Abstract
The control of calcium influx at the plasma membrane by endoplasmic reticulum (ER) calcium stores, a process common to invertebrates and vertebrates, is central to physiological calcium signalling and cellular calcium balance. STIM1 is a calcium sensor and regulatory protein localized to the ER. ORAI1 is a calcium channel in the plasma membrane (PM). In outline, STIM1 senses an ER-luminal calcium decrease, relocalizes to ER-PM junctions, and recruits and gates ORAI1 channels. Recent work, reviewed here, has offered detailed insight into the process of sensing and communicating ER calcium-store depletion, and particularly into the STIM1 conformational change that is the basis for communication between the ER and the PM.
Graphical Abstract
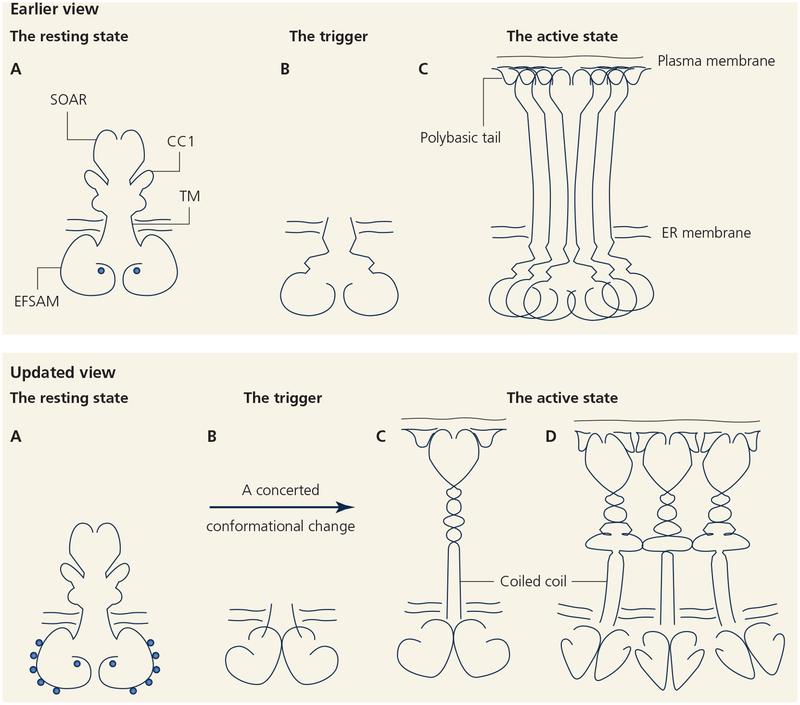
A shift in perspective on STIM calcium sensing and the mechanism of STIM activation. The ER-luminal domains of the calcium sensors, STIM1 and STIM2, bind multiple calcium ions per monomer in the resting state, and the cytoplasmic domains are folded back near the ER membrane. STIM proteins are activated when all bound calcium ions dissociate. This results in luminal domain dimerization and a concerted conformational change that triggers release of the resting CC1–SOAR/CAD interaction and extension of the STIM cytoplasmic domain. This model revises earlier proposed mechanisms of STIM activation that were centered around a single calcium-binding site per monomer and activation driven by unfolding and oligomerization of STIM ER-luminal domains.
Introduction
Electrophysiological studies carried out nearly thirty years ago established that the CRAC channel, now known to be an ORAI channel, is gated in response to physiological decreases in the level of ER calcium stores [Hoth and Penner 1992; Zweifach and Lewis 1993]. This finding framed two major questions about the cellular mechanisms that control the channel— first, how is a decrease in ER calcium sensed? and, second, how is a signal communicated from the ER to the PM CRAC channel? A first outline of the answers came in 2005 in the papers identifying STIM as an essential link in gating the channel— specifically, STIM has an EF-hand calcium sensor in the ER lumen, and STIM relocates to ER-PM junctions upon calcium-store depletion [Roos et al 2005; Liou et al 2005; Zhang et al 2005]. Work from many laboratories has since provided a more detailed picture. This review traces the experiments that led to the current understanding of the mechanism of STIM activation and notes some areas that remain to be explored.
STIM conserved regions and their functions
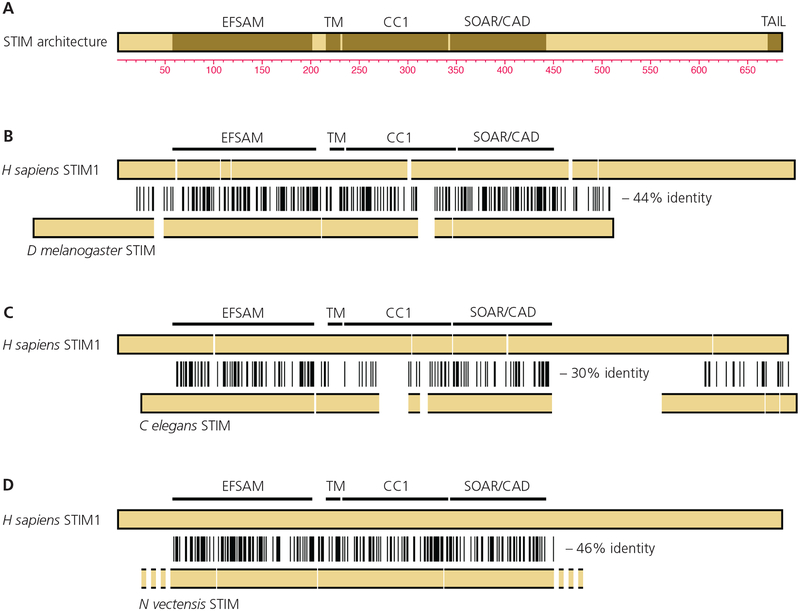
It may be helpful to begin by reviewing the evidence that physiological functions can be associated with specific domains or regions in STIM proteins [FIGURE 1A]. The ability to sense ER calcium depletion was assigned, as might have been expected, to the ER-luminal domain. A D76A replacement in the STIM1 luminal EF-hand or other mutations that would appreciably lower its affinity for calcium— in effect, making STIM insensitive to changes in calcium at concentrations encountered in the ER— rendered STIM1 constitutively active [Liou et al 2005; Zhang et al 2005; Spassova et al 2006]. Further reinforcing its connection to calcium sensing, the isolated luminal domain fragment STIM1(58–203) bound 45Ca with a Kd roughly estimated as 600 μM, and responded with structural alterations detected by measurements of intrinsic fluorescence and far-UV circular dichroism (CD) to changes in calcium in the concentration range 100 μM–1 mM [Stathopulos et al 2006]. The NMR structures of calcium-bound STIM1 and STIM2 luminal domains revealed paired EF-hands, only one of which binds calcium, embracing a sterile α motif (SAM) domain in much the way calmodulin EF-hands embrace their targets [Stathopulos et al 2008; Zheng et al 2011]. STIM1(58–203) is therefore referred to as ‘EFSAM’. The isolated STIM1 luminal fragment aggregated in the absence of calcium, precluding structure determination of the calcium-free form with that protein construct. The structure of the calcium-free active conformation of the STIM luminal domain still has not been solved.
FIGURE 1.
STIM functional organization and sequence alignments.
A, STIM protein domains or regions discussed in the text. The EFSAM domain in the ER lumen is connected by the transmembrane helix with the CC1 region, SOAR/CAD, and polybasic tail in the cytoplasm. The residue numbering is for human STIM1.
B, Alignment of D melanogaster STIM with human STIM1. The locations of EFSAM, the transmembrane helix, CC1, and SOAR/CAD in human STIM1 are marked. Identities are indicated by vertical lines, and gaps have been introduced as necessary to maintain the alignment.
C, Alignment of C elegans STIM with human STIM1.
D, Alignment of N vectensis STIM with human STIM1. The N- and C-terminal sequences of N vectensis STIM have not been annotated.
In (B)–(D), the stated percent identity refers to the region from EFSAM through SOAR/CAD.
The STIM domain that communicates with the CRAC channel was identified in two steps. An initial study found that the cytoplasmic region of STIM, STIM1CT, activates ORAI channels when expressed in cells [Huang et al 2006]. STIM1CT lacks the STIM1 luminal domain and transmembrane segment, and hence it is a soluble cytoplasmic protein, unregulated by store depletion. Engineered truncations then delineated the minimal region that gated ORAI channels as STIM1(344–442), termed SOAR (STIM1 Orai activating region) or CAD (CRAC activation domain) [Yuan et al 2009; Park et al 2009; Kawasaki et al 2009]. SOAR/CAD or other STIM cytoplasmic fragments decorated the PM if and only if ORAI was coexpressed at sufficient levels [Muik et al 2008; Yuan et al 2009; Park et al 2009; Muik et al 2009; Kawasaki et al 2009; Zhou et al 2010]. Moreover, FRET was observed between appropriately labelled ORAI1 and STIM1 after store depletion, and constitutive FRET occurred between ORAI1 and SOAR/CAD or other STIM1 cytoplasmic fragments [Muik et al 2008; Barr et al 2008; Navarro-Borelly et al 2008; Calloway et al 2009]. These observations implied that STIM binds either to ORAI or to a closely associated protein. Later, the functional reconstitution of calcium flux with recombinant STIM1CT and ORAI1 confirmed that a direct STIM–ORAI interaction underlies channel gating [Zhou et al 2010; Gudlur et al 2014].
The protein-protein interaction filled in only a part of the picture. STIM is anchored in the ER, and ORAI is in the PM, so the two can communicate directly only at close appositions of the two membranes termed ‘ER-PM junctions’. Because the ER–PM spacing is ~15 nm [Wu et al 2006], and because STIM was able to gate even channels with short ORAI cytoplasmic segments such as those formed by the truncated ORAI1(65–301) [Zhou et al 2010], STIM had to extend from the ER to position SOAR/CAD near the PM. It was recognized early that the predicted coiled coil 1 region (CC1) would be of sufficient length to place SOAR/CAD in the vicinity of the PM, and demonstrated later that an artificial spacer of 30 glycine residues between the transmembrane helix and SOAR/CAD sufficed to allow channel activation [Fahrner et al 2014]. It was less obvious initially that CC1 makes an important regulatory contribution to maintenance of the inactive state of STIM [Korzeniowski et al 2010; Muik et al 2011; Yang et al 2012; Zhou et al 2013; Fahrner et al 2014], a point considered in detail below.
The last region in this list is a short polybasic segment near the STIM C terminus that targets STIM to ER-PM junctions, presumably by interacting with PM lipids [Liou et al 2007]. PM phosphoinositides have been favored candidates for this STIM interaction, but the issue is not settled [Liou et al 2007; Ercan et al 2009; Korzeniowski et al 2009; Walsh et al 2010]. Early studies did not support a major role for STIM plasma-membrane targeting in STIM–ORAI signalling, since deletion of the polybasic tail had at most a modest effect of slowing the kinetics of ORAI channel activation when STIM and ORAI were overexpressed [Li et al 2007]. This observation was explained by the subsequent finding that STIM can also be targeted to ER-PM junctions solely by its interaction with overexpressed ORAI [Park et al 2009]. It is plausible that the targeting to PM lipids might be more consequential at native levels of STIM and ORAI expression, which are much lower than in overexpression experiments, but whether this is true has not been determined. An alternative role for the polybasic tail might be in directing STIM–ORAI complexes to PM lipid subdomains within the ER-PM junction [Maléth et al 2014].
Drosophila melanogaster and Caenorhabditis elegans store-dependent CRAC currents [Yeromin et al 2004; Lorin-Nebel et al 2007] have figured prominently in the investigation of the STIM–ORAI pathway. RNAi screening in D melanogaster cells linked STIM and ORAI to calcium entry [Roos et al 2005; Zhang et al 2005; Feske et al 2006; Zhang et al 2006; Vig et al 2006], and engineered proteins from both species have yielded structural insights [Yang et al 2012; Hou et al 2012]. EFSAM and SOAR/CAD domains are easily recognized in D melanogaster STIM and C elegans STIM, which exhibit 30%–44% pairwise sequence identity with the human STIM sequence spanning from EFSAM through SOAR/CAD [FIGURE 1B, 1C]. Within the central region, parts of CC1 are less well conserved, and the N and C termini are least conserved. The polybasic tail is absent in D melanogaster STIM, but a polybasic segment is present at the C terminus in the conceptually translated STIM sequences of some insects and of C elegans. Since these different STIM and ORAI proteins have been cited to draw conclusions about STIM and ORAI mechanisms, it is critical to ask how closely the mechanisms of STIM activation are conserved across the species despite the sequence differences. We will return later to this question.
Early views on the basis for STIM activation
Historically, inferences were drawn about the mechanism leading to STIM activation from the effects of engineered mutations or truncations on STIM relocalization to ER-PM junctions. The initial finding that either store depletion or a D76A mutation in the STIM1 EF-hand resulted in STIM1 relocalization to ER-PM junctions was taken as evidence for a conformational change—
‘In a plausible model, the loss of luminal Ca2+ binding by STIM proteins triggers a conformational change that leads to its translocation …’
With time there was a blurring between a mechanism dependent on STIM1 conformational change and a mechanism driven by STIM1 oligomerization. The facts that FRET between N-terminally labelled CFP-STIM1 and YFP-STIM1 increased before STIM relocalization to ER-PM junctions, and that STIM1ΔK lacking the polybasic tail exhibited a FRET change without relocalization, were interpreted as indicating that
‘The oligomerization of STIM1 exposes a C-terminal polybasic PM-targeting motif, which leads to the recruitment of STIM1 oligomers to nearby ER–PM junctions …’
Subsequent hypotheses on the STIM activation mechanism incorporated oligomerization, but differed in whether the oligomerization by itself was proposed to render STIM active, for example by increasing the avidity for PM lipids [Collins and Meyer 2011], or whether oligomerization was thought to trigger a change in conformation from inactive STIM to active STIM [Korzeniowski et al 2010; Muik et al 2011].
The ready acceptance that STIM luminal domain oligomerization was a primary event in activation can be traced to three lines of evidence. The increased FRET between STIM luminal domains in cells after calcium store depletion, which took place within seconds, could plausibly be explained by oligomerization [Liou et al 2007; Muik et al 2008; Covington et al 2010]. Aggregates of isolated EFSAM in vitro in the absence of calcium, detected by size-exclusion chromatography–multi-angle light scattering (SEC–MALS) and in negatively stained samples by electron microscopy, could be interpreted as a protein-biochemical correlate of oligomerization [Stathopulos et al 2006; Stathopulos et al 2008]. And, in a seemingly decisive test, the artificial oligomerization of STIM1 dimers in the ER, by chemically induced crosslinking of FKBP12 and FRB domains engineered in place of the STIM luminal domain, activated STIM1 [Luik et al 2008].
In retrospect there were grounds to question whether luminal domain oligomerization was the physiological driver of initial activation. The truncated protein STIM1ΔC, consisting of the STIM1 luminal domain and transmembrane segment only, localized to the ER when expressed in cells, and CFP-STIM1ΔC and YFP-STIM1ΔC exhibited FRET after store depletion [Covington et al 2010]. However, the FRET was not above the level observed for full-length STIM1 before store depletion— inclusion of SOAR/CAD in the protein construct was necessary to achieve higher levels of FRET— suggesting that the STIM1 luminal domain on its own might not oligomerize above the level of STIM1 in resting cells. At the protein level, the only nonaggregated oligomeric form of isolated EFSAM observed in vitro was the dimer [Stathopulos et al 2006; Stathopulos et al 2008; Furukawa et al 2014]. And the size of the isolated EFSAM aggregates visualized by electron microscopy— 10–100 nm or larger [Stathopulos et al 2008]— implied an improbably large number of ~3 nm-diameter monomers.
There was a possible alternative source of the STIM–STIM FRET signal. Since resting STIM is a constitutive dimer through SOAR/CAD–SOAR/CAD pairing in the cytoplasm [Muik et al 2009; Yuan et al 2009; Covington et al 2010; Yang et al 2012], and EFSAM is able to undergo a monomer-dimer transition controlled by a reduction in calcium, it was conceivable that store depletion brought about increased FRET through an intradimer rearrangement of the luminal domains. This possibility was tested in recent experiments with a chimera containing the STIM1 luminal domain, transmembrane helix, and CC1, but with SOAR/CAD replaced by a distinct dimerizing domain (GrpE) [Gudlur et al 2018]. The GrpE dimer does not further oligomerize in vitro, yet the chimera displayed store-dependent FRET in cells comparable to that of wildtype STIM1, strongly supporting the idea that an intradimer rearrangement, rather than oligomerization, is the initial step in activation. Of course oligomerization— not necessarily driven by the luminal domains— could still make a separate contribution to the STIM–STIM FRET signal observed in cells. For example, the portion of the STIM–STIM FRET change that occurs within 5 s [Liou et al 2007] could reflect mainly luminal domain rearrangement, whereas the later and smaller FRET change might reflect mainly oligomerization. A reassessment of the FKBP12–FRB oligomerization result [Luik et al 2008] concluded that it was compatible with interpretations that STIM activation is driven by intradimer crosslinking, or by interdimer crosslinking, or by both [Prakriya and Lewis 2015]. Given the evidence that STIM oligomerization, like constitutive STIM dimerization, is through SOAR/CAD–SOAR/CAD interactions [Covington et al 2010; Fahrner et al 2014], the most reasonable interpretation is that the initial step in activation exposes the relevant surfaces of SOAR/CAD, and any oligomerization is a subsequent step.
A concerted STIM conformational change
The findings that single mutations or small clusters of mutations could activate STIM [Korzeniowski et al 2010; Muik et al 2011] were most easily interpreted in terms of a STIM conformational change. The constitutive activity of an 318EEELE322 > AAALA mutant STIM1 led to the proposal that residues 318–322 of wildtype CC1 might interact with SOAR/CAD and damp its activity in resting cells [Korzeniowski et al 2010]. A STIM structure that included parts of C elegans CC1 and SOAR/CAD prompted the suggestion that a larger inhibitory helix, residues 310–337 in human STIM1, which contains the EEELE segment, might interact with SOAR/CAD in resting cells and release SOAR/CAD upon physiological stimulation [Yang et al 2012]. However, whatever the role of this region in the overall STIM conformational change, residues 318–322 or 310–337 are not part of the principal CC1–SOAR/CAD interface underlying the inactive–active transition in human STIM1, as discussed in detail in [Zhou et al 2013; Fahrner et al 2014].
The discovery and characterization of the constitutively active L251S and L248S mutants [Muik et al 2011] directed attention to the N-terminal part of CC1, which is immediately adjacent to the ER membrane in full-length STIM1. Introduction of either mutation into STIM1 led to constitutive currents through coexpressed ORAI1 channels. Likewise, the cytoplasmic fragment STIM1(233–474), or OASF, with either replacement was physically extended compared to wildtype OASF, as assessed in cells by YFP-OASF-CFP FRET measurements, and it exhibited increased binding to ORAI1. Quantitative distance measurements on isolated recombinant STIM1CT, by Tb3+–acceptor resonance energy transfer, showed that wildtype STIM1CT was folded back on the base of CC1 in a way that would keep SOAR/CAD near the ER membrane in cells. On the other hand, STIM1CT carrying an L251S replacement was extended by a minimum of 4–5 nm, which could place SOAR/CAD near the PM in cells [Zhou et al 2013]. The extended conformation was not a result of oligomerization, since size-exclusion chromatography established that neither STIM1(234–491)-L251S nor STIM1CT-L251S oligomerized beyond the constitutive dimer [Muik et al 2011; Zhou et al 2013].
The evidence that the extended configuration of STIM1-L251S was an active conformation of STIM naturally raised the question whether and how wildtype STIM1 could be induced to assume a similar extended configuration. The answer came in experiments where physical extension of the purified recombinant cytoplasmic domain STIM1CT was triggered in vitro [Zhou et al 2013]. The CC1 N termini in folded-back wildtype STIM1CT were not close together, as determined in FRET measurements, but bringing them together by crosslinking engineered N-terminal cysteine residues drove wildtype STIM1CT into an extended conformation. The mechanism was further dissected by examining subfragments of STIM1CT. Purified CC1 by itself was partially helical and partially unstructured, and monomeric. Forced dimerization, by crosslinking CC1 monomers through engineered N-terminal cysteines, increased the CC1 α-helical structure detected by CD measurements. The accompanying appearance of a cooperative thermal melting transition not observed with the monomer, along with other data, suggested the assembly of a coiled coil spanning the initial part of CC1. Whereas the CC1 monomer bound to MBP-SOAR/CAD, the forced dimer showed only weak binding. This diminished CC1–SOAR/CAD interaction when CC1 was crosslinked at the N terminus was proposed to be a direct correlate of the release of SOAR/CAD and the physical extension of the cytoplasmic domain elicited by crosslinking the N termini of STIM1CT.
Importantly, release of noncovalently bound SOAR/CAD from CC1 was also triggered physiologically by store depletion, in cells coexpressing a STIM ER-membrane-bound fragment truncated within or at the end of CC1 and a soluble SOAR/CAD fragment [Ma et al 2015; Korzeniowski et al 2017]. The two fragments were closely associated in resting cells, as judged by FRET between the fluorescently labelled STIM fragments or by SOAR/CAD localization to the ER, but came apart upon store depletion. Engineered mutations in the CC1 construct, together with data from earlier experiments, defined residues L248, L251, L258, and L261 in CC1 as part of the CC1–SOAR/CAD interface [Muik et al 2011; Ma et al 2015]. Similarly, L416, V419, and L423 in SOAR/CAD were implicated in the interface [Muik et al 2011; Ma et al 2015]. The possibility that other regions of SOAR/CAD participate in the CC1–SOAR/CAD interaction was not tested [Ma et al 2015].
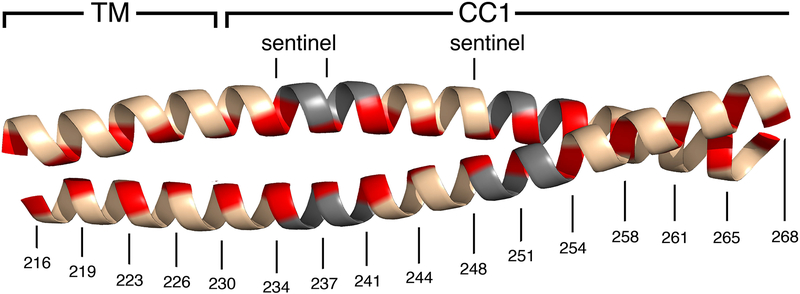
The last critical element of the mechanism was put in place with a direct demonstration that store depletion does, in fact, bring together the STIM CC1 segments. STIM1-A230C in isolated ER membranes could be efficiently disulfide crosslinked at the introduced cysteine in the absence of calcium, but not in the presence of 2 mM calcium [Hirve et al 2018]. The concentration dependence of crosslinking matched what had been reported for STIM1 activation in cells [Brandman et al 2007; Luik et al 2008]. The crosslinking approach was then applied to a panel of STIM1 proteins with single cysteines engineered into the transmembrane and CC1 regions, in order to demarcate the residues that come into close apposition after loss of calcium from STIM1. The experiments documented calcium depletion-dependent assembly of a coiled coil in the region adjacent to the ER membrane, extending at least to the CC1–SOAR/CAD interface at residues 248 and 251 [Hirve et al 2018]. In silico predictions suggested that the coiled coil would extend farther, to residue 268, although this prediction was not tested experimentally. There were imperfections in the STIM1 heptad repeat that would arguably facilitate the switch between inactive and active conformations, and ‘sentinel’ residues that were permissive for coiled-coil formation when it was directed by EFSAM–EFSAM pairing but that imposed a sufficient energy barrier to prevent spontaneous activation [Hirve et al 2018] [FIGURE 2]. The barrier imposed by sentinel residues was convincingly illustrated by the effect of the combined N234L/S237L replacements. Residues N234 and S237 are not part of the CC1–SOAR/CAD interface, since the N234L/S237L replacements did not interfere with the CC1–SOAR/CAD interaction when assayed in the isolated STIM1CT fragment. Yet the same replacements— designed to perfect the active-state coiled coil in the initial part of CC1— tipped the balance in favor of SOAR/CAD release in full-length STIM1.
FIGURE 2.
Cartoon representation of the transmembrane helices (TM) and the initial portions of CC1 pairing in a coiled coil, as in the STIM active conformation. The coiled-coil core residues (red), discontinuities in the heptad repeat (grey), and sentinel residues are marked. These features are conserved across most invertebrates and all vertebrates, and collectively constitute the hallmarks of an ancestral STIM activation mechanism [Hirve et al 2018]. The cartoon does not portray the local distortions in the coiled coil that will necessarily occur at the heptad discontinuities. Residue numbering for human STIM1 is shown for reference. The figure was created using PyMOL [Schrödinger LLC 2010].
Tying together these converging threads, SOAR/CAD and the polybasic tail are tethered near the ER at rest. STIM activation is initiated by association (or reorientation) of the paired luminal domains within a preexisting STIM dimer. This step is coupled to coiled-coil formation in the transmembrane helices and the initial region of CC1, releasing SOAR/CAD and the polybasic tail. The initial conformational change presumably also exposes the surfaces that underpin the CC3–CC3 interactions characterized by [Fahrner et al 2014] and that could support higher-order oligomerization of STIM at a subsequent step. At native levels of STIM1, it is unclear whether oligomerization occurs deep in the ER or only as STIM1 becomes concentrated in ER-PM junctions.
The STIM activation mechanism described here has an ancient origin. Its signature is imprinted in the CC1 sequence of the starlet sea anemone Nematostella vectensis— an invertebrate whose lineage diverged from the vertebrate lineage ~600 million years ago— and in the CC1 sequence across a broad range of invertebrates [Hirve et al 2018]. Simple aligment of the N vectensis STIM sequence with the human STIM1 sequence shows 46% identity in the central EFSAM–SOAR/CAD sequences [FIGURE 1D]. However, the conclusion that the activation mechanism is preserved across most invertebrate lineages is not based on sequence identity— there is considerable sequence variability in CC1 across species— but rather on the precisely conserved pattern of predicted coiled-coil core residues, placement of heptad discontinuities, and recognizable sentinel residues. Nematodes contrast with most invertebrates in having a lineage-specific pattern of predicted coiled-coil residues at the beginning of CC1. This lineage-specific pattern is arguably still consistent with a mechanism in which EFSAM–EFSAM dimerization brings the CC1 segments together in a coiled coil, but it suggests that activation might be implemented differently in detail. Some caution is therefore appropriate in extrapolating findings about the C elegans STIM mechanism to mammalian STIM1 and STIM2.
Sensing the level of ER-luminal calcium
The narrative to this point makes it clear that luminal domain dimerization upon ER calcium-store depletion is critical in initiating STIM activation. What can we say regarding the structural basis for dimerization? One specific proposal, pertaining to isolated EFSAM and based on indirect evidence, is that a small structured dimer interface exists in an otherwise poorly structured EFSAM region [Furukawa et al 2014]. An alternative possibility would be that EFSAM is stabilized in full-length STIM and less subject to unfolding, and therefore might have a more extensive dimer interface. This alternative has been tested using a fusion protein in which EFSAM was linked to the long paired N-terminal helices of Thermus thermophilus GrpE, arguably in a similar configuration to full-length STIM in the ER except that the EFSAM-GrpE fusion protein was soluble [Gudlur et al 2018]. This maneuver apparently stabilized the structure of calcium-free EFSAM. In previous estimates from CD measurements, the α-helix content of isolated EFSAM fell upon removal of calcium from 61% > 15% [Stathopulos et al 2008] or from 50% > 12% [Furukawa et al 2014]. In contrast, CD measurements indicated that the EFSAM domain within EFSAM-GrpE retained nearly all of its α-helical secondary structure in the absence of calcium [Gudlur et al 2018]. Furthermore, hydrogen-deuterium exchange–mass spectrometry (HDX–MS) found that there was actually less deuterium exchange in the absence of calcium across nearly the entire EFSAM domain, which is inconsistent with protein unfolding. Instead, because the protection of backbone amides from hydrogen-deuterium exchange in the calcium-bound form is due in large part to helix-backbone hydrogen bonds, the HDX–MS result buttresses the conclusion that the helical regions retain their structure upon loss of calcium. It remains arguable that neither isolated EFSAM nor EFSAM-GrpE perfectly replicates the conformational change of full-length STIM. However, the experiments on EFSAM-GrpE establish that EFSAM can undergo a conformational change between two well folded structures upon loss of calcium, and, as described below, that the conformational change leads to increased EFSAM–EFSAM FRET.
An unexpected offshoot of the experiments characterizing EFSAM-GrpE was that the EFSAM monomer had 5–6 calcium-binding sites, detectable by isothermal titration calorimetry (ITC) and independently by a fluorescence competition assay for calcium binding [Gudlur et al 2018]. A single high-affinity site per EFSAM-GrpE monomer— presumably the EF-hand— bound calcium at low micromolar concentrations, and the other sites bound calcium in the range of ER-luminal calcium concentrations. EFSAM-GrpE N-terminally labelled with fluorescein and Alexa Fluor 594 showed an increase in intradimer FRET when calcium was reduced to low micromolar concentrations [Gudlur et al 2018]. The simplest interpretation is that the EF-hand site controls the conformational change both in EFSAM-GrpE and in full-length STIM, but that the concentration dependence for calcium binding to the EF-hand is shifted to lower calcium concentrations in EFSAM-GrpE because the simpler construct uncouples calcium binding from a concerted active > inactive conformational change of the full-length STIM protein. This interpretation and an alternative interpretation have been fully discussed in [Gudlur et al 2018]. Mutational analyses indicated that the additional calcium-binding sites regulated calcium sensing by full-length STIM1. The multiple binding sites could offer a simple explanation for the steep calcium concentration dependence of STIM activation in cells, whose Hill coefficient is in excess of what could be explained by a dimer-monomer transition [Brandman et al 2007; Luik et al 2008]. Cooperative calcium regulation of the dimer-monomer transition of isolated EFSAM was observed previously, but a complicated explanation was proposed to reconcile the observations with the belief that there was a single calcium-binding site per EFSAM monomer [Furukawa et al 2014]. The recent data offer a simpler explanation. There is still important work to be done in this area, since calcium binding has been measured only in isolated EFSAM and in EFSAM-GrpE, not in full-length STIM.
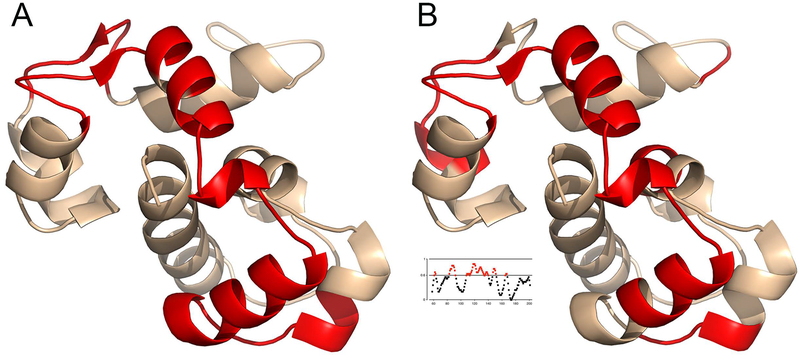
Does the EFSAM protein sequence itself carry the fingerprints of an ancestral calcium-sensing mechanism? The question cannot be addressed by looking for the hallmarks of a defining conformational change, as for the conformational extension of STIM cytoplasmic domain, since the calcium-free EFSAM structure and the conformational change underlying EFSAM–EFSAM dimerization have yet to be determined. It is nonetheless possible to give a tentative answer. Mapping separately the regions in EFSAM linked experimentally to calcium signalling and the segments of EFSAM where sequence identities between H sapiens STIM1 and N vectensis STIM are clustered reveals a remarkable correspondence [FIGURE 3]. Thus the detailed calcium-sensing mechanism, too, may have an ancient origin.
FIGURE 3.
Tentative evidence that the calcium-sensing mechanism is ancestral.
A, Regions experimentally linked to function in human EFSAM [PDB ID 2K60]. Red highlighting marks the EF-hands, where mutations are known to disrupt calcium sensing [Liou et al 2005; Zhang et al 2005; Spassova et al 2006; Stathopulos et al 2008], and the EFSAM segments most affected by the presence or absence of calcium in HDX–MS experiments [Gudlur et al 2018].
B, Segments of EFSAM with a notable clustering of sequence identities between H sapiens STIM1 and N vectensis STIM. H sapiens–N vectensis identities were mapped as a binary function to human EFSAM and smoothed with a triangle function of width 9, which amounts to assigning each residue in EFSAM a weighted sum of the binary map values that fall within four residues. Red highlighting marks residues for which this weighted sum equals or exceeds 0.6 [see inset].
The figure was created using PyMOL [Schrödinger LLC 2010].
Concluding note
STIM calcium sensing and STIM activation are the core mechanisms controlling STIM–ORAI signalling. This review has outlined our current understanding of these initial steps in store-dependent calcium entry [FIGURE 4], and has highlighted a few remaining questions regarding the STIM luminal-domain conformational change and STIM cytoplasmic-domain oligomerization. Parallel advances have delineated the mechanisms of STIM–ORAI interaction at ER-PM junctions. With a portrait of the basic machinery nearly complete, future challenges will be to determine how STIM–ORAI-dependent calcium entry is modulated, globally and locally, and how it is coordinated with other cellular processes.
FIGURE 4.
Two models for the STIM1 activation mechanism.
Upper, An earlier view. A, The resting state of STIM has a well structured ER-luminal (EFSAM) domain with calcium bound at a single EF-hand site. B, Store depletion triggers loss of calcium from the single calcium-binding site and unfolding of the luminal domain. C, Oligomerization of the unfolded EFSAM domains drives higher-order oligomerization of STIM and relocalization to ER-PM junctions.
Lower, An updated view based on the findings reviewed here. A, The resting state of STIM has a well structured EFSAM domain with 5–6 calcium-binding sites. The cytoplasmic domain is folded back on the CC1 region. B and C, store depletion triggers a concerted change in STIM involving dissociation of calcium from all EFSAM sites, rearrangement of the paired luminal domains to a well folded dimer, and assembly of a coiled coil in the initial region of CC1. The SOAR/CAD domain and polybasic tail are released from the restraints that hold them near the ER membrane in resting STIM, and the STIM cytoplasmic domain extends toward the PM. D, Extended STIM dimers may oligomerize further through interactions of their cytoplasmic domains.
FUNDING
This work was funded by National Institutes of Health grants AI084167, AI040127, and AI109842. AEZ was supported in part by postdoctoral fellowship 2016/12505–8 from the São Paulo Research Foundation (FAPESP).
Footnotes
COMPETING INTERESTS
PGH is a founder of CalciMedica, Inc, and a member of its scientific advisory board. CalciMedica had no voice in writing this review, and does not stand to profit from its publication, but we are disclosing this affiliation in accordance with NIH guidelines. The other authors declare no competing interests.
REFERENCES
- Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ, Sommers CL, Oh-hora M, Rao A, Samelson LE (2008) Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai 1 in activated T-cells: puncta and distal caps. Mol Biol Cell 19, 2802–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloway N, Vig M, Kinet JP, Holowka D, Barid B (2009) Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Bil Cell 20, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SR, Meyer T (2011) Evolutionary origins of STIM1, and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol 21, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington ED, Wu MM, Lewis RS (2010) Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell 21, 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M (2009) A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic 10, 1802–1818. [DOI] [PubMed] [Google Scholar]
- Fahrner M, Muik M, Schindl R, Butorac C, Stathopulos P, Zheng L, Jardin I, Ikura M, Romanin C (2014) A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1). J Biol Chem 289, 33231–33244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Teraguchi S, Ikegami T, Dagliyan O, Jin L, Hall D, Dokholyan NV, Namba K, Akira S, Kurosaki T, Baba Y, Standley DM (2014) Intrinsic disorder mediates cooperative signal transduction in STIM1. J Mol Biol 426, 2082–2097. [DOI] [PubMed] [Google Scholar]
- Gudlur A, Quintana A, Zhou Y, Hirve N, Mahapatra S, Hogan PG (2014) STIM1 triggers a gating rearrangement at the extracellular mouth of the ORAI1 channel. Nat Commun 5:5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudlur A, Zeraik AE, Hirve N, Rajanikanth V, Bobkov AA, Ma G, Zheng S, Wang Y, Zhou Y, Komives EA, Hogan PG (2018) Calcium sensing by the STIM1 ER-luminal domain. Nat Commun 9:4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirve N, Rajanikanth V, Hogan PG, Gudlur A (2018) Coiled-coil formation conveys a STIM1 signal from ER lumen to cytoplasm. Cell Rep 22, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB (2012) Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF (2006) STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol 8, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Lange I, Feske S (2009) A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun 385, 49–54 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T (2009) Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem 284, 21027–21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Manjarrés IM, Varnia P, Balla T (2010) Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal 3, ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Wisniewski E, Baird B, Holowka DA, Balla T (2017) Molecular anatomy of the early events in STIM1 activation – oligomerization or conformational change? J Cell Sci 130, 2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lu J, Xu P, Xie X, Chen L, Xu T (2007) Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem 282, 29448–29456. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T (2007) Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA 104, 9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin-Nebel C, Xing J, Yan X, Strange K (2007) CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologues and is essential for ovulation and fertility. J Physiol 580, 67–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS (2008) Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454, 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P, Li S, Sun A, Bi Y, Zhong L, Si H, Shen Y, Li M, Lee MS, Zhou W, Wang J, Wang Y, Zhou Y (2015) Inside-out Ca2+ signalling prompted by STIM1 conformational switch. Nat Commun 6:7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maléth J, Choi S, Muallem S, Ahuja M (2014) Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat Commun 5:5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C (2008) Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 283, 8014–8022. [DOI] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C (2009) A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem 284, 8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Rominin C (2011) STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J 30, 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS (2015) Store-operated calcium channels. Physiol Rev 95, 1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger LLC (2010) The PyMOL molecular graphics system, version 1.4.
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL (2006) STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA 103, 4040–4045 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M (2006) Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem 281, 35855–35862. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M (2008) Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135, 110–122. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD (2010) Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J 425, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Jin H, Cai X, Li S, Shen Y (2012) Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 109, 5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Roos J, Stauderman KA, Cahalan MD (2004) A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol 123, 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S (2009) SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD (2006) Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA 103, 9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Stathopulos PB, Schindl R, Li GY, Romanin C, Ikura M (2011) Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci USA 108, 1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG (2010) STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol 17, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos P, Ikura M, Rao A, Hogan PG (2013) Initial activation of STIM1, the regulator of store-operated calcium entry. Nat Struct Mol Biol 20, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS (1993) Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA 90, 6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]