Abstract
INTRODUCTION:
This study aimed to determine if later birth year influences trajectory of age-related cognitive decline across racial/ethnic groups and to test whether years of school, childhood socio-economic status (CSES), and cardiovascular disease (CVD) burden explain such secular trends.
METHODS:
We compared cognitive trajectories of global cognition and subdomains in two successive racially/ethnically and educationally diverse birth cohorts of a prospective cohort study.
RESULTS:
Later birth year was associated with higher initial cognitive levels for Whites and Blacks, but not Hispanics. Later birth year was also associated with less rapid rate of decline in all three racial/ethnic groups. More years of education and higher CSES, and to a smaller extent greater CVD burden, accounted for higher intercepts in the later-born cohort, but did not account for attenuated slope of cognitive decline.
DISCUSSION:
Later birth year is related to a slower rate of age-related decline in some cognitive domains in some racial/ethnic groups. Our analyses suggest that racial/ethnic and social inequalities are part of the mechanisms driving secular trends in cognitive aging and dementia.
Keywords: time trend, cohort studies, rate of change, cognitive aging, race, ethnicity, education, socio-economic status
Introduction
Due to prolonged life expectancy and aging baby boomers in developed nations, the net number of individuals with dementia is exponentially increasing [1, 2], and is projected to negatively affect society and the economy on a large scale [3–6]. While the trends in prevalence of dementia are under debate [7], a consistent picture is emerging from cohort studies showing that the incidence of dementia is declining in high-income countries [8–10]. Typically studied in white, well-educated cohorts, these patterns have been explained by factors such as better educational attainment and improved treatment of antecedent vascular risk factors [11, 12]. Noble et al. [13] showed that the associations between these factor and dementia incidence vary as a function of race/ethnicity. Regarding cognitive change, however, most prior studies on secular trends have not accounted for early life exposures and were unable to test whether cohort effects are uniform across different racial/ethnic groups. Early life exposures, such as childhood socio-economic status (CSES) that includes parental occupation and education, have been demonstrated to negatively affect late-life cognition [14], including among diverse race/ethnicity groups [15]. Importantly, it is unclear if secular trends also influence cognitive change over time, and if differences in trajectories between cohorts differ across racial/ethnic groups and are influenced by social factors.
We addressed these gaps in the literature by modeling longitudinal change in global cognition as well as subdomains of memory, language, and visuospatial ability in a racial/ethnically and educationally diverse cohort of non-Hispanic White, non-Hispanic Black, and Hispanic older adults. Participants were enrolled at two recruitment waves, seven years apart, and follow-up was modeled up to 17 years.
We aimed to investigate if later birth year influenced the trajectory of age-related cognitive decline across racial/ethnic groups and to test whether years of school, CSES, and cardiovascular disease (CVD) burden can explain such secular trends. Based on previous literature [13, 16], we hypothesized that later birth year would be paired with higher baseline scores as well as a slower rate of decline over time, but that the extent of the effect would be higher in Whites than in Blacks and Hispanics. Furthermore, we hypothesized that socio-demographic factors of improved CVD treatment, higher educational attainment, and higher CSES in a later birth cohort are mechanisms that contribute to these secular trends in cognitive aging in both baseline scores and trajectories. As these socio-demographic factors differ across race/ethnicity groups, we expected their explanatory value to vary as a function of minority status.
Methods
Study population
Initial selection of the study sample was identical to the one described in detail by Noble et al. [13]. Participants were part of the Washington Heights Inwood Columbia Aging Project (WHICAP), a prospective community-based longitudinal study of cognitive aging and dementia in the racially and ethnically diverse neighborhoods of northern Manhattan. Independent cohorts were recruited in 1992 (n = 2,125) and 1999 (n = 2,183) and followed over time with reevaluations approximately every 18 to 30 months.
One of the aims for the first recruitment wave in 1992 was to compare prevalence of dementia in this neighborhood, while the aims of the 1999 recruitment wave focused on incident dementia. Therefore, if individuals indicated during screening in the 1999 wave that they had serious subjective memory complaints or a diagnosis of dementia, they were excluded from the sample. After enrollment, individuals in both waves were asked about subjective memory complaints for memory in general, and for names, lists, and finding words (described in detail elsewhere [17]). As expected given the recruitment goals, the 1999 cohort had fewer subjective memory complaints than the 1992 cohort.
All individuals included in this study were free of dementia diagnosis at baseline as established in a consensus case conference based on neurological, neuropsychological, functional, medical, and psychiatric evaluation, and following standard criteria for all-cause dementia [18]. Individuals with insufficient data to determine dementia status (n = 354), with race/ethnicity other than non-Hispanic White, African-American, or Caribbean Hispanic (n = 44), with prevalent dementia at baseline (n = 563), and who were aged older than 86 at baseline and had more than 16 years of follow-up (n = 490; in order to ensure comparable baseline age and duration of follow-up for the two cohorts) were excluded from this study [13]. Additionally, from this sample reported by Noble et al., ten individuals were excluded due to missing neuropsychological data and seven due to missing time information. All participants gave written consent in accordance with the Institutional Review Boards of Columbia University Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
The total number of 2840 participants was divided into two birth year cohorts, with the first cohort being born between 1905–1920 (n = 1034) and the second cohort between 1921–1935 (n = 1806). Participant characteristics for each cohort across all participants and by race/ethnicity groups (Whites, Blacks, Hispanics) are presented in Table 1.
Table 1.
Demographic characteristics
| Characteristic | Overall | White | African-American | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
| Cohort | 1905–1920 | 1921–1935 | 1905–1920 | 1921–1935 | 1905–1920 | 1921–1935 | 1905–1920 | 1921–1935 |
| N | 1034 | 1806 | 254 | 514 | 383 | 573 | 397 | 719 |
| Birth year (m, SD) | 1916 (3.4) | 1926 (3.9)* | 1916 (3.3) | 1926 (3.8)* | 1916 (3.4) | 1926 (3.9)* | 1916 (3.3) | 1926 (3.9)* |
| Baseline age (years; m, SD) | 79.6 (3.9) | 72.5 (3.7)* | 80.5 (3.6) | 72.7 (3.7)* | 79.4 (4.0) | 72.7 (3.8)* | 79.2 (3.9) | 72.3 (3.7)* |
| Number of visits (m, SD) | 3.2 (1.8) | 3.2 (1.8) | 3.0 (1.7) | 3.4 (1.8)* | 3.1 (1.7) | 3.2 (1.7) | 3.3 (1.8) | 3.2 (1.9) |
| Time between visits (m, SD) | 2.5 (1.0) | 3.2 (1.2)* | 2.6 (1.3) | 3.2 (1.1)* | 2.4 (0.8) | 3.0 (1.1)* | 2.5 (0.9) | 3.2 (1.4)* |
| Sex (n, % female) | 719, 69.5 | 1161, 64.3* | 166, 65.4 | 302, 58.8 | 282, 73.6 | 379, 66.1* | 271, 68.3 | 480, 66.8 |
| Baseline memory complaints (m, SD) | 1.6 (1.6) | 1.3 (1.6)* | 1.3 (1.4) | 1.2 (1.4) | 1.6 (1.6) | 1.2 (1.5)* | 1.8 (1.7) | 1.5 (1.7)* |
| Education (m, SD) | 9.1 (4.7) | 10.3 (4.9)* | 12.4 (3.8) | 13.7 (3.5)* | 10.1 (3.6) | 11.7 (3.6)* | 6.1 (4.3) | 6.8 (4.3)* |
| CVD burden (m, SD) | 1.5 (1.0) | 1.6 (1.0) | 1.4 (1.1) | 1.5 (1.0) | 1.5 (1.0) | 1.7 (1.0)* | 1.6 (1.0) | 1.6 (1.1) |
| Diabetes Mellitus (n, % yes) | 229, 22.1 | 479, 26.5* | 37, 14.6 | 92, 17.9 | 84, 21.9 | 165, 28.8* | 108, 27.2 | 222, 30.9 |
| n, %missing | 55, 5.3 | 63, 3.5 | 16, 6.3 | 18, 3.5 | 16, 4.2 | 14, 2.4 | 23, 5.8 | 31, 4.3 |
| Heart Disease (n, % yes) | 367, 35.5 | 675, 37.4 | 96, 37.8 | 222, 43.2 | 130, 33.9 | 218, 38.0 | 141, 35.5 | 235, 32.7 |
| n, %missing | 55, 5.3 | 62, 3.4 | 16, 6.3 | 17, 3.3 | 16, 4.2 | 14, 2.4 | 23, 5.8 | 31, 4.3 |
| Stroke (n, % yes) | 168, 16.2 | 266, 14.7* | 35, 13.8 | 65, 12.6 | 60, 15.7 | 88, 15.4 | 73, 18.4 | 113, 15.7* |
| n, %missing | 210, 20.3 | 162, 9.0 | 46, 18.1 | 40, 7.8 | 83, 21.7 | 45, 7.9 | 81, 20.4 | 77, 10.7 |
| Hypertension (n, % yes) | 735, 71.1 | 76.6* | 169, 66.5 | 365, 71.0 | 277, 72.3 | 462, 80.6* | 289, 72.8 | 557, 77.5 |
| n, %missing | 60, 5.8 | 62, 3.4 | 17, 6.7 | 17, 3.3 | 17, 4.4 | 14, 2.4 | 26, 6.5 | 31, 4.3 |
| CSES (m, SD) | −.10 (.64) | .00 (.68)* | .16 (.61) | .36 (.64)* | −.07 (.51) | .05 (.56)* | −.29 (.70) | −.31 (.65) |
| Father’s education n, %no formal education | 84, 8.1 | 136, 7.5 | 10, 3.9 | 11, 2.1 | 12, 3.1 | 18, 3.1 | 62, 15.6 | 107, 14.9 |
| n, %grades 1–8 | 134, 13.0 | 313, 17.3 | 30, 11.8 | 75, 14.6 | 56, 14.6 | 109, 19.0 | 48, 12.1 | 129, 17.9 |
| n, %grades 9–11 | 13, 1.3 | 54, 3.0 | 7, 2.8 | 29, 5.6 | 5, 1.3 | 14, 2.4 | 1, .3 | 11, 1.5 |
| n, %high school | 77, 7.4 | 181, 10.0 | 29, 11.4 | 94, 18.3 | 25, 6.5 | 56, 9.8 | 23, 5.8 | 31, 4.3 |
| n, %some college | 11, 1.1 | 38, 2.1 | 3, 1.2 | 17, 3.3 | 5, 1.3 | 16, 2.8 | 3, .8 | 5, .7 |
| n, %college graduate | 24, 2.3 | 56, 3.1 | 11, 4.3 | 31, 6.0 | 5, 1.3 | 13, 2.3 | 8, 2.0 | 12, 1.7 |
| n, %beyond college graduate | 11, 1.1 | 56, 3.1 | 4, 1.6 | 36, 7.0 | 1, .3 | 7, 1.2 | 6, 1.5 | 13, 1.8 |
| n, %missing | 680, 65.8 | 972, 53.8 | 160, 63.0 | 221, 43.0 | 274, 71.5 | 340, 59.3 | 246, 62.0 | 411, 57.2 |
| Mother’s education n, %no formal education | 102, 9.9 | 158, 8.7 | 16, 6.3 | 11, 2.1 | 13, 3.4 | 16, 2.8 | 73, 18.4 | 131, 18.2 |
| n, %grades 1–8 | 184, 17.8 | 391, 21.7 | 37, 14.6 | 95, 18.5 | 79, 20.6 | 131, 22.9 | 68, 17.1 | 165, 22.9 |
| n, %grades 9–11 | 28, 2.7 | 53, 2.9 | 12, 4.7 | 23, 4.5 | 12, 3.1 | 21, 3.7 | 4, 1.0 | 9, 1.3 |
| n, %high school | 95, 9.2 | 225, 12.5 | 40, 15.7 | 114, 22.2 | 30, 7.8 | 87, 15.2 | 25, 6.3 | 24, 3.3 |
| n, %some college | 9, .9 | 37, 2.0 | 4, 1.6 | 21, 4.1 | 5, 1.3 | 15, 2.6 | 0, .0 | 1, .1 |
| n, %college graduate | 16, 1.5 | 43, 2.4 | 5, 2.0 | 18, 3.5 | 8, 2.1 | 19, 3.3 | 3, .8 | 6, .8 |
| n, %beyond college graduate | 6, .6 | 13, .7 | 3, 1.2 | 8, 1.6 | 1, .3 | 4, .7 | 2, .5 | 1, .1 |
| n, %missing | 594, 57.4 | 886, 49.1 | 137, 53.9 | 224, 43.6 | 235, 61.4 | 280, 48.9 | 222, 55.9 | 382, 53.1 |
| Parental occupation2 n, %low | 354, 34.2 | 698, 38.6 | 42. 16.5 | 133, 25.9* | 160, 41.8 | 249, 43.5 | 152, 38.3 | 316, 43.9 |
| n, %medium | 216, 20.9 | 413, 22.9 | 79, 31.1 | 127, 24.7 | 71, 18.5 | 129, 22.5 | 66, 16.6 | 157, 21.8* |
| n, %high | 175, 16.9 | 326, 18.1 | 70, 27.6 | 175, 34.0 | 36, 9.4 | 56, 9.8 | 69, 17.4 | 95, 13.2 |
| n, %missing | 289, 27.9 | 369, 20.4 | 63, 24.8 | 79, 15.4 | 116, 30.3 | 139, 24.3 | 110, 27.7 | 151, 21.0 |
| Siblings (n, % having more than 4) | 10, 1.0 | 94, 32.9 | 1, 0.4 | 11, 2.1 | 4, 1.0 | 23, 4.0 | 5, 1.3 | 60, 8.3 |
| n, %missing | 1004, 97.5 | 1514, 84.1 | 248, 97.6 | 422, 82.1 | 374, 97.7 | 501, 87.4 | 386, 97.2 | 597, 83.0 |
| Baseline cognition3 | ||||||||
| Global cognition (m, SD) | .10 (.53) | .33 (.53)* | .39 (.46) | .70 (.42)* | .14 (.48) | .34 (.48)* | −.12 (.51) | .05 (.47)* |
| Memory (m, SD) | .10 (.67) | .36 (.67)* | .29 (.69) | .69 (.63)* | .09 (.67) | .32 (.68)* | −.01 (.63) | .16 (.60)* |
| Language (m, SD) | .02 (.62) | .29 (.63)* | .35 (.65) | .72 (.55)* | .09 (.53) | .34 (.55)* | −.25 (.56) | −.06 (.53)* |
| Visuospatial (m, SD) | .14 (.62) | .31 (.58)* | .52 (.39) | .69 (.38)* | .20 (.55) | .35 (.49)* | −.17 (.66) | .02 (.61)* |
| Lost to follow-up (n, % of group total) | ||||||||
| Time 2 | 245, 23.7 | 427, 23.6 | 63, 24.8 | 101, 19.6 | 87, 22.7 | 133, 23.2 | 95, 23.9 | 193, 26.8 |
| Time 3 | 435, 42.1 | 734, 40.6 | 114, 44.9 | 192, 37.4 | 164, 42.8 | 229, 40.0 | 157, 39.5 | 313, 43.5 |
| Time 4 | 622, 60.2 | 1056, 58.5 | 166, 65.4 | 277, 53.9* | 231, 60.3 | 354, 61.8 | 225, 56.7 | 425, 59.1 |
| Time 5 | 771, 74.6 | 1312, 72.6 | 202, 79.5 | 367, 71.4* | 295, 77.0 | 434, 75.7 | 274, 69.0 | 511, 71.1 |
| Time 6 | 864, 83.6 | 1472, 81.5 | 224, 88.2 | 408, 79.4* | 321, 83.8 | 485, 84.6 | 319, 80.4 | 579, 80.5 |
Note. Chi-square and t-tests were used to compare cohorts
p < .05; m = mean, SD = standard deviation, CVD = cardiovascular disease (sum of diabetes, heart disease, hypertension, and stroke at baseline), CSES = childhood socio-economic status;
low: housewife/unskilled/semi-skilled, medium: skilled/clerical, high: manager/professional;
Cognitive factor scores are unadjusted for demographics
Cognitive outcomes
At every visit, participants were evaluated with cognitive, functional, and health measures in their preferred language (i.e., English or Spanish). The neuropsychological battery, including tests of memory, language, and visuospatial ability, has been described in detail elsewhere [19, 20]. Memory tests included total recall, delayed recall, and delayed recognition on the Selective Reminding Test [21]. Language tests included the 15-item Boston Naming Test [22], letter and category fluency, the Similarities subtest from the Wechsler Adult Intelligence Scale-Revised [23], and the Repetition and Comprehension subtests of the Boston Diagnostic Aphasia Evaluation [24], Visuospatial tests included the Recognition and Matching tasks on the Benton Visual Retention Test [25], the Rosen Drawing Test [26], and the Identities and Oddities subtest from the Mattis Dementia Rating Scale [27]. Composite scores for each domain were created based on a factor analysis [28], by creating z-scores for each indicator (at baseline) within each domain, and averaging those scores to create a composite. A global cognition score was calculated by averaging the composite scores of the three domains.
Covariates
Sex/gender and years of formal education (0–20 years) were self-reported. Individuals self-identified as either non-Hispanic White, non-Hispanic Black, or Hispanic based on U.S. Census criteria [29]. The CVD burden variable was the sum of four self-reported conditions at baseline: diabetes, heart disease, hypertension, and stroke [30]. CSES was calculated by a factor analysis score based on mother’s and father’s years of education, parental occupational level, and number of siblings. These variables have been used as proxies for CSES in previous literature [31, 32]. Father’s and mother’s education was categorized into no formal education, grades 1–8, grades 9–11, high school, some college, college graduate, and beyond college graduate. Parental occupation was categorized into low (housewife/unskilled/semi-skilled), medium (skilled/clerical), or high (manager/professional) based on the highest value of either parent. Number of siblings was dichotomized as 0–4 siblings or more than 4.
Because of the different recruitment goals between the 1992 and 1999 cohorts, baseline memory complaints were included (on a scale from 0–5) as a covariate in the current study in which the sample was divided by birth year. The number of individuals that were cognitively normal at baseline but endorsed serious memory complaints (5 out of 5 complaints) did not differ between the 1905–1920 (n = 56) and 1921–1935 (n = 87) birth year cohorts (χ2 (1, N = 2836) = .513, p = .477).
Statistical analysis
Descriptive statistics and comparisons between each cohort for demographic variables and baseline cognitive outcomes were performed using general linear models and chi-square analyses in SPSS Version 23.
To identify differences in the cognitive trajectories of the cohorts born between 1905–1920 and 1921–1935, we used latent growth curve modeling with full information maximum likelihood estimation in Mplus Version 8. To maximize covariance coverage, the trajectory was modeled up to six visits (follow-up up to 17 years). Loss of follow-up with each visit overall and by race/ethnicity group per birth year cohort is presented in Table 1. We parameterized time as years from study entry and used the time score option in Mplus to accommodate individual differences in intervals between re-evaluations.
All growth curve models used cohort as the primary predictor of interest, in which the 1905–1920 cohort was the reference category. We estimated models for the entire sample and using a multiple-group modeling approach with race/ethnicity as the grouping variable to investigate the effects within and between racial/ethnic groups.
First, we built unadjusted models for each of the four cognitive outcomes, in which cognitive outcome was predicted by cohort without adjusting for covariates. Linear and quadratic effects of time and age were examined for each cognitive outcome separately; using nested models, Bayesian information criterion (BIC) values indicated that linear models provided the best fit. To explore practice effects, we entered a latent retest factor with its loading fixed at 0 for baseline and 1 for follow-up visits (retest effect with a peak at the second assessment) and a latent retest factor with its loading fixed at 0 for baseline and second visit and 1 for subsequent follow-up visits (retest effect with a peak at the third assessment). The retest effect with a peak at the third visit improved model fit in the memory outcome (BICs: no retest factor: 15836.430; peak second visit: 15842.790; peak third visit: 15827.401). The retest effect with a peak in the second visit improved model fit in the language outcome (BICs: no retest factor: 9566.226; peak second visit: 9542.269; peak third visit: 9574.499). In the global cognition and visuospatial outcomes, latent retest factors did not improve model fit above the fit of a model without a retest effect. Retest effects for memory and language were included in subsequent analyses.
In addition to cohort as the predictor, adjusted models first included age, sex/gender, race/ethnicity and baseline memory complaints as covariates in Step 1, then followed sequentially by CVD burden in Step 2, education in Step 3, and CSES in Step 4. We included CSES last to explore whether experiences earlier in life could explain the cohort effect over and above proximal life exposures. Within each cohort, education and CSES were correlated with each other in the overall sample and per race/ethnicity group, but only to a moderate extent (r ≤ .495). The model steps were repeated for each cognitive outcome. Multiple-group models to investigate effects within and between groups used race/ethnicity as grouping variable instead of covariate. All continuous covariates (i.e., age, memory complaints, CVD burden, years of education, and CSES) were centered at their grand mean for parameter interpretation; additionally, categorical covariates were centered at their grand mean only to report intercept and slope effects. Model fit improved with the addition of each covariate as observed by a lower BIC value.
In exploratory models, to analyze who benefitted from secular effects, we estimated the cohorts’ cognitive trajectories separately for lowest-quartile performers and highest-quartile performers at baseline. Quartiles were defined per cohort within each racial/ethnic group. We estimated models for all cognitive outcomes in adjusted model Step 1 in a multiple-group model to investigate effects within and between the racial/ethnic groups. Subsequently, we analyzed to what extent the lowest and highest-quartile performers differed with regard to CSES, education, and CVD burden using general linear models.
Attrition from a longitudinal study due to dropout and death may result in biased estimation of trajectories, as previous cognitive scores of those who dropout or die are typically poorer than those of their counterparts who do not attrite [16, 33, 34]. We accommodated dropout due to death by jointly modeling cognitive trajectories with the survival process in the adjusted model Step 1 (i.e., covaried for age, sex/gender, race/ethnicity, and memory complaints). Missing data indicators were used to denote whether a single non-repeatable event (death) occurred prior to a specific time point. Missing data indicators were coded as data being observed or missing due to death. Once death was observed, missing data indicators for subsequent visits were coded as missing. A discrete-time survival model included a latent hazard function that represented the missing data indicator distribution. This hazard function is the conditional probability that an individual will die at a specific time point, given that they did not die or dropout at an earlier time point [35]. Data visualization was performed in RStudio Version 1.1.453.
Results
Sample characteristics
An overview of sample characteristics overall and per race/ethnicity group is presented in Table 1. Mean age overall and within each racial/ethnic group, was approximately 79.5 years in the 1905–1920 cohort and 72.5 years in the 1921–1935 cohort, and 60–70% were women. The number of follow-ups was approximately equal overall and across racial/ethnic groups, but the time between visits was shorter in the 1905–1920 than 1905–1920 cohort. The 1905–1920 cohort had more memory complaints at baseline than the 1921–1935 cohort, except in Whites. Compared to the 1905–1920 cohort, the 1921–1935 cohort had higher baseline cognitive test scores. The 1921–1935 cohort had more years of education overall and across race/ethnicity groups, and higher CSES across all but the Hispanic group than the 1905–1920 cohort; only Blacks had higher CVD burden in the 1921–1935 cohort compared to the 1905–1920 cohort. Loss to follow-up was not different between the two birth cohorts in general, or when stratified by race/ethnicity groups, except for slightly more loss to follow-up in Whites as of visit 4 in the 1921–1935 cohort. Loss to follow-up percentages were comparable across race/ethnicity groups.
Cognitive trajectories
First, we ran unadjusted models with each of the four cognitive outcomes (Supplementary Table 1). Across the whole sample and for all cognitive outcomes, the 1921–1935 cohort had a higher intercept and slower rate of decline than the 1905–1920 cohort. Results were the same in multiple-group models grouped by race/ethnicity, with higher intercepts and slower rates of decline in the 1921–1935 cohort than the 1905–1920 cohort.
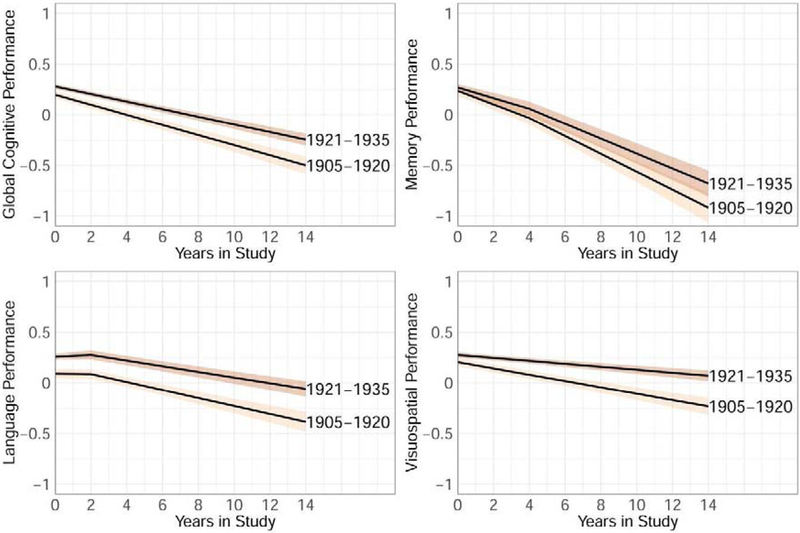
We then adjusted the model to account for demographic factors (age, sex/gender, race/ethnicity) and baseline memory complaints (Table 2; Supplementary Table 1). In these adjusted models in the whole sample, the pattern of the cohort effect remained the same, namely higher intercepts and less steep slopes in the 1921–1935 than the 1905–1920 cohort across all cognitive outcomes but memory, which showed neither an intercept nor slope effect (Figure 1). Per race/ethnicity group, a model adjusted for age, sex/gender, and baseline memory complaints showed in Whites significant intercept but not slope effects across outcomes, in Blacks significant intercept effects for global cognition, language, and visuospatial abilities, and a slope effect in visuospatial abilities, and in Hispanics no intercept or slope effects, except for a steeper slope for visuospatial abilities in the 1905–1920 cohort than the 1921–1935 cohort.
Table 2.
Cohort effects on intercept and slope across adjusted models per race/ethnicity group
| Step 1 (age, sex/gender, memory complaints) | Step 2 (Step 1+ CVD) | Step 3 (Step 2 + education) | Step 4 (Step 3 + CSES) | ||
|---|---|---|---|---|---|
| Global cognition | Cohort effect | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] |
| intercept | .151** [.055; .247] | .108* [.014; .201] | .045 [−.042; .133] | .050 [−.034; .135] | |
| slope | .013 [.000; .026] | .018** [.005; .031] | .017** [.004; .030] | .016* [.002; .029] | |
| Blacks | intercept | .112** [.028; .197] | .100* [.008; .191] | −.012 [−.095; .071] | −.047 [−.137; .043] |
| slope | .011 [−.002; .024] | .012 [−.001; .026] | .011 [−.003; .025] | .015 [.000; .029] | |
| Hispanics | intercept | .018 [−.060; .097] | −.005 [−.091; .081] | −.024 [−.099; .051] | −.024 [−.104; .055] |
| slope | .012 [.000; .025] | .016* [.003; .028] | .015* [.002; .028] | .015* [.002; .029] | |
| Memory | |||||
| Whites | intercept | .158* [.018; .297] | .107 [−.036; .251] | .045 [−.091; .182] | .034 [−.104; .173] |
| slope | .014 [−.012; .040] | .012 [−.012; .036] | .013 [−.012; .037] | .014 [−.012; .040] | |
| Blacks | intercept | .045 [−.072; .163] | .027 [−.101; .155] | −.059 [−.184; .066] | −.106 [−.244; .031] |
| slope | .022 [−.003; .046] | .030* [.004; .056] | .023 [−.003; .050] | .031* [.004; .059] | |
| Hispanics | intercept | −.048 [−.144; .048] | −.072 [−.176; .032] | −.086 [−.187; .014] | −.091 [−.197; .016] |
| slope | .004 [−.018; .026] | .012 [−.010; .034] | .012 [−.011; .034] | .013 [−.011; .037] | |
| Language | |||||
| Whites | intercept | .230** [.088; .372] | .144* [.017; .272] | .059 [−.060; .178] | .077 [−.041; .196] |
| slope | .016 [−.001; .033] | .014 [−.002; .030] | .013 [−.003; .029] | .007 [−.008; .022] | |
| Blacks | intercept | .227*** [.134; .319] | .219*** [.119; .319] | .073 [−.016; .161] | .045 [−.049; .139] |
| slope | .007 [−.007; .022] | .004 [−.011; .019] | .004 [−.011; .020] | .004 [−.012; .021] | |
| Hispanics | intercept | .074 [−.012; .161] | .048 [−.047; .142] | .026 [−.055; .106] | .044 [−.040; .128] |
| slope | .011 [−.004; .025] | .011 [−.003; .026] | .011 [−.004; .025] | .010 [−.005; .025] | |
| Visuospatial | |||||
| Whites | intercept | .083* [.016; .15] | .074* [.002; .146] | .028 [−.045; .101] | .029 [−.040; .099] |
| slope | .007 [−.004; .019] | .009 [−.003; .021] | .008 [−.004; .020] | .007 [−.005; .018] | |
| Blacks | intercept | .093* [.003; .182] | .091 [−.006; .187] | −.014 [−.103; .076] | −.045 [−.139; .050] |
| slope | .018** [.006; .03] | .019** [.007; .032] | .018** [.006; .030] | .019** [.007; .032] | |
| Hispanics | intercept | .052 [−.044; .149] | .047 [−.059; .152] | .020 [−.071; .111] | .003 [−.092; .098] |
| slope | .020** [.006; .035] | .023** [.008; .037] | .022** [.008; .037] | .024** [.009; .038] |
Note. 95% CI = 95% confidence interval; CVD = cardiovascular disease (sum of diabetes, heart disease, hypertension, and stroke at baseline), CSES = childhood socio-economic status;
p < .05,
p < .01,
p <.001
Figure 1.
Mean global cognitive performance trajectories (95% confidence interval) for global cognitive performance and its three subdomains in the whole sample adjusted for age, sex/gender, race, and baseline memory complaints
Between-group comparisons (Table 3) revealed that after adjusting for age, sex/gender, and memory complaints, intercepts in both 1905–1920 and 1921–1935 cohorts were higher for Whites than Blacks and Hispanics in all four cognitive outcomes, and higher for Blacks than Hispanics in all cognitive outcomes but memory. The cohort effect on intercept for Hispanics was smaller than Whites in global cognition and memory, and smaller than Blacks in language. Slope did not differ across race/ethnicity in either the 1905–1920 or 1921–1935 cohort, and there were no differences in cohort effect on slope between the race/ethnicity groups.
Table 3.
Multiple-group comparisons per cohort and between cohorts
| initial level (intercept) | linear change (slope) | |||||
|---|---|---|---|---|---|---|
| 1905–1920 | 1921–1935 | cohort effect | 1905–1920 | 1921–1935 | cohort effect | |
| Global cognition | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] |
| −.314*** [−.408; −.219] | −.352*** [−.413; −.292] | −.039 [−.166; .089] | −.004 [−.018; .010] | −.006 [−.016; .004] | −.002 [−.020; .017] | |
| Whites vs. Hispanics | −.538*** [−.632; −.443] | −.577*** [−687; −.466] | −.133* [−.256; −.009] | .001 [−.013; .016] | .001 [−.008; .010] | −.001 [−.019; .018] |
| Blacks vs. Hispanics | −.224*** [−.307; −.141] | −.224*** [−.307; −.141] | −.094 [−.209; .022] | .006 [−.008; .019] | .007 [−.003; .017] | .001 [−.017; .019] |
| Memory | ||||||
| Whites vs. Blacks | −.275*** [−.408; −.143] | −.388*** [−.476; −.300] | −.112 [−.295; .070] | −.012 [−.035; .012] | −.004 [−.025; .016] | .007 [−.026; .041] |
| Whites vs. Hispanics | −.372*** [−.501; −.243] | −.485*** [−.635; −.334] | −.206* [−.375; −.036] | −.004 [−.027; .019] | −.015 [−.034; .004] | −.010 [−.042; .021] |
| Blacks vs. Hispanics | −.097 [−.204; .010] | −.097 [−.204; .010] | −.093 [−.245; .058] | .007 [−.013; .028] | −.010 [−.031; .010] | −.018 [−.048; .013] |
| Language | ||||||
| Whites vs. Blacks | −.331*** [−.460; −.202] | −.334*** [−.409; −.259] | −.003 [−.172; .166] | .007 [−.010; .024] | −.002 [−.014; .010] | −.009 [−.031; .013] |
| Whites vs. Hispanics | −.605*** [−.733; −.476] | −.608*** [−.733; −.482] | −.155 [−.321; .011] | .014 [−.003; .031] | .008 [−.002; .019] | −.006 [−.027; .016] |
| Blacks vs. Hispanics | −.274*** [−.366; −.182] | −.274*** [−.366; −.182] | −.152* [−.279; −.026] | .007 [−.007; .021] | .010 [−.001; .022] | .003 [−.016; .023] |
| Visuospatial | ||||||
| Whites vs. Blacks | −.340*** [−.423; −.257] | −.330*** [−.386; −.274] | .010 [−.102; .122] | −.012 [−.025; .002] | −.001 [−.010; .008] | .011 [−.006; .028] |
| Whites vs. Hispanics | −.643*** [−.732; −.554] | −.633*** [−.754; −.513] | −.030 [−.148; .087] | −.013 [−.027; .001] | .000 [−.009; .009] | .013 [−.005; .031] |
| Blacks vs. Hispanics | −.303*** [−.401; −.205] | −.303*** [−.401; −.205] | −.040 [−.172; .092] | −.001 [−.016; .013] | .001 [−.008; .010] | .002 [−.016; .021] |
Note. Estimates are adjusted for age, sex/gender, and baseline memory complaints; 95% CI = 95% confidence interval;
p < .05,
p < .01,
p <.001
Effects of life exposures
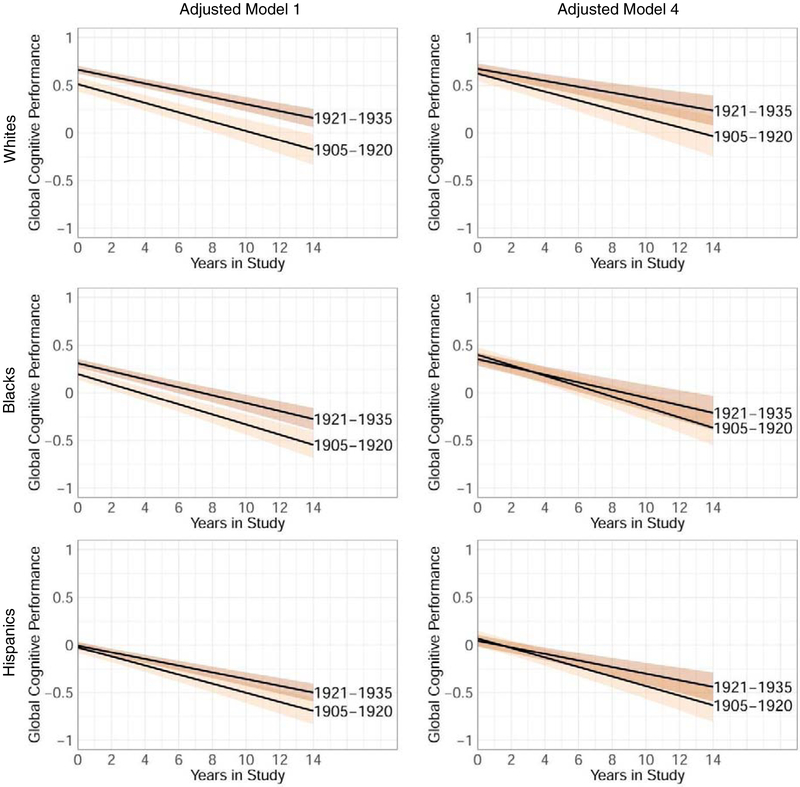
Effects of life exposures were investigated for each race/ethnicity group separately (Table 2; Figure 2). Successively adding CVD burden, education, and CSES to the model attenuated the higher intercept in the 1921–1935 cohort compared to the 1905–1920 cohort that was observed in the basic model. The raw change in intercept between successive models was relatively small when adding CVD burden and largest when adding education. While adjusting for these factors narrowed the difference in intercepts between the two cohorts, the adjustment either did not affect the difference in slopes or even enlarged the differences in slopes, with a less steep decline for the 1921–1935 cohort compared to the 1905–1920 cohort.
Figure 2.
Global cognitive performance trajectory (95% confidence interval) per ethnic group in the adjusted model Step 1 (adjusted for age, sex/gender, baseline memory complaints) versus the adjusted model Step 4 (additionally adjusted for cardiovascular disease, education, and childhood socio-economic status)
Upper and lower quartiles of performance
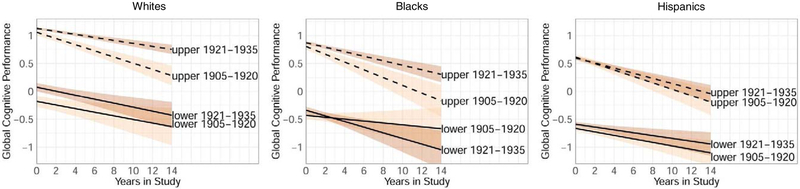
Intercept and cohort effects were calculated per cohort within each race/ethnicity group, which demonstrated that in general a slower rate of change for the later birth cohort was observed in the upper quartile of performers but not in the lower quartile (Table 4; Figure 3).
Table 4.
Cohort effects for upper and lower quartiles by race/ethnicity groups
| Whites | Blacks | Hispanics | |||||
|---|---|---|---|---|---|---|---|
| Intercept cohort effect |
Slope cohort effect |
Intercept cohort effect |
Slope cohort effect |
Intercept cohort effect |
Slope cohort effect |
||
| Quartile | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | Estimate [95% CI] | |
| lower | .256** [.108; .403] | −.004 [−.034; .027] | .092 [−.012; .197] | −.034 [−.078; .011] | .074* [.006; .142] | .006 [−.018; .030] | |
| upper | .069* [.006; .133] | .029** [.012; .045] | .067* [.006; .128] | .029* [.005; .054] | −.014 [−.076; .047] | .012 [−.009; .033] | |
| Memory | lower | .164* [.008; .321] | −.003 [−.049; .043] | −.058 [−.184; .069] | .022 [−.039; .083] | .053 [−.063; .169] | −.023 [−.063; .017] |
| upper | .047 [−.051; .145] | .007 [−.040; .053] | −.016 [−.112; .081] | .024 [−.021; .068] | −.080 [−.171; .010] | .037 [−.006; .079] | |
| Language | lower | .542** [.235; .849] | .028 [−.028; .083] | .210*** [.100; .320] | −.005 [−.060; .050] | .068 [−.031; .167] | −.001 [−.041; .038] |
| upper | .106** [.036; .175] | .038** [.013; .062] | .218*** [.137; .299] | .026 [−.001; .053] | .100* [.019; .181] | .001 [−.023; .025] | |
| Visuospatial | lower | .128* [.014; .242] | −.016 [−.043; .011] | .199** [.071; .327] | −.001 [−.042; .041] | .142* [.030; .254] | .004 [−.018; .026] |
| upper | −.029 [−.077; .020] | .037*** [.019; .055] | .037 [−.023; .097] | .015 [−.004; .033] | .046 [−.016; .108] | .034* [.005; .062] | |
Note. Estimates are adjusted for age, sex/gender, and baseline memory complaints; 95% CI = 95% confidence interval;
p < .05,
p < .01,
p <.001
Figure 3.
Trajectories of upper versus lower quartiles of global cognitive performance at baseline (95% confidence interval) per ethnic group, adjusted for age, sex/gender, baseline memory complaints
In the lowest quartile at baseline, adjusted for age, sex/gender, and baseline memory complaints, intercepts of all four cognitive outcomes were higher for the 1921–1935 cohort than 1905–1920 cohort among Whites. For the other two race/ethnicity groups, this pattern was also observed for language and visuospatial abilities among Blacks, and for global cognition and visuospatial abilities among Hispanics. In the lowest quartile, no cohort effects on slope were observed for any of the race/ethnicity groups.
Individuals who performed in the upper quartile at baseline, adjusted for age, sex/gender, and baseline memory complaints, had higher intercepts in the 1921–1935 compared to the 1905–1920 cohort in global cognition (Whites and Blacks) and language (Whites, Blacks, and Hispanics). Individuals who performed in the upper quartile had a slower rate of change in global cognition, language, and visuospatial abilities (Whites), global cognition (Blacks), and visuospatial abilities (Hispanics) in the 1921–1935 compared to the 1905–1920 cohort.
Using general linear models, we examined which variables distinguished those who scored in the upper quartile compared to the lower quartile of overall cognitive test performance. The lower and upper quartiles did not differ in their CVD burden in either the 1905–1920 cohort (Whites: F(1, 108) = .196, p = .659; Blacks: F(1, 172) = 1.048, p = .307; Hispanics: F(1, 200) = .142, p = .707) or 1921–1935 cohort (Whites: F(1, 244) = 1.539, p = .216; Blacks: F(1, 288) = .197, p = .657), except for Hispanics in the 1921–1935 cohort (F(1, 346) = 5.370, p = .021). While the upper quartile was higher educated in the 1905–1920 cohort (Whites: F(1, 108) = 11.507, p = .001; Blacks: F(1, 172) = 77.942, p < .001; Hispanics: F(1, 200) = 73.991, p < .001), it was not in the 1921–1935 cohort (Whites: F(1, 244) = .367, p = .545; Blacks: F(1, 288) = 2.472, p = .117), except for Hispanics in the 1921–1935 cohort (F(1, 346) = 201.976, p < .001). The upper quartile did have higher CSES scores than the lower quartile in both the 1905–1920 cohort (Whites: F(1, 108) = 9.248, p = .003; Blacks: F(1, 172) = 4.301, p = .040; Hispanics: F(1, 200) = 5.712, p = .018) and 1921–1935 cohort (Whites: F(1, 244) = 8.973, p = .003; Blacks: F(1, 288) = 4.112, p = .044; Hispanics: F(1, 346) = 5.914, p = .016).
Attrition bias
The latent hazard factor in the joint model showed that those who were lost to follow-up had lower initial level (estimate = −.416, 95% CI [−.548; −.284]) and a steeper slope of decline (estimate = −15.959, 95% CI [−19.480; −12.437]) versus those who remained in the study. Similar to findings in the previous model, the 1921–1935 cohort started at a higher level on the overall cognition composite (cohort effect estimate = .087, 95% CI [.037; .136]) and had a less steep decline (cohort effect estimate = .010, 95% CI [.003; .018]) compared to the 1905–1920 cohort after adjusting for age, sex/gender, race/ethnicity, and baseline memory complaints. While death affected overall intercept and trajectories, the effect of cohort remained largely unchanged for both level and slope as observed in the largely overlapping confidence intervals in the joint adjusted model Step 1 versus the regular adjusted model Step 1. This observation indicates that the effect of cohort was not substantially biased by attrition.
Discussion
We identified secular trends in cognitive trajectories in two birth cohorts of educationally and racially/ethnically diverse older adults, showing higher baseline abilities and less steep decline for the later-born cohort. The cohort effect on initial level of performance was accounted for by social and medical exposures across the life course; however, these factors did not directly account for the cohort effect on rate of cognitive decline. The participants in this study are distinct from participants in the majority of other large cohorts in two ways: both birth cohorts included people whose education ranged from zero to 20 years, and they were racially and ethnically diverse. This diversity allowed us to expose that later birth year benefitted those with high childhood socioeconomic circumstances and those who were highly educated, compared to those with less education and lower CSES.
This paper expands on the findings by Noble et al. [13]. The authors demonstrated that the decline in dementia incidence with later birth year, and its association with higher education and better treatment of antecedent vascular risk factors, vary as a function of race/ethnicity. To expand, we now demonstrated that the rate of cognitive decline in individuals that are cognitively healthy at baseline was reduced in the later-born cohort, and that in addition to education and CVD burden, CSES plays an important role in accounting for baseline differences between cohorts. These findings are in line with the concept of cognitive reserve, namely that cognitively challenging activities and acquisition of skills and knowledge with schooling increase the capacity to maintain cognitive function despite high risk for impairment [36]. We confirmed that, similar to the previous results with incidence dementia, cohort differences in cognitive trajectories and the influence of life exposures on these trajectories vary as a function of race/ethnicity. These results are in agreement with previous reports on racial/ethnic differences in cognitive performance at baseline but not in change over time [37].
Our primary result of a benefit for later birth year fits with the majority of reports on secular trends in late-life cognition. Prior research focused on dementia incidence [8–10, 13] or conducted multiple cross-sectional comparisons of cognition over time [16, 38–40]. Only a handful of studies investigated secular trends in longitudinal cognitive trajectories [41]. Gerstorf and colleagues [42] found a benefit for later-born individuals on baseline abilities and rate of change on four out of five cognitive abilities (i.e., on spatial orientation, inductive reasoning, word fluency, and verbal meaning, but not on number ability). Dodge et al. [43] reported that slower cognitive decline in those born later did not attenuate after adjusting for education. Benefits of later birth on cognition are not ubiquitous [44, 45], potentially due to the younger age of participants in some studies [46].
Later birth year was associated with higher baseline abilities and less rapid cognitive decline across cognitive outcomes and across race/ethnicity. By dividing the sample into high performers and low performers at baseline, we showed that the cohort effect seems to be driven by the higher performing individuals, who were higher educated and had higher CSES. This inequality in secular trends was observed across race/ethnicity in the full sample as well: the benefit of later birth year on baseline global cognitive abilities was highest in Whites, followed by a smaller benefit in Blacks, and no benefit among Hispanics. These observations mark the critical importance of investigating interindividual differences in intraindividual change within subgroups of the population [41].
Cardiovascular factors are related to the onset and rate of change of cognitive impairment [47–50]. The rising treated prevalence of CVD in combination with steady clinical prevalence [51] suggests that management of the negative effects of CVD burden is improving over time. In our models of cognitive performance, however, accounting for CVD burden had minimal effect on the cohort effect. The small impact of CVD burden on cohort effects contrasts to the much larger explanatory effects of educational attainment and CSES. We deliberately added life exposures to the models in reverse chronological order, starting with CVD, to determine if proximal exposures could account for benefits of later birth year. CSES consistently had a larger impact on secular trends at baseline than CVD burden, as differences in intercept largely decreased or even disappeared in Whites and Blacks (whose CSES rose between cohorts) in Step 4 compared to Step 2, but not in Hispanics (whose CSES did not differ between cohorts). However, none of the covariates substantially affected rate of change.
One limitation of the current study is that CVD burden is only an indirect proxy of improved CVD treatment. Future research that includes biological indicators of cardiovascular health should explore the extent to which improved management of CVD explains secular trends in cognitive trajectories. Another limitation may be that other sociocultural factors associated with cognition (e.g., early life stress, family networks) were unaccounted for in the current study. Future research should aim to further investigate socio-demographic changes across birth cohorts that pass on to cognitive aging. An additional limitation is that our neuropsychological battery included tests that tap executive function, including fluency and abstract reasoning, but does not include an executive functioning factor as both of these tests involve verbal skills and loaded on to a language factor in a confirmatory factor analysis. Therefore, we did not include a separate construct of executive functioning, while this domain is known to strongly decline with cognitive aging and dementia. It would also be of interest to investigate during which period in life socio-demographic changes are most influential to late-life cognitive functioning. In our study, we cannot distinguish when these changes occurred, as they may be happening in middle-age. In particular, additional research is required to evaluate the role of the causal path of early life factors on individuals’ subsequent life exposures, and if intervention in the expected path may moderate secular trends in cognitive aging.
In conclusion, later year of birth beneficially affects the level of cognitive performance and attenuates rate of change in cognitive functioning. These trends at baseline, but not in rate of change, can be attributed to better educational and childhood socio-economic circumstances across generations. Our results support that socio-cultural inequalities are part of the mechanisms driving secular trends in cognitive aging and dementia.
Supplementary Material
Research in context.
SYSTEMATIC REVIEW: We reviewed the literature using Google Scholar and PubMed. Multiple studies found lower prevalence and incidence of dementia with later birth year, but few studies discussed secular trends in longitudinal cognitive trajectories. The role of socio-demographic factors including race/ethnicity and socio-economic status on secular trends in cognitive aging remains unclear.
INTERPRETATION: Level and rate of cognitive change over time is influenced by birth cohort, and socio-cultural inequalities contribute to these secular trends.
FUTURE DIRECTIONS: Future studies should extent the scope of socio-demographic factors in investigating the mechanisms of secular trends in cognitive aging. More research is needed to evaluate the causal path of early life factors on individuals’ subsequent life exposures, to determine when interventions may be most effective.
Acknowledgments
Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP, PO1AG07232, R01AG037212, RF1AG054023) funded by the National Institute on Aging (NIA). This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. We thank Rich Jones, Douglas Tommet, and Alden Gross for providing Mplus and R syntax together with their helpful feedback on data visualization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9:63–75. e2. [DOI] [PubMed] [Google Scholar]
- [2].Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s research & therapy. 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wimo A, Jönsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9:1–11. e3. [DOI] [PubMed] [Google Scholar]
- [4].Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. The Lancet Neurology. 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- [5].Xu J, Zhang Y, Qiu C, Cheng F. Global and regional economic costs of dementia: a systematic review. The Lancet. 2017;390:S47. [Google Scholar]
- [6].Torti FM Jr, Gwyther LP, Reed SD, Friedman JY, Schulman KA. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Disease & Associated Disorders. 2004;18:99–109. [DOI] [PubMed] [Google Scholar]
- [7].Wu Y-T, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HC, et al. The changing prevalence and incidence of dementia over time—current evidence. Nature Reviews Neurology. 2017;13:327. [DOI] [PubMed] [Google Scholar]
- [8].Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–63. [DOI] [PubMed] [Google Scholar]
- [9].Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–94. [DOI] [PubMed] [Google Scholar]
- [10].Grasset L, Brayne C, Joly P, Jacqmin-Gadda H, Peres K, Foubert-Samier A, et al. Trends in dementia incidence: evolution over a 10-year period in France. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12:272–80. [DOI] [PubMed] [Google Scholar]
- [11].Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed research international. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kosteniuk JG, Morgan DG, O’Connell ME, Kirk A, Crossley M, Teare GF, et al. Simultaneous temporal trends in dementia incidence and prevalence, 2005–2013: a population-based retrospective cohort study in Saskatchewan, Canada. International psychogeriatrics. 2016;28:1643–58. [DOI] [PubMed] [Google Scholar]
- [13].Noble JM, Schupf N, Manly JJ, Andrews H, Tang M-X, Mayeux R. Secular Trends in the Incidence of Dementia in a Multi-Ethnic Community. Journal of Alzheimer’s Disease. 2017;60:1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64:750–7. [DOI] [PubMed] [Google Scholar]
- [15].Haan MN, Zeki Al-Hazzouri A, Aiello AE. Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: The Sacramento Area Latino Study on Aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66:i102–i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular trends in cognitive performance in older black and white US adults, 1993–2012: Findings from the Chicago Health and Aging Project. The Journals of Gerontology: Series B. 2018;73:S73–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing Diagnostic Criteria and Estimating Frequency of Mild Cognitive Impairment in an Urban Community. Archives of Neurology. 2005;62:1739–46. [DOI] [PubMed] [Google Scholar]
- [18].American Psychiatric A. Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition Washington, DC: American Psychiatric Press Inc; 1987. [Google Scholar]
- [19].Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of Neurology. 1992;49:453–60. [DOI] [PubMed] [Google Scholar]
- [20].Siedlecki KL, Honig LS, Stern Y. Exploring the structure of a neuropsychological battery across healthy elders and those with questionable dementia and Alzheimer’s disease. Neuropsychology. 2008;22:400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–25. [DOI] [PubMed] [Google Scholar]
- [22].Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- [23].Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- [24].Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- [25].Benton AL. The Benton Visual Retention Test. New York: The Psychological Corporation; 1955. [Google Scholar]
- [26].Rosen W. The Rosen Drawing Test. Bronx, NY: Veterans Administration Medical Center; 1981. [Google Scholar]
- [27].Mattis S, Bellak L, Karasu TB. Mental Status examination for organic mental syndrome in the elderly patient. New York: Grune & Stratton; 1976. [Google Scholar]
- [28].Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang M-X, Stern Y. Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology. 2010;24:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].U.S. Census Bureau. 2000 Census of Population and Housing Population and Housing Unit Counts. PHC-3–50 West Virginia Washington, D.C: 2003. [Google Scholar]
- [30].Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. in press. [DOI] [PubMed] [Google Scholar]
- [31].Hill EM, Ross LT, Mudd SA, Blow FC. Adulthood functioning: the joint effects of parental alcoholism, gender and childhood socio-economic stress. Addiction. 1997;92:583–96. [PubMed] [Google Scholar]
- [32].Lee M, Khan MM, Wright B. Is Childhood Socioeconomic Status Related to Coronary Heart Disease? Evidence From the Health and Retirement Study (1992–2012). Gerontology and geriatric medicine. 2017;3:2333721417696673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cooney TM, Schaie KW, Willis SL. The relationship between prior functioning on cognitive and personality dimensions and subject attrition in longitudinal research. Journal of Gerontology. 1988;43:P12–P7. [DOI] [PubMed] [Google Scholar]
- [34].Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal data with follow-up truncated by death: match the analysis method to research aims. Statistical science: a review journal of the Institute of Mathematical Statistics. 2009;24:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Singer JD, Willett JB. It’s about time: Using discrete-time survival analysis to study duration and the timing of events. Journal of educational statistics. 1993;18:155–95. [Google Scholar]
- [36].Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weuve J, Barnes LL, de Leon Mendes C, Rajan KB, Beck T, Aggarwal NT, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gerstorf D, Hülür G, Drewelies J, Eibich P, Duezel S, Demuth I, et al. Secular changes in late-life cognition and well-being: towards a long bright future with a short brisk ending? Psychology and aging. 2015;30:301. [DOI] [PubMed] [Google Scholar]
- [39].Choi H, Schoeni RF, Martin LG, Langa KM. Trends in the prevalence and disparity in cognitive limitations of Americans 55–69 years old. The Journals of Gerontology: Series B. 2018;73:S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van den Kommer TN, Deeg DJ, van der Flier WM, Comijs HC. Time Trend in Persistent Cognitive Decline: Results From the Longitudinal Aging Study Amsterdam. The Journals of Gerontology: Series B. 2018;73:S57–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schaie KW, Willis SL, Pennak S. An historical framework for cohort differences in intelligence. Research in human development. 2005;2:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gerstorf D, Ram N, Hoppmann C, Willis SL, Schaie KW. Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Developmental psychology. 2011;47:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dodge HH, Zhu J, Lee C-W, Chang C-CH, Ganguli M. Cohort effects in age-associated cognitive trajectories. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2013;69:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Cohort differences in trajectories of cognitive aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:P286–P94. [DOI] [PubMed] [Google Scholar]
- [45].Bowles RP, Grimm KJ, McArdle JJ. A structural factor analysis of vocabulary knowledge and relations to age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:P234–P41. [DOI] [PubMed] [Google Scholar]
- [46].Zelinski EM, Kennison RF. Not your parents’ test scores: Cohort reduces psychometric aging effects. Psychology and Aging. 2007;22:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Archives of neurology. 2009;66:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The Role of APOEϵ 4 in Modulating Effects of Other Risk Factors for Cognitive Decline in Elderly Persons. Jama. 1999;282:40–6. [DOI] [PubMed] [Google Scholar]
- [49].Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. Bmj. 1994;308:1604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Archives of neurology. 2008;65:1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Roehrig CS, Rousseau DM. The growth in cost per case explains far more of US health spending increases than rising disease prevalence. Health Affairs. 2011;30:1657–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.