Abstract
Objective:
As the perceived risk of cannabis use continues to decline among youths and access continues to increase, it becomes more important to synthesize the rapidly growing literature on the effects of cannabis on neurocognition. Hundreds of studies examining associations between cannabis use and neurocognitive functioning have been published in recent decades. However, results often differ across individual studies, particularly when sample sizes are small. Meta-analytic methods help to make sense of this literature and have been increasingly applied to studies on cannabis use and neurocognition.
Methods:
A systematic literature search using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was conducted to identify peer-reviewed meta-analyses of neurocognitive or functional neuroimaging data that examined associations between cannabis use and non-acute effects on neurocognitive functioning (n = 8).
Results:
Current findings suggest that regular healthy cannabis users, regardless of age, display poorer neurocognitive functioning relative to nonusers of small to medium effect sizes across many neurocognitive domains, as well as functional brain alterations when compared to non-users. Adverse effects are not uniform across neurocognitive domains and evidence for adolescent-onset users having poorer neurocognitive outcomes remains equivocal based on these studies. However, less is known about cannabis effects on neurocognition among clinical samples, as findings from specific clinical samples revealed mixed results.
Conclusions:
Meta-analyses have played an important role in helping to grasp the totality of results from a large body of literature on cannabis effects on neurocognition, yet more research (particularly large-scale longitudinal studies) is needed to identify critical periods or patterns of use that are more likely to result in negative outcomes.
Keywords: cannabis, neuropsychology, neurocognition, review, meta-analysis
Introduction:
Cannabis is the most commonly used psychoactive substance worldwide (WHO, 2018). Within the United States (U.S.), changes in state legislature have allowed for medicinal and recreational cannabis use to rapidly expand in recent years. In 1996, California became the first state to legalize medical cannabis use, with 32 additional states and the District of Columbia amending their laws for medical consumption as of November 2018 (Hasin, 2018). In addition to the changes in state legislation, public interest on the effects cannabinoids has grown considerably. Thus, while public interest has brought cannabis to the forefront of national media discussions, disentangling the effects of cannabis use on neurocognition at varying ages continues to generate scientific debate.
In recent years, neurocognitive research investigating the effects of cannabis use has grown rapidly. To illustrate this, a PubMed search using keywords “cannabis OR marijuana” AND “neuropsych*” yielded a total of 254 publications prior to 2005 and 1,239 as of November 2018. This growth in research is motivated, in part, by concerns about increasing cannabis use among youth given recent declines in perceived risk (The Monitoring the Future study, the University of Michigan [2016]). Additionally, trends to reduce or eliminate penalties for cannabis possession along with public perceptions of cannabis as medicine (Hoffman & Weber, 2010) have fueled debate across many countries. Although the extant literature has progressed our understanding on the effects of cannabis use on neurocognition, it has not been immune to problems of replication, with disparate and at times contradictory results. Cross-sectional designs, small sample sizes, publication bias, and poorly characterized samples with suboptimal control of potential confounds have all likely contributed to this situation.
Additionally, an accumulating body of research suggests mixed findings regarding poorer outcomes with early and persistent use of cannabis during adolescence. Thus, contrasting findings make it difficult to clearly delineate the lasting impact and magnitude of adverse cannabis effects on brain function and neurocognition across the lifespan. During adolescence, the brain is undergoing extensive neurobiological changes (Casey, 2008), including protracted development of white matter and increased neuronal pruning, which may make it more susceptible to neurotoxic effects of cannabis (Lubman, Cheetham, & Yücel, 2015). Findings from preclinical work suggest that cannabinoid exposure during early life in rodents, when compared to exposure during adulthood, has been associated with greater memory deficits and hippocampal alterations influencing both brain and behavior, with effects lasting into adulthood (O’Shea, Singh McGregor and Mallet, 2004; Tapert et al., 2008). Similarly, findings from human subjects research not only suggest that adolescent cannabis use is associated with neurocognitive deficits related to learning and memory, but also indicate possible macrostructural brain alterations and atypical neural functioning which may contribute to lasting neurocognitive impairments (Jacobus and Tapert, 2014; Tapert, Schweinsburg, & Brown, 2008). These impairments may represent adverse effects of exogenous cannabinoids on neuromaturation in regions rich in cannabinoid receptors, such as the dorsolateral prefrontal cortex, basal ganglia, and hippocampus (Iversen, 2003). Unsurprisingly, these structures are also heavily implicated in neurocognitive functions reported to be affected by cannabis use. As a result, evidence from rodent and human subject studies suggests that use during adolescence may have the more deleterious effects on the brain and neurocognitive functioning than among adults. However, other studies have found no effect of age of onset on neurocognition (Slomiak, Jones, Rosen, Moore, & Gur, 2018; Tait, Mackinnon, & Christensen, 2011).
To date, numerous narrative reviews have attempted to synthesize and reconcile current findings (e.g., Broyd, Van Hell, Beale, Yuecel, & Solowij, 2016; Gonzalez, Pacheco-Colón, Duperrouzel, & Hawes, 2017; Ganzer et.al, 2016; Lorenzetti et al., 2016; Volkow et al., 2016; Crane, Schuster, Fusar-Poli, & Gonzalez, 2013). There has been a notable degree of consensus that users, on average, show poorer neurocognitive performance (i.e., memory, attention, and executive functions) than non-users. However, there has been much less agreement about individual differences (i.e., age of onset or frequency of use) that may account for the large variability in neurocognitive performance observed among users. Meta-analytic approaches are well-suited to address these lingering questions. Meta-analyses utilize statistical techniques that quantitatively synthesize prior findings across studies, thereby increasing overall sample size and statistical power to detect small effects. This can provide more clarity in interpretation than single studies with disparate findings, particularly when sample sizes in individual studies are relatively small. Additionally, with a substantially large number of studies, meta-analyses are able to examine the effects of study-specific factors (e.g., age of participants, length of abstinence) on observed effect size differences between cannabis users and non-users. In recent years, several meta-analyses have been published on this topic. In this review article, we present results from these studies and discuss their implications for our understanding of the current literature on the non-acute effects of cannabis on neurocognitive functioning.
Methods
Literature Search:
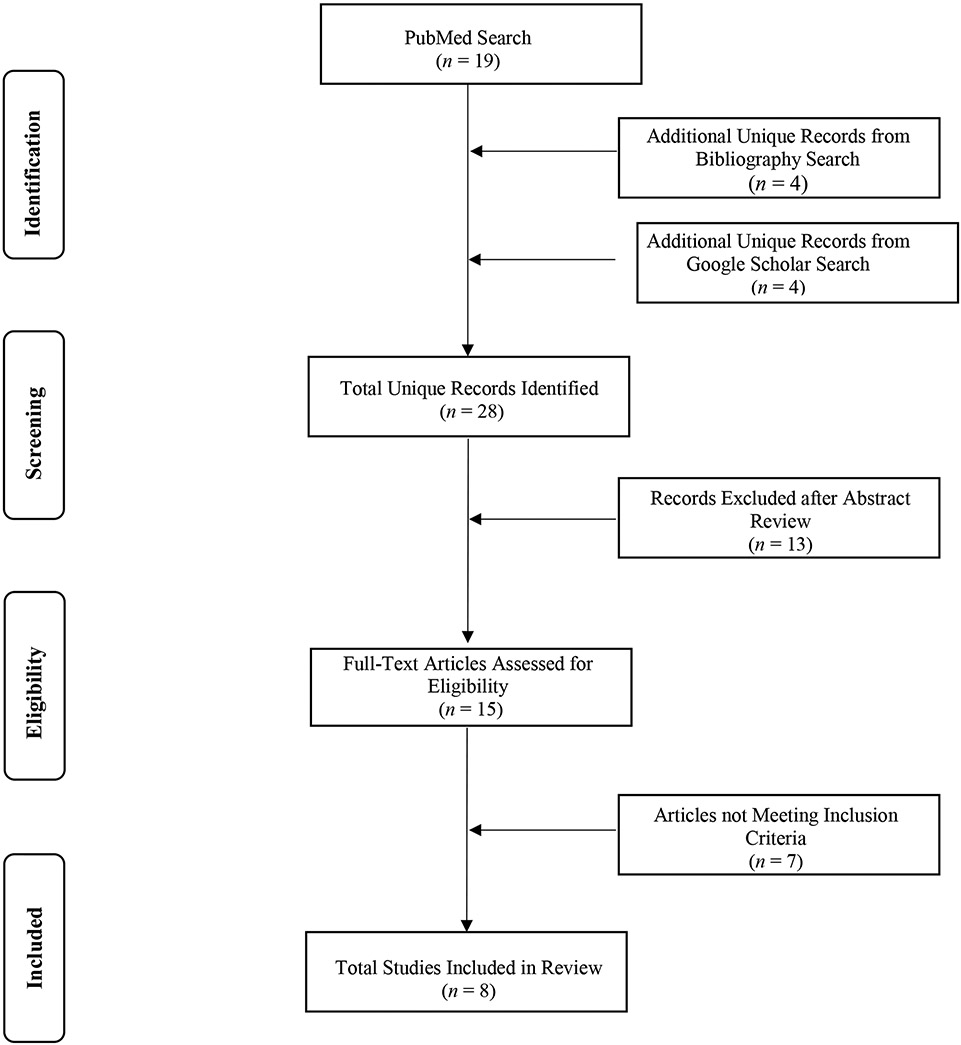
Literature searches were conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2015) and Figure 1 illustrates our article screening process. Our initial search of peer-reviewed meta-analyses investigating the effects of cannabis use on neurocognitive functioning in adolescent and/or adult samples was conducted in PubMed in April 2019, using the combination of terms: ((“cannabis” OR “marijuana”) AND (“cognition” OR “neurocog*” OR “neuropsych*”)), in conjunction with the PubMed article filter set to “meta-analyses” only. We then reviewed the reference sections of these studies for additional relevant studies and augmented our search with GoogleScholar. We included peer-reviewed English-language meta-analyses that assessed neurocognitive functioning via neuropsychological testing (i.e., decision-making, episodic memory, attention etc.) or used functional neuroimaging task-based methods to examine non-acute effects of cannabis on neurocognition in human participants. Additional inclusion criteria were a main focus on effect sizes comparing a cannabis-using group to an appropriate non-cannabis using control group, cannabis user status for all participants in the cannabis-using group, and cannabis as the primary substance used by individuals in the cannabis-using group. Studies examining the effects of alcohol and nicotine use in conjunction with cannabis were retained due to the known frequent comorbidity of use among cannabis users. We excluded studies where cannabis was not the primary substance of interest, studies that included individuals who did not currently use cannabis in their cannabis-using group, studies that included cannabis users in the control group, studies that did not include data on neurocognitive functioning, studies that focused on structural neuroimaging findings, studies that included participants with substance use disorders for drugs other than alcohol, nicotine, or cannabis in their cannabis-using group, and studies with a non-meta-analytic primary focus. This search process resulted in eight studies that fulfilled all the above-mentioned criteria. Study characteristics are listed in Tables 1 & 2 by year of publication.
Figure 1.
PRISMA flow diagram of meta-analyses considered and selected for review.
Table 1.
Characteristics of Reviewed Behavioral Meta-Analyses
| Behavioral Meta-Analyses | |||||
|---|---|---|---|---|---|
| Study | Sample Characteristics | # of Studies Included |
Years Covered |
Cannabis User Group Characteristics | Groups |
| Grant, et al. (2003, a) | N = 1,032 | 11 | 1977-2002 | Cannabis users, abstinent day of testing | Cannabis users n = 623 Non/Minimal users n = 409 |
| Grant, et al. (2003, b) | N = 1,188 | 15 | 1973-2002 | Cannabis users | Cannabis users n = 704 Controls n = 484 |
| Rabin, et al.(2011) |
N = 942 Cannabis users x̅ age = 28.7 Non-users x̅ age = 32.4 |
8 | 2005-2010 | Schizophrenia diagnosis with comorbid cannabis use | Schizophrenia with cannabis use n = 356 Schizophrenia without cannabis use n = 586 |
| Schreiner & Dunn (2012, a) | N = 1,849 | 33 | 2000-2011 | Cannabis users, abstinent day of testing | Cannabis users n = 1,010 Controls n = 839 |
| Schreiner & Dunn (2012, b) | N = 775 | 13 | 2000-2011 | Abstinent (≥ 25 days) cannabis users | Abstinent cannabis users n = 388 Controls n = 387 |
| Bogaty, et al. (2018) | N = 1,430 | 14 | 2006-2016 | Psychosis diagnosis with comorbid cannabis use (≤ 1x week in past 6 months) | Psychosis with cannabis use n = 529 Psychosis without cannabis use n = 901 |
| Scott, el al. (2018) |
N = 8,727 Cannabis users x̅ age = 20.6 Controls x̅ age = 20.8 |
69 | 1973-2017 | Heavy, frequent, and/orproblematic cannabis use | Cannabis users n = 2,152 Controls n = 6,575 |
| Study | Additional Substance Use Exclusions |
Neurocognitive Domains Examined | Neurocognitive Domains Adversely Impacted by Cannabis Use |
||
| Grant, et al. (2003, a & b) | Polydrug/other drug use | Simple reaction time, attention, verbal/language, abstraction/executive, perceptual/motor, motor, learning, and forgetting. | Learning, forgetting, and overall neurocognitive performance. | ||
| Rabin, et al.(2011) | Other drug, alcohol, or polydrug use disorder. | General cognitive ability and intelligence, Selective, sustained and divided attention, Executive abilities, Working memory and learning, Retrieval and recognition, Receptive and expressive language abilities, Visuo-spatial and construction abilities. | (All domains showed better neurocognitive functioning.) | ||
| Schreiner & Dunn (2012, a) | Other drug use. | Abstraction/executive, attention, forgetting/retrieval, learning, motor, perceptual-motor, simple reaction time, verbal/language. | Global Neurocognitive performance, Abstraction/executive, attention, forgetting/retrieval, learning, motor, and verbal/language. | ||
| Schreiner & Dunn (2012, b) | Other drug use. | Abstraction/executive, attention, forgetting/retrieval, learning, motor, perceptual-motor, simple reaction time, verbal/language. | None. | ||
| Bogaty, et al. (2018) | Polydrug use. | Processing speed, sustained attention, cognitive flexibility, working memory (verbal), verbal learning, verbal memory, conceptual set-shifting, verbal fluency, motor inhibition, current IQ, premorbid IQ. | Premorbid IQ, current IQ, verbal learning, working memory (verbal), and motor inhibition. (Conceptual set shifting showed better performance). |
||
| Scott, et al. (2018) | Other drug use. | Attention, learning, delayed memory, speed of information processing, verbal/language, visuospatial, motor functioning, and executive functioning (abstraction/shifting, inhibition, and updating/working memory). | Attention, learning, delayed memory, speed of information processing, and executive functioning (abstraction/shifting, inhibition, and updating/working memory). | ||
Table 2.
Characteristics of Reviewed Neuroimaging Meta-Analyses
| Neuroimaging Meta-Analyses | |||||
|---|---|---|---|---|---|
| Study | Sample Characteristics | # of Studies Included |
Years Covered |
Cannabis User Group Characteristics | Groups |
| Blest-Hopley, et al. (2019) | N = 755 | 12 | 2004-2015 | Cannabis users, ≤ 48 hours of abstinence | Current cannabis users n = 361 Controls n = 394 |
| Blest-Hopley, et al. (2018, a) | N = 1,553 | 20 (13 adult, 7 adolescent studies) |
2004-2015 | Current (≤ 1x week) and abstinent (> 48 hours) cannabis user groups | Adult cannabis users n = 530 Adult controls n = 580 Adolescent cannabis users n = 219 Adolescent controls n = 224 |
| Blest-Hopley, et al. (2018, b) |
N = 204 x̅ age= 17.8 |
3 | 2004-2015 | Abstinent (≥ 25 days) cannabis users | Abstinent cannabis users n = 98 Controls n = 106 |
| Yanes, et al. (2018) |
N = 1,272 Cannabis users x̅ age = 24.0 Controls x̅ age = 24.3 |
35 | 2002-2016 | N/A | Cannabis users n = 647 Controls n = 625 |
| Neuroimaging Meta-Analyses | |||||
| Study | Additional Substance Use/Imaging Exclusions | Functional Brain Alterations Observed in Cannabis Users | |||
| Blest-Hopley, et al. (2019) | Other drug use (excluding alcohol, nicotine, and cannabis use); ROI-only analyses | Greater activation in medial frontal gyrus, right insula. Lower activation in left cuneus, occipital gyri, and right precentral gyrus. |
|||
| Blest-Hopley, et al. (2018, a) | Other drug use (exception of alcohol, nicotine, and cannabis use); region-of-interest ROI-only analyses |
Adults: Greater activation in left superior temporal gyrus, right inferior frontal gyrus, left posterior transverse temporal gyrus. Lower activation in left striate area, left area piriformis insulae and right middle frontal gyrus. Adolescents: Greater activation in right inferior parietal gyrus, and right putamen. |
|||
| Blest-Hopley, et al, (2018, b) | Other drug use (excluding alcohol, nicotine, and cannabis use); ROI-only analyses | Greater activation in precuneus, middle frontal gyrus, superior frontal gyrus, and angular gyrus. | |||
| Yanes, et al. (2018) | Co-use of other substances other than alcohol, nicotine, or cannabis; ROI-only analyses | Greater activation in striatum. Lower activation in anterior cingulate cortex, and dorsolateral prefrontal cortex. |
|||
Note. Several meta-analyses included subset analyses with multiple calculations of between-group differences and characteristics for each of these analyses is presented above. ROI = region of interest.
Results
Review of Meta-Analyses on Cannabis Use and Neurocognitive Functioning:
To date, four meta-analyses have attempted to quantitatively synthesize research examining the non-acute effects of cannabis use on neurocognitive performance. The first, conducted by Grant, Gonzalez, Carey, Natarajan, and Wolfson (2003), explored non-acute (residual) effects of cannabis use in 11 studies with a total of 623 adult long-term users and 409 non-or minimal users. Study inclusion criteria consisted of samples with a cannabis-only group, a non-drug or minimal cannabis use control group, valid neurocognitive testing, information to calculate an effect size for between-group differences in neurocognitive performance, cannabis abstinence on day of testing, and relevant data on duration of cannabis abstinence, substance co-use, and neurological and psychological history. Within their analyses, neurocognition was assessed across eight domains including simple reaction time, attention, verbal/language, abstraction/executive, perceptual/motor, motor, learning, and forgetting. Standardized mean difference scores (i.e., effect sizes) and respective variances for each neurocognitive measure were generated for the control and cannabis use groups, and confidence intervals were compared to each other. A global neurocognitive effect size was also calculated by pooling across all neurocognitive domains and comparing between groups. The results revealed small adverse effects of cannabis on global neurocognition (ES = −0.15, 99% CI [−0.29, −0.02]), learning (ES = −0.21, 99% CI = [−0.39, −0.02]) and forgetting (ES = −0.27, 99% CI [−0.49, −0.04]), suggesting that regular users performed more poorly when compared to matched controls. Meta-regressions examining associations between neurocognition and amount of use or age of onset were not possible due to the relatively small number of studies included in the analyses that had such data.
Almost a decade later, Schreiner and Dunn (2012) conducted a meta-analysis that expanded upon Grant et al. (2003), in order to synthesize data from additional studies published since the original meta-analysis. Their analyses included 33 new studies, for a total of 1,010 cannabis users and 839 controls with limited or no cannabis use. The analyses used the same guidelines as Grant et al. (2003), creating eight neurocognitive domains for analyses. The global effect size for all assessed neurocognitive domains indicated a significant negative effect of cannabis on neurocognition, ES = −0.29, 95% CI [0.46, 0.12]. Examining effects on individual domains across the 33 samples included in the meta-analysis revealed significant differences between users and non-users on six of the eight neurocognitive domains (i.e., all except perceptual-motor and simple reaction time). Significant effect sizes were as follows: abstraction/executive ES = −0.21, 95% CI [−0.38, −0.05], attention ES = −0.36, 95% CI [−0.56, −0.16], forgetting/retrieval ES = −0.25, 95% CI [−0.47, −0.02], learning ES = −0.35, 95% CI [−0.55, −0.15], motor ES = −0.34, 95% CI [−0.57, −0.11], and verbal/language ES = −0.23, 95% CI [−0.47, −0.001].
The Schreiner and Dunn (2012) meta-analysis was able to take advantage of the rapidly growing cannabis literature and make use of substantially more data than was available at the time that the Grant et al. (2003) meta-analysis was published, thus improving their statistical power and the precision of confidence intervals. Schreiner and Dunn (2012) found effects of cannabis use across a greater number of neurocognitive domains than Grant et al. (2003), suggesting more widespread neurocognitive problems. However, the magnitude of the observed effects was similar, hovering around a quarter to a third of a standard deviation.
Schreiner and Dunn (2012) were also able to take advantage of a growing number of studies that had data on neurocognitive performance after participants underwent supervised abstinence from cannabis. They had a subset of 13 samples from the 33 included in the meta-analysis that specifically measured neurocognitive performance after at least one month of abstinence. This second analysis included 388 cannabis users, and 387 controls with limited or no cannabis use. Results from the second analysis found no significant effects on global cognition, or on any of the eight neurocognitive domains. This suggests that after approximately one month of abstinence no lasting residual effects of cannabis use on neurocognitive performance could be observed. Additional meta-regression analyses suggested that neither participant age nor duration of use were significant moderators of these associations. Their findings moved forward those of Grant et al (2003) by replicating the observed adverse, albeit modest, effects of cannabis on overall neurocognitive functioning. Importantly, their results provided strong evidence that adverse effects of cannabis use were reversible with abstinence. In contrast, there was no evidence for poorer outcomes associated with earlier cannabis use onset. The two aforementioned meta-analyses focused on studies of adult cannabis users. In order to better understand the effects that cannabis may have specifically on adolescents and young adults, Scott, Slomiak, Jones, Rosen, Moore, & Gur, (2018) published another meta-analysis on the non-acute effects of cannabis focusing on younger participants. Given the continued and rapid growth of research in this area, the Scott et al. (2018) meta-analysis is the largest to date. They analyzed 69 cross-sectional studies on adolescents and young adults with a mean age of samples ≤ 26 years. Study inclusion criteria were similar to those of the prior two meta-analyses, and included heavy cannabis use among users and a limited cannabis using group, relevant neurocognitive testing, and an adolescent or young adult age range. Studies were excluded if participants had past history of psychosis, prenatal exposure to cannabis, or were acutely intoxicated. Manuscripts with insufficient data, non-cross-sectional designs, IQ-only data measurements, or measures only administered during neuroimaging were excluded. Comparable to Grant et al. (2003), the data were separated into eight different domains: attention, learning, delayed memory, speed of information processing, verbal/language, visuospatial, motor functioning, and executive functioning. The increased data available allowed a more fine-grained subdivision of the domain of executive function (i.e., abstraction/shifting; inhibition; updating/working memory). Analyses were completed using a two-level mixed-effects multivariate model. The overall meta-analysis was composed of 8,727 participants. Cannabis users (n = 2,152) had a mean age of 20.6, were 68% male, and a mean age of 15.2 for cannabis use initiation. Comparison participants (n = 6,575) had minimal cannabis use, a mean age of 20.8, and were 55.8% male. Overall mean neurocognitive effect size was ES = −0.25, 95% CI [−0.32, −0.17], reflecting poorer neurocognitive functioning among cannabis users. Effect sizes were significant in the domains of learning ES = −0.33, 95% CI [−0.42, −0.24], executive functioning- abstraction/shifting ES = −0.30, 95% CI [−0.40, −0.20], speed of information processing ES = −0.26, 95% CI [−0.38, −0.16], delayed memory ES = −0.26, 95% CI [−0.35, −0.16], executive functioning-inhibition ES = −0.25, 95% CI [−0.38, −0.13], executive functioning-updating/working memory ES = −0.22, 95% CI= [−0.31, −0.12], and attention ES = −0.21, 95% CI [−0.31, −0.12], but not verbal/language, visuospatial, and motor functioning (p > .05). Thus, the overall magnitude of effects was similar to that observed in the prior meta-analyses, suggesting significant but modest adverse effects of cannabis on neurocognition, despite the younger age of participants.
Scott et al. (2018) also conducted additional analyses to determine what factors may influence the magnitude of observed effects. Importantly, the average age of participants in studies was not linked to the magnitude of effects sizes, and there were no relationships observed between age of cannabis use onset and neurocognitive performance. Additionally, follow-up analyses revealed that studies with treatment-seeking samples (n = 581; ES = −0.43, 95% CI [−.06, −0.24]) yielded larger effect sizes than non-treatment-seeking samples (n = 8,146; ES = −0.22, 95% CI [−0.29, −0.14]). This finding suggests that more problematic cannabis use may be associated with greater deficits in neurocognition than regular use. Finally, another set of analyses addressed the duration of adverse cannabis effects by stratifying studies based on the length of abstinence from cannabis use required at study entry. Those studies that required an abstinence period longer than 72 hours (n = 928) had an overall effect size that was non-significant (ES = −0.08, 95% CI [−0.22, 0.07]), and smaller than that observed in studies that required shorter durations of abstinence prior to testing. This suggested that even with a minimum of about three days of abstinence from cannabis, differences between users and non-users on neurocognition quickly diminished, thus arguing for the reversibility of adverse cannabis effects on neurocognition, even among younger subjects. Furthermore, results implied that a shorter length of abstinence and treatment-seeking status (presumably spurred by a cannabis use disorder) contributed to greater adverse effects of cannabis, rather than age of cannabis use onset or the age of the study sample.
Although numerous meta-analyses have been conducted with clinical samples, Rabin, Zakzanis, & George (2011) conducted a meta-analysis in which they examined associations between neurocognitive functioning and cannabis consumption among patients with schizophrenia. Unlike the prior meta-analyses reviewed, which relied on generally healthy controls, this study provides insights into effects of cannabis on a neurocognitively vulnerable clinical population. Furthermore, their study was unique in that, unlike similar meta-analyses involving clinical populations, it examined studies whose cannabis-using group consisted exclusively of users who were not abusing or dependent on other substances. Their search criteria resulted in eight studies analyzed for a total sample size of 942 patients of which 356 were cannabis-users and 586 non-users. Neurocognitive domains assessed included general cognitive ability and intelligence, selective, sustained, and divided attention, executive abilities, working memory and learning, retrieval and recognition, receptive and expressive language abilities, and visuo-spatial and construction abilities. Overall their results revealed that cannabis-using patients had better neurocognitive functioning than nonusers across all domains (general cognitive ability and intelligence ES = .48, selective, sustained, and divided attention ES = .35, executive abilities ES = .14, working memory and learning ES = .07, retrieval and recognition ES = .12, receptive and expressive language abilities ES = .06, and visuo-spatial and construction abilities ES = .33). Although the authors did not report confidence intervals, they did note that all effects were within the small to modest range. Although these results suggest that cannabis use may be associated with better neuropsychological functioning among individuals with schizophrenia, it is difficult to determine if these results generalize across other clinical populations.
Lastly, another recent meta-analysis explored cannabis effects on neurocognition in samples of adult patients with psychosis. Bogaty, Lee, Hickie, and Hermens (2018) identified studies on cannabis and neurocognition that included patients with a variety of psychosis- spectrum disorders. Inclusionary criteria consisted of diagnosis of a psychotic disorder according to the DSM or ICD; patients under the age of 25; groups comparing a psychotic cannabis-using group to a non-using clinical control group; cannabis used as the primary substance used by patients; and assessment of neurocognitive functioning by reliable and valid tests. Exclusionary criteria consisted of acute intoxication at time of testing, use of synthetic cannabis, investigation of solely tetrahydrocannabinol (THC) or cannabidiol (CBD), and/or a diagnosis of a substance/medication-induced psychotic disorder. This meta-analysis included 14 studies with 1,430 patients with psychosis (n = 529 with comorbid cannabis use and n = 901 without comorbid cannabis use). Several neurocognitive domains were examined including current and premorbid IQ, processing speed, cognitive flexibility, sustained attention, verbal learning, verbal memory, verbal working memory, conceptual set-shifting, motor inhibition, and verbal fluency. Findings revealed that patients who used cannabis performed worse across tests of premorbid IQ ES = −.40, 95% CI [ −0.59, −0.20] and current IQ ES = −.17, 95% CI [−0.34, 0.00], verbal learning ES = −.39, 95% CI [−0.8, .004], verbal working memory ES = −.76, 95% CI [−1.30, −0.22], and motor inhibition ES = −.19, 95% CI [−0.40, −0.02] than non-using patients. Surprisingly, the authors noted there was a trend (p < .10) suggesting that patients who used cannabis performed better in set-shifting when compared to non-users. Despite this finding, further research is needed to determine whether select neurocognitive domains may benefit from cannabis use within clinical samples. Overall, however, evidence indicates greater neurocognitive deficiencies in psychosis patients that use cannabis compared to those who do not. The magnitude of these effects are in a range consistent with those in the aforementioned meta-analyses, but appear to be slightly larger.
Taken together, results from three independent meta-analyses reveal consistent evidence that cannabis use is associated with poorer neurocognitive functioning overall and across most neurocognitive ability areas among non-clinical populations. On the other hand, two studies with samples including individuals with schizophrenia and psychotic disorders respectively came to different conclusions on the effects cannabis has on neurocognitive domains. It is clear that our understanding on the non-acute effects of cannabis on neurocognition would benefit from more meta-analyses focusing on specific clinical populations, as it appears that effects found with healthy samples may not generalize to clinical samples. Despite statistically significant findings, the magnitude of these effects is relatively modest. Furthermore, although individual manuscripts, narrative reviews, and theoretical evidence have suggested that an earlier age of cannabis use onset may be more deleterious (Crane, Schuster, Mermelstein, & Gonzalez, 2015; Meier et al., 2018; Schneider, 2008; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001; Shono, Edwards, Ames, & Stacy, 2018) for neurocognitive outcomes, the results emerging from the meta-analyses do not support this contention. The results from these meta-analyses also support the idea that adverse cannabis-related neurocognitive effects are likely reversible. Finally, the meta-analysis by Bogaty et al. (2018) suggests that results from otherwise healthy samples of cannabis users are comparable to a clinical sample with psychosis, but may not generalize to patients with a diagnosis of schizophrenia (Rabin et al., 2011). The growing body of literature and interest in this area will facilitate future meta-analyses that will have sufficient data to better examine study-level and participant-level characteristics that may influence the magnitude of observed cannabis effects in otherwise healthy samples and among specific clinical samples.
Review of Neuroimaging Meta-Analyses on Cannabis Use and Neurocognitive Functioning:
The aforementioned meta-analyses only include studies that employed behavioral neurocognitive measures to examine the possibility of adverse effects of cannabis on neurocognition. Such tests are typically administered via paper and pencil or via computer, with poorer performance on the task suggesting neurocognitive deficits. However, there is a rich functional neuroimaging literature examining the adverse effects of cannabis on brain functioning. Functional neuroimaging studies are able to provide additional information on specific brain structures that may be affected by use of cannabis and may be responsible for differences in neurocognitive performance. Indeed, such studies may reveal differences in brain activity between cannabis users and non-users even in the absence of differences in performance on neurocognitive tasks.
Several narrative reviews have summarized findings from functional neuroimaging studies of cannabis users (e.g., Batalla et al., 2013; Martin-Santos et al., 2010; Quickfall & Crockford, 2017). Here we present results from three meta-analyses that have been conducted specifically with functioning neuroimaging data. The first functional neuroimaging meta-analysis, conducted by Blest-Hopley, Giampetro, & Bhattacharyya (2018), investigated the effects of prolonged cannabis use (> 50 times of lifetime use) on brain function in adolescents and adults. Their literature search identified 13 manuscripts with adult samples (n = 530 cannabis users and n = 580 controls) and seven with adolescent samples (n = 219 cannabis users and n = 224 controls) in which cannabis users and cannabis-naïve healthy controls completed a neurocognitive task in the scanner (e.g., Go/No-Go, Iowa Gambling Task, Attention Network Task) during functional magnetic resonance imaging (fMRI) acquisition. Exclusion criteria consisted of minimal levels of cannabis use (< 50 times), use of positron emission tomography (PET) imaging, and comparisons between current and abstinent cannabis users only. Across individual studies, task stimuli did not involve cannabis-related cues and all findings were reported using whole-brain imaging analyses. All participants also abstained from consuming cannabis a minimum > 3 hours prior to scanning. To conduct the meta-analysis, seed-based d mapping (SDM; Sdmproject.com, 2017) was used to compute peak-based estimates for convolved anisotropic kernels (i.e., a matrix applied around voxels; Radua et al., 2014) and compare across participant groups. In other words, differences in brain activation were examined by incorporating voxel location, t-statistic values, and participant sample size to determine whole-brain activation differences across groups. Separate meta-analyses were employed for adult and adolescent users. Findings from the adult analyses revealed different patterns of activation for users when compared to non-users. Specifically, cannabis-using adults had greater activation in the left superior temporal gyrus, middle temporal gyrus, and right inferior frontal gyrus compared to controls during neurocognitive performance. Alternatively, users showed decreased activation in the cuneus. Comparably, adolescent findings revealed different patterns of activation in users relative to controls, with users having greater activation in the right inferior parietal gyrus and right putamen than non-users during neurocognitive task completion. Additionally, the authors explored whether differential task performance influenced activation differences by assessing a sub-sample of four studies reporting no performance differences between users and controls. Results with adult samples (n = 3) revealed greater activation in the posterior transverse gyrus and less activation in the middle occipital gyrus, postcentral gyrus, insula, and middle frontal gyrus compared to controls. The equivalent analysis conducted with an adolescent study (n = 1), found activation patterns previously reported remained unchanged. These findings suggest that prolonged cannabis use, regardless of age, is associated with altered neurobiological functioning compared to minimal or non-users. It is important to note that a wide variety of tasks were used to examine blood oxygenated level dependent (BOLD) differences between groups, resulting in significant task heterogeneity. Nonetheless, it appears that cannabis is associated with neurofunctional alterations in areas that are commonly recruited for higher order cognition including the frontal, parietal, and temporal lobe regions.
A follow-up meta-analysis to complement the above study was conducted by the same authors. Blest-Hopley, Giampietro, and Bhattacharyya (2019) examined how various lengths of abstinence affected the above-mentioned results. Utilizing the same literature search criteria as the aforementioned study, they identified and compared 12 studies that included “current” cannabis users abstinent for 48 hours or less, and three studies with users who were abstinent from cannabis for 600 or more hours. Similarly, SDM meta-analytic methods were employed and all voxels with peak-coordinates had an effect size and variance computed for analysis. Overall, three sets of analyses were conducted, which compared differences between current cannabis users (n = 361) versus non-users (n = 394); abstinent cannabis users (n = 98) versus non-users (n = 106; adolescent only sample); and current cannabis users (n = 361) versus abstinent cannabis users (n = 98). An important caveat is that the studies with abstinent (600+ hours) cannabis users consisted of an adolescent-only sample. Findings from the meta-analysis comparing current adult and adolescent cannabis users to non-users revealed that current users had increased activation in the medial frontal gyrus and right insula during neurocognitive task performance, as well as decreased activation in left cuneus, occipital gyri, and right precentral gyrus. These findings, which are consistent with the prior meta-analysis, suggest that current cannabis users display altered patterns of brain activity during neurocognitive task performance. When adolescent abstinent cannabis users were compared to non-users findings revealed greater activation in central executive and default mode networks, which include the precuneus, middle frontal gyrus, superior frontal gyrus, and angular gyrus during task completion. These networks were activated more in cannabis users when compared to controls during task completion. These findings suggest that greater neural activation across networks may be a neurological compensation mechanism for comparable task performance. Unlike current users, the abstinent cannabis users did not show decreased brain activation when compared to non-users but rather showed increased activation in several structures including bilateral inferior parietal lobule, right middle frontal gyrus, right middle occipital gyrus, right precuneus, and right inferior frontal gyrus. Lastly, prolonged abstinence (> 600 hours) was associated with increased activation in the precuneus, lingual gyrus, and inferior parietal lobule during neurocognitive task performance relative to current use.
In order to further delineate the effects of current use and prolonged abstinence among adolescent-only samples, the authors conducted two follow-up analyses. These included contrasts to compare current adolescent cannabis users with abstinent adolescent cannabis users and non-users. When comparing these adolescent-only samples, no differences in brain activation were found between current and abstinent users. However, when current adolescent users were compared to non-users, increased activation was reported in the frontal gyrus and occipital gyrus. Overall, the results presented by Blest-Hopley et al. (2018, 2019) further emphasize that cannabis use is associated with neurofunctional alterations across developmental ages. However, it appears that use during adolescence may be associated with lasting functional differences in central executive and default mode networks, despite extended periods of abstinence. These observable differences, which persist even after THC metabolites are no longer detected in urine, suggest that early use may be associated with lasting functional impairments. Although the authors did not examine functional abstinence differences with task performance, these results stand in contrast to the conclusions of the meta-analyses of behavioral data previously discussed, which suggested a return to “normal” function with abstinence.
Using a different meta-analytic approach than the prior two neuroimaging meta-analyses, Yanes et al. (2018) compared within-group and between-group activation using an activation likelihood estimation (ALE) meta-analysis framework (Laird et al., 2005). This approach utilizes BrainMap statistical software via the GingerAle v2.3.6 (http://brainmap.org/ale/) package and allows for the assessment of convergence of reported foci from different studies by modeling them as spatial probability (Gaussian) distributions at respective coordinates. In other words, this approach identified areas of brain activation that show agreement across studies. Inclusionary criteria consisted of neuroimaging studies that included comparisons between cannabis users and non-users, functional imaging acquisition, whole-brain voxel contrast, and completion of a neurocognitive, social neurocognitive, affective, perceptual and/or motor task performance during image collection. Additionally, any studies reporting only region of interest (ROI) or functional resting-state only were excluded. Thirty-five studies published prior to December 2016 met all criteria, resulting in data from 474 cannabis users, 466 non-users, and 202 extracted foci from 88 task-based contrasts. Contrasts of cannabis users compared to non-users during neurocognitive task completion revealed cannabis-related decreased activation in the anterior cingulate cortex and dorsolateral prefrontal cortex, with increased co-activation in the striatum and insula. These findings were interpreted to suggest that alterations in brain networks associated with cognition and reward sensitivity may underlie task performance disruptions among cannabis users.
Additionally, a functional decoding assessment was conducted by Yanes et al. (2018) to determine which neurocognitive domains may be most impacted based on their meta-analytic results. This approach estimates the likelihood that a voxel, network, or region will be activated by a neurocognitive process (e.g., working memory, fear) through forward- and reverse- inference analyses centered on specified regions of interest. Findings from these analyses revealed that the neurobiological pattern of differences observed between users and non-users appears to negatively impact learning, memory, reward, and pain processing.
It has only been in recent years that meta-analytic techniques have been applied to functional neuroimaging data in order to better understand the effects of cannabis use on neurocognition at the neurobiological level. Neuroimaging studies can greatly benefit from meta-analytic approaches, as they historically have suffered from smaller sample sizes and disparate findings (Button et al., 2013). Because these approaches are still relatively new, meta-analytic methods still vary across studies. Also, cross-study convergence in brain activation is usually examined in the context of many disparate neurocognitive tasks known to be dependent on different underlying brain structures. Therefore, less consensus was observed across the neuroimaging meta-analyses than the neurobehavioral studies. Nonetheless, the evidence does support differences in brain function between cannabis users and non-users which seem to seem to persist beyond abstinence. The specificity of these differences, their functional impact, and whether they predate or follow cannabis use remains to be determined.
Discussion
Over the last several decades, hundreds of studies have been published on associations between cannabis use and neurocognitive functioning. Their results, oftentimes conflicting, have been the subject of numerous narrative reviews. With the advancement of meta-analytic techniques, this rich body of work has been leveraged to pool results across individual studies, resulting in more highly powered analyses and precise calculation of effect sizes. Overall, results of the reviewed meta-analyses support evidence for adverse effects of cannabis use on neurocognitive functioning, which are detectable even after the effects of acute intoxication subside. This is supported by the results from meta-analyses of functional neuroimaging studies, which also provide evidence of differences in brain activity between cannabis users and non-users. However, these findings do not appear to translate to all clinical populations as the effects of cannabis use on neurocognitive performance remain mixed.
The results of the meta-analyses also shed some light on the pervasiveness of cannabis effects across neurocognitive domains and brain regions. This first meta-analysis conducted on this topic (Grant et al. 2003) identified significantly poorer performance among cannabis users only in the domains of learning and recall. Schreiner and Dunn (2012) and Scott et al. (2018) were able to make use of numerous additional studies published since Grant et al. (2003). Perhaps because of the increased power afforded to them, they found significant effects across a broader range of neurocognitive domains (i.e., motor, learning, recall, attention, processing speed, and executive functions). Taken together, their findings suggest that, with the exception of visuospatial and verbal abilities, cannabis use appears to be deleterious to neurocognitive functioning. Furthermore, across meta-analyses, decrements in the learning of new information appears to consistently have the largest effect sizes when compared to other neurocognitive domains. That said, it is important to highlight that across meta-analyses, overall and domain- level effect-sizes are relatively modest in magnitude and range between a quarter to a third of a standard deviation. Finally, the results from the meta-analyses suggest that recovery from the adverse effects of cannabis on neurocognition can be experienced within a month of abstinence, although recovery of functional brain alterations (as reported in the neuroimaging studies) may not occur. This is plausible, as differences in brain activation and connectivity patterns are often observed in the absence of differences in neurocognitive performance, suggesting possible compensatory mechanisms (Jager, Kahn, Van Der Brink, Van Ree, & Ramsey, 2006; Padula, Schweinsburg, & Tapert, 2007). Alternatively, functional brain alterations may display a different time-course for recovery, or may represent pre-existing differences among individuals who go on to use cannabis regularly.
Although results from the neuroimaging meta-analyses are consistent in reporting altered brain activation among cannabis users compared to non-users, there is a lack of consistency on the specific brain regions affected. Nonetheless, there appears to be evidence of substantial involvement in prefrontal regions and the insula. Several factors may contribute to the lack of consistency across the neuroimaging studies, including the relatively recent development of methods for meta-analyzing functional neuroimaging data, relatively fewer neuroimaging studies, and the use of various neurocognitive paradigms in the scanner during data acquisition.
Another area where consensus was not reached across meta-analyses is on evidence for greater detrimental effects on neurocognition with an earlier age of cannabis use onset. As previously discussed, there are numerous neurodevelopmental reasons why greater adverse effects would be expected if exogenous cannabinoids are introduced into the brain during a time of critical and rapid brain development. Several individual studies suggest that this is the case (Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Pope et al., 2001; Pope et al., 2003). However, the most comprehensive examination of this issue in a meta-analysis is that by Scott et al. (2018), which did not find evidence in support of this claim. At this time, it is prudent to say that a definitive conclusion has yet to be reached.
As we have noted, meta-analyses offer many advantages when synthesizing a body of research centered on a particular question. However, they are not without limitations and the overall conclusions of the current review must be interpreted with this in mind (Walker, Hernanadez, & Kattan, 2008). First, it is important to highlight that meta-analyses predominantly make use of data from published studies, which subject them to the “file drawer effect.” This refers to a known bias for studies with statistically significant results to be more likely to be published than those without significant results, which inflates the Type 1 error (i.e., false positive) rate (Rosenthal, 1979). Although methods to correct for this issue are often-times employed, it is difficult to know the true extent of publication bias. Secondly, although meta-analytic inclusion criteria are determined a priori in order to standardize study selection, some bias may enter study selection. More importantly, such criteria can serve to overly narrow the scope of the conclusions that can be made and limit generalizability. For example, such criteria may exclude based on participant age, use of other substances, methods of consumption, and neurocognitive task selection. Overly strict inclusion/exclusion criteria can result in samples of participants that do not accurately represent the average cannabis user. On the other hand, authors must be careful to avoid combining effect sizes from heterogeneous studies, as this may impact the validity of observed effects. Given that the neuroimaging meta-analyses combine data on functional brain activity across disparate neurocognitive tasks, they are likely more susceptible to these issues. Future functional imaging meta-analyses would benefit from examining effect sizes across studies that use the same neurocognitive task or tasks. Moreover, another source of variability across studies is the quantification and control of confounds. Finally, many outstanding meta-analyses were not captured within our inclusion criteria (Luijten et al., 2017; Potvin et al., 2008; & Yücel et al., 2010). We recommend that the reader explore these to gain a more integrated understanding of the effects of addiction on neurocognition.
Another important consideration is that the results from all but two of the meta-analyses presented here are from studies of otherwise healthy human subjects. Particularly with the growing accessibility to medical cannabis, it becomes even more important to understand if particular clinical populations may benefit or are harmed neurocognitively from use of cannabis. The meta-analyses by Rabin et al. (2011) and Bogaty et al. (2018) takes a step in this direction by specifically examining effects among individuals with schizophrenia and psychosis, respectively. Although these two meta-analyses were not the first to examine the effects of cannabis use on clinical populations, previous meta-analyses included individuals that did not currently use cannabis within their cannabis comparison group. Finally, it is important to consider that the majority of the studies included within the meta-analyses consisted of cross-sectional studies. Thus, attempts to examine changes in neurocognition that occur after onset of use or with abstinence are not evaluated in the meta-analyses at the within-subject level. Indeed, none of the evidence from the meta-analyses address causal relationships between cannabis use and neurocognition. This also limits conclusions that can be drawn from the meta-analyses pertaining to age of onset. Such issues are currently best addressed with large-scale longitudinal studies.
Longitudinal studies with large sample sizes and strong within-subject designs that begin before the onset of cannabis use can provide important information that is not gleaned from the extant meta-analytic literature. An important component of such studies is determining whether the neurocognitive differences reported between users and non-users predate cannabis use among participants. Longitudinal studies on cannabis use and neurocognitive functioning were recently reviewed (Gonzalez et al. 2017). The authors concluded that longitudinal studies revealed evidence of declines in neurocognitive functioning after initiation of cannabis use, that the magnitude of these effects are relatively modest and typically observed among the heaviest of users, and that control of numerous relevant confounds often attenuates the magnitude of observed effects. However, evidence emerging from the few twin studies conducted to date support evidence of shared risk factors (separate from cannabis use) that may account for the observed associations (Jackson et al., 2016; Meier et al., 2018). However, such studies are rare and may not have large numbers of heavy cannabis users that have a non-using twin-pair.
Currently, there are several projects funded through NIH aiming to tackle such goals. The Collaborative Research on Addiction (CRAN) is an initiative set forth to advance substance use, abuse, and addiction research. First funded in 2013, CRAN focuses on investigating genetic, epigenetic, molecular, neurobiological, behavioral, and environmental factors that underlie substance problems from epidemiological and clinical trials. One of the landmark initiatives under CRAN is the national Adolescent Brain Cognitive Development (ABCD) Study, which began in 2015 and aims to assess a large representative sample of over 11,000 youth longitudinally, with the goal of identifying what factors influence substance use trajectories. Participants began the study at nine to ten years of age and will be followed for ten years, through puberty and into young adulthood. The ABCD study makes use of brain, genetic, hormonal, developmental, social, cultural, neurocognitive, behavioral, health, and other relevant variables to understand how they interact over time to shape individual life trajectories (e.g., neurodevelopmental, neurocognitive, emotional, academic). The data from this project will be well-suited for uncovering factors that contribute to cannabis-associated neurocognitive declines, as well as their time course and the many factors that may influence their magnitude. For example, at this time, meta-analyses are not able to determine how different types of cannabis products of varying potency may differentially affect neurocognition. Individual studies will continue to pave the way for the testing of new hypotheses. When complemented with results from meta-analyses, findings from large-scale longitudinal studies will help depict a clearer picture of the replicability and robustness of observed effects.
Acknowledgments
This work was supported by grants R01 DA031176, R01 DA033156, and CNS-1532061 (PI: Gonzalez).
Footnotes
Disclosure
The authors report no conflict of interest.
References
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogue S, … & Martin-Santos R (2013). Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PloS One, 8(2), e55821. doi: 10.1371/journal.pone.0055821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest-Hopley G, Giampietro V, & Bhattacharyya S (2018). Residual effects of cannabis use in adolescent and adult brains—A meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 88, 26–41. [DOI] [PubMed] [Google Scholar]
- Blest-Hopley G, Giampietro V, & Bhattacharyya S (2019). Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: Results from a complementary meta-analysis. Neuroscience and Biobehavioral Reviews, 96, 45–55. doi: 10.1016/j.neubiorev.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaty SE, Lee RS, Hickie IB, & Hermens DF (2018). Meta-analysis of neurocognition in young psychosis patients with current cannabis use. Journal of Psychiatric Research, 99, 22–32. doi: 10.1016/j.jpsychires.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yuecel M, & Solowij N (2016). Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biological Psychiatry, 79(7), 557–567. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, & Munafò MR (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cohen R (2018, October). When adolescents give up pot, their cognition quickly improves. National Public Radio. Retrieved from https://www.nytimes.com/2018/10/27/style/cbd-benefits.html [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, & Gonzalez R (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. doi: 10.1007/s11065-012-9222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Mermelstein RJ, & Gonzalez R (2015). Neuropsychological sex differences associated with age of initiated use among young adult cannabis users. Journal of Clinical and Experimental Neuropsychology, 37(4), 389–401. doi: 10.1080/13803395.2015.1020770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, & Doody GA (2012). Effect of illegal substance use on cognitive function in individuals with a psychotic disorder, a review and meta-analysis. Neuropsychology, 26(6), 785. doi: 10.1037/a0029685 [DOI] [PubMed] [Google Scholar]
- Ganzer F, Broening S, Kraft S, Sack PM, & Thomasius R (2016). Weighing the evidence: A systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychology Review, 26(2), 186–222. doi: 10.1007/s11065-016-9316-2 [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Pacheco-Colón I, Duperrouzel JC, & Hawes SW (2017). Does cannabis use cause declines in neuropsychological functioning? A review of longitudinal studies. Journal of the International Neuropsychological Society, 23(9–10), 893–902. doi: 10.1017/S1355617717000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, & Wolfson T (2003). Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society, 9(5), 679–689. doi: 10.1017/S1355617703950016 [DOI] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, & Lukas SE (2012). Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors, 26(3), 496. doi: 10.1037/a0026269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018). US epidemiology of cannabis use and associated problems. Neuropsychopharmacology, 43(1), 195. doi: 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L (2003). Cannabis and the brain. Brain, 126(6), 1252–1270. doi: 10.1093/brain/awg143 [DOI] [PubMed] [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, & Baker LA (2016). Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proceedings of the National Academy of Sciences, 113(5), E500–E508. doi: 10.1073/pnas.1516648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, & F Tapert S (2014). Effects of cannabis on the adolescent brain. Current Pharmaceutical Design, 20(13), 2186–2193. doi: 10.2174/13816128113199990426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Kahn RS, Van Den Brink W, Van Ree JM, & Ramsey NF (2006). Long-term effects of frequent cannabis use on working memory and attention: An fMRI study. Psychopharmacology, 185(3), 358–368. doi: 10.1007/s00213-005-0298-7 [DOI] [PubMed] [Google Scholar]
- Kraan T, Velthorst E, Koenders L, Zwaart K, Ising HK, van den Berg D, ... & van der Gaag M (2016). Cannabis use and transition to psychosis in individuals at ultra-high risk: Review and meta-analysis. Psychological Medicine, 46(4), 673–681. doi: 10.1017/S0033291715002329 [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, & Fox PT (2005). ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25(1), 155–164. doi: 10.1002/hbm.20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, & Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society, 18(4), 678–688. doi: 10.1017/S1355617712000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM (2013). Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry, 4, 53. doi: 10.3389/fpsyt.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Alonso-Lana S, J Youssef G, Verdejo-Garcia A, Suo C, Cousijn J, ... & Solowij N (2016). Adolescent cannabis use: What is the evidence for functional brain alteration? Current Pharmaceutical Design, 22(42), 6353–6365. doi: 10.2174/1381612822666160805155922 [DOI] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, & Yücel M (2015). Cannabis and adolescent brain development. Pharmacology & Therapeutics, 148, 1–16. doi: 10.1016/j.pharmthera.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MW, & Sescousse G (2017). Disruption of reward processing in addiction: An image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry, 74(4), 387–398. doi: 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, & McGuire P (2010). Neuroimaging in cannabis use: A systematic review of the literature. Psychological Medicine, 40(3), 383–398. doi: 10.1017/S0033291709990729 [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S, Poulton R, & Moffitt T (2000). A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction, 95(4), 491–503. doi: 10.1046/j.1360-0443.2000.9544912.x [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, & Moffitt TE (2018). Associations between adolescent cannabis use and neuropsychological decline: A longitudinal co‐twin control study. Addiction, 113(2), 257–265. doi: 10.1111/add.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, ... & Stewart LA (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews, 4(1), 1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Conference of State Legislature. (2018, November 8). State Medical Marijuana Laws. Retrieved from http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- O’shea M, Singh ME, McGregor IS, & Mallet PE (2004). Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology, 18(4), 502–508. doi: 10.1177/0269881104047277 [DOI] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, & Tapert SF (2007). Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors, 21(4), 478. doi: 10.1037/0893-164X.21.4.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, & Yurgelun-Todd D (2001). Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry, 58(10), 909–915. doi: 10.1001/archpsyc.58.10.909 [DOI] [PubMed] [Google Scholar]
- Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, & Yurgelun-Todd D (2003). Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence, 69(3), 303–310. doi: 10.1016/S0376-8716(02)00334-4 [DOI] [PubMed] [Google Scholar]
- Potvin S, Joyal CC, Pelletier J, & Stip E (2008). Contradictory cognitive capacities among substance-abusing patients with schizophrenia: A meta-analysis. Schizophrenia Research, 100(1–3), 242–251. doi: 10.1016/j.schres.2007.04.022 [DOI] [PubMed] [Google Scholar]
- Quickfall J, & Crockford D (2006). Brain neuroimaging in cannabis use: A review. The Journal of Neuropsychiatry and Clinical Neurosciences, 18(3), 318–332. doi: 10.1176/jnp.2006.18.3.318 [DOI] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, & George TP (2011). The effects of cannabis use on neurocognition in schizophrenia: A meta-analysis. Schizophrenia Research, 128(1–3), 111–116. doi: 10.1016/j.schres.2011.02.017 [DOI] [PubMed] [Google Scholar]
- Radua J, Rubia K, Canales EJ, Pomarol-Clotet E, Fusar-Poli P, & Mataix-Cols D (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in Psychiatry, 5, 13. doi: 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, & Thorsteinsson EB (2008). Implicit cognition and substance use: A meta-analysis. Addictive Behaviors, 33, 1314–1328. doi: 10.1016/j.addbeh.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638. doi: 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- Schneider M (2008). Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction Biology, 13(2), 253–263. doi: 10.1111/j.1369-1600.2008.00110.x [DOI] [PubMed] [Google Scholar]
- Schreiner AM, & Dunn ME (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Experimental and Clinical Psychopharmacology, 20(5), 420. doi: 10.1037/a0029117 [DOI] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, & Gur RC (2018). Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry, 75(6), 585–595. doi: 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono Y, Edwards MC, Ames SL, & Stacy AW (2018). Trajectories of cannabis-related associative memory among vulnerable adolescents: Psychometric and longitudinal evaluations. Developmental Psychology, 54(6), 1148–1158. doi: 10.1037/dev0000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, & Christensen H (2011). Cannabis use and cognitive function: Eight year trajectory in a young adult cohort. Addiction, 106(12), 2195–2203. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, & Brown SA (2008). The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews, 1(1), 99–111. doi: 10.2174/1874473710801010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Monitoring the Future study, the University of Michigan (2016). Figures 3 and 4, Marijuana: Trends in annual and daily use in Grades 8, 10 and 12. University of Michigan News Service. Available at: http://www.monitoringthefuture.org/data/16data.html Accessed 22 November 2018. [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, & Baler R (2016). Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry, 73(3), 292–297. doi: 10.1001/jamapsychiatry.2015.3278 [DOI] [PubMed] [Google Scholar]
- Walker E, Hernandez AV, & Kattan MW (2008). Meta-analysis: Its strengths and limitations. Cleveland Clinic Journal of Medicine, 75(6), 431. doi: 10.3949/ccjm.75.6.431 [DOI] [PubMed] [Google Scholar]
- Williams A (2018, October 27) Why Is CBD Everywhere? The New York Times. Retrieved from https://www.nytimes.com/2018/10/27/style/cbd-benefits.html. [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, & Brown SA (2014). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society, 20(8), 784–795. doi: 10.1017/S1355617714000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes JA, Riedel MC, Ray KL, Kirkland AE, Bird RT, Boeving ER, & Sutherland MT (2018). Neuroimaging meta-analysis of cannabis use studies reveals convergent functional alterations in brain regions supporting cognitive control and reward processing. Journal of Psychopharmacology, 32(3), 283–295. doi: 10.1177/0269881117744995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, ... & McGorry PD (2010). The impact of cannabis use on cognitive functioning in patients with schizophrenia: A meta-analysis of existing findings and new data in a first-episode sample. Schizophrenia Bulletin, 38(2), 316–330. doi: 10.1093/schbul/sbq079 [DOI] [PMC free article] [PubMed] [Google Scholar]