Abstract
BACKGROUND:
Few studies have examined how adherence to antihypertensive medications varies across different regions or how neighborhood-level factors were related to individuals’ medication-taking behaviors in patients.
OBJECTIVE:
To explore local variation in medication adherence and examine environmental and individual influences on adherence to angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) among elderly hypertensive patients with chronic kidney disease (CKD) in the United States.
METHODS:
The Medicare 5% sample claim data (2006-2013), American Community Survey 5-Year Data (2005-2009) and the Health Resources and Services Administration Primary Care Service Area data (2007). The primary outcome was medication adherence, measured by Proportion of Days Covered (PDC). Geographically weighted regression (GWR) and linear mixed-effects models were used to investigate the relationship between environmental factors, individual risk factors and medication adherence.
RESULTS:
A total of 70,201 hypertensive CKD patients residing in 2,981 counties of the US were selected. Significant spatial autocorrelation was observed in ACEIs/ARBs PDC. The West North Central and New England regions demonstrated higher adherence compared to the East South Central and West South Central regions. Residing in Medically Underserved Areas, counties with high deprivation scores, and not receiving Part D Low-income Subsidy were associated with poor medication adherence.
CONCLUSIONS:
Medication adherence is geographically differentiated across the US. Environmental and individual factors identified may be helpful in the design of local interventions focused on improving patient outcomes from a population perspective.
Keywords: Medication adherence, Chronic Kidney Disease (CKD), geospatial analysis, environment, hypertension
Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) are recommended by practice guidelines as preferred anti-hypertensive agents for Chronic Kidney Disease (CKD) patients because of their additional protective renal benefits 1,2. Adherence to anti-hypertensive treatment is crucial for patients with hypertensive CKD, as poor medication adherence may result in uncontrolled blood pressure, and further, accelerate the rate of CKD progression and increase the risk of hospitalization, cardiovascular conditions, and death3-6. Previous research using nationally representative data has shown that approximately only one-third of CKD patients in the United States had their blood pressure under control7. Despite the importance of anti-hypertensive regimens, adherence to these agents remains suboptimal in this population. Previous studies of medication adherence have found that approximately 65% - 83% of CKD patients were adherent to their prescribed anti-hypertensive agents, while studies using self-report measures demonstrated somewhat better adherence rates than those using prescription refill measures (67%-83% versus 65%-70%)3-5,8,9.
Reasons for poor adherence to anti-hypertensive treatments in CKD patients vary from study to study and have been attributed to distinct characteristics of investigated medications and populations. For example, individuals’ social and demographic factors such as younger age, male sex, lower level of income and education were associated with increased risks of poor adherence in some studies but not in others3,4,8,10. With regards to patient health status factors, being depressed, having more hospitalizations, and unable to self-administer medications have been associated with poor adherence4,5,8. Inconsistent relationships between medication adherence and renal function have been observed in previous research5,10,11. Interview-based and survey-based studies have found that forgetfulness was the most common reason for nonadherence reported by CKD patients3,4,12. Adherence with anti-hypertensive treatments in CKD patients has shown to be influenced by other subjective factors, such as, patients’ perceived need for medication, perceived efficacy of medication, concerns about side effects, as well as physician-patient communication12,13. When treatment related characteristics were examined, medication side effects, complexity of regimens, and overall pill burden were associated with poor medication adherence8,14.
Although many studies have explored predictors of poor cardiovascular medication adherence, very few have examined how medication adherence varies across different regions or how neighborhood-level factors may be related to individuals’ medication-taking behaviors. A recently published study by Erickson et al. found geographical clustering in adherence to statins in the state of Michigan in the United States15. Similarly, another study by Hoang et al. observed spatial clustering in medication adherence among 1081 patients residing in southeastern Michigan who were discharged with acute coronary syndrome conditions16. A study by Couto et al. found that across the United States, adherence rates were highest in New England and the West North Central region, and followed by the East North Central and the Middle Atlantic region17, while the entire southern section of the United States, including the West South Central, the East South Central, and the South Atlantic region had relatively poor adherence. Moreover, similar geographical variation was observed in both Medicare beneficiaries and commercial insurance beneficiaries, and the variation was stable across different therapeutic drug classes (antidiabetics, antihypertensives, and antilipidemics). However, these studies did not investigate local characteristics that might contribute towards the geographic differences in medication adherence.
According to the Behavioral model of health services use proposed by Andersen, patients’ utilization of health care is influenced by not only characteristics of patients, but also environmental factors, such as structures of the health system and neighborhood socioeconomics18,19. Identifying environmental risk factors of medication nonadherence could be helpful in designing population-based strategies to impact health promotion. Therefore, the aim of this study was to explore local variations in medication adherence of ACEIs/ARBs, a commonly used class of recommended antihypertensive medications and examine environmental and individual influences on medication adherence. We hypothesized that medication-taking behaviors, defined in this study as adherence to prescribed ACEIs/ARBs, are associated with both patients’ individual characteristics and the characteristics of the neighborhood they live in. Moreover, we expected that the adherence rate of ACEIs/ARBs would vary across regions in the United States. It was also expected that the relationship between environmental factors and medication adherence would vary across the United States. In this study, a Geographically Weighed Regression (GWR) model was used to test the working hypotheses.
Methods
Data source
This study used the Medicare 5% sample files from the United States Renal Data System (USRDS) database. These files contain comprehensive information on demographic characteristics, Medicare enrollment status, diagnoses, procedures, and filled prescriptions for a random 5% sample of Medicare beneficiaries across the United States over time. To assess the relationship between environmental factors and medication-taking behaviors, Medicare claims were further merged with external data resources by state code and county code to obtain environmental information. Information from the U.S. Census Bureau American community Survey (ACS) 5-Year Estimates data (2009-2013) and the Health Resources and Services Administration (HRSA) Primary Care Service Area (PCSA) data (2007) were extracted to measure environmental characteristics and primary care resources at the county level. This unique linked dataset also included spatial information of population center, extracted from the Map of Centers of Population from the U.S. Census Bureau (2008)20. The information contained in the final dataset for statistical analyses in this study was completely de-identified.
Study design
A retrospective cohort study of Medicare Part D beneficiaries with hypertension and CKD in the United States was conducted. Elderly Medicare beneficiaries who had both diagnosis of hypertension and CKD during the period from 01/01/2006 to 12/31/2007 were selected in this study. Diagnosis of conditions were defined by the ICD-9 code in Medicare claims and following a previously validated algorithm: having at least one inpatient claims or at least two outpatient claims21. Eligible patients were followed at the beginning of the year 2008. To capture a complete history of medication use, the subjects were restricted to Medicare fee-for-service beneficiaries with continuous prescription drug coverage. Moreover, to assess the one-year medication adherence of ACEIs/ARBs, hypertensive CKD patients who did not have any ACEIs/ARBs claim, those whose first ACEIs/ARBs claim was after 12/31/2012, as well as those who died within one year period from their first ACEIs/ARBs claim, were excluded.
Measures
Medication-related outcome measures
One-year ACEIs/ARBs adherence was measured by PDC, which was defined as the proportion of days covered by ACEIs/ARBs in a fixed one-year refill interval, starting from the first date of dispensing ACEIs/ARBs. In this study, ACEIs and ARBs were treated as the same. A threshold of 80% PDC was applied to define good medication adherence, with higher PDC associated with greater adherence22.
Individual factors
Demographic information such as age, gender and race were extracted from the Medicare 5% files. Age was calculated at the baseline, and race was classified as white, black, Asian, others and unknown. Part D coverage was classified into three categories based on their enrollment of Low-income Subsidy (LIS) program in 2008, including Part D with deemed LIS, Part D with non-deemed LIS, and Part D without LIS. The LIS provides subsidies for monthly premium, annual deductible and copayments to help low income beneficiaries pay their prescription drugs. Patients who are dual eligible for Medicare and Medicaid can automatically enroll in LIS (Part D with deemed LIS), while the remaining eligible LIS beneficiaries whose income level is below 150% of the Federal Poverty Level need to apply to receive a subsidy (Part D with non-deemed LIS). Deemed LIS qualify for a 100% subsidy for the monthly premium, no deductible, and a lower fixed copay amount compared to non-deemed LIS. All LIS enrollees do not have a prescription coverage gap, also known as the “donut-hole” compared to Part D enrollees without LIS. Comorbid conditions were assessed at baseline following the same algorithm as diagnosis of hypertension and CKD. Separate indicators were generated for conditions associated with kidney dysfunction, including diabetes, cardiovascular conditions, and cardiovascular procedures. An adapted Deyo-Charlson comorbidity index (CCI) with exclusion of diabetes, renal and cardiovascular conditions was calculated23. A higher value of CCI indicated a heavier burden of illness.
Environmental factors
Characteristics of environmental factors were assessed at the county level. Socioeconomic status was measured by the Townsend deprivation index, a composite score derived from four census statistics: the percentage of households without car ownership and home ownership, the percentage of household overcrowding (more than one person per room), and unemployment rate among people aged 16 and over24. A higher Townsend index indicates a higher average level of deprivation. In addition, available primary care resources for each county were measured by the rate of general physicians per 10,000 persons and the percentage of the population residing in Medically Underserved Areas (MUAs). The MUAs are designated by HRSA as geographic areas with a shortage of primary care health services, high infant mortality, high poverty level and high proportion of older population. Percentage of residents covered by Medicare was also assessed as a measurement of predisposing characteristics in the county.
Statistical analyses
Study subjects were categorized into two groups based on their medication adherence: a PDC of at least 80% vs. a PDC below 80%. Patients with PDC above 80% were considered as being adherent to prescribed ACEIs/ARBs. Descriptive analyses of individual characteristics and environmental factors were conducted at baseline. The means and standard deviation for continuous variables and percentages for categorical variables are presented in Table 1. Group differences in these factors were examined using two-tailed t-tests for continuous variable and chi-square tests for categorical variables. A linear mixed effects model with normal distribution and identity link was used to assess the association between individual factors, environmental factors, and ACEIs/ARBs PDC, controlling for county random effects.
Table 1.
Descriptive Statistics of individual demographic characteristics and environmental factors among elderly patients with hypertension and chronic kidney disease (CKD) by status of ACEIs/ARRs adherence (n=70,201)
| Variable | PDC above 80% (n=42,733) |
PDC below 80%(n=27,468) |
All (n=70,201) |
|
|---|---|---|---|---|
| Baseline characteristics | Mean (SD)/% | Mean/% | Mean/% | |
| Age (years) | 78.6 (7.1) | 78.6 (7.1) | 78.6 (7.1) | |
| Age (years) | ||||
| <70 | 12.7 | 12.7 | 12.7 | |
| 70-75 | 22.7 | 22.7 | 22.7 | |
| 75-80 | 23.2 | 23.3 | 23.3 | |
| >80 | 41.4 | 41.4 | 41.4 | |
| Male | 38.9 | 41.3 | 39.9 | *** |
| Race | *** | |||
| White | 87.9 | 85.1 | 86.8 | |
| Black | 8.8 | 11.3 | 9.8 | |
| Asians | 1.7 | 1.6 | 1.7 | |
| Others | 1.5 | 1.8 | 1.6 | |
| Unknown | 0.1 | 0.1 | 0.1 | |
| Prescription coverage at baseline | *** | |||
| Part D without LIS | 64.3 | 63.8 | 64.1 | |
| Part D with non-deemed LIS | 2.4 | 2.1 | 2.3 | |
| Part D with deemed LIS | 33.3 | 34.2 | 33.7 | |
| Diabetes mellitus (DM) | 45.3 | 45.8 | 45.5 | |
| Charlson comorbidity index $ | 1.2 (1.7) | 1.4 (1.8) | 1.3 (1.7) | *** |
| Cardiovascular Comorbidities | ||||
| ASHD | 48.5 | 53.7 | 50.6 | *** |
| AMI | 11.4 | 13.7 | 12.3 | *** |
| CHF | 30.9 | 35.9 | 32.9 | *** |
| CVA-TIA | 29.7 | 33.3 | 31.1 | *** |
| PAD | 36.8 | 40.5 | 38.3 | *** |
| AFIB | 20.6 | 21.8 | 21.1 | *** |
| SCA/VA | 5.5 | 6.5 | 5.9 | *** |
| Other Cardiovascular diseases | 45.0 | 49.2 | 46.6 | *** |
| Cardiovascular Procedures | ||||
| PCI | 3.6 | 4.5 | 3.9 | *** |
| CABG | 1.3 | 1.4 | 1.4 | |
| ICD/CRT-D | 0.8 | 1.0 | 0.9 | ** |
| Environmental Factors | ||||
| Number of General physicians per 10,000 persons | 8.3 (3.2) | 8.3 (3.2) | 8.3 (3.2) | ** |
| Percent of Medicare Beneficiaries in Total Population | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | *** |
| Proportion of People Residing in Medically Underserved Areas | 0.4 (0.4) | 0.4 (0.4) | 0.4 (0.4) | ** |
| Deprivation score# | 1.5 (3.9) | 1.8 (4.0) | 1.6 (3.9) | *** |
p<0.05,
p<0.01,
P<0.001
Adapted Charlson comorbidity index was calculated by excluding diabetes, kidney diseases and cardiovascular diseases
Deprivation score was measured by Townsend index, which incorporates unemployment rate, percentage of households without care ownership, home ownership and overcrowding.
Abbreviations: ASHD, atherosclerotic heart disease; AMI, acute myocardial infarction; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; PAD, peripheral arterial disease; AFIB, atrial fibrillation; SCA/VA, sudden cardiac arrest and ventricular arrhythmias; PCI, percutaneous coronary interventions; CABG, coronary artery bypass grafting; ICD/CRT-D, implantable cardioverter defibrillators/cardiac resynchronization therapy with defibrillator devices.
Descriptive statistics of environmental characteristics at the county level are reported in Table 3, where patients’ one-year PDC was aggregated to county level. A total of 2,981 counties, out of 3,124 potential counties, were selected in this study. Counties were excluded due to missing information in the environmental characteristics. The spatial distribution of medication across counties in the United States was examined. Counties with less than 10 patients were suppressed due to the confidentiality concerns.
Table 3.
Descriptive Statistics of ACEIs/ARBs adherence (PDC) environmental characteristics at county level (n=2,981)
| Variable$ | Mean | SD |
|---|---|---|
| ACEIs/ARBs adherence (PDC) | 0.76 | 0.12 |
| Numbers of patients per county | 23.50 | 57.36 |
| Number of General physicians per 10,000 persons | 6.96 | 4.33 |
| Percent of Medicare Beneficiaries in Total Population | 0.14 | 0.04 |
| Proportion of People Residing in Medically Underserved Areas | 0.56 | 0.45 |
| Deprivation score# | −0.01 | 2.79 |
A total of 70,201 elderly patients with hypertension and chronic kidney disease resided in 2,981 counties.
Deprivation score was measured by Townsend index, which incorporating unemployment rate, percentage of households without care ownership, home ownership and overcrowding.
GWR was utilized to investigate the relationship between environmental factors and medication adherence at the county level. In the GWR model, counties with less than 10 patients were excluded due to the reliability of county-level medication adherence measurement. A total of 1,445 counties, out of 2,981 potential counties were excluded (n=1,522). A multivariate linear regression model was first developed, which has been referred to as the global model, to establish a properly specified model for GWR. The redundancy of the explanatory variables was assessed using VIF value, with a cut off at 2. Next, the GWR model was run and the increase in goodness-of-fit checked through R2, AIC and Bayesian Information Criterion (BIC). The GWOR model performed better than the global model in term of R2 (0.09 vs 0.05) and AIC (−4,166.4 vs −4,151.3), but not in term of BIC (−4,038.9 vs −4119.4). The statistical performance between the global model and the GWR model was also compared through the GWR ANOVA test. The GWR model performed better than the global model in term of F statistic (F=1.95, df= (26, 1491) p=0.0029). Lastly, the geographical variability test for each explanatory variable was conducted, which indicated whether the local estimates of the specified variable demonstrated significant spatial variability. The results of GWR model were displayed as maps of local parameter estimates. It was noteworthy that the statistics reported in the GWR model was the local t value. The da Silva and Fotheringham correction was used to calculate the adjusted p-value25, and the significant parameter estimates were identified26. The statistical significance level was set at p<0.05. SAS 9.3 was used for data management and statistical analysis. The GWR 4 software package was used for GWR model27.
Results
The study cohort consisted of 70,201 aged Medicare beneficiaries with hypertension and CKD who used ACEIs/ARBs during the study period. These patients resided in a total of 2,981 counties in the United States, with an average of 24 persons per county. Approximately 61% of them had one-year PDC of at least 80% (mean=0.9, SD=0.1) while the rest of them had poor adherence (mean=0.5, SD=0.2). Table 1 reports the descriptive statistics of individual and environmental characteristics between groups. There was no difference in the distribution of age and having diabetes between patients with good adherence or poor adherence to ACEIs/ARBs. Nevertheless, fewer male and more whites were observed in the good adherence group (male: 38.9% vs. 41.3%; white: 87.9% vs.85.1%). In addition, slightly fewer beneficiaries in the good adherence group were deemed LIS beneficiaries (33.3% vs.34.2%), compared to the poor adherence group. In term of comorbid conditions, patients with poor adherence demonstrated a higher CCI scores compared to those being adherent to ACEIs/ARBs (1.4 vs. 1.2). Assessing patients’ history of cardiovascular related conditions and procedures, patients classified into the poor adherence group showed a significantly higher percentage in almost all of investigated conditions and procedures except coronary artery bypass grafting (CABG).
Results from the mixed effects model indicated that predictors of increased medication adherence included: female, receiving LIS either deemed or non-deemed, having diabetes, having AFIB (atrial fibrillation), residing in a county with proportionately greater number of general medicine physicians, and residing in a county with an older population. Meanwhile, heavy comorbidity burden, having cardiovascular conditions, living in a high deprivation area and a region lacking medical resources were associated with poor adherence to ACEIs/ARBs (Table 2).
Table 2.
The association between individual, environmental characteristics and ACEIs/ARBs adherence at individual level using Mixed model (n=70,201)
| Variable | Estimate | Standard Error | Pr > ∣t∣ | |
|---|---|---|---|---|
| Age (years) | 0.000 | 0.000 | 0.924 | |
| Charlson comorbidity index $ | −0.005 | 0.001 | <.0001 | *** |
| Female | 0.010 | 0.002 | <.0001 | *** |
| Race (White) | ||||
| Black | −0.034 | 0.004 | <.0001 | *** |
| Asians | 0.001 | 0.008 | 0.8788 | |
| Others | −0.019 | 0.008 | 0.0186 | * |
| Unknown | −0.044 | 0.031 | 0.1462 | |
| Prescription coverage at baseline (Part D without LIS) | ||||
| Part D with non-deemed LIS | 0.018 | 0.007 | 0.0066 | ** |
| Part D with deemed LIS | 0.012 | 0.002 | <.0001 | *** |
| Diabetes mellitus (DM) | 0.011 | 0.002 | <.0001 | *** |
| ASHD | −0.010 | 0.002 | <.0001 | *** |
| AMI | −0.008 | 0.003 | 0.0135 | * |
| CHF | −0.016 | 0.003 | <.0001 | *** |
| CVA-TIA | −0.009 | 0.002 | <.0001 | *** |
| PAD | −0.007 | 0.002 | 0.0013 | ** |
| AFIB | 0.006 | 0.003 | 0.0352 | * |
| SCA/VA | −0.002 | 0.005 | 0.7011 | |
| Other Cardiovascular diseases | −0.010 | 0.002 | <.0001 | *** |
| PCI | −0.010 | 0.005 | 0.065 | |
| CABG | 0.010 | 0.009 | 0.2743 | |
| ICD/CRT-D | −0.002 | 0.011 | 0.8431 | |
| Number of General physicians per 10,000 persons | 0.001 | 0.000 | 0.0035 | ** |
| Percent of Medicare Beneficiaries in Total Population | 0.081 | 0.033 | 0.0123 | * |
| Proportion of People Residing in Medically Underserved Areas | −0.013 | 0.003 | <.0001 | *** |
| Deprivation score# | −0.001 | 0.000 | 0.0002 | *** |
p<0.05,
p<0.01,
P<0.001
Adapted Charlson comorbidity index was calculated by excluding diabetes, kidney diseases and cardiovascular diseases
Deprivation score was measured by Townsend index, which incorporates unemployment rate, percentage of households without care ownership, home ownership and overcrowding.
Table 3 summarizes the descriptive statistics of the average PDC of ACEIs/ARBs and environmental characteristics at county level. The average PDC of ACEIs/ARBs in a one-year fixed interval was 0.76. The average number of general physician per 10,000 patients was 7. On average, about 14% of the total population living in the studied counties had Medicare coverage and approximately 56% of people resided in MUAs. The mean value of the deprivation score among the investigated counties was −0.01, where a higher positive score indicate greater deprivation.
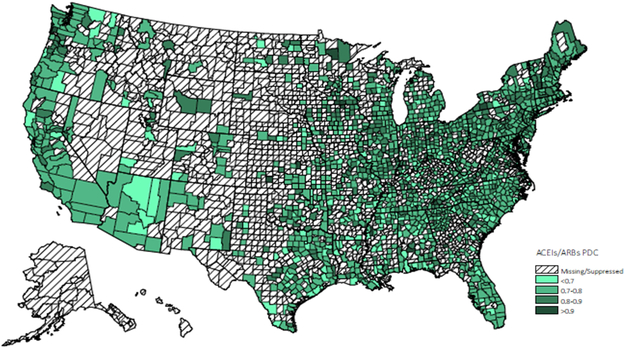
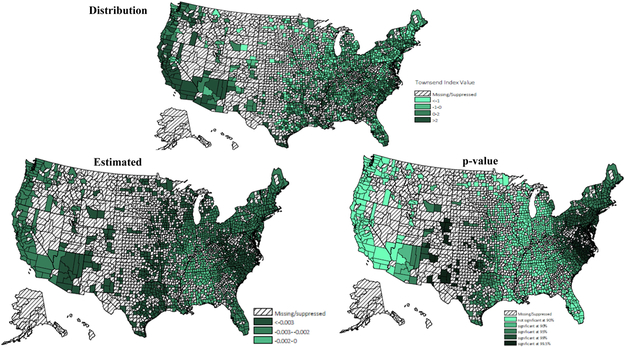
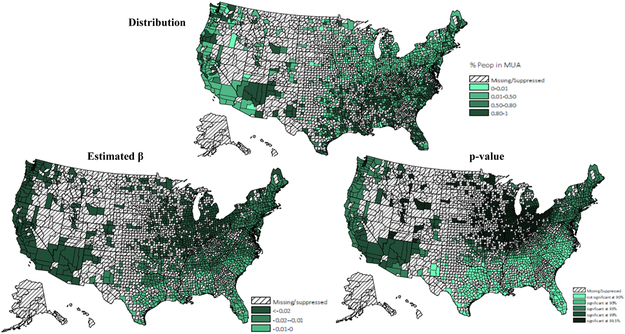
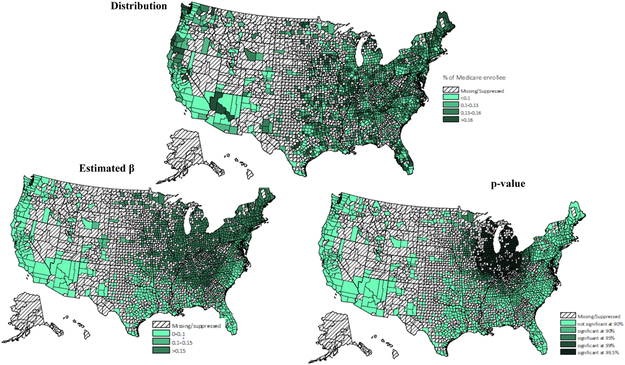
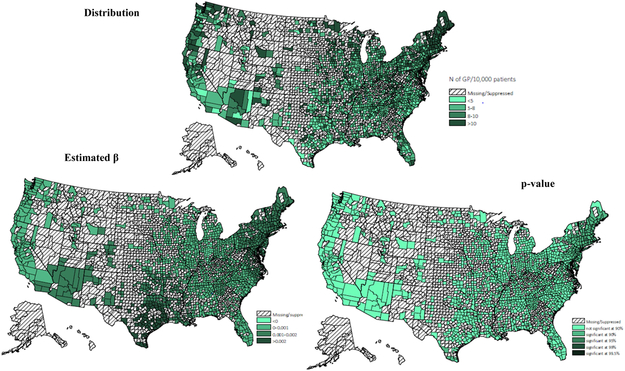
Adherence rates for ACEIs/ARBs at the county level were geographically clustered, and the map of medication adherence displayed geographic variation across the United States (Figure 1). Results of the GWR model have been summarized in Table 4. Overall, medication adherence was negatively associated with deprivation score and more people residing in MUAs, positively associated with the number of available physicians per capita and the percentage of older residents. Maps of the estimated relationship between medication adherence and predictors, which reflect these geographic differences (Appendix A - D) were drawn. Figure A reveals the West Mountain region, the West South Central region and the Northeast region had a significantly moderate to strong negative association between deprivation score and rate of medication adherence. Figure B displays the varied association between adherence rate and proportion of population residing in MUAs across the United States. A significantly strong negative association was observed in the Northeast region, the Midwest region, and the West region. Figure C and D indicate that both the percentage of older residents and the number of available physicians per patient were positively associated with medication adherence, but these associations were only significant in the Great Lake region and the New England region. By comparing the goodness of fit statistics, it was concluded that the GWR model fits the data better than the OLS model.
Figure 1.
Distribution of 1 year ACEIs/ARBs adherence
* Values for counties with 10 or fewer patients were suppressed.
Table 4.
The association between environmental characteristics and average ACEIs/ARBs adherence at county level using Geographically Weighted Regression (n=1,522)
| County level Variable* |
Mean of Estimate |
Standard Error |
Min | Lower Quartile |
Median | Upper Quartile |
Max |
|---|---|---|---|---|---|---|---|
| Number of General physicians per 10,000 persons | 0.0008 | 0.0006 | 0.0000 | 0.0003 | 0.0006 | 0.0013 | 0.0026 |
| Percent of Medicare Beneficiaries in Total Population | 0.1738 | 0.0911 | 0.0126 | 0.0932 | 0.1608 | 0.2415 | 0.3609 |
| Proportion of People Residing in Medically Underserved Areas | −0.0178 | 0.0075 | −0.0278 | −0.0252 | −0.0189 | −0.0116 | −0.0007 |
| Deprivation score# | −0.0028 | 0.0008 | −0.0057 | −0.0032 | −0.0028 | −0.0023 | −0.0013 |
Values for counties with 10 or fewer patients were excluded.
Deprivation score was measured by Townsend index, which incorporated unemployment rate, percentage of households without care ownership, home ownership and overcrowding.
Discussion
This study is, to the best of the authors’ knowledge, the first nationally representative study to examine the spatial variation in adherence to ACEIs/ARBs among CKD patients and investigate the spatial association between environmental factors and medication adherence across the United States. Approximately 61% of study subjects were adherent to their prescribed ACEIs/ARBs, defined as having a PDC at least 80%, which is lower than the adherence rate demonstrated in previous studies using claims data or pill counts (from 65% to 70%). The observed difference may be due to the older age and the greater comorbidity burden in the population we studied. The average proportion of days covered by ACEIs/ARBs was 76%. Consistent with previous studies, there was spatial clustering in adherence to ACEIs/ARBs in the study hypertensive CKD cohort15,16. Moreover, comparable geographic variation to Couto’s study17 was observed. In this study, the Northeast region and the Midwest region demonstrated better population adherence compared to the South region.
The findings of GWR models indicated adherence to ACEIs/ARBs was related to the characteristics of patients’ place of residence. Previous studies have found that accessibility to healthcare was related to neighborhood socioeconomic status, distance and travel time to facilities, and availability of healthcare resources28-31. Consistent with previous research, a negative association was found between medication adherence and living in regions with a higher deprivation level and lack of primary care resources across the United States. Additionally, by displaying the estimated local relationship in maps, the magnitude and significance of the relationship varied across regions. For example, deprivation score was significantly related to lower medication adherence in the Northeast region, the West South Central region and the West Mountain region. The proportion of population residing in MUSs reflects the availability of primary care resources. Lack of primary care resources were significantly associated with decreased adherence in all regions except the South, despite the fact that the South had a more severe shortage of healthcare resources. The study results indicated that in the West region, the Midwest region, and the Northeast region, interventions for enhancing medication adherence may consider targeting CKD patients living in places lacking primary care resources. While in the West Mountain region, the West South Central region and the Northeast region, interventions for medication adherence are recommended to target patients residing in high deprivation counties. Moreover, it is worth noting that neither the deprivation score nor lack of primary care resources explained the poor medication adherence in the East South Central and the South Atlantic. Other environmental factors that were not captured in this study may drive geographic differences in medication adherence, like treatment strategies and healthcare quality32-34. For example, a study of high-risk medication use found Part D enrollees who lived in the Southern United Sates were at higher risk of receiving regimens with high-risk medication, compared to those who lived in the New England area35.
Besides the environmental factors, the study found patients’ adherence to their prescribed medication was also associated with their demographic characteristics, medical conditions, and health plan coverage. In this study, a significant relationship between age and medication adherence was not established. This may be attributable to the relatively older age of the study population and limited variation (SD=7.1 mean=78.6). A review by DiMatteo evaluated 568 adherence studies from 1949 to 1988 and found inconsistent relationships between age, gender and treatment adherence36. The results of the current study also found patients with heavy comorbidity burden had lower adherence to their prescribed medication, and this may be because they have the greater pill burden and more complex regimens8,14. Moreover, as with previous studies, the onset of diabetes was associated with better adherence to ACEIs/ARBs, possibly because ACEIs/ARBs are first-line therapy for patients with diabetic CKD1. Additionally, the presence of AFIB was associated with better adherence to ACEIs/ARBs, which may be attributable to the protective effect of these RAS inhibiting agents on the recurrence of AFIB37. With regards to the status of Part D coverage, receiving the low-income subsidy from Part D was associated with better adherence. Beneficiaries with LIS may have a lower risk of cost-related medication nonadherence because they are not only receiving the subsidy for premium and copayment but also are not limited by the coverage gap. A study of patients with kidney failure found patients reduced their medication use when they fell into the coverage gap38.
This study has several limitations. First, as a retrospective cohort study, it cannot draw causal inferences but only provide associative information. Second, internal validity may be threatened due to the characteristics of administrative claims data, selection bias and omitted variables bias. Although the study subjects are all Part D enrollees, it may still not be possible to fully capture all pharmacy claims, in particular among those who have multiple prescription drug coverages, patients enrolled in long term care institutions, and dual eligible patients covered by both Medicare and Medicaid. This may result in underestimation of medication adherence. Additionally, patients with hypertension may have a higher risk of experiencing cardiovascular events, such as hospitalization because of coronary disease, heart failure, stroke or peripheral arterial disease. Therefore, further studies could exclude days of hospitalization from the denominator when calculating medication adherence. Moreover, administrative claims data do not sufficiently reveal actual medication adherence. It is possible that the refills dispensed by pharmacies are not taken by patients, and lead to overestimating medication consumption. The findings from this study should be interpreted with caution, especially when generalizing these results to practice. Although a large cohort of ACEIs/ARBs users with hypertension and CKD was extracted from a nationally representative dataset, sicker patients who died within one year period after filling ACEIs/ARBs were excluded. Meanwhile, patients were required to continuously enroll in Part D. These inclusion and exclusion criteria may cause selection bias. Additionally, because of the reliability concerns, counties with less than 10 CKD patients were not included in the GWR analysis, which causes rural areas to be under-represented. Third, this study lacks self-reported measures of factors such as patients’ perceived need for medications, perceived benefits of medications, side effects and effective physician-patient communications. These subjective factors are related to patients’ medication-taking behaviors. Fourth, it was not possible to directly observe prescriptions written, so the adherence measure necessarily relies on the assumption that discontinuation of ACEIs/ARBs reflects patients’ decisions not to fill rather than providers’ decisions to discontinue the prescription.
Future research is warranted to understand local barriers to appropriate medication use and medication adherence. Meanwhile, researchers can perform spatial analyses with both individual characteristics and environmental characteristics in small local regions. Then, researchers and healthcare providers could potentially design population-based strategies in the local area to promote medication adherence in CKD patients.
Conclusion
Medication adherence to ACEIs/ARBs varied across the United States, with the Northeast and Midwest showing better adherence than the South. Different risk factors at both the individual level and the environmental level have been detected in this study. Additionally, the study displays the geographic variation of the relationship between environmental characteristics and medication adherence, which helps to better clarify the place effects behind these relationships. This information has valuable policy implications, especially for researchers and practitioners seeking to implement local interventions to improve medication use among hypertensive CKD patients in the United States.
Acknowledgments
Funding/Disclosure: This research is funded through the United States Renal Data System (USRDS). The USRDS is funded by NIDDK, through NIH contract HHSN276201400001C. The USRDS Coordinating Center is located at the Kidney Epidemiology and Cost Center, University of Michigan, in partnership with Arbor Research Collaborative for Health, Ann Arbor, Michigan. None of the authors have any conflicts of interest to declare.
Appendix
Figure A.
Townsend deprivation score (distribution, β & p-value)
* Values for counties with 10 or fewer patients were excluded.
Figure B.
Proportion of population residing in MUAs (distribution, β & p-value)
* Values for counties with 10 or fewer patients wereexcluded.
Figure C.
Proportion of Medicare beneficiaries (distribution, β & p-value)
* Values for counties with 10 or fewer patients were excluded.
Figure D.
Number of general physicians/10,000 population (distribution, β, and p value)
* Values for counties with 10 or fewer patients were excluded.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004. May;43(5 Suppl 1):S1–290. [PubMed] [Google Scholar]
- 2.Qaseem A, Hopkins RH Jr, Sweet DE, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013. December 17;159(12):835–47. [DOI] [PubMed] [Google Scholar]
- 3.Tangkiatkumjai M, Walker DM, Praditpornsilpa K, Boardman H. Association between medication adherence and clinical outcomes in patients with chronic kidney disease: a prospective cohort study. Clin Exp Nephrol. 2017. June;21(3):504–512. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Judd SE, Krousel-Wood M, McClellan WM, Safford MM. Low medication adherence and hypertension control among adults with CKD: data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2010. September;56(3):447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt KE, Edie CF, Laflam P, Simbartl LA, Thakar CV. Adherence to antihypertensive agents and blood pressure control in chronic kidney disease. Am J Nephrol. 2010;32(6):541–8. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004. September 23;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 7.Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, Shlipak MG. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005. June;45(6):1119–24. [DOI] [PubMed] [Google Scholar]
- 8.Magacho EJ, Ribeiro LC, Chaoubah A, Bastos MG. Adherence to drug therapy in kidney disease. Braz J Med Biol Res. 2011. March;44(3):258–62. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Greene PG, Douglas M, Grim C, Kirk KA, Kusek JW, Milligan S, Smith DE, Whelton PK. Appointment attendance, pill counts, and achievement of goal blood pressure in the African American Study of Kidney Disease and Hypertension Pilot Study. Control Clin Trials. 1996. August;17(4 Suppl):34S–39S. [DOI] [PubMed] [Google Scholar]
- 10.Hong K, Muntner P, Kronish I, Shilane D, Chang TI. Medication adherence and visit-to-visit variability of systolic blood pressure in African Americans with chronic kidney disease in the AASK trial. J Hum Hypertens. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang TI, Desai M, Solomon DH, Winkelmayer WC. Kidney function and long-term medication adherence after myocardial infarction in the elderly. Clin J Am Soc Nephrol. 2011. April;6(4):864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AF, Manias E, Walker R. Adherence to multiple, prescribed medications in diabetic kidney disease: A qualitative study of consumers' and health professionals' perspectives. Int J Nurs Stud. 2008. December;45(12):1742–56. [DOI] [PubMed] [Google Scholar]
- 13.Rifkin DE, Laws MB, Rao M, Balakrishnan VS, Sarnak MJ, Wilson IB. Medication adherence behavior and priorities among older adults with CKD: a semistructured interview study. Am J Kidney Dis. 2010. September;56(3):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKillop G, Joy J. Patients' experience and perceptions of polypharmacy in chronic kidney disease and its impact on adherent behaviour. J Ren Care. 2013. December;39(4):200–7. [DOI] [PubMed] [Google Scholar]
- 15.Erickson SR, Lin YN. Geospatial analysis of statin adherence using pharmacy claims data in the state of Michigan. J Manag Care Spec Pharm. 2014. December;20(12):1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang C, Kolenic G, Kline-Rogers E, Eagle KA, Erickson SR. Mapping geographic areas of high and low drug adherence in patients prescribed continuing treatment for acute coronary syndrome after discharge. Pharmacotherapy. 2011. October;31(10):927–33. [DOI] [PubMed] [Google Scholar]
- 17.Couto JE, Panchal JM, Lal LS, Bunz TJ, Maesner JE, O'Brien T, Khan T. Geographic variation in medication adherence in commercial and Medicare part D populations. J Manag Care Spec Pharm. 2014. August;20(8):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips KA, Morrison KR, Andersen R, Aday LA. Understanding the context of healthcare utilization: assessing environmental and provider-related variables in the behavioral model of utilization. Health Serv Res. 1998. August;33(3 Pt 1):571–96. Review. [PMC free article] [PubMed] [Google Scholar]
- 19.Babitsch B, Gohl D, von Lengerke T. Re-revisiting Andersen's Behavioral Model of Health Services Use: a systematic review of studies from 1998-2011. Psychosoc Med. 2012;9:Doc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Census Bureau Geography Reference. Centers of Population. http://www.census.gov/geo/reference/centersofpop.html. Accessed June 18, 2019.
- 21.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018. March;71(3S1):A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005. July;11(7):449–57. Review. [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992. June;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 24.Townsend deprivation index. http://www.restore.ac.uk/geo-refer/36229dtuks00y19810000.php. Accessed June 18, 2019.
- 25.da Silva AR and Fotheringham AS (2016), The Multiple Testing Issue in Geographically Weighted Regression. Geogr Anal, 48: 233–247. [Google Scholar]
- 26.Mennis J (2006) Mapping the Results of Geographically Weighted Regression, The Cartographic Journal, 43:2, 171–179 [Google Scholar]
- 27.Nakaya T, Charlton M, Lewis P, C. B, Yao J, Fotheringham S. https://geodacenter.asu.edu/drupal_files/gwr/GWR4manual.pdf. Published 2014.
- 28.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005. March;46(1):15–31. [DOI] [PubMed] [Google Scholar]
- 29.Comber AJ, Brunsdon C, Radburn R. A spatial analysis of variations in health access: linking geography, socio-economic status and access perceptions. Int J Health Geogr. 2011. July 25;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagheri N, Holt A & Benwell GL Appl. Spatial Analysis (2009) 2: 177. [Google Scholar]
- 31.Andersen RM, Yu H, Wyn R, Davidson PL, Brown ER, Teleki S. Access to medical care for low-income persons: how do communities make a difference? Med Care Res Rev. 2002. December;59(4):384–411. [DOI] [PubMed] [Google Scholar]
- 32.King WD, Minor P, Ramirez Kitchen C, Oré LE, Shoptaw S, Victorianne GD, Rust G. Racial, gender and geographic disparities of antiretroviral treatment among US Medicaid enrolees in 1998. J Epidemiol Community Health. 2008. September;62(9):798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, Simoni-Wastila L, Sullivan SD. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006. November;44(11):1005–10. [DOI] [PubMed] [Google Scholar]
- 34.Sargen MR, Hoffstad OJ, Wiebe DJ, Margolis DJ. Geographic variation in pharmacotherapy decisions for U.S. Medicare enrollees with diabetes. J Diabetes Complications. 2012. Jul-Aug;26(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qato DM, Trivedi AN. Receipt of high risk medications among elderly enrollees in Medicare Advantage plans. J Gen Intern Med. 2013. April;28(4):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004. March;42(3):200–9. [DOI] [PubMed] [Google Scholar]
- 37.Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005. June 7;45(11):1832–9. [DOI] [PubMed] [Google Scholar]
- 38.Park Haesuk. The impact of Medicare Part D coverage on medication adherence and health outcomes in end-stage renal disease (ESRD) patients. Diss. 2013. https://repositories.lib.utexas.edu/handle/2152/27152. Accessed June 18, 2019.