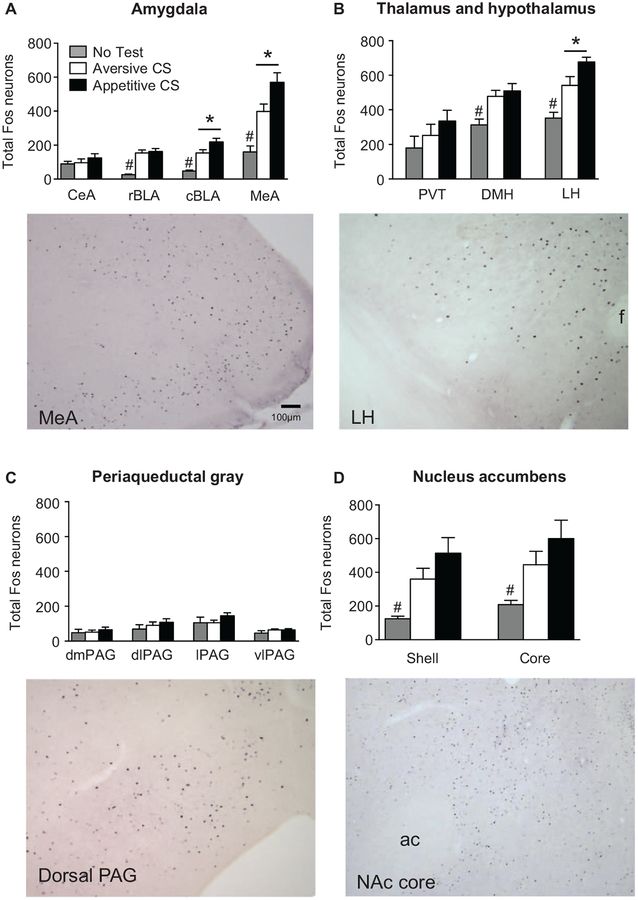
Our initial analysis using the within-subjects factor brain region (DMH, LH, CeA, BLA, MeA, PVT, NAc, PAG) and the between-subjects factor test CS showed a significant interaction between the two factors (F24, 156 = 2.9, p < .0001). Each brain region was subsequently analyzed with one-way ANOVAs. In LH, caudal BLA, and MeA, the number of Fos-positive cells in the appetitive CS group was significantly higher than the aversive CS group (p = .020, p = .026, p = .021, respectively; Figure 3A,B). In the DMH, LH, rostral BLA, caudal BLA, and MeA, total Fos in the appetitive CS and the aversive CS groups was significantly higher than the no-test group (p ≤ .05; Figure 3A,B). In the NAc core and shell, total Fos was significantly higher in the appetitive CS versus the no-test group (p < .05), but there was no significant difference between the aversive CS and the no-test groups (p > .05; Figure 3D). There was no significant effect of test CS on total Fos in CeA, PVT, or any subregion of the PAG (p > .05; Figure 3A–C).
Figure 3.
Fos expression after cue-induced food seeking after punishment. (A) In the caudal basolateral amygdala (cBLA) and the medial amygdala (MeA), rats tested with the appetitive conditioned stimulus (CS) had significantly higher numbers of Fos-positive cells compared to rats tested with the aversive CS. In the rostral BLA (rBLA), cBLA, and MeA, no-test rats had lower numbers of Fos-positive neurons compared to rats exposed to the appetitive and aversive CS. No differences in the number of Fos-positive cells across treatment groups was observed in the central amygdala (CeA). (B) Rats tested with the appetitive CS had significantly increased numbers of Fos-positive cells in the lateral hypothalamus (LH) compared to rats exposed to the aversive CS. Additionally, no-test rats had significantly lower numbers of Fos-positive cells in both the LH and dorsomedial hypothalamus (DMH) compared to rats tested with either an appetitive or aversive CS. (C) There were no significant differences in the number of Fos-positive cells across treatment group in the paraventricular thalamus (PVT). (D) There were no significant differences across treatment groups in the number of Fos-positive neurons in any subregion of the periaqueductal gray (PAG), including the dosomedial (dmPAG), dorsolateral (dlPAG), lateral (lPAG), and ventrolateral (vlPAG) regions. In the nucleus accumbens shell and core, the no-test group had lower numbers of Fos-positive cells compared to the appetitive CS group. * p < .05, # p < .05, no-test group significantly different from both the appetitive and aversive CS groups; $ p < .05, no-test group significantly different from the appetitive CS group. Appetitive CS group, n = 9; aversive CS group, n = 8; no-test group, n = 4; f = fornix; ac = anterior commissure.
Correlations Between Fos Expression and Additional Behavioral Measures During Cue-Induced Food Seeking After Punishment
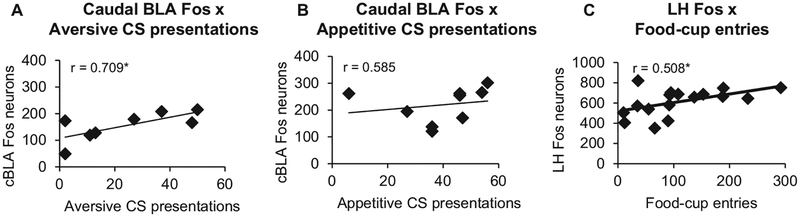
Correlation analyses showed a significant, positive correlation between the number of aversive CS presentations and total Fos in caudal BLA, r = .709, p = .049; Figure 4A. Correlations between aversive CS presentations and total Fos in LH, DMH, CeA, rostral BLA, PAG, PVT, MeA, and NAc were not significant (p > .05). Additionally, the number of appetitive CS presentations did not significantly correlate with total Fos in caudal BLA, r = .212, p = .585; Figure 4B. The only significant correlation between appetitive CS presentations and total Fos was a negative correlation in the lateral periaqueductal grey (LPAG), r = −0.714, p = .031; all other brain regions were not statistically significant (p > .05). We also observed a significant positive correlation between total food-cup entries during test (for both groups of animals) and total Fos in LH, r = .508, p = .037; Figure 4C. Finally, total food-cup entries did not correlate with total Fos in any other brain region examined (p > .05).
Figure 4.
Correlations between behavior on test and total Fos. (A) A significant, positive correlation was found between caudal basolateral amygdala (cBLA) Fos and aversive conditioned stimuli (CS) presentations (B). There was no significant relationship between cBLA Fos and appetitive CS presentations (C). Lateral hypothalamus (LH) Fos was positively correlated with the number of food-cup entries in all rats. * p < .05. Appetitive CS group, n = 8; aversive CS group, n = 8.
Specific Recruitment of Hypothalamic Orexin Cells in Response to Cue-Induced Food Seeking After Punishment
There were no differences observed in the total number of orexin-positive cells in either the DMH or LH (F2, 18 = 0.7, p = .490; Figure 5B.). Analysis of the number of Fos-positive orexin cells with a within-subjects factor of brain region (DMH, LH) and a between-subjects factor of test CS showed a significant main effect of test CS (F2, 18= 35.4, p < .001). Post hoc analyses revealed that in the LH, the number of Fos-positive orexin cells in the appetitive CS group was significantly higher than both the aversive CS and no-test groups (p < .001; Figure 5C). In the DMH, the number of Fos-positive orexin cells in the appetitive and aversive CS groups was significantly higher than the no-test group (p < .001; Figure 5C).
Expression of Fos in LH VGAT Neurons in Response to Cue-Induced Food Seeking After Punishment
Consistent with the previous LH Fos data, we found that rats presented with the appetitive CS had higher total Fos (n = 580) compared to rats presented with the aversive CS (n = 477). Our in situ hybridization assay revealed that this increase was driven in part by recruitment of LH GABAergic neurons in the appetitive CS group. A linear mixed model revealed that the proportion of GABAergic neurons also positive for Fos was higher in the appetitive CS group compared to the aversive CS group (significant fixed effect of test CS, F2, 18 = 7.545, p < .05). Accordingly, 58.94% ± 5.96% (n = 350/580) of Fos-positive neurons in the appetitive CS group were positive for VGAT mRNA compared to 42.58% ± 4.21% in the aversive CS group (n = 221/447) (Figure 6E). Further, 11.1% ± 1.4% of all neurons positive for VGAT mRNA in the appetitive CS group were also Fos positive, while only 8.4% ± 2.4% of VGAT neurons in the aversive CS group were Fos positive.
Discussion
In this report, we describe a procedure where cue-induced food seeking after punishment-imposed suppression of food taking occurs when rats are tested for conditioned reinforcement (presentation of the appetitive CS) compared to rats tested for conditioned punishment (presentation of the aversive CS). We have also shown that a discrete cue signaling punishment of a food-reinforced operant response can become associated with punishment, causing suppressed food seeking during a conditioned-punishment test. Cue-induced food seeking was associated with increased expression of Fos-protein in LH, caudal BLA, and MeA. Furthermore, our characterization of the phenotype of Fos-positive neurons in LH suggested that both orexin neurons and GABA neuron are activated by an appetitive CS.
Cue-Induced Relapse to Palatable Food Seeking After Punishment-Imposed Abstinence
The reinstatement model has been used extensively to study relapse to drugs of abuse (Bossert et al., 2013; Shaham et al., 2003;Venniro et al., 2016), and recent studies have begun to examine this model in the context of natural rewards, such as palatable food pellets (Calu et al., 2014; Nair et al., 2009). In this study, we used footshock punishment to suppress food taking, a method previously established in our lab for alcohol relapse studies (Marchant et al., 2013;Marchant et al., 2014; Marchant et al., 2016). However, whereas in our alcohol relapse studies we used different contexts to signal reinforcement and punishment, here we used response-contingent discrete cues to signal reinforcement or punishment. In our study, food reinforcement was preceded by a 10-s CS, which is similar to other studies looking at cue-induced reinstatement of extinguished food seeking (Floresco, McLaughlin, & Haluk, 2008; McLaughlin & Floresco, 2007). The use of discrete cues in this procedure, as opposed to immediate food reinforcement or shock punishment, allowed us to assess the role of appetitive or aversive cues in cue-induced relapse. Our test results show that rats tested with the appetitive CS displayed increased lever pressing and head entries during the CS compared to rats tested with the aversive CS. Therefore, we have shown that discrete cues can become associated with either reward or punishment and that such cues can be used to promote or inhibit relapse to food seeking after punishment-imposed suppression of food taking.
Role of LH in Cue-Induced Food Seeking After Punishment
LH function is typically associated with generating motivational drive for natural and drug reward seeking (Marchant, Millan, & McNally, 2012; Stuber & Wise, 2016). For example, early stimulation and lesion studies identified LH as critical for feeding (Anand & Brobeck, 1951; Margules & Olds, 1962; Olds & Milner, 1954). These data have been confirmed and extended with recently developed cellular and molecular tools. For example, both chemo-and optogenetic activation of LH GABA neurons increases feeding (Jennings et al., 2015). A role for LH in mediating reward seeking induced by conditioned stimuli (e.g., contexts) is also well established (Hamlin, Blatchford, & McNally, 2006; Hamlin, Newby, & McNally, 2007; Harris, Wimmer, & Aston-Jones, 2005; Marchant, Hamlin, & McNally, 2009; Marchant et al., 2014). Furthermore, cue-potentiated feeding (Weingarten, 1983) is disrupted by disconnection of the BLA-LH neural circuitry (Petrovich et al., 2002). In the present study, we found that cue-induced food seeking after punishment is associated with increased Fos-protein activity in the LH and that the number of food-cup entries on test (regardless of the tested CS) was positively correlated with LH Fos-protein expression. Therefore, our data further demonstrate that LH is involved in mediating cue-reward associations that have the ability to promote food-seeking responses.
The hypothalamic peptide orexin (hypocretin) has been implicated in reward seeking for both drug and natural rewards (Aston-Jones et al., 2010; Harris et al., 2005). Here we observed increased Fos-protein expression in LH orexin neurons in rats tested with the appetitive CS compared to rats tested with the aversive CS. Studies using cue-potentiated feeding have also demonstrated a role for LH orexin neurons in promoting food intake. Petrovich et al. (2012) found that orexin neurons in the perifornical area are activated in response to food-associated cues and have recently demonstrated that systemic injections of the orexin-1 receptor antagonist, SB-334867, reduces cue-induced feeding behavior in sated rats (Cole, Mayer, & Petrovich, 2015). Interestingly, Nair et al. (2008) reported that systemic SB-334867 injections decreased food self-administration but had no effect on pellet priming or yohimbine-induced reinstatement of extinguished food seeking, suggesting that LH orexin may specifically represent the motivational significance of food-associated cues. However, while several studies have shown that systemic SB-334867 injections reduce reinstatement of drug seeking, but not food seeking, elicited by discriminative cues (James, Yeoh, Graham, & Dayas, 2012; Martin-Fardon & Weiss, 2014), other studies have shown that cue-induced reinstatement of extinguished saccharin seeking is reduced by systemic SB-334867 injections (Cason & Aston-Jones, 2013).
With respect to LH GABA neurons, recent studies have identified a key role for these neurons in consummatory behaviors (Jennings, Rizzi, Stamatakis, Ung, & Stuber, 2013; Jennings et al., 2015; Navarro et al., 2016). Our in situ hybridization assay revealed increased Fos-protein expression in LH GABA neurons (identified by VGAT mRNA expression) in rats tested with the appetitive CS versus the aversive CS (see Figure 6). It is interesting to note that in the rats tested with the appetitive CS, we observed increased Fos-protein in LH GABA neurons but also observed increased Fos-protein in orexin neurons, which predominantly express Vglut mRNA (a marker of glutamatergic neurons; Rosin, Weston, Sevigny, Stornetta, & Guyenet, 2003). This raises an interesting question: Why does the appetitive CS recruit activation in both glutamatergic and GABAergic neurons? Both populations project to the ventral tegmental area (VTA; Balcita-Pedicino & Sesack, 2007; Nieh et al., 2016). Optical stimulation of LH GABA → VTA projections increases consummatory and reward behaviors (Barbano, Wang, Morales, & Wise, 2016; Nieh et al., 2016), and orexin in VTA plays a role in food reward (Borgland et al., 2009; Borgland, Taha, Sarti, Fields, & Bonci, 2006). Further, it was recently shown that LH neurons target glutamate, GABA, and dopamine neurons in the VTA (Faget et al., 2016). Therefore, LH glutamate and GABA may converge on different populations of VTA neurons to produce increased consummatory behaviors. Future studies are required to describe the presynaptic-postsynaptic relationships by which glutamate/orexin and GABA from LH can both cause increased motivated behavior.
Role of BLA in Cue-Induced Food Seeking After Punishment
While there is a wealth of evidence implicating BLA in fear conditioning (LeDoux, 2007), appetitive conditioning and instrumental behavior are also critically dependent on BLA function (Balleine & Killcross, 2006; Cador, Robbins, & Everitt, 1989; Gallagher & Holland, 1994; Janak & Tye, 2015; Robbins, Cador, Taylor, & Everitt, 1989). In this study, we found that both rostral and caudal BLA had significantly higher Fos-protein expression in rats tested with either CS compared to rats not tested. We propose that different populations of BLA neurons are activated by the appetitive or aversive CS. While our methodology (Fos-protein immunohistochemistry) does not allow us to answer this question directly, our finding of a significant positive correlation between caudal BLA Fos and aversive CS presentations but not appetitive CS presentations (see Figure 4) suggests that the pattern of activation is different depending on the motivational valence of the CS. Indeed, recent evidence has identified that specific, largely nonoverlapping, BLA neuronal populations encode either positive or negative outcomes (Beyeler et al., 2016; Namburi et al., 2015). Additional studies, such as those specifically manipulating activated neurons (e.g., Bossert et al., 2011; Cruz et al., 2013; Koya et al., 2009), are required to resolve this issue.
In caudal BLA, we found a significant increase in Fos expression in rats tested with the appetitive CS compared to rats tested with the aversive CS, suggesting a role for caudal BLA in cue-induced relapse to food seeking after punishment. However, several studies have shown that caudal BLA is involved in behavioral suppression (Jean-Richard-Dit-Bressel & McNally, 2015; McLaughlin & Floresco, 2007; Millan, Reese, Grossman, Chaudhri, & Janak, 2015). While we did not observe specific Fos activation in caudal BLA in rats tested with the aversive CS compared to the appetitive CS, the finding of increased Fos in this group compared to rats not tested shows that the aversive CS recruited the caudal BLA. We propose that one potential reason for this difference is that divergent projections from caudal BLA to LH or NAc shell exert opposing effects on food seeking. In support of this hypothesis, there are dense projections from caudal BLA to LH (Hamlin, Clemens, & McNally, 2008; Yoshida, McCormack, Espana, Crocker, & Scammell, 2006) and to NAc shell (Brog, Salyapongse, Deutch, & Zahm, 1993; McDonald, 1991). BLA→LH projections have been demonstrated as critical for cue-potentiated feeding (Petrovich et al., 2002; Petrovich et al., 2005). Furthermore, Sun et al. (2015) have shown in humans that food-associated cues in sated but not hungry subjects increased activity in the amygdala, which was linked to activity in the hypothalamus. In contrast, Millan et al. (2015) demonstrated that inactivation of caudal BLA or NAc shell causes increased alcohol seeking in the absence of conditioned cues, suggesting that the caudal BLA→NAc shell pathway is important for inhibition of reward seeking.
Role of MeA in Cue-Induced Food Seeking After Punishment
Research has traditionally focused on the role of the basolateral and central nuclei of the amygdala in cue-induced food seeking, with limited research examining the MeA. Previous research suggests that the MeA is activated when an animal experiences psychological stress such as loud noise or restraint (Dayas, Buller, Crane, Xu, & Day, 2001), and lesion studies have determined that MeA is necessary for the hypothalamic-pituitary-adrenal axis response to psychological stressors (Dayas, Buller, & Day, 1999). In our study, we observed increased Fos-protein expression in the MeA in rats tested with the appetitive CS. One possibility is that because cue-induced relapse testing occurred under extinction conditions, MeA recruitment by the appetitive CS group was due to psychological stress or frustration (Amsel, 1958; Konorski, 1967), caused by the nonreinforced extinction test. However, Blair (2004), suggested that modulation of the hypothalamus by the MeA can occur as a function of appetitive cues. Indeed, there is accumulating evidence of a role for the MeA in food seeking (Padilla et al., 2016; Xu et al., 2015). Further, MeA neurons synapse onto LH orexin neurons (Sakurai et al., 2005), providing anatomical connectivity for such a relationship. We propose that future research should not discount the importance of MeA and its projections in reward- and stress-related behaviors.
Conclusions
In summary, we have described a procedure where response-contingent discrete cues predictive of reward or punishment can promote or inhibit food seeking after punishment-imposed suppression of food taking. We propose that this procedure can be used to examine the neural substrates of cue-induced relapse to palatable food seeking after punishment-imposed abstinence. Using expression of Fos as a marker of neuronal activity, we show that cue-induced food seeking after punishment is associated with increased activation of caudal BLA, LH, and MeA. Furthermore, in LH, we observed increased activation of both LH orexin and GABA neurons, further implicating these systems in food seeking maintained by food-associated stimuli.
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program funds to the Neurobiology of Relapse Section (principal investigator: Yavin Shaham) and Neuronal Networks Section (principal investigator: Marisela Morales). Nathan J. Marchant received support from Early Career Fellowship 1053308 by the National Health and Medical Research Council. The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this article. We thank Yavin Shaham for his help in the conceptualization of the project and the write-up of the article.
Contributor Information
Erin J. Campbell, University of Newcastle and the Hunter Medical Research Institute
David J. Barker, National Institute on Drug Abuse, Baltimore, Maryland
Helen M. Nasser, University of Maryland, Baltimore
Konstantin Kaganovsky, National Institute on Drug Abuse, Baltimore, Maryland.
Christopher V. Dayas, University of Newcastle and the Hunter Medical Research Institute
Nathan J. Marchant, National Institute on Drug Abuse, Baltimore, Maryland, and University of Melbourne
References
- Amsel A (1958). The role of frustrative nonreward in noncontinuous reward situations. Psychological Bulletin, 55, 102–119 10.1037/h0043125 [DOI] [PubMed] [Google Scholar]
- Anand BK, & Brobeck JR (1951). Localization of a “feeding center” in the hypothalamus of the rat. Proceedings of the Society for Experimental Biology and Medicine, 77, 323–325. 10.3181/00379727-77-18766 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, & Richardson KA (2010). Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Research, 1314, 74–90. 10.1016/j.brainres.2009.09.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrin NH, & Holz WC (1966). Punishment. In Honig WK (Ed.), Operant behavior: Areas of research and application (pp. 380–447). Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Balcita-Pedicino JJ, & Sesack SR (2007). Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. Journal of Comparative Neurology, 503, 668–684. 10.1002/cne.21420 [DOI] [PubMed] [Google Scholar]
- Balleine BW, & Killcross S (2006). Parallel incentive processing: An integrated view of amygdala function. Trends in Neurosciences, 29, 272–279. http://dx.doi.org/10.1016Zj.tins.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Barbano MF, Wang HL, Morales M, & Wise RA (2016). Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. Journal of Neuroscience, 36,2975–2985 10.1523/JNEUR0SCI.3799-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, … Tye KM (2016). Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron, 90, 348–361. 10.1016/j.neuron.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, & Johnson S (1989). Conditioned meal initiation in young children. Appetite, 13, 105–113. 10.1016/0195-6663(89)90108-6 [DOI] [PubMed] [Google Scholar]
- Blair RJ (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition, 55, 198–208. 10.1016/S0278-2626(03)00276-8 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, & Blanchard DC (1969). Crouching as an index of fear. Journal of Comparative and Physiological Psychology, 67, 370–375. 10.1037/h0026779 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, … Bonci A (2009). Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. Journal of Neuroscience, 29, 11215–11225. 10.1523/JNEUROSCI.6096-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, & Bonci A (2006). Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron, 49, 589–601. 10.1016/j.neuron.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, & Shaham Y (2013). The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology, 229, 453–476. 10.1007/s00213-013-3120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, & Shaham Y (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature Neuroscience, 14, 420–422. 10.1038/nn.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, & Zahm DS (1993). The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. Journal of Comparative Neurology, 338, 255–278. 10.1002/cne.903380209 [DOI] [PubMed] [Google Scholar]
- Brownell KD, & Kramer FM (1989). Behavioral management of obesity. The Medical Clinics of North America, 73, 185–201. 10.1016/S0025-7125(16)30698-8 [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, & Everitt BJ (1989). Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience, 30, 77–86. 10.1016/0306-4522(89)90354-0 [DOI] [PubMed] [Google Scholar]
- Calu DJ, Chen YW, Kawa AB, Nair SG, & Shaham Y (2014). The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology, 76, 395–406. 10.1016/j.neuropharm.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi ED (Ed.). (1996). Why we eat what we eat: The psychology of eating. Washington, DC: American Psychological Association. [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, … Shaham Y (2015). Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of meth-amphetamine craving after prolonged voluntary abstinence in a rat model. Biological Psychiatry, 78, 463–473. 10.1016/j.biopsych.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, & Aston-Jones G (2013). Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology, 228, 499–507. 10.1007/s00213-013-3051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, & Petrovich GD (2015). Orexin/hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Scientific Reports, 5, 16143 10.1038/srep16143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CE, Rodin J, & Weingarten H (1989). Stimulus-induced eating when satiated. Physiology & Behavior, 45, 695–704. http://dx.doiorg/10.1016/0031-9384(89)90281-3 [DOI] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, & Hope BT (2013). New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nature Reviews Neuroscience, 14, 743–754. 10.1038/nrn3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, & Day TA (2001). Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary nor-adrenergic cell groups. European Journal of Neuroscience, 14, 1143–1152. 10.1046/j.0953-816x.2001.01733.x [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, & Day TA (1999). Neuroendocrine responses to an emotional stressor: Evidence for involvement of the medial but not the central amygdala. European Journal ofNeuroscience, 11,2312–2322 10.1046/j.1460-9568.1999.00645.x [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, … Sutcliffe JG (1998). The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America, 95,322–327 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, & Shaham Y (2006). Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology, 189, 1–16. 10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, … Hnasko TS (2016). Afferent inputs to neurotransmitter-defined cell types in the ventral tegmental area. Cell Reports, 15, 2796–2808. 10.1016/j.celrep.2016.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, & Haluk DM (2008). Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience, 154, 877–884. 10.1016/j.neuroscience.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Gallagher M, & Holland PC (1994). The amygdala complex: Multiple roles in associative learning and attention. Proceedings of the National Academy of Sciences of the United States of America, 91, 11771–11776. 10.1073/pnas.91.25.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, & Shaham Y (2006). The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacology, 31, 2188–2196. 10.1038/sj.npp.1300964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, & McNally GP (2006). Renewal of an extinguished instrumental response: Neural correlates and the role of D1 dopamine receptors. Neuroscience, 143, 25–38. 10.1016/j.neuroscience.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, & McNally GP (2008). Renewal of extinguished cocaine-seeking. Neuroscience, 151, 659–670. 10.1016/j.neuroscience.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, & McNally GP (2007). The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience, 146, 525–536. 10.1016/j.neuroscience.2007.01.063 [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437, 556–559. 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, & Gallagher M (2002). The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior, 76, 117–129. 10.1016/S0031-9384(02)00688-1 [DOI] [PubMed] [Google Scholar]
- James MH, Yeoh JW, Graham BA, & Dayas CV (2012). Insights for developing pharmacological treatments for psychostimulant relapse targeting hypothalamic peptide systems. Addiction Research and Therapy, S4(008). 10.4172/2155-6105.S4-008 [DOI] [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–292 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, & McNally GP (2015). The role of the basolateral amygdala in punishment. Learning & Memory, 22, 128–137. 10.1101/lm.035907.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, & Stuber GD (2013). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science, 341, 1517–1521. 10.1126/science.1241812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, … Stuber GD (2015). Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell, 160, 516–527. http://dx.doi.org/10.10167j.cell.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayman S, Bruvold W, & Stern JS (1990). Maintenance and relapse after weight loss in women: Behavioral aspects. American Journal of Clinical Nutrition, 52, 800–807.http://ajcn.nutrition.org/content/52/57800.long [DOI] [PubMed] [Google Scholar]
- Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X,… the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. (2004). Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation, 110, 2952–2967. 10.1161/01.CIR.0000145546.97738.1E [DOI] [PubMed] [Google Scholar]
- Konorski J (1967). Integrative activity of the brain. Chicago, IL: University of Chicago Press. [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, … Hope BT (2009). Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature Neuroscience, 12, 1069–1073. 10.1038/nn.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Current Biology, 17, R868–R874. 10.1016/j.cub.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Little TJ, Horowitz M, & Feinle-Bisset C (2007). Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: Implications for the pathophysiology of obesity. American Journal of Clinical Nutrition, 86, 531R868–541 http://ajcn.nutrition.org/content/86/3Z531.long [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, … Shaham Y (2016). Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. Journal of Neuroscience, 36, 3281–3294. 10.1523/JNEUROSCI.4299-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, & McNally GP (2009). Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. Journal of Neuroscience, 29, 1331–1342. 10.1523/JNEUROSCI.5194-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, & Shaham Y (2013). Context-induced relapse to alcohol seeking after punishment in a rat model. Biological Psychiatry, 73, 256–262. 10.1016/j.biopsych.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, & McNally GP (2012). The hypothalamus and the neurobiology of drug seeking. Cellular and Molecular Life Sciences, 69, 581–597. 10.1007/s00018-011-0817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, & Shaham Y (2014). A critical role of lateral hypothalamus in context-induced relapse to alcohol seeking after punishment-imposed abstinence. Journal of Neuroscience, 34, 7447–7457. 10.1523/JNEUR0SCI.0256-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margules DL, & Olds J (1962). Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science, 135, 374–375. 10.1126/science.135.3501.374 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, & Weiss F (2014). N-(2-methyl-6-benzoxazolyl)-N=−1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: Comparison with natural reward seeking. Addiction Biology, 19, 233–236 10.1111/j.1369-1600.2012.00480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1991). Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience, 44, 15–33. 10.1016/0306-4522(91)90248-M [DOI] [PubMed] [Google Scholar]
- McGuire MT, Wing RR, Klem ML, Lang W, & Hill JO (1999). What predicts weight regain in a group of successful weight losers? Journal of Consulting and Clinical Psychology, 67, 177–185. 10.1037/0022-006X.67.2.177 [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, & Floresco SB (2007). The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience, 146, 1484–1494. 10.1016/j.neuroscience.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Millan EZ, Reese RM, Grossman CD, Chaudhri N, & Janak PH (2015). Nucleus accumbens and posterior amygdala mediate cue-triggered alcohol seeking and suppress behavior during the omission of alcohol-predictive cues. Neuropsychopharmacology, 40, 2555–2565. 10.1038/npp.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, & Shaham Y (2009). The neuropharmacology of relapse to food seeking: Methodology, main findings, and comparison with relapse to drug seeking. Progress in Neurobiology, 89, 18–45. 10.1016/j.pneurobio.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, & Shaham Y (2008). Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. British Journal of Pharmacology, 154, 406–416. 10.1038/bjp.2008.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, … Tye KM (2015). A circuit mechanism for differentiating positive and negative associations. Nature, 520, 675–678. 10.1038/nature14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser HM, & McNally GP (2012). Appetitive-aversive interactions in Pavlovian fear conditioning. Behavioral Neuroscience, 126, 404–422. 10.1037/a0028341 [DOI] [PubMed] [Google Scholar]
- Nasser HM, & McNally GP (2013). Neural correlates of appetitive-aversive interactions in Pavlovian fear conditioning. Learning & Memory, 20, 220–228. 10.1101/lm.029744.112 [DOI] [PubMed] [Google Scholar]
- Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE,… Thiele TE (2016). Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology, 41, 1505–1512. 10.1038/npp.2015.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, … Tye KM (2016). Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron, 90, 1286–1298. 10.1016/j.neuron.2016.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, & Milner P (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology, 47, 419–427 10.1037/h0058775 [DOI] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, … Palmiter RD (2016). Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nature Neuroscience, 19, 734–741. 10.1038/nn.4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CB, & Mitchell JE (1999). Psychosocial and pharmacological treatment of eating disorders: A review of research findings. Journal of Clinical Psychology, 55, 685–697. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, & Gallagher M (2007). Control of food consumption by learned cues: A forebrain-hypothalamic network. Physiology & Behavior, 91, 397–403. 10.1016/j.physbeh.2007.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Hobin MP, & Reppucci CJ (2012). Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience, 224, 70–80. 10.1016/j.neuroscience.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, & Gallagher M (2005). Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. Journal of Neuroscience, 25, 8295–8302. 10.1523/JNEUR0SCI.2480-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, & Gallagher M (2002). Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. Journal of Neuroscience, 22, 8748–8753. http://www.jneurosci.org/content/22/19/8748.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience, 18, 9996–10015. http://www.jneurosci.org/content/18/23/9996.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, & Everitt BJ (1989). Limbic-striatal interactions in reward-related processes. Neuroscience and Biobehavioral Reviews, 13, 155–162. 10.1016/S0149-7634(89)80025-9 [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, & Morales M (2014). Single rodent mesohabenu-lar axons release glutamate and GABA. Nature Neuroscience, 17, 1543–1551. 10.1038/nn.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, & Guyenet PG (2003). Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. Journal of Comparative Neurology, 465, 593–603. 10.1002/cne.10860 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Mu-raki Y, … Yanagisawa M (2005). Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron, 46, 297–308. http://dx.doi.org/10.1016Zj.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, & Stewart J (2003). The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology, 168, 3–20. 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM Jr., & Foreyt JP (1996). Comparison of 2-year weight loss trends in behavioral treatments of obesity: Diet, exercise, and combination interventions. Journal of the American Dietetic Association, 96, 342–346. 10.1016/S0002-8223(96)00096-X [DOI] [PubMed] [Google Scholar]
- Stuber GD, & Wise RA (2016). Lateral hypothalamic circuits for feeding and reward. Nature Neuroscience, 19, 198–205. 10.1038/nn.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR,… Small DM (2015). Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. Journal of Neuroscience, 35, 7964–7976. 10.1523/JNEUR0SCI.3884-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, & Nowson CA (2007). Relationship between stress, eating behavior, and obesity. Nutrition, 23, 887–894. 10.1016/j.nut.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Progress in Brain Research, 224, 25–52. 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Weingarten HP (1983). Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science, 220, 431–433 10.1126/science.6836286 [DOI] [PubMed] [Google Scholar]
- Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, … Xu Y (2015). Estrogen receptor-a in medial amygdala neurons regulates body weight. Journal of Clinical Investigation, 125, 2861–2876. 10.1172/JCI80941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, & Morales M (2007). Glutamatergic neurons are present in the rat ventral tegmental area. European Journal of Neuroscience, 25, 106–118. 10.1111/j.1460-9568.2006.05263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, & Morales M (2011). Mesocorticolimbic glutamatergic pathway. Journal ofNeuroscience, 31, 8476–8490. 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, & Scammell TE (2006). Afferents to the orexin neurons of the rat brain. Journal of Comparative Neurology, 494, 845–861 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]