Abstract
Chronic alcohol consumption causes increased intestinal permeability and changes in the intestinal microbiota composition which contribute to the development and progression of alcohol-related liver disease. In this setting, little is known about commensal fungi in the gut. We studied the intestinal mycobiota in a cohort of patients with alcoholic hepatitis, patients with alcohol use disorder and non-alcoholic controls using fungal-specific internal transcribed spacer (ITS) amplicon sequencing of fecal samples. We further measured serum anti–Saccharomyces cerevisiae antibodies (ASCA) as systemic immune response to fungal products or fungi. Candida was the most abundant genus in the fecal mycobiota of the two alcohol groups whereas genus Penicillium dominated the mycobiome of non-alcoholic controls. We observed a lower diversity in the alcohol groups compared to controls. Antibiotic or steroid treatment was not associated with a lower diversity. Patients with alcoholic hepatitis had significantly higher ASCA levels compared to patients with alcohol use disorder and to non-alcoholic controls. Within the alcoholic hepatitis patient cohort, patients with levels of ≥34 IU/ml, had a significantly lower 90-day survival (60%) compared to those with ASCA levels <34 IU/ml (80%) with an adjusted hazard ratio of 3.13 (95% CI 1.11–8.82, p=0.031). In conclusion, patients with alcohol-associated liver disease have a lower fungal diversity with an overgrowth of Candida compared to controls. Higher serum ASCA was associated with increased mortality in alcoholic hepatitis patients. Intestinal fungi may serve as a therapeutic target to improve survival and ASCA may be useful to predict the outcome in patients with alcoholic hepatitis.
Keywords: microbiome, microbiota, mycobiome, alcoholic liver disease
Introduction
Alcohol-associated liver disease is one of the most prevalent liver diseases globally and the main cause of liver-related mortality (1, 2). It follows a pattern of disease progression beginning with simple steatosis and resulting in an hepatic inflammatory response with fibrosis and ultimately cirrhosis in a subgroup of patients (3). Some patients develop alcoholic hepatitis, an acute-on-chronic liver disease with prominent cholestasis, with high mortality rates of 20–40% within 6 months (4, 5). There is an urgent need to develop prognostic biomarkers and to identify new therapeutic targets for this deadly disease.
Chronic alcohol consumption does not only lead to liver injury via ethanol-mediated hepatocyte damage and changes in lipid metabolism, it also causes increased intestinal permeability and changes in the intestinal microbiota composition. Intestinal dysbiosis contributes to alcohol-associated liver disease by inducing translocation of bacterial products and bacteria from the gut to the liver (6, 7). Transplantation of gut microbiota from patients with alcoholic hepatitis exacerbates ethanol-induced liver injury in mice (8), indicating that susceptibility to alcohol-associated liver disease is, in part, transmissible. Additionally, a recent study suggests that fecal microbiota transfer (FMT) improved survival in steroid-ineligible alcoholic hepatitis patients (9).
Whereas most of the studies investigating the gut-liver axis focus on bacteria and bacterial products, little is known about another integral component of the gut microbiota, the commensal fungi in the gut (also called mycobiota). Invasive fungal infections, mainly with Candida albicans and Aspergillus occur in patients with alcoholic hepatitis and are associated with a high mortality (10-12).
Fungal surface components such as ß-glucan are recognized by a number of immune receptors and induce an inflammatory response (13, 14). We have recently shown that chronic ethanol administration results in intestinal fungal overgrowth and increased plasma ß-glucan level in mice. The inflammatory cytokine IL-1β plays a key role in the development of alcohol-associated liver disease (15); in our previous study, ß-glucan induced secretion of IL-1β from Kupffer cells via CLEC7A signaling, which contributes to hepatocyte damage (16). We also observed intestinal overgrowth of Candida in a small cohort of patients with alcohol-associated liver disease. Intestinal fungal dysbiosis has also been described in patients with inflammatory bowel disease (17-19), obesity (20) and liver cirrhosis (21).
In the current study, we focus on the mycobiota in a well described cohort of patients with alcoholic hepatitis and compared it to that of patients with alcohol use disorder with various stages of liver disease and to non-alcoholic controls. We further investigated the role of anti-Saccharomyces cerevisiae antibodies (ASCA) as a predictor of clinical outcome in patients with alcoholic hepatitis.
Patients and Methods
A total of 163 patients with alcoholic hepatitis, consecutively hospitalized in 12 centers that form part of the InTeam Consortium (ClinicalTrials.gov identifier number: NCT02075918) in the period between June of 2014 and April of 2017 for whom serum and/or stool samples were available. Cases were compared to 40 subjects with alcohol use disorder with various stages of liver disease and 23 non-alcoholic subjects. ASCA, zonulin and lipopolysaccharide binding protein (LPS-BP) was measured in the serum sample collected at admission. In a subgroup of 59 alcoholic hepatitis patients, 15 patients with alcohol use disorder and 11 controls, fungal internal transcribed spacer (ITS) amplicon sequencing of fecal samples was performed. 11 of these patients were also part of the fecal mycobiome analysis of our previous study (16). Patients fulfilling the DSM IV criteria (22) of alcohol dependence and with active alcohol consumption (self-reported >60g/day) were recruited in a clinic with an alcohol treatment program and compared to individuals without alcohol dependency (non-alcoholic controls; social drinkers consuming less than 20g/day). Non-alcoholic controls or patients with alcohol use disorder did not take antibiotics or immunosuppressive medication during the two months preceding enrollment. Other exclusion criteria were diabetes, inflammatory bowel disease, known liver disease of any other etiology, and clinically significant cardio-vascular, pulmonary or renal co-morbidities. The baseline characteristics of the cohort with alcoholic hepatitis are shown in Table 1A and 1B (subgroup for mycobiota analysis) and Table 2A and 2B (cohort for ASCA-analysis). Inclusion criteria for alcoholic hepatitis were: 1. Active alcohol abuse (>50 g/day for men and >40 g/day for women) in the last 3 months, 2. aspartate aminotransferase (AST) > alanine aminotransferase (ALT) and total bilirubin > 3 mg/dl in the past 3 months, 3. Liver biopsy and/or clinical picture consistent with alcoholic hepatitis. Exclusion criteria were: 1. Autoimmune liver disease (ANA > 1/320), 2. Chronic viral hepatitis, 3. Hepatocellular carcinoma, 4. Complete portal vein thrombosis, 5. Extrahepatic terminal disease, 6. Pregnancy, and 7. Lack of signed informed consent (23). In all patients, the clinical picture was consistent with alcoholic hepatitis and in patients who underwent liver biopsy, the histology was in line with the diagnosis of alcoholic hepatitis. Liver biopsies were only done if clinically indicated as part of routine clinical care for diagnostic purposes of alcoholic hepatitis. Mortality was evaluated at 30, 90 and 180 days after admission. During the study, four alcoholic hepatitis patients of the overall cohort underwent liver transplantation. These patients were considered as dead with transplant date as date of death. All demographic, clinical, laboratory parameters and follow-up data were collected. 24 patients with available day-90 serum samples were included in a longitudinal sub analysis. 18 of the 24 samples were collected at day-90, 6 had a longer follow up (between 90 and 154 days). In 7 patients with day-90 fecal samples, a longitudinal mycobiome analysis was performed. The Model for End-stage Liver Disease (MELD) score, sodium MELD score and Maddrey’s discriminant function (DF) were calculated for all patients were the required variables were available. The protocol was approved by the Ethics Committee of each participating center and written informed consent was obtained from each patient.
Table 1A:
Patient characteristics of the subgroup for the intestinal mycobiome analysis
| Non-alcoholic Controls |
Alcohol Use Disorder |
Alcoholic Hepatitis |
p value | |
|---|---|---|---|---|
| Clinical parameter | ||||
| Total n | 11 | 15 | 59 | |
| Age, years, n=85 | 45 (28-74) | 49 (28-67) | 51 (31-70) | 0.291 |
| Body Mass Index (BMI), kg/m2, n=78 | 26.3 (19.2-31.6) | 26.3 (17.9-37.0) | 26.1 (16.3-41.7) | 0.760 |
| Gender (male), n (%), n=85 | 7 (64) | 15 (100) | 42 (71) | 0.043* |
| Laboratory parameter | ||||
| Albumin (g/dl), n=71 | 4.1 (2.2-5.2) | 2.3 (1.3-3.9) | <0.001 | |
| Alkaline phosphatase (U/l), n=70 | 68 (33-225) | 192 (21-1153) | 0.003 | |
| ALT (IU/l), n=74 | 43 (9-150) | 47 (15-174) | 0.435 | |
| AST (IU/l), n=74 | 50 (20-141) | 134 (41-348) | <0.001 | |
| Total bilirubin (mg/dl), n=74 | 0.6 (0.2-3.7) | 14.1 (3.1-38.6) | <0.001 | |
| GGT (IU/l), n=41 | 145 (15-1131) | 219 (33-3145) | 0.119 | |
| Platelet counts (x10 9/l), n=73 | 217 (101-276) | 130 (21-447) | 0.114 | |
| Prothrombin time, s, n=49 | 20.6 (11-61) | |||
| Creatinine (mg/dl), n=73 | 0.82 (0.56-1.31) | 0.77 (0.3-8.1) | 0.320 | |
| Sodium (mEq/l), n=58 | 133 (118-143) | |||
| INR, n=72 | 1.0 (0.9-2.0) | 1.8 (1.0-4.4) | <0.001 | |
| Non-invasive fibrosis assessment | ||||
| FIB-4, n=73 | 2.1 (0.7-6.5) | 7.1 (1.5-69.4) | 0.007 | |
| <1.45 (F0-F1), n (%) | 4 (28.6) | 0 | <0.001 | |
| 1.45-3.25 (intermediate), n (%) | 6 (42.9) | 8 (13.6) | ||
| >3.25 (F3-F4), n (%) | 4 (28.6) | 51 (86.4) |
Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. In blank cells, patients from the respective group were not counted to missing numbers. One-Way ANOVA with Tukey’s post-hoc test for multiple comparisons for continuous variables and Chi-squared tests for categorical variables.
Post hoc p values Gender male: AUD vs. AH p= 0.991; Ctrl vs. AH p=0.617; Ctrl vs. AUD p=0.991. Bold font indicates significance (p value <0.05). ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUD, alcohol use disorder; AH, alcoholic hepatitis; Ctrl, non-alcoholic controls; BMI, body mass index; FIB-4, fibrosis-4 index; GGT, gamma-glutamyl-transferase; INR, international normalized ratio.
Table 1B:
Characteristics of the subgroup of alcoholic hepatitis patients for the mycobiome analysis
| Treatment at admission | Histology | ||||
| Steroids, n (%), n=58 | 20 (34) | Lobular fibrosis, n=34 | 0 | 2 (5.9) | |
| Pentoxifylline, n (%), n=57 | 7 (12) | 1 | 5 (14.7) | ||
| Steroids and pentoxifylline, n (%), n=58 | 1 (1.7) | 2 | 2 (5.9) | ||
| Antibiotics, n (%), n=58 | 15 (25.9) | 3 | 25 (73.5) | ||
| Prophylactic antibiotics, n (%), n=58 | 12 (20.7) | Pericellular fibrosis, n=35 | 0 | 4 (11.4) | |
| Proton pump inhibitors, n (%), n=28 | 2 (7) | 1 | 31 (88.6) | ||
| Antifungals n (%), n=28 | 0 (0) | Grade of steatosis, n=36 | 1 | 12 (33.3) | |
| 2 | 11 (30.6) | ||||
| Clinical characteristics | 3 | 13 (36.1) | |||
| Infection at admission, n (%), n=53 | 9 (17) | Mallory bodies, n=34 | 0 | 6 (17.6) | |
| Fungal infection (C. albicans), n=53 | 1 (1.9) | 1 | 28 (82.4) | ||
| Ascites | Bilirubinostasis, n=34 | 0 | 13 (38.2) | ||
| No | 17 (29) | 1 | 14 (41.2) | ||
| Small | 28 (47) | 2 | 0 (0.0) | ||
| Large | 14 (24) | 3 | 7 (20.6) | ||
| Ballooning, n=35 | 0 | 22 (62.9) | |||
| Clinical scores and outcome | 1 | 13 (37.1) | |||
| Model for end-stage liver disease (MELD), n=58 | 24 (12-40) | Giant mitochondria, n=31 | 0 | 28 (90.3) | |
| MELD>21, n (%), n=58 | 46 (79) | 1 | 3 (9.7) | ||
| Maddrey’s DF, n=46 | 50 (15-230) | PMN infiltration, n=35 | 0 | 6 (17.1) | |
| Maddrey’s DF >32, n=46 | 40 (87) | 1 | 16 (45.7) | ||
| 30 day mortality rate, n (%), n=56 | 5 (9) | 2 | 13 (37.1) | ||
| 90 day mortality rate, n (%), n=44 | 9 (21) | Inflammatory grade, n=36 | 0 | 12 (33.3) | |
| Follow-up, days, n=56 | 92 (7-762) | 1 | 21 (58.3) | ||
| 2 | 3 (8.3) | ||||
| Histology | |||||
| Liver biopsy available, n (%) | 36 (61) | ||||
| Stage of fibrosis, n=35 | 0 | 1 (2.9) | |||
| 1 | 0 (0.0) | ||||
| 2 | 4 (11.4) | ||||
| 3 | 7 (20.0) | ||||
| 4 | 23 (65.7) | ||||
Clinical characteristics of 59 alcoholic hepatitis patients. Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. Maddrey’s DF, Maddrey’s discriminant function. Fibrosis stage, 0 no fibrosis, 1 portal fibrosis, 2 expansive periportal fibrosis, 3 bridging fibrosis, 4 cirrhosis. Lobular fibrosis, 0 no fibrosis, 1 zone 3 (centrilobular) fibrosis, 2 zone 2+3 (midzonal) fibrosis, 3 panlobular fibrosis. Pericellular fibrosis, 0 absent, 1 present. Steatosis, 1 mild < 33%, 2 moderate < 33-66%, 3 marked > 66%. Mallory bodies, 0 absent, 1 present. Bilirubinostasis, 0 no, 1 hepato-canalicular, 2 cholangiolar, 3 both. Ballooning, 0 occasional hepatocellular, 1 marked hepatocellular, 2 none present. Megamitochondria, 0 absent, 1 present. PMN infiltration, 0 no, 1 mild, 2 severe. Inflammation, 0 no, 1 mild, 2 severe. PMN, polymorphonuclear infiltration.
Table 2A:
Patient characteristics for the ASCA analysis
| Non-alcoholic Controls |
Alcohol Use Disorder |
Alcoholic Hepatitis |
p value | |
|---|---|---|---|---|
| Clinical parameter | ||||
| Total n | 23 | 40 | 163 | |
| Age (years), n=224 | 46 (26-76) | 43 (27-63) | 49 (26-75) | 0.022* |
| Body Mass Index (BMI), kg/m², n=198 | 22.9 (18.8-42.3) | 23.6 (17.9-35.5) | 26.8 (16.3-44.6) | <0.001* |
| Male gender, %, n=226 | 87 | 82.5 | 66.5 | 0.033* |
| Laboratory parameter | ||||
| ASCA (IU/ml), n=226 | 10.7 (5.9-25.5) | 13.0 (4.5-37.0) | 26.0 (5.2-133.9) | <0.001* |
| Albumin (g/dl), n=182 | 4.5 (2.2-5.2) | 2.5 (0.2-4.2) | <0.001 | |
| Alkaline phosphatase (U/l), n=184 | 68 (33-225) | 170 (21-1153) | <0.001 | |
| ALT (IU/l), n=197 | 37.5 (9-184) | 44 (15-404) | 0.568 | |
| AST (IU/l), n=197 | 48 (15-283) | 130 (34-1858) | <0.001 | |
| Total bilirubin (mg/dl), n=194 | 0.5 (0.2-1.5) | 13.7 (2.5-51.8) | <0.001 | |
| GGT (IU/l), n=121 | 95 (15-1131) | 257 (33-3632) | <0.001 | |
| Platelet counts (x10 9/l), n=194 | 220 (21-434) | 122 (12-447) | <0.001 | |
| Prothrombin time, s, n=138 | 22.1 (9-141) | |||
| Creatinine (mg/dl), n=202 | 0.8 (0.2-1.3) | 0.8 (0.2-8.1) | 0.051 | |
| Sodium (mEq/l), n=158 | 134 (106-208) | |||
| INR, n=187 | 0.94 (0.8-1.1) | 1.8 (0.8-7.6) | <0.001 | |
| Non-invasive fibrosis diagnostic and ultrasound | ||||
| Steatosis in ultrasound, n (%), n=32 | 23 (72) | |||
| FIB-4, n=193 | 1.6 (0.3-21.4) | 7.6 (0.7-73.4) | <0.001 | |
| <1.45 (F0-F1), n (%) | 18 (47.4) | 3 (1.9) | <0.001 | |
| 1.45-3.25 (intermediate), n (%) | 14 (36.8) | 11 (7.1) | ||
| >3.25 (F3-F4), n (%) | 6 (15.8) | 141 (91) |
Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. In blank cells, patients from the respective group were not counted to missing numbers. One-Way ANOVA with Tukeýs post-hoc test.
Post hoc p values: Age: AUD vs. AH p=0.0226; Ctrl vs. AH p=0.419; Ctrl vs. AUD p=0.750. BMI: AUD vs. AH p= <0.001; Ctrl vs. AH p=0.023; Ctrl vs. AUD p=0.954. Gender male: AUD vs. AH p=0.058; Ctrl vs. AH p=0.062; Ctrl vs. AUD p=0.642. ASCA: AUD vs. AH p=<0.001; Ctrl vs. AH p=<0.001; Ctrl vs. AUD p= 0.863. ASCA: AUD vs. AH =<0.001; Ctrl vs. AH =<0.001; Ctrl vs. AUD p=0.864. Bold font indicates significance (p value <0.05). ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; AUD, alcohol use disorder; AH, alcoholic hepatitis; Ctrl, non-alcoholic controls; INR, international normalized ratio; FIB-4, fibrosis-4 index; GGT, gamma-glutamyl-transferase; BMI, body mass index.
Table 2B:
Characteristics of alcoholic hepatitis patients for the ASCA analysis
| Treatment at admission | Histology | ||||
| Steroids, n (%), n=156 | 58 (37.8) | Lobular fibrosis, n=83 | 0 | 6 (7.2) | |
| Pentoxifylline, n (%), n=138 | 8 (5.6) | 1 | 13 (15.7) | ||
| Steroids and pentoxifylline, n (%), n=138 | 1 (0.7) | 2 | 6 (7.2) | ||
| Antibiotics, n (%), n=157 | 43 (27.6) | 3 | 58 (69.9) | ||
| Prophylactic antibiotics, n (%), n=156 | 34 (21.8) | Pericellular fibrosis, n=84 | 0 | 19 (22.6) | |
| Proton pump inhibitors, n (%), n=77 | 13 (17) | 1 | 65 (77.4) | ||
| Antifungals n (%), n=77 | 0 | Grade of steatosis, n=85 | 1 | 31 (36.5) | |
| 2 | 24 (28.2) | ||||
| Clinical characteristics | 3 | 30 (35.3) | |||
| Infection at admission, n (%), n=121 | 26 (21.5) | Mallory bodies, n=83 | 0 | 15 (18.1) | |
| Fungal infection (C. albicans), n=121 | 1 (0.6) | 1 | 68 (81.9) | ||
| Ascites | Bilirubinostasis, n=83 | 0 | 29 (34.9) | ||
| No | 55 (34) | 1 | 34 (41.0) | ||
| Small | 68 (42) | 2 | 5 (6.0) | ||
| Large | 40 (25) | 3 | 15 (18.1) | ||
| Ballooning, n=83 | 0 | 51 (61.4) | |||
| Clinical scores and outcome | 1 | 32 (38.6) | |||
| Model for end-stage liver disease (MELD), n=156 | 24 (8-58) | Giant mitochondria, n=80 | 0 | 61 (76.2) | |
| MELD>21, n (%), n=156 | 116 (74) | 1 | 19 (23.8) | ||
| Maddrey’s DF, n=138 | 56 (−18-610) | PMN infiltration, n=84 | 0 | 18 (21.4) | |
| Maddrey’s DF >32, n=138 | 125 (91) | 1 | 39 (46.4) | ||
| 30 day mortality rate, n (%), n=140 | 20 (14) | 2 | 27 (32.1) | ||
| 90 day mortality rate, n (%), n=107 | 36 (34) | Inflammatory grade, n=85 | 0 | 20 (23.5) | |
| Follow-up, days, n=146 | 85 (1-814) | 1 | 56 (65.9) | ||
| 2 | 9 (10.6) | ||||
| Histology | |||||
| Liver biopsy available, n (%) | 87 (54.4) | ||||
| Stage of fibrosis, n=84 | 0 | 2 (2.4) | |||
| 1 | 2 (2.4) | ||||
| 2 | 10 (11.9) | ||||
| 3 | 18 (21.4) | ||||
| 4 | 52 (61.9) | ||||
Clinical characteristics of 163 alcoholic hepatitis patients. Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. Maddrey’s DF, Maddrey’s discriminant function. Fibrosis stage, 0 no fibrosis, 1 portal fibrosis, 2 expansive periportal fibrosis, 3 bridging fibrosis, 4 cirrhosis. Lobular fibrosis, 0 no fibrosis, 1 zone 3 (centrilobular) fibrosis, 2 zone 2+3 (midzonal) fibrosis, 3 panlobular fibrosis. Pericellular fibrosis, 0 absent, 1 present. Steatosis, 1 mild < 33%, 2 moderate < 33-66%, 3 marked > 66%. Mallory bodies, 0 absent, 1 present. Bilirubinostasis, 0 no, 1 hepato-canalicular, 2 cholangiolar, 3 both. Ballooning, 0 occasional hepatocellular, 1 marked hepatocellular, 2 none present. Megamitochondria, 0 absent, 1 present. PMN infiltration, 0 no, 1 mild, 2 severe. Inflammation, 0 no, 1 mild, 2 severe. PMN, polymorphonuclear infiltration.
Non-invasive fibrosis assessment
The Fibrosis-4 Index (FIB-4) is a non-invasive fibrosis score based on age, alanine aminotransferase, aspartate aminotransferase and platelet count; we used the published cut-offs to stratify patients in 3 groups (<1.45=low, 1.45–3.25=intermediate, >3.25=high) (24).
Fungal sequencing
To evaluate the human intestinal mycobiome, fungal ITS sequencing and analysis were conducted as previously described (16, 25-27). It is known that read2 (reverse) sequences tend lower quality than read 1 (forward) sequences, resulting in only a fraction of paired reads assembling together; therefore, more usable data can be obtained by using forward-only reads (28). Illumina MiSeq V3 kit, 150 cycles using primers containing the BITS and B58S3 sequences specific for the fungal ITS1 region (29) generated the ITS sequence data; however, ITS sequence data was generated and processed using just the read 1 (BITS) primer. Specifically, the 3 primers used are as follows (pad in regular type, linker in brackets, and targeting primer sequence in boldface): read 1 (ITSFWD, containing BITS), CATTATAGCT[CA]ACCTGCGGARGGATCA; I7 index, AACTTTYARCAAYGGATCTC[TG]CTGGATGACC; and read 2 (ITSREV, containing B58S3), GGTCATCCAG[CA]GAGATCCRTTGYTRAAAGTT. Nucleotides noted with an underscore were modified from our previous publication (16) to increase the Tm of Index 1 and read 2 primers, as recommended by Illumina. These changes did not alter the ITS1-targeting sequences. Index 2 was the standard Illumina Index 2 sequencing primer. PCR parameters included a 95°C denaturing step followed by 35 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, followed by a final extension step of 72°C for 5 minutes. Amplicons were purified using the Qiaquick PCR purification kit (Qiagen, Inc.). Purified amplicons were then quantified using SYBR Gold (Thermo Fisher Scientific), normalized, and pooled to generate a library of equimolar DNA molecules per sample (16). Fragments less than 200 bp were removed using BluePippin (Sage Scientific).
Accession Numbers
Sequence data were registered at the National Center for Biotechnology Information (NCBI) under BioProject PRJNA517994.
ELISA
ASCA-IgG levels were measured from serum samples using the ELISA kit ASG31-K01 from Eagle Biosciences according to the manufacturer’s protocol. As proposed in the manual, a 1:100 dilution of serum samples was used. Zonulin levels were measured from serum samples using the ELISA kit MBS2607261from MyBioSource.com, according to the manufacturer’s protocol. A 1:10 dilution of serum samples was used. Lipopolysaccharide binding protein (LPB-BP) levels were measured from serum samples using the ELISA kit HK315–02 from Hycult Biotech, according to the manufacturer’s protocol. As proposed in the manual, a 1:1000 dilution of serum samples was used.
Statistical analysis
Results are expressed as median and range for each continuous outcome, if not stated otherwise. A p value of equal or less than 0.05 was considered as statistically significant. Comparisons of baseline characteristics between the three groups were conducted using One-Way ANOVA with Tukeýs post-hoc test for multiple comparisons for continuous variables and Chi-squared tests for categorical variables. The fungal sequence reads were normalized to get the proportional, relative abundance of each fungus in each patient for further statistical analysis. To compare diversity, fungal abundance, ASCA, zonulin and LPS-BP levels between the groups, the Kruskal-Wallis test for nonparametric data and Dunn post-hoc test with adjustment for multiple comparisons using the false discovery rate (fdr) was used. In the longitudinal analysis, the paired t-test was used to compare the day-0 and day-90 values of the patients. Spearman’s correlation was conducted to correlate the relative fungi abundance at genus level with each of the clinical parameters. Additionally, a principal component analysis was performed to summarize outcomes of the relative abundance of all fungal genera between the three groups. One-Way ANOVA with Tukeýs post-hoc test was used to compare the principal components of the groups. Univariate linear regression analysis was performed to determine associations of ASCA and clinical parameters. A multivariate linear regression model to adjust for different variables was performed. Univariate Cox regression analysis was used to detect associations of ASCA and MELD within 90-, and 180-day mortality. Patients that were lost to follow-up were censored at the day they were last seen alive. Maximally selected rank statistic was used to determine the ASCA cut-off value that represents the maximum difference of two alcoholic hepatitis groups regarding 90-day survival (30). Kaplan-Meier curves were used to compare 90-, and 180-day survival between these groups. To test the diagnostic value of ASCA and to improve the performance of the MELD score by adding the variable ASCA, we modeled the binary 90-day mortality outcome using a logistic regression with MELD and log(ASCA) as the predictors. To help improve model fit, we used a generalized additive model (GAM) (31) to allow for potential non-linear forms of log(ASCA) and MELD in the model. GAM uses more flexible “base functions” to approximate nonlinear relationships in a regression setting. Base functions play the same role as polynomials, but they are selected to provide same or similar degrees of approximation accuracy using a minimum number of functions. Unlike analytic functions such as polynomials, based functions in GAM are data driven and cannot be expressed in an analytic form x or log(x). For our analysis, we used a procedure in R, GAM, which by default employs 9 thin plates as base functions to model the nonlinear relationships of each predictor (32), and obtained the following model:
where y denotes the binary outcome 90-day mortality with 1 for death and 0 otherwise, x denotes log(ASCA), z denotes MELD, pr(y=1∣x,z) denotes the conditional probability of y=1 given x and z, and denotes the logit link, and bk(x) ck(x) denote the thin plate base functions for log(ASCA) and MELD. As noted earlier, the base functions bk(x) and ck(x) cannot be expressed in analytic form, but can be readily evaluated numerically from their empirical forms when the fit model is used for prediction purposes. For comparison purposes, we also fit a GAM with only one predictor log(ASCA) (model estimates are not reported) or MELD (32). The AUROC values were compared using Delong’s method (33).
Statistical analysis was performed using R statistical software, R version 3.5.1, 2018 the R Foundation for Statistical Computing.
Results
Patient characteristics and laboratory parameters
The mycobiome analysis was performed from fecal samples that were available from 59 patients with alcoholic hepatitis, 15 patients with alcohol use disorder and 11 non-alcoholic controls. The median age and BMI did not differ between the three groups. The majority of the subjects were male (75%). As expected, compared to patients with alcohol use disorder, patients with alcoholic hepatitis had significantly lower albumin values, higher bilirubin and INR levels and a higher AST. The median MELD score in these patients was 24.
One third of patients with alcoholic hepatitis received treatment with steroids, 12% with pentoxifylline and 1.7% with both at the time of biospecimen collection. 26% were treated with antibiotics due to an infection and 21% with prophylactic antibiotics. No patient received antifungal treatment. In 61% of the patients, liver biopsy was performed. 66% of these patients had liver cirrhosis (Table 1A and 1B).
Patients with alcohol-associated liver disease have a low fungal diversity
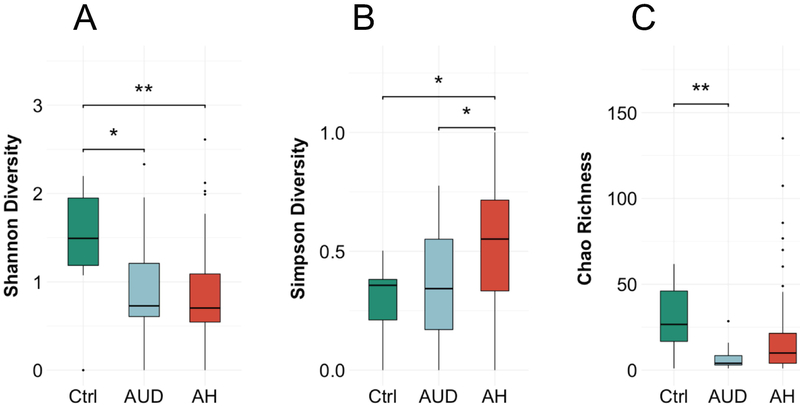
We initially investigated the diversity within the groups. We observed a lower Shannon-Index, which takes the number of observed species and the abundance into account, in the alcohol-associated groups compared to non-alcoholic controls (median (range) controls: 1.5 (0–2.2); alcohol use disorder: 0.7 (0–2.3); alcoholic hepatitis 0.7 (0–2.6), p value controls vs. alcoholic hepatitis = 0.0038, controls vs. alcohol use disorder 0.0356, alcohol use disorder vs. alcoholic hepatitis n.s., Figure 1A). The Simpson-Index, which expresses the probability that two species in one sample are from the same origin, was significantly higher in the alcoholic hepatitis group (controls: 0.36 (0–0.5); alcohol use disorder: 0.30 (0–0.8); alcoholic hepatitis: 0.56 (0–1), p value controls vs. alcoholic hepatitis = 0.015, alcohol use disorder vs. alcoholic hepatitis 0.046, controls vs. alcohol use disorder n.s., Figure 1B) and the Chao-richness, the predicted number of species, was lower in the alcohol-associated groups (27 (1–62); alcohol use disorder: 4 (1–29); alcoholic hepatitis: 10 (1–135), p value alcohol use disorder vs. alcoholic hepatitis n.s., controls vs. alcohol use disorder 0.0048, controls vs. alcoholic hepatitis = n.s., Figure 1C). Taken together, the alpha diversity is decreased in alcohol-dependent patients.
Figure 1. Patients with alcohol-associated liver disease have a low fungal diversity.
The fungal diversity and richness was calculated in 11 non-alcoholic controls, 15 patients with alcohol use disorder and 59 alcoholic hepatitis patients. (A) Shannon-Index. (B) Simpson-Index. (C) Chao-Richness. Kruskal-Wallis test for nonparametric data and Dunn post-hoc test. *p<0.05 p>0.01, **p<0.01 p>0.001. Ctrl, non-alcoholic controls; AUD, alcohol use disorder, AH, alcoholic hepatitis.
The fungal composition is altered in patients with alcohol-associated liver disease
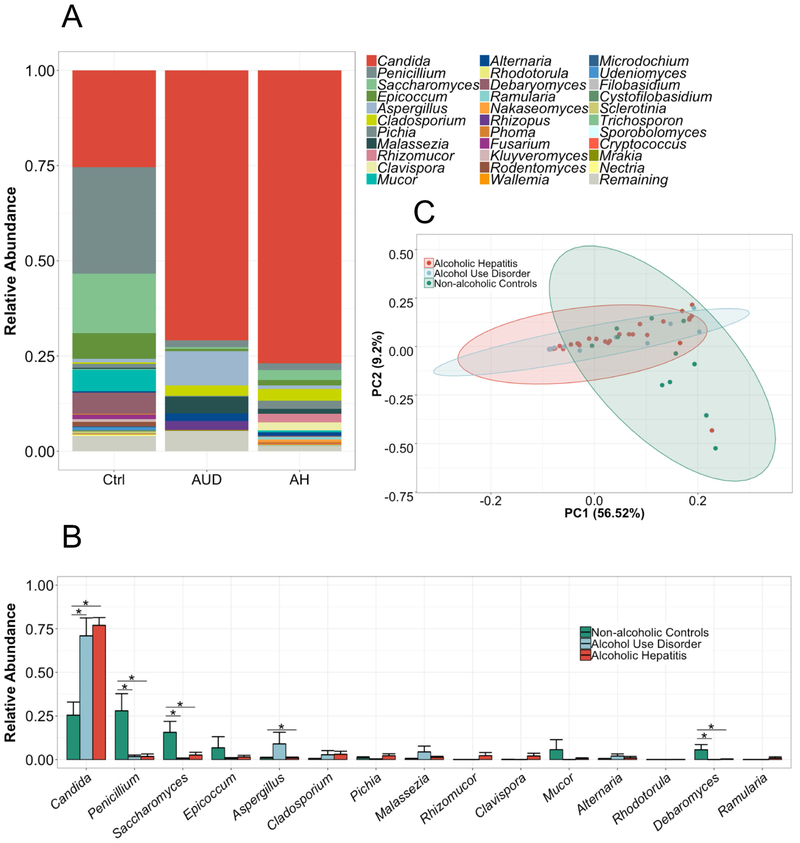
Next, we analyzed the intestinal fungal composition of each group. We observed a dramatic overgrowth of Candida in the alcohol-dependent patients whereas the genus Penicillium dominated the mycobiome of non-alcoholic controls (mean relative abundance (standard error of the mean) Candida: alcoholic hepatitis 77 (4.4)%; alcohol use disorder 71 (10) %; controls 25 (7.5)%, p value controls vs. alcoholic hepatitis = <0.001, controls vs. alcohol use disorder 0.005, alcohol use disorder vs. alcoholic hepatitis n.s.; Penicillium: controls 28 (9.8)%; alcohol use disorder 1.7 (0.8)%; alcoholic hepatitis 1.8 (1.4)%, p value controls vs. alcoholic hepatitis = <0.001, controls vs. alcohol use disorder 0.0025; alcohol use disorder vs. alcoholic hepatitis n.s., Figure 2A-B). Interestingly, despite less liver damage in alcohol use disorder patients, we observed similarities between the alcohol-dependent groups in the PCA analysis (Figure 2C). The main principal components clustered significantly different between non-alcoholic controls and alcohol use disorder or alcoholic hepatitis but not between alcohol use disorder and alcoholic hepatitis (Supplementary Table S1). Supplementary Figure S1 shows relative abundance of fungi on the phylum-, class-, order- and family level.
Figure 2. The fungal composition is altered in patients with alcohol-associated liver disease.
ITS sequencing of fecal samples from 11 non-alcoholic controls, 15 patients with alcohol use disorder and 59 alcoholic hepatitis patients. (A-B) The graphs demonstrate the mean relative abundance of sequence reads in each genus for each group. 0-1 corresponds to 0-100% abundance. A total of 81 different genera were detected. Shown are only genera that cover in total at least 95% abundance of all genera. In (B), the standard error of the mean is shown as error bar. *p<0.05, Kruskal-Wallis test for nonparametric data and Dunn post-hoc test. (C) Principal component analysis (PC) was used to show ß-diversity between the groups based on the abundance of 81 fungi at the genus level. The axes represent the two most discriminating axes using the euclidean distance metric. 56.5% of the variances are explained by PC1 and 9.2% by PC2. Ctrl, non-alcoholic controls; AUD, alcohol use disorder, AH, alcoholic hepatitis.
Intestinal fungi correlate with clinical and histological parameters in alcoholic hepatitis
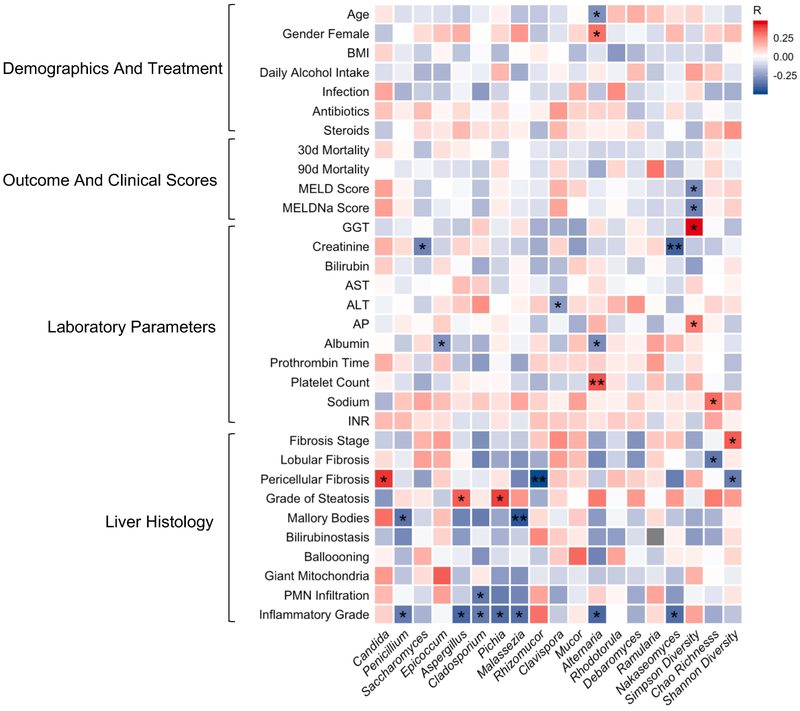
The heat map analysis demonstrates Spearman Rhós correlation of clinical parameters in the alcoholic hepatitis group with the most abundant fungi at the genus level and diversity (Figure 3). A high abundance of Candida was positively correlated with pericellular fibrosis, and the abundance of Penicillium was negatively correlated with inflammatory grade and Mallory-Denk bodies on liver biopsy. Interestingly, we did not observe any significant correlations between the abundance or diversity of fungi and treatment with antibiotics or steroids. Also, mortality was also not correlated with the dominating genus Candida. Significant correlations of all detected genera with clinical parameters are summarized in Supplementary Table S2.
Figure 3. Intestinal fungi correlate with clinical parameters in alcoholic hepatitis.
Heat map representing color-coded spearman’s correlations of clinical parameters. Red color indicates positive- and blue color negative correlation. All variables are coded from low to high- i.e. red color in antibiotics means antibiotic use is positively correlated with the respective fungi. *p<0.05 p>0.01, **p<0.01 p>0.001. Fibrosis stage, 0 no fibrosis, 1 portal fibrosis, 2 expansive periportal fibrosis, 3 bridging fibrosis, 4 cirrhosis. Lobular fibrosis, 0 no fibrosis, 1 zone 3 (centrilobular) fibrosis, 2 zone 2+3 (midzonal) fibrosis, 3 panlobular fibrosis. Pericellular fibrosis, 0 absent, 1 present. Steatosis, 1 mild < 33%, 2 moderate < 33–66%, 3 marked > 66%. Mallory bodies, 0 absent, 1 present. Bilirubinostasis, 0 no, 1 hepato-canalicular, 2 cholangiolar, 3 both. Ballooning, 0 occasional hepatocellular, 1 marked hepatocellular, 2 none present. Megamitochondria, 0 absent, 1 present. PMN infiltration, 0 no, 1 mild, 2 severe. Inflammation, 0 no, 1 mild, 2 severe. PMN, polymorphonuclear infiltration; INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl-transferase; AP, alkaline phosphatase; BMI, body mass index. MELD, Model for End-stage Liver Disease, MELDNa, Sodium Model for End-stage Liver Disease.
ASCA are increased in alcoholic hepatitis and associated with increased mortality
Next, we investigated the host immune response to fungi by measuring serum anti–Saccharomyces cerevisiae IgG antibodies (ASCA), with Candida albicans being an important immunogen for ASCA (34). In this cohort, 38% received treatment with steroids, 28% with antibiotics and 6% with pentoxifylline. 22% of the patients had an infection at admission; one third of these patients had a urinary tract infection. In one patient, C. albicans was found as pathogen causing the infection. 34% of the patients died within 90 days. For 54%, liver biopsy was performed (Table 2B). All clinical characteristics reported in Table 1 and 2 did not significantly differ among both datasets in alcoholic hepatitis patients and controls. AUD patients in the dataset for the mycobiome analysis had significantly lower albumin levels (median (range) AUD dataset for mycobiome analysis 4.1 g/dl (2.2–5.2) vs. 4.5 g/dl (2.2–5.2) in the ASCA dataset, p=0.026) and higher bilirubin levels (median (range) AUD dataset for mycobiome analysis 0.6 mg/dl (0.2–3.7) vs. 0.5 mg/dl (0.2–1.5) in the ASCA dataset, p=0.045).
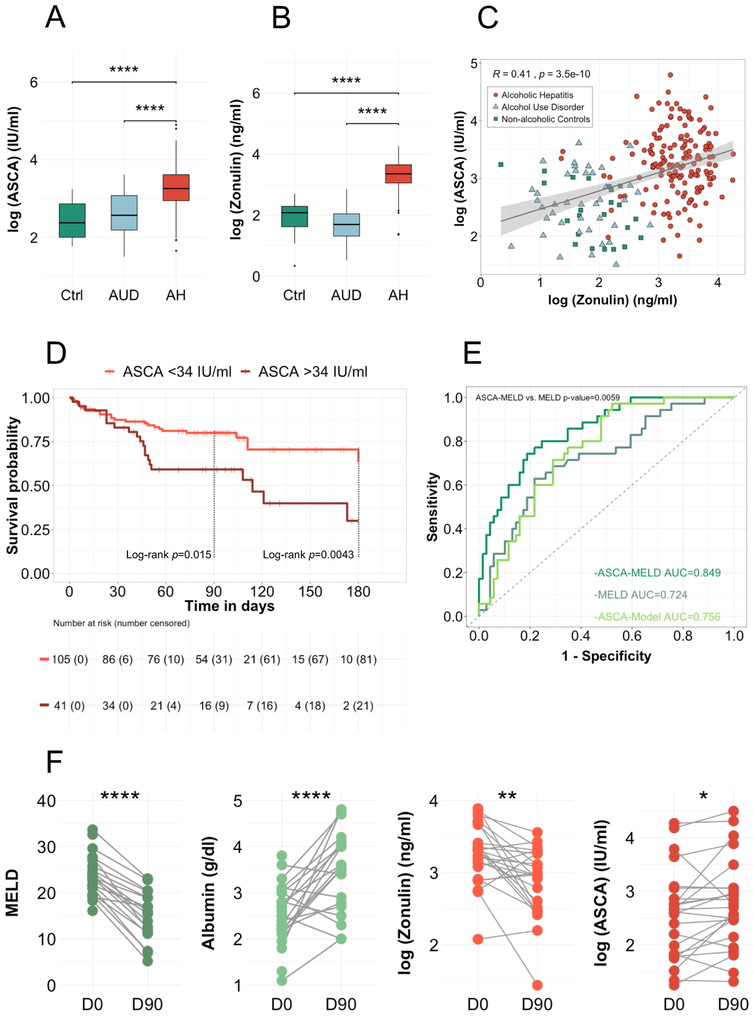
Patients with alcoholic hepatitis had significantly higher ASCA levels compared to patients with alcohol use disorder and non-alcoholic controls (median (range) controls 10.7 (5.9–25.5) IU/ml , alcohol use disorder 13.0 (4.5–37.0) IU/ml, alcoholic hepatitis 25.6 (5.2–133.9) IU/ml, p value controls vs. alcoholic hepatitis = <0.001, alcohol use disorder vs. alcoholic hepatitis <0.001, controls vs. alcohol use disorder n.s., Figure 4A), indicating an increased immune response to intestinal fungi in patients with alcoholic hepatitis.
Figure 4. ASCA are increased in alcoholic hepatitis and associated with increased mortality.
(A) Logarithmic ASCA levels were compared in 23 non-alcoholic controls, 40 patients with alcohol use disorder and 163 alcoholic hepatitis patients. Kruskal-Wallis test for nonparametric data and Dunn post-hoc test. *p<0.05 p>0.01, **p<0.01 p>0.001, ****p<0.001. (B) Logarithmic zonulin levels were compared and correlated (Spearman’s correlation) with ASCA levels (C) in 23 non-alcoholic controls, 40 patients with alcohol use disorder and 156 alcoholic hepatitis patients. (D) Kaplan-Meier curve of 180-day mortality for patients with alcoholic hepatitis. Patients were grouped according to their serum levels of ASCA. Patients that were lost to follow-up were censored at the time they were last seen alive. The number of patients at risk is indicated as well as the number of censored patients in brackets. At day-180, all remaining patients were censored, even though they had a longer survival, resulting in the high number of censored patients. (E) Receiver operating curves with area under the curve and Delong’s p value for the comparison of ASCA-MELD and MELD-score. (F) Longitudinal analysis of clinical parameter and serum marker of 24 alcoholic hepatitis patients. Measurement at day zero (D0, after admission) and around ninety-day follow-up visit (D90, follow-up measurement around ninety days after day zero, 18 of the 24 samples were collected at day-90, 6 had a longer follow up (between 90 and 154 days)). Paired t-test. Ctrl, non-alcoholic controls; AUD, alcohol use disorder; AH, alcoholic hepatitis. MELD; ASCA, anti–Saccharomyces cerevisiae antibodies; Model for End-stage Liver Disease.
In the univariate analysis, high ASCA levels were positively associated with the presence of liver cirrhosis and the stage of lobular fibrosis; this remained significant after adjusting for infection, MELD score, treatment with steroids, antibiotics and pentoxifylline (Supplementary Table S3). Further, antibiotic treatment was associated with higher ASCA levels in the univariate linear regression analysis (Supplementary Table S3). The presence of any infection at admission or the use of steroids and/or pentoxifylline was not associated with higher ASCA levels (data not shown).
To elaborate the relationship of ASCA with intestinal permeability, we further measured serum zonulin levels. In line with the ASCA levels, serum zonulin was only increased in alcoholic hepatitis patients (median (range) controls 8.0 (1.4–14.9) ng/ml, alcohol use disorder 5.4 (1.7–17.4) ng/ml, alcoholic hepatitis 28.6 (3.9–70.5) ng/ml, p value controls vs. alcoholic hepatitis = <0.001, alcohol use disorder vs. alcoholic hepatitis <0.001, controls vs. alcohol use disorder n.s., Figure 4B). ASCA levels correlated with zonulin levels within the overall study cohort (Figure 4C). However, within alcoholic hepatitis patients, ASCA levels did not correlate significantly with zonulin levels (Spearman’s R=0.035, p=0.66).
Using the maximally selected rank statistic, we found that within the alcoholic hepatitis patient cohort, those patients with levels of ≥34 IU/ml, had a significantly lower 90-day survival (60%) and 180-day survival (30%) compared to those with ASCA levels <34 IU/ml (90-day survival 80%, 180-day survival 63%), with a hazard ratio of 2.25 (95% CI 1.16–4.35, p=0.016) for 90-day survival and 2.34 (95% CI 1.29–4.25, p=0.005) for 180-day survival (Figure 4D and Table 3). This remained statistically significant after adjusting for confounding factors, such as presence of any infection, treatment with antibiotics, steroids, pentoxifylline and the MELD score (Table 3).
Table 3:
High ASCA levels are associated with increased 90- and 180-Day mortality in patients with alcoholic hepatitis
| Dependent: 90 Day Mortality |
Survival, % (95% CI) |
HR (univariable), (95% CI, p value) |
HR (multivariable), (95% CI, p value*) |
HR (multivariable), (95% CI, p value**) |
|
|---|---|---|---|---|---|
| ASCA | <34 IU/ml | 80.0 (72.4-88.2) | - | ||
| ≥34 IU/ml | 59.1 (45.5-76.8) | 2.22 (1.15-4.30, p=0.017) | 3.27 (1.21-8.86, p=0.020) | 3.13 (1.11-8.82, p=0.031) | |
| MELD | 1.11 (1.07-1.16, p<0.001) | 1.13 (1.07-1.19, p<0.001) | 1.13 (1.07-1.19, p<0.001) | ||
| Dependent: 180 Day Mortality |
|||||
| ASCA | <34 IU/ml | 63.4 (48.4-83.1) | |||
| ≥34 IU/ml | 29.9 (13.9-64.6) | 2.32 (1.28-4.21, p=0.006) | 3.48 (1.50-8.10, p=0.004) | 3.07 (1.30-7.28, p=0.011) | |
| MELD | 1.10 (1.06-1.15, p<0.001) | 1.13 (1.08-1.18, p<0.001) | 1.12 (1.07-1.17, p<0.001) | ||
p value adjusted for MELD score, infection, antibiotics, steroids, pentoxifylline.
p value adjusted for MELD score, infection, antibiotics, steroids, pentoxifylline and LPS-BP. Likelihood ratio test. Bold font indicates significance (p value <0.05). HR, hazard ratio; CI, confidence interval; ASCA, anti-Saccharomyces cerevisiae antibodies; LPS-BP, lipopolysaccharide binding protein; MELD, Model for End-stage Liver Disease.
The association of high ASCA levels with mortality is independent of bacterial translocation
We further measured serum LPS-BP to differentiate the effect of bacterial translocation from host immune response to fungi. Patients with alcoholic hepatitis had significantly higher LPS-BP levels compared to controls (median (range) controls 7.6 (2.5–18.9) ng/ml, alcohol use disorder 11.6 (0.0–27.5), alcoholic hepatitis 17.0 (0.0–72.7), p value controls vs. alcoholic hepatitis = <0.001, alcohol use disorder vs. alcoholic hepatitis <0.001, controls vs. alcohol use disorder n.s., Supplementary Figure S3A). ASCA ≥34 IU/ml remained significantly associated with mortality even after additional adjustment for LPS-BP in the Cox regression model (Table 3).
Adding ASCA to MELD score improves diagnostic performance regarding mortality
To test the diagnostic value of ASCA and to improve the performance of the MELD score by adding the variable ASCA, we conducted a generalized additive model since we observed a nonlinear association of ASCA and 90-day mortality. 90-day survival was used as outcome variable and ASCA alone as well as MELD and ASCA were used as predictors. The resulting AUĆs were 0.756 (95% CI 0.66–0.85) for the ASCA-Model alone and 0.85 for the ASCA-MELD (95% CI 0.74–0.90) compared to 0.724 (95% CI 0.62 −0.83) for the MELD score, with a significant improvement of ASCA-MELD compared to the MELD score (Delong p value=0.0059, Figure 4E). When we compared the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at cut-offs, defined by the Youden Index, the diagnostic performance of the ASCA-MELD was the most precise (ASCA-MELD, sensitivity 74%, specificity 81%, PPV 67%, NPV 86%, MELD score, sensitivity 63%, specificity 76%, PPV 58%, NPV 80%, ASCA-Model sensitivity 97%, specificity 47%, PPV 49%, NPV 97%). Taken together, adding ASCA, significantly improved the established MELD score to predict 90-day mortality.
Mycobiome profile and ASCA levels among alcohol use disorder patients
To investigate differences in the gut mycobiota composition and ASCA levels among alcohol use disorder patients, we built three subgroups according to the non-invasive fibrosis-4 (FIB-4) index. Patients in the intermediate (n=6) and high FIB-4 (n=4) subgroup had a higher Candida abundance as compared with the low subgroup (n=4), but the differences were not significantly different (Supplementary Figure S2G-H). Diversity indices among the subgroups are shown in Supplementary Figure S2A-C. Correlations of clinical parameters with diversity and fungal abundance at genus level are shown in Supplementary Figure S2D and with ASCA in Supplementary Figure S2E. ASCA levels did not differ in alcohol use disorder patients with low, intermediate or high FIB-4 score (Supplementary Figure S2F). However, this analysis is limited by the relative small number of patients in each subgroup and requires confirmation in larger patient cohorts.
Longitudinal analysis
For a subset of 24 alcoholic hepatitis patients, longitudinal follow-up serum samples were available. For 7 patients, day-90 follow-up fecal samples were available. Clinical characteristics of these subgroups are reported in Supplementary Table S4 (longitudinal mycobiome analysis) and Supplementary Table S5 (longitudinal serum analysis). Except for one patient, all patients were abstinent from alcohol abuse at day-90 (Supplementary Table S4 and S5). Despite clinical improvement in clinical parameters as evidenced by an increase in albumin, a decrease in MELD and improved intestinal permeability based on zonulin levels, ASCA levels slightly increased (Figure 4F). Serum LPS-BP levels did not significantly change in day-90 follow-up (Supplementary Figure S3B). Within alcoholic hepatitis patients, ASCA did not correlate with zonulin levels at baseline. This indicates that ASCA is not simply a marker for increased intestinal permeability but other factors such as immune dysfunction might play a role in the regulation of the response to fungal products. Further, the fungal microbiota composition and diversity did not change uniformly (Supplementary Figure S4).
Discussion
This study describes the intestinal mycobiome and the host immune response to fungi and fungal products in patients with alcoholic hepatitis. A reduced fungal diversity with an overgrowth of Candida was observed in patients with alcohol-associated liver disease. Furthermore, patients with alcoholic hepatitis had higher serum ASCA levels compared with non-alcoholic controls and patients with alcohol use disorder, indicating more translocation through the gut barrier and an increased systemic immune response to fungi. High ASCA levels were associated with increased 90- and 180-day mortality and most importantly, this effect was independent from the MELD score, the presence of an infection or treatment with steroids, antibiotics or pentoxifylline as well as from LPS-BP as marker for bacterial translocation.
Chronic excess consumption of alcohol damages the gut mucosal barrier leading to translocation of microbial products from the intestine into the portal blood stream and thence into the liver, and thereafter translocation of pathogens to the systemic circulation. We have shown that dysbiosis-induced intestinal inflammation increases intestinal permeability in a preclinical model of alcoholic liver disease (6). Patients with liver cirrhosis are frequently colonized with fungi (35) and are at higher risk for fungal infections (36, 37). With a concomitant dysregulated immune regulation in advanced liver diseases, an invasive mycosis has a poor prognosis. Spontaneous fungal peritonitis is a severe complication in liver cirrhosis (38) and invasive aspergillosis is a frequent complication in patients with alcoholic hepatitis that carries a very high risk of mortality (11) .
Similar to our study, Bajaj et al. described fungal dysbiosis with an overgrowth of Candida in 143 patients with liver cirrhosis, 34% of whom had alcohol-related liver disease (21). In their study, prior treatment with antibiotics, whether non-absorbable rifaximin or absorbable cephalosporins, increased fungal dysbiosis (21). We did not observe a similar effect in patients with alcoholic hepatitis. Furthermore, in our study, treatment with corticosteroids or pentoxifylline had no effect on the abundance of Candida or fungal diversity.
To our knowledge, the present study is the first description of an association of ASCA with mortality in alcoholic hepatitis. Interestingly, high ASCA levels were not associated with the presence of any or specific fungal infection at admission in our study and the association with mortality was observed independently of the presence of an infection. Fungal infections however might be underreported in our study since the incidence in our cohort was lower than described in the literature (10, 39) and in most of the cases, no pathogen causing the infection was found in culture.
High ASCA levels were observed exclusively in the alcoholic hepatitis cohort. This may indicate that an increased ASCA level is not only a consequence of persistent fungal overgrowth with Candida, but also requires dysregulated intestinal barrier function, perhaps as a result of recent exposure to ethanol. This was reflected by increased zonulin levels only in alcohol hepatitis patients, similar to ASCA. Furthermore, patients with alcoholic hepatitis exhibit multimodal activation of immune processes, in addition to disruption of the mucosal barrier (40), so that defective mucosal phagocytosis of fungi could result in an enhanced systemic immune response to fungi. Patients with alcohol use disorder are also affected by increased intestinal permeability (41), a functioning mucosal phagocytosis, however, might lead to a lower systemic immune response in these patients.
Intestinal fungal dysbiosis has also been observed in other diseases with defects in the intestinal barrier function such as IBD (17). Moreover, increased ASCA level are observed in IBD (42) and celiac disease, which is associated with a different mucosal barrier disruption (43).
Whereas a causative role of fungi driving disease severity in IBD has not been clearly demonstrated (44), fungal dysbiosis contributes to ethanol-induced steatohepatitis in mice. In our previous studies, reducing the fungal mycobiota using non-absorbable antifungal drugs ameliorated liver injury and resulted in lower systemic levels of 1,3-ß-D-glucan, a cell wall component of many fungi (16). We further showed that patients with liver cirrhosis due to alcohol abuse had higher ASCA levels compared to patients with chronic hepatitis B related liver cirrhosis and to controls. Finally, we showed that the mortality was greater in patients with alcohol-associated cirrhosis and high ASCA values (16).
One strength of the present study is the multicenter design with subjects from 6 countries in North America and Europe with different ethnic backgrounds. Limitations are the relatively small patient numbers in the respective groups. An independent, prospective cohort is required to validate our findings and predictive model. One third of our alcoholic hepatitis patients received treatment with corticosteroids and one fourth were treated with antibiotics, this might have had an influence on the fungal composition even though we did not observe a lower diversity or higher Candida abundance.
In summary, patients with alcohol-dependent liver disease have lower fungal diversity with an overgrowth of Candida compared to controls. Patients with alcoholic hepatitis, but not patients with alcohol use disorder or non-alcoholic controls, demonstrate significantly elevated ASCA levels, and an elevated serum ASCA level is associated with increased mortality. Intestinal fungi may serve as a therapeutic target to improve survival in alcoholic hepatitis and ASCA in combination with MELD may be useful to predict the outcome in patients with alcoholic hepatitis.
Supplementary Material
Acknowledgements:
This study was supported in part by the Deutsche Forschungsgemeinschaft (DFG) grant LA 4286/1–1 (to S.L.), NIH grants R01 AA020703, R01 AA24726, U01 AA021856, U01 AA026939 and by Award Number BX004594 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to B.S.) and NIH grant UL1TR001442 (to X.M.T).
Abbreviations:
- AH
alcoholic hepatitis
- AUD
alcohol use disorder
- Ctrl
non-alcoholic controls
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- AUROC
area under the receiver operating curve
- FIB-4
fibrosis-4 Index
- GGT
gamma-glutamyl-transferase
- INR
international normalized ratio
- BMI
body mass index
- LPS-PB
lipopolysaccharide binding protein
- MELD
Model for End-stage Liver Disease
- MELDNa
Sodium Model for End-stage Liver Disease
- Maddrey’s DF
Maddrey’s discriminant function
- PPV
positive predictive value (PPV)
- NPV
negative predictive value
- n.s.
not significant
Footnotes
Conflict of interest: D.S.: Advisory Boards: Shionogi and Norgine; Paid Lectures: Falk Pharma and Norgine; Funding: Norgine. B.S. is consulting for Ferring Research Institute. Other authors have no conflicting financial interests.
References
- 1.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013;59:160–168. [DOI] [PubMed] [Google Scholar]
- 2.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 3.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995;346:987–990. [DOI] [PubMed] [Google Scholar]
- 4.Mathurin P, Abdelnour M, Ramond MJ, Carbonell N, Fartoux L, Serfaty L, Valla D, et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology 2003;38:1363–1369. [DOI] [PubMed] [Google Scholar]
- 5.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015;61:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starkel P, Schnabl B. Bidirectional Communication between Liver and Gut during Alcoholic Liver Disease. Semin Liver Dis 2016;36:331–339. [DOI] [PubMed] [Google Scholar]
- 8.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–839. [DOI] [PubMed] [Google Scholar]
- 9.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, Kumar G, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol 2017;15:600–602. [DOI] [PubMed] [Google Scholar]
- 10.Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, Forrest EH, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017;152:1068–1077.e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustot T, Maillart E, Bocci M, Surin R, Trepo E, Degre D, Lucidi V, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol 2014;60:267–274. [DOI] [PubMed] [Google Scholar]
- 12.Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int 2016;36:721–728. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007;8:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012;336:1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122:3476–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, Bluemel S, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest 2017;127:2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, et al. Fungal microbiota dysbiosis in IBD. Gut 2017;66:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, Baldassano RN, et al. Fungal Signature in the Gut Microbiota of Pediatric Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis 2015;21:1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, Drews O, et al. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand J Gastroenterol 2008;43:831–841. [DOI] [PubMed] [Google Scholar]
- 20.Mar Rodriguez M, Perez D, Javier Chaves F, Esteve E, Marin-Garcia P, Xifra G, Vendrell J, et al. Obesity changes the human gut mycobiome. Sci Rep 2015;5:14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj JS, Liu EJ, Kheradman R, Fagan A, Heuman DM, White M, Gavis EA, et al. Fungal dysbiosis in cirrhosis. Gut 2018;67:1146–1154. [DOI] [PubMed] [Google Scholar]
- 22.Ball SA, Tennen H, Poling JC, Kranzler HR, Rounsaville BJ. Personality, temperament, and character dimensions and the DSM-IV personality disorders in substance abusers. J Abnorm Psychol 1997;106:545–553. [DOI] [PubMed] [Google Scholar]
- 23.Brandl K, Hartmann P, Jih LJ, Pizzo DP, Argemi J, Ventura-Cots M, Coulter S, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol 2018;69:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 25.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011;53:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fouts DE, Szpakowski S, Purushe J, Torralba M, Waterman RC, MacNeil MD, Alexander LJ, et al. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS One 2012;7:e48289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bokulich NA, Thorngate JH, Richardson PM, Mills DA. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci U S A 2014;111:E139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor DL, Walters WA, Lennon NJ, Bochicchio J, Krohn A, Caporaso JG, Pennanen T. Accurate Estimation of Fungal Diversity and Abundance through Improved Lineage-Specific Primers Optimized for Illumina Amplicon Sequencing. Appl Environ Microbiol 2016;82:7217–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokulich NA, Mills DA. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol 2013;79:2519–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003;43:121–137. [Google Scholar]
- 31.Hastie TJ, Tibshirani RJ. Generalized Additive Models: Taylor & Francis, 1990. [Google Scholar]
- 32.Wood SN. Thin plate regression splines. Journal of the Royal Statistical Society Series B 2003;65:95–114. [Google Scholar]
- 33.Kowalski J, Tu XM. Modern Applied U-Statistics: Wiley, New York, 2007. [Google Scholar]
- 34.Standaert-Vitse A, Jouault T, Vandewalle P, Mille C, Seddik M, Sendid B, Mallet JM, et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006;130:1764–1775. [DOI] [PubMed] [Google Scholar]
- 35.Lahmer T, Messer M, Mayr U, Saugel B, Noe S, Schultheiss C, Thies P, et al. Fungal “colonisation” is associated with increased mortality in medical intensive care unit patients with liver cirrhosis. Mycopathologia 2015;179:63–71. [DOI] [PubMed] [Google Scholar]
- 36.Park WB, Choe YJ, Lee KD, Lee CS, Kim HB, Kim NJ, Lee HS, et al. Spontaneous cryptococcal peritonitis in patients with liver cirrhosis. Am J Med 2006;119:169–171. [DOI] [PubMed] [Google Scholar]
- 37.Bartoletti M, Giannella M, Caraceni P, Domenicali M, Ambretti S, Tedeschi S, Verucchi G, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol 2014;61:51–58. [DOI] [PubMed] [Google Scholar]
- 38.Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, Yoon JH, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis 2014;33:259–264. [DOI] [PubMed] [Google Scholar]
- 39.Parker R, Im G, Jones F, Hernandez OP, Nahas J, Kumar A, Wheatley D, et al. Clinical and microbiological features of infection in alcoholic hepatitis: an international cohort study. J Gastroenterol 2017;52:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhanda AD, Collins PL. Immune dysfunction in acute alcoholic hepatitis. World J Gastroenterol 2015;21:11904–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 2012;26:911–918. [DOI] [PubMed] [Google Scholar]
- 42.Vermeire S, Peeters M, Vlietinck R, Joossens S, Den Hond E, Bulteel V, Bossuyt X, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis 2001;7:8–15. [DOI] [PubMed] [Google Scholar]
- 43.Granito A, Muratori L, Muratori P, Guidi M, Lenzi M, Bianchi FB, Volta U. Anti-saccharomyces cerevisiae antibodies (ASCA) in coeliac disease. Gut 2006;55:296. [PMC free article] [PubMed] [Google Scholar]
- 44.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017;14:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.