Abstract
More than one hundred years have passed since the development of the first microbial inoculant for plants. Nowadays, the use of microbial inoculants in agriculture is spread worldwide for different crops and carrying different microorganisms. In the last decades, impressive progress has been achieved in the production, commercialization and use of inoculants. Nowadays, farmers are more receptive to the use of inoculants mainly because high-quality products and multi-purpose elite strains are available at the market, improving yields at low cost in comparison to chemical fertilizers. In the context of a more sustainable agriculture, microbial inoculants also help to mitigate environmental impacts caused by agrochemicals. Challenges rely on the production of microbial inoculants for a broader range of crops, and the expansion of the inoculated area worldwide, in addition to the search for innovative microbial solutions in areas subjected to increasing episodes of environmental stresses. In this review, we explore the world market for inoculants, showing which bacteria are prominent as inoculants in different countries, and we discuss the main research strategies that might contribute to improve the use of microbial inoculants in agriculture.
Keywords: Biological nitrogen fixation, Plant-growth-promoting bacteria, Azospirillum, PGPB, PGPR, Inoculation, Rhizobia, Chemical fertilizers
Introduction
Humanity has always been concerned about food production to attend the increasing population and, for a long time, the solution was to expand agriculture to new areas. However, this scenario has changed in recent decades, first due to limitations of unexplored cultivable land, but also reinforced by the development of new technologies that allow higher yields, in addition to increasing environmental concerns, leading to agricultural practices aiming at achieving sustainable production. Therefore, although the global demand for food continues to increase, the concepts of agriculture sustainability, recovery of degraded areas, and mitigation of environmental impacts are gaining more respect (Canfield et al. 2010; Godfray et al. 2010). In this context, microbial inoculants—denominated as biofertilizers in some countries—have received increasing attention, gaining prominence and market scale in agriculture.
Inoculants are products that have in their composition living microorganisms capable of benefiting the development of different plant species. The most antique microorganisms used as inoculants are the “rhizobia”, diazotrophic bacteria able to colonize the rhizosphere and establish nodules in the roots of their host plants, composed by several species of the Fabaceae family. The symbiosis legumes-rhizobia leads to the process of biological nitrogen fixation (BNF), which very often can fully supply the plant´s demands on N. Moreover, other diazotrophic bacteria, such as Azospirillum, establish less straight relationships with the host plant, but are also able to supply, at least partially, the plant’s demands on N. Both Azospirillum and rhizobia, among other diazotrophic and non-diazotrophic bacteria are named as plant-growth-promoting bacteria (PGPB) or plant-growth-promoting rhizobacteria (PGPR), as they may benefit the plants by a variety of single or combined processes, including the production of phytohormones, siderophores, phosphate solubilization, induction of plant intrinsic systemic resistance to abiotic and biotic stresses, among others (Bhattacharyya and Jha 2012; Malusá and Vassilev 2014; Fukami et al. 2017, 2018a, b). Other microorganisms have also been increasingly used in agriculture for biological control of pests and diseases (Ciancio et al. 2016; Berg et al. 2017; Singh et al. 2017; Xiang et al. 2017), but this review will only deal with inoculants carrying strains that facilitate plant growth. Moreover, we will name all rhizobia and other bacteria carrying different mechanisms that promote plant growth as PGPB.
Currently, soybean (Glycine max (L.) Merr.) is the most inoculant-consuming crop worldwide, carrying bacteria belonging to the genus Bradyrhizobium. Brazil is probably the global leader in the use of inoculants for the soybean crop (Hungria and Mendes 2015; Okon et al. 2015; ANPII 2016) where approximately 78% of the copping area—nowadays 36.5 million hectares—is inoculated yearly (ANPII 2018). Additionally, inoculation of common beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata (L.) Walp.), maize (Zea mays L.) and co-inoculation of soybean and common bean with rhizobia and Azospirillum have also increased in Brazil (Hungria et al. 2010, 2015), so that the number of doses commercialized in the last years has impressively grown (Fig. 1). Other top countries in the use of inoculants are Argentina and India (Mazid and Khan 2014; Hungria and Mendes 2015; Okon et al. 2015; Sruthilaxmi and Babu 2017).
Fig. 1.
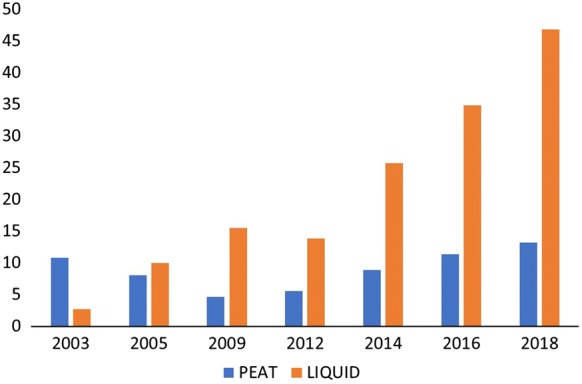
Market of microbial inoculants in Brazil in the last 15 years (million doses)
However, there are limiting factors that restrict the use of inoculants in some areas. Biotic and abiotic stresses may affect the effectiveness of the product, making them inefficient in cases such as nutrient-poor or unbalanced soils, salinity, water stress, increasing temperatures, pests and diseases, among others (Bashan et al. 2014; Das et al. 2017; Khan et al. 2017; Thilakarathna and Raizada 2017; Samago et al. 2018). To circumvent these factors, several studies have been addressed to gain better knowledge on the intrinsic properties of PGPB, seeking at understanding their optimum growth conditions and interaction with the host plants (Flores-Félix et al. 2018; Goulart-Machado et al. 2018; Jiménez-Gómez et al. 2018). Efforts have also been applied to improve the efficiency of microorganisms already available and in the identification of new elite strains to be used as inoculants under unfavorable and stressful environmental conditions, such as areas frequently experiencing drought, soils with low nutrient availability or with salinity, among others (Benidire et al. 2017; Koskey et al. 2017; Youseif et al. 2017). There is an increasing number of studies aiming to isolate, identify and evaluate the capacity of plant-growth promotion of bacteria with a variety of plant species, with potential to be transformed into new microbial inoculants in a near future (Yanni et al. 2016; Koskey et al. 2017; Manasa et al. 2017; Muleta et al. 2017).
Another technology with increasing application relies on the use of mixed inoculants, aiming to promote plant growth by combining distinct mechanisms of different microorganisms. Mixed inoculants can provide excellent results and show the great potential of being increasingly used by the farmers (Juge et al. 2012; Hungria et al. 2013, 2015; Chibeba et al. 2015; Bulegon et al. 2017; Ferri et al. 2017).
The objective of this short review is to explore the current market of inoculants, highlighting what has been produced and marketed lately in several countries, and the impact on agricultural sustainability. We also explore new ideas, new objectives and new strategies that are needed to generate information for the development of new products, breaking down barriers needed to expand the use of microbial inoculants in agriculture.
Inoculant carriers
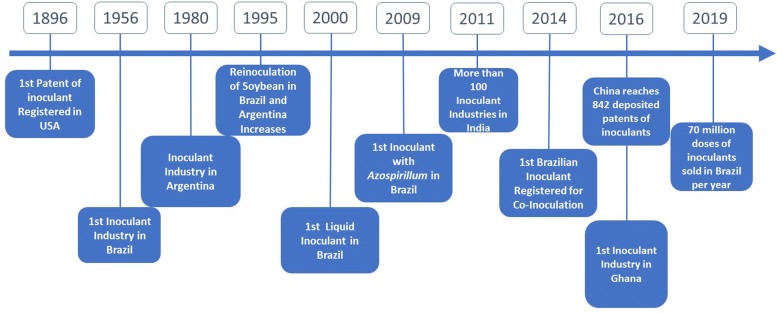
Since the beginning of the manufacturing of inoculants, the industry has been concerned about generating increasingly efficient products, at a low cost, whose handling attends to the needs and the quality required by farmers. An important aspect is the choice of the carrier for the microorganisms, which should, among other things, provide long cellular viability and be of easy application. In 1896, in the USA, the first inoculant commercially produced, “Nitragin” (Fig. 2), used gelatin, and later, nutrient medium was employed as carrier for bacterial cells. Due to the high mortality rate, these carriers were soon replaced by peat, which remained as the “gold” carrier until the end of the 1990s, when the scenario began to change (Fig. 2) (Williams 1984).
Fig. 2.
Chronology of some important steps in the development of microbial inoculants
Peat is a solid material, consisting of organic soil naturally occurring in specific environments and formed after a long geological period. The choice of peat as carrier for inoculants is due to its richness in organic matter, which serves as an important source of nutrients for the microorganisms. The peaty matrix also provides physical protection to the microorganisms against soil adversities and allows better cell survival in conditions of water restriction and high temperatures (Hungria et al. 2000a, 2005). In the process of seed inoculation with peat it is essential to use adhesives to help to stick the peaty matrix to the seeds; for example, in Brazil the most popular adhesive is 10% sucrose solution (Hungria et al. 2000a). The peat-based inoculant must be packed in sterilized polyethylene or polypropylene bags, with thickness of 0.06–0.38 mm, which preserves moisture but guarantees gas exchange with the external medium (Hungria et al. 2005).
Concerns about the use of peat as inoculant carrier rely on the exploitation of peat bogs, which may cause serious environmental impacts, including the destruction of habitats and CO2 emissions. In addition, in countries such as Brazil, where there are few peat bogs, importation of this material is required, increasing the production costs (Ribeiro et al. 2013). Due to these limitations, inoculants based on liquid formulations began to gain space, especially from the late 1990s onwards. In Brazil, the first liquid inoculant was approved by the Ministry of Agriculture for commercial used in 2000, and a decade later almost 80% of the inoculants sold in the country were in liquid formulations (Fig. 2); similar proportion is found in Argentina (ANPII 2018). Liquid inoculants consist of microbial cultures suspended in liquid medium rich in nutrients and cell protectors. They are easily handled and compatible with mechanized sowing, offering an advantage over solid inoculants at sowing. Another advantage is the easiness of sterilization, facilitating the absence of contaminants and, consequently, allowing higher cell concentration (Bashan et al. 2014; Cassán et al. 2015).
In addition to seed inoculation, liquid inoculants allow alternative application methods, such as in-furrow, and sprayed on soil or by “foliar” application (Campo et al. 2010; Fukami et al. 2016; Moretti et al. 2018). Alternative methods of application may be advantageous in some cases, for example, the inoculation in-furrow, to alleviate the impact of pesticides used for seed treatments in contact with the bacteria (Campo et al. 2010).
Other vehicles and methods for carrying microorganisms, such as agricultural and industrial residues, lyophilized bacteria and polymers for cell encapsulation, have been researched to develop more efficient and stable products. According to Bashan et al. (2014), industrial residues and agricultural by-products such as sugarcane bagasse, sawdust or brewery waste can be used as carriers for inoculation of microorganisms. However, the major limitation for the use of these raw materials is their poorly consistent composition, and often difficulties for sterilization.
As one of the challenges for inoculant production is to maintain cell viability for long period, lyophilization and freezing of microbial cells have emerged as possibilities to overcome this problem. The process of lyophilization consists of removing the intracellular water, reducing the metabolic activity and increasing microbial lifetime. The dry cell culture must be mixed with a liquid or gel formulation at sowing. The great barrier to the commercial production of inoculants with lyophilized microorganisms is the high production cost because it requires specialized equipment and skilled labor (Williams 1984; Hungria et al. 2005). Besides, the time and conditions needed for cell recovering in liquid or gel formulations represent barriers for the adoption of inoculants by farmers, especially high large areas are cropped, such as for the soybean crop in South America.
The encapsulation of living microbial cells with polymers, such as alginate and polyacrylamide has also been increasingly mentioned. For the encapsulation, the liquid inoculant containing bacterial culture is mixed with an adjuvant polymer, capable of causing solidification. The most used method consists of mixing dropwise the microbial culture in a solution containing calcium chloride, resulting in solid beads with high cell concentration. The spheres are placed in contact with the seeds at sowing time and the bacteria are slowly released. These spheres are biodegradable and do not cause environmental impact. Encapsulation confers protection to the cells for high temperature and environmental stresses and are also easy to handle. Once again, the economic factors have always represented the main obstacle for scaling the industrial production (Bashan 1986; Bashan et al. 2002; Date 2001).
Great efforts have been applied by several industries to develop new products able to attend the new requirements of the market and compatible with new technologies. The trend for this next decade is to apply considerable investment in innovation, searching for new inoculant formulations to hit the ever growing market.
Inoculants containing mixes of bacteria
The great majority of the first manufactured inoculants contained only one species of microorganism, and in general one strain, the one with the best inoculation results for a particular crop. Exceptions included a maximum of two microorganisms “of the same type”, for example, two Bradyrhizobium strains or species for soybean. The use of two strains in the same inoculant would increase the chances that at least one would nodulate and perform well with the legume. For example, in Brazil, the combination of two Bradyrhizobium strains for the soybean crop has been preferentially used by the farmers since the 1950s (Hungria et al. 1994; Hungria and Mendes 2015).
Particularly in the last decade, the use of inoculants containing microorganisms of “different type” has expanded. The idea is of combining strains or species acting in different microbial processes, so that the combined benefits of each one would result in higher benefits and, ultimately, yields. Examples of mixed inoculant are those combining microorganisms whose major processes are BNF (e.g. Bradyrhizobium spp., Rhizobium spp.) and phytohormone production (e.g. Azospirillum spp., Pseudomonas spp.), solubilization of phosphate (e.g. Bacillus spp.), or biological control (e.g. Pseudomonas spp., Bacillus spp.). If the microorganisms cannot be combined in a single product, they are manufactured separately and the bags containing each one are sold in the same package.
The application of mixed inoculants is usually called co-inoculation or mixed inoculation and it is currently possible to find co-inoculants for several crops in the market. The efficiency of co-inoculation is closely related to the appropriate selection of strains, the cellular concentration of each one, method of inoculation (applied to the seeds, leaf-spray, in-furrow), and to the plant genotype. Therefore, research is needed to generate knowledge aiming at the production of new formulations for commercial inoculants with mixed bacteria (Cassán et al. 2015), and on alternative methods of application of inoculants and microbial molecules (Campo et al. 2010; Fukami et al. 2016).
In Brazil, co-inoculation of A. brasilense with Bradyrhizobium spp. for the soybean crop and with Rhizobium tropici for the common beans was launched in 2014 and impressive increases in grain yield have been reported (Hungria et al. 2013, 2015; Souza and Ferreira 2017; Nogueira et al. 2018). Even in areas with high population of compatible rhizobia for both crops (> 104 cells of compatible rhizobia/g soil), for the soybean crop single inoculation of Bradyrhizobium resulted in mean increases of 8.4% in grain yield compared with the naturalized population, whereas the co-inoculation with A. brasilense promoted an “upgrade” to 16.1%; for common beans, single inoculation with R. tropici increased yield by 8.3%, whereas the co-inoculation improved the yield by 19.6% (Hungria et al. 2013) (Table 1). Since them, other benefits attributed to the co-inoculation of soybean with Bradyrhizobium and Azospirillum in Brazil are the promotion of early nodulation (Chibeba et al. 2015), and increased tolerance to moderate water restriction (Cerezini et al. 2016; Silva et al. 2019).
Table 1.
Examples of studies comprising inoculation of various plant species with specific bacterial strains resulting in increased grain yield
| Crop | Microorganism | Strains | Increase in grain yield compared with the non-inoculated control (%) | References |
|---|---|---|---|---|
| Soybean | Bradyrhizobium japonicum | – | 4.5 | Hungria et al. (2001) |
| B. japonicum | SEMIA 5079 and SEMIA 5080 | 8.4 | Hungria et al. (2013) | |
| B. japonicum | 532 C and USDA 110 | 12–19 | Ulzen et al. (2016) | |
| B. japonicum | – | 1.6–6.3 | Leggett et al. (2017) | |
| Common beans | Rhizobium tropici | SEMIA 4080 (= PRF 81) | 31.6–36 | Hungria et al. (2000b) |
| R. tropici | SEMIA 4080 | 8.3 | Hungria et al. (2013) | |
| R. tropici | CPAO 12.5 L2 | 66 | Mercante et al. (2017) | |
| Rhizobium leguminosarum sv. phaseoli | HB-429 | 48 | Samago et al. (2018) | |
| Cowpea | B. japonicum | BR 3267 | 38.1 | Ulzen et al. (2016) |
| Bradyrhizobium liaoningense | VIBA-1 | 54.8 | Padilla et al. (2016) | |
| Bradyrhizobium yuanmingense | VIBA-2 | 38.3 | Padilla et al. (2016) | |
| Faba beans | R. leguminosarum sv. viciae | NGB-FR 126 | 46.8–81.4 | Youseif et al. (2017) |
| R. leguminosarum sv. vicieae | NSFBR-30 and HUFBR-15 | 5–75 | Argawa and Mnalku (2017) | |
| Maize | Azospirillum brasilense | Ab-V5 and Ab-V6 | 27 | Hungria et al. (2010) |
| A. brasilense | Ab-V5 | 29 | Ferreira et al. (2013) | |
| A. brasilense | Ab-V5 and Ab-V6 | 14.3 | Galindo et al. (2019) | |
| Pseudomonas fluorescens | – | 29–31 | Sandini et al. (2019) | |
| Wheat | Bacillus polymyxa | Bp 4317 | 13.6–19.5 | Rodriguez-Caceres et al. (1996b) |
| A. brasilense | Sp246 | 14.7 | Ozturk et al. (2003) | |
| A. brasilense | Ab-V5 and Ab-V6 | 31 | Hungria et al. (2010) | |
| A. brasilense | – | 18 | Karimi et al. (2018) | |
| Rice | Burkholderia vietnamiensis | TVV75 | 22 | Tran et al. (2000) |
| B. vietnamiensis | MGK3 | 12.1 | Govindarajan et al. (2007) | |
| Tomato | A. brasilense | Sp-7 | 11 | Alfonso et al. (2005) |
| P. fluorescens | SS5 | 57 | Ahirwar et al. (2015) | |
| Co-inoculation | ||||
| Soybean | A. brasilense and B. japonicum | Ab-V5 and Ab-V6; SEMIA 5079 and SEMIA 5080 | 14.1 | Hungria et al. (2013) |
| A. brasilense* and B. japonicum* | Ab-V5 and Ab-V6; SEMIA 5019 and SEMIA 5079 | 81.9 | Ferri et al. (2017) | |
| Common beans | A. brasilense* and R. tropici | Ab-V5 and Ab-V6; SEMIA 4080 | 19.6 | Hungria et al. (2013) |
| Wheat | Serratia marcescens, Microbacterium arborescens, and Enterobacter sp. | – | 24 | Kumar et al. (2017) |
| Rice | Klebsiella pneumoniae, P. fluorescens, and Citrobacter freundii | 4P, 1N and 3C | 17.5 | Nguyen et al. (2003) |
| P. fluorescens, Bacillus subtilis, Bacillus amyloliquafaciens and Candida tropicalis | 1N, B9, E19 and HY | 26.7 | Nguyen (2008) | |
| A. brasilense and P. fluorescens | – | 20.2 | de Salamone et al. (2012) | |
All experiments were carried out under field conditions with seed inoculation, except those marked (*), which inoculation occurred in-furrow. Yield increase varied between studies because of specific cropping conditions such as soil composition, temperature, site and environmental conditions
In addition to Azospirillum spp., several other PGPB have been reported as successful in co-inoculation trials with soybean, as Pseudomonas sp. (Egamberdieva et al. 2017; Pawar et al. 2018), Actinomyces sp. (Nimnoi et al. 2014), Bacillus sp. (Atieno et al. 2012; Subramanian et al. 2014; Petkar et al. 2018). Improvements in yields have also been reported with the co-inoculation of rhizobia presenting different mechanisms of action. For example, Jesus et al. (2018) verified benefits by the co-inoculation of common bean with R. tropici CIAT 899, Bradyrhizobium diazoefficiens USDA 110 and Bradyrhizobium elkanii 29w; according to the authors, Bradyrhizobium spp. would improve the symbiosis efficiency of Rhizobium, resulting in greater number of nodules, biomass production and N accumulation. The suggested mechanism is that Bradyrhizobium sp. co-inoculated produces signaling molecules, such as nodulation factors (Nod factors) and surface polysaccharides that stimulate root nodulation by R. tropici, improving the efficiency of BNF.
Co-inoculation has also been shown to be efficient under several limiting conditions, such as in low phosphate soils. Generally, the BNF is compromised under these situations, but the co-inoculation with phosphate-solubilizing microorganisms can make it available for plant nutrition and, in the case of legumes, help to ensure the benefits of BNF (Jorquera et al. 2008; Morel et al. 2012; Shiri-Janagard et al. 2012; Korir et al. 2017). For example, Korir et al. (2017) evaluated the effects of co-inoculation in common beans grown in a soil with low P and observed that plants inoculated with Rhizobium strain IITA-PAU 987 and Bacillus megaterium increased nodulation, shoot dry weight and had 31% increase in BNF when compared with the single inoculation with Rhizobium.
Main inoculated crops
Soybean
Soybean is an annual herbaceous dicotyledonous, originally grown in the eastern region of Asia (Aliyev and Mirzoyev 2010). Until the nineteenth century, its cultivation remained restricted to the eastern countries, and spread to other continents, as America and Africa, only at the end of this period (Dall´Agnol et al. 2007; Aliyev and Mirzoyev 2010). Nowadays, the main soybean producers are the USA, Brazil, and Argentina.
Soybean is probably the most successful example of crop benefiting from the application of microbial inoculants, more specifically, carrying Bradyrhizobium spp. strains. South American countries lead soybean inoculation. In contrast, in the USA, estimates are that only 15% of the area cropped with soybean has been inoculated, what might be related to the low cost of N-fertilizer marketed in the country (Chang et al. 2015). The low cost of N-fertilizer may also have implied in lower interest in innovation of technologies updated with new agricultural practices.
The Brazilian research for the production and commercialization of inoculants is very advanced and the country has one of the most complete legislation in this area. Common resolutions for inoculants commercialization were defined in 1998 for the Mercosur, the common market including Brazil, Argentina, Uruguay and Paraguay. Following, in Brazil, a legislation of 2004 included definitions and norms on specifications, guarantees, registrations, packaging and labeling of inoculants, as well as the list of the microorganisms that could be used in commercial inoculants in the country; the document was updated in 2011 (MAPA 2004, 2011). Nowadays, four strains of Bradyrhizobium are authorized for the production of soybean inoculants in the country (Bradyrhizobium japonicum SEMIA 5079 (= CPAC 15), B. diazoefficiens SEMIA 5080 (= CPAC 7), B. elkanii SEMIA 5019 (= 29w) and SEMIA 587). The legislation still establishes a minimum concentration of viable cells (1 × 109 viable cells/g or mL) of the inoculant until the expiration date, which must be at least 6 months, and void of contaminants at the 1 × 10−5 dilution (Hungria et al. 2010; MAPA 2011). The technical recommendation in Brazil indicates a dose that allows at least 1.2 million viable cells/seed to guarantee a successful nodulation (Hungria et al. 2017; Hungria and Nogueira 2019). The credibility of the inoculant market in Brazil relies on strict legal regulation. Interestingly, the legislation was created based mainly on the Australian legislation, where nowadays the regulation relies on an agreement between partners, as a voluntary control (Bullard et al. 2005; AIRG 2010).
In Brazil, the inoculation of soybean with elite Bradyrhizobium spp. strains can fully supply the crop’s demand on N, dismissing the use of N-fertilizers. Probably as a result of breeding for BNF, the symbiosis with soybean is very sensitive to N-fertilizers, drastically reducing nodulation (Hungria et al. 2007; Hungria and Mendes 2015). Soybean cropping without any N-fertilizer has generated an annual economy that today is estimated at about 20 billion dollars.
In Brazil, Argentina and in other South American countries, successful results have been achieved with the re-inoculation of soybean, i.e., the yearly inoculation even in soils presenting well-established compatible rhizobial population from previous inoculations (Hungria et al. 2001; Hungria and Mendes 2015). This practice led to the commercialization of over 70 million doses of inoculants for soybean in Brazil in the last crop season. Estimates in Brazil are that re-inoculation increases soybean grain yield by 8% in average (Hungria and Mendes 2015) and by 6.8% (Leggett et al. 2017) to 14% (Hungria et al. 2016) in Argentina. In the USA, re-inoculation is traditionally not recommended, based on results from a former study showing that rhizobial populations as low as 10 cells/g would inhibit the nodule formation by inoculant strains (Thies et al. 1991a, 1995). However, mean yield increases due to inoculation considering areas of traditional soybean cropping have been recently estimated at 1.67% (Leggett et al. 2017), but could probably be higher if high N-fertilizer levels were not applied to the crops comprising the soybean agricultural systems (Chang et al. 2015). Amazingly, even the most recent studies on the quantification of soybean BNF in the USA take into consideration a large number of sites, soil fertility, and application of mineral N, but not the re-inoculation component (Córdova et al. 2019). Certainly, the annual re-inoculation is responsible for the high contribution of BNF to the soybean N nutrition in Brazil, with values as high as 94% of the aboveground N accumulation (Hungria et al. 2006), while in the USA these values range from 23 to 65% (Córdova et al. 2019).
The Sub-Saharan Africa (SSA) region has developed, over the years, strategies for the use of beneficial microorganisms in soybean adapted to local environment and social characteristics. As consequence of the lack of local production and difficulties in the importation of inoculants in the 1970s, the International Institute of Tropical Agriculture (IITA) launched a breeding program aiming at developing high-yielding tropical soybean varieties capable of nodulating with indigenous rhizobial strains. These new varieties were named “TGx” (tropical Glycine cross) or “promiscuous” soybeans (Kueneman et al. 1984; Pulver et al. 1985), and contributed to the expansion of soybean production in the SSA.
Because the usually acidic, saline, and low organic matter of the SSA soils, the average soybean yield is usually well below the world average (Thuita et al. 2012; Muleta et al. 2017). Therefore, in addition to the soybean genetic breeding, further studies have been carried out aiming at increasing yields. For example, in Ethiopia, Muleta et al. (2017) searched for acid-tolerant rhizobia as strategy to increase soybean performance. A local isolate was able to improve soybean yield, indicating that search for indigenous or naturalized elite isolates might represent an interesting strategy to be adopted in other African countries. Impressive yield increases have also been observed by combining application of P-fertilizer and rhizobial inoculant in Nigeria (Ronner et al. 2016), and along with other studies suggest that P is probably the main limiting factor to the BNF in Africa (Vanlauwe et al. 2019).
In Mozambique, the majority of soybean cropping was with promiscuous varieties without inoculation; however, due to the increased demand on exportation of grains and poultry industry, the cultivation of non-promiscuous and more-productive cultivars associated with inoculation has increased (Dias and Amane 2011). As the agroclimatic conditions of the soybean production areas in Mozambique are similar to the main areas of soybean cultivation in the Brazilian savanna, Chibeba et al. (2018) evaluated and confirmed that elite strains identified in Brazil could have a successful performance in Mozambique with non-promiscuous soybean genotypes. The feasibility of transferring inoculation technologies between countries is of outstanding importance, as it can accelerate the establishment of sustainable cropping systems, saving time, labor and money. However, it is always desirable to search for indigenous or adapted strains, and promising local soybean strains have been identified in Mozambique (Chibeba et al. 2017), in a near future, their performance should be compared with the imported strains under field conditions.
Common beans
Similar to soybeans, common beans (Phaseolus vulgaris L.) are cropped worldwide, representing the most important source of protein in several countries, especially in South and Central America and Africa (Hungria et al. 2000b, 2013; Ribeiro et al. 2013). Although Brazil is one of the main producers (3.17 million hectares in the 2017/2018 crop season) and consumer of common beans worldwide, grain yields are usually low, bellow 1000 kg/ha (Hungria et al. 2007; CONAB 2019). Therefore, many strategies have been considered to improve yield, concomitantly to the tolerance to environmental stresses, at low cost.
Studies carried out in Brazil identified two strains of the “R. tropici group” for common bean that show high BNF rates, competitiveness, tolerance to environmental stresses and genetic stability (Hungria et al. 2000a, 2003; Mostasso et al. 2002). The strains PRF 81 (= SEMIA 4080) of R. freirei and H 12 (= SEMIA 4088) of R. tropici have been used in commercial inoculants in Brazil since 1998 and 2004, respectively, in addition to R. tropici CIAT 899, originally isolated in Colombia by Dr. Peter H. Graham (Hungria et al. 2000a, 2003; Gomes et al. 2015). Interestingly, CIAT 899 has been recognized as an outstanding strain in several countries (Gomes et al. 2015; Vanlauwe et al. 2019).
The use of inoculants for common bean favors yields, but there are reports indicating that BNF might not replace N-fertilizers completely, especially in soils where the N concentration is very low. Studies suggest that the application of 15 or 20 kg N/ha along with inoculation at sowing might improve grain yield (Soares et al. 2016), but higher doses of N at sowing may lead to reduced nodulation (Hungria et al. 2003). Noteworthy, Mercante et al. (2017), in a series of field trials performed in the Brazilian Cerrados verified that, in comparison with the indigenous population, the mean increase in grain yield by inoculating R. tropici CIAT 899 was of 410 kg/ha, but decreased to 365 kg/ha with the application of 20 kg of N/ha at sowing; a new identified elite strain resulted in outstanding mean increases of 665 kg/ha in grain production (Table 1).
The African continent also stands out in the production and consumption of common beans. Estimates are that 25% of the total world area cropped with common beans are in Africa, where the legume is part of the diet of more than 100 million people (Aserse et al. 2012; Beebe et al. 2013), with Tanzania, Kenya, Uganda and South Africa been the main producers (USDA 2012). Similar to South America, African researchers are studying different ways of increasing common bean yield by using BNF, especially in situations where the efficiency of nodulation by Rhizobium is compromised, such as dry conditions, low P concentrations, soil salinity and high temperatures (Yanni et al. 2016; Samago et al. 2018). In order to identify rhizobia capable of tolerating drought and salinity stresses, Yanni et al. (2016) selected indigenous strains in the eastern and western regions of the Nile delta, and identified elite strains with good performance under saline and water stress conditions, promising for the use as inoculants (Kanonge-Mafaune et al. 2018).
The approach of selecting adapted indigenous strains with high capacity of BNF was also investigated by Koskey et al. (2017) in soils of low fertility in Kenya. Regarding the symbiotic efficiency, four indigenous isolates showed good symbiotic performance, one being able to increase grain yield by 30% in comparison to the commercial inoculum for beans, Biofix (strains not informed). The importance of P for the symbiotic performance of common bean was highlighted under field conditions in Nigeria (Ronner et al. 2016) and Ethiopia (Samago et al. 2018).
Cowpea
Originated from the African continent, cowpea (Vigna unguiculata L. Walp.) is the major legume cropped in many African countries, responsible for more than 95% of the world’s production (Silva et al. 2016). In Brazil, cowpea was introduced in the sixteenth century and has been cultivated mainly in the North and Northeast regions. Despite the still modest yield, Brazil has exported cowpea grains to some countries such as India, Egypt and Pakistan (Silva et al. 2016).
Cowpea is usually tolerant to high temperatures, low soil fertility and water restriction; grain yield can be limited by N availability, which can be supplied by BNF. Interestingly, African countries with climate and humidity conditions similar to the North and Northeast of Brazil have tested and observed positive responses to inoculation with elite Bradyrhizobium strains from Brazil. Boddey et al. (2016) and Ulzen et al. (2016) observed significant increases in nodulation and yield of cowpea inoculated with Brazilian rhizobia in northern Mozambique and northern Ghana.
Other indigenous microorganisms have also been identified, selected and proved to increase cowpea yield. A study carried out in a saline soil in Cuba demonstrated the efficacy of two indigenous strains (Bradyrhizobium liaoningense VIBA-1 and Bradyrhizobium yuanmingense VIBA-2) (Padilla et al. 2016) (Table 1). In another study, in Bangladesh, one strain isolated from cowpea nodules was identified as Rhizobium sp. SOY7 and presented excellent results of nodulation and plant growth, when compared with the non-inoculated control (Nushair et al. 2017).
Faba beans
Used in Chinese cooking for at least 5000 years, the origin of faba beans (Vicia faba L.) is still controversial (Duc 1997). Currently, the crop is produced and consumed in several countries, due to its adaptation to various climatic zones. The main producers are China, Italy, Spain, United Kingdom, Egypt, Ethiopia, Morocco, Russia, Mexico and Brazil (Duc et al. 2010; Lavania et al. 2015). However, there has been a considerable decline in the cropped area worldwide, mainly due to susceptibility to environmental stresses, affecting yield stability (Rubiales 2010).
In relation to the capacity of BNF, many soils favor the development of compatible rhizobial strains (Köpke and Nemecek 2010). The identification of rhizobia from nodules of faba beans indicate that the most common species are Rhizobium leguminosarum bv. viciae, Rhizobium fabae, Rhizobium laguerreae and Rhizobium anhuiense (Mutch and Young 2004; Tian et al. 2008; Saïdi et al. 2014; Zhang et al. 2015). Because of the high population of rhizobia in areas cropped with the legume for a long time, inoculation is usually not adopted. However, in regions where faba beans are not intensively cropped, or under stressful conditions, inoculation can benefit plant development (Köpke and Nemecek 2010; Youseif et al. 2017).
Faba beans are one of the most consumed grains in Egypt. Despite the predominantly low-fertility soils, inoculation is usually not performed and grain production is low, not attending the country’s demand. However, the potential of response to inoculation has been demonstrated in some studies, e.g. Youseif et al. (2017) evaluated 17 indigenous rhizobial strains from different regions of Egypt, and observed that seed inoculation increased grain yield (Table 1) and N accumulation, reaching up to 155 kg ha of N in grains.
In saline soils in Morocco, Benidire et al. (2017) reported two indigenous strains of R. leguminosarum (RhOF34 and RhOF125) that induced plant protection against salinity, leading to increases in nodulation, plant biomass and N content, confirming that indigenous species may have excellent results when inoculated in fava beans.
Other legumes
Legumes are generally part of the food base of people and animals throughout the world. In addition to soybeans and various types of beans, other crops are also important sources of protein and nutrients and serve as raw materials for many industrialized products. Therefore, raising the yield of these crops under a variety of environments, by means of inoculation with elite rhizobial strains has been the subject of several studies in several countries.
In Brazil, Bradyrhizobium sp. strain SEMIA 6144, originally from Africa, has been used in commercial inoculants for peanut (Arachis hypogaea); however, inoculation is not a common practice for this crop in the country, attributed to the lack of response, due to the high population of indigenous rhizobia. Indeed, peanut is a very promiscuous species capable of nodulating with a broad range of soil rhizobia (Thies et al. 1991b). However, an efficient contribution of the BNF may require specific elite strains, adapted to local biotic and abiotic conditions and may vary with the plant genotype. For example, Marcondes et al. (2010) evaluated the BNF efficiency of isolates from two peanut varieties (IAC 886 Runner and IAC Tatu ST) and verified that the bacteria performance varied with the plant genotype.
In 2017 the first inoculant was produced for peanuts in Africa, 1 year after the establishment of the first industrial plant for inoculant production in Ghana, in a partnership with Brazil (Fig. 2). Although it is still in the testing phase, the results are promising and peanut growers are expected to benefit from inoculation in the coming years.
Chickpea (Cicer arietinum) is a highly nutritive legume cropped mainly in India, but also in more than fifty other countries (Jukanti et al. 2012). Bacteria of the genus Mesorhizobium sp. are commonly found in association with chickpea (Laranjo et al. 2014) and Mesorhizobium ciceri has already been indicated for the production of inoculants. In Australia, M. ciceri strain CC1192 has been used in inoculants since the 1970s (Bullard et al. 2005). Besides, several studies have been carried out to identify indigenous strains capable of nodulating and promoting chickpea growth, even in unfavorable environments, such as low-fertility soils (Tena et al. 2016; Pandey et al. 2018).
Guar (Cyamopsis tetragonolobus L.) is a legume that has gained prominence in global agriculture due to several industrial uses, as their seeds are rich in galactomannan gum, which can be used as lubricant, binder, thickener and emulsifier. It is cultivated in several semi-arid regions such as in India, Pakistan and the United States (Ibrahim et al. 2016; Thapa et al. 2018). Similar to other legumes, guar has the potential to associate with rhizobia, but the process of nodulation with rhizobia is still not well known (Abidi et al. 2015); therefore, studies have been performed to identify elite rhizobial strains (Ibrahim et al. 2016; Khandelwal and Sindhu 2012). Thapa et al. (2018) evaluated two guar varieties inoculated with two rhizobial inoculants, one composed by a complex mixture of Rhizobium and the other carrying Rhizobium USDA 3385, on two soils of different textures, and promising results were found, as abundant nodulation, incentivizing further experiments.
An increasing number of yields increase have been reported for important crops such as soybeans, common beans and chickpeas inoculated with elite rhizobial strains, leading to interest in using microbial inoculants for several other legumes. However, it has also increased the interest for the use of other plant-growth promoting bacteria in non-legumes.
Maize
Maize (Zea mays L.) is a native grass from Central America (Doebley 1990a, b), and currently the third most cultivated cereal in the world. The interest in maize production is due to its versatility and broad use, ranging from human and animal feed to the production of biofuel, and also as an input in the manufacture of many products (Awika 2011). The main producers and consumers are the USA and China, followed by Brazil (DERAL 2019).
Maize can associate with PGPB, particularly those belonging to the genus Azospirillum, which are currently used as inoculants for this crop worldwide. Mexico was one of the first countries to commercialize inoculants for maize carrying Azospirillum in 2002 (Reis 2007), followed by Argentina.
Brazil has a long tradition in studies with Azospirillum, carried initially by Dr. Johanna Döbereiner. She described the capacity of Azospirillum, originally named as Spirillum, to perform BNF when associated with grasses. In 1978 the species Spirillum lipoferum, initially described by Beijerinck (1925), was reclassified as Azospirillum, with the prefix “azo” added as a reference to the term “azote,” nomenclature given by Lavoisier to nitrogen. At that time, the genus comprised two species, Azospirillum lipoferum and Azospirillum brasilense (Tarrand et al. 1978). Other species of Azospirillum were described in the following years, so that in 2019 the genus comprises 21 species (DSMZ 2019).
However, it was only in 2009 that the first commercial strains of A. brasilense, Ab-V5 and Ab-V6, were released for the use in commercial inoculants for maize and wheat (Triticum aestivum L.) in Brazil (Hungria et al. 2010; MAPA 2011). In maize, these strains resulted in increases in grain yield that reached 27%, compared with the non-inoculated control (Hungria et al. 2010) (Table 1). Since the release of the first commercial inoculant for grasses in Brazil, in 2009 (Fig. 2), the number of sold doses of inoculants carrying A. brasilense has grown significantly, reaching about 7 million doses in the 2017/18 crop season. In Argentina, the market of Azospirillum has started before Brazil, with the commercial strain A. brasilense Az39 selected in the 1980s and able to increase maize and wheat yields from 13 to 33% (Cassán et al. 2015; Cassán and Diaz-Zorita 2016).
In addition to its ability for BNF, numerous studies have demonstrated other properties of Azospirillum, the most important being the capacity for synthesizing phytohormones. Many of these molecules are related to root development, positively influencing their growth, resulting in greater absorption of nutrients and water from soil (Bashan and De-Bashan 2010; Ardakani and Mafakheri 2011; Fukami et al. 2017, 2018a, b). Therefore, grasses associated with Azospirillum present root structure capable of absorbing larger amounts of nutrients and water (Bashan and De-Bashan 2010). Auxins (Fallik et al. 1989; Fukami et al. 2017), gibberellins (Janzen et al. 1992; Cohen et al. 2009), ethylene (Perrig et al. 2007), cytokinins (Strzelczyk et al. 1994; Abbasi et al. 2015) and salicylic acid (Perrig et al. 2007; Cohen et al. 2009; Fukami et al. 2017) are the most commonly cited molecules.
Turan et al. (2012) emphasized the capacity of P solubilization by some strains of Azospirillum, increasing P availability in the soil and yields of wheat. Some strains of Azospirillum may also attenuate damages caused by abiotic stress, such as salinity and drought, as well as biotic stresses, like plant resistance against pathogens (Bashan and De-Bashan 2010; Fukami et al. 2018a).
Despite the benefits of Azospirillum in cereals, the bacterium is not able to supply all N demand, requiring the application of complementary doses of N. However, the amount of N-fertilizer to achieve high yields can be reduced by 25 to 50% (Hungria et al. 2010; Piccinin et al. 2013; Fukami et al. 2016).
Although Azospirillum is mainly inoculated on the seeds due to easiness and low doses (Cassán et al. 2015), the seed treatment with pesticides is potentially harmful and may impair the survival and metabolism of the inoculated cells. To overcome such problem, alternative methods of inoculation via foliar, in-furrow or soil spraying can be used. Fukami et al. (2016) evaluated the responses of maize inoculated with Azospirillum in-furrow, via soil spraying at sowing or via leaf spraying after seedlings had emerged, in comparison seed inoculation. Positive results were obtained with both alternative methods of inoculation, but higher doses were required than inoculation via seeds.
Besides Azospirillum, other groups of PGPB have been studied in inoculation of maize, such as Pseudomonas spp. (Burr et al. 1978; Ahirwar et al. 2015; Thirumal et al. 2017; Sandini et al. 2019). Pseudomonas are able to produce siderophores, which are molecules capable of capturing insoluble iron from the environment (Fe3+), and convert it to a soluble form (Fe2+) available for plants (Sharma and Johri 2003; Sah et al. 2017). Considering that iron is essential for metabolism and consequently, for plant development, the siderophores-producing microorganisms can positively improve plant development in Fe-deficient environments.
The production of siderophores by P. aeruginosa strains RSP5 and RSP8 was demonstrated in iron sufficient and iron-deficient soil (Sah et al. 2017). The strain RSP5 produced more siderophores in both soils and improved the Fe uptake by maize, in addition to increases in shoot and root length, number of spikes and number of grains. However, we must emphasize that many PGPB may also be highly pathogenic to humans, animals and plants. Therefore, it is critical to evaluate the non-pathogenicity of the strains before thinking about any use as inoculant and, certainly, P. aeruginosa is not a proper candidate for a commercial inoculant.
The use of Bacillus strains as inoculants is also increasing, in replacement to fertilizers. In Brazil, strains have been selected that improve P mobilization, by mechanisms as phytohormones production and P solubilization, this last one attribute to acid production by the bacteria (de Abreu et al. 2017). In Brazil, elite strains of Bacillus proved to improve P uptake production of grasses (Ribeiro et al. 2018), and the first commercial inoculant carrying P-solubilizing bacteria (Bacillus subtilis and B. megaterium) was released in 2019, with great acceptance by the farmers.
Wheat
Wheat is a cereal of global importance for human and animal feeding and can also benefit from inoculation with A. brasilense (Bashan et al. 2004; Hungria et al. 2010). In the 1980s an important study was carried out in Mexico on the inoculation of wheat with Azospirillum. The concentration of the inoculant was 3–5 × 108 CFU/g and the dose applied of 15 g/kg seed. Inoculation caused significant increases in yield, from 23 to 63% in 1986, and from 24 to 43% in 1987. The best results were obtained with strain Cd and with a local A. brasilense strain isolated from the rhizosphere of Brachiaria mutica (UAP-55) (Caballero-Mellado et al. 1992).
In the following decade, in Argentina, many studies were carried out with inoculation of Azospirillum. In 1992–1993 two experiments were carried out with inoculation of strains Az39 and Cd on wheat under greenhouse conditions using soil from a semiarid region of Argentina. Az39 and Cd strains increased the grain yield by 30% and 16%, respectively, and both increased the root dry weight compared with the non-inoculated control (Rodriguez-Caceres et al. 1996a). Nowadays, Az39 is the major strain used in commercial inoculants in Argentina (Okon et al. 2015).
In Brazil, Hungria et al. (2010) observed 13 to 18% increase in grain yield of wheat inoculated with A. brasilense Ab-V1, Ab-V5, Ab-V6 and Ab-V8 strains. When the strains Ab-V5 and Ab-V6 were combined, wheat yields increased by 31%; therefore, inoculant industries have mixed both strains in wheat inoculants (Hungria et al. 2010) (Table 1).
Further beneficial action of A. brasilense has been reported on wheat, such as the photo-protection of photosynthetic pigments and increase of proton efflux of roots, positively affecting plant development (Bashan et al. 1989, 2005).
Successful wheat inoculation with Azospirillum has also being reported in Israel (Kapulnik et al. 1983, 1985), England (Harris et al. 1989), Egypt (El-Lattief 2012), and Pakistan (Zaheer et al. 2019). Unfortunately, despite numerous studies proving the benefits of wheat inoculation, this practice is poorly adopted, especially in the major wheat-producing countries such as European Union, Russia, China, India and the United States.
Rice
The origin of rice (Oryza sativa) is estimated at least 130 million years ago in Asia and has spread over the years all over the planet (Khush 1997), representing about 11% of the global cropped area. This cereal represents the primary source of food for more than one-third of the world’s population; unlike other crops, rice is consumed almost exclusively by humans (Khush 1997; Singh et al. 2018).
More than 90% of the world’s rice is grown and consumed in Asia, where it accounts for 35 to 60% of the calories consumed by 3 billion people, 60% of the worlds’ population (Khush 1997; Seck et al. 2012; Singh et al. 2018). The main producers are China, India, Indonesia and Bangladesh, with the production of 145.5; 103.5; 36.3 and 34.6 million tons, respectively (Gadal et al. 2019).
Similar to the grasses earlier mentioned, rice can also benefit from the inoculation with PGPB. Although rice is typically grown in wetland, upland cropping is very important in several countries. In wetland, rice can be associated with aerobic and anaerobic PGPB (Choudhury and Kennedy 2004). Many bacterial species have been evaluated over the years, single or associated, for growth promotion of rice, e.g. A. lipoferum (Watanabe and Lin 1984; Mirza et al. 2000), A. brasilense (de Salamone et al. 2012; Zhang et al. 2017) Pseudomonas spp. (Watanabe and Lin 1984; de Salamone et al. 2012; Zhang et al. 2017), Herbaspirillum spp. (Baldani et al. 2000; Mirza et al. 2000), Burkholderia spp. (Baldani et al. 2000; Tran et al. 2000; Govindarajan et al. 2007), Bradyrhizobium sp. (Greetatorn et al. 2019).
One of the most important studies related to inoculants for rice was carried out in Vietnam from 1999 to 2001 (Nguyen et al. 2003) and resulted in a commercial inoculant named “Biogro”. Three bacterial strains isolated from soils cropped with rice were selected and their inoculation promoted increase in grain yield compared with the non-inoculated control, reaching yields of 6.7; 6.0 and 6.2 t/ha in 1999, 2000 and 2001, respectively, when 111 kg/ha of biofertilizer were applied; the overall mean increase over the non-inoculated control was of 15% (728 kg/ha), ranging from 8.3 to 30.7%. (Nguyen et al. 2017). Similar results were obtained 1 year later in Australia, using the same mix of bacteria (Williams and Kennedy 2002).
Before 2005, the strains in “Biogro” were Klebsiella pneumoniae (4P), Pseudomonas fluorescens (1N) and Citrobacter freundii (3C) (Kecskes et al. 2008). From 2005 on, the inoculant was reformulated with the strains P. fluorescens (1N), B. subtilis (B9), Bacillus amyloliquafaciens (E19) and a soil yeast, Candida tropicalis (HY) (Nguyen et al. 2017). In addition to BNF, the pool of microorganisms also improved the P mobilization from soil. In field trials the new inoculant applied at a rate of 50 kg/ha promoted grain yield of 6.91 t/ha (Nguyen 2008; Nguyen et al. 2017) (Table 1). This inoculant was also efficient in rice grown on a degraded soil in the south of Vietnam (Phan and Tran 2008).
Sugarcane
An economically important Poaceae is sugarcane. Belonging to the genus Saccharum, it is native from the tropical region of South and Southeast Asia (Mukherjee 1957). After many taxonomic revisions that occurred mainly during the twenty ninth century, currently the genus Saccharum has six species: S. officinarum, S. spontaneum, S. robustum, S. sinense, S. barberi e S. edule. Current sugarcane varieties are hybrids originating from interspecific crosses involving mainly 90% of S. officinarum and 10% of S. spontaneum. These hybrids are cited as Saccharum spp. (Ming et al. 2006).
America and Asia are the main sugarcane producing regions, such that in 2017 accounted for 55.7% and 37.2% of world sugarcane production, respectively (FAOSTAT 2019). The largest sugarcane producing country is Brazil, producing 758 Mt in 2017, about 41% of the world production. India, China, Thailand, Pakistan and Mexico are also important producers, contributing with 306, 104, 103, 73 e 57 Mt of sugarcane, respectively (FAOSTAT 2019).
The economic importance of this culture is related to its various purposes. Sugarcane is a raw material in the production of ethanol, biofuel widely used mainly in Brazil, in addition to the production of sugar and cane molasses, products for the food and feed industry; the vast market of products keeps its production growing continuously (Silalertruksa and Gheewala 2019).
Sugarcane is able to associate with a great diversity of diazotrophic plant growth-promoting bacteria, including species of the genera Azospirillum (Reis Junior et al. 2000; Tejera et al. 2005), Azotobacter (Tejera et al. 2005), Burkholderia (Perin et al. 2006; Antonio et al. 2016; Silva et al. 2016; Leite et al. 2018a, b), Herbaspirillum (Baldani et al. 1996; Reis Junior et al. 2000), Pantoeae (Taulé et al. 2012; Fischer et al. 2012, Silva et al. 2016), and the species Gluconacetobacter diazotrophicus (basonym Acetobacter diazotrophicus) (Cavalcante and Döbereiner 1988; Munõz-Rojas and Caballero-Mellado 2003; Restrepo et al. 2017), among others.
After the isolation and description of sugarcane-associated diazotrophic bacteria, and in view of the observed benefits of bacterial/plant association for other cultures, research has been intensified in Brazil. Dos Santos et al. (2018) observed the effects of inoculating a mix of diazotrophic bacteria (G. diazotrophicus PAL5T, Herbaspirillum rubrisubalbicans HCC10, Herbaspirillum seropedicae HRC54, Nitrospirillum amazonense CBAmC and Paraburkholderia tropica PPe4T) on sugarcane growth. After 15 days of planting, a 50% increase in dry mass of inoculated roots was observed.
The same group of bacteria was used in hydroponic sugarcane cultivation for 59 days under different concentrations of N. Two varieties of sugarcane were used: RB867515 (adapted to low fertility soils) and IACSP95-5000 (adapted to medium to high fertility soils). The authors reported that the two sugarcane varieties, when inoculated with the bacterial mix, presented different results regarding the activity of enzymes related to the assimilation of N. Under low N concentration, nitrate reductase activity was increased in RB867515 by 26% in the shoots, and by 48% in the roots, while glutamine synthetase activity was 21% higher than the control. For the IACSP95-5000 under low N concentration, nitrate reductase activity decreased by 62% in roots, and glutamine synthetase activity was increased by 16% (Dos Santos et al. 2019). This information corroborates with Schultz et al. (2017), who analyzed yield parameters in two field sites and with two sugarcane varieties (RB867515 and RB72454) inoculated or the same bacterial mix. For variety RB867515 the inoculation promoted increases in stem yield by 22.3 Mg ha−1 in the first site and 38.0 Mg ha−1 in the second site compared to the control. The variety RB72454 showed increases of 16.7 and 37.5 Mg ha−1, respectively.
Optimum yield results via inoculation with the same bacterial mix suggest reduced N-fertilizer application. Pereira et al. (2018) consider that inoculation coupled with the application of a low dose of N (50 kg N ha−1) can raise productivity with economy. In 2019 the first commercial inoculant for the sugarcane was released in Brazil, carrying Nitrospirillum amazonense strain.
Pastures with grasses and legumes
Estimates are that the global pasture area covers 26% of the ice-free land surface, but in many of these places, the pastures are degraded and insufficient to provide nutrients to the animals, demanding new areas (Steinfeld et al. 2006; Fonte et al. 2014). The major problem in increasing pasture areas is that they often occur in detriment of forests, leading to deforestation, decrease in biodiversity and other environmental damages (Steinfeld et al. 2006; Don et al. 2011).
In order to improve the development of grasses in degraded pastures, the use of PGPB is once again a viable strategy. The idea is to increase soil fertility, yield and nutritional quality of grasses, decreasing the pressures on native forests (Monk et al. 2009; Campos et al. 2012; Hungria et al. 2016).
Grasslands in Brazil are estimated in 180 million ha, of which over 60 million ha are classified as degraded (LAPIG 2018), with Brachiaria (= Urochloa) representing the main component (Hungria et al. 2016). Strains Ab-V5 and Ab-V6 of A. brasilense have been evaluated as inoculants for Urochloa spp. in different sites of Brazil and the combination with N-fertilizer (40 kg ha of N) increased biomass production by 15% and of protein by 25% in comparison to the control receiving only N-fertilizer (Hungria et al. 2016). Other studies confirmed the good performance of these strains of A. brasilense with brachiarias (Bulegon et al. 2016; Guimarães et al. 2016; Leite et al. 2018a, b), and also with another important pasture in Brazil, panicum Panicum maximum, = Megathyrsus maximus) (Leite et al. 2019). In addition to A. brasilense, positive results were reported for brachiaria inoculated with Bacillus sp. isolated from the rhizosphere of Urochloa brizantha (Araujo et al. 2012).
In New Zealand, Monk et al. (2009) isolated bacteria capable of colonizing the roots of tall fescue (Festuca arundinacea) grasses with promising characteristics for pastures. The isolated bacteria were studied in vitro and selected for their plant-growth promotion properties, such as the production of auxins, siderophores and P solubilization.
In Colombia, Pennisetum clandestinum (kikuyo) was inoculated with two PGPB strains of Stenotrophomonas sp. and Pseudomonas sp. able to synthesize indole compounds, to fix nitrogen and to solubilize phosphate in vitro. Under greenhouse conditions, significant increases in the biomass and root dry weight were observed in comparison to the non-inoculated control.
Pastures with legumes are also spread all over the world, and Trifolium spp., Arachis pintoi, Medicago sativa L., Stylosanthes spp. are important examples. Dozens of studies have been performed with PGPB with those legumes. Trifolium repens and Trifolium pratense are two clovers species broadly used in pastures in Uruguay. To ensure good development there is a recommendation, since 1967, of inoculation of both clovers with R. leguminosarum sv. trifolii strain U204, a commercial inoculant strain introduced from the USA (Tartaglia et al. 2019).
Alfalfa (Medicago sativa L.) is present in pastures in temperate and subtropical, and arid and semi-arid areas. Buntić et al. (2019) developed a liquid-formulated inoculant containing Sinorhizobium (= Ensifer) meliloti strain L3Si allowing better shelf life, pre-inoculation and performance in alfalfa, as there were no liquid inoculants available with this strain. Shoot N content of plants originated from seeds pre-inoculated 1 month before sowing ranged from 3.72 to 4.19%, whereas the control with N-fertilizer had 4.03%; the highest SDW value was of 27.12 mg/plant in the inoculated plants, higher than the control with N-fertilizer (20.20 mg/plant), indicating a high effectiveness of the liquid formulation (Buntić et al. 2019).
Interest in increasing alfalfa production has also growing in Saudi Arabia. Daur et al. (2018) isolated, identified and exploited the PGPR potential of 17 bacterial isolates belonging to the genus Bacillus, Acinetobacter and Enterobacter from the Saudi Arabia desert and evaluated their effects on alfalfa yield. The strains were single inoculated in alfalfa seeds and sown in the fields under desert conditions. All strains improved plant relative water content, chlorophyll (a and b), carotenoids, N, P and K contents, plant height, leaf-to-stem ratio and fresh and dry weight in comparison to the non-inoculated control. However, one major consideration in this and in several other studies is the need of regulation to avoid potentially pathogenic strains in microbial inoculants, such as Acinetobacter, Enterobacter and even some species of Bacillus.
In Brazil, forage peanuts (A. pintoi) and Stylosanthes spp. are the most commonly used legumes in pastures. For A. pintoi, two Bradyrhizobium spp. strains are used in commercial inoculants, SEMIA 6439 (= MGAP 13) and SEMIA 6440 (= NC 230). In a field experiment that resulted in the selection of these two strains, they increased shoot dry weight, in comparison to the non-inoculated controls, without and with N-fertilizer, by 63 and 47%, respectively (Purcino et al. 2003). More recently, estimates of BNF in A. pintoi under field conditions were up to 65% of the total N in plants in the spring period (Carvalho et al. 2019).
Despite the widespread use of Stylosanthes spp. in Brazil, there are still few studies about the diversity and symbiotic efficiency of nitrogen-fixing bacteria associated to this plant. Two strains have been used in commercial inoculants, B. japonicum SEMIA 6155 (= BR 502) and SEMIA 6154 (= BR 446); recently, SEMIA 6154 was recognized as the type strain of a new species, Bradyrhizobium stylosanthis (Delamuta et al. 2016). da Chaves et al. (2016) reported that two bacterial species isolated from Stylosanthes (strains ERR 1178 and ERR 942 of Bradyrhizobium spp.) in savanna areas in Roraima, Brazil, increased the shoot biomass and N of Stylosanthes capitata cv. Lavradeiro under greenhouse conditions.
Australia has a long-time tradition in selecting strains and inoculating forage legumes, with emphasis on Trifolium spp. (Brockwell et al. 1982; Collins et al. 2002; Yates et al. 2005). More recently, in the inland areas of central Queensland, Leucaena has been sown and provided excellent results as forage in animal production (Buck et al. 2019); however, the inoculation of this legume is still little studied in the country.
Vegetables
Vegetables can highly benefit from several PGPB, but this market niche is still not well explored. Taken as an example, tomato (Solanum lycopersicum L.) takes part in the diet of million people, consumed in salads, as ingredient of hot dishes and with great application in the industry as raw material in the manufacture of many products, mainly sauces (Subramanian 2016). Due to its versatility, tomatoes are one of the most produced vegetables worldwide. China accounts for one-quarter of world’s tomato production, followed by India and the USA (Heuvelink 2018).
Tomatoes may respond to inoculation with Azospirillum (Alfonso et al. 2005; Mangmang et al. 2015a; Lima et al. 2018). In Colombia, inoculation with A. brasilense resulted in better seedling growth, plant nutritional status, and yield 11% higher than the non-inoculated control (Alfonso et al. 2005) (Table 1).
In India, PGPB of the genera Bacillus and Azotobacter were isolated from the rhizosphere of tomatoes and tested as inoculants for this crop (Prashar et al. 2014). Previous reports from Cuba show that inoculation of tomatoes seeds with Azotobacter chroococcum increased plant dry weight (Puertas and Gonzales 1999). In Brazil, positive effects of inoculation of two tomatoes cultivars with Bacillus amyloliquefaciens subsp. plantarum FZB42 have also been reported (Szilagyi-Zecchin et al. 2015), increasing shoot growth, chlorophyll a, b and total, and favoring the synthesis of indole compounds and siderophores.
Several other vegetables have been reported as responsive to microbial inoculants, including lettuce (Lactuca sativa) (Flores-Félix et al. 2013; Mangmang et al. 2014; Fasciglione et al. 2015), carrot (Daucus carota L.) (Flores-Félix et al. 2013; Clemente et al. 2016) and cucumber (Cucumis sativus L.) (Mangmang et al. 2015b). The increasing demands of the population on organic products may also stimulate the use of microbial inoculants for the production of vegetables.
Some of the actual threats for the use of microbial inoculants
Attention should be paid to some threats that appear from the increased scientific and commercial interest on microbial inoculants. Several studies are reporting plant-growth promoting benefits in studies with bacteria that may be harmful to plants, animals and humans. Analyzing these studies, there is no doubt that several strains of Enterobacter spp., of the Burkholderia cepacia complex, Pseudomonas aeruginosa, among others, can be isolated from soils and have the capacity of promoting plant growth (e.g. Adesemoye et al. 2008; Daur et al. 2018; Jung et al. 2018; Rojas-Rojas et al. 2019; Roychowdhury et al. 2019). However, they cannot be used as inoculants. Therefore, before proceeding with studies to verify the plant performance with such isolates, priority should be given to determine their taxonomic position.
In relation to agronomic practices, the compatibility with agrochemicals used for seeds treatments, with an emphasis on pesticides represents a major limitation to the survival of bacteria (e.g. Campo et al. 2009), and the problem has increased with the use of pre-inoculated seeds stored for long periods in contact with pesticides (Hungria and Mendes 2015). Priority should be given to the search for compatible agrochemicals and cell protectors (Hungria et al. 2005), or alternative technologies of application, such as the application of inoculants in-furrow to avoid the direct contact with the products used for seed treatment (Campo et al. 2010).
Amazingly, information about the benefits of microorganisms on plant growth is leading some farmers to the production of their own microbial inoculants and products for biological control. It is not difficult to perceive the threat that such practice can result to the agriculture. Production of microbial inoculants require specific requirements not easily followed even under specialized conditions (Hungria et al. 2005). Therefore, plant, human and animal pathogens have been found as predominant microorganisms in farmers´ products (Valicente et al. 2018; Hungria and Nogueira 2019) and may jeopardize the benefits of high-quality products.
Perspectives for the future
Research on inoculants and inoculation with rhizobia and legumes raised great interest from researchers and companies in the 1970s. In the following decades, although several reports of benefits of new PGPB and the advances achieved at the inoculant industry, modest interest from research and industry has been observed. However, nowadays, increased demand for food, interest in sustainable agriculture and increasing reports on pests and pathogens resistance to agrochemicals are exponentially raising the global interest on microbial inoculants. Based on the information presented in this brief review, it is possible to perceive the increased number of studies that have been carried out about the development of new inoculants (Santos et al. 2017; Gundi et al. 2018), identification of new strains, and new inoculation methods, e.g. Zvinavashe el al. (2019), who developed a protein-based biomaterial capable of encapsulating and protecting rhizobacteria inoculated into seeds even after sowing, improving the effects of inoculation. According to information from the Web of Science database, between 2015 and 2019, 68 papers (excluding revisions) were published using the keywords “inoculant” or “biofertilizer” followed by “production” or “development”. Therefore, it is expected that in the following years innovation will be presented, encompassing both microorganisms and technologies. China currently leads the number of registered patents related to inoculation, more than 800, and India already has more than 100 inoculant industries (Fig. 2). It is expected that these numbers will also increase in other countries.
One challenge to the development of new inoculants relies on the increasing concerns about climate changes. The expected increases in temperature and dry periods in the next years will have major impacts on agriculture. According to Ramirez-Villegas and Thornton (2015), in tropical areas, maize and rice yields may decrease by 5–10% and 2–5%, respectively, for each degree of temperature increase. Climate changes will decrease the available areas for cultivation. It is therefore mandatory to search for microbial inoculants more effective under stressful conditions; on the other hand, microbial inoculants can also help to mitigate the effects of climate changes and other related abiotic stresses, such as salinity (e.g. Cerezini et al. 2016; Fukami et al. 2018b; Leite et al. 2018a, b). With increased availability of high-quality products, in addition to commitments from the governments towards more sustainable agricultural systems, the use of microbial inoculants is expected to dramatically increase in the following years.
Acknowledgements
Research group supported by the INCT-Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq 465133/2014-2, Fundação Araucária-STI 043/2019, CAPES), and CNPq-Universal (400468/2016-6). M.S.S. acknowledges a Ph.D. fellowship from Araucaria Foundation of support to the Scientific and Technological Development of the State of Paraná.
Abbreviations: All abbreviations have been cited in their complete forms when mentioned for the first time in the manuscript.
Authors’ contributions
MSS, MAN, MH participated in all stages of writing the manuscript. All authors read and approved the final manuscript.
Funding
Financed by INCT-Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq 465133/2014-2, Fundação Araucária-STI 043/2019, CAPES); CNPq-Universal (400468/2016-6), Embrapa.
Availability of data and materials
All data and materials cited in the manuscript are freely available for the scientific community.
Ethics approval and consent to participate
Authors declare no ethical problems. The study has not involved any human or animal participation or data.
Consent for publication
All authors gave the consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mariana Sanches Santos, Email: mari_sanches_s@hotmail.com.
Marco Antonio Nogueira, Email: marco.nogueira@embrapa.br.
Mariangela Hungria, Email: mariangela.hungria@embrapa.br, Email: biotecnologia.solo@hotmail.com.
References
- Abbasi K, Mir-Mahmoodi T, Jalilnezhad N. Effects of Azospirillum bacteria and cytokinin hormone on morphology, yield and yield components of corn (Zea mays L.) Int J Biol Sci. 2015;6:378–386. doi: 10.12692/ijb/6.3.378-386. [DOI] [Google Scholar]
- Abidi N, Liyanage S, Auld D, Imel RK, Norman L, Grover K, Angadi S, Singla S, Trostle C. Challenges and opportunities for increasing guar production in the United States to support unconventional oil and gas production. In: Uddameri V, Morse A, Tindle KJ, editors. Hydraulic fracturing impacts and technologies. 1. Boca Raton: CRC Press; 2015. pp. 207–226. [Google Scholar]
- Adesemoye AO, Obini M, Ugoji EO. Comparison of plant growth-promotion with Pseudomonas aeruginosa and Bacillus subtilis in three vegetables. Braz J Microbiol. 2008;39(3):423–426. doi: 10.1590/S1517-83822008000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahirwar NK, Gupta G, Singh V, Rawlley RK, Ramana S. Influence on growth and fruit yield of tomato (Lycopersicon esculentum Mill.) plants by inoculation with Pseudomonas fluorescence (SS5): possible role of plant growth promotion. Int J Curr Microbiol Appl Sci. 2015;4:720–730. doi: 10.1016/j.sjbs.2012.10.004. [DOI] [Google Scholar]
- AIRG (Australian Inoculants Research Group) (2010) National code of practice and quality trademark for legume microbial inoculant products used in Australian crops and pastures. https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0008/361295/Web-Version-of-the-NATIONAL-CODE-OF-PRACTICE-and-TRADE-MARK-LOGO-2Nov2010_final.pdf. Accessed 16 Sept 2019
- Alfonso ET, Leyva A, Hernández A. Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon esculentum, Mill) Rev Colomb Biotecnol. 2005;7:47–54. [Google Scholar]
- Aliyev JA, Mirzoyev RS. Photosynthesis and productivity of soybean [Glycine max (L.) Merrill] Proc ANAS (Biol Sci) 2010;65(5–6):60–70. [Google Scholar]
- ANPII—Associação Nacional dos Produtores e Importadores de Inoculantes (2016) Congresso debate microrganismos no futuro da lavoura. http://www.anpii.org.br/congresso-debate-microrganismos-no-futuro-da-lavoura. Accessed 15 Jan 2019
- ANPII—Associação Nacional dos Produtores e Importadores de Inoculantes (2018) Levantamento do uso de Inoculantes no Brasil. VIII Congresso Brasileiro de Soja, Goiânia, 2018
- Antonio CS, Rouws LFM, Teixeira KRS, Reis VM. Diazotrophic bacteria associated to sugarcane varieties cropped at Northeast Region of Brazil. Rev Bras Ciênc Agrár. 2016;4:272–280. doi: 10.5039/agraria.v11i4a5393. [DOI] [Google Scholar]
- Araujo FF, Guaberto LM, Silva IF. Bioprospecção de bactérias promotoras de crescimento em Brachiaria brizantha. R Bras Zootec. 2012;41:521–527. doi: 10.1590/S1516-35982012000300007. [DOI] [Google Scholar]
- Ardakani M, Mafakheri S. Designing a sustainable agroecosystem for wheat (Triticum aestivum L.) production. J Appl Environ Biol Sci. 2011;1(10):401–413. [Google Scholar]
- Argawa A, Mnalku A. Effectiveness of native Rhizobium on nodulation and yield of faba bean (Vicia faba L.) in Eastern Ethiopia. Arch Agron Soil Sci. 2017;63:1390–1403. doi: 10.1080/03650340.2017.1287353. [DOI] [Google Scholar]
- Aserse AA, Räsänen LA, Assefa F, Hailemariam A, Lindström K. Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst Appl Microbiol. 2012;35:120–131. doi: 10.1016/j.syapm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Atieno M, Herrmann L, Okalebo R, Lesueur D. Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J Microbiol Biotechnol. 2012;28:2541–2550. doi: 10.1007/s11274-012-1062-x. [DOI] [PubMed] [Google Scholar]
- Awika JM. Major cereal grains production and use around the world. In: Awika JM, Piironen V, Bean S, editors. Advances in cereal science: implications to food processing and health promotion. 1. Washington, DC: American Chemical Society; 2011. pp. 1–13. [Google Scholar]
- Baldani JI, Pot B, Kirchhof G, Falsen E, Baldani VLD, Olivares FL, Hoste B, Kersters K, Hartmann A, Gillis M, Dobereiner J. Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a mild plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Evol Microbiol. 1996;46:802–810. doi: 10.1099/00207713-46-3-802. [DOI] [PubMed] [Google Scholar]
- Baldani DVL, Baldani JI, Döbereiner J. Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol Fertil Soils. 2000;30:485–491. doi: 10.1007/s003740050027. [DOI] [Google Scholar]
- Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol. 1986;51:1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y, De-Bashan LE. How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron. 2010 doi: 10.1016/s0065-2113(10)08002-8. [DOI] [Google Scholar]
- Bashan Y, Levanony H, Mitiku G. Changes in proton efflux of intact wheat roots induced by Azospirillum brasilense Cd. Can J Microbiol. 1989;35:691697. doi: 10.1139/m89-113. [DOI] [Google Scholar]
- Bashan Y, Hernandez J, Leyva LA, Bacilio M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol Fertil Soils. 2002;35:359–368. doi: 10.1007/s00374-002-0481-5. [DOI] [Google Scholar]
- Bashan Y, Holguin G, de-Bashan LE. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003) Can J Microbiol. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- Bashan Y, Bustillos JJ, Leyva LA, Hernandez JP, Bacilio M. Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol Fertil Soils. 2005;42:279–285. doi: 10.1007/s00374-005-0025-x. [DOI] [Google Scholar]
- Bashan Y, De-Bashan L, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Beebe SE, Rao IM, Blair MW, Acosta-Gallegos JA. Phenotyping common beans for adaptation to drought. Front Physiol. 2013;4:1–20. doi: 10.3389/fphys.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijerinck MW. Uber ein Spirillum, welches freien Stickstoff binden kann? Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt. 1925;2:353–359. [Google Scholar]
- Benidire L, Lahrouni M, El Khalloufi F, Göttfert M, Oufdou K. Effects of Rhizobium leguminosarum inoculation on growth, nitrogen uptake and mineral assimilation in Vicia faba plants under salinity stress. JAST. 2017;19(4):889–901. [Google Scholar]
- Berg G, Köberl M, Rybakova D, Müller H, Grosch R, Smalla K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol Ecol. 2017;93:1–9. doi: 10.1093/femsec/fix050. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Boddey RM, Fosu M, Atakora WK, Miranda CHB, Boddey LH, Guimarães AP, Ahiabor BDK. Cowpea (Vigna unguiculata) crops in Africa can respond to inoculation with Rhizobium. Exp Agric. 2016;53:578–587. doi: 10.1017/S0014479716000594. [DOI] [Google Scholar]
- Brockwell J, Gault RR, Zorin M, Roberts MJ. Effects of environmental variables on the competition between inoculum strains and naturalized populations of Rhizobium trifolii for nodulation of Trifolium subterraneum L. and on rhizobia persistence in the soil. Aust J Agric Res. 1982;33(5):803–815. doi: 10.1071/AR9820803. [DOI] [Google Scholar]
- Buck S, Rolfe J, Lemin C, English B. Establishment of Leucaena in Australia. Trop Grassl-Forrajes Trop. 2019;7:104–111. doi: 10.17138/TGFT(7)104-111. [DOI] [Google Scholar]
- Bulegon LG, Guimarães VF, Laureth JCU. Azospirillum brasilense affects the antioxidant activity and leaf pigment content of Urochloa ruziziensis under water stress. Pesq Agropec Trop. 2016;46:343–349. doi: 10.1590/1983-40632016v4641489. [DOI] [Google Scholar]
- Bulegon LG, Guimarães VF, Klein J, Batisttus AG, Inagaki AM, Offmann LC, Souza AKP. Enzymatic activity, gas exchange and production of soybean co-inoculated with Bradyrhizobium japonicum and Azospirillum brasilense. Aust J Crop Sci. 2017;11:888–896. doi: 10.21475/ajcs.17.11.07.pne575. [DOI] [Google Scholar]
- Bullard GK, Roughley RJ, Pulsford DJ. The legume inoculant industry and inoculant quality control in Australia: 1953–2003. Aust J Exp Agric. 2005;45:127–140. doi: 10.1071/EA03159. [DOI] [Google Scholar]
- Buntić AV, Stajković-Srbinović OS, Knežević MM, Kuzmanović DZ, Rasulić NV, Delić DI. Development of liquid rhizobial inoculants and pre-inoculation of alfalfa seeds. Arch Biol Sci. 2019;71:379–387. doi: 10.2298/ABS181008062B. [DOI] [Google Scholar]
- Burr TJ, Schroth MN, Suslow T. Increased potato yields by treatment of seed pieces with specific strains of Pseudomonas fluorescens and P. putida. Phytopathology. 1978;68:1377–1383. doi: 10.1094/Phyto-68-1377. [DOI] [Google Scholar]
- Caballero-Mellado J, Carcano-Montiel M, Mascarua Esparza MA. Field inoculation of wheat (Triticum aestivum) with Azospirillum brasilense under temperate climate. Symbiosis. 1992;13:243–253. [Google Scholar]
- Campo RJ, Araujo RS, Hungria M. Nitrogen fixation with the soybean crop in Brazil: compatibility between seed treatment with fungicides and bradyrhizobial inoculants. Symbiosis. 2009;48:154–163. doi: 10.1007/BF03179994. [DOI] [Google Scholar]
- Campo RJ, Araujo RS, Mostasso FL, Hungria M. In-furrow inoculation of soybeans as alternative for fungicides and micronutrients seed treatment and inoculation. Rev Bras Ciênc Solo. 2010;34:1103–1112. doi: 10.1590/S0100-06832010000400010. [DOI] [Google Scholar]
- Campos PJC, Obando M, Sánchez ML, Bonilla R. Efecto de bacterias promotoras de crecimiento vegetal (PGPR) asociadas a Pennisetum clandestinum en el altiplano cundiboyacense. Cien Tecnol Agropecuaria. 2012;13(2):189–195. [Google Scholar]
- Canfield DE, Glazer AN, Falkowski PG. The evolution and future of earth’s nitrogen cycle. Science. 2010;330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- Carvalho LR, Pereira LET, Hungria M, Camargo PB, da Silva SC. Nodulation and biological nitrogen fixation (BNF) in forage peanut (Arachis pintoi) cv. Belmonte subjected to grazing regimes. Agric Ecosyst Environ. 2019;278:96–106. doi: 10.1016/j.agee.2019.02.016. [DOI] [Google Scholar]
- Cassán F, Diaz-Zorita M. Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem. 2016;103:117–130. doi: 10.1016/j.soilbio.2016.08.020. [DOI] [Google Scholar]
- Cassán F, Okon Y, Creus CM. Handbook for Azospirillum: technical issues and protocols. Cham: Springer International Publishing; 2015. [Google Scholar]
- Cavalcante VA, Döbereiner J. A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil. 1988;108:23–31. doi: 10.1007/BF02370096. [DOI] [Google Scholar]
- Cerezini P, Kuwano BH, Santos MB, Terassi F, Hungria M, Nogueira MA. Strategies to promote early nodulation in soybean under drought. Field Crop Res. 2016;196:160–167. doi: 10.1016/j.fcr.2016.06.017. [DOI] [Google Scholar]
- Chang WS, Lee HI, Hungria M. Soybean production in the Americas. In: Lugtenberg B, editor. Principles of plant-microbe interactions. Cham: Springer International Publishing; 2015. pp. 393–400. [Google Scholar]
- Chibeba AM, Guimarães MDF, Brito OR, Nogueira MA, Araujo RS, Hungria M. Co-Inoculation of soybean with Bradyrhizobium and Azospirillum promotes early nodulation. Am J Plant Sci. 2015;6:1641–1649. doi: 10.4236/ajps.2015.610164. [DOI] [Google Scholar]
- Chibeba AM, Kyei-Boahen S, Guimarães MF, Nogueira MA, Hungria M. Isolation, characterization and selection of indigenous Bradyrhizobium strains with outstanding symbiotic performance to increase soybean yields in Mozambique. Agric Ecosyst Environ. 2017;246:291–305. doi: 10.1016/j.agee.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibeba AM, Kyei-Boahen S, Guimarães MDF, Nogueira MA, Hungria M. Feasibility of transference of inoculation-related technologies: a case study of evaluation of soybean rhizobial strains under the agro-climatic conditions of Brazil and Mozambique. Agric Ecosyst Environ. 2018;261:230–240. doi: 10.1016/j.agee.2017.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury ATMA, Kennedy IR. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils. 2004;39:219–227. doi: 10.1007/s00374-003-0706-2. [DOI] [Google Scholar]
- Ciancio A, Pieterse CMJ, Mercado-Blanco J. Editorial: harnessing useful rhizosphere microorganisms for pathogen and pest biocontrol. Front Microbiol. 2016;7:1–5. doi: 10.3389/fmicb.2016.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente JM, Cardoso CR, Vieira BS, Flor LM, Costa RL. Use of Bacillus spp. as growth promoter in carrot crop. Afr J Agric Res. 2016;11:3355–3359. doi: 10.5897/AJAR2016.11316. [DOI] [Google Scholar]
- Cohen AC, Travaglia CN, Bottini R, Piccoli PN. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany. 2009;87:455–462. doi: 10.1139/b09-023. [DOI] [Google Scholar]
- Collins MT, Thies JE, Abbott LK. Diversity and symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii isolates from pasture soils in south-western Australia. Aust J Soil Res. 2002;40(8):1319–1329. doi: 10.1071/SR01052. [DOI] [Google Scholar]
- CONAB—Companhia Nacional de Abastecimento (2019) Acompanhamento da safra brasileira grãos, vol 6, safra 2018/2019. Conab, Brasília. ISSN: 2318-6852
- Córdova SC, Castellano MJ, Dietzel R, Licht MA, Togliatti K, Martínez-Feria R, Archontoulis SV. Soybean nitrogen fixation dynamics in Iowa, USA. Field Crops Res. 2019;236:165–176. doi: 10.1016/j.fcr.2019.03.018. [DOI] [Google Scholar]
- da Chaves JS, Baraúna AC, Mosqueira CA, Gianluppi V, Zillid JE, da Silva K. Stylosanthes spp. from Amazon savanna harbour diverse and potentially effective rhizobia. Appl Soil Ecol. 2016;108:54–61. doi: 10.1016/j.apsoil.2016.08.003. [DOI] [Google Scholar]
- Dall´Agnol A, Roessing AC, Lazzarotto JJ, Hirakuri MH, Oliveira AB (2007) O complexo agroindustrial da soja brasileira. Londrina: Embrapa Soja (Embrapa Soja. Circular Técnica, n. 43)
- Das K, Prasanna R, Saxena AK. Rhizobia: a potential biocontrol agent for soil-borne fungal pathogens. Folia Microbiol. 2017;62:425–435. doi: 10.1007/s12223-017-0513-z. [DOI] [PubMed] [Google Scholar]
- Date RA. Advances in inoculant technology: a brief review. Aust J Exp Agric. 2001;41:321–325. doi: 10.1071/ea00006. [DOI] [Google Scholar]
- Daur I, Saad MM, Eida AA, Ahmad S, Shah ZH, Ihsan MZ, Muhammad Y, Sohrab SS, Hirt H. Boosting alfalfa (Medicago sativa L.) production with rhizobacteria from various plants in Saudi Arabia. Front Microbiol. 2018;9:477. doi: 10.3389/fmicb.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Abreu CS, Figueiredo JE, Oliveira CA, Dos Santos VL, Gomes EA, Ribeiro VP, Barros BA, Lana UG, Marriel IE. Maize endophytic bactéria as mineral phosphate solubilizers. Genet Mol Res. 2017;16:1. doi: 10.4238/gmr16019294. [DOI] [PubMed] [Google Scholar]
- de Salamone IEG, Funes JM, Di Salvo LP, Escobar-Ortega JS, D’Auria F, Ferrando L, Fernandez-Scavino A. Inoculation of paddy rice with Azospirillum brasilense and Pseudomonas fluorescens: impact of plant genotypes on rhizosphere microbial communities and field crop production. Appl Soil Ecol. 2012;61:196–204. doi: 10.1016/j.apsoil.2011.12.012. [DOI] [Google Scholar]
- Delamuta JRM, Ribeiro RA, Araújo JLS, Rouws LFM, Zilli JE, Parma MM, Melo IS, Hungria M. Bradyrhizobium stylosanthis sp. nov., comprising nitrogen-fixing symbionts isolated from nodules of the tropical forage legume Stylosanthes spp. Int J Syst Evol Microbiol. 2016;66:3078–3087. doi: 10.1099/ijsem.0.001148. [DOI] [PubMed] [Google Scholar]
- Deral—Departamento de Economia Rural (2019) Milho Análise da Conjuntura. http://www.agricultura.pr.gov.br/modules/conteudo/conteudo.php?conteudo=240. Accessed 04 Mar 2019
- Dias D, Amane M (2011) Yield response of soybean genotypes to different planting dates in Mozambique. In: African crop science conference 10:539–541. ISSN: 1023-070X/2011
- Doebley J. Molecular evidence and the evolution of maize. Econ Bot. 1990;44:6–27. doi: 10.1007/BF02860472. [DOI] [Google Scholar]
- Doebley J. Molecular evidence for gene flow among Zea species. BioSience. 1990;40:443–448. doi: 10.2307/1311391. [DOI] [Google Scholar]
- Don A, Schumacher J, Freibauer A. Impact of tropical land-use change on soil organic carbon stocks—a meta-analysis. Glob Change Biol. 2011;17:1658–1670. doi: 10.1111/j.1365-2486.2010.02336.x. [DOI] [Google Scholar]
- dos Santos SG, Chaves VA, Ribeiro FS, Alves GC, Reis VM. Rooting and growth of pre-germinated sugarcane seedlings inoculated with diazotrophic bacteria. Appl Soil Ecol. 2018 doi: 10.1016/j.apsoil.2018.08.015. [DOI] [Google Scholar]
- dos Santos SG, Ribeiro FS, Alves GC, Santos LA, Reis VM. Inoculation with five diazotrophs alters nitrogen metabolism during the initial growth of sugarcane varieties with contrasting responses to added nitrogen. Plant Soil. 2019 doi: 10.1007/s11104-019-04101-1. [DOI] [Google Scholar]
- DSMZ—Deutsche Sammiung von Mikroorganismen und Zellkulturen GmbH (2019) Prokaryotic nomenclature up to date. https://www.dsmz.de/bacterial-diversity/prokaryotic-nomenclature-up-to-date/prokaryotic-nomenclature-up-to-date.html. Accessed 29 Apr 2019
- Duc G. Faba bean (Vicia faba L.) Field Crop Res. 1997;53:99–109. doi: 10.1016/s0378-4290(97)00025-7. [DOI] [Google Scholar]
- Duc G, Bao S, Baum M, Redden B, Sadiki M, Suso ML, Vishniakova M, Zong X. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crop Res. 2010;115:270–278. doi: 10.1016/j.fcr.2008.10.003. [DOI] [Google Scholar]
- Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interact. 2017;12:100–107. doi: 10.1080/17429145.2017.1294212. [DOI] [Google Scholar]
- El-Lattief AEA (2012) Improving bread wheat productivity and reduce use of mineral nitrogen by inoculation with Azotobacter and Azospirillum under arid environment in upper Egypt. In: International conference on applied life sciences, Turkey, pp 393–398. 10.5772/intechopen.84110
- Fallik E, Okon Y, Epstein E, Goldman A, Fischer M. Identification and quantification of IAA and IBA in Azospirillum brasilense-inoculated maize roots. Soil Biol Biochem. 1989;21:147–153. doi: 10.1016/0038-0717(89)90024-2. [DOI] [Google Scholar]
- FAOSTAT—Food and Agriculture Organization of the United Nations: Statistics Division (2019) http://www.fao.org/faostat/en/#data/QC. Accessed 03 Oct 2019
- Fasciglione G, Casanovas EM, Quillehauquy V, Yommi AK, Goñi MG, Roura SI, Barassi CA. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci Hortic. 2015;195:154–162. doi: 10.1016/j.scienta.2015.09.015. [DOI] [Google Scholar]
- Ferreira AS, Pires RR, Rabelo PG, Oliveira RC, Luz JMQ, Brito CH. Implications of Azospirillum brasilense inoculation and nutrient addition on maize in soils of the Brazilian cerrado under greenhouse and field conditions. Appl Soil Ecol. 2013;72:103–108. doi: 10.1016/j.apsoil.2013.05.020. [DOI] [Google Scholar]
- Ferri GC, Braccini AL, Anghinoni FBG, Pereira LC. Effects of associated co-inoculation of Bradyrhizobium japonicum with Azosprillum brasilense on soybean yield and growth. Afr J Agric Res. 2017;12:6–11. doi: 10.5897/AJAR2016.11711. [DOI] [Google Scholar]
- Fischer D, Pfitzner B, Schmid M, Simões-Araujo JL, Reis VM, Pereira W, Ormenõ-Orrillo E, Hai B, Hofmann A, Schloter M, Martinez-Romero E, Baldani JI, Hartmann A. Molecular characterisation of the diazotrophic bacterial community in uninoculated and inoculated field-grown sugarcane (Saccharum sp.) Plant Soil. 2012;356:83–99. doi: 10.1007/s11104-011-0812-0. [DOI] [Google Scholar]
- Flores-Félix JD, Menéndez E, Rivera LP, Marcos-García M, Martínez-Hidalgo P, Mateos PF, Rivas R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J Plant Nutr Soil Sci. 2013;176:876–882. doi: 10.1002/jpln.201300116. [DOI] [Google Scholar]
- Flores-Félix JD, Velázquez E, García-Fraile P, González-Andrés F, Silva LR, Rivas R. Rhizobium and Phyllobacterium bacterial inoculants increase bioactive compounds and quality of strawberries cultivated in field conditions. Food Res Int. 2018;111:416–422. doi: 10.1016/j.foodres.2018.05.059. [DOI] [PubMed] [Google Scholar]
- Fonte SJ, Nesper M, Hegglin D, Velásquez JE, Ramirez B, Rao IM, Bernasconi SM, Bünemann EK, Frossard E, Oberson A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils. Soil Biol Biochem. 2014;68:150–157. doi: 10.1016/j.soilbio.2013.09.025. [DOI] [Google Scholar]
- Fukami J, Nogueira MA, Araujo RS, Hungria M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express. 2016;6:1–13. doi: 10.1186/s13568-015-0171-y). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami J, Ollero FJ, Megías M, Hungria M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express. 2017;7:153. doi: 10.1186/s13568-017-0453-7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami J, Cerezin P, Hungria M. Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express. 2018;8:1–12. doi: 10.1186/s13568-018-0608-1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami J, De La Osa C, Ollero FJ, Megías M, Hungria M. Co-inoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct Plant Biol. 2018;45(3):328–339. doi: 10.1071/FP17167. [DOI] [PubMed] [Google Scholar]
- Gadal N, Shrestha J, Poudel MN, Pokharel B. A review on production status and growing environments of rice in Nepal and in the world. Arch Agric Environ Sci. 2019;4:83–87. doi: 10.26832/24566632.2019.0401013. [DOI] [Google Scholar]
- Galindo FS, Teixeira Filho MCM, Buzetti S, Pagliari PH, Santini JMK. Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense. Agron J. 2019;111:1985–1997. doi: 10.2134/agronj2018.07.0481. [DOI] [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Gomes DF, Ormeño-Orrillo E, Hungria M. Biodiversity, symbiotic efficiency and genomics of Rhizobium tropici and related species. In: de Bruijn FJ, editor. Biological nitrogen fixation. New Jersey: Hoboken; 2015. pp. 747–756. [Google Scholar]
- Goulart-Machado R, Saccol De Sá EL, Hahn L, Pilatti Sant´Ana WL. Inoculation of plant growth promoting rhizobia in Sudan grass (Sorghum sudanense (Piper) Stapf cv Sudanense) and millet (Pennisetum glaucum (L.) R. Br. cv. BRS1501) Acta Agron. 2018;67:135–141. doi: 10.15446/acag.v67n1.55849. [DOI] [Google Scholar]
- Govindarajan M, Balandreau J, Kwon SW, Weon HY, Lakshminarasimhan C. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb Ecol. 2007;55:21–37. doi: 10.1007/s00248-007-9247-9. [DOI] [PubMed] [Google Scholar]
- Greetatorn T, Hashimoto S, Sarapat S, Tittabutr P, Boonkerd N, Uchiumi T, Teaumroong N. Empowering rice seedling growth by endophytic Bradyrhizobium sp. SUTN9-2. Lett Appl Microbiol. 2019;68(3):258–266. doi: 10.1111/lam.13114. [DOI] [PubMed] [Google Scholar]
- Guimarães SL, Santos CSA, Bonfim-Silva EM, Polizel AC, Batista ER. Nutritional characteristics of marandu grass (Brachiaria brizantha cv. marandu) subjected to inoculation with associative diazotrophic bacteria. Afr J Microbiol Res. 2016;10:873–882. doi: 10.5897/ajmr2016.7951. [DOI] [Google Scholar]
- Gundi JS, Santos MS, Oliveira ALM, Nogueira MA, Hungria M. Development of liquid inoculants for strains of Rhizobium tropici group using response surface methodology. Afr J Biotechnol. 2018;17:411–421. doi: 10.5897/AJB2018.16389. [DOI] [Google Scholar]
- Harris JM, Lucas JA, Davey MR, Lethbridge G, Powell KA. Establishment of Azospirillum inoculant in the rhizosphere of winter wheat. Soil Biol Biochem. 1989;21:59–64. doi: 10.1016/0038-0717(89)90011-4. [DOI] [Google Scholar]
- Heuvelink E, editor. Tomatoes: crop production science in horticulture. Boston: CABI Publishing; 2018. [Google Scholar]
- Hungria M, Mendes IC. Nitrogen fixation with soybean: the perfect symbiosis? In: De Bruijn FJ, editor. Biological nitrogen fixation. New Jersey: Hoboken; 2015. pp. 1009–1023. [Google Scholar]
- Hungria M, Nogueira MA (2019) Tecnologias de inoculação da cultura da soja: Mitos, verdades e desafios. In: Boletim de Pesquisa 2019/2020. Fundação MT, Rondonópolis, pp 50–62. (Fundação MT. Boletim, 19)
- Hungria M, Vargas MAT, Suhet AR, Peres JRR. Fixação biológica do nitrogênio em soja. In: Araujo RS, Hungria M, editors. Microrganismos de Importância Agrícola. Brasília: EMBRAPA-SPI; 1994. pp. 9–89. [Google Scholar]
- Hungria M, Andrade DS, Chueire LMO, Probanza A, Guitierrez-Manero FJ, Megías M. Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem. 2000;21:1515–1528. doi: 10.1016/S0038-0717(00)00063-8. [DOI] [Google Scholar]
- Hungria M, Vargas MAT, Campo RJ, Chueire LMO, de Andrade DS. The brazilian experience with the soybean (Glycine max) and common bean (Phaseolus vulgaris) symbioses. Curr Plant Sci Biotechnol Agric. 2000 doi: 10.1007/0-306-47615-0_292. [DOI] [Google Scholar]
- Hungria M, Campo RJ, Mendes IC (2001) Fixação biológica do nitrogênio na cultura da soja. Londrina: Embrapa Soja, p 48. (Embrapa Soja. Circular Técnica, n.35; Embrapa Cerrados. Circular Técnica, n.13)
- Hungria M, Campo RJ, Mendes IC. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol Fertil Soils. 2003;39:88–93. doi: 10.1007/s00374-003-0682-6. [DOI] [Google Scholar]
- Hungria M, Loureiro FM, Mendes IC, Campo RJ, Graham PH. Inoculant preparation, production and application. In: Werner D, Newton WE, editors. Nitrogen fixation agriculture, forestry, ecology and the environment. Dordrecht: Springer; 2005. pp. 224–253. [Google Scholar]
- Hungria M, Campo RJ, Mendes IC, Graham PH. Contribution of biological nitrogen fixation to the N nutrition of grain crops in the tropics: the success of soybean (Glycine max L. Merr.) in South America. In: Singh RP, Shankar N, Jaiwal PK, editors. Nitrogen nutrition and sustainable plant productivity. Houston: Studium Press; 2006. pp. 43–93. [Google Scholar]
- Hungria M, Campo RJ, Mendes IC (2007) A importância do processo de fixação biológica do nitrogênio para a cultura da soja: componente essencial para a competitividade do produto brasileiro. Londrina: Embrapa Soja, p 80 (Embrapa Soja. Documentos, 283)
- Hungria M, Campo RJ, Souza EM, Pedrosa FO. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 2010;331:413–425. doi: 10.1007/s11104-009-0262-0. [DOI] [Google Scholar]
- Hungria M, Nogueira MA, Araujo RS. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol Fertil Soils. 2013;49:791–801. doi: 10.1007/s00374-012-0771-5. [DOI] [Google Scholar]
- Hungria M, Nogueira MA, Araujo RS. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. Am J Plant Sci. 2015;6:811–817. doi: 10.4236/ajps.2015.66087. [DOI] [Google Scholar]
- Hungria M, Nogueira MA, Araujo RS. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Agric Ecosyst Environ. 2016;221:125–131. doi: 10.1016/j.agee.2016.01.024. [DOI] [Google Scholar]
- Hungria M, Araujo RS, Silva Júnior EB, Zilli JE. Inoculum rate effects on the soybean symbiosis in new or old fields under tropical conditions. Agron J. 2017;109:1–7. doi: 10.2134/agronj2016.11.0641. [DOI] [Google Scholar]
- Ibrahim KA, Naeim EAM, El Naim AM, Elsheikh MA. Response of guar (Cyamopsis teteragonolopa L.) to Bradyrhizobium inoculations in semi-arid Environment. Int J Agric For. 2016;6(4):137–141. doi: 10.5923/j.ijaf.20160604.01. [DOI] [Google Scholar]
- Janzen RA, Rood SB, Dormaar JF, McGill WB. Azospirillum brasilense produces gibberellin in pure culture on chemically defined medium and in co-culture on straw. Soil Biol Biochem. 1992;24:1061–1064. doi: 10.1016/0038-0717(92)90036-w. [DOI] [Google Scholar]
- Jesus EC, Leite RA, Bastos RA, Aragão OOS, Araújo AP. Co-inoculation of Bradyrhizobium stimulates the symbiosis efficiency of Rhizobium with common bean. Plant Soil. 2018;425:201–215. doi: 10.1007/s11104-017-3541-1. [DOI] [Google Scholar]
- Jiménez-Gómez A, Flores-Félix JD, García-Fraile P, Mateos PF, Menéndez E, Velázquez E, Rivas R. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-017-18632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorquera MA, Hernández MT, Rengel Z, Marschner P, Mora ML. Isolation of culturable phosphobacteria with both phytate mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils. 2008;44:1025–1034. doi: 10.1007/s00374-008-0288-0. [DOI] [Google Scholar]
- Juge C, Prévost D, Bertrand A, Bipfubusa M, Chalifour FP. Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium, Azospirillum and arbuscular mycorrhizae. Appl Soil Ecol. 2012;61:147–157. doi: 10.1016/j.apsoil.2012.05.006. [DOI] [Google Scholar]
- Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Brit J Nutr. 2012;108:11–26. doi: 10.1017/S0007114512000797. [DOI] [PubMed] [Google Scholar]
- Jung BK, Hong S-J, Park G-S, Kim M-C, Shin J-H. Isolation of Burkholderia cepacia JBK9 with plant growth-promoting activity while producing pyrrolnitrin antagonistic to plant fungal diseases. Appl Biol Chem. 2018;61(2):173–180. doi: 10.1007/s13765-018-0345-9. [DOI] [Google Scholar]
- Kanonge-Mafaune G, Chiduwa MS, Chikwari E, Pisa C. Evaluating the effect of increased rates of rhizobial inoculation on grain legume productivity. Symbiosis. 2018;75:217–227. doi: 10.1007/s13199-018-0550-7. [DOI] [Google Scholar]
- Kapulnik Y, Sarig SI, Okon Y. Effect of Azospirillum inoculation on yield of field-grown wheat. Can J Microbiol. 1983;29:895–899. doi: 10.1139/m83-145. [DOI] [Google Scholar]
- Kapulnik Y, Okon Y, Henis Y. Changes in root morphology of wheat caused by Azospirillum inoculation. Can J Microbiol. 1985;31:881–887. doi: 10.1139/m85-165. [DOI] [Google Scholar]
- Karimi N, Zarea MJ, Mehnaz S. Endophytic Azospirillum for enhancement of growth and yield of wheat. Environ Sustain. 2018;1:149–158. doi: 10.1007/s42398-018-0014-2. [DOI] [Google Scholar]
- Kecskes ML, Rose MT, Tran TKC, Nguyen KO, Michel E, Lauby B, Rakotondrainibe M, Casteriano AV, Palagyi A, Krishnen G, Kennedy IR (2008) Identification and quality control of BioGro inoculant Biofertiliser strains. In: Kennedy IR, Choudhury ATMA, Kecskes ML, Rose MT (eds) Efficient nutrient use in rice production in Vietnam achieved using inoculant biofertilisers. ACIAR Proceedings, Canberra, pp 117–125
- Khan MR, Mohiddin FA, Ahamad F. Inoculant rhizobia suppressed root-knot disease, and enhanced plant productivity nutrient uptake and of some field-grown food legumes. Acta Agric Scand Sect B Soil Plant Sci. 2017;68(2):166–174. doi: 10.1080/09064710.2017.1374448. [DOI] [Google Scholar]
- Khandelwal A, Sindhu SS. Expression of 1-aminocyclopropane-1-carboxylate deaminase in rhizobia promotes nodulation and plant growth of clusterbean (Cyamopsis tetragonoloba L.) Res J Microbiol. 2012;7:158–170. doi: 10.3923/jm.2012.158.170. [DOI] [Google Scholar]
- Khush GS. Origin, dispersal, cultivation and variation of rice. Plant Mol Biol. 1997;35:25–34. doi: 10.1007/978-94-011-5794-0_3. [DOI] [PubMed] [Google Scholar]
- Köpke U, Nemecek T. Ecological services of faba bean. Field Crop Res. 2010;115:217–233. doi: 10.1016/j.fcr.2009.10.012. [DOI] [Google Scholar]
- Korir H, Mungai NW, Thuita M, Hamba Y, Asso C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front Plant Sci. 2017;8:1–10. doi: 10.3389/fpls.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskey G, Mburu SW, Njeru EM, Kimiti JM, Ombori O, Maingi JM. Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of eastern Kenya. Front Plant Sci. 2017;8:1–12. doi: 10.3389/fpls.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueneman EA, Root WR, Dashiell KE, Hohenberg J. Breeding soybean for the tropics capable of nodulating effectively with indigenous Rhizobium spp. Plant Soil. 1984;83:387–396. doi: 10.1007/BF02184276. [DOI] [Google Scholar]
- Kumar A, Maurya BR, Raghuwanshi R, Meena VS, Tofazzal IM. Co-inoculation with Enterobacter and rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under indo-gangetic plain of India. J Plant Growth Regul. 2017;36:608–617. doi: 10.1007/s00344-016-9663-5. [DOI] [Google Scholar]
- LAPIG—Laboratório de Processamento de Imagem e Geoprocessamento (2018) Atlas digital das pastagens brasileiras. https://pastagem.org/atlas/map. Accessed 06 May 2019
- Laranjo M, Alexandre A, Oliveira S. Legume growth-promoting rhizobia: an overview on the Mesorhizobium genus. Microbiol Res. 2014;169:2–17. doi: 10.1016/j.micres.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Lavania D, Siddiqui MH, Al-Whaibi MH, Singh AK, Kumar R, Grover A. Genetic approaches for breeding heat stress tolerance in faba bean (Vicia faba L.) Acta Physiol Plant. 2015;37:1–9. doi: 10.1007/s11738-014-1737-z. [DOI] [Google Scholar]
- Leggett M, Diaz-Zorita M, Koivunen M, Bowman R, Pesek R, Stevenson C, Leister T. Soybean response to inoculation with Bradyrhizobium japonicum in the United States and Argentina. Agron J. 2017;109:1031–1038. doi: 10.2134/agronj2016.04.0214. [DOI] [Google Scholar]
- Leite RC, dos Santos JGD, Silva EL, Alves CRCR, Hungria M. Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. Crop Pasture Sci. 2018;70:61–67. doi: 10.1071/CP18105. [DOI] [Google Scholar]
- Leite MCBS, Pereira APA, Souza AJ, Andrade PAM, Barbosa MV, Andreote FD, Freire FJ, Sobral JK. Potentially diazotrophic endophytic bacteria associated to sugarcane are effective in plant growth-promotion. J Exp Agric Int. 2018;21:1–15. doi: 10.9734/JEAI/2018/39963. [DOI] [Google Scholar]
- Leite RC, Santos AC, dos Santos JGD, Leite RC, Oliveira LBT, Hungria M. Mitigation of Mombasa grass (Megathyrsus maximus) dependence on nitrogen fertilization as a function of inoculation with Azospirillum brasilense. Rev Bras Ciênc Solo. 2019;43:180–234. doi: 10.1590/18069657rbcs20180234. [DOI] [Google Scholar]
- Lima NSA, Vogel GF, Fey R. Rates of application of Azospirillum brasilense in tomato crop. Rev Agric Neotrop. 2018;5:81–87. doi: 10.32404/rean.v5i4.3142. [DOI] [Google Scholar]
- Malusá E, Vassilev N. A contribution to set a legal framework for biofertilisers. Appl Microbiol Biotechnol. 2014;98:6599–6607. doi: 10.1007/s00253-014-5828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasa K, Reddy RS, Triveni S, Kumar BK, Priya NG. Characterization of Rhizobium isolates and their potential PGPR characteristics of different rhizosphere soils of Telangana region, India. Int J Curr Microbiol Appl Sci. 2017;6:2808–2813. doi: 10.20546/ijcmas.2017.605.316. [DOI] [Google Scholar]
- Mangmang JS, Deaker R, Rogers G. Effects of plant growth promoting rhizobacteria on seed germination characteristics of tomato and lettuce. J Trop Crop Sci. 2014;1(2):35–40. doi: 10.29244/jtcs.1.2.35-40. [DOI] [Google Scholar]
- Mangmang JS, Deaker R, Rogers G. Azospirillum brasilense enhances recycling of fish effluent to support growth of tomato seedlings. Horticulturae. 2015;1:14–26. doi: 10.3390/horticulturae1010014. [DOI] [Google Scholar]
- Mangmang JS, Deaker R, Rogers G. germination characteristics of cucumber influenced by plant growth–promoting rhizobacteria. Int J Veg Sci. 2015;22:66–75. doi: 10.1080/19315260.2014.938850. [DOI] [Google Scholar]
- MAPA—Ministério da Agricultura, Pecuária e Abastecimento (2004) Instrução Normativa No 5, de 6 de agosto de 2004. Diário Oficial da União da República Federativa do Brasil
- MAPA—Ministério da Agricultura, Pecuária e Abastecimento (2011) Instrução Normativa No 13, de 24 de março de 2011. Diário Oficial da União da República Federativa do Brasil
- Marcondes J, Ferraudo AS, Scaquitto DC, Alves LMC, Lemos EGM. Efetividade na fixação biológica do nitrogênio de bactérias nativas isoladas de plantas de amendoim. Cien Tecnol. 2010;1:21–32. [Google Scholar]
- Mazid M, Khan TA. Future of bio-fertilizers in Indian agriculture: an overview. Int J Agric Food Res. 2014;3:10–23. [Google Scholar]
- Mercante FM, Otsubo AA, Brito OR. New native rhizobia strains for inoculation of common bean in the Brazilian savanna. Rev Bras Ciênc Solo. 2017;41:e0150120. doi: 10.1590/18069657rbcs20150120. [DOI] [Google Scholar]
- Ming R, Moore PH, Wu K, D’Hont A, Glaszmann JC, Tew TL. Sugarcane improvement through breeding and biotechnology. Hoboken: Wiley; 2006. [Google Scholar]
- Mirza MS, Rasul G, Mehnaz S, Ladha JK, So RB, Ali S, Malik KA. Beneficial effects of inoculated nitrogen-fixing bacteria on rice. In: Ladha JK, Reddy PM, editors. The quest for nitrogen fixation in rice. Los Banos: International Rice Research Institute; 2000. pp. 191–204. [Google Scholar]
- Monk J, Gerard E, Young S, Widdup K, O’Callaghan M. Isolation and identification of plant growth-promoting bacteria associated with tall fescue. Proc New Zeal Grassland Assoc. 2009;71:211–216. [Google Scholar]
- Morel MA, Braña V, Castro-Sowinski S (2012) Legume crops, importance and use of bacterial inoculation to increase production. In: Goyal A (ed) Crop Plant 2:218–240. InTech. http://www.intechopen.com/books/crop-plant/legume-crops-importance-and-use-ofbacterial-inoculation-to-increase-production, http://www.intechopen.com/books/crop-plant/legume-crops-importance-and-use-ofbacterial-inoculation-to-increase-production
- Moretti LG, Lazarini E, Bossolani JW, Parente TL, Caioni S, Araujo RS, Hungria M. Can additional inoculations increase soybean nodulation and grain yield? Agron J. 2018;110:715–721. doi: 10.2134/agronj2017.09.0540. [DOI] [Google Scholar]
- Mostasso L, Mostasso FL, Vargas MAT, Hungria M. Selection of beans (Phaseolus vulgaris) rhizobial strains for the Brazilian cerrados. Field Crop Res. 2002;73:121–132. doi: 10.1016/S0378-4290(01)00186-1. [DOI] [Google Scholar]
- Mukherjee SK. Origin and distribution of Saccharum. Bot Gaz. 1957;119:55–61. doi: 10.1086/335962. [DOI] [Google Scholar]
- Muleta D, Ryder MH, Denton MD. The potential for rhizobial inoculation to increase soybean grain yields on acid soils in Ethiopia. Soil Sci Plant Nutr. 2017;63:1–11. doi: 10.1080/00380768.2017.1370961. [DOI] [Google Scholar]
- Munõz-Rojas J, Caballero-Mellado J. Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb Ecol. 2003;46:454–464. doi: 10.1007/s00248-003-0110-3. [DOI] [PubMed] [Google Scholar]
- Mutch LA, Young JPW. Diversity and specificity of Rhizobium leguminosarum biovar viciae on wild and cultivated legumes. Mol Ecol. 2004;3:2435–2444. doi: 10.1111/j.1365-294X.2004.02259.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TH (2008) The product BioGro and improvements in its performance. In: Kennedy IR, Choudhury ATMA, Kecskés ML, Rose MT (eds) Efficient nutrient use in rice production in Vietnam achieved using inoculant biofertilisers. ACIAR Proceedings, Canberra, pp 15–23
- Nguyen TH, Deaker R, Kennedy IR, Roughley RJ. The positive yield response of field grown rice to inoculation with a multi-strain biofertiliser in the Hanoi area, Vietnam. Symbiosis. 2003;35:231–245. [Google Scholar]
- Nguyen TH, Phan TC, Choudhury ATMA, Rose MT, Deaker RJ, Kennedy IR. BioGro: a plant growth-promoting biofertilizer validated by 15 years’ research from laboratory selection to rice farmer’s fields of the Mekong Delta. Agro-Environ Sustain. 2017 doi: 10.1007/978-3-319-49724-2_11. [DOI] [Google Scholar]
- Nimnoi P, Pongsilp N, Lumyong S. Co-inoculation of soybean (Glycine max) with Actinomycetes and Bradyrhizobium japonicum enhances plant growth, nitrogenase activity and plant nutrition. J Plant Nutr. 2014;37:432–446. doi: 10.1080/01904167.2013.864308. [DOI] [Google Scholar]
- Nogueira MA, Prando AM, Oliveira AB, Lima D, Conte O, Harger N, Oliveira FT, Hungria M (2018) Ações de transferência de tecnologia em inoculação/coinoculação com Bradyrhizobium e Azospirillum na cultura da soja na safra 2017/18 no estado do Paraná. Embrapa Soja, Londrina, pp 15. (Embrapa Soja. Circular Técnica, 143). ISSN 1516-7860. https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1095314/acoes-de-transferencia-de-tecnologia-em-inoculacaocoinoculacao-com-bradyrhizobium-e-azospirillum-na-cultura-da-soja-na-safra-201718-no-estado-do-parana
- Nushair AM, Saha AK, Rahman A, Mohanta MK, Haque F. genotypic characterization of indigenous Rhizobium strain from cultivated cowpea (Vigna unguiculata L.) in Bangladesh. Int J Curr Microbiol Appl Sci. 2017;6:2493–2502. doi: 10.20546/ijcmas.2017.610.293. [DOI] [Google Scholar]
- Okon Y, Labandera-Gonzales C, Lage M, Lage P. Agronomic applications of Azospirillum and other PGPR. In: De Bruijn FJ, editor. Biological nitrogen fixation. Hoboken: Wiley; 2015. pp. 925–936. [Google Scholar]
- Ozturk A, Caglar O, Sahin F. Yield response of wheat and barley to inoculation of plant growth promoting rhizobacteria at various levels of nitrogen fertilization. J Plant Nutr Soil Sci. 2003;166:262–266. doi: 10.1002/jpln.200390038. [DOI] [Google Scholar]
- Padilla EG, Ruiz-Díez B, Fernández-Pascual M, López Sánchez R, Bloem E, Eichler-Löbermann B. Inoculation with native bradyrhizobia strains improved growth of cowpea plants cultivated on a saline soil. Commun Soil Sci Plant Anal. 2016;47:2218–2224. doi: 10.1080/00103624.2016.1228950. [DOI] [Google Scholar]
- Pandey RP, Srivastava AK, Gupta VK, O’Donovan A, Ramteke PW. Enhanced yield of diverse varieties of chickpea (Cicer arietinum L.) by different isolates of Mesorhizobium ciceri. Environ Sustain. 2018;1:425–435. doi: 10.1007/s42398-018-00039-9. [DOI] [Google Scholar]
- Pawar PU, Kumbhar CT, Patil VS, Khot GG. Effect of co-inoculation of Bradyrhizobium japonicum and Pseudomonas fluorescens on growth, yield and nutrient uptake in soybean [Glycine max (L.) Merrill] Crop Res. 2018;53:57–62. doi: 10.5958/2454-1761.2018.00009.8. [DOI] [Google Scholar]
- Pereira W, Sousa JS, Schultz N, Reis VM. Sugarcane productivity as a function of nitrogen fertilization and inoculation with diazotrophic plant growth-promoting bacteria. Sugar Tech. 2018 doi: 10.1007/s12355-018-0638-7. [DOI] [Google Scholar]
- Perin L, Martínez-Aguilar L, Paredes-Valdez G, Baldani JI, Estrada-de los Santos P, Reis VM, CaballeroMellado J. Burkholderia silvatlantica sp. nov., a diazotrophic bacterium associated with sugar cane and maize. Int J Syst Evol Microbiol. 2006;56:1931–1937. doi: 10.1099/ijs.0.64362-0. [DOI] [PubMed] [Google Scholar]
- Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassán FD, Luna MV. Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol. 2007;75:1143–1150. doi: 10.1007/s00253-007-0909-9. [DOI] [PubMed] [Google Scholar]
- Petkar VV, Deshmukh TT, Jadhav AN. effect of dual inoculation of Bacillus subtilis and Bradyrhizobium japonicum on growth parameters of soybean (Glycine max L.) Int J Curr Microbiol Appl Sci. 2018;7:563–567. doi: 10.20546/ijcmas.2018.710.062. [DOI] [Google Scholar]
- Phan TC, Tran DD (2008) Interaction effects of BioGro with nitrogen and phosphorus on grain yield and nutrient uptake of rice in light-textured soils of southern Vietnam. In: Kennedy IR, Choudhury ATMA, Kecskés ML, Rose MT (eds) Efficient nutrient use in rice production in Vietnam achieved using inoculant biofertilisers. ACIAR Proceedings, Canberra, pp 24–31
- Piccinin GG, Braccini AL, Dan LG, Scapim CA, Ricci TT, Bazo GL. Efficiency of seed inoculation with Azospirillum brasilense on agronomic characteristics and yield of wheat. Ind Crop Prod. 2013;43:393–397. doi: 10.1016/j.indcrop.2012.07.052. [DOI] [Google Scholar]
- Prashar P, Kapoor N, Sachdeva S. Plant growth promoting activities of rhizobacteria associated with tomato in semi-arid region. Adv Life Sci Health. 2014;1:43–54. doi: 10.15764/ALSH.2014.01006. [DOI] [Google Scholar]
- Puertas A, Gonzales LM. Aislamiento de cepas nativas de Azotobacter chroococcum en la provincia Granmay evaluacion de su actividad estimuladora en plantulas de tomate. Cell Mol Life Sci. 1999;20:5–7. [Google Scholar]
- Pulver EL, Kueneman EA, Ranga-Rao V. Identification of promiscuous nodulating soybean efficient in N2 fixation. Crop Sci. 1985;25:660–663. doi: 10.2135/cropsci1985.0011183X002500040019x. [DOI] [Google Scholar]
- Purcino HMA, Sá NMH, Viana MCM, Scotti MR, Mendes IC, Vargas MAT (2003) Response of Arachis pintoi to inoculation with selected rhizobia strains in Brazilian Cerrado soils under field conditions. Pasturas Tropicales 25(2):26–29. http://www.tropicalgrasslands.info/public/journals/4/Elements/DOCUMENTS/2003-vol25-rev1-2-3/Vol_25_rev2_03_pags_26-29.pdf
- Ramirez-Villegas J, Thornton PK. Climate change impacts on African crop production. Copenhagen: Research Program on Climate Change, Agriculture and Food Security; 2015. [Google Scholar]
- Reis VM (2007) Uso de Bactérias Fixadores de Nitrogênio omo Inoculante para Aplicação em Gramíneas. Seropédica: Embrapa Agrobiologia, p 22. (Embrapa Agrobiologia. Documentos 232). ISSN 1517-8498
- Reis Junior FB, Reis VM, Urquiaga S, Dobereiner J. Influence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugarcane (Saccharum spp.) Plant Soil. 2000;219:153–159. doi: 10.1023/A:1004732500983. [DOI] [Google Scholar]
- Restrepo GM, Sánchez OJ, Marulanda SM, Galeano NF, Taborda G. Afr J Biotechnol. 2017;16:1619–1629. doi: 10.5897/AJB2017.16016. [DOI] [Google Scholar]
- Ribeiro RA, Ormeño-Orrillo E, Dall’agnol RF, Graham PH, Martinez-Romero E, Hungria M. Novel Rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res Microbiol. 2013;164:740–748. doi: 10.1016/j.resmic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Ribeiro VP, Marriel IE, Sousa SM, Lana UGP, Mattos BB, Oliveira CA, Gomes EA. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz J Microbiol. 2018;49(Suppl 1):40–46. doi: 10.1016/j.bjm.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Caceres EA, Di Ciocco C, Basurco JCP. Influencia de la inoculación con Azospirillum brasiliense en trigo cultivado en suelos de la provincia de La Pampa, Argentina. Cien Suelo. 1996;14:110–112. [Google Scholar]
- Rodriguez-Caceres EA, Anta GG, López JR, Di Ciocco CA, Basurco JCP, Parada JL. Response of field-grown wheat to inoculation with Azospirillum brasilense and Bacillus polymyxain the semiarid region of Argentina. Arid Soil Res Rehab. 1996;10:13–20. doi: 10.1080/15324989609381416. [DOI] [Google Scholar]
- Rojas-Rojas FU, López-Sánchez D, Meza-Rdilla G, Méndez-Canarios A, Ibarra JA, Estrada-de Los Santos P. The controversial Burkholderia cepacia complex, a group of plant growth promoting species and plant, animals and human pathogens. Rev Argent Microbiol. 2019;51(1):84–92. doi: 10.1016/j.ram.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Ronner E, Franke AC, Vanlauwe B, Dianda M, Edeh E, Ukem B, Bala A, van Heerwaarden J, Giller KE. Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers’ fields in northern Nigeria. Field Crops Res. 2016;186:133–145. doi: 10.1016/j.fcr.2015.10.023. [DOI] [Google Scholar]
- Roychowdhury R, Qaiser TF, Mukherjee P, Roy M. Isolation and characterization of a Pseudomonas aeruginosa strain PGP for plant growth promotion. Proc Natl Acad Sci India Sect B Biol Sci. 2019;89:353. doi: 10.1007/s40011-017-0946-9. [DOI] [Google Scholar]
- Rubiales D. Faba beans in sustainable agriculture. Field Crop Res. 2010;115:201–202. doi: 10.1016/j.fcr.2009.11.002. [DOI] [Google Scholar]
- Sah S, Singh N, Singh R. Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3 Biotech. 2017;7:1–7. doi: 10.1007/s13205-017-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saïdi S, Ramírez-Bahena MH, Santillana N, Zúniga D, Alvarez-Martínez E, Peix A, Mhamdi R, Vel AE. Rhizobium laguerreae sp. nov. nodulates Vicia faba on several continents. Int J Syst Evol Microbiol. 2014;64:242–247. doi: 10.1099/ijs.0.052191-0. [DOI] [PubMed] [Google Scholar]
- Samago TY, Anniye EW, Dakora FD. Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis. 2018;75:245–255. doi: 10.1007/s13199-017-0529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandini IE, Pacentchuk F, Hungria M, Nogueira MA, Cruz SP, Nakatani AS, Araujo RS. Seed inoculation with Pseudomonas fluorescens promotes growth, yield and reduces nitrogen application in maize. Int J Agric Biol. 2019 doi: 10.17957/IJAB/15.1210. [DOI] [Google Scholar]
- Santos MS, Hungria M, Nogueira MA. Production of polyhydroxybutyrate (PHB) and biofilm by Azospirillum brasilense aiming at the development of liquid inoculants with high performance. Afr J Biotechnol. 2017;16:1855–1862. doi: 10.5897/AJB2017.16162. [DOI] [Google Scholar]
- Schultz N, Pereira W, Silva PA, Baldani JI, Boddey RM, Alves MJR, Urquiaga S, Reis VM. Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod Sci. 2017;20:366–374. doi: 10.1080/1343943X.2017.1374869. [DOI] [Google Scholar]
- Seck PA, Diagne A, Mohanty S, Wopereis MCS. Crops that feed the world 7: rice. Food Secur. 2012;4:7–24. doi: 10.1007/s12571-012-0168-1. [DOI] [Google Scholar]
- Sharma A, Johri BN. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol Res. 2003;158:243–248. doi: 10.1078/0944-5013-00197. [DOI] [PubMed] [Google Scholar]
- Shiri-Janagard M, Raei Y, Gasemi-Golezani G, Aliasgarzad N. Influence of Bradyrhizobium japonicum and phosphate solubilizing bacteria on soybean yield at different levels of nitrogen and phosphorus. Int J Agron Plant Prod. 2012;3:544–549. [Google Scholar]
- Silalertruksa T, Gheewala SH. Competitive use of sugarcane for food, fuel, and biochemical through the environmental and economic factors. Int J Life Cycle Assess. 2019 doi: 10.1007/s11367-019-01664-0. [DOI] [Google Scholar]
- Silva KJD, Rocha MM, Menezes Júnior JAN. Socioeconomia. In: Bastos EA, editor. A Cultura do Feijão-Caupi no Brasil. 1. Teresina: Embrapa Meio-Norte; 2016. pp. 6–12. [Google Scholar]
- Silva ER, Zoz J, Oliveira CES, Zuffo AM, Steiner F, Zoz T, Vendruscolo EP. Can co-inoculation of Bradyrhizobium and Azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.)? Arch Microbiol. 2019;201(3):325–335. doi: 10.1007/s00203-018-01617-5. [DOI] [PubMed] [Google Scholar]
- Singh N, Raina S, Singh D, Ghosh M, Heflish AI. Exploitation of promising native strains of Bacillus subtilis with antagonistic properties against fungal pathogens and their PGPR characteristics. J Plant Pathol. 2017;99(1):27–35. doi: 10.4454/jpp.v99i1.3809. [DOI] [Google Scholar]
- Singh PK, Venkatesan K, Swarnam TP. Rice genetic resources in tropical islands. In: Sivaperuman CA, Singh AA, Jaisankar I, editors. Biodiversity and climate change adaptation in tropical Islands. 1. Boca Raton: Academic Press; 2018. pp. 355–386. [Google Scholar]
- Soares BL, Ferreira PAA, Rufini M, Martins FAD, Oliveira DP, Reis RP, Andrade MJB, Moreirae FMDS. Agronomic and economic efficiency of common-bean inoculation with rhizobia and mineral nitrogen fertilization. Rev Bras Cienc Solo. 2016;40:1–13. doi: 10.1590/18069657rbcs20150235. [DOI] [Google Scholar]
- Souza JEB, Ferreira EPB. Improving sustainability of common bean production systems by co-inoculating rhizobia and azospirilla. Agric Ecosyst Environ. 2017;237:250–257. doi: 10.1016/j.agee.2016.12.040. [DOI] [Google Scholar]
- Sruthilaxmi CB, Babu C. Microbial bio-inoculants in Indian agriculture: ecological perspectives for a more optimized use. Agric Ecosyst Environ. 2017;242:23–25. doi: 10.1016/j.agee.2017.03.019. [DOI] [Google Scholar]
- Steinfeld H, Gerber P, Wassenaar T, Castel V, Rosales M, De Haan C. Livestock’s long shadow: environmental issues and options. Rome: United Nations Food and Agriculture Organization; 2006. [Google Scholar]
- Strzelczyk E, Kampert M, Li CY. Cytokinin-like substances and ethylene production by Azospirillum in media with different carbon sources. Microbiol Res. 1994;149:55–60. doi: 10.1016/S0944-5013(11)80136-9. [DOI] [Google Scholar]
- Subramanian R. India processing tomato segment: current status, trends and opportunities for engagement. Hyderabad: World Vegetable Center; 2016. [Google Scholar]
- Subramanian P, Kim K, Krishnamoorthy R, Sundaram S, Sa T. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul. 2014;76:327–332. doi: 10.1007/s10725-014-9993-x. [DOI] [Google Scholar]
- Szilagyi-Zecchin V, Mógor AF, Ruaro L, Röder C. Crescimento de mudas de tomateiro (Solanum lycopersicum) estimulado pela bactéria Bacillus amyloliquefaciens subsp. plantarum FZB42 em cultura orgânica. Rev Cienc Agrar. 2015;38:26–33. [Google Scholar]
- Tarrand JJ, Krieg NR, Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- Tartaglia C, Azziz G, Lorite MJ, Sanjuán J, Monza J. Phylogenetic relationships among introduced and autochthonous rhizobia nodulating Trifolium spp. in Uruguayan soils. Appl Soil Ecol. 2019;139:40–46. doi: 10.1016/j.apsoil.2019.03.014. [DOI] [Google Scholar]
- Taulé C, Mareque C, Barlocco C, Hackembruch F, Reis MV, Sicardi M, Battistoni F. The contribution of nitrogen fixation to sugarcane (Saccharum officinarum L.), and the identification and characterization of part of the associated diazotrophic bacterial community. Plant Soil. 2012;356:35–49. doi: 10.1007/s11104-011-1023-4. [DOI] [Google Scholar]
- Tejera N, Lluch C, Martínez-Toledo MV, González-López J. Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil. 2005;270:223–232. doi: 10.1007/s11104-004-1522-7. [DOI] [Google Scholar]
- Tena W, Wolde-Meskel E, Walley F. Response of chickpea (Cicer arietinum L.) to inoculation with native and exotic Mesorhizobium strains in southern Ethiopia. Afr J Biotechnol. 2016;15(35):1920–1929. doi: 10.5897/AJB2015.15060. [DOI] [Google Scholar]
- Thapa S, Adams CB, Trostle C. Root nodulation in guar: effects of soils, Rhizobium inoculants, and guar varieties in a controlled environment. Ind Crop Prod. 2018;120:198–202. doi: 10.1016/j.indcrop.2018.04.060. [DOI] [Google Scholar]
- Thies JE, Bohlool BB, Singleton PW. Subgroups of de Cawpea Miscellany: symbiotic specificity within Bradyrhizobium spp. For Vigna unhuiculata, Phaseolus lunatus, Arachis hypogaea and Macroptilum atroputpureum. Appl Environ Microbiol. 1991;57:1540–1545. doi: 10.1128/aem.57.5.1540-1545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies JE, Singleton PW, Bohlool BB. Influence of size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol. 1991;57(1):19–28. doi: 10.1128/aem.57.1.19-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies JE, Woomer PL, Singleton PW. Enrichment of Bradyrhizobium spp. populations in soil due to cropping of the homologous host plant. Soil Biol Biochem. 1995;27(4–5):633–636. doi: 10.1016/0038-0717(95)98643-3. [DOI] [Google Scholar]
- Thilakarathna MS, Raizada MN. A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol Biochem. 2017;105:177–196. doi: 10.1016/j.soilbio.2016.11.022. [DOI] [Google Scholar]
- Thirumal GR, Subhash RS, Triveni Y, Prasannakumar B. Screening of native rhizobia and pseudomonas strains for plant growth promoting activities. Int J Curr Microbiol Appl Sci. 2017;6:616–625. doi: 10.20546/ijcmas.2017.607.075. [DOI] [Google Scholar]
- Thuita M, Pypers P, Herrmann L, Okalebo RJ, Othieno C, Muema E, Lesueur D. Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol Fert Soil. 2012;48:87–96. doi: 10.1007/s00374-011-0611-z. [DOI] [Google Scholar]
- Tian CF, Wang ET, Wu LJ, Han TX, Chen WF, Gu CT, Gu JG, Chen WX. Rhizobium fabae sp nov., a bacterium that nodulates Vicia faba. Int J Syst Evol Microbiol. 2008;58:2871–2875. doi: 10.1099/ijs.0.2008/000703-0. [DOI] [PubMed] [Google Scholar]
- Tran VV, Berge O, Ke SN, Balandreau J, Heulin T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil. 2000;218:273–284. doi: 10.1023/A:1014986916913. [DOI] [Google Scholar]
- Turan M, Gulluce M, von Wirén N, Sahin F. Yield promotion and phosphorus solubilization by plant growth-promoting rhizobacteria in extensive wheat production in Turkey. J Plant Nutr Soil Sci. 2012;175:818–826. doi: 10.1002/jpln.201200054. [DOI] [Google Scholar]
- Ulzen J, Abaidoo RC, Mensah NE, Masso C, AbdelGadi AH. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in northern Ghana. Front Plant Sci. 2016;7:1–9. doi: 10.3389/fpls.2016.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA—United States Department of Agriculture (2012) Agricultural marketing services, livestock and grain market news. Greeley, Colorado
- Valicente FH, Lana UGP, Pereira ACP, Martins JLA, Tavares ANG (2018) Riscos à produção de biopesticida à base de Bacillus thuringiensis. Circular Técnica 239, Embrapa Milho e Sorgo, Sete Lagoas. p 20. ISSN 1679-1150
- Vanlauwe B, Hungria M, Kanampiu F, Giller KE. The role of legumes in the sustainable intensification of African smallholder agriculture: lessons learnt and challenges for the future. Agric Ecosyst Environ. 2019;284:106583. doi: 10.1016/j.agee.2019.106583). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Lin C. Response of wetland rice to inoculation with Azospirillum lipoferum and Pseudomonas sp. Soil Sci Plant Nutr. 1984;30:117–124. doi: 10.1080/00380768.1984.10434675. [DOI] [Google Scholar]
- Williams PM. Current use of legume inoculant technology. In: Alexander M, editor. Biological nitrogen fixation. New York: Plenum Press; 1984. pp. 173–200. [Google Scholar]
- Williams RL, Kennedy IR. A model for testing the effectiveness of biofertiliser for Australian rice production. In: Kennedy IR, Choudhury ATMA, editors. Biofertilisers in action. Canberra: Rural Industries Research and Development Corporation; 2002. pp. 112–114. [Google Scholar]
- Xiang N, Lawrence KS, Kloepper JW, Donald PA, Mcinroy JA. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS ONE. 2017;12:181–201. doi: 10.1371/journal.pone.0181201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni Y, Zidan M, Dazzo F, Rizk R, Mehesen A, Abdelfattah F, Elsadany A. Enhanced symbiotic performance and productivity of drought stressed common bean after inoculation with tolerant native rhizobia in extensive fields. Agric Ecosyst Environ. 2016;232:119–128. doi: 10.1016/j.agee.2016.07.006. [DOI] [Google Scholar]
- Yates RJ, Howieson JG, Real D, Reeve WG, Vivas-Marfisi A, O’Hara GW. Evidence of selection for effective nodulation in the Trifolium spp. symbiosis with Rhizobium leguminosarum biovar trifolii. Aust J Exp Agric. 2005;45:189–198. doi: 10.1071/EA03168. [DOI] [Google Scholar]
- Youseif SH, El-Megeed FHA, Saleh SA. Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low-fertility sandy soil. Agronomy. 2017;7:1–2. doi: 10.3390/agronomy7010002. [DOI] [Google Scholar]
- Zaheer MS, Raza MAS, Saleem MF, Khan IH, Ahmad S, Iqbal R, Manevski K. Investigating the effect of Azospirillum brasilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L.) Arch Agron Soil Sci. 2019 doi: 10.1080/03650340.2019.1566954. [DOI] [Google Scholar]
- Zhang YJ, Zheng WT, Everall I, Young JPW, Zhang XX, Tian CF, Sui XH, Wang ET, Chen WX. Rhizobium anhuiense sp.nov., isolated from effective nodules of Vicia faba and Pisum sativum. Int J Syst Evol Microbiol. 2015;65:2960–2967. doi: 10.1099/ijs.0.000365. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hussain S, Zhao F, Zhu L, Cao X, Yu S, Jin Q. Effects of Azospirillum brasilense and Pseudomonas fluorescens on nitrogen transformation and enzyme activity in the rice rhizosphere. J Soils Sediment. 2017;18:1453–1465. doi: 10.1007/s11368-017-1861-7. [DOI] [Google Scholar]
- Zvinavashe AT, Lima E, Suna H, Marellia B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. PNAS. 2019 doi: 10.1073/pnas.1915902116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials cited in the manuscript are freely available for the scientific community.