Summary
INTRODUCTION:
Multi-domain intervention for Alzheimer’s disease (AD) risk reduction is an emerging therapeutic paradigm.
METHODS:
Patients were prescribed individually-tailored interventions (education/pharmacologic/non-pharmacologic) and rated on compliance. Normal cognition/subjective cognitive decline/preclinical-AD were classified as Prevention. Mild cognitive impairment due to AD/mild-AD were classified as Early Treatment. Change from baseline to 18-months on the modified-Alzheimer’s Prevention Cognitive Composite (primary outcome) was compared against matched historical control cohorts. Cognitive aging composite (CogAging), AD/cardiovascular risk-scales, and serum biomarkers were secondary outcomes.
RESULTS:
174 were assigned interventions (age 25–86). Higher-compliance Prevention improved more than both historical cohorts (P=.0012,P<.0001). Lower-compliance Prevention also improved more than both historical cohorts (P=.0088,P<.0055). Higher-compliance Early Treatment improved more than lower-compliance (P=.0007). Higher-compliance Early Treatment improved more than historical cohorts (P<.0001,P=.0428). Lower-compliance Early Treatment did not differ (P=.9820,P=.1115). Similar effects occurred for CogAging. AD/cardiovascular risk-scales and serum biomarkers improved.
DISCUSSION:
Individualized multi-domain interventions may improve cognition and reduce AD/cardiovascular risk scores in patients at-risk for AD-dementia.
Keywords: Alzheimer’s disease prevention, Multi-domain interventions, Alzheimer’s Prevention Clinic, Personalized medicine, Preclinical Alzheimer’s disease
Introduction
Late-life Alzheimer’s disease (AD) develops over an extended preclinical period.1–4 Considering over 46 million people in the United States alone have preclinical AD, this pre-dementia period offers a unique opportunity for early intervention to address modifiable risk.5
Given the paucity of effective AD treatments, prevention or delay of dementia is essential. Further, AD drug trials may have been more successful if initiated earlier in the disease course.6 It is therefore important to evaluate the effectiveness of AD interventions across the disease spectrum, especially in at-risk individuals before clinically-evident decline.
Population-attributable risk models estimate that risk factor modification (e.g., hypertension, insulin resistance, physical inactivity, hearing loss, depression) may prevent up to one-third of AD cases.7,8 These targetable risk factors may influence AD pathological pathways (e.g., glucose hypometabolism, inflammation, oxidative stress, amyloid burden, trophic factors).8,9 The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study was the first large long-term randomized controlled trial (RCT) showing multi-domain interventions (nutrition/physical activity/cognitive training) can maintain cognitive function and reduce the risk of cognitive impairment among at-risk older adults from the general population.10,11 Other RCTs applying lifestyle modifications have demonstrated similar effects in mild cognitive impairment (MCI) participants and adults at-risk for cognitive decline.12,13 However, encouraging data from RCTs require translation to clinical practice, including verification of how patient compliance (or “dose response”) affects outcomes.14
Considering the heterogeneity of AD pathology, the application of precision medicine allows for interventions that can be targeted for individual patients.12,15 The National Institutes of Health defines precision medicine as “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person”.16 An overall structure of how precision medicine may be achieved in the future will be through convergence of technological advances (e.g., big data, genomic sequencing, “-omics” technologies, systems biology, integrated disease modeling) as it is hypothesized that deconstructing the disease into multiple subsets that exist within a heterogeneous population, and tailoring therapies accordingly, may be preferentially effective based on individual biological make-up (protein-protein interactions, epigenetic modifications, metabolic pathways).17,18 A term that has been used to adapt this approach, using currently available clinical assessments in everyday practice,19 is clinical precision medicine, where medical history (e.g., lifestyle patterns, life-course events), physical/neurological examination, anthropometrics, commercially-available blood biomarkers (including genetics), and cognitive assessments inform a multi-modal management plan.20,21 Patients are followed longitudinally to evaluate the effectiveness of, and further refine, personally-tailored interventions. In 2013, an Alzheimer’s Prevention Clinic (APC) was established in New York, with research collaboration in Puerto Rico.21,22 APC’s mission is to mitigate late-life AD dementia risk by applying individualized clinical management strategies toward primary, secondary, and tertiary AD prevention while simultaneously studying its comparative effectiveness (Figure S1).23
In this proof-of-concept trial, we investigated effects of multi-domain evidence-based individually-tailored interventions on cognition, AD/cardiovascular risk scores, and AD-risk biomarkers in real-world clinical practice.22,24
2. Methods
2.1. Study design and participants
In this prospective comparative effectiveness trial, all patients requesting an APC clinical consultation between March 12, 2015 and January 10, 2018 were initially screened via telephone (Figure S2) for participation to achieve a pre-specified sample of at least 150 participants with baseline and post-intervention assessments (powered to detect a 3.5-point difference [SD 6.5] on the primary outcome with 90% power and a sample size of 75 participants in each compliance group; See Figure S3 for study design, Appendix A for power calculation). Inclusion criteria assessed via initial telephone screen were a family history of AD and no/minimal cognitive complaints. Exclusion criteria assessed during an in-person evaluation included a diagnosis of moderate-to-severe AD dementia or other dementia; disorders affecting safe engagement in interventions (e.g., malignant disease, major depression, psychotic disorder); or coincident participation in another trial. Participants with a clinical diagnosis of MCI or early mild dementia with negative Amyloid neuroimaging were also excluded (n=7). See CONSORT diagram for additional details (Figure S2).
Institutional Review Board approval was obtained on February 16th, 2015 and patients were consented to participate in the Comparative Effectiveness Dementia & Alzheimer’s Registry (Protocol #1408015423). See Appendix B for consent procedures.
2.2. Procedures
Participants underwent a comprehensive screening evaluation: detailed clinical history, physical examination, anthropometrics, blood biomarkers, apolipoprotein-ε4 (APOE-e4) genotyping, and cognitive assessment (Table S1 and detailed in prior publication).22 Additional assessments were ordered in symptomatic patients (incorporating American Academy of Neurology Guidelines25), when indicated. Amyloid positron emission tomography (PET) or cerebrospinal fluid biomarkers were used to confirm/exclude AD pathology in participants with a clinical diagnosis of MCI or early mild dementia. Participants were diagnosed as normal cognition, subjective cognitive decline (SCD), preclinical AD (PRE), MCI due to AD, or early mild AD dementia incorporating the 2011 National Institutes of Health and the Alzheimer’s Association diagnostic criteria (Appendix C).1,22,26,27
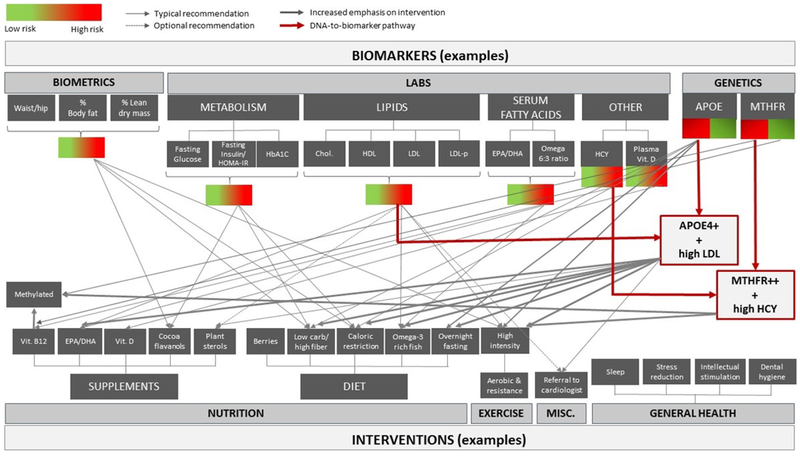
Enrolled participants were given individualized, multi-domain intervention recommendations informed by clinical and biomarker data (methods previously described)22, and received a mean of 21 recommendations by a neurologist or family nurse practitioner (Figure 1). Categories of recommendations included patient education/genetic counseling, pharmacological approaches (medications/vitamins/supplements), non-pharmacological approaches (customized recommendations for exercise, nutrition, vascular risk, sleep, cognitive engagement, stress, general medical care), and others based on methods previously published.22 Longitudinal follow-up occurred every 6-months with continual refinement of interventions for each participant. Upon follow-up, each participant was assessed as “compliant” or “not compliant” with each individual recommendation. A compliance score was calculated as a percentage of recommendations adhered to on a scale of 1–10 (1 represents 0–10% of recommendations, etc.) as independently assessed by two clinicians based on patient report at the visit and patient Likert-scale ratings. Clinicians then assigned an overall compliance score by consensus before review of any follow-up data. Higher-compliance participants were pre-specified as following >60% of all recommendations given, versus lower-compliance participants (≤60%).28
Figure 1: Example Biomarker to Intervention Paradigm.
NOTE. Each data point collected during the initial clinical intake and evaluation, as well as at each follow-up visit, is used to inform which precision medicine interventions are recommended per participant.
As an example of the application of the previously published method of an individualized clinical approach, a peri-menopausal 59-year-old woman (apolipoprotein E4 [APOE ε3/ε4] heterozygote) without subjective cognitive complaints and a past medical history of untreated “borderline” hypertension (~140s/80s), hyperlipidemia and abdominal obesity, elevated waist-to-hip ratio (.93), elevated visceral body-fat, insulin resistance, elevated homocysteine and normal (albeit sub-optimal) memory function received 25 individualized recommendations.22 These included patient education about potential risks/benefits of long-term hormone replacement therapy, genetic counseling, referral to a preventative cardiologist for blood pressure control (goal 120s/70s) and consideration of a coronary calcium scan for cardiovascular risk stratification, exercise counseling including a targeted amount/type of aerobic-versus-resistance training (geared for body-fat reduction), nutrition advice centered on Mediterranean-style diet (emphasis on fatty fish and extra-virgin olive oil consumption to address elevated LDL and low HDL-cholesterol), while limiting high-glycemic foods (considering insulin resistance) and optimizing B-complex (B12/folate/B6) vitamin intake (considering elevated homocysteine) and cocoa flavanols (considering insulin resistance, elevated blood pressure and lower-than-expected memory performance), as well as several other detailed recommendations such as sleep hygiene, cognitive engagement/training strategies, stress management, ongoing care with primary care physician (Figure 1), and information on AD prevention clinical trials which she may soon qualify for based on age/genotype.22 An introductory course on AD prevention (10 lessons, 2+ hours of interactive-multimedia content) that has been shown to increase knowledge and willingness to participate in AD prevention clinical trials is also recommended via the online learning portal AlzU.org.29 On follow-up, she was given a compliance score of 8 based on clinical consensus, and was thus classified as a higher-compliance Prevention participant (based on following 71–80% of the 25 recommendations).
Adverse events were recorded during each follow-up, with the treating clinician asking all participants whether they experienced any side effects/harm related to assigned interventions. Trial registered at ClinicalTrials.gov ().
2.3. Outcomes
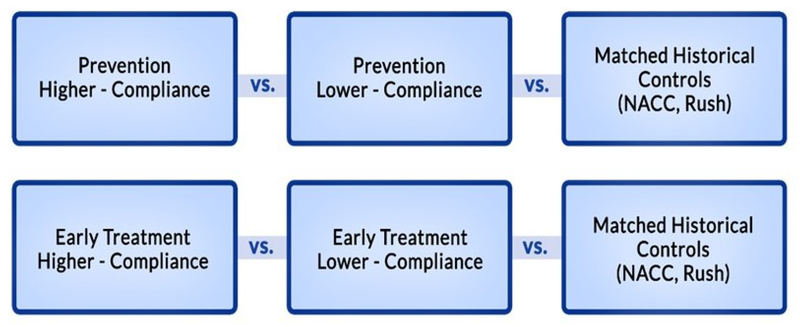
The primary outcome was change in performance on the modified Alzheimer’s Prevention Initiative Cognitive Composite (m-APCC) from baseline to 18-months.30 Statistical comparisons were performed between higher- and lower-compliance groups within each diagnostic classification and against matched historical control cohorts: National Alzheimer’s Coordinating Center (NACC) and Rush University Memory and Aging Project (Rush) (Figure 2).
Figure 2: Comparison Groups.
NOTE. Participants were classified to reflect the different biological phases along the AD continuum (Figure S1) and level of compliance into one of the following four analysis groups: Higher-compliance Prevention, Lower-compliance Prevention, Higher-compliance Early Treatment, and Lower-compliance Early Treatment. Each group was compared to two matched historical control cohorts, NACC and Rush (n=38836 and n=3289, respectively)
The original APCC was empirically determined to document progression of preclinical cognitive decline related to AD progression, and was selected due to its concurrent use in two AD prevention clinical trials (Alzheimer’s Prevention Initiative Generation Program, Autosomal-Dominant AD Trial).31,32 Similar to other trials,33,34 we refined the APCC based on the selection of tests administered (Table S2 and prior publication of neuropsychological measures used in our clinic).24 Tests comprising the m-APCC were selected to represent the same cognitive domains as those used in the APCC.24
Secondary outcomes included changes on a composite of neuropsychological tests associated with non-pathological cognitive aging (CogAging, Appendix D), two AD risk scales (Australian National University–AD Risk Index [ANU-ADRI], Cardiovascular Risk Factors, Aging and Incidence of Dementia [CAIDE]), two cardiovascular risk scores (American College of Cardiology/American Heart Association [ACC/AHA], Multi-Ethnic Study of Atherosclerosis [MESA]), and risk biomarkers (Table S1).35–38
See Table S1, S9–S12/Appendix E for exploratory outcomes/results.
2.4. Statistical Analyses
2.4.1. General
Participants were classified based on clinical diagnosis and level of compliance (Figures 2, S1). Two-sided P-values were used for all comparisons with no correction for multiplicity due to the a priori intent to investigate the primary outcome separately within the diagnosis groups. Secondary analyses may be considered hypothesis-generating and not confirmatory.
2.4.2. Mixed Model Repeated Measures (MMRM)
Change from baseline in all outcomes was analyzed at 6, 12, and 18 months for the Full Analysis Set (FAS) using MMRM that included all available data for participants with at least one follow-up visit. Least squares mean (LSMEANs) estimates at each visit were reported and groups were compared with least squares differences (LSDIFFs). The primary model included diagnostic classification (Prevention/Early Treatment) and compliance (Lower/Higher) with Diagnosis×Compliance interaction, as well as age, baseline score, baseline Mini-Mental State Examination, and visit. LSMEANS estimates from the Diagnosis×Compliance interaction are shown for the primary analysis. The interaction between quantitative compliance and diagnosis group was used to assess whether compliance affected diagnosis groups differently. SAS® V9.4 PROC MIXED was used.
2.4.3. Historical Comparison
NACC (n=38836) and Rush (n=3289) were the two data repositories used to derive comparisons (as neither cohort received therapeutic interventions). See Table S3 for demographic comparisons. Participants were matched for age and m-APCC score at baseline within diagnosis category (Appendix F). However, MCI diagnoses in each cohort were not amyloid-confirmed unlike our cohort. Since the NACC dataset had APOE genotype, additional analyses were performed in APOE4 carriers which were matched as a proxy for increased likelihood of amyloid positivity and potentially more comparable rates of decline to our amyloid-confirmed participants.39,40 The Rush cohort included data from the Religious Orders Study, Memory and Aging Project, and Minority Aging Research Study.41,42 Since the youngest Rush participant was >50, only our participants aged 50+ were used for this comparison in addition to using age for matching.
2.4.4. Compliance Adjusted Model
Since participant characteristics may affect compliance levels, predictors of compliance were assessed by fitting a stepwise regression model, with compliance as the outcome variable, and including APOE-e4 carrier status, age, gender, diagnostic classification, baseline cognitive scores, baseline blood biomarkers, baseline biometrics, and baseline risk scores as predictors. To assess the specific impact of compliance, significant baseline predictors of compliance (at α<.05) were identified and corrected for as covariates in the adjusted MMRM, which also included a term for Baseline×Time interaction. This adjusted model is compared to the primary model in Table 1.
Table 1:
Comparison of Prevention vs. Treatment Groups for Lower- vs. Higher-Compliance
| Compliance | Lower- | Higher- | Higher- vs. Lower- | ||||
|---|---|---|---|---|---|---|---|
| Prevention | |||||||
| Mo. 18 | 4.5 (1.2) | 4.6 (0.8) | 0.1 (1.4) | ||||
| Mo. 18 | 4.5 (1.2) | 4.7 (0.9) | 0.2 (1.3) | ||||
| Mo. 18 | −3.4 (0.8) | −2.6 (0.6) | 0.8 (1.0) | ||||
| ANU-ADRI | Mo. 6 | −1.2 (0.6) | −2.8 (0.5) | −1.6 (0.8) | |||
| CAIDE | Mo. 18 | 0.0 (0.1) | −0.1 (0.1) | 0.0 (0.2) | |||
| ACC/AHA | Mo. 18 | −2.8 (0.4) | −3.8 (0.4) | −0.9 (0.4) | |||
| MESA | Mo. 18 | −1.4 (0.1) | −1.7 (0.2) | −0.3 (0.2) | |||
| Early Treatment | |||||||
| Mo. 18 | −6.0 (2.4) | 4.8 (3.0) | 10.8 (3.1) | ||||
| Mo. 18 | −7.6 (3.1) | 3.9 (3.2) | 11.5 (3.5) | ||||
| Mo. 18 | 5.9 (1.8) | −2.0 (2.3) | −7.9 (2.2) | ||||
| ANU-ADRI | Mo. 6 | −3.9 (1.7) | −5.9 (2.1) | −2.0 (1.5) | |||
| CAIDE | Mo. 18 | −0.7 (0.3) | −0.9 (0.3) | −0.1 (0.4) | |||
| ACC/AHA | Mo. 18 | −13.0 (2.4) | −10.4 (3.0) | 2.6 (3.0) | |||
| MESA | Mo. 18 | −2.7 (1.0) | −2.7 (0.7) | 0.1 (0.9) | |||
2.4.5. Exploratory Analyses
Change in each AD-risk biomarker was assessed for correlation with m-APCC and CogAging to assess whether biomarker improvements were associated with corresponding improvements in cognition.
3. Results
3.1. Disposition
202 patients were screened via telephone and were scheduled for an in-person evaluation. Of these, 10 scheduled a visit but did not come and 18 did not meet inclusion/exclusion criteria (7 excluded due to clinical diagnosis of MCI or early mild dementia with negative amyloid imaging, 8 due to clinical diagnosis of mild to moderate AD, 2 due to history of major depression, and 1 due to diagnosis/ongoing treatment of multiple myeloma. Of the remaining 174 patients (ages 25–86), all were assigned interventions (Table S4). 154 participants (88.5%) had at least one post-baseline assessment and were included in the FAS analysis (Figure S2). Study discontinuation rate was 22.1% at 12-months and 26.6% at 18-months (Figure S2/Table S4). Of those allocated to treatment, 24 (15.6%) discontinued because the treating physician left the practice (relocation), while 17 (11.0%) were lost to follow-up. See Table S4 for disposition at each time-point.
3.2. Demographics and Baseline Characteristics
Baseline characteristics are reported in Table 3/Appendix G. There were no differences at baseline between the 20 participants who were assigned interventions but did not follow-up compared to those with at least one post-baseline assessment (Table S5). Of those who followed-up, >20% were born outside the United States and over one-third reside outside the New York-metropolitan area. Higher- and lower-compliance early treatment participants exhibited significant differences in m-APCC and CogAging at baseline, with no differences between Prevention compliance groups.
Table 3: Patient Demographics and Baseline Characteristics*.
NOTE: Denominator for percentages is the number of subjects with observed data for the variable within each category. SD=Standard Deviation, Min=Minimum, Max=Maximum. Due to missing data, sample sizes for the cognitive outcomes listed in Table 1 ranged from 110–119 in the prevention group and 32–34 in the treatment group at baseline.
| Prevention | Early Treatment | |||||
|---|---|---|---|---|---|---|
| Variable | Subcategory or statistic | Lower- Compliance | Higher- Compliance | Lower- Compliance | Higher- Compliance | Total N=154 |
| Male | 17 (31.5%) | 32 (49.2%) | 12 (60%) | 7 (46.7%) | 68 (44.2%) | |
| Subjective Cognitive Decline | 17 (31.5%) | 17 (26.2%) | 34 (22.1%) | |||
| Age>Median (61) | ||||||
| Non-Carriers | 28 (52.8%) | 34 (52.3%) | 5 (25%) | 8 (53.3%) | 75 (49%) | |
| Missing | 3 (5.6%) | 2 (3.1%) | 3 (15%) | 3 (20%) | 11 (7.1%) | |
| Diff. (p-value) | 3.67 (0.0906) | 1.28 (0.6019) | ||||
| Diff. (p-value) | 0.26 (0.6971) | 0.93 (0.5374) | ||||
| Diff. (p-value) | 0.16 (0.5822) | 0.33 (0.6779) | ||||
| Cognitive Scores | ||||||
| Diff. (p-value) | 1.25 (0.4595) | 12.95 (0.0035) | ||||
| Diff. (p-value) | 1.47 (0.2271) | 6.26 (0.0400) | ||||
| Diff. (p-value) | 0.17 (0.4050) | 1.27 (0.1255) | ||||
| Risk scores | ||||||
| Diff. (p-value) | 2.05 (0.2024) | 6.68 (0.2536) | ||||
| Diff. (p-value) | 0.46 (0.7829) | 1.68 (0.6765) | ||||
| CAIDE | Diff. (p-value) | 0.30 (0.5155) | 0.35 (0.5985) | |||
| Diff. (p-value) | 1.29 (0.0220) | 1.58 (0.5467) | ||||
| Biomarkers | ||||||
| Diff. (p-value) | 0.02 (0.4833) | 0.02 (0.8493) | ||||
| Diff. (p-value) | 13.83 (0.2329) | 19.21 (0.5059) | ||||
| Diff. (p-value) | 0.08 (0.2130) | 0.25 (0.0127) | ||||
| Diff. (p-value) | 3.78 (0.2764) | 3.70 (0.7072) | ||||
| Diff. (p-value) | 0.14 (0.7531) | 0.51 (0.5947) | ||||
| Diff. (p-value) | 0.26 (0.4109) | 0.63 (0.4564) | ||||
| Diff. (p-value) | 0.09 (0.8616) | 2.02 (0.7010) | ||||
| LDL Cholesterol Direct | Diff. (p-value) | 13.64 (0.0657) | 17.19 (0.4207) | |||
| Diff. (p-value) | 4.36 (0.6813) | 0.67 (0.9628) | ||||
| Diff. (p-value) | 13.04 (0.1757) | 22.0 (0.2388) | ||||
| Diff. (p-value) | 3.03 (0.2357) | 3.95 (0.3865) | ||||
| Biometrics/vital signs | ||||||
| Body fat percentage | Diff. (p-value) | 1.08 (0.4987) | 1.32 (0.6271) | |||
| Dry lean mass percentage | Diff. (p-value) | 0.67 (0.1400) | 0.10 (0.8978) | |||
| Diff. (p-value) | 0.00 (0.7967) | 0.06 (0.2912) | ||||
| Diff. (p-value) | 1.07 (0.6422) | 0.51 (0.8880) | ||||
| Diff. (p-value) | 3.61 (0.2236) | 5.58 (0.3828) | ||||
| Diastolic blood pressure | Diff. (p-value) | 2.81 (0.2057) | 4.14 (0.2935) | |||
One patient declined APOE testing.
Serum biomarkers differed between higher- and lower-compliance Early Treatment groups only for glycated hemoglobin (HbA1c), and none between Prevention groups. Biometric baseline values were similar between higher- and lower-compliance groups in Prevention and Early Treatment (Table 3).
3.3. MMRM for Primary Outcome - m-APCC
3.3.1. Compliance by Diagnosis Group (Prevention vs. Treatment) Interaction (Figure 3)
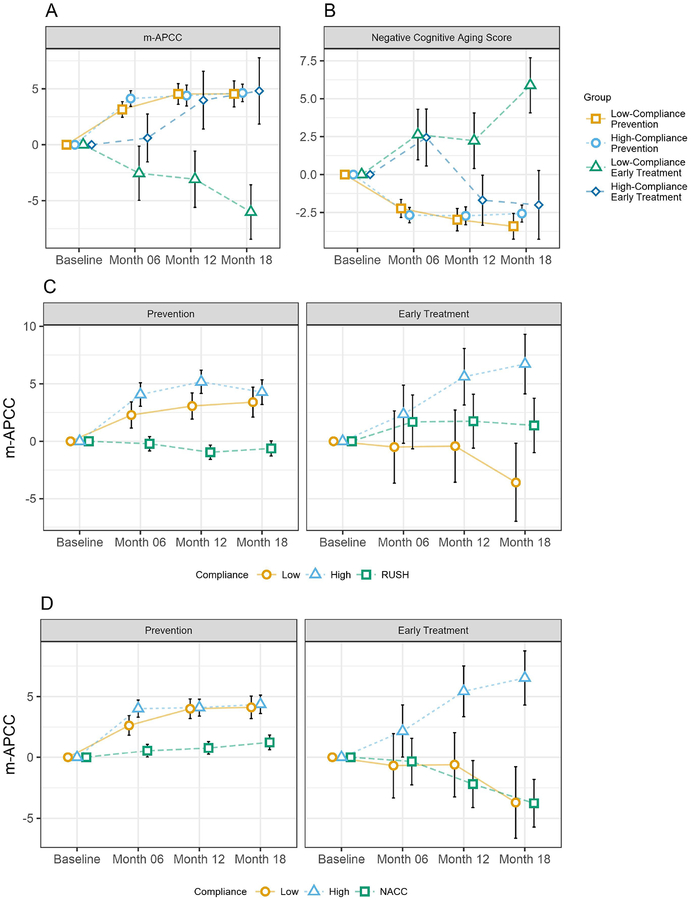
Figure 3: m-APCC(a) and Cognitive aging(b), NACC comparison(c), and Rush comparison(d).
NOTE: A) Change from Baseline on the m-APCC at 18 months amongst the four diagnosis x compliance groups. B) Change from Baseline on the non-pathological CogAging composite at 18 months amongst the four diagnosis x compliance groups. C) Comparison of change in m-APCC between higher-compliance, lower-compliance, and Rush control (matched for baseline m-APCC and age). D) Comparison of change in m-APCC between higher-compliance, lower-compliance, and NACC control (matched for baseline m-APCC and age)
In Prevention participants, higher- and lower-compliance groups showed significant improvements by 4.6 (95% CI=3.09–6.19, P<.0001) and 4.5 (2.24–6.84, P=.0002) points on the m-APCC, respectively. There was no difference between these groups (−2.79–2.61, P=.9488). In Early Treatment participants, the higher-compliance group increased by 4.8 points but this was not significant (−1.06–10.67, P =.1073). The lower-compliance Early Treatment group had significant worsening by 6.0 points (−10.83, −1.20, P =.0148). The difference between these groups (10.8 points) was significant (4.67–16.97, P =.0007).
3.3.2. Historical Comparison for the Primary Outcome (Table 2)
Table 2:
m-APCC comparison to Historic Controls
| Difference Between Groups in Change from Baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Statistic | Higher- Compliance | Higher- vs. Lower- Compliance | Higher- vs. Historic | ||||
| Prevention | ||||||||
| p-value | <0.0001 | 0.0385 | 0.8343 | 0.0012 | ||||
| p-value | 0.0092 | 0.343 | 0.5945 | <0.0001 | ||||
| Early Treatment | ||||||||
| p-value | 0.2064 | 0.0546 | 0.0011 | <0.0001 | ||||
| p-value | 0.2946 | 0.5611 | 0.0041 | 0.0428 | ||||
The higher-compliance Prevention group improved more than NACC by 3.1 (1.14–5.06, P=.0012) and Rush by 4.9 (2.55–7.25, P<.0001). The lower-compliance Prevention group improved more than NACC by 2.9 (0.74–5.06, P=.0088) and Rush by 4.0 (1.26–6.74, P=.0055). The higher-compliance Early Treatment group improved more than NACC by 10.3 (5.99–14.61, P<.0001) and Rush by 5.3 (0.20–10.40, P=.0428). Lower-compliance Early Treatment did not differ from NACC (P=.9820) or Rush (P=.1115).
See Table S6 for additional analyses matching our amyloid confirmed participants to enriched NACC participants who were APOE4 carriers.
See Figure S4 for additional details.
3.4. Adjustment for Baseline Factors Predictive of Compliance
3.4.1. Predictors of Compliance
The baseline compliance model identified three baseline parameters that significantly predicted compliance: baseline HbA1c (P<.0001), baseline ACC/AHA risk score (P<.0001), and baseline homocysteine (P=.0225). Each extra percentage of baseline HbA1c predicted a 32.5 percentage point increase in compliance on average. An increase of 10 points on the ACC/AHA risk scale predicted a 7 percentage point decrease in compliance on average. An increase of 1 μmol/L of homocysteine at baseline predicted a 2 percentage point increase in compliance on average. The interaction analysis for quantitative compliance and diagnosis resulted in a statistically significant interaction for compliance by diagnosis (p=0.0049) and compliance by diagnosis by visit (p=0.0003). Each extra point of compliance (complying with an additional 10% of recommendations) results in 0.06 point improvement in APCC at 18 months within the non-MCI group (p=0.8547), and 2.41 points of improvement in the MCI group (p=0.0003).
The adjusted model resulted in notably similar estimates of change on the m-APCC (see m-APCC[adjusted] in Table 1), suggesting that differences in m-APCC performance for lower- and higher-compliance groups were not explained by baseline characteristics predictive of compliance or differing rates of progression depending on baseline scores.
3.5. Secondary endpoints
3.5.1. Cognitive Aging Changes (Non-AD Specific)
For Prevention participants, CogAging showed a mean improvement of 2.6 (0.6) years for the higher-compliance group (1.47–3.67, P<.0001) and 3.4 (0.8) years for the lower-compliance group (1.73–5.09, P<.0001) (difference=−0.8 (−2.84–1.16, P=.4069)). Early Treatment participants improved by 2.0 (2.3) years in CogAging for the higher-compliance group but the change was not significant (−2.48–6.48, P=.3786), and worsened by 5.9 (1.8) years for the lower-compliance group (2.3–9.48, P=.0015) (difference=7.9 (3.52–12.26, P=.0005)).
3.5.2. Risk Scales (Table 1 and Figure S5).
For ANU-ADRI at 6-months, Prevention decreased by 2.8 (0.5) points for higher-compliance (1.76–3.75, P<.0001) and decreased by 1.2 (0.6) for lower-compliance (0.01–2.35, P=.0480) (difference=1.6 [−0.01–3.15, P=.0508]). Early Treatment decreased by 5.9 (2.1) for higher-compliance (1.73–10.11, P=.0060) and decreased by 3.9 (1.7) for lower-compliance (0.52–7.27, P=.0240) (difference=2.0 [−0.87–4.92, P=.1695]).
For CAIDE at 18-months, Prevention decreased by 0.1 (0.1) points for higher-compliance (−0.14–0.25, P=.6053) and did not change 0.0 (0.1) for lower-compliance (−0.26–0.33, P=.8247) (difference=0.0 [−0.33–0.37, P=.9177]). Early Treatment decreased by 0.9 (0.3) for higher-compliance (0.19–1.53, P=.0120) and decreased by 0.7 (0.3) for lower-compliance (0.14–1.35, P=.0170) (difference =0.1 [−0.59–0.83, P=.7389]).
For ACC/AHA cardiovascular at 18-months, Prevention decreased by 3.8 (0.4) points for higher-compliance (3.05–4.49, P<.0001), and decreased by 2.8 (0.4) for lower-compliance (2.06–3.60, P<.0001) (difference=0.9 [0.08–1.79, P=.0317]). Early Treatment decreased by 10.4 (3.0) for higher-compliance (4.54–16.30, P=.0006) and decreased by 13.0 (2.4) for lower-compliance (8.20–17.78, P<.0001) (difference=2.6 [−3.28–8.42, P=.3867]).
For MESA at 18-months, Prevention decreased by 1.7 (0.2) points for higher-compliance (1.39–1.99, P<.0001) and decreased by 1.4 (0.1) for lower-compliance (1.17–1.64, P<.0001) (difference=0.3 [0.04–0.61, P=.0891]). Early Treatment decreased by 2.7 (0.7) for higher-compliance (1.37–3.95, P<.0001) and decreased by 2.7 (1.0) for lower-compliance (0.73–4.68, P=.0076) (difference=0.1 [−1.73–1.86, P=.9557]).
3.5.3. Serum Risk Biomarkers
In Prevention participants, improvements were found in HDL-C (6.0, P<0.0001), hs-CRP (−1.3, P<0.0001), adiponectin (2.1, P<0.0001) and 25-hydroxy-vitamin D (4.5, P=0.0010). In Early Treatment participants, fibrinogen (−40.2, P=0.0269), homocysteine (−1.0, P=0.0416), HDL-C (10.0, P=0.0095), hs-CRP (−1.8, P=0.0006), adiponectin (5.1, P=0.0001) and Lp(a) Mass (14.6, P=0.0035) improved. No biomarker changes were significantly correlated with either change in m-APCC or change in CogAging across all patient groups, with the exception of cystatin C. A worsening in cystatin C of 0.1 point corresponded to greater improvement in CogAging by 1.2 years (P=0.0227). Table S7 shows the mean change in biomarkers from baseline to 18 months. These changes were compared between the diagnostic groups and correlated with change in cognitive outcomes. See Table S7 for all secondary and exploratory biomarker endpoints.
3.6. Safety Analysis
No serious adverse events were reported. Intervention-related adverse events occurred in 9.1% of participants (5.9% Prevention, 20% Early Treatment) (Table S8), including gastrointestinal complaints, myalgia/arthralgia, ankle sprain, irritability, insomnia, lethargy, fatigue, somnolence, nightmares, and anxiety (each <2%).
4. Discussion
To our knowledge, this is the first empirical trial in a clinical setting indicating that individualized AD risk factor management may improve cognitive function which may be related to AD pathology. In addition, secondary analyses demonstrated that multi-domain tailored interventions may reduce calculated AD and cardiovascular risk scores across a broad range of ages and diagnostic classifications, and may potentially have a cognitive-aging-modifying effect on non-pathological age-related cognitive decline. Within the Early Treatment group, cognitive improvements were seen only in the higher-compliance group, suggesting that close adherence to the interventions is needed to derive benefit within the context of definitive AD pathology. However, cognitive improvements were seen in both the higher- and lower-compliance Prevention participants, with both compliance groups demonstrating improvements compared to historical cohorts. Further, our population was easily and quickly recruited from a real-world clinical setting and the interventions were well-tolerated, adding to the translational value for practice (Appendix J). Additionally, while socio-economic factors will differ among varied cohorts, intervention-related costs negatively impacted adherence in 7.1% of participants (Appendix K).
Because m-APCC measures AD-related cognitive change, improvements may have resulted from targeting risk factors that lead to AD pathogenesis; however, direct evidence of changes in these pathways was not obtained. Additional evidence from longitudinal volumetric magnetic resonance imaging, fludeoxyglucose-PET and amyloid-PET would demonstrate whether observed improvements were related to disease modification. Neuroimaging data is forthcoming from a brain imaging substudy (n=135) begun in 2018.9 Furthermore, longitudinal measurement of potential key AD-related biomarkers, such as those related to neuroinflammation and synaptic dysfunction, may be incorporated into future studies to investigate the direct effects on AD pathology.
Cognitive aging composites indicated that the estimated delay of cognitive decline may be approximately three years in Prevention participants and two years in the higher-compliance Early Treatment group. Improvements in cognitive decline related to non-pathological cognitive aging may potentially be linked to reducing vascular dementia risk and/or targeting other factors (e.g., synaptic plasticity, alterations in neuronal structure, dysfunction of neuronal networks).43 However, due to lack of cognitive aging biomarkers, biological factors related to this potential response were not measured and are thus unclear. Treatment effects observed using both cognitive composite measures may suggest that treatment response is broad. Therefore, addressing risk factors that collectively impact overall health may assist in mitigating age-related cognitive decline, along with other potential health benefits stemming from treating comorbidities (e.g., cardiovascular risk).
We observed improvements in several AD-risk biomarkers. In Prevention participants, improvements were found in HDL-C, hs-CRP, adiponectin, and 25-hydroxy-vitamin D. In Early Treatment participants, fibrinogen, homocysteine, HDL-C, hs-CRP, adiponectin, and Lp(a) improved. However, none of these correlated with improvement in cognition. Unexpectedly, a worsening of cystatin C of 0.1 point corresponded to an improvement in CogAging. One possible explanation for lack of correlations between biomarkers and cognition is that such relationships likely involve multiple biomarker changes that may vary by person, as well as varied baseline values within a broad spectrum of reference ranges. A bayesian hierarchical analysis also did not identify an individual biomarker or category of biomarkers that was primarily associated with observed cognitive changes. See Appendix H/I for discussion.
While further study incorporating a host of biomarkers pre- vs. post-intervention may help to inform causality, we observed changes in efficacy outcomes such as serum biormarkers, anthropometrics and risk scales (Figure S6). These changes, along with the comparison of compliance groups, correction of multiple covariates, and matched historical comparisons, may suggest that these findings were potentially driven by the prescribed interventions.
Traditionally, treatment trials have attempted to isolate one effect at a time for single interventions, but the complexity of AD may require targeting multiple mechanisms simultaneously to affect disease progression. Our initial evidence of broad effects across risk scales, and measurements of cognition and biomarkers changing in expected directions, suggest this approach warrants further rigorous study.
Our study has several limitations. Our key limitation is the lack of a concurrent, randomized control group. Two considerations led to this design. A true control group may not have been possible, since well-informed participants actively enrolled in an AD risk reduction study may seek out and make lifestyle and/or other behavioral changes that impact outcomes. Additionally, given the setting of a real-world clinical practice where patients seek AD risk reduction care for modifiable risk factors in a clinic outside of a traditional solely research environment, it would not be feasible to withold treatment from a non-intervention randomized control group.
The disadvantage of an uncontrolled study is that it is unclear whether observed effects are due to baseline characteristics of participants or other aspects of general study conduct unrelated to treatment. In an attempt to mitigate these effects, we corrected the model for baseline predictors of compliance by including them as covariates to better ensure the improved outcomes were not primarily due to baseline characteristics. We also used historical comparison cohorts with similar demographics and matched each participant based on age and baseline m-APCC. We used historical comparisons to also help account for study procedure effect, such as practice effects. Compared to these matched historical comparisons, participants demonstrated greater improvements at similar time-points. Because these historical comparison groups were not part of any intervention, a response associated with intervention expectations may potentially explain part of the cognitive benefit that was observed in our study. However, the 18-month duration is longer than is usually expected for this type of effect. Furthermore, improvements found in lab biomarkers and AD and CV risk scales are less likely to be influenced by placebo effects.44 Future studies which include randomized non-intervention groups would allow for more definitive conclusions.
Because few NACC participants and no Rush participants had available amyloid biomarkers, we were unable to match on confirmed AD pathophysiology. After updating the matching algorithim to include APOE4 positivity as an enrichment strategy for NACC participants, our cohort continued to show cognitive benefit. While the lack of amyloid biomarkers is an important limitation, we would have expected the rate of cognitive decline in historical subjects without amyloid-confirmation to be slower than a matched population with amyloid confirmation, resulting in a more conservative estimate of the intervention effect. Unexpectedly, when enriching for APOE4, we observed less decline in the enriched population. Another limitation stems from the study environment of a real-world clinical practice and the challenges of rating compliance. There is a paucity of evidence on how to use compliance in comparative effectiveness studies as an outcome to differentiate treatment effects. While some studies have defined high compliance as following two-thirds of prescribed recommendations, we selected 60% for two reasons.45,46 An initial motivation was that a cut-point of 60% led to a roughly even number of patients in higher- and lower-compliance categorizations when care was previously provided in the clinic (from 2013–14 prior to initiating the comparative effectiveness study). Further, we applied categorizations from a prior study quantifying compliance into 4 groups: noncompliant (compliant to treatment schedule less than 20% of the time), low (20% to 59% of the time), moderate (60% to 79% of the time), and high (≥80% of the time) compliance.28 Based on this study framework, we divided our participants into higher (≥60%) and lower (<60%) compliance groups. See Appendix K/Table S13 for additional information.
It is important to note that because lower compliance is often related to disease severity, statistical corrections for baseline m-APCC, HbA1c, homocysteine, AHA/ACC, and age were applied to decrease the possibility of bias due to these potential confounders. Further, the separation of diagnosis and compliance groups was critical due to a strong compliance by diagnosis interaction effect.
While our sample size was modest and further stratification led to relatively small diagnostic and compliance groups, observed effects seen were of a large enough magnitude to still be detectable. Continued recruitment across additional sites globally (n=1000 planned) will allow for confirmation of these proof-of-concept results and more detailed analysis of patient subgroups (e.g., age, ethnicity), biomarkers, and intervention approaches. Expanded recruitment may enable deeper understanding of precision effects and more definitive conclusions, and allow assessment of the impact of medical comorbidities and concomitant medications.
Practice effects due to repeated cognitive test exposure is another potential concern. To mitigate this, we administered alternate test forms at each time-point and required that participants complete simulated at-home cognitive assessments prior to baseline. This also primed participants to testing conditions/procedures and mitigated test anxiety in effort to reduce practice effects. Also, practice effects on cognitive measures tend to occur at briefer test-retest intervals than those involved in this study, and the comparison to historical cohorts who took related measures repeatedly demonstrated improvement beyond what can reasonably be explained by practice effects.47
While the m-APCC was our primary outcome, there is no gold standard for which cognitive measures should be used (and how often), and the degree of benefit which should be expected.48 Cognitively normal patients at-risk may have a lower ceiling for benefit as they do not yet manifest cognitive decline. As such, assessment scales cannot be easily repeated from prior treatment trials, and novel composite measures sensitive to pre-dementia decline may hold promise.24,48 Because the study was conducted in the real-world clinical setting and one of the treating clinicians left the practice due to geographical relocation, 24 participants (58.6%) were lost to follow-up for this reason. Future studies should consider safeguards to account for similar factors that can substantially influence discontinuation rate. However, because the major contributing factor to discontinuation would not be expected to be related to response to treatment, it may be less likely that loss of these patients introduces bias in our results.
Furthermore, while patients who seek risk reduction care tend to be highly motivated, this approach may not be as effective in patient populations with lower motivation. Further discussion about factors related to compliance are detailed in Appendix K. Also, despite the study’s translational value, long-term effects are unknown. Longitudinal assessments are ongoing. Additionally, while the median age in our cohort was 61 years, and the mean age was 60 years, our cohort included a broad age-range due to younger, middle-aged, and older patient demand in a real-world clinical setting. Nevertheless, the majority of participants (~75%) were aged 50 years or older. Age was included as a continuous linear covariate in the primary model, and as such, all estimated changes were for an average aged person (60 years old). The Prevention group had 0.1 points less improvement per year of age, and the Early Treatment group had 0.2 points less improvement per year of age. As such, an older population may demonstrate less improvements in cognition and, similar to AD drug trials, this intervention may be more effective in younger and/or less impaired populations. Future studies are warranted to more deeply understand age effects of this intervention.
With a clear understanding of these limitations, we offer this framework as a tool for clinicians across a broad range of subspecialties, and clinician researchers, to approach patient care while further clarifying its effectiveness (see Figure S7 to visualize levels of personalization). Given the magnitude of disease, significant morbidity of late-life dementia, and growing interest in applying preventative neurology to clinical care, it is important to report these findings as larger studies are developed and while our own sample size grows. Overall, these results help extend prior RCT/observational findings into a clinic setting where individualized lifestyle modifications produced measurable benefits.
5. Conclusion
From a practical clinical perspective, individualized AD risk factor management maybe applied for care to tens of millions of patients at-risk for AD dementia.
Our study adds to the body of comparative effectiveness research by applying the same framework across distinct pre-dementia diagnostic classifications, and provides a reproducible model for future research.
Further study in a large, multi-site, international cohort study, merits consideration.
Supplementary Material
Research in Context.
Systematic Review
Authors searched ClinicalTrials.gov and World Health Organization’s International Clinical Trial Registry Platform to identify multidomain precision medicine intervention studies to delay cognitive decline in patients at-risk for AD. Search terms were “prevention of dementia OR prevention of Alzheimer’s” and “precision medicine OR personalized medicine.” While several randomized controlled trials utilizing multidomain interventions were found, no completed precision medicine studies were identified. One recruiting study investigating an individualized intervention () was found yet results are not available.
Interpretation
To our knowledge, this is the first empirical trial to demonstrate individualized multidomain interventions may improve cognitive function and reduce AD/cardiovascular risk scores in patients at risk for AD dementia in real-world clinical practice.
Future directions
Given the paucity of treatments and extended preclinical period, focus on AD risk reduction is essential. This study provides a feasible framework for studying AD risk reduction in clinical practice. Further research on individualized multi-domain interventions is warranted in larger cohorts across sites globally.
Acknowledgments
We are grateful to all the patients who participated in this study. We thank Dr. Islon Woolf, Dr. Arthur Agatston, Dr. Hannah Gardener, Dr. Tanja Rundek, Dr. Miia Kivipelto, Dr. Yakir Kaufman and Dr. Laurie Glimcher for their support and/or contributions to the development of our methodology and research program.
Funding
NIH/NCATS UL1TR002384 and NIH PO1AG026572; Zuckerman Family Foundation; Women’s Alzheimer’s Movement; Memories for Mary, Hilarity for Charity; Alzheimer’s Prevention Clinic patients.
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing the report. RSI, HH, NS, KH, SH, JM, JS, MF, PL, MT, CB, AR, SB, JMC, EC, GS, RC, PL, SD, MJH, OS, MM, MS, KN, CEG, PA, LM, RK had full access to all data in the study. The report was approved for submission by all authors. The corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors report no conflicts of interest or other relevant disclosures.
Data Sharing Statement
All de-identified data related to the specific outcomes of this study are sharable with a written data-sharing and publication agreement, and will be available (along with data dictionary) three months after publication. Requests can be made to Pentara Corporation at shendrix@pentaracorp.com. The study protocol, statistical analysis plan, and informed consent form will be available upon publication. Requests for these documents can be made to rii9004@med.cornell.edu. Neuroimaging data (e.g., MRI, FDG-PET, Amyloid PET) will be unavailable until full publication of those results in the future.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2011; 7(3): 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology 2010; 9(1): 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology 2013; 12(2): 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2018; 14(4): 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2018; 14(2): 121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobke B, McBride T, Nevin L, et al. Setbacks in Alzheimer research demand new strategies, not surrender. PLoS medicine 2018; 15(2): e1002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. The Lancet Neurology 2014; 13(8): 788–94. [DOI] [PubMed] [Google Scholar]
- 8.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet (London, England) 2017; 390(10113): 2673–734. [DOI] [PubMed] [Google Scholar]
- 9.Schelke MW, Attia P, Palenchar DJ, et al. Mechanisms of Risk Reduction in the Clinical Practice of Alzheimer’s Disease Prevention. Frontiers in aging neuroscience 2018; 10: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet (London, England) 2015; 385(9984): 2255–63. [DOI] [PubMed] [Google Scholar]
- 11.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2013; 9(6): 657–65. [DOI] [PubMed] [Google Scholar]
- 12.Rovner BW, Casten RJ, Hegel MT, Leiby B. Preventing Cognitive Decline in Black Individuals With Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA neurology 2018; 75(12): 1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal JA, Smith PJ, Mabe S, et al. Lifestyle and neurocognition in older adults with cognitive impairments. Neurology 2019; 92(3): e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical trials (London, England) 2014; 11(5): 532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council Committee on AFfDaNToD. The National Academies Collection: Reports funded by National Institutes of Health. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington (DC): National Academies Press (US) National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 16.What is precision medicine? - Genetics Home Reference - NIH. https://ghr.nlm.nih.gov/primer/precisionmedicine/definition (accessed Feb 20 2018).
- 17.Hampel H, O’Bryant SE, Castrillo JI, et al. PRECISION MEDICINE - The Golden Gate for Detection, Treatment and Prevention of Alzheimer’s Disease. The journal of prevention of Alzheimer’s disease 2016; 3(4): 243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampel H, O’Bryant SE, Durrleman S, et al. A Precision Medicine Initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric 2017; 20(2): 107–18. [DOI] [PubMed] [Google Scholar]
- 19.Reitz C. Toward precision medicine in Alzheimer’s disease. Ann Transl Med 2016; 4(6): 107-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schelke MW, Hackett K, Chen JL, et al. Nutritional interventions for Alzheimer’s prevention: a clinical precision medicine approach. Annals of the New York Academy of Sciences 2016; 1367(1): 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifan A, Isaacson R. The Alzheimer’s Prevention Clinic at Weill Cornell Medical College / New York - Presbyterian Hospital: Risk Stratification and Personalized Early Intervention. The journal of prevention of Alzheimer’s disease 2015; 2(4): 254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacson RS, Ganzer CA, Hristov H, et al. The clinical practice of risk reduction for Alzheimer’s disease: A precision medicine approach. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2018; 14(12): 1663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodes JF, Oakley CI, O’Keefe JH, et al. Alzheimer’s “Prevention” vs. “Risk Reduction”: Transcending Semantics for Clinical Practice. Frontiers in neurology 2018; 9: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackett K, Krikorian R, Giovannetti T, et al. Utility of the NIH Toolbox for assessment of prodromal Alzheimer’s disease and dementia. Alzheimer’s & dementia (Amsterdam, Netherlands) 2018; 10: 764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurology AAo. AAN guideline summary for clinicians: detection, diagnosis and management of dementia.
- 26.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2011; 7(3): 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2011; 7(3): 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutzelnigg A, Kopeinig M, Chen C-K, et al. Compliance as a stable function in the treatment course of bipolar disorder in patients stabilized on olanzapine: results from a 24-month observational study. International journal of bipolar disorders 2014; 2(1): 13-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaacson RS, Haynes N, Seifan A, et al. Alzheimer’s Prevention Education: If We Build It, Will They Come? www.AlzU.org. The journal of prevention of Alzheimer’s disease 2014; 1(2): 91–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Langbaum JB, Hendrix SB, Ayutyanont N, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s & Dementia 2014; 10(6): 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez Lopez C, Caputo A, Liu F, et al. The Alzheimer’s Prevention Initiative Generation Program: Evaluating CNP520 Efficacy in the Prevention of Alzheimer’s Disease. The journal of prevention of Alzheimer’s disease 2017; 4(4): 242–6. [DOI] [PubMed] [Google Scholar]
- 32.Tariot PN, Lopera F, Langbaum JB, et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimer’s & dementia (New York, N Y) 2018; 4: 150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malek-Ahmadi M, Chen K, Perez SE, Mufson EJ. Cerebral Amyloid Angiopathy and Neuritic Plaque Pathology Correlate with Cognitive Decline in Elderly Non-Demented Individuals. Journal of Alzheimer’s disease: JAD 2019; 67(1): 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G, Weinger JG, Lu ZL, Xue F, Sadeghpour S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Alzheimer’s disease: JAD 2016; 49(4): 971–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prevention science: the official journal of the Society for Prevention Research 2013; 14(4): 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2014; 10(5): 562–70. [DOI] [PubMed] [Google Scholar]
- 37.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. Jama 2014; 311(14): 1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). Journal of the American College of Cardiology 2015; 66(15): 1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012; 79(15): 1570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Annals of neurology 2010; 67(3): 308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. Journal of Alzheimer’s Disease 2018; 64(s1): S161–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Current Alzheimer research 2012; 9(6): 734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murman DL. The Impact of Age on Cognition. Seminars in hearing 2015; 36(3): 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews 2010; (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heesch KC, Mâsse LC, Dunn AL, Frankowski RF, Mullen PDJJoBM. Does Adherence to a Lifestyle Physical Activity Intervention Predict Changes in Physical Activity? 2003; 26(4): 333–48. [DOI] [PubMed] [Google Scholar]
- 46.Sjösten NM, Salonoja M, Piirtola M, et al. A multifactorial fall prevention programme in the community-dwelling aged: predictors of adherence. European Journal of Public Health 2007; 17(5): 464–70. [DOI] [PubMed] [Google Scholar]
- 47.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich HJBN. Practice effects in healthy adults: A longitudinal study on frequent repetitive cognitive testing. 2010; 11(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kivipelto M, Mangialasche F, Solomon A. Pointing the FINGER at multimodal dementia prevention – Authors’ reply. The Lancet 2015; 386(10004): 1627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.