Abstract
Background:
The extent to which obesity and genetics determine post-operative complications is incompletely understood.
Methods:
We performed a retrospective study using two population cohorts with electronic health record (EHR) data. The first included 736,726 adults with body mass index (BMI) recorded between 1990-2017 at Vanderbilt University Medical Center. The second cohort consisted of 65,174 individuals from 12 institutions contributing EHR and genome-wide genotyping data to the Electronic Medical Records & Genomics (eMERGE) Network. Pairwise logistic regression analyses were used to measure the association of BMI categories with postoperative complications derived from International Classification of Disease-9 codes, including postoperative infection, incisional hernia, and intestinal obstruction. A genetic risk score (GRS) was constructed from 97 obesity-risk single nucleotide polymorphisms for a Mendelian randomization study to determine the association of genetic risk for obesity on postoperative complications. Logistic regression analyses were adjusted for sex, age, site, and race/principal components.
Results:
Individuals with overweight or obese BMI (≥25 kg/m2) had increased risk for incisional hernia (Odds ratio [OR] 1.7-5.5, p<3.1×10−20), and people with obesity (BMI≥30 kg/m2) had increased risk for postoperative infection (OR 1.2-2.3, p<2.5×10−5). In the eMERGE cohort, genetically-predicted BMI was associated with incisional hernia (OR 2.1 [95% CI 1.8-2.5], p=1.4×10−6) and postoperative infection (OR 1.6 [95% CI 1.4-1.9], p=3.1×10−6). Association findings were similar after limitation of the cohorts to those who underwent abdominal procedures.
Conclusions:
Clinical and Mendelian randomization studies suggest that obesity, as measured by BMI, is associated with the development of postoperative incisional hernia and infection.
Keywords: obesity, postoperative complications, phenome-wide association studies
Introduction
Obesity, defined as a body-mass index (BMI) of 30.0 kg/m2 or greater, is known to be a strong predictor of cardiovascular morbidity and mortality.[1-3] Over two-thirds of the adult population in the United States have an overweight or obese BMI,[4] and there is significant burden of obesity on healthcare worldwide.[5, 6] In addition to the known cardiovascular morbidity associated with obesity, it is generally regarded that obesity is a risk factor for increased postoperative complications. This risk has growing significance in surgery, as the obesity epidemic has resulted in a rising prevalence of obesity-related diseases that require operative intervention, thus increasing the number of patients with obesity undergoing surgery.[7] Bariatric surgery has also become increasingly common and safe to perform with very low reported immediate post-operative complications.[8, 9] However, prior cohort studies have suggested an increased incidence of surgical site infections in individuals with obesity undergoing non-bariatric procedures.[10-23] The majority of these studies consist of cohorts undergoing a limited set of procedures such as vascular surgeries,[12, 13] oncologic resections,[14] gynecologic procedures,[15, 16] or colorectal resections.[17] Therefore, we aim to determine the influence obesity has on postoperative outcomes and if genetic risk for obesity impacts long-term surgical complications. This information can provide surgeons with more definitive data on a patient’s operative risk stratification.
Mendelian randomization (MR) is a method that uses single or sets of genetic variants associated with a phenotype of interest as an instrumental variable for association studies.[24] Prior studies have used MR to determine the association of obesity-risk single nucleotide polymorphisms (SNPs) with medical conditions such as ischemic heart disease,[25, 26] hypertension,[26] type 2 diabetes,[26] symptomatic cholelithiasis,[27] deep venous thrombosis,[28] and others.[29-37] However, prior studies have not investigated the association of obesity-risk SNPs with postoperative outcomes.
We leveraged a large electronic health record (EHR) population to identify specific postoperative complications associated with BMI. In a second cohort, we then used MR for obesity by estimating BMI-risk using 97 SNPs known to strongly correlate with BMI to investigate the relationship between genetic risk for obesity and postoperative complications.[38]
Methods
Vanderbilt Cohort
We conducted a retrospective study of all adult (≥18 years of age) individuals using the Vanderbilt University Medical Center (VUMC) Synthetic Derivative, a de-identified version of over 2.4 million VUMC patient health records.[39, 40] Inclusion criteria were at least one documented BMI, calculated as weight in kilograms divided by height in meters squared (kg/m2), where both weight and height were measured at a single encounter. The study protocol was designated as non-human subject research by the Institutional Review Board at VUMC.
All measured BMI values were extracted for each individual, with BMI data obtained during pregnancy or with clinically implausible values (less than 10 kg/m2 or greater than 70 kg/m2) excluded. Each individual was classified by his or her median BMI into one of 6 BMI categories, as defined by the World Health Organization (WHO), including underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obesity class 1 (30.0–34.9 kg/m2), class 2 (35.0–39.9 kg/m2), and class 3 (≥40.0 kg/m2).[41]
Evaluating the Vanderbilt Cohort for Postoperative Complications
We evaluated for three of the most prevalent postoperative outcomes in abdominal surgery (e.g., postoperative infection, incisional hernia, and intestinal obstruction) using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. These outcomes were chosen to not only capture immediate postoperative outcomes, but also potential long-term consequences of surgical interventions, including those not present on the initial admission. To do so, all distinct ICD-9-CM codes from each individuals’ record were captured and translated into PheWAS codes (phecodes).[42, 43] Phecodes are a hierarchical classification system for ICD-9-CM codes and have been previously shown to appropriately categorize diseases in clinical practice.[42, 44] Existing phecodes were reviewed by a team member with surgical expertise, and these six phecodes were selected as outcomes for this study because they represent well-defined surgical complications with explicit ICD-9 billing codes. To improve the accuracy of mapping ICD-9 codes to phenotypes, a minimum of 2 instances of a matching ICD-9 code on separate days was required to be translated to a phecode. In order to capture long-term sequelae and patients who underwent surgery at a separate institution, the full cohort was not limited to patients who had undergone surgery at Vanderbilt and no specific timepoint for the postoperative complication following abdominal surgery was defined.
We performed a sequence of logistic regression models adjusted for age, sex, and self-reported race, with the predictor being the BMI category and outcomes being each postoperative complication. Effect sizes for associations of BMI categories with postoperative outcomes are determined by comparison to those individuals with BMI in the normal range. All analyses were performed using the PheWAS code map version 1.2.[45] and PheWAS package for R statistical software, version 3.4.3.[46] Bonferroni correction for analyses with multiple comparisons was used to adjust the significance threshold to a two-sided p-value <0.003.
eMERGE Mendelian Randomization Cohort
The cohort utilized for MR analyses was obtained through the Electronic Medical Records and Genomics (eMERGE) Consortium, a national network organized and funded by the National Human Genome Research Institute (NHGRI).[47] This cohort included all individuals from institutions contributing data to the eMERGE network phases I-III. Inclusion criteria were age ≥18 years with extant genome-wide genotyping data and ICD-9-CM codes.
Genotyping and Imputation in the eMERGE Mendelian Randomization Cohort
Genotyping and imputation was performed to coalesce genetic results across 12 different sites and 78 genotype array batches in the eMERGE Consortium using the Michigan Imputation Server [48] and Haplotype Reference Consortium (HRC1.1).[49, 50] The resulting imputed genome wide set consists of approximately 40 million single nucleotide variant marker allele doses down to 0.1% minor allele frequency (MAF). Genotype array files were referenced to the build 37 genome position using the forward genome strand. Quality control included filtering for sample missingness <2.0% and SNP missingness <2.0% in data preprocessing. For duplicate samples on differing arrays, the sample with the most genotyped variants for that subject was selected for the merged dataset. Principal component analysis (PCA) using the first 10 principal components was performed to determine genetic ancestry using PLINK [51] with variants having >5% MAF. Single nucleotide variants with a missing rate >10% or not meeting the linkage disequilibrium threshold r2 < 0.7 were excluded in PCA. We performed identity by descent (IBD) analysis to identify related individuals using probability of zero alleles IBD (Z0) < 0.83 and the probability of having one allele IBD (Z1) > 0.10 to capture first through third-degree relatives. The oldest family member from each family was included in the cohort analysis. We excluded suspected monozygotic twins or duplicates.
Construction of the Obesity Genetic Risk Score (GRS)
The GRS was calculated from 97 SNPs (Supplementary Table 1) associated with BMI at genome-wide significance in a prior meta-analysis of genome-wide association studies conducted by the Genetic Investigation of ANthropometric Traits (GIANT) Consortium.[38] For the 97 SNPs, the minimum mean imputation r2 for any single SNP was 0.83 with an overall mean r2 of 0.95. Using the all-ancestry beta-coefficients reported by GIANT, we calculated a GRS for obesity for each individual in our cohort. This obesity GRS was calculated as a sum of risk allele dosages weighted by the effect estimates. The effect estimates are described by the GIANT consortium as beta-coefficients per 1-SD unit of BMI (4.8 kg/m2). We measured the association of the GRS with BMI by calculation of the BMI variance explained (adjusted R2) by the associated SNPs using linear regression models adjusted for site, age, sex, and the first 10 principal components.
Mendelian Randomization Analyses
We used MR to assess for association of genetic risk for obesity with the postoperative outcomes. We performed logistic regression, adjusted for site, age, sex, and the first 10 principal components, to calculate causal effect estimates for genetically-determined BMI on the postoperative complications. To adjust for multiple comparison analyses, we used a Bonferroni correction for association, giving a conservative significance threshold of p=0.017. Effect estimates are reported per 1-SD difference in BMI (derived from beta estimates and SD of 4.8 kg/m2 in a prior cohort of 449,472 individuals).[26] Among individuals with both a calculated GRS and reported BMI, the logistic regression analyses were performed with additional adjustment for median BMI to assess for residual association between the instrumental variable (GRS) with the outcomes (postoperative complications) through a pathway external to BMI. Such associations could indicate pleiotropic genes, or genes that can affect multiple, distinct phenotypes, were included in the BMI GRS.
Surgical Cohort Analyses
The analyses were also performed in subsets of the Vanderbilt and eMERGE populations who had documentation of undergoing a procedure. These two separate cohorts of surgical patients were captured by extracting Current Procedural Terminology (CPT) codes and mapping them to aggregated procedure categories including general, urologic, or gynecologic abdominal operations.[52] To further ensure that surgical patients undergoing hernia repair were not driving the associations with incisional hernia, the analysis was performed with exclusion of individuals with a CPT code corresponding to hernia repair. We also evaluated for the difference in complications in patients who underwent exploratory laparotomy versus laparoscopy.
Lastly, we evaluated for the association of obesity with 90-day postoperative mortality among all individuals at Vanderbilt who had undergone an abdominal surgical procedure. We performed a logistic regression to measure the association of BMI category with 90-day postoperative mortality, adjusting for age, gender, and race.
Results
Demographics of the Vanderbilt and eMERGE Cohorts
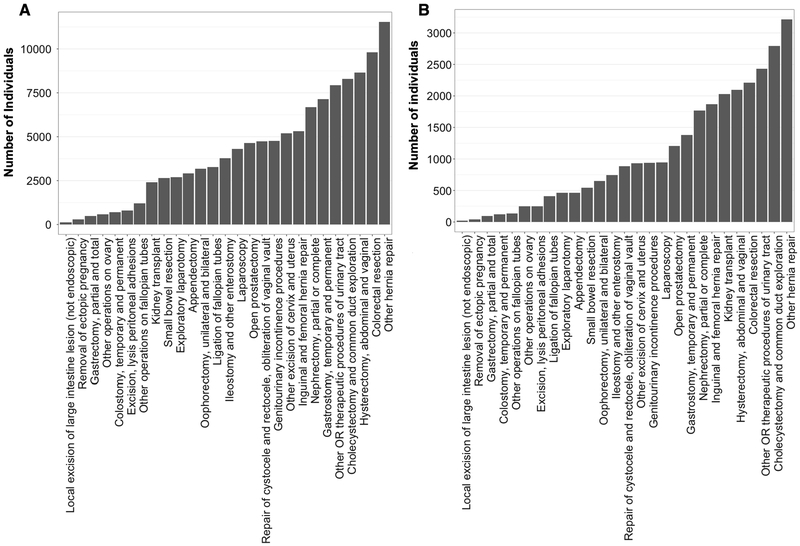
After exclusion of adult individuals with only BMI values recorded in pregnancy (12,588 individuals) or BMI values out of range (354 individuals), there were 736,726 individuals in the Vanderbilt cohort. Of these, 68,266 had undergone an abdominal surgical procedure for inclusion in the Vanderbilt surgical cohort (Figure 1A). Median follow-up of individuals who underwent a surgical procedure was 6.9 years (range 0–30.4 years). In the eMERGE MR cohort, 65,174 individuals had extant genotyping and ICD-9 codes for analysis in the entire cohort, of which 15,355 had a CPT code for abdominal surgery for inclusion in the eMERGE surgical cohort (Figure 1B). Table 1 describes the institutions contributing patients to the eMERGE cohorts. The majority of individuals in all cohorts were female and white or European ancestry (Table 2). Median BMI of the Vanderbilt individuals was 27.3 kg/m2 (IQR 23.6–32.0), which was similar to that in the eMERGE cohort and surgical subpopulations. Individuals with overweight or obese BMI comprised 65.2% (480,530 individuals) of the Vanderbilt cohort and 67.9% (35,722 individuals) of the eMERGE cohort.
Figure 1. Frequency of Surgical Procedures in Vanderbilt (A) and eMERGE (B) Surgical Cohorts.
Table 1.
eMERGE Sites and Numbers of Individuals Contributing Adult Data
| Site | Entire Cohort | Surgical Cohort |
|---|---|---|
| Number of subjects, n (%) (Total n = 65,174) |
Number of subjects, n (%) (Total n = 15,355) |
|
| Boston Children’s Hospital | 252 (0.4) | 0 |
| Children’s Hospital of Philadelphia | 4,649 (7.1) | 24 (0.2) |
| Cincinnati Children’s Hospital Medical Center | 1,331 (2.0) | 45 (0.3) |
| Columbia University | 1,680 (2.6) | 686 (4.5) |
| Geisinger | 2,772 (4.3) | 974 (6.3) |
| Harvard University | 9,689 (14.9) | 2,141 (13.9) |
| Kaiser Permanente Washington with the University of Washington and the Fred Hutchinson Cancer Research Center | 3,197 (4.9) | 763 (5.0) |
| Marshfield Clinic | 3,683 (5.7) | 1,711 (11.1) |
| Mayo Clinic | 8,199 (12.6) | 2,053 (13.4) |
| Mount Sinai | 5,701 (8.7) | 758 (4.9) |
| Northwestern University | 4,431 (6.8) | 848 (5.5) |
| Vanderbilt University | 19,590 (30.1) | 5,352 (34.9) |
Abbreviations: eMERGE, Electronic Medical Records and Genomics consortium
Table 2.
Demographics for Vanderbilt and eMERGE cohorts
| Clinical Variable | Vanderbilt Cohort (n = 736,726) |
Vanderbilt Surgical Cohort (n = 68,266) |
eMERGE Cohort (n = 65,174) |
eMERGE Surgical Cohort (n = 15,355) |
|---|---|---|---|---|
| Age, median (IQR), years | 49.0 (33.0 – 63.0) | 54.0 (40.0 – 66.0) | 67.0 (51.0 – 79.0) | 69.0 (56.0-80.0) |
| Sex, No. (%) | ||||
| Female | 434,266 (58.9) | 41,077 (60.2) | 35,997 (55.2) | 8,701 (56.7) |
| Unknown | 57 (0.1) | 0 | 0 | 0 |
| Race (Vanderbilt) or Genetic Ancestry (eMERGE), No. (%) | ||||
| White/European ancestry | 553,368 (75.1) | 55,694 (81.6) | 52,760 (81.0) | 13,068 (85.1) |
| Black/African ancestry | 70,409 (9.6) | 8,656 (12.7) | 11,323 (17.4) | 2,017 (13.1) |
| Asian | 11,998 (1.6) | 798 (1.2) | 1,091 (1.7) | 270 (1.8) |
| Other | 18,332 (2.5) | 1,942 (2.8) | 0 | 0 |
| Unknown | 82,619 (11.2) | 1,176 (1.7) | 0 | 0 |
| BMI (kg/m2), median (IQR) | 27.3 (23.6 – 32.0) | 27.8 (24.1 – 32.7) | 27.6 (23.9 – 32.1) | 28.3 (24.8 – 33.1) |
| BMI (kg/m2), mean (SD) | 28.5 (7.0) | 29.1 (7.3) | 28.6 (7.0) | 29.67 (7.0) |
| BMI category, No. (%) | ||||
| Underweight (<18.5) | 15,509 (2.1) | 1,564 (2.3) | 1,961 (3.7) | 221 (1.6) |
| Normal (18.5 – 24.9) | 240,676 (32.7) | 19,630 (28.8) | 14,926 (28.4) | 3,414 (25.1) |
| Overweight (25.0 – 29.9) | 229,630 (31.2) | 21,590 (31.6) | 17,088 (32.5) | 4,576 (33.7) |
| Obesity Class 1 (30.0 – 34.9) | 135,488 (18.4) | 13,317 (19.5) | 10,425 (19.8) | 2,827 (20.8) |
| Obesity Class 2 (35.0 – 39.9) | 64,539 (8.8) | 6,702 (9.8) | 4,802 (9.1) | 1,407 (10.4) |
| Obesity Class 3 (≥40.0) | 50,873 (6.9) | 5,463 (8.0) | 3,407 (6.5) | 1,146 (8.4) |
| No BMI reported | 0 | 0 | 12,565 | 1,764 |
Abbreviations: eMERGE, Electronic Medical Records and Genomics consortium; BMI, body mass index; IQR, interquartile range; SD, standard deviation
BMI Associations with Postoperative Complications
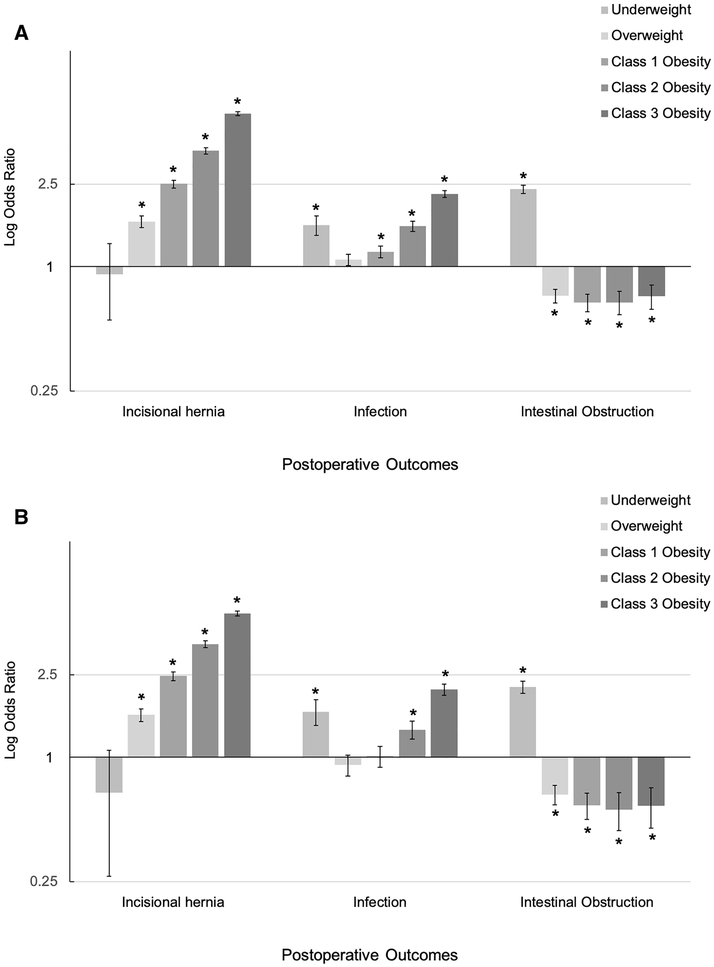
In the Vanderbilt cohort, we found that overweight or obesity was associated with incisional hernia and postoperative infection in both the full and surgical cohorts (Table 3). There was an increased association with these postoperative complications with increasing BMI (Figure 2A-B) in both the complete and surgical Vanderbilt cohorts. In the entire Vanderbilt cohort, OR for incisional hernia in individuals with overweight BMI was 1.7 (95% confidence interval [CI] 1.5–1.8, p=3.1×10−20) and increased to an OR of 5.5 (95% CI 5.4–5.6, p=2.2×10−172) in class 3 obesity, a 3.2-fold increase. The association of obesity with incisional hernia persisted in the Vanderbilt subpopulation of individuals who had undergone general, urologic, or gynecologic abdominal surgery, with OR in surgical patients with overweight BMI of 1.6 (95% CI 1.5–1.7, p=1.2×10−15) and surgical patients with class 3 obesity BMI of 4.9 (95% CI 4.8–5.1, p=2.5×10−117). Further exclusion of individuals who had undergone hernia repair showed persistent associations of obesity with incisional hernia in class 1 (OR 1.7 [95% CI 1.4–2.1], p=1.2×10−3), 2 (OR 3.5 [95% CI 3.1–3.8], p=1.6×10−12) and 3 (OR 3.9 [95% CI 3.5–4.3], p=1.2×10−12) obesity in the surgical patient population. In patients with both a low (<30 mg/kg2) and high BMI (≥30 mg/kg2), the large majority of incisional hernias presented themselves in the first 2 years following the index operation (Supplementary Figure 1).
Table 3.
Association of BMI with postoperative complications*
| Entire Vanderbilt Cohort (n = 736,726) | |||||
|---|---|---|---|---|---|
| Phenotype | Underweight <18.5 kg/m2 OR (95% CI) |
Overweight 25.0-29.9 kg/m2 OR (95% CI) |
Obesity Class 1 30.0-34.9 kg/m2 OR (95% CI) |
Obesity Class 2 35.0-39.9 kg/m2 OR (95% CI) |
Obesity Class 3 ≥40.0 kg/m2 OR (95% CI) |
| Postoperative infection (n = 6,228) |
1.59 (1.42-1.76) † | 1.08 (1.01-1.15) | 1.18 (1.10-1.26) † | 1.57 (1.48-1.66) † | 2.25 (2.16-2.33) † |
| Incisional hernia (n = 3,580) |
0.92 (0.55-1.29) | 1.65 (1.54-1.76) † | 2.51 (2.40-2.62) † | 3.62 (3.51-3.75) † | 5.48 (5.36-5.60) † |
| Intestinal obstruction (n = 8,525) |
2.37 (2.26-2.47) † | 0.72 (0.67-0.78) † | 0.67 (0.61-0.74) † | 0.67 (0.58-0.76) † | 0.72 (0.62-0.82) † |
| Vanderbilt Surgical Cohort (n = 68,266) | |||||
| Postoperative infection (n = 2,749) |
1.66 (1.43-1.89) † | 0.92 (0.81-1.02) | 1.01 (0.89-1.13) | 1.36 (1.22-1.49) † | 2.13 (2.00-2.26) † |
| Incisional hernia (n = 3,120) |
0.67 (0.27-1.08) | 1.60 (1.49-1.72) † | 2.47 (2.35-2.59) † | 3.52 (3.39-3.65) † | 4.95 (4.81-5.08) † |
| Intestinal obstruction (n = 5,389) |
2.19 (2.03-2.33) † | 0.66 (0.59-0.73) † | 0.59 (0.50-0.67) † | 0.56 (0.44-0.67) † | 0.58 (0.45-0.71) † |
| Vanderbilt Exploratory Laparotomy Cohort (n = 2,410) | |||||
| Postoperative infection (n = 432) |
0.95 (0.36-1.55) | 1.25 (0.96-1.53) | 1.14 (0.81-1.47) | 1.39 (0.97-1.80) | 2.95 (2.55-3.34) † |
| Incisional hernia (n = 296) |
0.91 (0.08-1.73) | 1.79 (1.42-2.15) † | 2.00 (1.59-2.40) † | 4.47 (4.03-4.92) † | 4.63 (4.16-5.09) † |
| Intestinal obstruction (n = 688) |
1.11 (0.63-1.59) | 0.91 (0.66-1.16) | 0.62 (0.32-0.92) † | 1.14 (0.75-1.53) | 0.66 (0.23-1.10) |
| Vanderbilt Laparoscopy Cohort (n = 3,841) | |||||
| Postoperative infection (n = 174) |
1.06 (0.00-2.26) | 1.06 (0.60-1.51) | 1.17 (0.70-1.65) | 1.19 (0.65-1.73) | 1.56 (1.10-2.03) |
| Incisional hernia (n = 250) |
0.89 (0.00-2.35) | 1.35 (0.91-1.79) | 2.15 (1.71-2.59) † | 3.09 (2.63-3.55) † | 3.54 (3.10-3.98) † |
| Intestinal obstruction (n = 327) |
0.37 (0.80-2.26) | 0.16 (0.44-1.05) | 0.18 (0.21-0.91) † | 0.25 (0.00-0.90) † | 0.20 (0.15-0.95) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio; CI, confidence interval
Reference odds ratio 1.0 represents normal median BMI. Logistic regressions adjusted for sex, age, and reported race.
Results significant to Bonferroni corrected p-value of p = 0.003 compared to individuals with normal range BMI.
Figure 2. Association of BMI with Postoperative Complications in Vanderbilt General (A) and Surgical (B) Cohorts.
Error bars represent 95% confidence interval. Significance threshold of p <0.003. BMI, body mass index; SD, standard deviation.
In the clinical cohort, both underweight (BMI <18.5 kg/m2) and class 1–3 obesity (BMI ≥ 30.0 kg/m2) demonstrated an association with postoperative infection (p<2.5×10−5). The strongest association of postoperative infection with BMI class was with class 3 obesity (OR 2.3 [95% CI 2.2–2.3], p=2.3×10−71) and this relationship persisted in the abdominal surgery subpopulation (OR 2.1 [95% CI 2.0–2.6], p=3.0×10−29).
In the full cohort, patients with underweight BMI had an increased risk of intestinal obstruction compared to patients with a BMI within the normal range (OR 2.4 [95% CI 2.3–2.5], p=4.6×10−57). In contrast, patients with a BMI in the overweight or obese range showed a decreased risk of intestinal obstruction in comparison to patients with a normal BMI (Table 3). The finding of an increase in obstruction in patients with an underweight BMI and decrease in obstruction in patients with BMI over the normal range persisted in the subset of individuals who had undergone an abdominal surgical procedure.
While patients with obesity who underwent exploratory laparotomy or laparoscopy both had increased risk for postoperative infection and incisional hernia, the risk was greatest in patients who underwent open laparotomy (Table 3). In class 3 obesity, the risk for postoperative infection for patients undergoing laparotomy was OR 3.0 (95% CI 2.6–3.3) compared to OR 1.6 (95% CI 1.1–2.0) in patients who underwent laparoscopy. Similarly, patients with class 3 obesity who underwent laparotomy had an OR of 4.6 (95% CI 4.2–5.1) for incisional hernia compared to OR 3.5 (95% CI 3.1–4.0) in those who underwent laparoscopy.
In comparison to individuals having normal BMI who underwent abdominal surgical procedure, those having an overweight or obese BMI had increased risk for mortality in the 90-day postoperative period. Increased BMI was associated with increased risk: overweight BMI had a OR of 1.02 (95% CI 1.0–1.0, p=0.04) while class 3 obesity had a OR of 1.12 (95% CI 1.1–1.2, p=2.5×10−11).
Mendelian Randomization for Obesity Associations with Postoperative Complications
In the eMERGE cohort, the obesity GRS was strongly correlated with mean BMI (p<2.0×10−16), aligning with findings from Locke et al.[38] Using a conservative p-value threshold of 0.017, the obesity GRS was associated with incisional hernia (OR 2.1 [95% CI 1.8–2.4], p=1.4×10−6) and postoperative infection (OR 1.6 [95% CI 1.4–1.9], p=3.1×10−6) in the entire eMERGE cohort (Table 4). Limiting to only those individuals who had undergone a general, urologic, or gynecologic abdominal surgery, the obesity GRS remained associated with both incisional hernia (OR 2.0 [95% CI 1.7–2.4], p=9.4×10−5) and postoperative infection (OR 1.5 [95% CI 1.2–1.8], p=0.01).
Table 4.
Mendelian randomization genetic risk for obesity association with postoperative complications *
| Entire eMERGE Cohort (n = 65,174) | ||
|---|---|---|
| Phenotype | OR per 1-SD BMI (95% CI) |
p-value |
| Incisional hernia † (n = 1,620) |
2.14 (1.83-2.45) | 1.4 × 10−6 |
| Postoperative infection † (n = 3,709) |
1.64 (1.42-1.86) | 3.1 ×10−6 |
| Intestinal obstruction (n = 4,523) |
1.05 (0.86-1.24) | 0.595 |
| eMERGE Surgical Cohort (n = 15,355) | ||
| Phenotype | OR per 1-SD BMI (95% CI) | p-value |
| Incisional hernia † (n =1,356) |
1.82 (1.66-2.36) | 9.4 × 10−5 |
| Postoperative infection (n = 1,938) |
2.01 (1.19-1.79) | 0.009 |
| Intestinal obstruction (n = 2,792) |
0.97 (0.71-1.23) | 0.801 |
Abbreviations: eMERGE, Electronic Medical Records and Genomics consortium; SE, standard error; OR, odds ratio
Logistic regression adjusted for site, sex, age, and first ten principal components. Odds ratio report per 1-SD (4.8 kg/m2) of BMI
Results significant to Bonferroni corrected p-value = 0.017.
The obesity GRS was not associated with intestinal obstruction in the complete (OR 1.1 [95% CI 0.9–1.2], p = 0.59) or surgical cohort (OR 1.0 [95% CI 0.7–1.2], p = 0.80).
Adjustment for median BMI in MR analyses to assess for residual association not attributable to BMI exposure showed attenuation of the associations with postoperative infection (p=0.126) and incisional hernia (p=0.038), suggesting that the association of the obesity-risk GRS with these postoperative complications is through BMI.
Discussion
This study found that obesity as measured by both BMI and genetic risk is associated with postoperative infections and incisional hernias in separate cohorts. The findings from this study are supported by prior reports in which overweight and obesity demonstrated an observed association with surgical site infections [10-23] and incisional hernias.[17, 53, 54] While these clinical associations have been demonstrated previously, the use of Mendelian randomization in this study suggests a possible causal role for obesity in the development of postoperative infections and incisional hernias.
It has long been studied whether it is obesity itself or the comorbidities found in obese patients, such as diabetes mellitus, are the driver for postoperative complications. There are many potential explanations for the association between obesity and postoperative infections and incisional hernias with the mechanism likely being multifactorial.[55] An increase in subcutaneous adipose tissue and local tissue trauma related to retraction could play a role. Subcutaneous tissue oxygenation is reduced in obese patients [56] and thus may reduce wound perfusion and predispose to wound infection and decreased healing, leading to both postoperative infections and incisional hernias. Lengthened operative time may also contribute to the increased incidence of surgical-site infections caused by obesity,[57] and surgical site infection itself is known to be a strong risk factor for incisional hernia formation.[58]
Because BMI itself is a strong predictor of postoperative complications, genetic variants are clinically unnecessary for estimation of the risk obesity plays in operative interventions. However, as genetic testing becomes less expensive and more common, it is another piece of data that can be leveraged in both research and clinical settings to provide for more accurate and validated predictions. Further, the use of genetic data to confirm clinical findings as we have demonstrated in this study substantiates the role obesity plays in development of postoperative incisional hernias and surgical site infections.
Interestingly, intestinal obstruction showed no association with obesity and, in the clinical cohort, was associated underweight BMI. This association is unclear and should be further investigated.
MR can be particularly useful because genetic variants are not subject to the same biases as traditional observational studies due to their random assortment during meiosis, thus allowing for potential causal inferences.[24, 59] Despite these advantages, MR and this study has several potential limitations. The method relies on BMI recorded in the EHR; however, this measure may not fully capture the true causal exposure of lifetime obesity exposure. Another limitation is that while the sensitivity analysis with adjustment of BMI suggested that pleiotropy of genetic variants did not play a significant role, pleiotropy is common and cannot be fully excluded. Lastly, the main limitation of this retrospective study is that it relies on both medical and procedural codes within the EHR, which can change over time, be inaccurate, and are often incomplete. While the use of ICD and CPT codes captures diagnoses and procedures at the study institution, we were unable to capture individuals who had surgery elsewhere or who presented to outside institutions with postoperative complications.
Conclusions
Genetic determinants of BMI suggest that obesity, aside from confounders or other metabolic diseases, is associated with the development of postoperative infection and incisional hernia. Thus, BMI represents an important risk factor for postoperative complication, warranting appropriate preoperative consideration and postoperative awareness.
Supplementary Material
Acknowledgements
Funding: JR Robinson receives support by the 5T15LM007450 training grant from the National Library of Medicine. Support for the research and personnel was also provided by the R01LM010685 grant from the National Library of Medicine. The eMERGE sites were funded through several series of grants from the National Human Genome Research Institute: U01HG8657, U01HG006375, U01HG004610 (Kaiser Permanente Washington/University of Washington); U01HG8685 (Brigham and Women’s Hospital); U01HG8672, U01HG006378, U01HG004608 (Vanderbilt University Medical Center); U01HG8666, U01HG006828 (Cincinnati Children’s Hospital Medical Center); U01HG6379, U01HG04599 (Mayo Clinic); U01HG8679, U01HG006382 (Geisinger Clinic); U01HG008680 (Columbia University Health Sciences); U01HG8684, U01HG006830 (Children’s Hospital of Philadelphia); U01HG8673, U01HG006388, U01HG004609 (Northwestern University); U01HG8676 (Partners Healthcare/Broad Institute); U01HG8664 (Baylor College of Medicine); U01HG006389 (Essentia Institute of Rural Health, Marshfield Clinic Research Foundation and Pennsylvania State University); U01HG006380 (Icahn School of Medicine at Mount Sinai); U01HG8701, U01HG006385, U01HG04603 (Vanderbilt University Medical Center serving as the Coordinating Center); eMERGE Genotyping Centers were also funded through U01HG004438 (CIDR) and U01HG004424 (the Broad Institute). Vanderbilt University Medical Center’s Synthetic Derivative and BioVU are supported by institutional funding and by the CTSA grant ULTR000445 from NCATS/NIH.
Footnotes
Conflicts of interest: None
References
- 1.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, et al. (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet Lond Engl 388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration, Whitlock G, Lewington S, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet Lond Engl 373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Wormser D, Kaptoge S, et al. (2011) Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet Lond Engl 377:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. (2017) Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet Lond Engl 387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawn MT, Bian J, Leeth RR, et al. (2005) Impact of obesity on resource utilization for general surgical procedures. Ann Surg 241:821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surve A, Cottam D, Zaveri H, et al. (2018) Does the future of laparoscopic sleeve gastrectomy lie in the outpatient surgery center? A retrospective study of the safety of 3162 outpatient sleeve gastrectomies. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 14:1442–1447. [DOI] [PubMed] [Google Scholar]
- 9.Poelemeijer YQM, Marang-van de Mheen PJ, Wouters MWJM, et al. (2019) Textbook Outcome: an Ordered Composite Measure for Quality of Bariatric Surgery. Obes Surg 29:1287–1294. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Muller MK, Weber M, Clavien P-A (2003) Obesity in general elective surgery. Lancet Lond Engl 361:2032–2035. [DOI] [PubMed] [Google Scholar]
- 11.Mullen JT, Moorman DW, Davenport DL (2009) The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 250:166–172. [DOI] [PubMed] [Google Scholar]
- 12.Giles KA, Hamdan AD, Pomposelli FB, et al. (2010) Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005–2007. Ann Vasc Surg 24:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles KA, Wyers MC, Pomposelli FB, et al. (2010) The impact of body mass index on perioperative outcomes of open and endovascular abdominal aortic aneurysm repair from the National Surgical Quality Improvement Program, 2005–2007. J Vasc Surg 52:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen JT, Davenport DL, Hutter MM, et al. (2008) Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 15:2164–2172. [DOI] [PubMed] [Google Scholar]
- 15.Bouwman F, Smits A, Lopes A, et al. (2015) The impact of BMI on surgical complications and outcomes in endometrial cancer surgery--an institutional study and systematic review of the literature. Gynecol Oncol 139:369–376. [DOI] [PubMed] [Google Scholar]
- 16.Wloch C, Wilson J, Lamagni T, et al. (2012) Risk factors for surgical site infection following caesarean section in England: results from a multicentre cohort study. BJOG Int J Obstet Gynaecol 119:1324–1333. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Wang J, Bian H, et al. (2017) BMI as a Predictor for Perioperative Outcome of Laparoscopic Colorectal Surgery: a Pooled Analysis of Comparative Studies. Dis Colon Rectum 60:433–445. [DOI] [PubMed] [Google Scholar]
- 18.Thelwall S, Harrington P, Sheridan E, Lamagni T (2015) Impact of obesity on the risk of wound infection following surgery: results from a nationwide prospective multicentre cohort study in England. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 21:1008.e1–8. [DOI] [PubMed] [Google Scholar]
- 19.Holley JL, Shapiro R, Lopatin WB, et al. (1990) Obesity as a risk factor following cadaveric renal transplantation. Transplantation 49:387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas EJ, Goldman L, Mangione CM, et al. (1997) Body mass index as a correlate of postoperative complications and resource utilization. Am J Med 102:277–283. [DOI] [PubMed] [Google Scholar]
- 21.Jeschke E, Citak M, Günster C, et al. (2018) Obesity Increases the Risk of Postoperative Complications and Revision Rates Following Primary Total Hip Arthroplasty: An Analysis of 131,576 Total Hip Arthroplasty Cases. J Arthroplasty. [DOI] [PubMed] [Google Scholar]
- 22.Galyfos G, Geropapas GI, Kerasidis S, et al. (2017) The effect of body mass index on major outcomes after vascular surgery. J Vasc Surg 65:1193–1207. [DOI] [PubMed] [Google Scholar]
- 23.Tjeertes EKM, Tjeertes EEKM, Hoeks SE, et al. (2015) Obesity--a risk factor for postoperative complications in general surgery? BMC Anesthesiol 15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GD, Ebrahim S (2003) “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32:1–22 [DOI] [PubMed] [Google Scholar]
- 25.Nordestgaard BG, Palmer TM, Benn M, et al. (2012) The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med 9:e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyall DM, Celis-Morales C, Ward J, et al. (2017) Association of Body Mass Index With Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol 2:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stender S, Nordestgaard BG, Tybjaerg-Hansen A (2013) Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatol Baltim Md 58:2133–2141. [DOI] [PubMed] [Google Scholar]
- 28.Lindström S, Germain M, Crous-Bou M, et al. (2017) Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian Randomization study. Hum Genet 136:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vimaleswaran KS, Berry DJ, Lu C, et al. (2013) Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Xu M, Xie L, et al. (2016) Obesity and peripheral arterial disease: A Mendelian Randomization analysis. Atherosclerosis 247:218–224. [DOI] [PubMed] [Google Scholar]
- 31.Mokry LE, Ross S, Timpson NJ, et al. (2016) Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med 13:e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianfrancesco MA, Glymour MM, Walter S, et al. (2017) Causal Effect of Genetic Variants Associated With Body Mass Index on Multiple Sclerosis Susceptibility. Am J Epidemiol 185:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thrift AP, Shaheen NJ, Gammon MD, et al. (2014) Obesity and risk of esophageal adenocarcinoma and Barrett’s esophagus: a Mendelian randomization study. J Natl Cancer Inst 106:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis D, Mitchell JS, Law PJ, et al. (2016) Mendelian randomisation analysis strongly implicates adiposity with risk of developing colorectal cancer. Br J Cancer 115:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon SC, Nagle CM, Thrift AP, et al. (2016) Adult body mass index and risk of ovarian cancer by subtype: a Mendelian randomization study. Int J Epidemiol 45:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee NA, Giulianini F, Geelhoed B, et al. (2017) Genetic Obesity and the Risk of Atrial Fibrillation: Causal Estimates from Mendelian Randomization. Circulation 135:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panoutsopoulou K, Metrustry S, Doherty SA, et al. (2014) The effect of FTO variation on increased osteoarthritis risk is mediated through body mass index: a Mendelian randomisation study. Ann Rheum Dis 73:2082–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locke AE, Kahali B, Berndt SI, et al. (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden DM, Pulley JM, Basford MA, et al. (2008) Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 84:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson JR, Wei W-Q, Roden DM, Denny JC (2018) Defining Phenotypes from Clinical Data to Drive Genomic Research. Annu Rev Biomed Data Sci 1:69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Defining Adult Overweight and Obesity ∣ Overweight & Obesity ∣ CDC. https://www.cdc.gov/obesity/adult/defining.html. Accessed 22 Oct 2017
- 42.Denny JC, Ritchie MD, Basford MA, et al. (2010) PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinforma Oxf Engl 26:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie MD, Denny JC, Crawford DC, et al. (2010) Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet 86:560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei W-Q, Bastarache LA, Carroll RJ, et al. (2017) Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PloS One 12:e0175508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll RJ, Bastarache L, Denny JC (2014) R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinforma Oxf Engl 30:2375–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 7 Dec 2017
- 47.Gottesman O, Kuivaniemi H, Tromp G, et al. (2013) The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med Off J Am Coll Med Genet 15:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michigan Imputation Server. https://imputationserver.sph.umich.edu/index.html. Accessed 24 Feb 2018
- 49.McCarthy S, Das S, Kretzschmar W, et al. (2016) A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48:1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanaway IB, Hall TO, Rosenthal EA, et al. (2019) The eMERGE genotype set of 83,717 subjects imputed to ~40 million variants genome wide and association with the herpes zoster medical record phenotype. Genet Epidemiol 43:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purcell S, Neale B, Todd-Brown K, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.HCUP-US Tools and Software Page CCS-Services and Procedures. https://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp. Accessed 10 Jan 2018
- 53.Weissler JM, Lanni MA, Hsu JY, et al. (2017) Development of a Clinically Actionable Incisional Hernia Risk Model after Colectomy Using the Healthcare Cost and Utilization Project. J Am Coll Surg 225:274–284.e1. [DOI] [PubMed] [Google Scholar]
- 54.Ooms LS, Verhelst J, Jeekel J, et al. (2016) Incidence, risk factors, and treatment of incisional hernia after kidney transplantation: An analysis of 1,564 consecutive patients. Surgery 159:1407–1411. [DOI] [PubMed] [Google Scholar]
- 55.Falagas ME, Kompoti M (2006) Obesity and infection. Lancet Infect Dis 6:438–446. [DOI] [PubMed] [Google Scholar]
- 56.Kabon B, Nagele A, Reddy D, et al. (2004) Obesity Decreases Perioperative Tissue Oxygenation. Anesthesiology 100:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng H, Chen BP-H, Soleas IM, et al. (2017) Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg Infect 18:722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray BW, Cipher DJ, Pham T, Anthony T (2011) The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am J Surg 202:558–560. [DOI] [PubMed] [Google Scholar]
- 59.Nitsch D, Molokhia M, Smeeth L, et al. (2006) Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. Am J Epidemiol 163:397–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.