Abstract
In the present study, influence of temperature and dairy industry waste water (DIWW) concentration on the growth of Chlorella pyrenoidosa has been done along with the thermodynamic analysis of different functions viz. change in enthalpy (∆H), change in entropy (∆S), free energy change (∆G), and activation energy (Ea) to study the impact on cell size distribution and morphological changes. Among the studied temperatures, higher biomass productivity was observed at 35 °C at 75% of DIWW. Thermodynamic analysis showed the spontaneous and exothermic nature of growth of C. pyrenoidosa. Experimental data have significantly proven the kinetic and thermodynamics functions with 35 °C temperature, ∆H (− 46.78 kJ mol−1), ∆S (− 0.10 kJ mol−1), ∆G (− 14.8 kJ mol-1), and Ea (49.28 kJ mol−1). At this temperature, size distribution showed maximum percentage (48%) cells were of 6540 nm, whereas the minimum percentage (3%) cells were of 2750 nm. SEM–EDX study revealed that increase in temperature leads to increase in roughness and elemental deposition of metal on cell surface.
Keywords: Wastewater, Temperature, Algal cell morphology, Thermodynamics, Kinetics
Introduction
Among the various environmental features, temperature is the factor which directly regulates the algal growth with light fluctuation and nutrient availability. It strongly affects the cellular composition, rate of uptake of nutrients, fixation of carbon dioxide, and rate of growth for every species of algae (Juneja et al. 2013; Yadala and Cremaschi 2014; Li et al. 2019). The growth rate increases up to an optimum temperature after which further increase will cause decrease in growth rate and will also impact on algal shape. Photosynthesis process is one of the most heat-sensitive processes in algae, and it is more affected by temperature in comparison to other stress factors (Larras et al. 2013; Mathur et al. 2014; Kang et al. 2018). Heat created by temperature stress disturbs the supply of energy and consumption of energy in photosynthetic algae, which affects the chloroplast efficiency in process of photosynthesis and consequently, reduces the temperature acclimation and stops the photosynthesis in algal cell (Tikkanen et al. 2012). Environmental temperature significantly influences the microbial community structure. Decrease in temperature can shift the structure of microalgal community due to difference in sensitivity uptake of nutrient from biological wastewater and deterioration of the structure of the community was also studied (Ashok et al. 2019). Temperature affects the microalgal process very much in ways as rate of reaction and pathway, death and yield of microorganism (Huang et al. 2015). Lower temperature of wastewater may also lead to physiological deterioration of process performance by microbial communities also studied by Zhou et al. (2018).
Singh and Singh (2014) reported that optimum range of temperature for Chlorella vulgaris is 25–30 °C which increases the biomass content and chlorophyll content at elevated carbon dioxide level (6%). Cassidy (2012) studied the growth of Scenedesmus sp. at temperatures of 15–36 °C and found that at low temperature the level of chlorophyll and protein get reduced whereas levels of carbohydrates and lipid are increased. Zhou et al. (2018) found that the structural and functional activity of microalgal cell directly depends on the low temperature and nutrients present, which affect cell membrane and structural composition. The relationship between morphology (size and shape) of algae and fluctuation in temperature has experienced a contemporary stimulation due to the distresses about changing the climate. Some recent studies have confirmed that at higher temperatures the algal cell size reduces (Yvondurocher et al. 2011). With the increased evidence of temperature, influence on size decline was observed, interest in the relative importance of direct and indirect temperature effects has emerged (Peter and Sommer 2015). Cell size increase and decrease depends on the catabolic and anabolic process which is affected by the change in temperature and demand of resources (Ras et al. 2013). The mechanism driving intraspecific and community level size reductions differs between systems temperature and nutrient limitation which promotes small size algae (Finkel et al. 2010; Peter and Sommer 2015) and higher sedimentation for algae (Piontek et al. 2009). During the harvesting process of algae, temperature plays a crucial role because the harvesting efficiency of flocculants depends on specific temperature ranges (Kothari et al. 2017a, b). Furthermore, Shurair et al. (2019) said that bio-flocculant conserved algal structure and chemical flocculant stressed and deformed algal cells. Moreover, temperature directly alters photosynthesis and respiration rates but this direct effect can be outweighed by other factors.
In this work, influence of temperature on Chlorella sp. was evaluated on the basis of morphological features taking diary industry wastewater as a nutrient media. The studies were focused on variation in growth rate, cell size and shape with reference to providing heat stress conditions in association with variation in concentrations (25%, 50%, 75%, and 100%) of wastewater selected as nutrients. Growth kinetics and thermodynamic studies were also assessed with selected temperature ranges.
Materials and methods
Algal strain (C. pyrenoidosa; NCIM-2738) was obtained from NCIM, Pune, India and cultured as per prescription in BG-11 medium (in gL−1): NaNO3 1.5; K2HPO4 0.04; MgSO4·7H2O 0.075; CaCl2·2H2O 0.036; citric acid 0.006; ferric ammonium citrate 0.006; EDTA (disodium salt) 0.001; Na2CO3, 0.02; 1 mL trace elements solution (in gL−1: H3BO3 2.86; MnCl2·4H2O 1.81; ZnSO4·7H2O 0.222; NaMoO4·2H2O 0.39; CuSO4·5H2O 0.079; Co(NO3)2·6H2O 0.0494), with a pH of 7.0 ± 1. Sterilization of media and flasks were done before use by 20 min autoclaving at 15 Psi and 121 °C. Similarly, sterilization of glasswares were also done at 120 °C for 6–7 h before use. Inoculation of algal strain was done at 25 ± 1 °C in a 12 h light (10 Wm−2)/dark cycle.
Collection of Dairy industry wastewater
Dairy industry wastewater (pH 7.2 ± 0.3; total dissolved solids 975 ± 12.5 mgL−1, chloride 310 ± 6.5 mgL−1, hardness 471.5 ± 7 mgL−1; nitrate 58.5 ± 3.5 mgL−1; phosphate 18.6 ± 2.4 mgL−1; alkalinity 983.5 ± 5 mg CaCO3mgL−1, BOD 618.75 ± 1.5 mgL−1, COD 1074.35 ± mgL−1) was collected from Producer Cooperative Milk Union Limited, Lucknow, Uttar Pradesh, India. This has the capacity to produce an average of 1MLD of wastewater per day. The wastewater samples were collected in sterilized sampling bottles and stored at 4 °C before use. The characterization of wastewater was performed according to standard methodologies (APHA, AWWA, WEF 2012). Various concentrations of wastewater were selected to optimize the growth at different temperatures.
Effect of temperature
To study temperature induced algal growth, size and morphological changes, thirty five Erlenmeyer Flasks (250 mL), each filled with 200 mL of different concentrations of DIWW were used as algae growth reactors. Each flask was inoculated with C. pyrenoidosa culture and was incubated for 12 h of lights (10 Wm−2)/dark cycle at different ranges of temperature. Agitation (mixing) was given to flask kept in the incubator shaker set at 100 rpm at different temperatures of 20 °C, 25 °C, 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C.
Measurement of algal growth
Microalgae growth was measured by observing the optical density (O.D.) using UV–Vis spectrophotometer (Shimadzu Model,) at 680 nm. All cultures were inoculated to the extent so that the initial O.D. was 0.1 at zero time point. Cells were concentrated and dried (60 °C) and expressed in terms of gL−1. From the calibration curve observed from O.D. and dry algal biomass, the regression equation obtained was y = 0.613x (R2 = 0.98), where y is the dry cell weight (gL−1) and x is the absorbance at 680 nm.
Measurement of algal cell size, cell shape and morphological characterization
Percentage distribution of cell size of algae measured was determined by the Malvern HYDRO 200MU, Laser particle size analyzer. Microalgal cells shape and size were viewed on an optical microscope (OLYMPUS) with digital camera. Algal cell surface morphological characterization and elemental composition were analyzed by the Scanning Electron Microscope coupled Energy Dispersive X-ray spectroscopy (SEM–EDX model: JSM-6490LV, Make: JEOL, Japan).
Algal growth kinetics
Most of the algal growth practices can be described by Teleken et al. (2018) and Logistic equations. Among them, Logistic growth equation was commonly applied to interpretate the relationship between the growth and density during nutrition-limited condition (Ahmad et al. 2018‚ 2019). Thus, Logistic model Eq. (1) was chosen for algal growth.
| 1 |
where dX/dt was the microalgae growth rate; k was the maximum specific growth rate of the microalgae, X was the biomass concentration of microalgae, and Xmax was the maximum biomass concentration.
Algal growth was evaluated daily by optical density measurements at 650 nm in three replicates, which was converted into dry cell weight per liter of culture by a regression equation derived previously. Specific growth rate (SGR) and volumetric biomass productivity (VBP) were calculated using the cell density (g/L). Specific growth rate (d−1) was calculated as follows:
| 2 |
Volumetric biomass productivity, r (gL−1 d−1) was calculated as follows:
| 3 |
where X1 and X2 were the biomass concentration (g L−1) on days t1 and t2, respectively.
Determination of thermodynamic functions
Investigation of different thermodynamic functions (enthalpy, entropy, Gibb’s free energy, and activation energy) for algal growth are complete by use of Eyring and Arrhenius equations over the parameters obtained from growth kinetic study with different ranges of selected temperature (Kothari et al. 2017a, b). Among these equations, Eyring equation provides the value of enthalpy and entropy whereas Arrhenius equation is helpful in analyzing the value of activation energy for the growth of algae at different range of temperatures. From these parameters, obtained Gibbs free energy was determined using Gibbs free energy equation.
Eyring equation
For this study, temperature based reaction’s rate variation describe using Eyring equation for kinetics. It allows the activation energy (Ea), enthalpy (ΔH), and entropy (ΔS), to be determined from the temperature which depends upon rate constant. For thermodynamic analysis of algal growth, batch experiments were conducted at different temperatures with the difference of 5 °C and initiating from 20 to 50 °C. The growth rate constant k was determined from the growth vs time profile study of the algal biomass. The graph plots in ln(K/T) versus 1/T to calculate the thermodynamic parameters using Eyring equation (Eqs. 4–6). To calculate the parameters on the basis of thermodynamic study, the slope and intercept of straight line equation (Eyring equation) were used with the help of standard enthalpy change (ΔH) and the standard enthalpy change (ΔS) (Liu et al. 2017).
| 4 |
| 5 |
| 6 |
where k is growth rate constant obtained from biomass vs time profile study, R is the universal gas constant (8.314 K−1 mol−1), T is temperature, Kb is Boltzmann constant (1.381 × 10−23m2kg s−2 K−2), and h is plank’s constant (6.626 × 10−34m2kgs−1).The Gibb’s free energy (ΔG) was calculated from the obtained value of ΔS and ΔH using equation Eq. (7)
| 7 |
Activation energy
According to recent experimental studies temperature plays an essential role in growth of algae. Various values of first order rate constant were observed for different temperatures and a graph was plotted between lnk vs 1/T. From this graph, Arrhenius equation (Eq. 8) was used to investigate the activation energy for the chemical reaction and growth. According to this equation, relationship between rate constant and temperature is:
| 8 |
| 9 |
In this equation, R is the universal gas constant (8.31 J mol−1 K−1), T is temperature (K), Ea/R is the slope of the regression line, and lnA is the y-intercept value (Lucia 2015).
Results and discussion
Microalgae photosynthesize, i.e., they assimilate the nutrients (organic and inorganic) to convert them into organic-type matter. Temperature is the crucial factor which drives the photosynthetic reaction (Mackey et al. 2013). Many experimental studies focused on the algal growth variation due to temperature, an important factor for energy source in algae. Here, in the present study the algal growth was conducted and observed for 30 days taking the nutrient as dairy wastewater test period for the given experimental conditions.
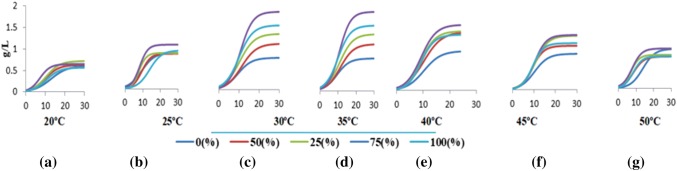
Major objective for this was to optimize the temperature range for algal growth with optimized concentration of wastewater used for this study very particular to C. pyrenoidosa. The measurements of algal growth under exposure of different temperatures have been shown in Fig. 1. It is observed that growth rates with selected temperature showed a linear relationship, which directly corresponds to high R2 values, i.e., linear growth trend. High R2 values support that the growth rate is in favour of linear trend with the given experimental conditions.
Fig. 1.
Logistic growth kinetics of algae with selected concentration of DIWW with temperature: a 20 °C; b 25 °C; c 30 °C; d 35 °C; e 40 °C; f 45 °C; g 50 °C
Logistic growth kinetics of algae at selected temperature ranges
Four different phases for algal growth are: (1) lag or acclimatization phase; (2) log growth phase; (3) declining growth phase; (4) stationary phase. Normal curve of development was observed at the 0%, 25%, and 50% medium composition, which closely resembles the growth curve. From Fig. 1a–g, it is evident that the lag phase for the algal growth occurred between 0 and 10 days. At 20 °C and 25 °C lag phase was 6 days but at 30°C and 35 °C it got reduced to 5 days. At 40 °C and 45 °C lag phase increases further which goes to 10 days at 50 °C. Exponential growth was observed between 6–10th days at 20 °C and 25 °C but at 30 °C and 35 °C exponential phase increases further by 5 days than previous which suddenly decreased with further increasing temperature up to 50 °C. Stationary phase was similar and of more duration at 20 °C and 25 °C with length shortening with rise in temperature to 30 °C and 35 °C. With an increment in temperature it was observed that the duration of stationary phase increases and was maximum at temperature 50 °C with different concentration of DIWW. According to literature, C. vulgaris had the best growth rates till temperature with 30 °C only. Malakootian et al. (2016), Bita and Gerats, (2013) said that temperatures above 30 °C affect the growth, and till 30 °C were the stress conditions for algal growth. Algae are able to change their shape, color, structure, composition, and mode of survival depending on their surrounding environment (temperature, pH, light, etc.). The statistical analysis of the experimental findings showed that the interactions between wastewater and temperature were significantly different (p > 0.05). Table 1 depicts the logistic growth kinetics variable for algal growth and data favours that 75% concentration of DIWW for biomass growth all selected temperature ranges. Direct relationship were observed with biomass yield and DIWW concentration from 0 to 75% but after that it showed inverse relationship for biomass yield with increase in temperature, concentration, and concentration of DIWW. Physiological adjustment found in Symbiodinium show optimum growth at > 32 °C (Sammarco and Strychar 2013). Specific growth rate (K) of alga was also found with the similar pattern and recorded with continuous increase up to 75% wastewater concentration. The specific growth rate constant was maximum and the value of doubling time was found minimum for the algal growth at 75% wastewater concentration. For 75% wastewater concentration, the value of doubling time decreased with increasing temperature up to its optimal value which was 35 °C after which doubling time increases due to decrease in the metabolic activity of algae with rise in temperature. Feasible temperature for feedstock utilization is well flourished under 25 and 32 °C (Shukla and Kumar 2018). According to Singh and Singh (2015) and Juneja et al. (2013) below optimal temperature, growth rate declines markedly with the species or strain specific in nature. Among all the concentrations, maximum doubling time was observed with 0% DIWW concentration which is obvious as there was no growth possible in the absence of media. The optimal temperature of cultivation for algal growth ranges from 15 to 30 °C, and beyond this temperature range algal cell damages may lead to dearth. Pithophora oedogonia and Cladophora glomerata showed good survival rate better survive at 10–28 °C (Ugwu et al. 2007). Another investigation revealed that 25 °C for optimal growth of C. pyrenoidosa with a growth rate 1.1 d−1 (Mondal et al. 2017). Growth rate of B. braunii strain become stable at 15–30 °C but cannot grow above 51 °C. Bazguj (2009) said that C. vulgaris show heat stress above 30 °C. On the other hand, Scenedesmus sp. has more consistent growth rate ranging from 20 to 40 °C. Lower temperature decreases the fluidity of cell membrane in algal cells.
Table 1.
Logistic growth of Chlorella pyrenoidosa with different concentrations of DIWW at different temperatures
| DIWW (%) | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k (d−1) | dt | k (d−1) | dt | k (d−1) | dt | k (d−1) | dt | k (d−1) | dt | K (d−1) | dt | k (d−1) | dt | |
| 0 | 0.19 | 3.4 | 0.19 | 3.83 | 0.20 | 5.32 | 0.21 | 4.11 | 0.20 | 4.45 | 0.19 | 4.31 | 0.20 | 4.41 |
| 25 | 0.21 | 4.7 | 0.22 | 3.89 | 0.25 | 4.95 | 0.25 | 4.6 | 0.22 | 4.32 | 0.18 | 4.85 | 0.18 | 4.49 |
| 50 | 0.21 | 5.01 | 0.25 | 4.35 | 0.27 | 4.94 | 0.24 | 4.26 | 0.22 | 4.45 | 0.22 | 4.46 | 0.19 | 4.42 |
| 75 | 0.23 | 4.94 | 0.25 | 4.34 | 0.27 | 4.51 | 0.28 | 4.31 | 0.24 | 4.02 | 0.23 | 4.37 | 0.20 | 5.24 |
| 100 | 0.22 | 4.37 | 0.23 | 4.19 | 0.25 | 5.51 | 0.27 | 3.99 | 0.21 | 3.97 | 0.21 | 4.57 | 0.17 | 4.37 |
Thermodynamic functions of algal growth at different concentration of DIWW at varying temperatures
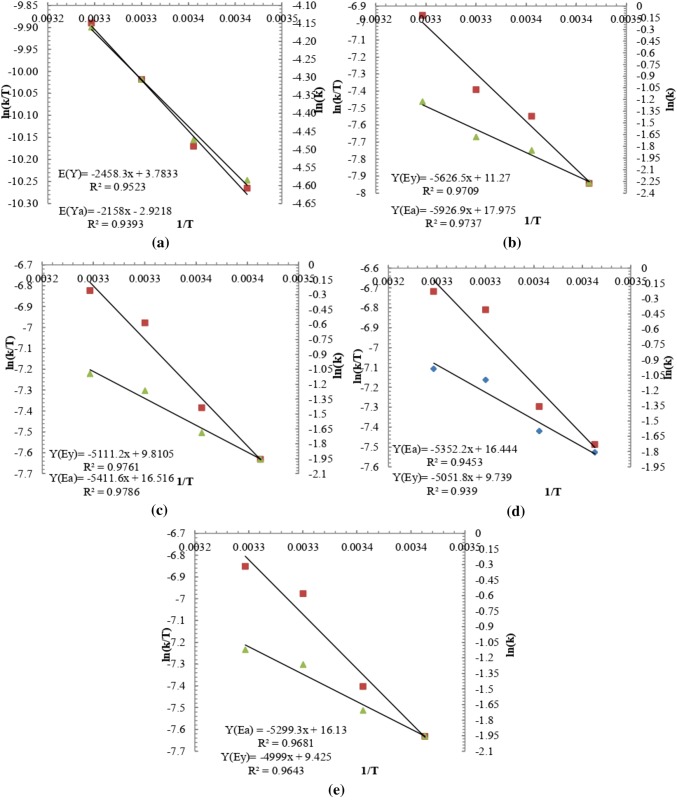
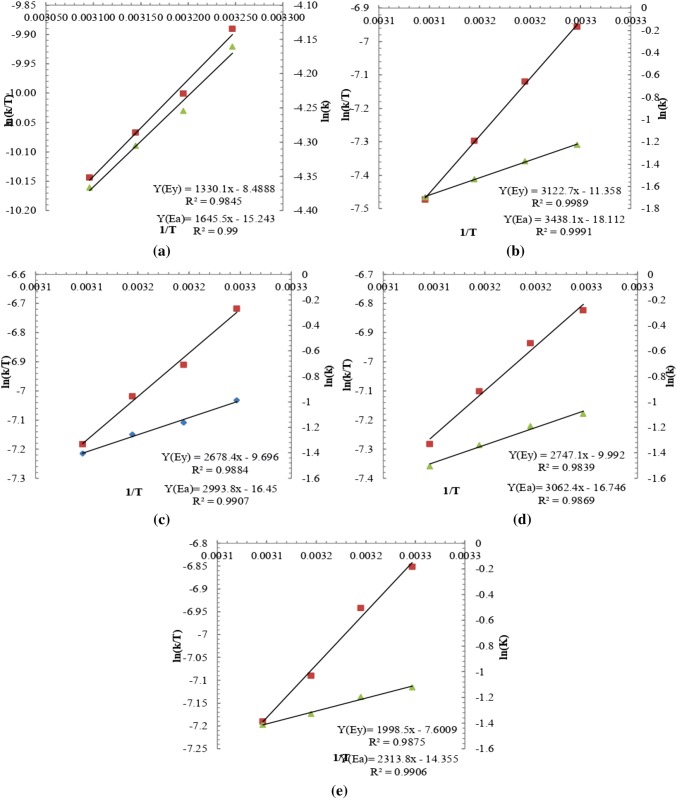
The specific growth rate of algae at different concentration of nutrient (0%, 25%, 50% 75%, and 100%) were obtained from their plot between biomass growth vs time curve (Fig. 1). From these plots growth rate constants were obtained and corresponding plot between (1/T) vs ln (k/T) were drawn to obtain different thermodynamics function ΔH, ΔS using the Eyring equation which was further extended to find the free energy ΔG involved in the growth kinetics (Kothari et al. 2017b). Using Arrhenius equation and the corresponding plot between (1/T) v/s ln (k) gives the activation energy of the system (Jayakumar et al. 2015). The increase in temperature accelerates the rate of biochemical reaction, which in turn increases the growth rate due to increase in the substrate utilization rate. Temperature coefficient represents a measure of the rate of change of the system, when the temperature is increased by 10 °C (Shukla and Kumar 2018). The values of ΔH and ΔS were calculated by Eyring plots with slope and intercepts as shown in Fig. 2a–e for temperature 20–35 °C and Fig. 3a–e for temperature 35–50 °C at different concentration of dairy industry wastewater (DIWW). Eyring plot and Arrhenius plot were plotted separately for temperature ranges 20–35 °C and from 35 to 50 °C as from changing temperature range from 20–35 °C to 35–50 °C had different impacts on the growth and thermodynamic properties due to which there was change in direction and pattern of the slope. So, these have been analyzed separately from 20 to 35 °C in Fig. 2a–e and 35 °C to 50 °C in Fig. 3a–e. From values of ΔH and ΔS obtained from the Eyring equation the corresponding value of the free energy change (ΔG) involved in the growth was calculated at selected temperatures (20–50 °C).
Fig. 2.
Erying and Arrhenius plot of algal growth for temperature (20–35 °C) with selected concentrations of DIWW: a 0%; b 25%; c 50%; d 75%; e 100%
Fig. 3.
Erying and Arrhenius plot of algal growth for temperature (35–50 °C) with selected concentration of DIWW: a 0%; b 25%; c 50%; d 75%; e 100%
Activation energy was obtained from the Arrhenius plot (lnK verses 1/T) which was found to be highest 49.28 kJmol−1 for 25% DIWW and minimum was 42.00 kJmol−1 for 100% DIWW from 20 to 35 °C shown in Table 2 (i). By increasing temperature from 35 to 50 °C drastic change was observed in the value of activation energy as shown in Table 2 (ii). Activation energy is the minimum energy required for growth of algal therefore the experiment study was done from 20 to 50 °C for study of growth characteristics.
Table 2.
Thermodynamic functions kJ mol−1 (ΔH, ΔS, ΔG and Ea) for growth of microalgae Chlorella sp. at temperature range (i) 20–35 °C; (ii) 35–50 °C
| Thermodynamic function | 0 (%) | 25 (%) | 50 (%) | 75 (%) | 100 (%) |
|---|---|---|---|---|---|
| (i) 20–35 °C | |||||
| ΔH | − 20.44 | − 46.78 | − 42.49 | − 41.99 | − 41.56 |
| ΔS | − 0.17 | − 0.10 | − 0.12 | − 0.12 | − 0.12 |
| ΔG(20 °C) | 28.22 | − 16.35 | − 8.51 | − 7.84 | − 6.64 |
| ΔG(25 °C) | 29.06 | − 15.83 | − 7.93 | − 7.26 | − 6.04 |
| ΔG(30 °C) | 29.89 | − 15.31 | − 7.35 | − 6.67 | − 5.45 |
| ΔG(35 °C) | 30.72 | − 14.80 | − 6.77 | − 6.09 | − 4.85 |
| Ea | − 17.94 | 49.28 | 44.99 | 44.06 | 42.00 |
| (ii) 35–50 °C | |||||
| ΔH | − 2.74 | − 25.96 | − 22.27 | − 22.84 | − 19.24 |
| ΔS | − 0.27 | − 0.29 | − 0.28 | − 0.28 | − 0.32 |
| ΔG(35 °C) | 79.84 | 63.96 | 63.40 | 63.59 | 78.31 |
| ΔG(40 °C) | 81.18 | 65.42 | 64.79 | 64.99 | 79.90 |
| ΔG(45 °C) | 82.52 | 66.88 | 66.18 | 66.40 | 81.48 |
| ΔG(50 °C) | 83.86 | 68.34 | 67.58 | 67.80 | 83.06 |
| Ea | − 3.68 | − 28.58 | − 24.89 | − 25.46 | − 16.62 |
Influence of temperature on size distribution of algal cell
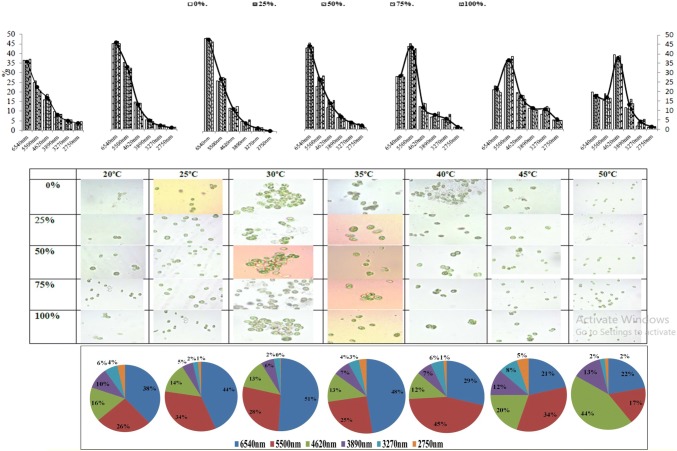
Figure 4 shows the impact of temperature ranges from 20 to 50 °C on distribution of cell size of C. pyrenoidosa. Three ranges of algae 6540 nm, 5500 nm, and 4260 nm were found in maximum percentage at different temperature ranges. It is evident from Fig. 4 that biomass grown showed the highest distribution of largest cell size, i.e., 6540 nm which increases with increasing temperature from 20 °C up to 35 °C. Besides this the maximum percentage of 4260 nm size algae were found in the higher temperature range 30–50 °C. Thus, moderate temperature range showed the production of large cell sized algal biomass.
Fig. 4.
Morphological characterstics and shape of the algae with selected temperature ranges with different concentration of DIWW
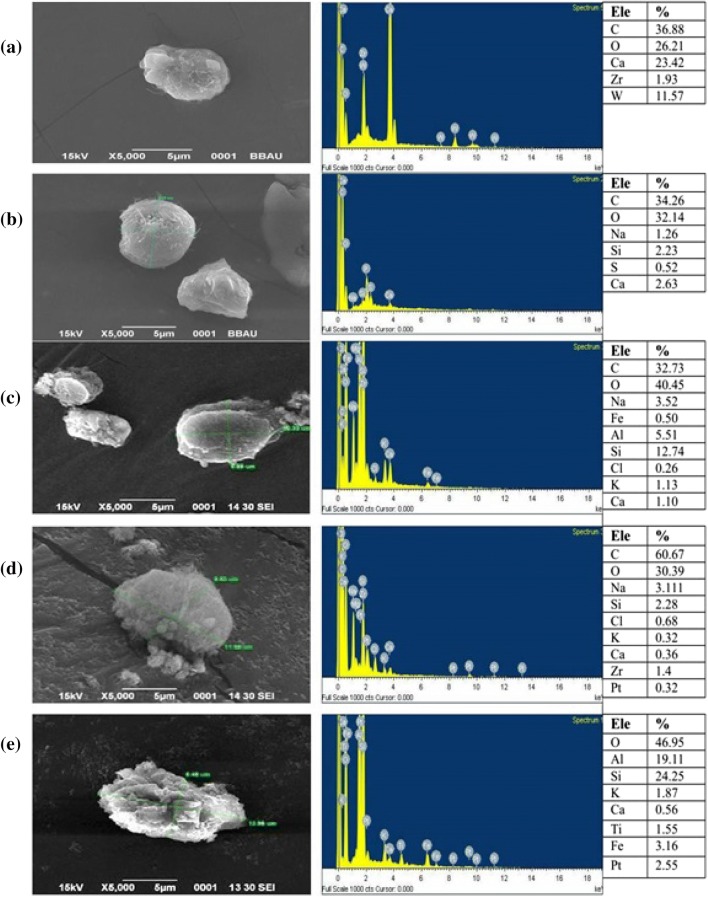
Temperature is one of the important process parameter for growth of algal biomass. It strongly influences directly to morphological features of cell and uptake of nutrients. The change in cell morphology during the treatments were analyzed using SEM–EDX generated micrograph of algal biomass (Fig. 5a–e at selected temperatures 20 °C, 30 °C, 40 °C, and 50 °C (10 °C difference in temperature). All temperatures were observed with or more significant morphological changes. There was a smooth cell surface without any elemental deposition noticed for control. Whereas, apparent differences were observed between the temperatures (20 °C, 30 °C, 40 °C, and 50 °C) during the experiment with 75% of DIWW with respect to cell size and the deposition observed at cell surface.
Fig. 5.
Surface morphology of algae and deposition of elemental materials with selected temperature: a control; b 20 °C; c 30 °C; d 40 °C; e 50 °C
Earlier investigations on cell size of green algae over different ranges of temperature also showed that the major physical factor for algal growth and its size was directly temperature. In this context, Rasconi et al. (2015) studied the effect of increasing water temperature on fresh water plankton. They reported that highest temperature favored the smaller size of plankton and lower temperature favored the higher size of plankton. Similar to the present study, Parmesan (2006) indicated that climatic condition with elevated temperature produced lower size of plankton while the climatic condition with lower temperature favors higher size of plankton. Gonzolez-Meler et al. (2004) found that temperature stress lead to slower growth rate, not only does temperature alter the shape of algal cell but it damages organelle (chloroplast, mitochondria, nuclei, etc). Temperature induced changes in algal cell size can be attributed to the physiological reasons. According to Simionato et al. (2013), nitrogen starvation or starvation leads to decreasing growth rate photosynthesis cell size and increase in lipid and carbohydrate content. A reference study also found by Campbell et al. (2017), suggested that temperature changes cause the alternation in anabolic and catabolic process, which ultimately affects the organism size. Brown et al. (2005) had attempted to predict that large scale ecological pattern affect the metabolic rate and body size. According to Bramburger et al. (2017) all species’ mean cell size significantly decreases with increasing temperature. Bisova and Zachleder (2014) proved that growth rate as well as size of microalgae was affected by temperature critical cell size changes significantly with different growth rates, suggesting that the size comprises more than one component.
Effect of temperature on algal surface morphology
Although, C. pyrenoidosa was selected here in this study to observe the impact of temperature on algal cell morphology and cell size but this strain also has been targeted in various other multiple studies like wastewater treatment and bio-oil option in our laboratory for last few years in integration with different types of wastewater concentration and temperature interaction being done for the first time. The total sizes of algal cells along with deposition at their surface were found to be increasing with the increase in temperature. However, it was observed that this increase in size was not due to the increase in cell size, but due to the depositions observed on cell surface. The maximum cell size (without deposition) was seen at 30 °C than at other temperatures which may be due to the swollenness of the cells. Increase in deposition at cell surface causes increase in roughness of the cell surface which was found highest at 50 °C. It was observed that results support to achieve the goal of the study, i.e., high temperature is responsible for roughness on algal cell surface morphology. It was also cited by researchers (Rhee 1982; Darley 1982; Harris 1986; Juneja et al. 2013) that high or low temperature differs from optimal ranges, results in growth with minimal cell size and utilization of carbon and nitrogen efficiency also decreases with this. Whereas, Pasaribu et al. (2016) also studied the effect of temperature on algal cell morphology by Symbiodium and cited that sometimes adaptive responses of algal responses favour the environmental stresses for high-value bio products also. EDX study of C. pyrenoidosa surface at different temperatures supported that increase in temperature causes decrease in cell size due to shrinking but increases the elemental deposition which leads to increase in roughness behavior of algal cell at high temperatures. Thus, the SEM microscopy of biomass obtained at 20 °C, 30 °C, 40 °C, and 50 °C revealed that the variations in size of algal cells have been found to be related with variation in physiological activity of algal cell at different range of temperatures.
Conclusion
The change in temperature affects growth and thermodynamic functions as well as causes the morphological changes in the cells of Chlorella sp. In our study, the optimal temperature was found to be 35 °C with 75% DIWW concentration for maximum growth. The experimental data was proven significantly by kinetic model and thermodynamic functions analysis of ∆H (− 46.78 kJ mol−1), ∆S (− 0.10 kJ mol−1), ∆G (− 14.8 kJ mol−1), and Ea (49.28 kJ mol−1). Activation energy was obtained from the Arrhenius plot (lnK verses 1/T) which was found to be highest as 49.28 kJmol−1 for 25% DIWW and minimum was 42.00 kJmol−1 for 100% DIWW. At this temperature the maximum percentage (48%) cells were of size 6540 nm. SEM–EDX study revealed that increase in temperature leads to increase in roughness and elemental deposition of metal on cell surface. The findings of the present study support the relative elasticity of C. pyrenoidosa towards selected temperature ranges, which affects the catabolic and anabolic activities of algal cell via directly influencing the shape, size and surface morphology. For future view, this research study will helpful to regulate derived growth of algal biomass with optimum temperature range, as per needs of application for value-added products.
Acknowledgements
The authors and co-authors of the article are very thankful to UGC, India for proving financial assistance and to Head, Department of Environmental Science and Director, USIC of BBAU, Lucknow, India.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Ahmad S, Pathak VV, Kothari R, Kumar A, Krishna SBN. Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech. 2018;8:326. doi: 10.1007/s13205-018-1342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Kothari R, Pathania D, Tyagi VV. Optimization of nutrients from wastewater using RSM for augmentation of Chlorella pyrenoidosa with enhanced lipid productivity, FAME content, and its quality assessment using fuel quality index. Biomass Convers Biorefin. 2019;1:18. doi: 10.1007/s13399-019-00443-z. [DOI] [Google Scholar]
- APHA, AWWA, WEF . Standard methods for examination of water and wastewater. 22. Washington: American Public Health Association; 2012. p. 1360. [Google Scholar]
- Ashok V, Shriwastav A, Bose P, Gupta SK. Phycoremediation of wastewater using algal-bacterial photobioreactor: effect of nutrient load and light intensity. Bioresour Technol Rep. 2019;7:100205. [Google Scholar]
- Bajguz A. Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J Plant Physiol. 2009;8:882–886. doi: 10.1016/j.jplph.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Bisova K, Zachleder V. Cell-cycle regulation in green algae dividing by multiple fission. J Exp Bot. 2014;10:2585–2602. doi: 10.1093/jxb/ert466. [DOI] [PubMed] [Google Scholar]
- Bita C, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramburger AJ, Reavie ED, Sgro GV, Estepp LR, Chraïbi VS, Pillsbury RW. Decreases in diatom cell size during the 20th century in the Laurentian Great Lakes: a response to warming waters. J Plankton Res. 2017;39:199–210. [Google Scholar]
- Brown JH, West GB, Enquist BJ. Yes, West, Brown and Enquist’s model of allometric scaling is both mathematically correct and biologically relevant. Funct Ecol. 2005;19:735–738. [Google Scholar]
- Campbell K, Herrera-Dominguez L, Correia-Melo C, Zelezniak A, Ralser M. Biochemical principles enabling metabolic cooperativity and phenotypic heterogeneity at the single cell level. Curr Opin Syst Biol. 2017;8:97–108. [Google Scholar]
- Cassidy KO (2012) Evaluating algal growth at different temperatures. Thesis, University of Kentucky, UK
- Gonzalez-Meler MA, Taneva LINA, Trueman RJ. Plant respiration and elevated atmospheric CO2 concentration: cellular responses and global significance. Ann Bot. 2004;94:647–656. doi: 10.1093/aob/mch189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley WM. Algal biology: a physiological approach. 9. Oxford: Blackwell; 1982. p. 1168. [Google Scholar]
- Finkel ZV, Beardall J, Flynn KJ, Quigg A, Rees TAV, Raven JA. Phytoplankton in a changing world: cell size and elemental stoichiometry. J Plankton Res. 2010;32:119–137. [Google Scholar]
- Harris GP. Phytoplankton ecology: structure, function and fluctuation. New York: Chapman and Hall; 1986. [Google Scholar]
- Huang W, Li B, Zhang C, Zhang Z, Lei Z, Lu B, Zhou B. Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresour Technol. 2015;179:187–192. doi: 10.1016/j.biortech.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Rajasimman M, Karthikeyan C. Optimization, equilibrium, kinetic, thermodynamic and desorption studies on the sorption of Cu (II) from an aqueous solution using marine green algae: Halimeda gracilis. Ecotox Environ Saf. 2015;121:199–210. doi: 10.1016/j.ecoenv.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Juneja A, Ceballos RM, Murthy GS. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies. 2013;6:4607–4638. [Google Scholar]
- Kang D, Kim K, Jang Y, Moon H, Ju D, Kwon G, Jahng D. Enhancement of wastewater treatment efficiency through modulation of aeration and blue light on wastewater-borne algal-bacterial consortia. Int Biodeterior Biodegrad. 2018;135:9–18. [Google Scholar]
- Kothari R, Pandey A, Ahmad S, Kumar A, Pathak VV, Tyagi VV. Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech. 2017;7:243. doi: 10.1007/s13205-017-0812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari R, Pathak VV, Pandey A, Ahmad S, Srivastava C, Tyagi VV. A novel method to harvest Chlorella sp. via low cost bioflocculant: influence of temperature with kinetic and thermodynamic functions. Bioresour Technol. 2017;225:84–89. doi: 10.1016/j.biortech.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Larras F, Lambert AS, Pesce S, Rimet F, Bouchez A, Montuelle B. The effect of temperature and a herbicide mixture on freshwater periphytic algae. Ecotoxicol Environ Saf. 2013;98:162–170. doi: 10.1016/j.ecoenv.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang T, Yang Z. Immobilizing unicellular microalga on pellet-forming filamentous fungus: can this provide new insights into the remediation of arsenic from contaminated water? Bioresour Technol. 2019;284:231–239. doi: 10.1016/j.biortech.2019.03.128. [DOI] [PubMed] [Google Scholar]
- Liu X, Ying K, Chen G, Zhou C, Zhang W, Zhang X, Tao Y. Growth of Chlorella vulgaris and nutrient removal in the wastewater in response to intermittent carbon dioxide. Chemosphere. 2017;186:977–985. doi: 10.1016/j.chemosphere.2017.07.160. [DOI] [PubMed] [Google Scholar]
- Lucia U. Bioengineering thermodynamics of biological cells. Theor Biol Med Model. 2015;12:29. doi: 10.1186/s12976-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey KR, Paytan A, Caldeira K, Grossman A, Moran D, McIlvin M, Saito M. Effect of temperature on photosynthesis and growth in marine Synechococcus. Plant Physiol. 2013;63(2):815–829. doi: 10.1104/pp.113.221937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakootian M, Hatami B, Dowlatshahi S, Rajabizadeh A. Growth and lipid accumulation in response to different cultivation temperatures in Nannochloropsis oculata for biodiesel production. Environ Health Eng Manag J. 2016;1:29–34. [Google Scholar]
- Mathur S, Agrawal D, Jajoo A. Photosynthesis: response to high temperature stress. J Photochem Photobiol B Biol. 2014;137:116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Mondal M, Shrayanti G, Ashmita G, Gunapati O, Tiwari ON, Papita D, Gayen K, Mandal MK, Halder GN. Production of biodiesel from microalgae through biological carbon capture: a review. 3 Biotech. 2017;7:99. doi: 10.1007/s13205-017-0727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- Pasaribu B, Li YS, Kuo PC, Lin IP, Tew KS, Tzen JT, Liao YK, Chen CS, Jiang PL. The effect of temperature and nitrogen deprivation on cell morphology and physiology of Symbiodinium. Oceanologia. 2016;58(4):272–278. [Google Scholar]
- Peter KH, Sommer U. Interactive effect of warming, nitrogen and phosphorus limitation on phytoplankton cell size. Ecol Evol. 2015;5:1011–1024. doi: 10.1002/ece3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek J, Handel N, Langer G, Wohlers J, Riebesell U, Engel A. Effects of rising temperature on the formation and microbial degradation of marine diatom aggregates. Aquat Microb Ecol. 2009;54:305–318. [Google Scholar]
- Ras M, Steyer JP, Bernard O. Temperature effect on microalgae: a crucial factor for outdoor production. Rev Env Sci Biotech. 2013;12(2):153–164. [Google Scholar]
- Rasconi S, Gall A, Winter K, Kainz M. Increasing water temperature triggers dominance of freshwater picoplankton. PLoS ONE. 2015;10(10):e0140449. doi: 10.1371/journal.pone.0140449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee GY. Effects of environmental factors and their interactions on phytoplankton growth. Adv Microb Ecol. 1982;6:33–74. [Google Scholar]
- Sammarco PW, Strychar KB. Responses to high seawater temperatures in Zooxanthellate Octocorals. PLoS ONE. 2013;8(2):54989. doi: 10.1371/journal.pone.0054989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M, Kumar S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew Sust Energ Rev. 2018;82:402–414. [Google Scholar]
- Shurair M, Almomani F, Bhosale R, Khraisheh M, Qiblawey H. Harvesting of intact microalgae in single and sequential conditioning steps by chemical and biological based–flocculants: effect on harvesting efficiency, water recovery and algal cell morphology. Bioresour Technol. 2019;281:250–259. doi: 10.1016/j.biortech.2019.02.103. [DOI] [PubMed] [Google Scholar]
- Simionato D, Block MA, La Rocca N, Jouhet J, Marechal E, Finazzi G, Morosinotto T. Response of Nannochloropsis gaditana to nitrogen starvation includes a de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids and a reorganization of the photosynthetic apparatus. Eukaryot Cell. 2013;12(5):665–676. doi: 10.1128/EC.00363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Singh P. Effect of CO2 concentration on algal growth: a review. Renew Sust Energ Rev. 2014;38:172–179. [Google Scholar]
- Singh SP, Singh P. Effect of temperature and light on the growth of algae species: a review. Renew Sust Energ Rev. 2015;50:431–444. [Google Scholar]
- Teleken JT, Galvao AC, Da Silva RW. Use of modified Richards model to predict isothermal and non-isothermal microbial growth. Braz J Microbiol. 2018;49(3):614–620. doi: 10.1016/j.bjm.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Nurmi M, Rantala M, Suorsa M, Aro EM. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos Trans R Soc. 2012;367:3486–3493. doi: 10.1098/rstb.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwu CU, Aoyagi H, Uchiyama H. Influence of irradiance, dissolved oxygen concentration, and temperature on the growth of Chlorella sorokiniana. Photosynthetica. 2007;2:309–311. [Google Scholar]
- Yadala S, Cremaschi S. Design and optimization of artificial cultivation units for algae production. Energy. 2014;78:23–39. [Google Scholar]
- Yvondurocher G, Montoya JM, Trimmer M, Woodward GUY. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob Change Biol. 2011;17:1681–1694. [Google Scholar]
- Zhou H, Li X, Xu G, Yu H. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci Total Environ. 2018;643:225–237. doi: 10.1016/j.scitotenv.2018.06.100. [DOI] [PubMed] [Google Scholar]